Abstract
Triple negative breast cancer (TNBC) represents approximately 10–15% of all breast cancers and has a poor outcome as it lacks a receptor target for therapy, and TNBC is frequently associated with a germline mutation of BRCA1. Poly (ADP-ribose) polymerase inhibitor (PARPi) drugs have demonstrated some effectiveness in treating BRCA1 or BRCA2 mutated breast and ovarian cancers but resistance to PARPi is common. Published results found that resistance to Olaparib, a PARPi, can be due to downregulation of EMI1 and the consequent upregulation of the RAD51 recombinase. Using a tissue culture-based cell viability assay, we extended those observations to another PARPi and to other chemotherapy drugs that affect DNA repair or the cell cycle. As we expected, EMI1 downregulation resulted in resistance to another PARPi drug, Talazoparib. EMI1 downregulation also led to resistance to other cytotoxic drugs, Cisplatin and CHK1 inhibitor. Notably, increasing the RAD51 protein expression only recapitulated some, but not all, of the effects of EMI1 depletion in conferring to the cell resistance to different PARPi and the other cytotoxic drugs. These results suggest that the downstream effects of EMI1 downregulation that contribute to PARPi resistance are increasing the concentration of RAD51 protein in the cell and blocking mitotic entry. We found that combining CHK1 inhibitor with olaparib results in restoration of sensitivity even when EMI1 expression is downregulated. This combination therapy may be a means to overcome the PARPi resistance in BRCA1-deficient TNBC cells.
Introduction
The prognosis of breast cancer depends on several characteristic features, namely, estrogen receptor (ER), progesterone receptor (PR), and HER2 receptor expression and mutation status. The phenotype of germline BRCA1 mutations are usually characterized by aggressiveness, high grade, and are more likely to be triple-negative (ER-, PR-, and HER2-) [1,2]. BRCA1 or BRCA2 mutant breast tumor cells are deficient in the repair of DNA double strand breaks (DSB) via the homology directed repair (HDR) mechanism. This repair mechanism is sequence conserving, and cells lacking the appropriate function of either of these genes have an increased rate of mutation. Poly (ADP-ribose) polymerase inhibitors (PARPi) block the base excision repair mechanism for single strand breaks, but PARPi in combination with a BRCA1 or BRCA2 mutation are synthetic lethal, and such cells are sensitive to PARPi [3,4]. Thus, tumors deficient in BRCA1 or BRCA2 are highly vulnerable to the effects of PARP inhibition [2,5]. PARP inhibitors have shown promise in cancer therapy via a mechanism dependent on synthetic lethality; the inhibition of PARP results in the accumulation of a significant amount of double-strand breaks (DSB) by interfering with replication fork progression at the site of DNA damage [6]. Since BRCA1 and BRCA2 are tumor suppressors, cells heterozygous for a BRCA mutation can repair DSB and are resistant to PARP inhibitors. Loss of the normal allele results in a cell only having a mutated copy of the BRCA gene and sensitivity to the PARPi [2,7]. Despite the promising preliminary results, prolonged treatment of breast or ovarian cancer with PARPi is frequently associated with acquired resistance to this therapy. There are multiple mechanisms of resistance to PARPi chemotherapy, some of them are targetable for therapy including the modulation of EMI1 and RAD51 expression levels [8,9].
HDR is a complex pathway that requires not only the efficient use of BRCA1 and BRCA2 proteins but a number of other related proteins including, among others, REV7, PTIP, RIF1 and RAD51 [10]. The key protein in HDR is the recombinase, RAD51; the activity and level of the RAD51 protein is regulated in part by p53 [11,12]. The level of RAD51 is biologically important in regulating DNA repair [13] and RAD51 overexpression, is associated with an increase in the spontaneous level of HDR with subsequent resistance to ionizing radiation [14].
Another mechanism for resistance to DNA damage in actively dividing cells is the activation of distinct cell checkpoint responses, which pause the cell from progressing to the next cell cycle, enabling DNA repair and promoting cell survival. Upon DNA damage, G2 arrest is triggered by p21-dependent EMI1 downregulation [15]. The EMI1 protein has a constant level of abundance in the human cell with the exception of its regulated degradation from mitotic entry to G1 phase. EMI1 inhibits the activity of the Anaphase Promoting Complex / Cyclosome (APC/C), and degradation of EMI1 by β-Trcp1 activates APC/C [16,17], which in turn degrades key substrates, such as securin and mitotic cyclins, driving the cell into anaphase [15]. Additionally, PARP1 deficient cells show a stronger G2 checkpoint response, which is regulated by the ATR/CHK1 pathway [18,19]. Combination of PARPi with ATRi leads to complete ovarian tumor regression in an HDR-deficient PDX model [19].
Published experiments [20] demonstrated that downregulation of EMI1 in BRCA1-deficient breast cancer cells led to a decrease in sensitivity of the cells to PARPi. They suggested that, in the absence of DNA damage, the E3 ubiquitin ligase activity of EMI1 regulates the stability of RAD51. Marzio et al showed that the resistance to PARPi conferred by decreased EMI1 concentration could be replicated by over-expressing RAD51. Marzio et al tested only olaparib as the PARPi and they suggested, but did not test, that the resistance conferred by low EMI1 concentration could be reversed by including a CHK1 inhibitor to their cell system. In the current study, we tested a second PARPi as well as other DNA-damaging or cytostatic drugs for resistance in cells with low EMI1 concentration, and we tested whether combining CHK1 inhibitor with the PARPi could rescue sensitivity in these cells even though they had low EMI1 concentrations.
Materials and methods
Cell culture
The identities of cell lines were confirmed by short tandem repeat fingerprinting by the OSU Comprehensive Cancer Center Genomics Shared Resource. Cell line MDA-MB-436 (ATCC HTB-130) was propagated in DMEM media supplemented with 10% FBS (VWR Seradigm Life Science), 1% L-glutamine and 1% sodium pyruvate (Life Technology- Thermo-Fisher). MDA-MB-231 (ATCC HTB-26) was propagated in DMEM media with 10% FBS, 1% L-glutamine and 1% sodium pyruvate and 1% penicillin/streptomycin. MCF7 propagated in DMEM media with 10% FBS, 1% L-glutamine and 1% sodium pyruvate, 1% penicillin/streptomycin and 0.01mg/ml bovine insulin. SUM149PT (Asterand Bioscience) was propagated in Ham’s F12/L-glutamine media supplemented with 5% FBS,10mM HEPES, 5 μg/ml insulin and 1 μg/ml hydrocortisone. Cell lines were confirmed by STR fingerprinting and tested periodically for mycoplasma contamination using PCR kit and MycoAlert TM Mycoplasma Detection Kit (Catalog #: LT07-418) and the results were negative.
Gene depletion by siRNA transfection
DNA and RNA oligonucleotides were purchased from IDT. Cells were transfected using either non-targeting siRNA (GL2) [21] that targets the luciferase gene and served as a negative control or siRNAs directed toward EMI1. (Sequences of siRNAs used in this study are provided in S1 Table).
Plasmids
RAD51 cDNA (gift of R. Fishel, Ohio State University) was inserted into a pcDNA3 vector and empty pcDNA3 vector was used as a negative control. Transfections were done using either oligofectamine for siRNA transfection (Invitrogen P/N 58303) using 50 pmol of each siRNA or Lipofectamine 2000 (Invitrogen P/N 52887) for RAD51 vector or Empty pcDNA3 vector, using 3 μg of each vector.
For MDA-MB-436, 5X105 cells were seeded per well in a 6 well plate, 24 h later transfections done, and after overnight incubation media was changed. 3 h later, a second transfection was applied. For MDA-MB-231, 3X105 cells were seeded per well of a 6 well plate, and a similar transfection protocol was applied.
Immunoblotting
Protein was isolated from packed cell pellets using protein lysis buffer (50 mM Tris PH 7.9, 300 mM NaCl, 2.5% NP40, 1 mM EDTA, 5% glycerol). Then samples were resolved on 8% SDS-PAGE gels and transferred to PVDF membrane. The following primary antibodies were used according to the manufacturer's recommendations: EMI1 3D2D6 (Thermo-Fisher (1:500)) or SAB2100793, (Sigma-Aldrich (1mg/ml)), BRCA1 (antibody prepared by Parvin laboratory 1:500) [22], RAD51 (GeneTex, Cat. No. GTX 70230 (1:500)). RNA Helicase A (RHA antibody prepared by Parvin laboratory; 1:20,000) [23] was used as loading control. Secondary antibodies were used according to the manufacturer's specification. anti-rabbit horseradish peroxidase (HRP) 1:5,000 (Cell Signaling Technology, cat. No. 7074), anti-mouse HRP 1:5,000 (Cell Signaling Technology, catalog no. 7076).
Cell viability assay
Different cell lines were transfected as described above, and 24 h after the second transfection, cells were plated by 2500 cells/well for each condition in 96 well plates. 24h later drug is added according to each experiment design. After incubation for 72 h for cisplatin and for 96 h for all other drugs, viability was measured using alamarBlue (Cat. No DAL1025, Thermo-Fisher Scientific) according to manufacturer specifications. The drugs olaparib (Cat. No. A10111-10), Talazoparib (BMN-673; Cat. No. A11243), CHK1 inhibitor (SB218078; Cat. No. A1548), and MEK inhibitor selumetinib (AZD6244; Cat. No. A10257) were purchased from AdooQ. Cisplatin was purchased from Enzo (Cat. No. ALX-400-040-M050).
Statistical analysis
We used the Excel for Mac -Version 16.16.27- to calculate the Student’s two tailed paired t test which determine the significance between the mean of at least three different biological repeats for each dose of drug used before and after EMI1 down regulation. Results are indicated as statistically significant if p<0.05 (*) or p<0.01 (**).The error bars represent the standard error of mean which were calculated by this equation; SEM = (STDEV(A1:A100)/SQRT(COUNT(A1:A100)).
Results
Reduction EMI1 expression and resistance to PARPi, cisplatin, and CHKi
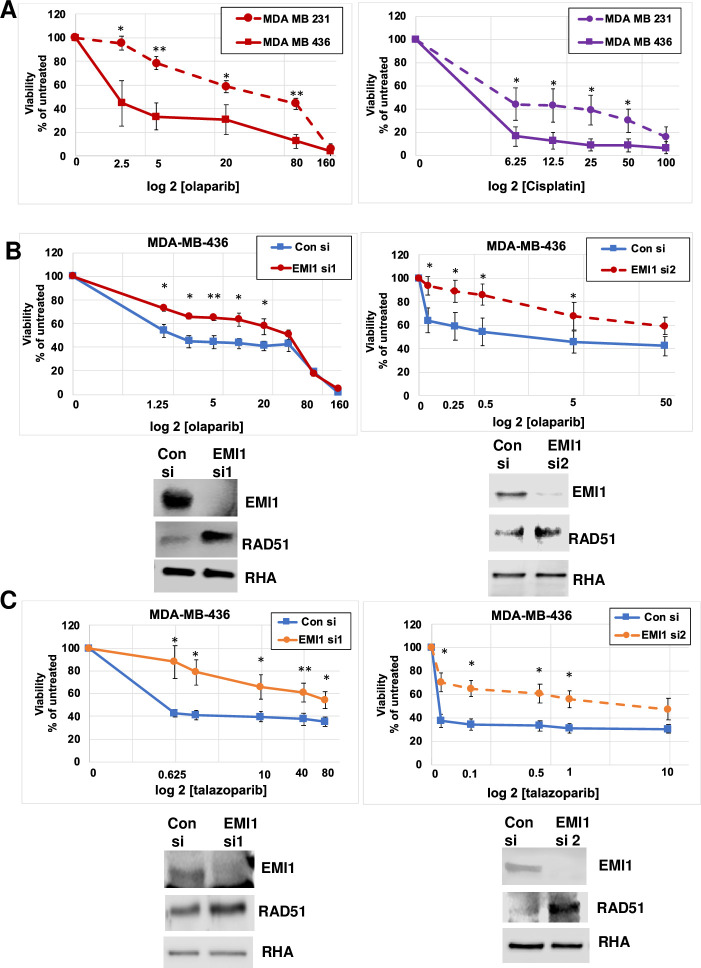
As a first step in extending the findings of Marzio et al, we compared two cell lines, BRCA1-mutated MDA-MB-436 to BRCA1-wild-type MDA-MB-231 cells. Both cell lines recapitulate the phenotypes of Triple Negative Breast Cancer (TNBC) cells. We found, consistent with expectations, that the MDA-MB-436 cells were much more sensitive to olaparib; at all datapoints from 2.5 μM to 80 μM olaparib there was a statistically significant difference in sensitivity to the drug. The IC50 was about 2.5 μM olaparib in MDA-MB-436 cells and about 80 μM olaparib in the BRCA1-wild-type MDA-MB-231 (Fig 1A, left). Similarly, the MDA-MB-436 cells were more sensitive to cisplatin than were MDA-MB-231 cells, though the change in IC50 was not as dramatic (Fig 1A, right). Consistent with the Marzio et al report, depletion of EMI1 in BRCA1 mutated MDA-MB-436 cells by transfection with either of two siRNAs reversed the sensitivity to olaparib and shifted the IC50 in this experiment from about 1.25 μM with normal EMI1 to about 40 μM when EMI1 was depleted (Fig 1B). Western blots revealed that the depletion of EMI1 with each siRNA was effective and resulted in an increase in the abundance of RAD51 protein. Immunoblots of RNA Helicase A (RHA) were used as a loading control. In experiments using the siRNA-1 targeting EMI1, the IC50 was reached at the lowest concentration of olaparib tested (1.25 μM) in the cells receiving the control siRNA; cells receiving the EMI1 specific siRNA were comparatively resistant at these concentrations. We thus tested concentrations lower than 1.25 μM olaparib when repeating this assay with a second siRNA targeting EMI1 (Fig 1B, right). Results of transfection experiments using the two siRNAs targeting EMI1 were exactly consistent and reveal that depletion of EMI1 results in resistance to olaparib.
Fig 1. Depletion of EMI1 in BRCA1 mutant MDA-MB-436 cells confers resistance to PARPi.
(A) Cell lines that mimic TNBC, MDA-MB-436 cells (BRCA1 mutant) and MDA-MB-231 cells (expressing wild-type BRCA1) were grown in medium containing olaparib (left) or cisplatin (right) at the indicated concentrations for 96 h for olaparib or 72 h for cisplatin when cells were assayed for proliferation. For each transfection, the proliferation in the absence of drug was set at 100%. The x-axis is in log10 and the datapoints from samples containing no drug (vehicle only) was placed on the y-intercept. Results represent the mean and SEM of three replicate experiments. Datapoints at each concentration of olaparib were analyzed by the two-sided student’s t test, and * indicates p<0.05, and ** indicates p<0.01. (B) MDA-MB-436 cells were transfected with a control siRNA or EMI1-specific siRNA1 (left) or control siRNA or EMI1 siRNA2 (right) and then grown in medium containing olaparib at the indicated concentrations for 96 h when cells were assayed for proliferation. Western blots are shown from one of the replicates used in the proliferation assay and stained for protein abundance of EMI1, RAD51, and RNA helicase A (RHA), as indicated. Results represent the mean and SEM of five replicate experiments testing EMI1 specific siRNA1 and four replicates for experiments testing siRNA2. (C) MDA-MB-436 cells were transfected as in panel B, and subjected to the indicated concentrations of talazoparib in the culture medium for 96 h when proliferation and protein expression was measured as described above. Results represent the mean and SEM of three replicate experiments.
Extending the results from Marzio et al to a second PARPi, talazoparib, which has a higher propensity than olaparib to have a bound moiety trapped on the DNA [24], we found that depletion of EMI1 by transfection of either of the two siRNAs from MDA-MB-436 cells rendered the cells resistant to talazoparib and the IC50 changed from less than 0.05 μM with normal EMI1 levels to about 10 μM with EMI1 levels reduced by transfection of specific siRNA (Fig 1C), consistent with the expectation of PARPi resistance when the EMI1 levels were depleted. As was observed in Fig 1B, in experiments with EMI1 siRNA1 the viability of cells that were treated with the control siRNA was below 50% at the lowest concentration of talazoparib tested (0.625 μM). We thus tested lower concentrations of talazoparib in experiments using the second EMI1-targeting siRNA (Fig 1C, right). Results clearly indicate that depletion of EMI1 leads to resistance to talazoparib in BRCA1-mutant MDA-MB-436 cells.
Strikingly, we found that low EMI1 levels in these BRCA1 mutated MDA-MB-436 cells can modulate the sensitivity to other cytotoxic drugs, such as cisplatin. Results in Fig 2A show a significant upward shift of the IC50 of cisplatin from lower than 0.5 μM with normal EMI1 levels to about 5 μM with EMI1 levels reduced by specific siRNA transfection. Since the titration of cisplatin when testing the sensitivity of MDA-MB-436 cells transfected with EMI1-specific siRNA-1 the lowest dose tested, 5 μM, was well below the IC50 (Fig 2A, left), we tested lower concentrations of cisplatin for EMI1-specific siRNA-2 (Fig 2A, right). We found that the lowest dose, 0.5 μM was still higher than the IC50 in control siRNA transfected cells, and in EMI1-depleted cells the IC50 was between 1.5 μM and 10 μM cisplatin.
Fig 2. Depletion of EMI1 in BRCA1 mutant MDA-MB-436 cells confers resistance to cytostatic drugs.
(A) MDA-MB-436 cells, transfected as in Fig 1, were subjected to the indicated concentrations of cisplatin for 72 h followed by a proliferation assay and an immunoblot of one of the replicates. The x-axis is in log10 and the datapoints from samples containing no drug (vehicle only) were placed on the y-intercept. Results represent the mean and SEM of three replicate experiments. Datapoints at each concentration of cisplatin were analyzed by the two-sided students t test, and * indicates p<0.05, and ** indicates p<0.01. (B) MDA-MB-436 cells were transfected as in Fig 1 and subjected to the CHK1 inhibitor (SB218078) in the growth medium for 96 h and analyzed as described above. Results represent the mean and SEM of three replicate experiments. (C) MDA-MB-436 cells were transfected with control siRNA and EMI1-specific siRNA2, followed by inclusion of the MEKi (selumetinib) in culture medium at the indicated concentrations for 96 h, followed by analysis for proliferation and protein abundance as described above. Results represent the mean and SEM of three replicate experiments.
We also found that reduction of EMI1 levels resulted in a decrease in sensitivity of CHK1 inhibitor (SB218078) when used as a monotherapy. The IC50 for the CHKi was about 2.5 μM for the control siRNA transfected MDA-MB-436 cells, and transfection of EMI1-specific siRNA-1 resulted in a relative resistance to the CHKi, with an IC50 of about 15 μM CHKi (Fig 2B left). Repeat of this experiment using EMI1-specific siRNA-2 revealed a similar trend, though there was a minor change in the IC50 for both control and EMI1-specific siRNAs.
We next asked if a cytostatic drug that blocks the cell cycle in G1 phase would be affected by EMI1 depletion. Depletion of EMI1 is known to arrest cells in mitosis [15], and MEKi blocks cells in G1 phase of the cell cycle [25,26]. Surprisingly, when we tested the effect of depleted EMI1 levels toward the cytostatic drug, MEK inhibitor (selumetinib AZD6244), we found that depletion of EMI1 resulted in a small shift in sensitivity to MEKi. However, the minor change in sensitivity was not statistically significant at any concentration of the drug, and we conclude that depletion of EMI1 does not confer resistance to the MEK inhibitor [15,25,26].
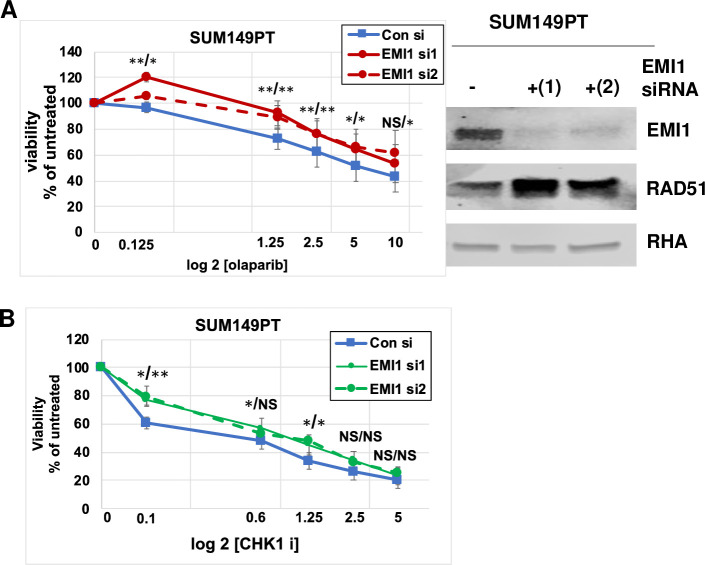
We tested a second BRCA1 mutated cell line, SUM149PT. We found that depletion of EMI1 by siRNA transfection diminished the sensitivity of each, olaparib and CHK1 inhibitor. RNAi depletion of EMI1, using ether of the two specific siRNAs in this cell line, was effective in reducing the expression of EMI1 and with the downstream effect of increasing RAD51 protein levels (Fig 3C). The effect of EMI1 depletion on IC50 was shifted from 5 μM to about more than 10 μM olaparib (Fig 3A) and from 0.6 μM to 1.25 μM CHK1i (Fig 3B). Although the effect of EMI1 depletion on resistance to these two drugs was not as dramatic as observed with MDA-MB-436 cells, the results were statistically significant at most concentrations of olaparib and at some concentrations of CHK1i, as indicated in the Figure. The trends in these observations using SUM-149PT cells were consistent with our previous findings using MDA-MB-436 cells and with published results [20]. Taken together with our other results, these results suggest that low expression of EMI1 can modulate the sensitivity to several chemotherapeutic drugs, not only PARPi, and this is a potential mechanism to cancer therapy resistance.
Fig 3. Depletion of EMI1 in BRCA1 mutant SUM149PT cells confers resistance to olaparib and CHK1i.
(A) SUM149PT cells were transfected with control siRNA, EMI1-specific siRNA1 or EMI1-specific siRNA2, followed by inclusion of olaparib in culture medium at the indicated concentrations for 96 h. Cells were then analyzed for proliferation, and the measure in samples without drug (vehicle only) were set at 100%. Results represent the mean and SEM of three replicate experiments. Datapoints at each concentration of olaparib were analyzed by the two-sided students t test, and * represents p<0.05, ** represents p<0.01, and NS represents p>0.05. At each concentration of drug, the first asterisks represent EMI siRNA1 versus control, and the second asterisks represent EMI1 siRNA2 versus control. Results represent the mean and SEM of three replicate experiments. (B) SUM149PT cells were transfected as in panel A and subjected to growth in medium with CHK1i (SB218078) at the indicated concentrations for 96 h followed by a proliferation assay. Results were analyzed as in panel A and represent the mean and SEM of three replicate experiments. (C) Protein lysates from one of the replicates used in panels A and B were analyzed by immunoblot and probed for EMI1, RAD51, and RHA protein abundance, as indicated.
Effect of EMI1 depletion on resistance to some chemotherapy drugs is not phenocopied by elevated RAD51 protein abundance
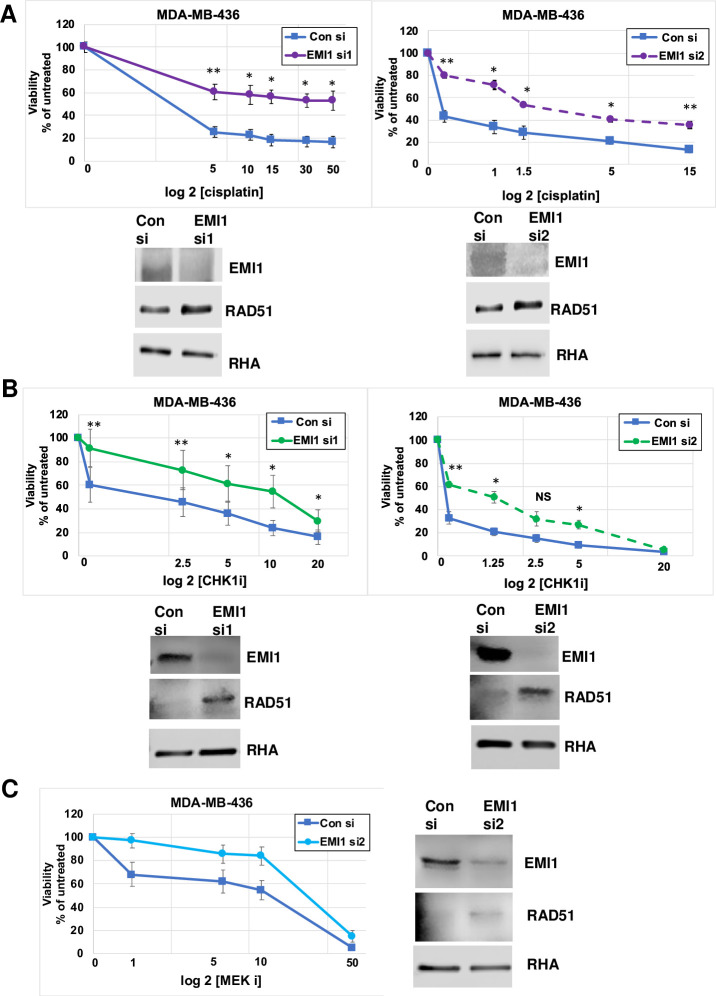
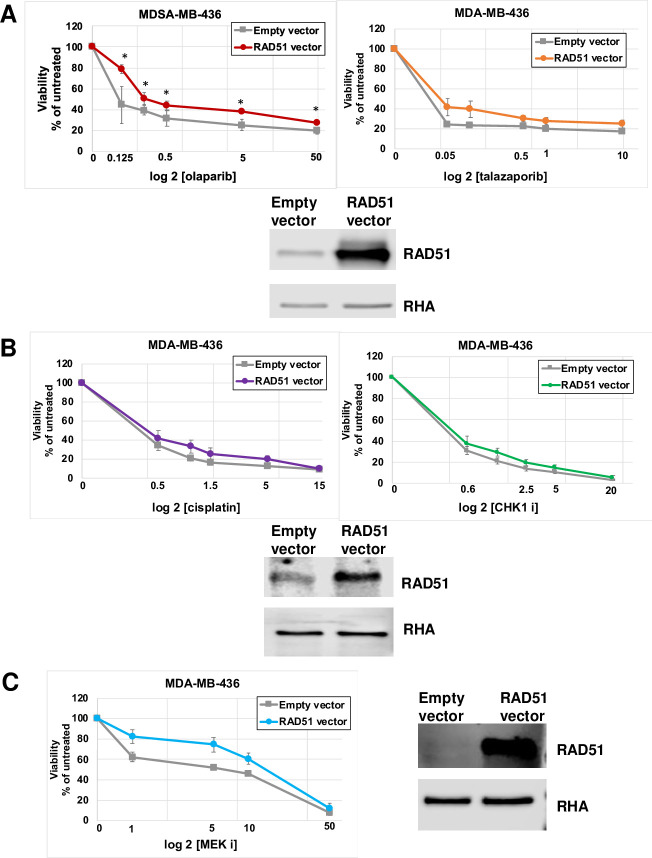
It had been observed that resistance to olaparib due to EMI1 depletion could be recapitulated by expressing RAD51 at higher levels [20]. The results of that study indicated that RAD51 protein was a substrate of the EMI1 ubiquitin ligase, and this regulatory event was the key downstream effect of low EMI1 levels. We tested the effect of RAD51 overexpression in BRCA1-mutant cells on sensitivity to different cytotoxic drugs including the PARPi. We prepared an expression vector for human RAD51 that can be used for transfection of mammalian cells and increase the expression of RAD51 protein in BRCA1-mutant MDA-MB-436 cells. As measured by immunoblot analysis, transient transfection of RAD51 in MDA-MB-436 cells resulted in a several-fold increase in RAD51 protein levels (Fig 4A). Similar to the results of Marzio et al, we found that the resistance to olaparib conferred by EMI1 depletion was largely recapitulated by RAD51 overexpression (Fig 4A, left). The IC50 for olaparib was shifted from lower than 0.125 μM with normal, endogenous RAD51 levels to about 0.25 μM with overexpressed RAD51 and, though the change was less dramatic than observed with EMI1 depletion (Fig 1B), it was statistically significant at each concentration of olaparib (Fig 4A left). By contrast, overexpression of RAD51 did shift the curve of the cells resistant to talazoparib, but for none of the data points were the differences statistically significant (Fig 4A, right). Similarly, overexpression of RAD51 resulted in a minor, and not statistically significant, shift of the curve for MDA-MBD-436 cells treated with cisplatin (Fig 4B left), CHK1 inhibitor (Fig 4B right) and MEK inhibitor (Fig 4C).These results indicated that high abundance of RAD51 protein, alone, cannot explain the resistance to these drugs by low expression of EMI1. Inactivation of EMI1 leads to premature APC/C activation in G2-phase, and consequently blocks cell entry into mitosis [27,28]. PARP-induced cytotoxicity has been attributed to repeated cycles of both replication and mitosis showing that forced mitotic bypass through EMI1 depletion could largely rescue viability of HDR-deficient cells upon PARP inhibition [29]. Taken together, we infer that two effects of EMI1 depletion, the increased abundance of RAD51 protein and a cell cycle block in G2, combine to overcome the effects of PARPi, cisplatin, and CHK1i.
Fig 4. RAD51 overexpression does not recapitulate all of the effects of EMI1 depletion on resistance to cytostatic drugs in MDA-MB-436 cells.
(A) MDA-MB-436 cells were transfected with pcDNA3 empty vector or with the same vector with the human RAD51 gene. Cells were grown for 96 h in medium containing the indicated concentration of olaparib (left) or talazoparib (middle) followed by a proliferation assay. Results represent the mean and SEM of three replicate experiments. Datapoints at each concentration of drug were analyzed by the two-sided student’s t test, and * represents p<0.05. Protein lysates from one replicate were analyzed by immunoblot for RAD51 protein abundance (right). (B) MDA-MB-436 cells were transfected as in panel A, and cells were grown in medium containing the indicated concentration of cisplatin (left) for 72 h or CHK1i (middle) for 96 h followed by a proliferation assay. Results were analyzed as in panel A and represent the mean and SEM of three replicate experiments. Protein lysates from one of the replicates were analyzed by immunoblot for RAD51 protein abundance (right). (C) MDA-MB-436 cells were transfected as in panel A, and cells were grown in medium containing the indicated concentration of MEKi (left) for 96 h followed by a proliferation assay. Results were analyzed as in panel A and represent the mean and SEM of three replicate experiments. Protein lysates from one of the replicates were analyzed by immunoblot for RAD51 protein abundance (right).
Combining CHK1 inhibitor with PARPi restores sensitivity to BRCA1 mutated breast cancer cells with low EMI1 expression
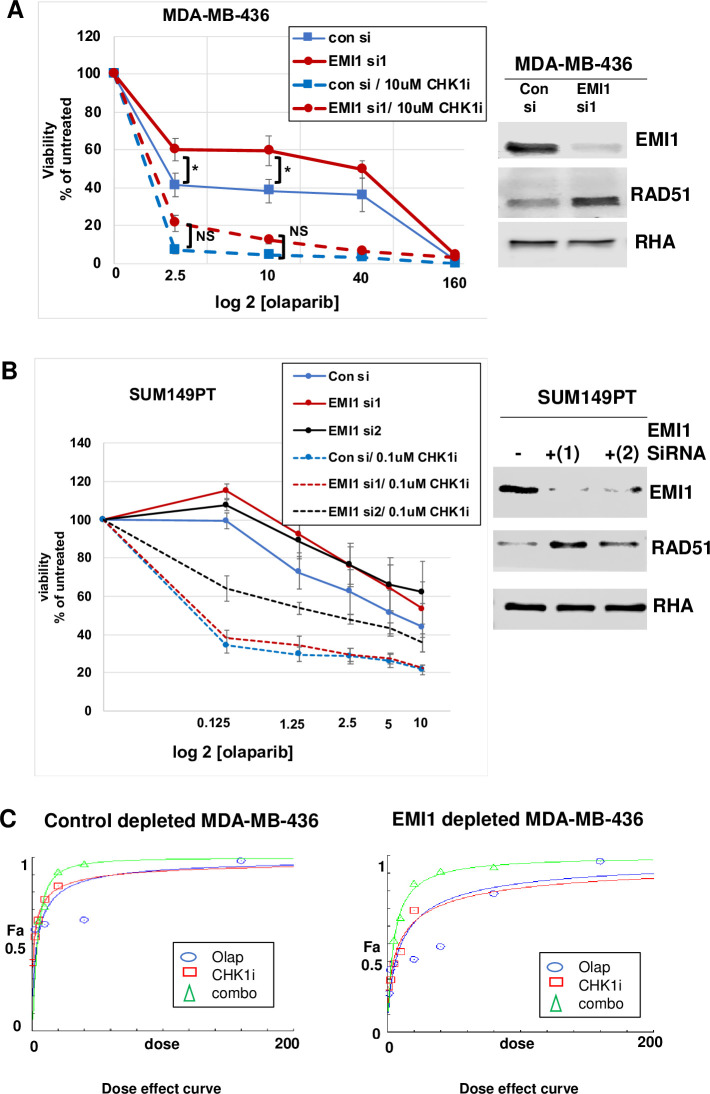
In their earlier publication, Marzio and his colleagues suggested, but did not test, that the resistance to olaparib conferred by low EMI1 concentration could be reversed by including a CHK1 inhibitor [20]. In addition, it was reported that a stronger DNA damage-induced G2 checkpoint dependent on CHK1 activation in the absence of PARP1 could be abolished by CHK1 siRNA, sensitizing PARP1-/- cells to IR-induced killing [18]. Taking into consideration these results, we tested the combination of CHK1 inhibitor and olaparib in BRCA1-mutated MDA-MB-436 and SUM149PT cells. MDA-MB-436 samples were subjected to 10 μM CHK1i, and the olaparib was titrated from 0 to 160 μM, while, SUM149PT samples were subjected to 0.1 μM CHK1i, and the olaparib was titrated from 0 to 10 μM. In the presence of both, CHK1i and olaparib, the resistance due to EMI1 depletion was reversed (Fig 5A). Consistent with our results from Fig 2, in the presence of one drug, olaparib, EMI1 depletion resulted in a significantly higher percentage of viable cells than control depletion (solid lines in Fig 5A). By contrast, inclusion in culture media of CHK1i resulted in a sensitivity of these cells to olaparib in both control and EMI1-depleted cells (dashed lines in Fig 5A). The IC50 for olaparib in EMI1 depleted cells changed from 40 μM to less than 2.5 μM when CHK1i was included in the culture medium, and the differences in cell viability comparing control depleted versus EMI1-depleted MDA-MB-436 cells under these conditions with two drugs was not statistically significant (p> 0.05), indicating that EMI1 depletion conferred no resistance to olaparib when in the presence of the CHK1i. Similarly, when testing the combination of olaparib and CHK1i in SUM149PT cells, we found that the combination rendered EMI1-depleted cells sensitive (Fig 5B). Just as in the MDA-MB-436 cells, SUM149PT cells depleted of EMI1 were relatively resistant to olaparib, and the proliferation of these cells dropped in the presence of both drugs. Results were similar when depleting EMI1 from these cells using siRNA2; the IC50 for olaparib in EMI1-siRNA2 depleted cells was greater than 10 μM and in the presence of both drugs was about 2 μM. Though the magnitude of the change in sensitivity to two drugs when transfecting with siRNA2 was not as large as with siRNA1, the results were consistent. The p-values for student’s t-test comparisons are given in the legend to Fig 5B. These results suggest that CHK1 inhibitor in combination with PARPi could be a viable treatment option in these tumor types when EMI1 depletion causes resistance.
Fig 5. Combination of olaparib and CHK1i restores sensitivity to EMI1-depleted MDA-MB-436 and SUM149PT cells.
(A) MDA-MB-436 cells were transfected with control siRNA or EMI1-specific siRNA1 and grown for 96 h in medium containing vehicle plus olaparib at the indicated concentrations (solid lines) or in medium containing 10 μM CHK1i plus olaparib at the indicated concentrations (dashed lines). Proliferation assays (n = 3) measured the growth of the cells under each condition. The growth of control siRNA transfected cells was compared to the growth of EMI1 depleted cultures grown in the absence of CHK1i and in the presence of CHK1i. P-values comparing the growth of EMI1-depleted cells versus control siRNA-transfected cells were less than 0.05 at olaparib concentrations of 2.5 μM and 10 μM in the presence of a single drug, but in the presence of both drugs, the p-values were not significant. Protein lysates from one replicate were analyzed by immunoblot for EMI1 and RAD51 protein abundance (right). (B) SUM149PT cells were transfected with control siRNA or EMI1-specific siRNA1 or siRNA2 and grown for 96 h in medium containing vehicle plus olaparib at the indicated concentrations (solid lines) or in medium containing 0.1μM CHK1i plus olaparaib at the indicated concentrations (dashed lines) and analyzed as in panel A. When comparing EMI1-siRNA1 to control siRNA, p-values were less than 0.05 at concentrations of olaparib of 0.125 μM, 1.25 μM, 2.5 μM, and 5 μM when testing the single drug, and was not significant (p > 0.05) at all concentrations when comparing EMI1 siRNA1 to control siRNA in two drugs. When comparing EMI1-siRNA2 to control siRNA, p-values were less than 0.05 at all concentrations of olaparib tested in the single drug and in the two drug conditions. (C) CompuSyn software analysis of additive effects of olaparib and CHK1i in MDA-MB-436 cells. The dose effect (x-axis) was plotted against the fraction of the total drug effect (Fa, y-axis) for olaparib alone (blue ovals), CHK1i alone (red rectangles) or the combination of both drugs (green triangles) in control siRNA transfected cells (left) and EMI1 siRNA1 depleted cells (right).
We analyzed if the CHK1i and olaparib were acting synergistically in MDA-MD-436 cells by applying the Chou-Talalay method [30] and using the computer software CompuSyn. We observed a synergistic effect of the two drugs increased in the EMI1-depleted MDA-MB-436 cells (Fig 5C). For Fig 5C, we used results from siRNA1 depletion of EMI1. This synergistic effect of the combination of olaparib and CHK1i in EMI1-depleted MDA-MB-436 cells is evident in the increase in separation of the curve for the drug combination from the curves for the single drugs.
Discussion
In this study, we discovered: 1) depletion of EMI1 made BRCA1-mutant cells resistant to talazaparib, cisplatin, and CHK1i in addition to the previously shown resistance to olaparib [20]; 2) only some of the effects of depleting EMI1 on drug resistance were recapitulated by over expression of RAD51, suggesting that EMI1 effects on cell cycle progression also contribute to the PARPi resistance; 3) combinations of EMI1 depletion and CHK1i restored sensitivity of the BRCA1-mutant cells toward PARPi; and 4) olaparib and CHK1i were a synergistic drug combination over a wide concentration range in BRCA1 mutant cells expressing a low level of EMI1 protein.
It is well established that the “BRCAness” phenotype of the breast cancer cells plays a crucial role toward PARPi sensitivity and conferring the concept of synthetic lethality, and our results are consistent with that concept. Partial restoration of the homologous recombination DNA repair pathway can lead to resistance to PARPi [31], and upregulation of RAD51 in BRCA1-defective cells is associated with resistance to PARPi [20]. It is worth noting that our results of olaparib resistance in different BRCA1 mutant cells due to EMI1 downregulation that were associated with high RAD51 levels are in line with previous results done either in BRCA1 mutant cancer cells [20] or BRCA2 mutant ones [29]. As expected, our results extended the findings of low EMI1 levels toward modulating the resistance to another PARPi, talazoparib, and strikingly, our results show resistance upon EMI1 downregulation to other cytotoxic drugs including cisplatin and CHK1i. We hypothesized that the RAD51 increase that accompanied the low EMI1 levels may explain the resistance as high RAD51 expression is reported to be associated with decreased cytotoxicity to DNA damage induced by chemical agents and/or ionizing radiation [14,32,33] and RAD51 downregulation can lead to chemo/radio-sensitizing effect [34]. However, RAD51 overexpression only recapitulated some aspects of the phenotype of resistance to PARPi due to low EMI1 levels, such as conferring to the cell resistance to olaparib, but the cells were still sensitive to talazaparib, cisplatin, and CHK1i. We infer from these differences that other effects of EMI1 on cell growth also contribute to the PARPi resistance such as the well-known requirement for EMI1 function for cells to enter mitosis. Normal EMI1 function inhibits APC/C and also inhibits DNA re-replication, promoting cell proliferation [35]. Inactivation of EMI1 leads to premature APC/C activation in G2-phase, interferes with cyclin B accumulation and consequently precludes mitotic entry. As a result, EMI1-depleted cells bypass mitosis and enter cycles of endoreplication [27,28,36]. We suggest that BRCA1 mutated breast cancer cells can develop PARPi resistance by downregulating the EMI1 gene which could be triggered via p21 [15]. Since EMI1 is an essential gene in vivo [37] and is also required for long-term growth in vitro [38–40], the downregulation of EMI1 must either be transient or retain residual activity sufficient for cell viability. The downregulation of EMI1 arrests cells in G2 phase of cell cycle, tumor cells that remain in G1- or G2-phase longer are less sensitive to PARP-inhibitor-induced cytotoxicity [29]. In addition, down regulation of EMI1 stabilizes RAD51 protein levels since EMI1 regulates the ubiquitin-mediated degradation of RAD51 [20]. Our results suggest that these combined effects are responsible for the chemotherapy resistance conferred by EMI1 depletion, and overexpression of RAD51 causes only partial resistance to some PARPi.
It had been predicted that combined effects of CHK1i and PARPi would restore sensitivity of cells that had reduced EMI1 expression levels [8,20]. Results presented in this study confirm this notion for BRCA1-mutant breast cancer cells. These results are consistent with recently published results for other BRCA1/2 mutant olaparib sensitive and olaparib resistant ovarian cancer cell lines [41]. These results suggest a potential treatment strategy toward overcoming PARPi resistance in BRCA1 associated breast cancer cells.
Supporting information
(DOCX)
(DOCX)
A constant-ratio drug combinations 1:1 of olaparib:CHK1 I was analyzed in all cells tested. When analyzing the proliferation of EMI1-depleted cells versus control siRNA transfected cells, the CI values for all concentrations tested were lower than 1, suggestive of a synergistic effect between the two drugs under these conditions.
(TIF)
(PDF)
Acknowledgments
We would like to thank the members working in Parvin lab for invaluable assistance, including, Aleksandra Adamovich, Tapahsama Banerjee, Xue Wu, Kathryn Duncan, Mariame Diabate, Alexander E. Hare and Gregory R. Nagy.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by grant CA228083 from the National Cancer Institute and Egyptian Ministry of Higher Education and scientific Research (Mission sector).
References
- 1.Atchley DP, Albarracin CT, Lopez A, Valero V, Amos CI, Gonzalez-Angulo AM, et al. Clinical and pathologic characteristics of patients with BRCA-positive and BRCA-negative breast cancer. Journal of Clinical Oncology. 2008;26(26):4282–8. 10.1200/JCO.2008.16.6231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dziadkowiec KN, Gasiorowska E, Nowak-Markwitz E, Jankowska A. PARP inhibitors: Review of mechanisms of action and BRCA1/2 mutation targeting. Przeglad Menopauzalny. 2016;15(4):215–9. 10.5114/pm.2016.65667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farmer H, McCabe N, Lord CJ, Tutt ANJ, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature [Internet]. 2005;434(7035):917–21. Available from: 10.1038/nature03445 [DOI] [PubMed] [Google Scholar]
- 4.Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature [Internet]. 2005;434(7035):913–7. Available from: 10.1038/nature03443 [DOI] [PubMed] [Google Scholar]
- 5.Rouleau Michèle Patel Anand, Hendzel MJ Kaufmann SH, Poirier GG. PARP inhibition: PARP1 and beyond Michèle. Nat Rev Cancer. 2010;10(4):293–301. 10.1038/nrc2812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anwar M, Aslam HM, Anwar S. PARP inhibitors. Hereditary Cancer in Clinical Practice. 2015;13(1):24–7. 10.1186/s13053-014-0024-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tutt ANJ, Lord CJ, McCabe N, Farmer H, Turner N, Martin NM, et al. Exploiting the DNA repair defect in BRCA mutant cells in the design of new therapeutic strategies for cancer. Cold Spring Harbor Symposia on Quantitative Biology. 2005;70:139–48. 10.1101/sqb.2005.70.012 [DOI] [PubMed] [Google Scholar]
- 8.Adamovich AI, Toland AE, Parvin JD. F-Box Protein-Mediated Resistance to PARP Inhibitor Therapy. Molecular Cell [Internet]. 2019;73(2):195–6. Available from: 10.1016/j.molcel.2018.12.019 [DOI] [PubMed] [Google Scholar]
- 9.Noordermeer SM, van Attikum H. PARP Inhibitor Resistance: A Tug-of-War in BRCA-Mutated Cells. Trends in Cell Biology. 2019;29(10):820–34. 10.1016/j.tcb.2019.07.008 [DOI] [PubMed] [Google Scholar]
- 10.D’Andrea AD. Mechanisms of PARP inhibitor sensitivity and resistance. DNA Repair [Internet]. 2018;71(August):172–6. Available from: 10.1016/j.dnarep.2018.08.021 [DOI] [PubMed] [Google Scholar]
- 11.Magnusson KP, Sandström M, Ståhlberg M, Larsson M, Flygare J, Hellgren D, et al. p53 splice acceptor site mutation and increased HsRAD51 protein expression in Bloom’s syndrome GM1492 fibroblasts. Gene. 2000;246(1–2):247–54. 10.1016/s0378-1119(00)00068-8 [DOI] [PubMed] [Google Scholar]
- 12.Xia SJ, Shammas MA, Shmookler Reis RJ. Elevated recombination in immortal human cells is mediated by HsRAD51 recombinase. Molecular and Cellular Biology. 1997;17(12):7151–8. 10.1128/mcb.17.12.7151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lundin C, Schultz N, Arnaudeau C, Mohindra A, Hansen LT, Helleday T. RAD51 is involved in repair of damage associated with DNA replication in mammalian cells. Journal of Molecular Biology. 2003;328(3):521–35. 10.1016/s0022-2836(03)00313-9 [DOI] [PubMed] [Google Scholar]
- 14.Vispé S, Cazaux C, Lesca C, Defais M. Overexpression of Rad51 protein stimulates homologous recombination and increases resistance of mammalian cells to ionizing radiation. Nucleic Acids Research. 1998;26(12):2859–64. 10.1093/nar/26.12.2859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jinho Lee, Kim JAH Barbier Valerie, Arun Fotedar, Rati Fotedar. Title:DNA Damage Triggers p21WAF1-dependent Emi1 Down-Regulation That Maintains G2 Arrest.pdf. Molecular Biology of the Cell. 2008;Vol. 20,:1891–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guardavaccaro D, Kudo Y, Boulaire J, Barchi M, Busino L, Donzelli M, et al. Control of meiotic and mitotic progression by the F box protein β-Trcp1 in vivo. Developmental Cell. 2003;4(6):799–812. 10.1016/s1534-5807(03)00154-0 [DOI] [PubMed] [Google Scholar]
- 17.Margottin-Goguet F, Hsu JY, Loktev A, Hsieh HM, Reimann JDR, Jackson PK. Prophase destruction of Emi1 by the SCFβTrCP/Slimb ubiquitin ligase activates the anaphase promoting complex to allow progression beyond prometaphase. Developmental Cell. 2003;4(6):813–26. 10.1016/s1534-5807(03)00153-9 [DOI] [PubMed] [Google Scholar]
- 18.Lu HR, Wang X, Wang Y. A stronger DNA damage-induced G2 checkpoint due to over-activated CHK1 in the absence of PARP-1. Cell Cycle. 2006;5(20):2364–70. 10.4161/cc.5.20.3355 [DOI] [PubMed] [Google Scholar]
- 19.Kim H, George E, Ragland RL, Rafail S, Zhang R, Krepler C, et al. Targeting the ATR/CHK1 axis with PARP inhibition results in tumor regression in BRCA-mutant ovarian cancer models. Clinical Cancer Research. 2017;23(12):3097–108. 10.1158/1078-0432.CCR-16-2273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marzio A, Puccini J, Kwon Y, Maverakis NK, Arbini A, Sung P, et al. The F-Box Domain-Dependent Activity of EMI1 Regulates PARPi Sensitivity in Triple-Negative Breast Cancers. Molecular Cell [Internet]. 2019;73(2):224–237.e6. Available from: 10.1016/j.molcel.2018.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ransburgh DJR, Chiba N, Ishioka C, Toland AE, Parvin JD. Identification of breast tumor mutations in BRCA1 that abolish its function in homologous DNA recombination. Cancer Research. 2010;70(3):988–95. 10.1158/0008-5472.CAN-09-2850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlegel BP, Starita LM, Parvin JD. Overexpression of a protein fragment of RNA helicase A causes inhibition of endogenous BRCA1 function and defects in ploidy and cytokinesis in mammary epithelial cells. Oncogene. 2003. February;22(7):983–91. 10.1038/sj.onc.1206195 [DOI] [PubMed] [Google Scholar]
- 23.Anderson SF, Schlegel BP, Nakajima T, Wolpin ES, Parvin JD. BRCA1 protein is linked to the RNA polymerase II holoenzyme complex via RNA helicase A. Nature genetics. 1998. July;19(3):254–6. 10.1038/930 [DOI] [PubMed] [Google Scholar]
- 24.Murai J, Huang S-YN, Renaud A, Zhang Y, Ji J, Takeda S, et al. Stereospecific PARP trapping by BMN 673 and comparison with olaparib and rucaparib. Molecular cancer therapeutics. 2014. February;13(2):433–43. 10.1158/1535-7163.MCT-13-0803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bartholomeusz C, Oishi T, Saso H, Akar U, Liu P, Kondo K, et al. MEK1/2 inhibitor selumetinib (AZD6244) inhibits growth of ovarian clear cell carcinoma in a PEA-15-dependent manner in a mouse xenograft model. Molecular Cancer Therapeutics. 2012;11(2):360–9. 10.1158/1535-7163.MCT-11-0400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zou Y, Wang J, Leng X, Huang J, Xue W, Zhang J, et al. The selective MEK1 inhibitor Selumetinib enhances the antitumor activity of everolimus against renal cell carcinoma in vitro and in vivo. Oncotarget. 2017;8(13):20825–33. 10.18632/oncotarget.15346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reimann JDR, Freed E, Hsu JY, Kramer ER, Peters JM, Jackson PK. Emi1 is a mitotic regulator that interacts with Cdc20 and inhibits the anaphase promoting complex. Cell. 2001;105(5):645–55. 10.1016/s0092-8674(01)00361-0 [DOI] [PubMed] [Google Scholar]
- 28.Machida YJ, Dutta A. The APC/C inhibitor, Emi1, is essential for prevention of rereplication. Genes and Development. 2007;21(2):184–94. 10.1101/gad.1495007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schoonen PM, Talens F, Stok C, Gogola E, Heijink AM, Bouwman P, et al. Progression through mitosis promotes PARP inhibitor-induced cytotoxicity in homologous recombination-deficient cancer cells. Nature Communications [Internet]. 2017. December 17;8(1):15981 Available from: http://www.nature.com/articles/ncomms15981. 10.1038/ncomms15981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Advances in Enzyme Regulation [Internet]. 1984;22(C):27–55. Available from: https://www.sciencedirect.com/science/article/abs/pii/0065257184900074. 10.1016/0065-2571(84)90007-4 [DOI] [PubMed] [Google Scholar]
- 31.Kim Y, Kim A, Sharip A, Sharip A, Jiang J, Yang Q, et al. Reverse the resistance to PARP inhibitors. International Journal of Biological Sciences. 2017;13(2):198–208. 10.7150/ijbs.17240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schild D, Wiese C. Overexpression of RAD51 suppresses recombination defects: A possible mechanism to reverse genomic instability. Nucleic Acids Research. 2009;38(4):1061–70. 10.1093/nar/gkp1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu Y, Chen K, Cai Y, Cheng C, Zhang Z, Xu G. Overexpression of Rad51 predicts poor prognosis and silencing of Rad51 increases chemo-sensitivity to doxorubicin in neuroblastoma. American Journal of Translational Research. 2019;11(9):5788–99. [PMC free article] [PubMed] [Google Scholar]
- 34.Du LQ, Wang Y, Wang H, Cao J, Liu Q, Fan FY. Knockdown of Rad51 expression induces radiation- and chemo-sensitivity in osteosarcoma cells. Medical Oncology. 2011;28(4):1481–7. 10.1007/s12032-010-9605-1 [DOI] [PubMed] [Google Scholar]
- 35.Yamano H. EMI1, a three-in-one ubiquitylation inhibitor. Nature Structural and Molecular Biology. 2013;20(7):773–4. 10.1038/nsmb.2626 [DOI] [PubMed] [Google Scholar]
- 36.Wang W, Kirschner MW. Emi1 preferentially inhibits ubiquitin chain elongation by the anaphase-promoting complex. Nature Cell Biology. 2013. July;15(7):797–806. 10.1038/ncb2755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee H, Lee DJ, Oh SP, Park HD, Nam HH, Kim JM, et al. Mouse emi1 Has an Essential Function in Mitotic Progression during Early Embryogenesis. Molecular and Cellular Biology. 2006;26(14):5373–81. 10.1128/MCB.00043-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hart T, Chandrashekhar M, Aregger M, Steinhart Z, Brown KR, MacLeod G, et al. High-Resolution CRISPR Screens Reveal Fitness Genes and Genotype-Specific Cancer Liabilities. Cell [Internet]. 2015;163(6):1515–26. Available from: 10.1016/j.cell.2015.11.015 [DOI] [PubMed] [Google Scholar]
- 39.Wang T, Birsoy K, Hughes NW, Krupczak KM, Post Y, Wei JJ, et al. Identification and characterization of essential genes in the human genome. Science. 2015;350(6264):1096–101. 10.1126/science.aac7041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blomen VA, Májek Peter, Jae LT, Bigenzahn JW, Nieuwenhuis Joppe, Staring Jacqueline, et al. Gene essentiality and synthetic lethality in haploid human cells. Science. 2015;350(6264):1092–6. 10.1126/science.aac7557 [DOI] [PubMed] [Google Scholar]
- 41.Burgess BT, Anderson AM, McCorkle JR, Wu J, Ueland FR, Kolesar JM. Olaparib combined with an ATR or Chk1 inhibitor as a treatment strategy for acquired olaparib-resistant BRCA1 mutant ovarian cells. Diagnostics. 2020;10(2). 10.3390/diagnostics10020121 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
A constant-ratio drug combinations 1:1 of olaparib:CHK1 I was analyzed in all cells tested. When analyzing the proliferation of EMI1-depleted cells versus control siRNA transfected cells, the CI values for all concentrations tested were lower than 1, suggestive of a synergistic effect between the two drugs under these conditions.
(TIF)
(PDF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.