Abstract
The compulsive, habitual behaviors that have been observed in individuals diagnosed with substance use disorders may be due to disruptions in the neural circuits that mediate goal-directed actions. The endocannabinoid system has been shown to play a critical role in habit learning, but the role of this neuromodulatory system in habit expression is unclear. Here, we investigated the role of the endocannabinoid system in established habitual actions using contingency degradation in male C57BL/6 mice. We found that administration of the endocannabinoid transport inhibitor AM404 reduced habitual responding for food and that antagonism of cannabinoid receptor type 1 (CB1), but not transient receptor potential cation subfamily V (TRPV1), receptors produced a similar reduction in habitual responding. Moreover, pharmacological stimulation of CB1 receptors increased habitual responding for food. Co-administration of an enzyme inhibitor that selectively increases the endocannabinoid 2-arachidonoyl glycerol (2-AG) with AM404 partially restored habitual responding for food. Together, these findings demonstrate an important role for the endocannabinoid system in the expression of habits and provide novel insights into potential pharmacological strategies for reducing habitual behaviors in mental disorders.
Keywords: AM404, 2-arachidonoyl glycerol, CB1 receptor, Mice, Goal-directed behaviors
Graphical Abstract
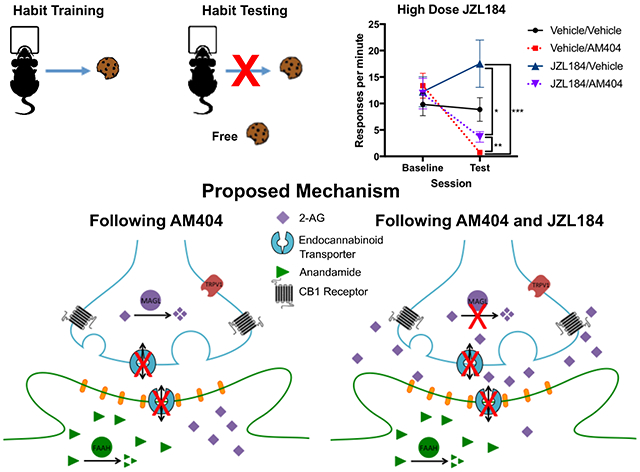
Mice were trained to habitually respond for food. Administration of the putative endocannabinoid transporter inhibitor, AM404, decreased habitual responding. This effect of AM404 was abrogated by pre-treatment with JZL184, a catabolic enzyme inhibitor that increases synaptic levels of the endocannabinoid 2-arachidonoyl glycerol. We propose that the mechanism by which AM404 reduces habitual responding is through the prevention of endocannabinoid release.
Introduction
Habitual actions are routine behaviors that are automatized, taken in response to antecedent environmental stimuli, and insensitive to changes in the desirability of the outcome. Conversely, goal-directed behaviors are deliberately taken for desirable outcomes and mediated by knowledge of the causal relationship between the action and outcome. Individuals with substance use disorders (SUD), including alcohol, methamphetamine, and cocaine use disorders, have been observed to have deficiencies in goal-directed behavior (Sebold et al., 2014; Voon et al., 2015; Ersche et al., 2016; McKim et al., 2016). A transition from goal-directed to habitual responding for drugs of abuse is thought to contribute to the compulsive patterns of drug-seeking and taking that are characteristic of SUD (Jentsch & Taylor, 1999; Ostlund & Balleine, 2008; Barker & Taylor, 2014; Everitt & Robbins, 2016). Exposure to drugs of abuse, including amphetamine, alcohol, or cannabis, can engender faster formation of habits (Nelson & Killcross, 2006; Nazzaro et al., 2012; Renteria et al., 2018). Identification of pharmacological tools that are able to reduce habitual and subsequently improve goal-directed control of behavior would, therefore, have substantial therapeutic benefit for disorders in which compulsive, habitual actions are a primary deficit.
The transition from flexible, goal-directed actions to inflexible, habitual responses corresponds to a progression of behavioral control from associative to sensorimotor cortico-basal-ganglia networks (Yin & Knowlton, 2006), that are highly conserved across species (Balleine & O’Doherty, 2010; Quinn et al., 2013). The sensorimotor striatum has high expression of presynaptic cannabinoid receptor type 1 (CB1) that contribute to forms of short and long term plasticity (Herkenham et al., 1991; Lovinger, 2010). It is hypothesized that these forms of plasticity are important for habit formation (Gerdeman et al., 2003). Impaired habit learning, i.e. preserved goal-directed behavior, has been observed in transgenic mice with global CB1 knockout, and in wildtype mice given CB1 antagonists specifically during habit learning (Hilario et al., 2007). Habit learning is also impaired when presynaptic CB1 receptors are only genetically removed from orbitofrontal cortical neurons that project to dorsal striatum (Gremel et al., 2016). Therefore, the reduction in CB1 receptor availability that has been observed in substance-dependent individuals (Ceccarini et al., 2014, 2015) may be the mechanism by which habitual drug-taking behaviors emerge in SUD. Moreover, the release of endocannabinoids may be an integral part of formation and expression of habits.
Activation of the CB1 receptor by exogenous substances, such as marijuana, have long been known to promote feeding behavior and an understanding of the complex neural circuits that underlie this behavior is an area of active research (for recent review: Lau et al., 2017). 2-arachidonoyl glycerol (2-AG) is one endogenous ligand for the CB1 receptor, the primary endocannabinoid receptor in neurons, where it acts as a full agonist (Soethoudt et al., 2017). Despite numerous studies implicating CB1 receptors in habit, the role of endocannabinoids in habit formation and expression is unknown. There is some evidence, however, that 2-AG is involved in appetitive behaviors that may suggest a role for 2-AG in appetitive habits. Release of 2-AG is dynamically regulated by feeding behavior: 2-AG release is high when fasted and reduced below baseline when fed in ventral forebrain tissue punches that include the nucleus accumbens (Kirkham et al., 2002). Transgenic mice that overexpress the main metabolic enzyme for 2-AG, monoacyl glycerol lipase (MAGL), have decreased 2-AG tone, and have reduced reward from high fat food and resistance to diet-induced obesity (Jung et al., 2012; Wei et al., 2016). Conversely, pharmacological inhibitors of MAGL, such as JZL184 (Long et al., 2008), increase motivation for food (Oleson et al., 2012). Heightened 2-AG signaling at the CB1 receptor may, therefore, be a mechanism by which habitual behavior persists.
Here, we investigated the role of endocannabinoid signaling in the expression of habitual behavior in mice. Using a contingency degradation paradigm, we show that AM404, a drug that inhibits the putative endocannabinoid transporter, reduces habitual responding. There is evidence that, under certain conditions, drugs that inhibit the endocannabinoid transporter can prevent the release of endocannabinoids (Melis et al., 2004; Ronesi et al., 2004; Straiker & Mackie, 2005; Chicca et al., 2012) but see (Nicolussi & Gertsch, 2015). AM404 additionally binds to and activates the TRPV1 receptor directly (Zygmunt et al., 2000). We report that administration of a CB1 antagonist, but not a TRPV1 antagonist, decreased habitual responding in a manner similar to that observed following administration of AM404, suggesting that the mechanism of AM404 on habitual responding was due to a reduction in CB1 receptor-mediated signaling. Additionally, we provide evidence that administration of a non-selective CB1 agonist increases habitual responding in mice following moderate amounts of operant training. Finally, we demonstrate that inhibition of MAGL partially rescued expression of habitual responding during AM404 treatment. Together, these data provide convergent evidence supporting a role for 2-AG in the expression of habitual responding for food.
Materials and Methods
Animals.
Adult male C57BL/6 mice (Charles River Laboratories; Wilmington, MA) were used in the current study. Males were selected because they were found to form food habits slower than females (Quinn et al., 2007), granting more dynamic behavioral range of study. Mice arrived at 7-8 weeks of age and were group housed in standard cages on ventilation racks (Tecniplast, West Chester, PA) within a climate-controlled vivarium, maintained on 12 h light/dark cycle (lights on at 0700 hours). Following 7 days of acclimation to the vivarium, food was restricted. Mice were maintained at 85-90% of free-feeding body weight for the duration of the experiment by feeding 2.0-3.0 g of standard rodent chow (2918 Teklad diet, Envigo, Huntingdon, United Kingdom) per mouse per day. All procedures were approved by the Yale University Institutional Animal Care and Use Committee and were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources.
Drugs.
The drugs used were the following: AM404 [R&D Systems (Minneapolis, MN) and Fisher Scientific (Waltham, MA)]; MRS1477 and AM251 [Fisher Scientific]; capsazepine [VWR (Radnor, PA) and Cayman Chemical (Ann Arbor, MI)]; CP-55,940 [Santa Cruz Biotechnology (Dallas, TX)]; JZL184 [Sigma-Aldrich (St. Louis, MO) and Cayman Chemical]. All drugs were dissolved in 5% DMSO, 15% Tween 80 in sterile physiological saline and injected intraperitoneal at 10 mL/kg. The doses of drugs used were based on previous studies: AM404 at 10 mg/kg (Gamaleddin et al., 2013), MRS1477 at 10 mg/kg (Kaszas et al., 2012), AM251 at 1 mg/kg (Chen et al., 2004), capsazepine at 5 mg/kg (dos-Santos-Pereira et al., 2016), CP-55,940 at 30 μg/kg (Vinod et al., 2008), and JZL184 at two doses: 2 mg/kg and 18 mg/kg (Oleson et al., 2012; Hartley et al., 2016).
Operant training, testing, and behavioral analyses.
All operant behavior was conducted in standard operant chambers within sound-attenuated boxes (Med Associates, St. Albans, VT) as detailed previously (Gourley et al, 2010). Briefly, chambers were equipped with three adjacent nosepoke apertures on the back wall and a magazine located in the center of the front wall. Apertures and magazine were each equipped with a light and a photobeam sensor. All entries into the apertures and magazine were recorded. Sucrose-sweetened grain pellet reinforcers (Bioserv F0071, Flemington, NJ) were dispensed into the magazine. A fan provided ventilation and background noise throughout sessions.
Mice underwent two days of magazine training where a single reinforcer was delivered once every 60 seconds. Entries into the magazine and apertures had no programmed consequence. Sessions terminated after 30 minutes. Following magazine training, mice underwent operant training.
Operant Training.
One aperture, either the left or right, was assigned to deliver reward (referred to as “active”) and the other two apertures had no programmed consequence (referred to as “inactive”). This assignment was counterbalanced across mice and maintained throughout the experiment. Operant sessions began with illumination of the active aperture and ended with the light extinguishing.
Active aperture entries were reinforced using a fixed ratio 1 (FR1) schedule, where each response resulted in a single reinforcer. FR1 sessions terminated after 30 min or when mice earned 60 reinforcers, whichever occurred first. Once individual mice earned 30 reinforcers in a single FR1 session (1-7 days), they were then reinforced using a variable interval (VI) schedule. The duration of each interval was randomly selected from an exponential list (Fleshler & Hoffman, 1962), with an average of 30 s for VI30, and 60 s for the VI60 schedule. The first active response made after the interval elapsed resulted in a reinforcer. The duration of the next interval was then randomly selected. Sessions terminated after 30 minutes. Mice received three VI30 sessions and were subsequently trained on the VI60 schedule for a minimum of 10 days.
Contingency degradation test.
Contingency degradation, rather than specific satiety or lithium chloride devaluation, was selected to index habitual responding for our pharmacological studies because it can be done repeatedly within subjects. Moreover, contingency degradation can be conducted in a single day whereas devaluation most often requires multiple sessions. Contingency degradation was performed using the same protocol that has previously been implemented for testing acute pharmacological and optogenetic manipulations (Barker et al., 2013, 2017). Briefly, sessions appeared similar to training sessions but reinforcers were non-contingently delivered. Active aperture responses had no programmed consequence. Reinforcers were delivered at equal intervals, matching the total number to reinforcers earned the day prior. Sessions terminated after 30 min.
For most experiments, the first contingency degradation test was administered following 10 days of VI60 training to confirm that responding was habitual prior to pharmacological challenge. For the experiment assessing whether CB1 agonism could increase habitual responding, VI60 training was reduced to three days to lessen the likelihood that mice had developed habitual responding. Following each test, mice received additional VI60 sessions. Cohorts that were not habitual after 10 days received additional VI60 training sessions (3-8 days), until response rates returned to levels comparable to before the contingency degradation test. Stability was confirmed when the total number of reinforcers earned did not deviate by more than 25% across two days of VI60 training. Once stability in the VI60 session was achieved, an additional contingency degradation test was administered, until habitual responding was confirmed.
Individual Logistic Regression Analysis.
Conventional statistical analyses for assessing habitual behavior compare the average response rate of a group of animals in a baseline operant session to that in a habit test. If the average response rate is significantly lower in the test than in the preceding operant session, behavior for all animals is classified as goal-directed. In contrast, if it is approximately equivalent, behavior is classified as habitual. Individual variability, however, is not well accounted for in such analyses, which can lead to classification errors – including goal-directed animals in a group classified as habitual, or all animals being classified as goal-directed when the statistical difference is driven by a small subset.
To address this, we applied a statistical analysis for classifying individual animals as goal-directed or habitual. The response rate for each mouse on the contingency degradation test was compared to that mouse’s response rate for all previous VI60 sessions using a generalized linear model (GLM) with a Poisson distribution, which allows for the analysis to take the individual animal’s variability across training days into account when determining if the pattern of responding during the contingency degradation test was significantly different from that using the VI60 schedule. A session-type regressor (i.e., VI60 session coded as 0, test coded as 1) and a linear covariate regressor were included. Mice with significant negative coefficients for the session-type regressor were classified as goal-directed, because this indicated that the decrease in responding on the test was beyond the normal range of variability for that animal. In contrast, mice with non-significant or positive coefficients for the session-type regressor were classified as habitual, because this indicated that responding had not substantially decreased on the test. The statistical significance of session-type regression coefficients was assessed by calculating a z statistic (regression coefficient/standard error) for each individual mouse. Mice with session-type z values that were less than or equal to −1.96 were determined to be significant. Only animals confirmed to be habitual were included in the pharmacological studies. Animals that were excluded were used in non-operant experiments reported elsewhere.
Pharmacological Studies.
Once responding on the VI60 schedule was stable following initial contingency degradation tests (2–9 days), the pharmacological studies began. The vehicle was administered to all mice in the VI60 session prior to these contingency degradation tests. This served as the non-degraded operant session (referred to as “Baseline”). If administration of vehicle dramatically reduced rewards earned compared to previous sessions, mice underwent additional training (2–11 days) until rewards earned were approximately equivalent in the Baseline compared to previous sessions, using the same stability criterion described above. The day after completing the Baseline, drug was administered and responding was assessed by contingency degradation. All injections were given 30 minutes prior to the session based on previous studies (e.g., Hilario et al., 2007; Gamaleddin et al., 2013), except for JZL184, which was administered 2 hours prior (Oleson et al., 2012; Hartley et al., 2016) because of the pharmacokinetic properties of enzyme inhibitors. Drug order was counterbalanced using a Latin square.
Behavioral Control Experiments
Locomotor behavior was assessed as previously described (Gourley et al., 2009) using photobeam equipped chambers (Med Associates) following administration of AM404 to determine if robust changes in activity could explain the observed reduction in habitual responding. Sequential beam breaks on two adjacent detectors were counted as one unit of activity. Using a within-subjects design, mice (n=12) were injected with AM404 or vehicle 30 minutes before being placed in the locomotor chamber and activity was recorded for 30 minutes to match the timing of the operant tests.
Conditioned place testing was conducted with AM404 to determine if non-specific aversion could explain the observed reductions in habitual responding. Standard two-chamber conditioned preference boxes (Med Associates) were used that had distinct walls and floors, see (Barker et al., 2013) with a middle neutral chamber. Mice (n=6) were placed in the neutral chamber with both doors retracted so that mice could freely explore all chambers for 30 minutes for a “baseline”. Photobeam detectors quantified the amount of time mice spent in each chamber and was used to determine which chamber would be paired with AM404 versus vehicle using an unbiased design. On conditioning days, 30 minutes following injection, mice were confined to the AM404-paired, or vehicle-paired chamber on alternate days for 30 min. Mice underwent three conditioning days per side, followed by a preference test that was identical to the baseline session. Conditioned place testing was assessed by comparing the time spent in the AM404-paired and vehicle-paired chambers during the preference test.
Statistical analysis
Data were analyzed using Prism 7 (Graphpad, San Diego, CA), SPSS 21 (IBM, Armonk, NY), and MATLAB 2016b (MathWorks, Natick, MA). Data are presented as the mean ± standard error of the mean, except where otherwise specified. Response rates across drugs and behavioral sessions were analyzed using repeated measures GLM with a Poisson distribution, because this distribution is the most appropriate for count data. Regression coefficients were tested with Wald χ2 to determine if they were significantly different from zero. Significant interaction effects were analyzed pairwise among drug conditions with a Sidak correction for multiple comparisons. Conditioned place preference and locomotor activity data were analyzed using two tailed, paired t tests.
Results
Logistic regression for classifying individual animals as goal-directed or habitual
To illustrate the considerable variability both between and within animals, we analyzed the response rate for mice (n=71) in the VI60 sessions across 10 days. The mean response rate across training varied between 2.5 and 30.4 responses per minute (Figure 1A). Notably, we also observed substantial within-animal variability. For example, Figure 1B plots the response rates of two mice with similar intra-individual variability (coefficient of variation; mouse 84: 12.3, mouse 83: 13.6) but with different mean response rates (mouse 84: 30.4, mouse 83: 8.9 responses per minute). Conversely, Figure 1C plots examples of two mice with similar mean response rates (mouse 83: 8.9 and mouse 313: 8.4 responses per minute) yet different intra-individual variability (coefficient of variation; mouse 83: 13.6, mouse 313: 55.5). This variability can erroneously impact the assessment of behavior. For example, a decrease of 10 responses per minute in a contingency degradation test would be considered relatively stable, i.e., habitual, for mouse 83 (high response rate) but it would be a significant decrease, i.e., goal-directed, for mouse 84 (low response rate). Moreover, the identical behavioral response of 10 fewer responses per minute would be expected from mice with high intra-individual variability (Figure 1C; mouse 313) than mice with stable response rates (Figure 1C; mouse 84), yet this change would be classified erroneously as goal-directed for both mice. Averaging across these dissimilar mice for single sessions (as in conventional habitual analyses) increases the likelihood of committing statistical errors and mischaracterizing animals as goal-directed or habitual.
Figure 1:
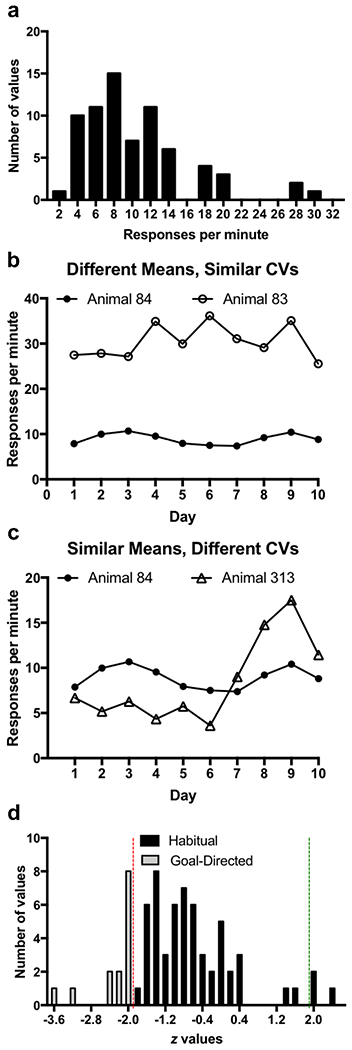
Habitual food seeking responses in mice are variable within and between subjects
a. Frequency histogram of average response rate across 10 days of responding for food on a VI60 schedule for n=71 mice included in the habit experiments in this paper
b. Response rate on 10 days of responding for food on a VI60 schedule for two subjects with different mean response rate yet similar coefficient of variation ((standard deviation/mean)*100).
c. Response rate across 10 days of responding for food on a VI60 schedule for two subjects with similar mean response rate yet different coefficient of variation.
d. Frequency histogram of z values for session-type regressor from logistic GLM with a Poisson distribution for all n=71 animals included in the habit experiments. Red line indicates cutoff for significant negative regression coefficients (p<0.05), indicating goal-directed behavior. Green line indicates cutoff for significant positive (p<0.05) regression coefficients.
Using our method, we compared the response rate of each individual mouse on the contingency degradation test to that on all previous VI60 sessions using a GLM with a Poisson distribution. Figure 1D is a frequency histogram of the z values for the session-type regression coefficients; 57 mice were characterized as habitual in the first contingency degradation test. Whereas 14 mice had coefficients that were below our critical value: 10 mice became habitual after more VI60 training and 4 mice were excluded because they never became habitual.
Demonstration of habitual behavior and impact of AM404 on responding
The experimental timeline for measuring the impact of AM404 on habitual responding is presented in Figure 2A. Active response rates increased across VI30 and VI60 sessions (Figure 2B). We then conducted a contingency degradation test and classified animals as goal-directed or habitual based on our individual regression analyses (Figure 2C; Supplementary table S1). At the group level, mice were confirmed to be habitual: response rates on the 10th VI60 day were not significantly different from the habit test (Figure 2D; χ2=2.2, p=0.14), thereby confirming our individual GLM with conventional behavioral analysis.
Figure 2:
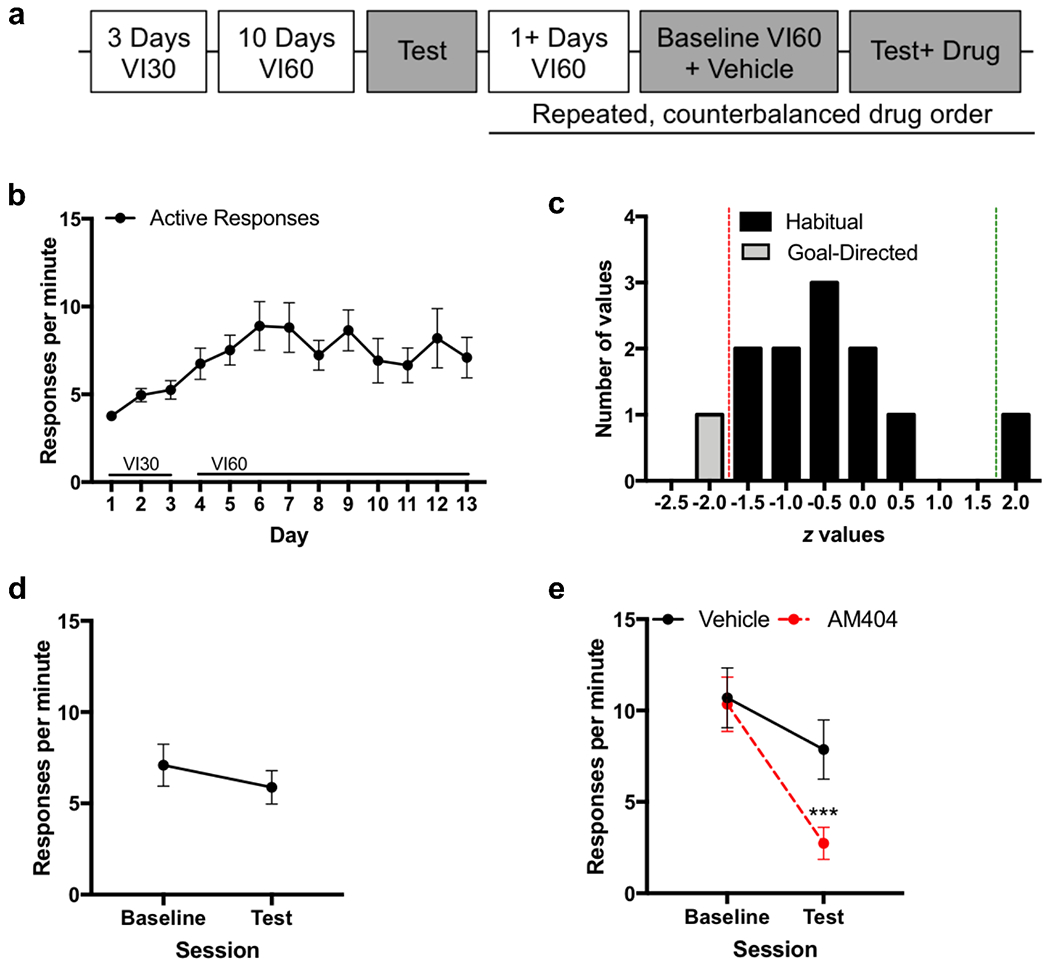
AM404 reduces habitual responding for food
a. Experimental timeline
b. Response rate across acquisition of habitual responding for food
c. Frequency histogram of z values for the session-type regressor for the logistic GLM with a Poisson distribution analyzing response rates during each individual animal’s VI60 training history compared to response rates on test for the n=12 mice in this specific experiment. Red line indicates cutoff for significant negative regression coefficients (p<0.05), indicating goal-directed behavior. Green line indicates cutoff for significant positive (p<0.05) regression coefficients.
d. Response rates on the baseline day compared to the contingency degradation test demonstrating that this cohort is habitual at the group level (n=11) with no significant difference in response rate compared to the baseline day.
e. Response rate across contingency degradation tests with AM404 or Vehicle, within subjects. ***p=0.001
AM404 administration significantly reduced habitual responding in the contingency degradation test compared to that of baseline (Figure 2E; main effect of session: χ2=23.7, p<0.001; main effect of drug: χ2=10.0, p=0.002; session-by-drug interaction: χ2=9.9, p=0.002). Responding on the test following AM404-treatment was significantly lower than that following vehicle-treatment (χ2=10.1, p=0.001). The observed reduction in habitual responding is unlikely due to non-specific motoric or aversive effects because we confirmed, in a separate cohort, that AM404 did not alter locomotor activity or induce conditioned preference or aversion (Figure S1), consistent with previous studies (Bortolato et al., 2006; Gamaleddin et al., 2013).
Dissociating the endocannabinoid receptor effects of AM404 on habitual responding
To determine the mechanism mediating the effect of AM404 on habitual responding (see Figure 3A timeline), a cohort of mice were trained on the VI schedules (Figure 3B) and confirmed to be habitual (Figure S2). If the mechanism of AM404 is to inhibit release of endocannabinoids, then it may result in decreased activation of the CB1 receptor, or increased activation of the TRPV1 receptor because the binding site is intracellular (De Petrocellis et al., 2001). Thus, a CB1 receptor antagonist (AM251) or a TRPV1 receptor positive allosteric modulator (MRS1477) was administered prior to contingency degradation. The response rates were significantly altered following drug administration (Figure 3C; main effect of session: χ2 =62.7, p<0.001; main effect of drug: χ2=39.9, p<0.001; session-by-drug interaction: χ2=35.3, p<0.001). AM251-treatment significantly reduced responding compared to vehicle (p<0.001) in a manner similar to that observed following AM404-treatment. Notably, MRS1477 did not significantly reduce responding on the test session compared to vehicle (p=0.95).
Figure 3:
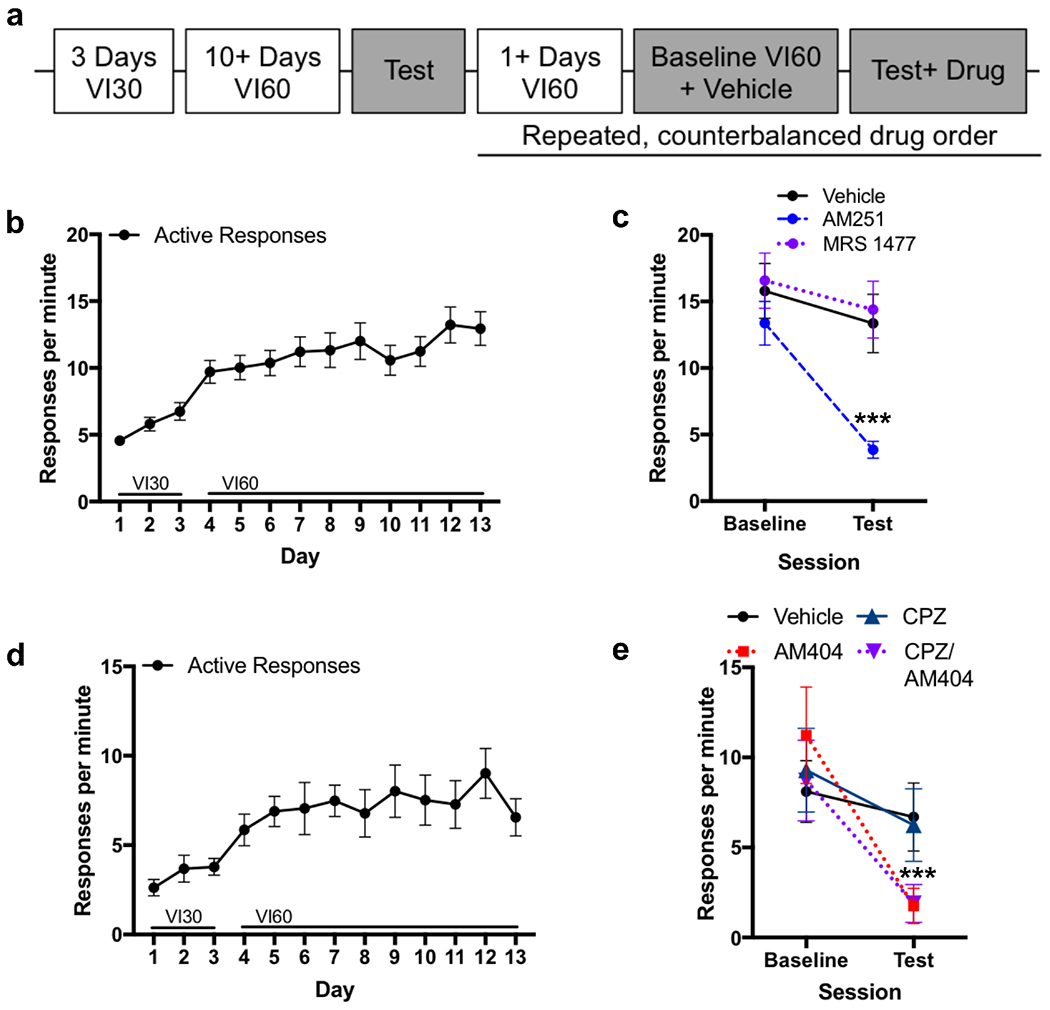
CB1 receptor, not TRPV1 receptor, likely mediates AM404 effect
a. Experimental timeline
b. Response rate across days showing acquisition for food for AM251 and MRS1477 experiment
c. Response rate on repeated, within-subjects contingency degradation testing with AM251, MRS1477, or vehicle (n=34). ***p<0.001
d. Response rate across days showing acquisition for food for capsazepine experiment.
e. Response rate on repeated, within-subjects contingency degradation testing with Capsazepine, AM404, Vehicle, or AM404 with Capsazepine (n=10). ***p<0.001
It is possible that the negative result with MRS1477 was due to it being a positive allosteric modulator, and not a full agonist at the TRPV1 receptor. If TRPV1 receptors mediate the mechanism of AM404’s effect on habitual responding, then blocking TRPV1 receptors concurrently with AM404 should prevent the reduction in responding. Additionally, there is some evidence that global knockout of TRPV1 receptors prevents habit formation (Shan et al., 2015), so it is possible that antagonism of TRPV1 receptors can prevent habit expression. To test this, a separate cohort of mice was trained (Figure 3D) and confirmed to be habitual (Figure S2). Mice received a single injection of vehicle, AM404, capsazepine (TRPV1 antagonist), or capsazepine combined with AM404 before contingency degradation. Responding was significantly affected following drug administration (Figure 3E; main effect of session: χ2=19.9, p<0.001; main effect of drug: χ2=20.1, p<0.001; session-by-drug interaction: χ2=15.2, p=0.002). Consistent with our first experiment, AM404-treatment reduced responding in the test compared to vehicle (p<0.001). Co-administration of capsazepine and AM404 also reduced responding in the test compared to vehicle (p<0.001), but there were no significant differences between AM404 in the presence or absence of capsazepine (p=0.8), demonstrating that capsazepine did not alter the AM404-induced reduction in responding. These results demonstrate that TRPV1 receptors do not mediate the effect of AM404 on habitual responding.
Because our data indicate that antagonism of CB1, but not TRPV1, receptors reduces habitual responding, we hypothesized that activation of CB1 receptors would promote habitual responding. To test this, we decreased the number of VI60 sessions (Figure 4A) to lessen the likelihood of habitual responding. Mice (n=26) were separated into two groups matched on response rates (Figure 4B). The high efficacy, non-selective CB1 receptor agonist, CP-55,940, or vehicle was administered prior to contingency degradation. A significant session-by-drug interaction was observed in response rates (Figure 4C; χ2=7.5, p=0.006). Mice given CP-55,940 increased responding in the test compared to vehicle-treated animals (χ2=4.4, p=0.04) indicating that enhanced signaling at the CB1 receptor can increase habitual responding.
Figure 4:
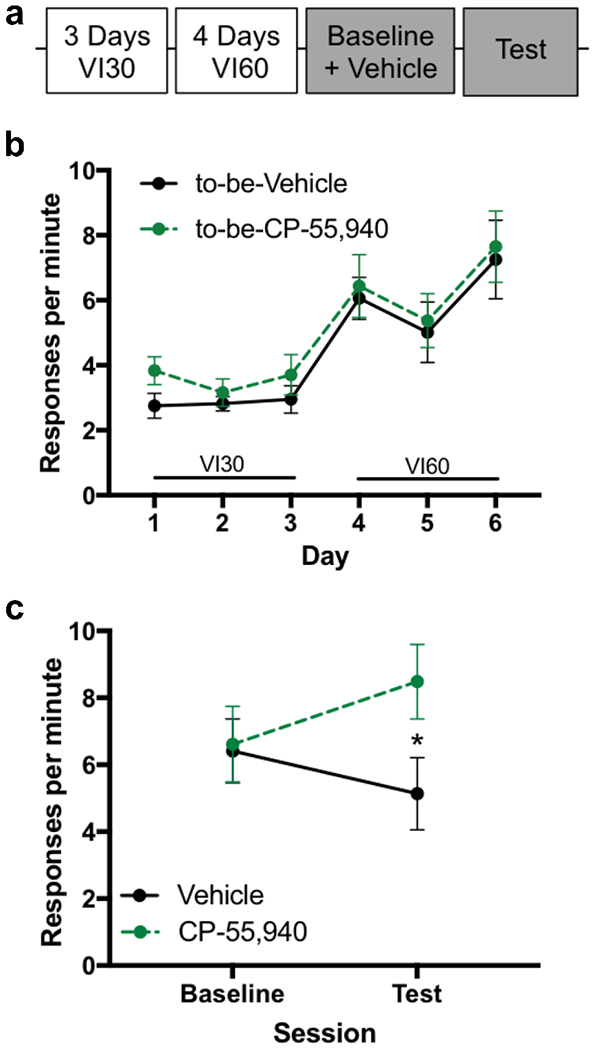
Endocannabinoid effect on habitual responding is bi-directional. CB1 agonist increases habitual responding for food after moderate training.
a. Experimental timeline
b. Response rate across days showing acquisition for food responding
c. Response rate across contingency degradation, between subjects CP-55,940 or Vehicle (n=13 each group). *p<0.05
The role of 2-AG in habitual responding
We hypothesized that AM404-mediated reductions in habitual responding were due to reductions in 2-AG tone, and subsequent reductions in signaling at the CB1 receptor. To test this hypothesis, we examined the effect of JZL184, a MAGL inhibitor that increases 2-AG tone, on habitual responding (see Figure 5A timeline). Response rate increased across VI sessions (Figure 5B), and mice were confirmed to be habitual (Figure 5C; Figure S3). Mice received JZL184 (2 mg/kg) or vehicle 2 hours before, and AM404 or vehicle 30 minutes before contingency degradation. This resulted in four drug conditions: 1) Vehicle/Vehicle, 2) Vehicle/AM404, 3) JZL184/Vehicle, and 4) JZL184/AM404.
Figure 5:
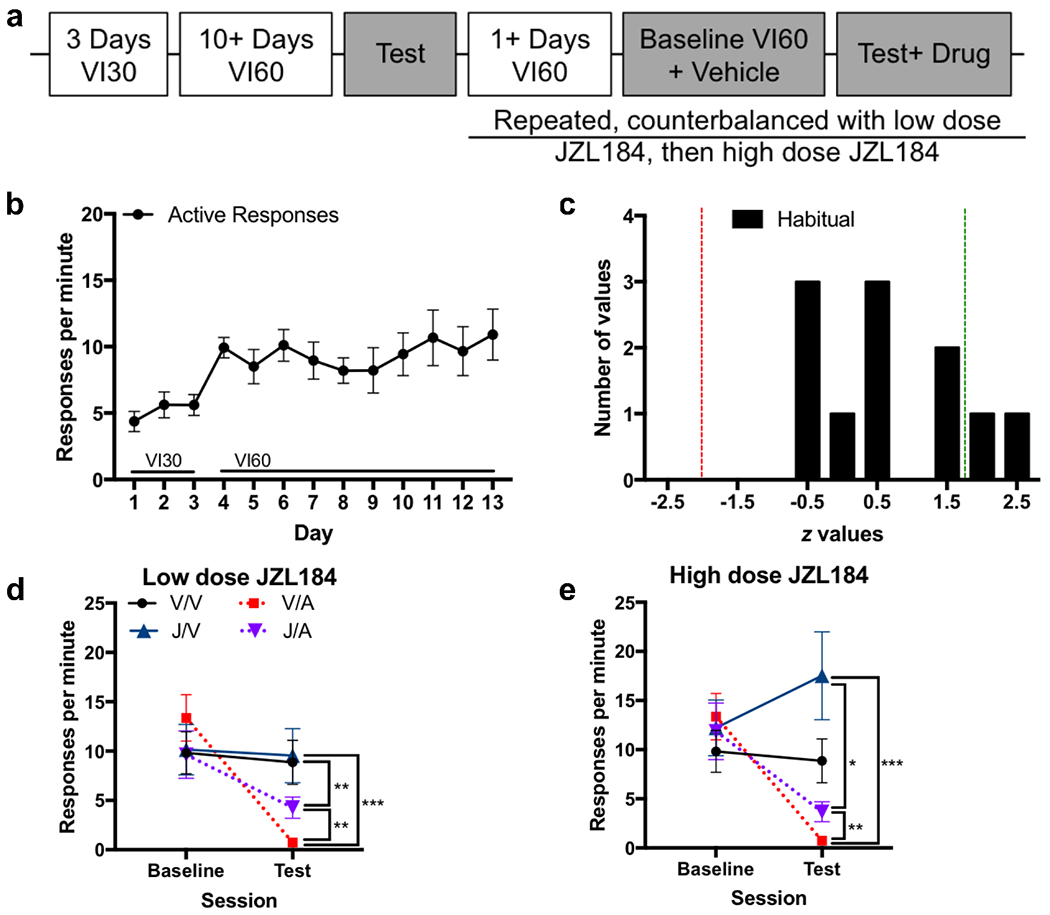
2AG enzyme inhibitor partially blocks AM404 effect
a. Experimental timeline
b. Response rate across days showing acquisition for food (n=11)
c. Frequency histogram of z values for the session-type regressor for the logistic GLM with a Poisson distribution analyzing response rates during each individual animal’s VI60 training history compared to response rates on contingency degradation test. Red line indicates cutoff for significant negative regression coefficients (p<0.05), indicating goal-directed behavior. Green line indicates cutoff for significant positive (p<0.05) regression coefficients.
d. Response rate on contingency degradation testing within subjects, low dose JZL184 (2 mg/kg). Conditions include Vehicle/Vehicle (V/V), Vehicle/AM404 (V/A), low dose JZL184/Vehicle (J/V), and low dose JZL184/AM404 (J/A).
e. Response rate on contingency degradation testing within subjects, high dose JZL184 (18 mg/kg). Conditions include Vehicle/Vehicle (V/V), Vehicle/AM404 (V/A), high dose JZL184/Vehicle (J/V), and high dose JZL184/AM404 (J/A).
Drug administration significantly affected responding (Figure 5D; main effect of session: χ2=62.5, p<0.001; main effect of drug: χ2=34.4, p<0.001; session-by-drug interaction: χ2=73.3, p<0.001). AM404 administration reduced responding in the test compared to vehicle (Vehicle/Vehicle vs. Vehicle/AM404: p=0.001), but JZL184 administration did not alter responding compared to vehicle (Vehicle/Vehicle vs. JZL184/Vehicle: p=0.99). Co-administration of JZL184 and AM404 decreased responding compared to vehicle (Vehicle/Vehicle vs. JZL184/AM404: p=0.01), yet responding was higher than when AM404 was administered alone (JZL184/AM404 vs. Vehicle/AM404: p=0.008), suggesting that JZL184 pretreatment partially restored the AM404-induced decrease in habitual responding.
Mice then went through two additional drug conditions using a higher dose of JZL184 (18 mg/kg) because this dose is known to cause tolerance and receptor desensitization (Schlosburg et al., 2010). Response rates were significantly altered following these drug conditions (Figure 5E; main effect of session: χ2=32.4, p<0.001; main effect of drug: χ2=71.0 p<0.001; session-by-drug interaction: χ2=74.7, p<0.001). Response rates following administration of high dose JZL184 were not significantly different from response rates following vehicle administration (high dose JZL184/Vehicle vs. Vehicle/Vehicle: p=0.14), and significantly increased responding on the test compared to both AM404 conditions (high dose JZL184/Vehicle vs. Vehicle/AM404: p=0.001; high dose JZL184/Vehicle vs. high dose JZL184/AM404: p=0.02). Consistent with our hypothesis, responding following administration of high dose JZL184 with AM404 was significantly higher than when AM404 was administered alone (high dose JZL184/AM404 vs. Vehicle/AM404: p=0.01), and not significantly different from responding following vehicle-treatment (high dose JZL184/AM404 vs. Vehicle/Vehicle: p=0.3), indicating that pretreatment with high dose JZL184 was able to restore habitual responding for food.
Discussion
We provide novel insights into the endocannabinoid mechanisms regulating expression of habitual behavior. By combining advanced behavioral analyses with pharmacology, we demonstrate that endocannabinoid activity at CB1 receptors is necessary for the expression of habitual behavior. Additionally, we have applied a statistical method for determining if an individual animal is goal-directed or habitual, which is useful because standard analyses using group means can suffer from imprecise classification. These studies are the first to our knowledge to employ endocannabinoid pharmacology during contingency degradation tests for habitual behavior. Our results have important implications for understanding the pathophysiology of mental disorders characterized by compulsive, habitual actions such as SUDs, and may suggest novel therapeutic approaches.
Here, we show that AM404 produces a robust and highly replicable reduction in habitual food responding. It has previously been reported that systemic AM404 administration fails to alter food intake or food-seeking responses (Reyes-Cabello et al., 2012; Gamaleddin et al., 2013). This discrepancy may be due to the fact that these studies were conducted under conditions of either free feeding or FR reinforcement schedules that are unlikely to develop habitual response strategies. Indeed, if AM404 were selective for habitual responding, this may be advantageous from a clinical perspective. Furthermore, a previous study observed increased feeding following intracerebroventricular infusion of AM404 in 70% DMSO (Reyes-Cabello et al., 2012). It is likely that the infusion procedures were stressful, which may have caused 2-AG release (for review: Morena et al, 2016), and if so, this 2-AG would have been prolonged in the synapse by AM404 blocking the transporter. Further research into the conditions under which AM404 and related endocannabinoid transport inhibitors block release or reuptake of endocannabinoids are warranted.
There is little consensus with regard to the transport mechanism for endocannabinoids (Nicolussi & Gertsch, 2015). The precise conditions under which AM404 and related compounds act as release or reuptake inhibitors are not fully characterized, but there is evidence from in vitro studies (Straiker & Mackie, 2005; Chicca et al., 2012), slice electrophysiology studies (Ronesi et al., 2004), and in vivo electrophysiology studies (Melis et al., 2004; Pillolla et al., 2007) that endocannabinoid transport inhibitors can prevent the release of endocannabinoids – that the putative endocannabinoid transport protein acts in a bi-directional manner. Our data are consistent, at least in the context of food habits, with the ability of AM404 to prevent endocannabinoid release to reduce habitual behavior. Since food habits can be reduced by AM404 and by direct antagonism of the CB1 receptor with AM251, but are increased by direct agonism of the CB1 receptor with CP-55,940, the most parsimonious explanation is that AM404 blocks endocannabinoid release. These results would appear paradoxical if AM404 were acting as an endocannabinoid reuptake inhibitor and thereby increased endocannabinoid tone. Indeed, if AM404 were acting exclusively as an endocannabinoid reuptake inhibitor, we would expect that CP-55,940 would decrease habitual food seeking – in fact, CP-55,940 increased habitual food seeking. Moreover, if AM404 were acting exclusively as a reuptake inhibitor, we would predict that AM251 would increase habitual food seeking – in fact, AM251 decreased habitual food seeking. It is important to note that CP-55,940 is a non-selective CB1 receptor agonist and has affinity for the CB2 receptor where it acts as an agonist (Soethoudt et al., 2017). However, it is unlikely that CB2 receptor agonism contributed to the observed behavioral effect of increased habitual food seeking because selective CB2 activation is known to decrease food intake (Onaivi et al., 2008). Nevertheless, additional studies using more selective CB1 and CB2 compounds in our food habit paradigm are warranted.
Furthermore, we provide evidence that AM404’s effect can be counteracted by pre-treatment with JZL184. If AM404 were acting as a reuptake inhibitor, then we would expect to see a potentiation of the reduction in habitual food seeking with co-administration of JZL184. However, consistent with our mechanistic hypothesis, increasing endogenous 2-AG tone with JZL184 before AM404 treatment restored habitual food responding. We suggest that our results are remarkably consistent with AM404 leading to reductions in release of 2-AG during food habits – an effect mediated through subsequent reduced activity at CB1 receptors. Previously, an endocannabinoid transport inhibitor decreased neural encoding of reward predicting cues and decreased motivated operant behavior (Oleson & Cheer, 2012), which also could potentially be explained as a reduction in 2-AG release (Oleson et al., 2012). However, we cannot rule out the possibility that this effect of AM404 may depend on reductions in release of anandamide. Indeed, transgenic knockout mice that do not express the metabolic enzyme for anandamide, fatty acid amide hydrolase (FAAH), have been reported to have increased motivation to work for food on a progressive ratio schedule (Touriño et al., 2010). However, this effect was not observed with pharmacological inhibitors of FAAH (Oleson et al., 2012). Future studies should determine whether anandamide release also contributes to the expression of habitual responding for food.
Our data indicate that AM404’s actions were not driven by TRPV1 receptor activation (Zygmunt et al., 2000), but possibly through action at the endocannabinoid transporter. We co-administered the selective TRPV1 antagonist (capsazepine) with AM404, and report that administration of capsazepine did not alter the AM404-mediated reduction in habitual responding. Moreover, administration of a TRPV1 positive allosteric modulator (MRS1477) did not reproduce the reduction in food habit expression observed with AM404. Therefore, these data suggest that the expression of habitual responding for food is not mediated by the TRPV1 receptor. It is likely, therefore, that the persistent goal-directed behavior observed in TRPV1 receptor knockout mice (Shan et al., 2015) is due to a specific requirement of TRPV1 receptors for the formation of habits and not the expression of habits.
Previous research has established a critical role for CB1 receptors in habit formation, particularly presynaptic CB1 receptors in orbitofrontal cortical projection neurons to the dorsal striatum (Hilario et al., 2007; Gremel et al., 2016). Our work extends these findings to include the necessity of CB1 receptor activation for the expression of habits. We hypothesize that our drug interventions affected cortico-basal-ganglia networks involved in habits. Future studies that infuse AM404 directly into the dorsal striatum would provide insight into whether this region is the key neuroanatomical locus of AM404’s effect. Nevertheless, the translational relevance of systemic AM404’s ability to reduce habitual responding is promising because AM404 is an active metabolite of acetaminophen/paracetamol (Högestätt et al., 2005) and, thus, could potentially be used clinically. Research into whether the effects of AM404 on habit expression can be also observed in acute or chronic stress models will be important because stress is a known regulator of habitual behavior (Dias-ferreira et al, 2009; Gourley et al, 2012; Schwabe and Wolf, 2009), of endocannabinoid release (Morena et al, 2016), and of relapse for SUDs (Mantsch et al., 2016). It would be imperative for endocannabinoid transport inhibitors to reduce habitual behaviors under conditions of stress for them to be effective therapeutic tools.
Together our data suggest that pharmacological strategies aimed at attenuating endogenous release of 2-AG – as we hypothesize occurs with AM404 – might reduce pathological habitual behaviors with less risk of significant on-target negative side effects, as has been observed with global CB1 receptor antagonism.
Supplementary Material
Acknowledgements
This study was supported by public health service grants AA012870, DA041480, and DA043443. Additional support was provided by a NARSAD award, the Charles B.G. Murphy Fund, and the State of CT Department of Mental Health Services.
Graphical abstract made with Motifolio drawing toolkits (www.motifolio.com). We thank Peter Harris for additional assistance with the graphical abstract.
Abbreviations
- 2-AG
2-arachidonoyl glycerol
- CB1
cannabinoid receptor type 1
- FAAH
fatty acid amide hydrolase
- FR1
fixed ratio 1
- GLM
generalized linear model
- MAGL
monoacyl glycerol lipase
- SUD
substance use disorder
- TRPV1
transient receptor potential cation subfamily V
- VI
variable interval
Footnotes
Conflict of Interest Statement
The authors declare no conflict of interest.
Data Accessibility Statement
Data are available via request from the authors.
References
- Balleine BW & O’Doherty JP (2010) Human and rodent homologies in action control: Corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology, 35, 48–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, Glen WB, Linsenbardt DN, Lapish CC, & Chandler LJ (2017) Habitual Behavior Is Mediated by a Shift in Response-Outcome Encoding by Infralimbic Cortex. Eneuro, 4, ENEURO.0337-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM & Taylor JR (2014) Habitual alcohol seeking: Modeling the transition from casual drinking to addiction. Neurosci. Biobehav. Rev, 47, 281–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, Torregrossa MM, & Taylor JR (2013) Bidirectional modulation of infralimbic dopamine D1 and D2 receptor activity regulates flexible reward seeking. Front. Neurosci, 7, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolato M, Campolongo P, Mangieri RA, Scattoni ML, Frau R, Trezza V, La Rana G, Russo R, Calignano A, Gessa GL, Cuomo V, & Piomelli D (2006) Anxiolytic-like properties of the anandamide transport inhibitor AM404. Neuropsychopharmacology, 31, 2652–2659. [DOI] [PubMed] [Google Scholar]
- Ceccarini J, Hompes T, Verhaeghen A, Casteels C, Peuskens H, Bormans G, Claes S, & Van Laere K (2014) Changes in cerebral CB1 receptor availability after acute and chronic alcohol abuse and monitored abstinence. J. Neurosci, 34, 2822–2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarini J, Kuepper R, Kemels D, van Os J, Henquet C, & Van Laere K (2015) [ 18 F]MK-9470 PET measurement of cannabinoid CB 1 receptor availability in chronic cannabis users. Addict. Biol, 20, 357–367. [DOI] [PubMed] [Google Scholar]
- Chen RZ, Huang R-RC, Shen C-P, MacNeil DJ, & Fong TM (2004) Synergistic effects of cannabinoid inverse agonist AM251 and opioid antagonist nalmefene on food intake in mice. Brain Res, 999, 227–230. [DOI] [PubMed] [Google Scholar]
- Chicca A, Marazzi J, Nicolussi S, & Gertsch J (2012) Evidence for bidirectional endocannabinoid transport across cell membranes. J. Biol. Chem, 287, 34660–34682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Petrocellis L, Bisogno T, Maccarrone M, Davis JB, Finazzi-Agrò A, & Di Marzo V (2001) The Activity of Anandamide at Vanilloid VR1 Receptors Requires Facilitated Transport across the Cell Membrane and Is Limited by Intracellular Metabolism. J. Biol. Chem, 276, 12856–12863. [DOI] [PubMed] [Google Scholar]
- Dias-ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ, Costa RM, & Sousa N (2009) Chronic Stress Causes Frontostriatal Reorganization and Affects Decision-Making. Science (80-. ), 325, 621–625. [DOI] [PubMed] [Google Scholar]
- dos-Santos-Pereira M, Da-Silva CA, Guimarães FS, & Del-Bel E (2016) Co-administration of cannabidiol and capsazepine reduces L-DOPA-induced dyskinesia in mice: Possible mechanism of action. Neurobiol. Dis, 94, 179–195. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Gillan CM, Jones PS, Williams GB, Laetitia HE, Luijten M, Wit S De, Sahakian BJ, Bullmore ET, & Robbins TW (2016) Carrots and sticks fail to change behavior in cocaine addiction. Science (80-. ), 352, 1468–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ & Robbins TW (2016) Drug Addiction: Updating Actions to Habits to Compulsions Ten Years On. Annu. Rev. Psychol, 67, 23–50. [DOI] [PubMed] [Google Scholar]
- Fleshler M & Hoffman HS (1962) A progression for generating variable-interval schedules. J. Exp. Anal. Behav, 5, 529–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamaleddin I, Guranda M, Scherma M, Fratta W, Makriyannis A, Vadivel SK, Goldberg SR, & Le Foll B (2013) AM404 attenuates reinstatement of nicotine seeking induced by nicotine-associated cues and nicotine priming but does not affect nicotine- and food-taking. J. Psychopharmacol, 27, 564–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdeman GL, Partridge JG, Lupica CR, & Lovinger DM (2003) It could be habit forming: drugs of abuse and striatal synaptic plasticity. Trends Neurosci, 26, 184–192. [DOI] [PubMed] [Google Scholar]
- Gourley SL, Koleske AJ, & Taylor JR (2009) Loss of dendrite stabilization by the Abl-related gene (Arg) kinase regulates behavioral flexibility and sensitivity to cocaine. Proc. Natl. Acad. Sci. U. S. A, 106, 16859–16864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Lee AS, Howell JL, Pittenger C, & Taylor JR (2010) Dissociable regulation of instrumental action within mouse prefrontal cortex. Eur. J. Neurosci, 32, 1726–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Swanson AM, Jacobs AM, Howell JL, Mo M, DiLeone RJ, Koleske AJ, & Taylor JR (2012) Action control is mediated by prefrontal BDNF and glucocorticoid receptor binding. Proc. Natl. Acad. Sci, 109, 20714–20719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremel CM, Chancey JH, Atwood BK, Luo G, Neve R, Ramakrishnan C, Deisseroth K, Lovinger DM, & Costa RM (2016) Endocannabinoid Modulation of Orbitostriatal Circuits Gates Habit Formation. Neuron, 90, 1312–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley ND, Gunduz-Cinar O, Halladay L, Bukalo O, Holmes A, & Patel S (2016) 2-arachidonoylglycerol signaling impairs short-term fear extinction. Transl. Psychiatry, 6, e749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, & Rice KC (1991) Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J. Neurosci, 11, 563–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilario MRF, Clouse E, Yin HH, & Costa RM (2007) Endocannabinoid signaling is critical for habit formation. Front. Integr. Neurosci, 1, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Högestätt ED, Jönsson BAG, Ermund A, Andersson DA, Björk H, Alexander JP, Cravatt BF, Basbaum AI, & Zygmunt PM (2005) Conversion of acetaminophen to the bioactive N-acylphenolamine AM404 via fatty acid amide hydrolase-dependent arachidonic acid conjugation in the nervous system. J. Biol. Chem, 280, 31405–31412. [DOI] [PubMed] [Google Scholar]
- Jentsch JD & Taylor JR (1999) Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl), 146, 373–390. [DOI] [PubMed] [Google Scholar]
- Jung KM, Clapper JR, Fu J, D’Agostino G, Guijarro A, Thongkham D, Avanesian A, Astarita G, DiPatrizio NV, Frontini A, Cinti S, Diano S, & Piomelli D (2012) 2-Arachidonoylglycerol signaling in forebrain regulates systemic energy metabolism. Cell Metab., 15, 299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaszas K, Keller JM, Coddou C, Mishra SK, Hoon MA, Stojilkovic S, Jacobson KA, & Iadarola MJ (2012) Small Molecule Positive Allosteric Modulation of TRPV1 Activation by Vanilloids and Acidic pH. J. Pharmacol. Exp. Ther, 340, 152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham TC, Williams CM, Fezza F, & Di Marzo V (2002) Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: Stimulation of eating by 2-arachidonoyl glycerol. Br. J. Pharmacol, 136, 550–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau BK, Cota D, Cristino L, & Borgland SL (2017) Endocannabinoid modulation of homeostatic and non-homeostatic feeding circuits. Neuropharmacology, 124, 38–51. [DOI] [PubMed] [Google Scholar]
- Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, Pavón FJ, Serrano AM, Selley DE, Parsons LH, Lichtman AH, & Cravatt BF (2008) Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat. Chem. Biol, 5, 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM (2010) Neurotransmitter roles in synaptic modulation, plasticity and learning in the dorsal striatum. Neuropharmacology, 58, 951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Baker DA, Funk D, Lê AD, & Shaham Y (2016) Stress-induced reinstatement of drug seeking: 20 years of progress. Neuropsychopharmacology, 41, 335–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKim TH, Bauer DJ, & Boettiger CA (2016) Addiction history associates with the propensity to form habits. J. Cogn. Neurosci, 28, 1024–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M, Perra S, Muntoni AL, Pillolla G, Lutz B, Marsicano G, Marzo V Di, Gessa, G.L., & Pistis, M. (2004) Prefrontal Cortex Stimulation Induces 2-Arachidonoyl-Glycerol-Mediated Suppression of Excitation in Dopamine Neurons. J. Neurosci, 24, 10707–10715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morena M, Patel S, Bains JS, & Hill MN (2016) Neurobiological Interactions Between Stress and the Endocannabinoid System. Neuropsychopharmacology, 41, 80–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazzaro C, Greco B, Cerovic M, Baxter P, Rubino T, Trusel M, Parolaro D, Tkatch T, Benfenati F, Pedarzani P, & Tonini R (2012) SK channel modulation rescues striatal plasticity and control over habit in cannabinoid tolerance. Nat. Neurosci, 15, 284–293. [DOI] [PubMed] [Google Scholar]
- Nelson A & Killcross S (2006) Amphetamine Exposure Enhances Habit Formation. J. Neurosci, 26, 3805–3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolussi S & Gertsch J (2015) Endocannabinoid transport revisited. Vitam. Horm, 98, 441–485. [DOI] [PubMed] [Google Scholar]
- Oleson EB, Beckert MV, Morra JT, Lansink CS, Cachope R, Abdullah RA, Loriaux AL, Schetters D, Pattij T, Roitman MF, Lichtman AH, & Cheer JF (2012) Endocannabinoids Shape Accumbal Encoding of Cue-Motivated Behavior via CB1 Receptor Activation in the Ventral Tegmentum. Neuron, 73, 360–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleson EB & Cheer JF (2012) Paradoxical effects of the endocannabinoid uptake inhibitor VDM11 on accumbal neural encoding of reward predictive cues. Synapse, 66, 984–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onaivi ES, Carpio O, Ishiguro H, Schanz N, Uhl GR, & Benno R (2008) Behavioral Effects of CB2 Cannabinoid Receptor Activation and Its Influence on Food and Alcohol Consumption. Ann. N. Y. Acad. Sci, 1139, 426–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund SB & Balleine BW (2008) On habits and addiction: an associative analysis of compulsive drug seeking. Drug Discov. Today Dis. Model, 5, 235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillolla G, Melis M, Perra S, Muntoni AL, Gessa GL, & Pistis M (2007) Medial forebrain bundle stimulation evokes endocannabinoid-mediated modulation of ventral tegmental area dopamine neuron firing in vivo. Psychopharmacology (Berl), 191, 843–853. [DOI] [PubMed] [Google Scholar]
- Quinn JJ, Hitchcott PK, Umeda EA, Arnold AP, & Taylor JR (2007) Sex chromosome complement regulates habit formation. Nat. Neurosci, 10, 1398–1400. [DOI] [PubMed] [Google Scholar]
- Quinn JJ, Pittenger C, Lee AS, Pierson JL, & Taylor JR (2013) Striatum-dependent habits are insensitive to both increases and decreases in reinforcer value in mice. Eur. J. Neurosci, 37, 1012–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renteria R, Baltz ET, & Gremel CM (2018) Chronic alcohol exposure disrupts top-down control over basal ganglia action selection to produce habits. Nat. Commun, 9, 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Cabello C, Alen F, Gomez R, Serrano A, Rivera P, Orio L, Rodriguez De Fonseca F, & Pavon FJ (2012) Effects of the anandamide uptake blocker AM404 on food intake depend on feeding status and route of administration. Pharmacol. Biochem. Behav, 101, 1–7. [DOI] [PubMed] [Google Scholar]
- Ronesi J, Gerdeman GL, & Lovinger DM (2004) Disruption of Endocannabinoid Release and Striatal Long-Term Depression by Postsynaptic Blockade of Endocannabinoid Membrane Transport. J. Neurosci, 24, 1673–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosburg JE, Blankman JL, Long JZ, Nomura DK, Pan B, Kinsey SG, Nguyen PT, Ramesh D, Booker L, Burston JJ, Thomas EA, Selley DE, Sim-Selley LJ, Liu QS, Lichtman AH, & Cravatt BF (2010) Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat. Neurosci, 13, 1113–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L & Wolf OT (2009) Stress Prompts Habit Behavior in Humans. J. Neurosci, 29, 7191–7198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebold M, Deserno L, Nebe S, Schad DJ, Garbusow M, Hägele C, Keller J, Jünger E, Kathmann N, Smolka M, Rapp MA, Schlagenhauf F, Heinz A, & Huys QJM (2014) Model-based and model-free decisions in alcohol dependence. Neuropsychobiology, 70, 122–131. [DOI] [PubMed] [Google Scholar]
- Shan Q, Christie MJ, & Balleine BW (2015) Plasticity in striatopallidal projection neurons mediates the acquisition of habitual actions. Eur. J. Neurosci, 42, 2097–2104. [DOI] [PubMed] [Google Scholar]
- Soethoudt M, Grether U, Perret C, Gils N Van, Petrocellis L De, Ullmer C, Rothenha B, Finlay D, Macdonald C, Chicca A, Gens MD, Stuart J, Vries H De, Mastrangelo N, Xia L, Alachouzos G, Baggelaar MP, Martella A, Mock ED, Deng H, Heitman LH, Connor M, & Marzo V Di (2017) Cannabinoid CB 2 receptor ligand profiling reveals biased signalling and off-target activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straiker A & Mackie K (2005) Depolarization-induced suppression of excitation in murine autaptic hippocampal neurones. J. Physiol, 569, 501–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson AM, Shapiro LP, Whyte AJ, & Gourley SL (2013) Glucocorticoid receptor regulation of action selection and prefrontal cortical dendritic spines. Commun. Integr. Biol, 6, e26068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touriño C, Oveisi F, Lockney J, Piomelli D, & Maldonado R (2010) FAAH deficiency promotes energy storage and enhances the motivation for food. Int. J. Obes, 34, 557–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinod KY, Yalamanchili R, Thanos PK, Vadasz C, Cooper TB, Volkow ND, & Hungund BL (2008) Genetic and pharmacological manipulations of the CB1 receptor alter ethanol preference and dependence in ethanol preferring and nonpreferring mice. Synapse, 62, 574–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V, Derbyshire K, Rück C, Irvine MA, Worbe Y, Enander J, Schreiber LRN, Gillan C, Fineberg NA, Sahakian BJ, Robbins TW, Harrison NA, Wood J, Daw ND, Dayan P, Grant JE, & Bullmore ET (2015) Disorders of compulsivity: A common bias towards learning habits. Mol. Psychiatry, 20, 345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei D, Lee DY, Li D, Daglian J, Jung KM, & Piomelli D (2016) A role for the endocannabinoid 2-arachidonoyl-sn-glycerol for social and high-fat food reward in male mice. Psychopharmacology (Berl), 233, 1911–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH & Knowlton BJ (2006) The role of the basal ganglia in habit formation. Nat. Rev. Neurosci, 7, 464–476. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Chuang HH, Movahed P, Julius D, & Högestätt ED (2000) The anandamide transport inhibitor AM404 activates vanilloid receptors. Eur. J. Pharmacol, 396, 39–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


