Abstract
Quercetin is a flavonoid compound widely present in plants and exhibits a variety of biological activities. Research on quercetin has shown its potential for medical application. In this research, we elucidate its antioxidant mechanism and the broad-spectrum antibacterial and antiparasite properties; summarise its potential application in antioncology and cardiovascular protection and anti-immunosuppression treatment; and demonstrate its ability to alleviate the toxicity of mycotoxins. This research is expected to offer some insights and inspirations for the further study of quercetin, its properties, and the scientific basis for its better application in clinical practice.
1. Introduction
Quercetin, the name coming from quercetum (oak forest), named after Quercus, has been applied since 1857. It is widely found in plants in nature, including apples, berries, brassica vegetables, capers, grapes, onions, spring onions, tea, and tomatoes, as well as in many seeds, nuts, flowers, bark, and leaves [1]. However, quercetin is also contained in medicinal plants, including Ginkgo biloba, Hypericum perforatum, and elderberry [2–4], and is mainly derived from onions, apples, and tea [5]. Its molecular formula is C15H10O7, and the chemical structural formula is illustrated in Figure 1. It is a naturally occurring polar auxin transport inhibitor [6]. Quercetin has a ketocarbonyl group in its molecule, and the oxygen atom on the first carbon is basic and can generate salts with strong acids. Its molecular structure contains four active groups, namely, a dihydroxy group between the A ring, o-dihydroxy group B, C ring C2, C3 double bond, and 4-carbonyl. The presence of a phenolic hydroxyl group and double bonds endows quercetin with a strong antioxidant activity. Its antioxidant and anti-inflammatory properties are closely related to the prevention and treatment of cardiovascular diseases and cancer. In addition, in vivo and in vitro studies have found that quercetin also has antibacterial activity and effectively reduces the formation of biofilms by inhibiting the expression of related genes, antitumour activity, antiangiogenic activity, etc. In addition, quercetin plays an important role in reducing mycotoxins, protecting cells from damage. We have selected and analysed the key aspects of the biological functions of quercetin and its potential applications in clinical medicine to reach a unified understanding of its various functions. This review is designed to help with further research, and its nature is to provide some insights and enlightenment, providing a scientific basis for its better clinical application.
Figure 1.
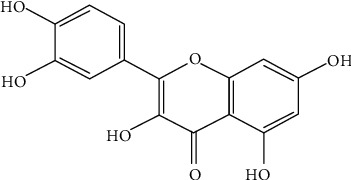
Structural formula of quercetin.
2. The Main Pharmacological Activity of Quercetin
2.1. Antioxidant
Free radicals are produced by the body during metabolism and are among the causes of many diseases. They can cause cell membrane damage and gene mutation, accelerate aging of the body, and induce various diseases, such as heart disease, liver damage, and diabetes [7, 8]. Hanasaki et al. [9] found that quercetin is the most effective free radical scavenger in the flavonoid family. By investigating the chemical structure of quercetin, it was found that there are four hydroxyl groups on the benzo-dihydropyran ring of the polyphenol, so quercetin has a strong antioxidant capacity, can eliminate free radicals produced in the body, and can help the body maintain a stable state.
The antioxidant mechanisms of quercetin in vitro mainly include the following:
Directly scavenging free radicals: Oh et al. [10] proved that quercetin had a strong antioxidant capacity, and it showed the highest antioxidant activity in all test samples. In addition, Manca et al. [11] found that quercetin adulterated with liposomes and glycerol nanoparticles could scavenge free radicals and protect human keratinocytes from hydrogen peroxide damage in vitro.
Chelating metal ions: related studies have confirmed that quercetin can induce Cu2+ and Fe2+ to play an antioxidant role through catechol in its structure. Tang et al. [12] fed adult male C57BL/6J mice to form a model of alcoholic liver disease and treated them with quercetin. The results suggested that quercetin could inhibit Fe2+-induced lipid peroxidation by binding Fe2+ and finally inhibit iron overload and oxidative damage in alcoholic liver disease. Babenkova et al. [13] undertook a chemiluminescence study to demonstrate that Fe2+ in compounds containing dihydroquercetin is inactive, unable to catalyse the decomposition of hydrogen peroxide, and unable to trigger further generation of hydroxyl free radicals. Therefore, quercetin can play the role of antioxidant stress through various cohorts and Fe2+.
Inhibiting lipid peroxidation: Lim et al. [14] confirmed that quercetin could inhibit the oxidative modification of low-density lipoprotein by observing the changes in the fluorescence intensity of thiobarbital, phosphatidylcholine hydroperoxides, and oxidised low-density lipoprotein, thus inhibiting the oxidative damage of LDL. Mbikay et al. [15] verified that, at low concentrations, quercetin can increase the expression of LDL-R, reduce the secretion of PCSK9, stimulate the uptake of LDL, and thus inhibit LDL oxidative damage.
The antioxidation mechanisms of quercetin in vivo are mainly such that the antioxidant capacity of quercetin is gradient-dependent and a high concentration of quercetin confers strong antioxidant capacity:
The antioxidant characteristics of quercetin: these are mainly manifested in the regulation of glutathione levels to enhance antioxidant capacity. When ROS are generated, SOD-2 will quickly capture O2- and convert it into H2O2. GSH-Px catalyses the degradation of H2O2 to water molecules, which requires glutathione to provide reducing hydrogen [16].
Effects on enzyme activities: Odbayar et al. [17] found that quercetin can increase the expression of some antioxidant enzymes, such as glutathione transferase and aldo-keto reductase. The level of expression is proportional to the amount of quercetin.
Impact on signal transduction pathway: Wang et al. [18] showed that quercetin had a protective effect on granulosa cells by upregulating the expression of some genes related to oxidative stress in vivo and in vitro. In addition, Granado-Serrano et al. and Kobori et al. [19, 20] verified that quercetin upregulates the expression of Nrf2 and nuclear transfer by activating the intracellular p38 MAPK pathway, increasing the level of intracellular GSH, and affecting antioxidant enzyme activities, so that the antioxidant capacity of the cell is improved.
2.2. Antimicrobial Properties
Studies have shown that quercetin has broad-spectrum antibacterial properties; it not only has a good inhibitory effect on bacteria but also has a significant inhibitory activity on fungi. Several experiments have found that quercetin has a good inhibitory effect on the growth of pathogenic bacteria such as Pseudomonas aeruginosa, Salmonella enteritidis, Staphylococcus aureus, Escherichia coli, Proteus, and Aspergillus flavus [21, 22]. Hossion et al. [23] found that novel, artificially designed and synthesised, quercetin acyl glucosides effectively inhibited the growth of E. coli, S. aureus, and P. aeruginosa. In addition, bayberry extract has significant antibacterial activities against Salmonella, Listeria, and Shigella with the minimum inhibitory concentration (MIC) values ranging from 2.07 to 8.28 mg/mL [24].
According to current research, the antibacterial mechanism of quercetin mainly includes destroying the cell wall of bacteria and changing the cell permeability, affecting protein synthesis and expression, reducing enzyme activities, and inhibiting nucleic acid synthesis. Wang et al. [22] used TEM images to demonstrate that quercetin could damage the cell wall and membrane of S. aureus (at 10 × MIC) and demonstrated that treatment of E. coli (at 50 × MIC) with quercetin eventually led to cavitation and death. Zhao et al. [25] found that sugarcane bagasse (with 470 mg quercetin/g polyphenol) extract showed bacteriostatic activities against the growth of S. aureus, L. monocytogenes, E. coli, and S. typhimurium. In addition, Plaper et al. [26] found that quercetin altered the activity of ATP, thereby affecting the growth of E. coli. Wang et al. [27] found that quercetin can protect rats from catheter-related S. aureus infection by inhibiting thrombin activities. The relevant experiments on quercetin in inhibiting bacteria in recent years are summarised in Table 1.
Table 1.
The inhibitory effect of quercetin on bacteria.
| Bacteria | Whether it has inhibitory effect | Mechanism |
|---|---|---|
| Aerobic bacteria [28] | Yes | Inhibits nucleic acid synthesis and destroys cell plasma membrane and energy metabolism |
|
| ||
| E. coli [22, 28, 29] | Yes | Inhibits nucleic acid synthesis and disrupts plasma membrane function |
|
| ||
| Pseudomonas aeruginosa [22, 28] | Yes | Inhibits nucleic acid synthesis and disrupts plasma membrane function |
|
| ||
| Salmonella typhimurium [22] | Yes | Inhibits nucleic acid synthesis and disrupts plasma membrane function |
|
| ||
| Staphylococcus aureus [22] | Yes | Inhibits nucleic acid synthesis and disrupts plasma membrane function |
|
| ||
| Drug-resistant E. coli [30] | Yes | By destroying bacterial cell walls and cell membranes |
|
| ||
| Bacillus subtilis [30] | Yes | By destroying bacterial cell walls and cell membranes |
|
| ||
| Enterococcus faecalis [31] | Yes | Inhibits the synthesis of Enterococcus faecalis naphthalate synthase |
|
| ||
| Mycobacterium [32] | Yes | Quercetin-3-O-β-D-glucoside inhibits glutamine synthetase |
|
| ||
| Aerobes [29] | Yes | Inducing antibacterial effects by inhibiting the supercoiled activity and DNA cleavage of bacterial gyrase |
|
| ||
| Bifidobacterium [29] | No | \ |
|
| ||
| Lactobacillus [29] | No | \ |
|
| ||
| Streptococcus mutans [33] | Yes | Reduces dry weight of biofilm and total protein |
|
| ||
| Carbapenem-resistant Pseudomonas aeruginosa [34] | Yes | Destroys cell wall integrity and changes cell morphology to exert bactericidal activity |
|
| ||
| Acinetobacter baumannii [34] | Yes | Destroys cell wall integrity and changes cell morphology to exert bactericidal activity |
|
| ||
| Carbapenem-resistant Pseudomonas aeruginosa [35] | Yes | Through alteration of blaVIM, ompC expression, and cellular morphology of bacteria |
|
| ||
| Klebsiella pneumoniae [35] | Yes | Through alteration of blaVIM, ompC expression, and cellular morphology of bacteria |
In addition, quercetin can prevent bacterial adhesion, inhibit quorum sensing pathways, destroy or change the plasma membrane, inhibit efflux pumps, and block nucleic acid synthesis. Wang et al. [18] confirmed that quercetin inhibits the formation of Streptococcus pneumoniae biofilms. Qayyum et al. [36] found that quercetin was effective against Enterococcus faecalis MTCC 2729 at the subminimal inhibitory concentration (sub-MIC), and scanning electron microscopy (SEM) and confocal laser scanning microscopy (CLSM) were used to elucidate that quercetin inhibited 95% of biofilm formation at 1/2 × MIC (256 g/mL). Kim et al. [37] found that a quercetin-pivaloxymethyl conjugate (Q-POM) at 5 μg/mL inhibited 70% of biofilm establishment by a vancomycin-resistant E. faecium isolate. Vazquez-Armenta et al. [38] found that quercetin would hinder the abiotic surface colonisation of Listeria monocytogenes at concentrations below the MIC. In addition, Lee et al. [39] obtained that quercetin has an inhibitory effect on genes related to bacterial adhesion. Cho et al. [40] found that quercetin could significantly inhibit the production of biofilms of a methicillin-sensitive S. aureus strain (MSSA) ATCC 6538 after 24 h at concentrations of 20 μg/mL and 50 μg/mL. Table 2 summarises the antibiofilm effect of quercetin on bacteria.
Table 2.
Antibiofilm effect of quercetin on bacteria.
| Biofilm-producing strains | Quercetin/quercetin conjugate | Effect |
|---|---|---|
| Bacillus subtilis strain FB17 [41] | Quercetin | Quercetin diminished biofilm formation |
|
| ||
| Enterococcus faecalis MTCC 2729 [36] | Quercetin | At submic concentrations, quercetin inhibits biofilm formation. Compared with the control group, 10 and 9 proteins were overexpressed and effective after quercetin treatment |
|
| ||
| A vancomycin-resistant Enterococcus Faecalis [37] | Quercetin–pivaloxymethyl conjugate (Q-POM) | Q-POM efficiently hampered biofilm formation in a dose-dependent manner |
|
| ||
| Staphylococcus aureus ATCC 6538 [39] | Quercetin | Quercetin not only abolished the biofilm forming and hemolytic S. aura but also suppresses the expression of adhesion-related, quorum sensing, and virus-regulatory genes |
|
| ||
| Staphylococcus aureus ATCC 25923 [39] | Quercetin | Quercetin not only abolished the biofilm forming and hemolytic S. aura but also suppresses the expression of adhesion-related, quorum sensing, and virus-regulatory genes |
|
| ||
| A clinical isolate of Staphylococcus aureus [42] | Quercetin-AgNP hybrid | Quercetin-AgNP hybrid significantly reduced the formation of biofilms and the production of extracellular polymers |
|
| ||
| MSSA ATCC 29213, MRSA ATCC 33591, and clinical isolates of Staphylococcus aureus [43] | Quercetin | Quercetin (at MIC and sub-MICs) inhibited around 50% of biofilm establishment |
|
| ||
| Streptococcus mutans strain Ingbritt [44] | Quercetin-doped adhesive groups | Compared with the control group, the binder group doped with quercetin showed antibacterial activity, acceptable biocompatibility, inhibition of matrix metalloproteinases, and an effective bonding interfacial seal |
|
| ||
| Clinical isolates of Pseudomonas Aeruginosa [45] | Quercetin | Quercetin basically inhibits biofilm formation and twitching movements |
|
| ||
| Proteus mirabilis HI4320 [46] | Quercetin | Quercetin dose dependently increased biofilm establishment |
|
| ||
| Streptococcus pneumoniae strain D39 [47] | Quercetin | Quercetin reduced biofilm formation and CFUs in a dose-dependent manner |
Quercetin has a broad inhibitory effect on bacteria, but as far as the current research on the fungal inhibitory effect is concerned, its fungal inhibitory effect is not as obvious as that on bacteria. Quercetin has no antifungal effect on Clostridium neospora when used alone, but when used together with AmB (amphotericin B), the antifungal activity is greatly improved. This implies that quercetin is a potential adjuvant drug for antifungal treatment of AmB [48]. Gao et al. found that quercetin is a beneficial antifungal drug in the clinical management of Candida vaginitis caused by Candida albicans biofilms and is a promising synergistic agent with fluconazole [49]. Quercetin enhances fluconazole-resistant Candida albicans-induced apoptosis by regulating quorum sensing [50]. The relevant experiments of the inhibitory effect of quercetin on fungi in recent years are summarised in Table 3.
Table 3.
The inhibitory activity of quercetin against fungi.
| Fungi | Synergistic effect of quercetin | MIC |
|---|---|---|
| Aspergillus flavus [51] | NO | 505 μg/mL |
| Candida tropicalis resistant to fluconazole [52] | Fluconazole | 128 μg/mL of flavonoids, combined with fluconazole (16 μg/mL) |
| Actinobacillus actinomycetemcomitans (Aa) [53] | NO | 0.1 g/mL |
| Porphyromonas gingivalis (Pg) [53] | NO | 0.0125 g/mL |
| Candida albicans [53] | NO | No effect |
| Rhizopus azygosporus [54] | NO | No effect |
| Candida parapsilosis [55] | NO | 0.5 μg/mL |
| Cryptococcus neoformans ATCC 90012 [48] | Amphotericin B | 0.125 μg/mL |
3. Applications of Quercetin
3.1. Antitumour
Many studies have shown that quercetin can exert antitumour effects through various mechanisms, which has been confirmed in various tumour in vivo and in vitro models. Quercetin can significantly prevent the cell cycle, promote cell apoptosis, and inhibit blood vessel generation and transfer. Lee et al. [56] found that in human leukaemia U937 cells, quercetin induces cell cycle arrest at G2 (late DNA synthesis phase). Suh et al. [57] found that quercetin can also induce G0/G1 (pre-DNA synthesis) phase changes in 232B4 chronic lymphocytic leukaemia cells and HOS osteosarcoma cells. In addition, Chou et al. [58] have proved that quercetin also affects the regulation of p53-related pathways in the tumour cell cycle. Their experiments discovered that quercetin can induce ER stress and promote the release of p53, thereby inhibiting the activities of CDK2, cyclin A, and cyclin B, thereby causing MCF-7 breast cancer cells to stagnate in the S phase. In addition, Hamidullah et al. [59] found that in PC-3 and DU145 prostate cancer cell lines, a certain dose of quercetin-6-C-β-D-glucopyranoside treatment can lead to cell cycle arrest in the G0/G1 phase. This phenomenon may be related to the downregulation of cyclins E and D, PNCA, and Cdk-2 protein expression and increased expressions of p21 and p27 (Table 4).
Table 4.
Inhibitory effect of quercetin on different cancer cells.
Quercetin can affect the cancer cell apoptosis pathway and induce tumour cell death. Experiments have shown that a reasonable dose of quercetin can increase the expression of proapoptotic protein and reduce the expression level of antiapoptotic protein. Granato et al. [60] found that quercetin inhibited the PI3K/AKT/mTOR and STAT3 pathways in PEL, which downregulated the expression of survival cell proteins such as c-FLIP, cyclin D1, and cMyc. Deng et al. [61] found that quercetin induced MCF-7 cell apoptosis and inhibited the proliferation of MCF-7 breast cancer cells in a time and concentration-dependent manner, thereby inhibiting breast cancer cells. In addition, Teekaraman and others [62] studied the role of quercetin apoptosis in the human metastatic ovarian cancer PA-1 cell line. The results showed that quercetin induced the mitochondrial-mediated apoptosis pathway, thereby inhibiting metastatic ovarian cancer cell growth. Seo et al. [63] showed through experiments that quercetin induced apoptosis at concentrations in excess of 20 μM by inhibiting STAT3 signalling and could be used as a useful compound for the prevention or treatment of breast cancer overexpressing HER2 (Table 5).
Table 5.
Effects of quercetin on apoptosis of different tumour cells and its mechanism.
With the development of clinical trials, the great potential of quercetin in the treatment of cancer has been further confirmed; however, there remain some limitations in the scope and number of clinical trials involved, and more comprehensive clinical trials are needed to confirm its therapeutic effect on tumours.
3.2. Anti-Inflammatory and Immunosuppressive Effects
Quercetin has been confirmed to be a long-acting anti-inflammatory substance in flavonoids [64, 65]. Both in animal and in human models, quercetin can show significant anti-inflammatory potential in different cell types [66, 67]. The plant extract of quercetin is used as the main component of many potential antiallergic drugs. Compared with Cromolin (the antiallergic drug disodium cromoglycate), its ability to inhibit IL-8 is stronger and can inhibit IL-6 and increase cytosolic calcium levels [68]. Its anti-inflammatory and antiallergic properties have been validated in the treatment of respiratory and food allergies [69, 70]. In addition to a wide range of biochemical and pharmacological activities, quercetin has been repeatedly shown to exert anti-inflammatory effects on endothelial and monocyte/macrophage systems in vitro [71, 72].
Li et al. [73] conducted experiments in different animal models and found that quercetin inhibited the production of tumour necrosis factor alpha (TNF-α) induced by lipopolysaccharide (LPS) in macrophages [66] and lung A549 cells LPS-induced IL-8 production [67]. Furthermore, it has even been shown in glia cells that quercetin can suppress LPS-induced mRNA levels of TNF-α and interleukin- (IL-) 1α: neuronal cell death is also reduced [74]. Quercetin can inhibit the enzymes that produce inflammation (cyclooxygenase (COX) and lipoxygenase (LOX)) [75].
According to several studies on the correlation between quercetin and its immunomodulatory effects, quercetin can reduce disease after strenuous exercise. Nieman et al. showed that, among well-trained cyclists, supplementing quercetin and epigallocatechin-3-gallate (Q-EGCG) for two weeks could enhance GOBA granulocytes and resist inflammation after three days of heavy exercise [75]. In addition, in clinical trials, quercetin and resveratrol, EGCG, and genistein have been found to enhance cellular and humoral immune functions [76].
3.3. Cardiovascular Protection
The quercetin exerts beneficial effects on cardiovascular diseases, such as hypertension, atherosclerosis, ischemia-reperfusion injury, or cardiotoxicity [77–79], which are closely associated with the anti-inflammatory and antioxidant properties of quercetin. The protective mechanism of quercetin on the cardiovascular system includes (1) reducing systolic blood pressure, diastolic blood pressure, and mean arterial pressure. (2) The levels of ST segment, lipid peroxidation in the plasma and heart, free fatty acid, phospholipid, total cholesterol, and triglyceride in serum were decreased. (3) It can regenerate blood vessels and reduce blood sugar. (4) It can effectively decrease the thickness of the aortic wall. Edwards et al. [80] found that, among patients with stage 1 hypertension, those who took 730 mg of quercetin for 28 days had a decrease in their systolic, diastolic, and mean arterial pressure. Quercetin presents significant heart-inhibiting effects on LDL oxidation and endothelium-dependent vasodilation [81] and reduces the effects of adhesion molecules and other inflammation markers. In addition, a study of 93 overweight or obese subjects at high risk of metabolic syndrome who were given a daily dose of 150 mg quercetin for six weeks showed significant reductions in plasma concentrations of LDL oxidised by systolic blood pressure and atherosclerosis [82]. The protective effect refers to the effects of nitrogen oxide (NO) and endothelial function and the prevention of oxidative inflammatory damage of neurons and the antiaggregation effect of platelets. Wei et al. [83] found that quercetin has a potential for use in treating heart disease as quercetin treatment is found to be capable of reducing LPS-induced cardiac abnormalities in mice.
Quercetin can control dyslipidaemia, and changes in fatty liver functions are essential for controlling serum fat levels. Gnoni et al. investigated the effect of quercetin on rat hepatocyte fat production [84]. The experiment found that the addition of quercetin to liver cells at a concentration of 25 μM, within 30 minutes could inhibit the synthesis of fatty acids. Tian et al. [85] found that 50 μM 7-O-sialic acid (QA) can protect human umbilical vein endothelial cells. In addition, study showed that quercetin (10 mg/kg) orally administered to rats for seven consecutive days protected them from experimental myocardial infarction [86]. Kleemann et al. [87] demonstrated that quercetin could downregulate the expression of C-reactive protein and cardiovascular risk factors (SAA, fibrinogen) in mice. These results indicated that quercetin might have cardiovascular protective effects.
Quercetin also protected mice fed a high-fat diet from endothelial dysfunction caused by oxidants and protected apolipoprotein E-knockout mice from atherosclerosis [88]. Some studies have shown that quercetin positively influences the development of the embryo, foetus, and placenta. Since this flavonoid has no teratogenic and miscarriage effects, it is generally considered safe. Therefore, in this risk group, its potential use in the prevention and treatment of pregnancy-induced hypertension syndrome has received much research attention [89–91].
3.4. Quercetin Relieves Mycotoxin Toxicity
According to multiple studies, quercetin can alleviate the toxicity of mycotoxins. Quercetin alleviates mycotoxin toxicity due to its antioxidant and anti-inflammatory properties. Quercetin alleviates mycotoxins by protecting cells from endoplasmic reticulum stress and apoptosis induced by mycotoxins, increasing the level of glutathione peroxidase, enhancing the activity of oxide dismutase, increasing the activity of catalase, reducing the lipid peroxidation reaction, and decreasing the level of ROS (Table 6). Ben et al. [92] found that the antioxidant activity of quercetin and saffron can decrease the level of ROS produced by ZEN, inhibit ER stress, and protect HCT116 and HEK293 cells from ZEN-induced apoptosis. Further research by Ben et al. [93] proved that quercetin could prevent a/b-ZOL-induced ROS generation in HCT116, prevent a-ZOL and b-ZOL-induced ER stress, and reduce a-ZOL and b-ZOL-induced apoptosis. Their experiments show that quercetin protects HCT116 cells from a-ZOL and b-ZOL-induced apoptotic cell death. This is in good agreement with the existing literature on quercetin as an antioxidant in various types of oxidative damage [94, 95].
Table 6.
Protect effects of quercetin on some main mycotoxin toxicity and its mechanism.
| Mycotoxin | Mechanism | Effect |
|---|---|---|
| ZEN [92] | Antioxidant activity, ROS production ↓, ER256 ↓ | Protecting HCT116 and HEK293 cells and inhibit cell apoptosis |
|
| ||
| a/b-ZOL [93] | ROS production ↓, inhibit a-zol, b-zol endoplasmic reticulum stress | Protecting cells from damage |
|
| ||
| AFB1 [98] | Reversing the negative regulation of GSTA1, increase GSH level ↑ | Inhibiting AFB1 biotransformation |
|
| ||
| AFB1 [105] | ↑ Increased the level of glutathione peroxidase, increase the activity of oxide dismutase, increased the activity of catalase, and ↓ reduced the lipid peroxidation reaction | Improved brain cognition and spatial memory, increased anxiety and drowsiness disorders |
|
| ||
| AFB1 [106] | ↓ Reduced ROS generation, ↑ antioxidant enzyme activity | Improved the learning and memory impairment of mice |
|
| ||
| AFB1 [107] | Cross the blood-brain barrier | Quercetin could be a potential neuroprotective approach to slow degenerative disease progression |
|
| ||
| Ochratoxin A [108] | / | Protecting cells from damage |
|
| ||
| Deoxynivalenol cytotoxicity [104] | / | Protecting intestinal caco-2 cells from damage |
|
| ||
| AFB1 [109–112] | Inhibited CYP1A-mediated 7-ethoxyresorufin O-deethylase (EROD) activity in liver microsomes | Affects AFB1 biotransformation remains |
|
| ||
| Citrinin (CTN), patulin (PAT), and zearalenol (ZEAR) [113] | ↓ Decreased cell viability and ↑ increased LDH activity | Protecting the cell lines from cytotoxicity |
|
| ||
| AFB1 [114] | ↓ Decreasing the rate of ROS formation, lipid peroxidation and improved cell viability, mitochondrial membrane potential and glutathione level and reducing levels of aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase | Hepatoprotective effect |
Aflatoxin B1 (AFB1) is a common mycotoxin found in feed, which has a variety of toxic effects. The neurotoxicity of AFB1 can lead to memory disorder. Quercetin plays a preventive role in antioxidant stress by promoting the antioxidant defence system and limiting lipid peroxidation. Studies have shown that quercetin can increase the level of glutathione peroxidase (GSH) and the activity of superoxide dismutase (SOD) and catalase (CAT) in the brain and reduce the lipid peroxidation of AFB1-treated mice. This is consistent with the effect of quercetin on behavioural and cognitive impairment in a Parkinson's disease model [96] and a chronic cerebral ischemia model [97]. Quercetin can significantly reduce the synthesis of AFB1. In recent years, it has been found that quercetin in tea polyphenols can hinder the conversion of aflatoxin AFB1 to the carcinogenic product AFB1-8,9-epoxide [98], which matches the findings in a study by Ghadiri et al. [99]. Resveratrol and quercetin (both 5 μM) (to a lesser extent) significantly offset the impaired cell viability mediated by AFB1 (concentration range: 96-750 μM). There are toxicological implications associated with AFB1 intake such as hepatotoxicity and carcinogenicity. Quercetin can detoxify AFB1 by regulating the activity of glutathione and SOD; also, the participation of mitochondria and lysosomes in AFB1-induced cytotoxicity might be a possible proposed mechanism thereof.
Quercetin pretreatment can inhibit aflatoxin-induced cytotoxicity and oxidative stress, mainly by activating Nrf2 pathway to regulate changes to the antioxidant defence system induced by Aspergillus. In addition, quercetin also shows antigenic toxicity potential by reducing DNA damage and micronucleus (MN) damage induced by the Aspergillus toxin. Ramyaa et al. [100, 101] first found that quercetin pretreatment can inhibit ochratoxin-induced cytotoxicity and oxidative stress. Schoneberg et al. [102] found that the contents of NO, TNF-α, IL-6, and IL-8 of ochratoxin were significantly reduced in samples pretreated with quercetin, indicating that quercetin had anti-inflammatory effects. It has been proved that quercetin has a cytoprotective effect on ochratoxin-induced oxidative stress, genotoxicity, and lymphocyte inflammation [103]. Bollina and Kushalappa [104] found that the addition of quercetin at a concentration of 2.95 mM reduced the production of deoxynivalenol (DON) by Fusarium graminearum in vitro, but no obvious concentration response was found in mycotoxins. The protective effects of quercetin on key mycotoxin toxicities and their mechanism are summarised in Table 6.
3.5. Other Functions
Currently, quercetin extract is widely used as a nutritional supplement and therapeutic ingredient for many diseases, such as diabetes, which is associated with obesity and circulatory dysfunction (including inflammation and emotional distress) [115]. Previous experiments showed that quercetin can inhibit fat production and benefit obese people [116]. The mechanism of action of quercetin is pleiotropic, involving inhibition of intestinal glucose absorption, insulin secretion, and insulin sensitisation activities, and improvement of glucose utilisation in peripheral tissues [78]. In addition, quercetin helps reduce lipid peroxidation, platelet aggregation, and capillary permeability and may be used in the treatment of obesity and type 2 diabetes [117, 118]. Quercetin also reduces obesity-induced skeletal muscle atrophy by inhibiting inflammatory receptors and their signalling pathways. Quercetin is used to prevent obesity-induced muscle inflammation and sarcopenia [119]. Ying et al. [120] suggested that quercetin can decrease the levels of malondialdehyde (MDA) and NO by changing the activity of antioxidant enzymes, activating the expression of PI3K/PKB signalling pathway-related genes, regulates glucose metabolism, reduces oxidative damage, and has a protective effect on ascorbic acid therapy.
Quercetin has been shown to be important in the fight against parasites and has been demonstrated in different clinical trials, such as those against Leishmania, Trypanosoma, and Plasmodium. The antiparasitic effect is related to the destruction of mitochondrial function and the inhibition of different important enzymes and molecules, including heat-shock protein (HSP), acetylcholinesterase, DNA topoisomerase, and kinase (Table 7).
Table 7.
Inhibitory mechanism of quercetin on several parasites.
| Parasite | Mechanism of action |
|---|---|
| Leishmania donovani [121] | Low selectivity to parasite DNase I |
| Trypanosoma brucei [122] | Cause a loss of mitochondrial membrane potential and marked DNA degradation |
| Plasmodium falciparum [123] | Antiplasmodial potential |
| Encephalitozoon intestinalis [124] | Antiparasitic activity |
| Leishmania mexicana [125] | Inhibition of parasite cathepsin L |
In addition, quercetin can reverse cognitive impairment and enhance memory in the ageing process. Quercetin has the protective effects of antioxidant damage and neuroinflammation, so it is a potential therapeutic candidate for the treatment of neurological diseases and is helpful for the treatment of cognitive impairment [126, 127]. Multiple experiments have shown that quercetin has a neuroprotective effect [128]. Ishisaka et al. and Das et al. [129, 130] found that rodents can be protected from various forms of neurotoxic damage after oral administration of quercetin (0.5-50 mg/kg). Quercetin can also protect nerve damage caused by heavy metals, such as lead and mercury [131–133]. In addition, quercetin can also reduce nerve damage caused by chemicals, such as the insecticide endosulfan [134, 135].
4. Summary and Future Prospects
Quercetin has shown good therapeutic activities against various diseases. Through continuous research, quercetin is expected to become a new drug that can prevent and treat various diseases. Its powerful antioxidant, anti-inflammatory, and antitumour effects have great prospects in clinical application. At this stage, the antioxidants added to animal feed have carcinogenic, teratogenic, mutagenic, and other side effects on humans and animals. Quercetin is a safe, natural antioxidant and can be used in animal feed. At the same time, when quercetin exerts antioxidant activities in the body, it can also improve physical functions and reduce stress reactions. The author believes that the level and effect of quercetin in different animal feeds need further in-depth discussion.
The broad-spectrum antimicrobial properties of quercetin can be used in the prevention and treatment of various infectious bacterial diseases and can provide treatment options to reduce the use of antibiotics, which has important implications for the safety and sustainable development of human and animal health: however, at present, research into the antibacterial effect of quercetin is mainly focused on the antibacterial activity of quercetin, but there is little research on the antifungal effect. Whether the antibacterial mechanism of quercetin is akin to those of fungi and bacteria or whether it has inhibitory effects on different types of fungi still needs further experimental research.
According to the broad-spectrum antimicrobial properties, application as a preservative is expected. In addition, quercetin antioxidant treatment may help to prevent mycotoxin toxicity in food and feed industry. However, in terms of the present study, the absorption of quercetin in the human body and the metabolic mechanism are not clear. Further research into quercetin is needed before pharmacological application.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (grant nos. 31972746, 31772809, and 31872538) and Liaoning Provincial Natural Fund Guidance Program Project (2019-ZD-0708).
Contributor Information
Miao Long, Email: longjlau@126.com.
Peng Li, Email: lipeng2018@syau.edu.cn.
Data Availability
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare no competing financial interest.
Authors' Contributions
Dengyu Yang wrote the paper. Tiancheng Wang revised the paper. Miao Long and Peng Li revised and supervised the paper.
References
- 1.Anand David A. V., Arulmoli R., Parasuraman S. Overviews of biological importance of quercetin: a bioactive flavonoid. Pharmacognosy Reviews. 2016;10(20):84–89. doi: 10.4103/0973-7847.194044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hakkinen S. H., Karenlampi S. O., Heinonen I. M., Mykkanen H. M., Torronen A. R. Content of the flavonols quercetin, myricetin, and kaempferol in 25 edible berries. Journal of Agricultural and Food Chemistry. 1999;47(6):2274–2279. doi: 10.1021/jf9811065. [DOI] [PubMed] [Google Scholar]
- 3.Williamson G., Manach C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. The American Journal of Clinical Nutrition. 2005;81(1):243S–255S. doi: 10.1093/ajcn/81.1.243S. [DOI] [PubMed] [Google Scholar]
- 4.Wiczkowski W., Romaszko J., Bucinski A., et al. Quercetin from shallots (Allium cepa L. var. aggregatum) is more bioavailable than its glucosides. The Journal of Nutrition. 2008;138(5):885–888. doi: 10.1093/jn/138.5.885. [DOI] [PubMed] [Google Scholar]
- 5.Manach C., Williamson G., Morand C., Scalbert A., Remesy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. The American Journal of Clinical Nutrition. 2005;81(1):230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 6.Fischer C., Speth V., Fleig-Eberenz S., Neuhaus G. Induction of zygotic polyembryos in wheat: influence of auxin polar transport. Plant Cell. 1997;9(10):1767–1780. doi: 10.1105/tpc.9.10.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ullah F., Iqbal N., Ayaz M., et al. DPPH, ABTS free radical scavenging, antibacterial and phytochemical evaluation of crude methanolic extract and subsequent fractions of Chenopodium botrys aerial parts. Pakistan Journal of Pharmaceutical Sciences. 2017;30(3):761–766. [PubMed] [Google Scholar]
- 8.Ghosh N., Chakraborty T., Mallick S., et al. Synthesis, characterization and study of antioxidant activity of quercetin-magnesium complex. Spectrochimica Acta. Part A, Molecular and Biomolecular Spectroscopy. 2015;151:807–813. doi: 10.1016/j.saa.2015.07.050. [DOI] [PubMed] [Google Scholar]
- 9.Hanasaki Y., Ogawa S., Fukui S. The correlation between active oxygens scavenging and antioxidative effects of flavonoids. Free Radical Biology & Medicine. 1994;16(6):845–850. doi: 10.1016/0891-5849(94)90202-x. [DOI] [PubMed] [Google Scholar]
- 10.Oh W. Y., Ambigaipalan P., Shahidi F. Preparation of quercetin esters and their antioxidant activity. Journal of Agricultural and Food Chemistry. 2019;67(38):10653–10659. doi: 10.1021/acs.jafc.9b04154. [DOI] [PubMed] [Google Scholar]
- 11.Manca M. L., Castangia I., Caddeo C., et al. Improvement of quercetin protective effect against oxidative stress skin damages by incorporation in nanovesicles. Colloids and Surfaces B: Biointerfaces. 2014;123:566–574. doi: 10.1016/j.colsurfb.2014.09.059. [DOI] [PubMed] [Google Scholar]
- 12.Tang Y., Li Y., Yu H., et al. Quercetin attenuates chronic ethanol hepatotoxicity: implication of "free" iron uptake and release. Food and Chemical Toxicology. 2014;67:131–138. doi: 10.1016/j.fct.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 13.Babenkova I. V., Osipov A. N., Teselkin Y. O. The effect of dihydroquercetin on catalytic activity of Iron (II) ions in the Fenton reaction. Bulletin of Experimental Biology and Medicine. 2018;165(3):347–350. doi: 10.1007/s10517-018-4167-x. [DOI] [PubMed] [Google Scholar]
- 14.Lim B., Yu B., Cho S., Her E., Park D. The inhibition by quercetin and ganhuangenin on oxidatively modified low density lipoprotein. Phytotherapy Research. 1998;12(5):340–345. doi: 10.1002/(sici)1099-1573(199808)12:5<340::aid-ptr316>3.0.co;2-u. [DOI] [Google Scholar]
- 15.Mbikay M., Sirois F., Simoes S., Mayne J., Chretien M. Quercetin-3-glucoside increases low-density lipoprotein receptor (LDLR) expression, attenuates proprotein convertase subtilisin/kexin 9 (PCSK9) secretion, and stimulates LDL uptake by Huh7 human hepatocytes in culture. FEBS Open Bio. 2014;4(1):755–762. doi: 10.1016/j.fob.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu D., Hu M. J., Wang Y. Q., Cui Y. L. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules. 2019;24(6):p. 1123. doi: 10.3390/molecules24061123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Odbayar T. O., Kimura T., Tsushida T., Ide T. Isoenzyme-specific up-regulation of glutathione transferase and aldo-keto reductase mRNA expression by dietary quercetin in rat liver. Molecular and Cellular Biochemistry. 2009;325(1-2):121–130. doi: 10.1007/s11010-009-0026-4. [DOI] [PubMed] [Google Scholar]
- 18.Wang J., Qian X., Gao Q., et al. Quercetin increases the antioxidant capacity of the ovary in menopausal rats and in ovarian granulosa cell culture in vitro. Journal of Ovarian Research. 2018;11(1):p. 51. doi: 10.1186/s13048-018-0421-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Granado-Serrano A. B., Martin M. A., Bravo L., Goya L., Ramos S. Quercetin modulates Nrf2 and glutathione-related defenses in HepG2 cells: involvement of p38. Chemico-Biological Interactions. 2012;195(2):154–164. doi: 10.1016/j.cbi.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Kobori M., Takahashi Y., Akimoto Y., et al. Chronic high intake of quercetin reduces oxidative stress and induces expression of the antioxidant enzymes in the liver and visceral adipose tissues in mice. Journal of Functional Foods. 2015;15:551–560. doi: 10.1016/j.jff.2015.04.006. [DOI] [Google Scholar]
- 21.Qin X. R., Zhang M. J., Gao X. N., Lin Y., Li M. A., Si-Yi H. E. Study on the antibacterial activity of quercetin. Chemistry & Bioengineering. 2009;26:55–57. [Google Scholar]
- 22.Wang S., Yao J., Zhou B., et al. Bacteriostatic effect of quercetin as an antibiotic alternative in vivo and its antibacterial mechanism in vitro. Journal of Food Protection. 2018;81(1):68–78. doi: 10.4315/0362-028X.JFP-17-214. [DOI] [PubMed] [Google Scholar]
- 23.Hossion A. M., Zamami Y., Kandahary R. K., et al. Quercetin diacylglycoside analogues showing dual inhibition of DNA gyrase and topoisomerase IV as novel antibacterial agents. Journal of Medicinal Chemistry. 2011;54(11):3686–3703. doi: 10.1021/jm200010x. [DOI] [PubMed] [Google Scholar]
- 24.Yao W. R., Wang H. Y., Wang S. T., Sun S. L., Zhou J., Luan Y. Y. Assessment of the antibacterial activity and the antidiarrheal function of flavonoids from bayberry fruit. Journal of Agricultural and Food Chemistry. 2011;59(10):5312–5317. doi: 10.1021/jf200211m. [DOI] [PubMed] [Google Scholar]
- 25.Zhao Y., Chen M., Zhao Z., Yu S. The antibiotic activity and mechanisms of sugarcane (Saccharum officinarum L.) bagasse extract against food-borne pathogens. Food Chemistry. 2015;185:112–118. doi: 10.1016/j.foodchem.2015.03.120. [DOI] [PubMed] [Google Scholar]
- 26.Plaper A., Golob M., Hafner I., Oblak M., Solmajer T., Jerala R. Characterization of quercetin binding site on DNA gyrase. Biochemical and Biophysical Research Communications. 2003;306(2):530–536. doi: 10.1016/s0006-291x(03)01006-4. [DOI] [PubMed] [Google Scholar]
- 27.Wang L., Li B., Si X., et al. Quercetin protects rats from catheter-related Staphylococcus aureus infections by inhibiting coagulase activity. Journal of Cellular and Molecular Medicine. 2019;23(7):4808–4818. doi: 10.1111/jcmm.14371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hendra R., Ahmad S., Sukari A., Shukor M. Y., Oskoueian E. Flavonoid analyses and antimicrobial activity of various parts of Phaleria macrocarpa (Scheff.) Boerl fruit. International Journal of Molecular Sciences. 2011;12(6):3422–3431. doi: 10.3390/ijms12063422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu H. N., Liu Y., Hu L. L., et al. Effects of dietary supplementation of quercetin on performance, egg quality, cecal microflora populations, and antioxidant status in laying hens. Poultry Science. 2014;93(2):347–353. doi: 10.3382/ps.2013-03225. [DOI] [PubMed] [Google Scholar]
- 30.Yang X., Zhang W., Zhao Z., et al. Quercetin loading CdSe/ZnS nanoparticles as efficient antibacterial and anticancer materials. Journal of Inorganic Biochemistry. 2017;167:36–48. doi: 10.1016/j.jinorgbio.2016.11.023. [DOI] [PubMed] [Google Scholar]
- 31.Das S., Batra S., Gupta P. P., et al. Identification and evaluation of quercetin as a potential inhibitor of naphthoate synthase from Enterococcus faecalis. Journal of Molecular Recognition. 2019;32(11, article e2802) doi: 10.1002/jmr.2802. [DOI] [PubMed] [Google Scholar]
- 32.Safwat N. A., Kashef M. T., Aziz R. K., Amer K. F., Ramadan M. A. Quercetin 3-O-glucoside recovered from the wild Egyptian Sahara plant, Euphorbia paralias L., inhibits glutamine synthetase and has antimycobacterial activity. Tuberculosis. 2018;108:106–113. doi: 10.1016/j.tube.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 33.Zeng Y., Nikitkova A., Abdelsalam H., Li J., Xiao J. Activity of quercetin and kaemferol against Streptococcus mutans biofilm. Archives of Oral Biology. 2019;98:9–16. doi: 10.1016/j.archoralbio.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pal A., Tripathi A. Quercetin potentiates meropenem activity among pathogenic carbapenem-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Journal of Applied Microbiology. 2019;127(4):1038–1047. doi: 10.1111/jam.14388. [DOI] [PubMed] [Google Scholar]
- 35.Pal A., Tripathi A. Demonstration of bactericidal and synergistic activity of quercetin with meropenem among pathogenic carbapenem resistant Escherichia coli and Klebsiella pneumoniae. Microbial Pathogenesis. 2020;143, article 104120 doi: 10.1016/j.micpath.2020.104120. [DOI] [PubMed] [Google Scholar]
- 36.Qayyum S., Sharma D., Bisht D., Khan A. U. Identification of factors involved in Enterococcus faecalis biofilm under quercetin stress. Microbial Pathogenesis. 2019;126:205–211. doi: 10.1016/j.micpath.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 37.Kim M. K., Lee T. G., Jung M., Park K. H., Chong Y. In vitro synergism and anti-biofilm activity of quercetin-pivaloxymethyl conjugate against Staphylococcus aureus and Enterococcus species. Chemical and Pharmaceutical Bulletin. 2018;66(11):1019–1022. doi: 10.1248/cpb.c18-00380. [DOI] [PubMed] [Google Scholar]
- 38.Vazquez-Armenta F. J., Bernal-Mercado A. T., Tapia-Rodriguez M. R., et al. Quercetin reduces adhesion and inhibits biofilm development by Listeria monocytogenes by reducing the amount of extracellular proteins. Food Control. 2018;90:266–273. doi: 10.1016/j.foodcont.2018.02.041. [DOI] [Google Scholar]
- 39.Lee J. H., Park J. H., Cho H. S., Joo S. W., Cho M. H., Lee J. Anti-biofilm activities of quercetin and tannic acid against Staphylococcus aureus. Biofouling. 2013;29(5):491–499. doi: 10.1080/08927014.2013.788692. [DOI] [PubMed] [Google Scholar]
- 40.Cho H. S., Lee J. H., Cho M. H., Lee J. Red wines and flavonoids diminish Staphylococcus aureus virulence with anti-biofilm and anti-hemolytic activities. Biofouling. 2015;31(1):1–11. doi: 10.1080/08927014.2014.991319. [DOI] [PubMed] [Google Scholar]
- 41.Bordeleau E., Mazinani S., Nguyen D., Betancourt F., Yan H. Abrasive treatment of microtiter plates improves the reproducibility of bacterial biofilm assays. RSC Advances. 2018;8(57):32434–32439. doi: 10.1039/C8RA06352D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vanaraj S., Keerthana B. B., Preethi K. Biosynthesis, characterization of silver nanoparticles using quercetin from Clitoria ternatea L to enhance toxicity against bacterial biofilm. Journal of Inorganic and Organometallic Polymers and Materials. 2017;27(5):1412–1422. doi: 10.1007/s10904-017-0595-8. [DOI] [Google Scholar]
- 43.Junior S. D. D. C., Santos J. V. D. O., Campos L. A. D. A., Pereira M. A., Magalhaes N. S. S., Cavalcanti I. M. F. Antibacterial and antibiofilm activities of quercetin against clinical isolates of Staphyloccocus aureus and Staphylococcus saprophyticus with resistance profile. International Journal of Environment, Agriculture and Biotechnology. 2018;3(5, article 266213):1948–1958. doi: 10.22161/ijeab/3.5.50. [DOI] [Google Scholar]
- 44.Yang H., Li K., Yan H., Liu S., Wang Y., Huang C. High-performance therapeutic quercetin-doped adhesive for adhesive-dentin interfaces. Scientific Reports. 2017;7(1):p. 8189. doi: 10.1038/s41598-017-08633-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vipin C., Mujeeburahiman M., Ashwini P., Arun A. B., Rekha P. D. Anti-biofilm and cytoprotective activities of quercetin against Pseudomonas aeruginosa isolates. Letters in Applied Microbiology. 2019;68(5):464–471. doi: 10.1111/lam.13129. [DOI] [PubMed] [Google Scholar]
- 46.Aygul A., Ozturk I., Cilli F. F., Ermertcan S. Quercetin inhibits swarming motility and activates biofilm production of Proteus mirabilis possibly by interacting with central regulators, metabolic status or active pump proteins. Phytomedicine. 2019;57:65–71. doi: 10.1016/j.phymed.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 47.Wang J., Song M., Pan J., et al. Quercetin impairs Streptococcus pneumoniae biofilm formation by inhibiting sortase A activity. Journal of Cellular and Molecular Medicine. 2018;22(12):6228–6237. doi: 10.1111/jcmm.13910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oliveira V. M., Carraro E., Auler M. E., Khalil N. M. Quercetin and rutin as potential agents antifungal against Cryptococcus spp. Brazilian Journal of Biology. 2016;76(4):1029–1034. doi: 10.1590/1519-6984.07415. [DOI] [PubMed] [Google Scholar]
- 49.Gao M., Wang H., Zhu L. Quercetin assists fluconazole to inhibit biofilm formations of fluconazole-resistant Candida albicans in in vitro and in vivo antifungal managements of vulvovaginal candidiasis. Cellular Physiology and Biochemistry. 2016;40(3-4):727–742. doi: 10.1159/000453134. [DOI] [PubMed] [Google Scholar]
- 50.Singh B. N., Upreti D. K., Singh B. R., et al. Quercetin sensitizes fluconazole-resistant Candida albicans to induce apoptotic cell death by modulating quorum sensing. Antimicrobial Agents and Chemotherapy. 2015;59(4):2153–2168. doi: 10.1128/AAC.03599-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li X. M., Li Z. Y., Wang Y. D., Wang J. Q., Yang P. L. Quercetin inhibits the proliferation and aflatoxins biosynthesis of Aspergillus flavus. Toxins. 2019;11(3):p. 154. doi: 10.3390/toxins11030154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.da Silva C. R., de Andrade Neto J. B., de Sousa Campos R., et al. Synergistic effect of the flavonoid catechin, quercetin, or epigallocatechin gallate with fluconazole induces apoptosis in Candida tropicalis resistant to fluconazole. Antimicrobial Agents and Chemotherapy. 2014;58(3):1468–1478. doi: 10.1128/AAC.00651-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Geoghegan F., Wong R. W., Rabie A. B. Inhibitory effect of quercetin on periodontal pathogens in vitro. Phytotherapy Research. 2010;24(6):817–820. doi: 10.1002/ptr.3014. [DOI] [PubMed] [Google Scholar]
- 54.Gonzales G. B., Smagghe G., Wittevrongel J., Huynh N. T., Van Camp J., Raes K. Metabolism of quercetin and naringenin by food-grade fungal inoculum, Rhizopus azygosporus Yuan et Jong (ATCC 48108) Journal of Agricultural and Food Chemistry. 2016;64(49):9263–9267. doi: 10.1021/acs.jafc.6b04124. [DOI] [PubMed] [Google Scholar]
- 55.Rocha M., Sales J. A., da Rocha M. G., et al. Antifungal effects of the flavonoids kaempferol and quercetin: a possible alternative for the control of fungal biofilms. Biofouling. 2019;35(3):320–328. doi: 10.1080/08927014.2019.1604948. [DOI] [PubMed] [Google Scholar]
- 56.Lee T. J., Kim O. H., Kim Y. H., et al. Quercetin arrests G2/M phase and induces caspase-dependent cell death in U937 cells. Cancer Letters. 2006;240(2):234–242. doi: 10.1016/j.canlet.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 57.Suh D. K., Lee E. J., Kim H. C., Kim J. H. Induction of G1/S phase arrest and apoptosis by quercetin in human osteosarcoma cells. Archives of Pharmacal Research. 2010;33(5):781–785. doi: 10.1007/s12272-010-0519-4. [DOI] [PubMed] [Google Scholar]
- 58.Chou C. C., Yang J. S., Lu H. F., et al. Quercetin-mediated cell cycle arrest and apoptosis involving activation of a caspase cascade through the mitochondrial pathway in human breast cancer MCF-7 cells. Archives of Pharmacal Research. 2010;33(8):1181–1191. doi: 10.1007/s12272-010-0808-y. [DOI] [PubMed] [Google Scholar]
- 59.Hamidullah, Kumar R., Saini K. S., et al. Quercetin-6- C - β -D-glucopyranoside, natural analog of quercetin exhibits anti-prostate cancer activity by inhibiting Akt-mTOR pathway via aryl hydrocarbon receptor. Biochimie. 2015;119:68–79. doi: 10.1016/j.biochi.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 60.Granato M., Rizzello C., Gilardini Montani M. S., et al. Quercetin induces apoptosis and autophagy in primary effusion lymphoma cells by inhibiting PI3K/AKT/mTOR and STAT3 signaling pathways. The Journal of Nutritional Biochemistry. 2017;41:124–136. doi: 10.1016/j.jnutbio.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 61.Deng X. H., Song H. Y., Zhou Y. F., Yuan G. Y., Zheng F. J. Effects of quercetin on the proliferation of breast cancer cells and expression of survivin in vitro. Experimental and Therapeutic Medicine. 2013;6(5):1155–1158. doi: 10.3892/etm.2013.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Teekaraman D., Elayapillai S. P., Viswanathan M. P., Jagadeesan A. Quercetin inhibits human metastatic ovarian cancer cell growth and modulates components of the intrinsic apoptotic pathway in PA-1 cell line. Chemico-Biological Interactions. 2019;300:91–100. doi: 10.1016/j.cbi.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 63.Seo H. S., Ku J. M., Choi H. S., et al. Quercetin induces caspase-dependent extrinsic apoptosis through inhibition of signal transducer and activator of transcription 3 signaling in HER2-overexpressing BT-474 breast cancer cells. Oncology Reports. 2016;36(1):31–42. doi: 10.3892/or.2016.4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Read M. A. Flavonoids: naturally occurring anti-inflammatory agents. The American Journal of Pathology. 1995;147(2):235–237. [PMC free article] [PubMed] [Google Scholar]
- 65.Orsolic N., Knezevic A. H., Sver L., Terzic S., Basic I. Immunomodulatory and antimetastatic action of propolis and related polyphenolic compounds. Journal of Ethnopharmacology. 2004;94(2-3):307–315. doi: 10.1016/j.jep.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 66.K R. M., Ghosh B. Quercetin inhibits LPS-induced nitric oxide and tumor necrosis factor-α production in murine macrophages. International Journal of Immunopharmacology. 1999;21(7):435–443. doi: 10.1016/s0192-0561(99)00024-7. [DOI] [PubMed] [Google Scholar]
- 67.Geraets L., Moonen H. J., Brauers K., Wouters E. F., Bast A., Hageman G. J. Dietary flavones and flavonoles are inhibitors of poly(ADP-ribose)polymerase-1 in pulmonary epithelial cells. The Journal of Nutrition. 2007;137(10):2190–2195. doi: 10.1093/jn/137.10.2190. [DOI] [PubMed] [Google Scholar]
- 68.Mlcek J., Jurikova T., Skrovankova S., Sochor J. Quercetin and its anti-allergic immune response. Molecules. 2016;21(5):p. 623. doi: 10.3390/molecules21050623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Juríková T., Mlček J., Sochor J., Hegedűsová A. Polyphenols and their mechanism of action in allergic immune ResponseImmune response. Global Journal of Allergy. 2015;1:37–39. doi: 10.17352/2455-8141.000008. [DOI] [Google Scholar]
- 70.Gabor M. Anti-inflammatory and anti-allergic properties of flavonoids. Progress in Clinical and Biological Research. 1986;213:471–480. [PubMed] [Google Scholar]
- 71.Boesch-Saadatmandi C., Loboda A., Wagner A. E., et al. Effect of quercetin and its metabolites isorhamnetin and quercetin-3-glucuronide on inflammatory gene expression: role of miR-155. The Journal of Nutritional Biochemistry. 2011;22(3):293–299. doi: 10.1016/j.jnutbio.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 72.Lotito S. B., Frei B. Dietary flavonoids attenuate tumor necrosis factor α-induced adhesion molecule expression in human aortic endothelial cells. The Journal of Biological Chemistry. 2006;281(48):37102–37110. doi: 10.1074/jbc.M606804200. [DOI] [PubMed] [Google Scholar]
- 73.Li Y., Yao J., Han C., et al. Quercetin, inflammation and immunity. Nutrients. 2016;8(3):p. 167. doi: 10.3390/nu8030167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bureau G., Longpre F., Martinoli M. G. Resveratrol and quercetin, two natural polyphenols, reduce apoptotic neuronal cell death induced by neuroinflammation. Journal of Neuroscience Research. 2008;86(2):403–410. doi: 10.1002/jnr.21503. [DOI] [PubMed] [Google Scholar]
- 75.Nieman D. C., Henson D. A., Maxwell K. R., et al. Effects of quercetin and EGCG on mitochondrial biogenesis and immunity. Medicine & Science in Sports & Exercise. 2009;41(7):1467–1475. doi: 10.1249/MSS.0b013e318199491f. [DOI] [PubMed] [Google Scholar]
- 76.Jantan I., Ahmad W., Bukhari S. N. Plant-derived immunomodulators: an insight on their preclinical evaluation and clinical trials. Frontiers in Plant Science. 2015;6:p. 655. doi: 10.3389/fpls.2015.00655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Terao J. Factors modulating bioavailability of quercetin-related flavonoids and the consequences of their vascular function. Biochemical Pharmacology. 2017;139:15–23. doi: 10.1016/j.bcp.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 78.Haddad P., Eid H. The antidiabetic potential of quercetin: underlying mechanisms. Current Medicinal Chemistry. 2017;24(4):355–364. doi: 10.2174/0929867323666160909153707. [DOI] [PubMed] [Google Scholar]
- 79.Gormaz J. G., Quintremil S., Rodrigo R. Cardiovascular disease: a target for the pharmacological effects of quercetin. Current Topics in Medicinal Chemistry. 2015;15(17):1735–1742. doi: 10.2174/1568026615666150427124357. [DOI] [PubMed] [Google Scholar]
- 80.Edwards R. L., Lyon T., Litwin S. E., Rabovsky A., Symons J. D., Jalili T. Quercetin reduces blood pressure in hypertensive subjects. The Journal of Nutrition. 2007;137(11):2405–2411. doi: 10.1093/jn/137.11.2405. [DOI] [PubMed] [Google Scholar]
- 81.Brüll V., Burak C., Stoffel-Wagner B., et al. Acute intake of quercetin from onion skin extract does not influence postprandial blood pressure and endothelial function in overweight-to-obese adults with hypertension: a randomized, double-blind, placebo-controlled, crossover trial. European Journal of Nutrition. 2017;56(3):1347–1357. doi: 10.1007/s00394-016-1185-1. [DOI] [PubMed] [Google Scholar]
- 82.Egert S., Bosy-Westphal A., Seiberl J., et al. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: a double-blinded, placebo-controlled cross-over study. The British Journal of Nutrition. 2009;102(7):1065–1074. doi: 10.1017/S0007114509359127. [DOI] [PubMed] [Google Scholar]
- 83.Wei X., Meng X., Yuan Y., Shen F., Li C., Yang J. Quercetin exerts cardiovascular protective effects in LPS-induced dysfunction in vivo by regulating inflammatory cytokine expression, NF-κB phosphorylation, and caspase activity. Molecular and Cellular Biochemistry. 2018;446(1-2):43–52. doi: 10.1007/s11010-018-3271-6. [DOI] [PubMed] [Google Scholar]
- 84.Gnoni G. V., Paglialonga G., Siculella L. Quercetin inhibits fatty acid and triacylglycerol synthesis in rat-liver cells. European Journal of Clinical Investigation. 2009;39(9):761–768. doi: 10.1111/j.1365-2362.2009.02167.x. [DOI] [PubMed] [Google Scholar]
- 85.Tian H., Liu Q., Qin S., et al. Synthesis and cardiovascular protective effects of quercetin 7-O-sialic acid. Journal of Cellular and Molecular Medicine. 2017;21(1):107–120. doi: 10.1111/jcmm.12943. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 86.Prince P. S., Sathya B. Pretreatment with quercetin ameliorates lipids, lipoproteins and marker enzymes of lipid metabolism in isoproterenol treated cardiotoxic male Wistar rats. European Journal of Pharmacology. 2010;635(1-3):142–148. doi: 10.1016/j.ejphar.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 87.Kleemann R., Verschuren L., Morrison M., et al. Anti-inflammatory, anti-proliferative and anti-atherosclerotic effects of quercetin in human in vitro and in vivo models. Atherosclerosis. 2011;218(1):44–52. doi: 10.1016/j.atherosclerosis.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 88.Shen Y., Ward N. C., Hodgson J. M., et al. Dietary quercetin attenuates oxidant-induced endothelial dysfunction and atherosclerosis in apolipoprotein E knockout mice fed a high-fat diet: a critical role for heme oxygenase-1. Free Radical Biology & Medicine. 2013;65:908–915. doi: 10.1016/j.freeradbiomed.2013.08.185. [DOI] [PubMed] [Google Scholar]
- 89.Vanhees K., Godschalk R. W., Sanders A., van Waalwijk van Doorn-Khosrovani S. B., van Schooten F. J. Maternal quercetin intake during pregnancy results in an adapted iron homeostasis at adulthood. Toxicology. 2011;290(2-3):350–358. doi: 10.1016/j.tox.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 90.Maschio B. H., Gentil B. C., Caetano E., et al. Characterization of the effects of the shiitake culinary-medicinal mushroom, Lentinus edodes (Agaricomycetes), on severe gestational diabetes mellitus in rats. International Journal of Medicinal Mushrooms. 2017;19(11):991–1000. doi: 10.1615/IntJMedMushrooms.2017024498. [DOI] [PubMed] [Google Scholar]
- 91.Liu W., Zhang M., Feng J., Fan A., Zhou Y., Xu Y. The influence of quercetin on maternal immunity, oxidative stress, and inflammation in mice with exposure of fine particulate matter during gestation. International Journal of Environmental Research and Public Health. 2017;14(6):p. 592. doi: 10.3390/ijerph14060592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ben Salem I., Prola A., Boussabbeh M., et al. Crocin and quercetin protect HCT116 and HEK293 cells from Zearalenone-induced apoptosis by reducing endoplasmic reticulum stress. Cell Stress & Chaperones. 2015;20(6):927–938. doi: 10.1007/s12192-015-0613-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ben Salem I., Prola A., Boussabbeh M., et al. Activation of ER stress and apoptosis by α- and β-zearalenol in HCT116 cells, protective role of Quercetin. NeuroToxicology. 2016;53:334–342. doi: 10.1016/j.neuro.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 94.Gitika B., Sai Ram M., Sharma S. K., Ilavazhagan G., Banerjee P. K. Quercetin protects C6 glial cells from oxidative stress induced by tertiary-butylhydroperoxide. Free Radical Research. 2006;40(1):95–102. doi: 10.1080/10715760500335447. [DOI] [PubMed] [Google Scholar]
- 95.Sakanashi Y., Oyama K., Matsui H., et al. Possible use of quercetin, an antioxidant, for protection of cells suffering from overload of intracellular Ca2+: a model experiment. Life Sciences. 2008;83(5-6):164–169. doi: 10.1016/j.lfs.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 96.Sriraksa N., Wattanathorn J., Muchimapura S., Tiamkao S., Brown K., Chaisiwamongkol K. Cognitive-enhancing effect of quercetin in a rat model of Parkinson's disease induced by 6-hydroxydopamine. Evidence-based Complementary and Alternative Medicine. 2012;2012:9. doi: 10.1155/2012/823206.823206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yao Y., Han D. D., Zhang T., Yang Z. Quercetin improves cognitive deficits in rats with chronic cerebral ischemia and inhibits voltage-dependent sodium channels in hippocampal CA1 pyramidal neurons. Phytotherapy Research. 2010;24(1):136–140. doi: 10.1002/ptr.2902. [DOI] [PubMed] [Google Scholar]
- 98.Chirumbolo S. Quercetin in cancer prevention and therapy. Integrative Cancer Therapies. 2013;12(2):97–102. doi: 10.1177/1534735412448215. [DOI] [PubMed] [Google Scholar]
- 99.Ghadiri S., Spalenza V., Dellafiora L., et al. Modulation of aflatoxin B1 cytotoxicity and aflatoxin M1 synthesis by natural antioxidants in a bovine mammary epithelial cell line. Toxicology In Vitro. 2019;57:174–183. doi: 10.1016/j.tiv.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 100.Ramyaa P., Padma V. V. Ochratoxin-induced toxicity, oxidative stress and apoptosis ameliorated by quercetin--modulation by Nrf2. Food and Chemical Toxicology. 2013;62:205–216. doi: 10.1016/j.fct.2013.08.048. [DOI] [PubMed] [Google Scholar]
- 101.Ramyaa P., Krishnaswamy R., Padma V. V. Quercetin modulates OTA-induced oxidative stress and redox signalling in HepG2 cells – up regulation of Nrf2 expression and down regulation of NF-κB and COX-2. Biochimica et Biophysica Acta. 2014;1840(1):681–692. doi: 10.1016/j.bbagen.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 102.Schoneberg T., Kibler K., Sulyok M., et al. Can plant phenolic compounds reduce Fusarium growth and mycotoxin production in cereals? Food Additives & Contaminants: Part A. 2018;35(12):2455–2470. doi: 10.1080/19440049.2018.1538570. [DOI] [PubMed] [Google Scholar]
- 103.Periasamy R., Kalal I. G., Krishnaswamy R., Viswanadha V. Quercetin protects human peripheral blood mononuclear cells from OTA-induced oxidative stress, genotoxicity, and inflammation. Environmental Toxicology. 2016;31(7):855–865. doi: 10.1002/tox.22096. [DOI] [PubMed] [Google Scholar]
- 104.Bollina V., Kushalappa A. In vitro inhibition of trichothecene biosynthesis in Fusarium graminearum by resistance-related endogenous metabolites identified in barley. Mycology. 2011;2:291–296. doi: 10.1080/21501203.2011.604354. [DOI] [Google Scholar]
- 105.Gugliandolo E., Peritore A. F., D’Amico R., Licata P., Crupi R. Evaluation of neuroprotective effects of quercetin against aflatoxin B1-intoxicated mice. Animals. 2020;10(5):p. 898. doi: 10.3390/ani10050898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jin U. H., Park H., Li X., et al. Structure-dependent modulation of aryl hydrocarbon receptor-mediated activities by flavonoids. Toxicological Sciences. 2018;164(1):205–217. doi: 10.1093/toxsci/kfy075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Youdim K. A., Qaiser M. Z., Begley D. J., Rice-Evans C. A., Abbott N. J. Flavonoid permeability across an in situ model of the blood-brain barrier. Free Radical Biology & Medicine. 2004;36(5):592–604. doi: 10.1016/j.freeradbiomed.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 108.Tao Y., Xie S., Xu F., et al. Ochratoxin A: toxicity, oxidative stress and metabolism. Food and Chemical Toxicology. 2018;112:320–331. doi: 10.1016/j.fct.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 109.Pilipenko N., Ropstad E., Halsne R., Zamaratskaia G. Effect of naringenin, quercetin, and sesamin on Xenobiotica-metabolizing CYP1A and CYP3A in mice offspring after maternal exposure to persistent organic pollutants. BioMed Research International. 2017;2017:8. doi: 10.1155/2017/8472312.8472312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kuo I., Chen J., Chang T. K. Effect of Ginkgo biloba extract on rat hepatic microsomal CYP1A activity: role of ginkgolides, bilobalide, and flavonols. Canadian Journal of Physiology and Pharmacology. 2004;82(1):57–64. doi: 10.1139/y03-133. [DOI] [PubMed] [Google Scholar]
- 111.Ekstrand B., Rasmussen M. K., Woll F., Zlabek V., Zamaratskaia G. In vitro gender-dependent inhibition of porcine cytochrome p450 activity by selected flavonoids and phenolic acids. BioMed Research International. 2015;2015:7. doi: 10.1155/2015/387918.387918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sakalli S., Burkina V., Pilipenko N., Zlabek V., Zamaratskaia G. In vitro effects of diosmin, naringenin, quercetin and indole-3-carbinol on fish hepatic CYP1A1 in the presence of clotrimazole and dexamethasone. Chemosphere. 2018;192:105–112. doi: 10.1016/j.chemosphere.2017.10.106. [DOI] [PubMed] [Google Scholar]
- 113.Yang C., Bahar E., Adhikari S. P., Kim S. J., Kim H., Yoon H. Precise modeling of the protective effects of quercetin against mycotoxin via system identification with neural networks. International Journal of Molecular Sciences. 2019;20(7):p. 1725. doi: 10.3390/ijms20071725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Eftekhari A., Ahmadian E., Panahi-Azar V., Hosseini H., Tabibiazar M., Maleki Dizaj S. Hepatoprotective and free radical scavenging actions of quercetin nanoparticles on aflatoxin B1-induced liver damage:in vitro/in vivo studies. Artif Cells Nanomed Biotechnol. 2017;46(2):411–420. doi: 10.1080/21691401.2017.1315427. [DOI] [PubMed] [Google Scholar]
- 115.D'Andrea G. Quercetin: a flavonol with multifaceted therapeutic applications? Fitoterapia. 2015;106:256–271. doi: 10.1016/j.fitote.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 116.Zhao Y., Chen B., Shen J., et al. The beneficial effects of quercetin, curcumin, and resveratrol in obesity. Oxidative Medicine and Cellular Longevity. 2017;2017:8. doi: 10.1155/2017/1459497.1459497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen S., Jiang H., Wu X., Fang J. Therapeutic effects of quercetin on inflammation, obesity, and type 2 diabetes. Mediators of Inflammation. 2016;2016:5. doi: 10.1155/2016/9340637.9340637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Portillo M. Beneficial effects of quercetin on obesity and diabetes. The Open Nutraceuticals Journal. 2011;4(1):p. 189. doi: 10.2174/1876396001104010189. [DOI] [Google Scholar]
- 119.le N. H., Kim C. S., Park T., et al. Quercetin protects against obesity-induced skeletal muscle inflammation and atrophy. Mediators of Inflammation. 2014;2014:10. doi: 10.1155/2014/834294.834294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ying L., Chaudhry M. T., Xiao F., et al. The effects and mechanism of quercetin dietary supplementation in streptozotocin-induced hyperglycemic arbor acre broilers. Oxidative Medicine and Cellular Longevity. 2020;2020:11. doi: 10.1155/2020/9585047.9585047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jean-Moreno V., Rojas R., Goyeneche D., Coombs G. H., Walker J. Leishmania donovani: differential activities of classical topoisomerase inhibitors and antileishmanials against parasite and host cells at the level of DNA topoisomerase I and in cytotoxicity assays. Experimental Parasitology. 2006;112(1):21–30. doi: 10.1016/j.exppara.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 122.Worthen C., Jensen B. C., Parsons M. Diverse effects on mitochondrial and nuclear functions elicited by drugs and genetic knockdowns in bloodstream stage Trypanosoma brucei. PLoS Neglected Tropical Diseases. 2010;4(5, article e678) doi: 10.1371/journal.pntd.0000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ganesh D., Fuehrer H. P., Starzengrüber P., et al. Antiplasmodial activity of flavonol quercetin and its analogues in Plasmodium falciparum: evidence from clinical isolates in Bangladesh and standardized parasite clones. Parasitology Research. 2012;110(6):2289–2295. doi: 10.1007/s00436-011-2763-z. [DOI] [PubMed] [Google Scholar]
- 124.Mead J., McNair N. Antiparasitic activity of flavonoids and isoflavones against Cryptosporidium parvum and Encephalitozoon intestinalis. FEMS Microbiology Letters. 2006;259(1):153–157. doi: 10.1111/j.1574-6968.2006.00263.x. [DOI] [PubMed] [Google Scholar]
- 125.de Sousa L. R., Wu H., Nebo L., et al. Natural products as inhibitors of recombinant cathepsin L of Leishmania mexicana. Experimental Parasitology. 2015;156:42–48. doi: 10.1016/j.exppara.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 126.Jakaria M., Azam S., Jo S. H., Kim I. S., Dash R., Choi D. K. Potential therapeutic targets of quercetin and its derivatives: its role in the therapy of cognitive impairment. Journal of Clinical Medicine. 2019;8(11):p. 1789. doi: 10.3390/jcm8111789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Suganthy N., Devi K. P., Nabavi S. F., Braidy N., Nabavi S. M. Bioactive effects of quercetin in the central nervous system: focusing on the mechanisms of actions. Biomedicine & Pharmacotherapy. 2016;84:892–908. doi: 10.1016/j.biopha.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 128.Ossola B., Kaariainen T. M., Mannisto P. T. The multiple faces of quercetin in neuroprotection. Expert Opinion on Drug Safety. 2009;8(4):397–409. doi: 10.1517/14740330903026944. [DOI] [PubMed] [Google Scholar]
- 129.Ishisaka A., Ichikawa S., Sakakibara H., et al. Accumulation of orally administered quercetin in brain tissue and its antioxidative effects in rats. Free Radical Biology & Medicine. 2011;51(7):1329–1336. doi: 10.1016/j.freeradbiomed.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 130.Das S., Mandal A. K., Ghosh A., Panda S., Das N., Sarkar S. Nanoparticulated quercetin in combating age related cerebral oxidative injury. Current Aging Science. 2008;1(3):169–174. doi: 10.2174/1874609810801030169. [DOI] [PubMed] [Google Scholar]
- 131.Hu P., Wang M., Chen W. H., et al. Quercetin relieves chronic lead exposure-induced impairment of synaptic plasticity in rat dentate gyrus in vivo. Naunyn-Schmiedeberg's Archives of Pharmacology. 2008;378(1):43–51. doi: 10.1007/s00210-008-0301-z. [DOI] [PubMed] [Google Scholar]
- 132.Barcelos G. R., Grotto D., Serpeloni J. M., et al. Protective properties of quercetin against DNA damage and oxidative stress induced by methylmercury in rats. Archives of Toxicology. 2011;85(9):1151–1157. doi: 10.1007/s00204-011-0652-y. [DOI] [PubMed] [Google Scholar]
- 133.Sachdeva S., Pant S. C., Kushwaha P., Bhargava R., Flora S. J. Sodium tungstate induced neurological alterations in rat brain regions and their response to antioxidants. Food and Chemical Toxicology. 2015;82:64–71. doi: 10.1016/j.fct.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 134.Lv C., Hong T., Yang Z., et al. Effect of Quercetin in the 1-Methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine- Induced Mouse Model of Parkinson's Disease. Evidence-based Complementary and Alternative Medicine. 2012;2012:6. doi: 10.1155/2012/928643.928643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lakroun Z., Kebieche M., Lahouel A., Zama D., Desor F., Soulimani R. Oxidative stress and brain mitochondria swelling induced by endosulfan and protective role of quercetin in rat. Environmental Science and Pollution Research International. 2015;22(10):7776–7781. doi: 10.1007/s11356-014-3885-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.


