Abstract
Objective
To define the effect of DOCK8 deficiency on thymic tolerance in mice.
Methods
Thymocytes from wild‐type (Dock8+/+) and DOCK8‐deficient (Dock8pri/pri) mice were examined by flow cytometry. Some mice had transgenic expression of the BCL2 anti‐apoptotic protein in haemopoietic cells. Some mice expressed the transgenic 3A9 T‐cell receptor (TCR), which triggers thymocyte deletion in mice also expressing hen egg lysozyme under the insulin promoter.
Results
In Dock8pr/pri mice, the proportion of thymocytes induced to acquire tolerance at the immature CCR7− stage was normal. Deletion of strongly self‐reactive CD4+ thymocytes occurred efficiently in Dock8pri/pri mice in a TCR‐transgenic model that requires self‐antigen transfer from epithelial cells to bone marrow (BM)‐derived antigen‐presenting cells. Thymic Foxp3+ T‐regulatory cells (TREG) and Helios+ Foxp3− TREG precursors were decreased in Dock8pri/pri mice, including when apoptosis was inhibited by BCL2 transgene expression. Dock8pri/pri thymic TREG expressed CD25 and CTLA‐4 at normal levels. The results suggest that DOCK8 deficiency does not affect the function of BM‐derived antigen‐presenting cells in the thymus, the TCR self‐reactivity threshold that activates tolerance mechanisms in thymocytes or the apoptotic deletion of these thymocytes. However, DOCK8 is required to prevent a subset of developing TREG cells from undergoing cell death via a mechanism that is distinct from apoptosis.
Conclusion
DOCK8 deficiency diminishes TREG development in the thymus without compromising thymocyte deletion.
Keywords: dedicator of cytokinesis 8 (DOCK8), T‐cell deletion, T‐cell selection, T‐cell tolerance, thymus, T‐regulatory cells
Deleterious mutations in DOCK8 cause a severe immune deficiency syndrome, but whether thymic self‐tolerance mechanisms are affected has been unclear. In this study, we found that DOCK8‐deficient mice have normal clonal deletion of self‐reactive T cells at two stages of development in the thymus. However, DOCK8 deficiency conferred a T‐cell‐intrinsic defect in the intrathymic differentiation of Foxp3+ T‐regulatory cells.
Introduction
Deleterious homozygous mutations in DOCK8 cause a combined immunodeficiency characterised by susceptibility to allergies, infections and malignancies, with a poor prognosis in the absence of haemopoietic stem cell transplantation. 1 , 2 , 3 These manifestations arise, at least in part, from dysregulated immune responses to foreign antigens caused by defects in T‐cell and dendritic cell (DC) migration and in memory T‐ and B‐cell survival and function. 4 , 5 , 6 , 7 Autoimmunity is not a common consequence of DOCK8 deficiency, 8 but several patients have developed autoimmune haemolytic anaemia and/or vasculitis. 3 In studied DOCK8‐deficient patients, B‐cell tolerance induction in the bone marrow was normal, but the mature naïve B‐cell receptor repertoire was enriched in autoreactive antibodies. 9 While the thymic TREG population is diminished in DOCK8‐deficient mice, 10 whether and how DOCK8 deficiency affects T‐cell tolerance induction in the thymus has not been examined.
In T cells, DOCK8 is required for actin polymerisation and immunological synapse formation induced by T‐cell receptor (TCR) engagement. 4 , 10 Defective immunological synapse formation may alter the TCR self‐reactivity threshold at which thymocytes activate tolerance mechanisms, including apoptotic deletion and TREG differentiation. 11 The transcription factor, Helios, is upregulated in thymocytes that have activated tolerance mechanisms and is downregulated in thymocytes maturing into naïve T cells. 12 Thymic TREG differentiation has been characterised as a two‐step process whereby strong TCR signalling induces the formation of CD25+ Foxp3− TREG precursors (pre‐TREG) in which IL‐2 signalling induces upregulation of Foxp3. 13 As DOCK8 is required for immunological synapse formation and for IL‐2 signalling, 10 , 14 DOCK8 deficiency may perturb one or both of these steps.
The missense Dock8pri allele (Dock8S1827P) was identified in a genetic screen for decreased antibody recall responses 5 and has been extensively used as a mouse model of human DOCK8 deficiency. 4 , 5 , 15 It displays defects in actin reorganisation and T‐cell homing to lymph nodes that are indistinguishable from Dock8 −/− mice. 15 Here, we used Dock8pri/pri mice to assess the effect of DOCK8 deficiency on the induction of T‐cell tolerance in the thymus.
Results
DOCK8 deficiency perturbs tolerance induction in CCR7+ CD4+ CD8− thymocytes
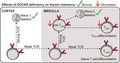
Distinct tolerance mechanisms are induced in thymocytes at two stages of development, which are distinguishable using molecular markers (Figure 1a). In normal mice, approximately half of the thymocytes that receive a detectable αβ TCR signal undergo apoptosis before they upregulate CCR7. 12 This process is called wave 1 deletion. 16 Strongly TCR‐signalled CCR7− CD24+ thymocytes destined for wave 1 deletion upregulate Helios and PD‐1. 12 To test whether DOCK8 deficiency affects the scale of wave 1 deletion, we used flow cytometry to examine thymocytes from Dock8+/+ and Dock8pri/pri mice. Dock8pri/pri mice had a small but consistent reduction in Helios+ PD‐1+ cell frequencies among CCR7− CD24+ thymocytes (Figure 1b, c). To measure more accurately the scale of wave 1 deletion, we examined Dock8+/+ and Dock8pri/pri mice expressing a BCL2 transgene (BCL2‐tg), which inhibits apoptosis. 17 On the BCL2‐tg background, Dock8pri/pri mice had Helios+ PD‐1+ cell frequencies among CCR7− CD24+ thymocytes similar to Dock8+/+ controls (Figure 1c). These data show that DOCK8 deficiency has little or no effect on the scale of wave 1 deletion in the thymus.
Figure 1.
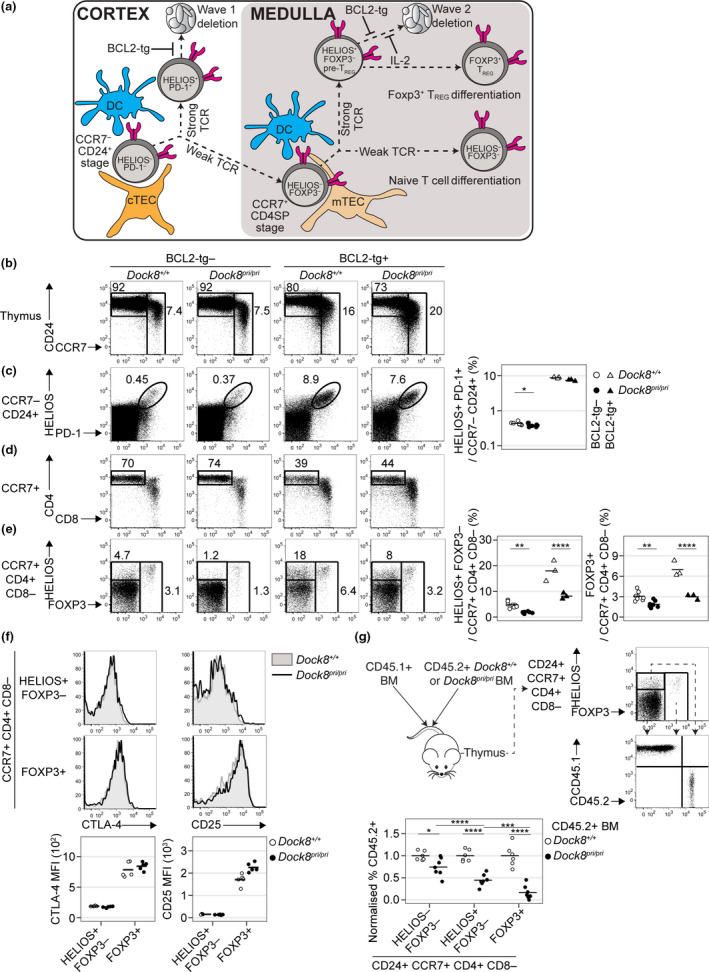
DOCK8 deficiency perturbs tolerance induction in CCR7+ CD4SP thymocytes. (a) Approach to the quantification of thymic tolerance. Cortical thymic epithelial cells (cTEC) and DC induce a subset of strongly self‐reactive immature CCR7− CD24+ thymocytes to upregulate Helios and PD‐1 before undergoing apoptosis (wave 1 deletion). Upon encountering medullary thymic epithelial cells (mTEC) and DC at the subsequent CCR7+ CD4SP stage, strong TCR signalling induces additional thymocytes to upregulate Helios to produce Helios+ Foxp3− pre‐TREG that are poised for apoptosis (wave 2 deletion) or Foxp3 upregulation (TREG differentiation). Foxp3 upregulation marks the onset of the requirement for IL‐2 to inhibit wave 2 deletion. BCL2‐tg expression inhibits apoptosis, enabling measurement of the scale of wave 1 deletion and wave 2 deletion. Thymocytes that undergo weak TCR signalling at both stages differentiate into naïve T cells. (b) CD24/CCR7 phenotype of thymocytes from mice with the indicated genotypes (top) with a gate for the CD24+ CCR7− population, which was analysed for (c) Helios and PD‐1 expression to enumerate the Helios+ PD‐1+ subset (right). (d) CD4/CD8 phenotype of CCR7+ thymocytes, with a gate for the CD4SP population, which was (e) analysed for Helios and Foxp3 expression to enumerate the Helios+ Foxp3− and Foxp3+ subsets (right). Data in c and e were compiled from two independent experiments with n = 8 Dock8+/+, n = 8 Dock8pri/pri, n = 3 Dock8+/+BCL2‐tg+ and n = 3 Dock8pri/priBCL2‐tg+ mice. (f) Histogram overlays show CTLA4 (left) and CD25 (right) expression on the Helios+ Foxp3− and Foxp3+ subsets of CCR7+ CD4SP thymocytes in concatenated FACS files from a single experiment. Black line represents Dock8pri/pri and grey shading Dock8+/+ littermate controls. Graphs (below) show mean fluorescence intensities (MFI) for n = 5 mice per group, representative of two independent experiments. (g) Irradiated mice were reconstituted with 50:50 mixtures of CD45.1 wild‐type BM plus CD45.2 Dock8+/+ BM or CD45.2 Dock8pri/pri BM eight weeks before analysis. Plots show CD24+ CCR7+ CD4SP thymocytes divided into three subsets based on Helios and Foxp3 expression, each of which was analysed for the frequency of CD45.2+ cells. To facilitate statistical comparisons, the frequency of CD45.2+ cells in each CCR7+ CD24+ CD4SP subset was divided by the frequency of CD45.2+ cells at the precursor CD4+ CD8+ double‐positive stage in the same sample, then divided by the mean of the Dock8+/+ group for each subset. Transplants were performed on a single day, and chimeric mice were analysed in two separate experiments with concordant results. Results for one experiment with n = 6 CD45.1 WT/CD45.2 Dock8+/+ BM recipients and n = 7 CD45.1 WT/CD45.2 Dock8pri/pri BM recipients are shown. Each symbol represents a measurement from 1 mouse, and horizontal bars show the group mean. Statistical analyses used 2‐way ANOVA with Sidak’s multiple comparisons test. P‐values: * < 0.05, ** < 0.01, *** < 0.001 and **** < 0.0001.
Helios is also upregulated in strongly TCR‐signalled CCR7+ thymocytes destined to undergo apoptosis at the CD4+ CD8− single‐positive (CD4SP) stage (wave 2 deletion). 12 , 18 Moreover, as most nascent thymic TREG are Helios+, the CCR7+ CD4SP Helios+ Foxp3− thymocyte population contains pre‐TREG. 18 , 19 In Dock8pri/pri mice, the Helios+ Foxp3− cell frequency among CCR7+ CD4SP thymocytes was 35% of that observed in Dock8+/+ mice (1.7% in the Dock8pri/pri group versus 4.8% in the Dock8+/+ group) (Figure 1d, e). Similarly, in the presence of BCL2‐tg expression, the frequency of pre‐TREG cells in Dock8pri/pri mice was 45% of that in the Dock8+/+ group (8.2% versus 18.0%; Figure 1d, e). Other markers have also been described to identify pre‐TREG populations – analysis for CD25+ Foxp3− cells 13 , 20 and CD25− Foxp3+ cells 20 , 21 in the CD4SP gate also showed a decrease in pre‐TREG in Dock8pri/pri mice compared with wild type (Supplementary figure 1a, b).
DOCK8 deficiency reduces the size of the thymic Foxp3+ TREG population, 10 which is composed of nascent and non‐nascent subsets. 22 Nascent TREG have a CCR7+ CD4SP phenotype. 23 In Dock8pri/pri mice, the Foxp3+ cell frequency among CCR7+ CD4SP thymocytes was 60% of that observed in Dock8+/+ mice when apoptosis was normal (1.9% versus 3.1%) and 40% in the presence of BCL2‐tg expression (3.0% versus 7.0%) (Figure 1e). DOCK8 deficiency thus diminishes the thymic pre‐TREG and TREG populations via a defect that is not corrected by BCL2‐tg expression. Furthermore, DOCK8 deficiency diminished the non‐nascent CCR7− CD4SP subset of TREG in the thymus 23 (Supplementary figure 1a, c).
During thymic TREG development, the transition from the Helios+ Foxp3− stage to the Foxp3+ stage is accompanied by upregulation of CTLA‐4 and CD25. 19 We found no evidence that DOCK8 deficiency decreased CTLA‐4 or CD25 upregulation during this transition (Figure 1f).
To test whether a thymocyte‐intrinsic defect contributes to the abnormalities observed in Dock8pri/pri mice, mixed BM chimeras were made by reconstituting irradiated mice with CD45.1+ wild‐type BM mixed with either CD45.2+ Dock8+/+ BM or CD45.2+ Dock8pri/pri BM. To exclude host‐derived thymic TREG cells, most of which have a CD24− CCR7− phenotype, 18 we gated on the CCR7+ CD24+ CD4SP thymocyte population, which was divided into subsets defined by Helios and Foxp3 expression (Figure 1g). This revealed that Dock8pri/pri thymocytes have a moderate defect in becoming Helios− Foxp3− cells, a severe defect in becoming Helios+ Foxp3− pre‐TREG and the most severe defect in becoming Foxp3+ TREG (Figure 1g).
DOCK8 is not required for deletion of strongly self‐reactive CD4SP thymocytes
To study the effect of DOCK8 deficiency on thymic tolerance in a well‐characterised model, we used TCR‐transgenic mice. In I‐Ak+ mice bearing the 3A9 TCR transgene (3A9+) alone, developing thymocytes receive a weak TCR signal and develop efficiently into naïve CD4+ T cells. 24 Compared with Dock8+/+ 3A9+ mice, Dock8pri/pri 3A9+ mice had a lower frequency of TCR3A9+ Foxp3− CD4SP thymocytes (Figure 2a, b). DOCK8 deficiency moderately impairs naïve CD4+ T‐cell development in 3A9+ mice, as in non‐transgenic mice. 4
Figure 2.
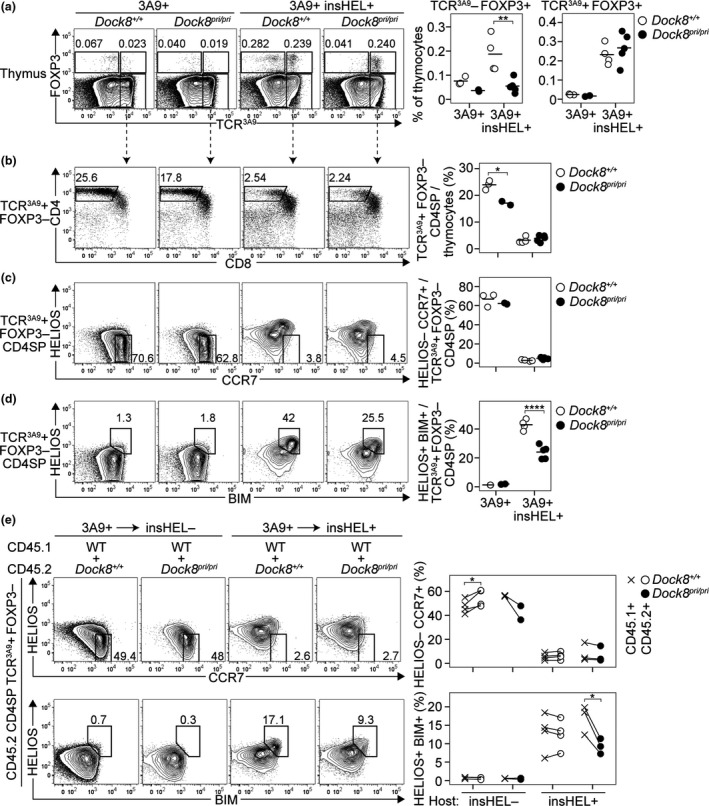
DOCK8 is not required for deletion of strongly self‐reactive CD4SP thymocytes. For thymocytes from mice of the indicated genotypes (top), plots show (a) expression of Foxp3 versus TCR3A9 with numbers representing the percentage of cells in the adjacent gate, enumerated in the summaries (right). (b) CD4/CD8 phenotype of TCR3A9+ Foxp3− thymocytes with a summary showing the percentage of TCR3A9+ Foxp3− CD4SP cells among all thymocytes (right). TCR3A9+ Foxp3− CD4SP thymocytes were analysed for (c) Helios/CCR7 phenotype, with a gate for antigen‐inexperienced Helios− CCR7+ cells, and (d) Helios/Bim phenotype, with a gate for antigen‐experienced Helios+ Bim+ cells, enumerated in the summaries (right). In a–d, each symbol in a summary graph represents a measurement from 1 mouse (n = 3 Dock8+/+3A9+, n = 2 Dock8pri/pri3A9+, n = 4 Dock8+/+3A9+insHEL+ and n = 5 Dock8pri/pri3A9+insHEL+), and horizontal bars show the group mean compiled from 2 experiments. (e) Mixed chimeras made by reconstituting irradiated insHEL− or insHEL+ hosts with mixtures of CD45.1 wild‐type 3A9+ BM plus CD45.2 Dock8+/+ or Dock8pri/pri 3A9+ BM were analysed 8 weeks after BM transplantation. Plots show CD45.2+ TCR3A9+ Foxp3− CD4SP thymocytes analysed for Helios/CCR7 (top) or Helios/Bim (bottom) phenotypes, with summaries (right) showing the frequency of subsets gated on the plots. Each line joins frequencies for the CD45.1+ and CD45.2+ subsets of TCR3A9+ Foxp3− CD4SP thymocytes from an individual mouse (n = 4 CD45.1Dock8+/+: CD45.2 Dock8+/+ into insHEL−; n = 2 CD45.1Dock8+/+: CD45.2 Dock8pri/pri into insHEL−; n = 4 CD45.1Dock8+/+: CD45.2 Dock8+/+ into insHEL+; and n = 3 CD45.1Dock8+/+: CD45.2 Dock8pri/pri into insHEL+). Statistical analyses used 2‐way ANOVA with Sidak’s multiple comparisons test (a–d) or paired Student’s t‐tests (e); P‐values: * < 0.05, ** < 0.01 and **** < 0.0001.
In TCR‐transgenic 3A9+ mice that also express a transgene encoding the hen egg lysozyme (HEL) antigen under the control of the insulin promoter (insHEL), most thymocytes undergo deletion or TREG differentiation as a result of strong TCR signalling. 24 Surprisingly, the TCR3A9+ Foxp3+ thymocyte frequency was normal in Dock8pri/pri 3A9+ insHEL+ mice (Figure 2a), indicating that DOCK8 deficiency permits differentiation of thymic TREG in TCR‐transgenic thymocytes encountering HEL/I‐Ak complexes. However, consistent with the results in non‐transgenic mice, DOCK8 deficiency did decrease the frequency of TCR3A9− Foxp3+ thymocytes in the same mice (Figure 2a).
TCR3A9+ Foxp3− CD4SP thymocytes downregulate Helios and upregulate CCR7 as they mature into naïve CD4+ T cells in 3A9+ mice, whereas they upregulate Helios and Bim before they undergo deletion in 3A9+ insHEL+ mice. 12 In wild‐type and Dock8pri/pri 3A9+ insHEL+ mice, there was no difference in the frequency of TCR3A9+ Foxp3− CD4SP thymocytes, including those with a Helios− CCR7+ antigen‐inexperienced phenotype (Figure 2b, c). In this model, DOCK8 deficiency does not increase T‐cell escape from deletion in the thymus. In fact, the frequency of Helios+ Bim+ cells in Dock8pri/pri 3A9+ insHEL+ mice was only 56% of that observed in Dock8+/+ 3A9+ insHEL+ mice (24.1% versus 43.0%) (Figure 2d), suggesting that the DOCK8‐deficient thymocytes may be deleted more efficiently than in control mice. These effects were also observed in mixed BM chimeras, demonstrating that the decrease in antigen‐experienced thymocytes was a cell‐intrinsic property of DOCK8‐deficient lymphocytes (Figure 2e).
Discussion
DOCK8 mutations cause immunodeficiency and allergy, but the effects of DOCK8 deficiency on thymic tolerance mechanisms have not been examined. By analysing Dock8pri/pri mice, we found that DOCK8 deficiency does not impair the deletion of strongly self‐reactive thymocytes. However, DOCK8 deficiency causes a defect in the survival of CCR7+ CD4SP Helios+ Foxp3− thymocytes that have begun to differentiate towards the TREG lineage. This defect is present at a stage that precedes the requirement for IL‐2 signalling in thymic TREG differentiation and was not corrected by BCL2‐tg expression, suggesting that DOCK8 is necessary to prevent a cell death mechanism that is distinct from previously characterised causes of apoptosis in developing TREG cells.
The data suggest that DOCK8 deficiency does not impair self‐antigen presentation by BM‐derived cells in the thymus, nor does it alter the sensitivity of thymocytes to activation by self‐antigens. The scale of wave 1 deletion was normal in Dock8pri/pri mice, unlike in mice lacking MHC expression in BM‐derived cells, in which the scale of wave 1 deletion was reduced by 45%. 16 Likewise, DOCK8 deficiency did not impair thymic deletion in 3A9+ insHEL+ mice, in which deletion is absolutely dependent on self‐antigen presentation by BM‐derived cells. 25 Self‐reactive CD4SP thymocytes in Dock8pri/pri 3A9+ insHEL+ mice were induced to upregulate Helios and Bim, as expected, but these cells died prematurely. This suggests the reduction in CCR7+ CD4SP Helios+ Foxp3− thymocytes in Dock8pri/pri mice reflects decreased survival, rather than decreased induction, of self‐reactive thymocytes. This conclusion aligns CD4SP thymocytes with DOCK8‐deficient B cells, naïve T cells and NKT cells, in which the initial response to antigen appears normal but the survival of antigen‐activated lymphocytes is reduced. 4 , 5 , 26 We conclude that the deletion of self‐reactive thymocytes was not impaired in DOCK8‐deficient mice. This finding may provide an explanation for the low rate of autoimmunity in DOCK8‐deficient patients compared with some other primary immunodeficiency syndromes. 8
DOCK8 deficiency is known to impair the differentiation of thymocytes towards the naïve CD4+ T‐cell, naïve CD8+ T‐cell, NKT and TREG lineages. 4 , 10 , 26 While low BCL2 expression suggested that DOCK8‐deficient thymocytes may have heightened susceptibility to apoptosis, 26 , 27 thymic pre‐TREG and TREG populations remain diminished in BCL2‐tg+ Dock8pri/pri mice compared with BCL2‐tg+ Dock8+/+ controls. IL‐2 prevents deletion at the transition between the Helios+ Foxp3− and Foxp3+ stages of thymic TREG differentiation. 19 Consistent with a requirement for DOCK8 in IL‐2 signalling within TREG, 10 , 14 mixed chimera experiments revealed that the Dock8pri/pri genotype impairs this transition. However, the Helios+ Foxp3− pre‐TREG population is also diminished, indicating that a requirement for DOCK8 exists in thymic TREG differentiation preceding the requirement for IL‐2. The absence of IL‐2 signalling reduces CTLA‐4 and CD25 expression within thymic TREG, 19 but these parameters were normal in Dock8pri/pri thymic TREG. To explain this finding, the available IL‐2 might be sufficient to sustain the relatively small thymic TREG populations in Dock8pri/pri mice. Unexpectedly, the size of the thymic TCR3A9+ Foxp3+ population was normal in Dock8pri/pri 3A9+ insHEL+ mice, suggesting that DOCK8 is dispensable for the development of some thymic TREG in a manner that might be determined by variation in TCR signalling.
The available data implicate a role for DOCK8 in preventing the death of developing TREG by a mechanism that is distinct from inhibiting apoptosis or promoting IL‐2 signalling. DOCK8‐deficient T cells display a defect in withstanding shape deformations as they migrate through dense tissues and are prone to die via cytothripsis (cell shattering). 6 Self‐reactive CD4SP thymocytes do undergo shape deformations as they interact with antigen‐presenting cells in the thymic medulla. 28 Regardless of the cell death mechanism prevented by DOCK8, our data establish that DOCK8 deficiency diminishes TREG differentiation without detectably affecting deletion in the thymus.
Methods
Mice
The generation of Dock8pri/pri mice by ENU mutagenesis was previously described. 5 Dock8pri/pri BCL2‐tg mice were obtained from a colony of Dock8pri/pri.BCL2‐tg.swHEL mice (derived from BCL2‐tg mice described by Ogilvy 17 ), but were mice that did not have a transgenic B‐cell receptor. Mice bearing the Dock8pri allele were backcrossed to mice bearing the 3A9 transgene 29 and/or insHEL transgene 30 on the B10.BR (H2k) genetic background for 3 generations before intercrossing to produce experimental animals. Bone marrow chimeras were generated by irradiating recipient mice with X‐rays (two doses of 4.5 Gy given 4 h apart) and injecting at least 2 × 106 bone marrow cells intravenously on the same day. All procedures were approved by the Animal Experimentation Ethics Committee of the Australian National University.
Flow cytometry
All antibodies were obtained from BioLegend (San Diego, California, USA) unless otherwise indicated. The detection of CCR7 on thymocytes was as previously described. 18 Briefly, cells were incubated at 37°C for 60 min in FACS wash buffer (PBS containing 2% v/v heat‐inactivated bovine serum and 0.01% m/v sodium azide) containing PE‐ or biotin‐conjugated anti‐CCR7 antibody (clone 4B12). In some experiments, to label cells expressing the 3A9 TCR (TCR3A9), cells were then pelleted and incubated for 30 min at 4°C in hybridoma supernatant containing the 1G12 monoclonal antibody. 24 Cells were then pelleted and incubated for 30 min at 4°C in FACS wash buffer containing the rest of the primary antibodies specific for cell‐surface proteins, including PE‐Cy7‐conjugated anti‐CD24 (M1/69), PerCP‐Cy5.5‐conjugated anti‐CD25 (PC61.5, eBioscience (San Diego, California, USA)), A700‐conjugated anti‐CD4 (RM4‐5), APC‐Cy7‐conjugated anti‐CD8 (53‐6.7), PE‐Cy5‐ (BD Pharmingen, San Diego, California, USA) or PerCP‐Cy5.5‐conjugated anti‐CD45.2 (clone 104), APC‐, AF700‐ or AF647‐conjugated anti‐CD45.1 (A20), PE‐conjugated anti‐PD1 (J43, eBioscience) and PE‐ or biotin‐conjugated anti‐mouse IgG1 to detect TCR3A9+ cells. After washing in FACS buffer, cells were fixed and permeabilised using the eBioscience Foxp3 staining kit (Cat. no: 71‐5775‐40), then incubated with FITC‐ or e450‐conjugated anti‐Foxp3 (FJK‐165, eBioscience), FITC‐ or Pacific Blue‐conjugated anti‐Helios (22F6), APC‐conjugated anti‐CTLA‐4 (UC10‐4B9, eBioscience) and Qdot 605 streptavidin to detect TCR3A9+ cells. Data were acquired on an LSRII flow cytometer (BD) and analysed using FlowJo software (BD).
Conflicts of interest
The authors have no conflicts of interest to declare.
Author Contribution
Katrina Randall: Conceptualization; Data curation; Formal analysis; Funding acquisition; Methodology; Project administration; Supervision; Writing‐original draft; Writing‐review & editing. Hsei Di Law: Formal analysis; Investigation; Writing‐review & editing. Andrew Ziolkowski: Formal analysis; Investigation; Writing‐review & editing. Rushika Wirasinha: Formal analysis; Investigation; Writing‐review & editing. Christopher Goodnow: Conceptualization; Resources; Supervision; Writing‐review & editing. Stephen Robert Daley: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Supervision; Writing‐original draft; Writing‐review & editing.
Supporting information
Supplementary figure 1
Acknowledgments
This work was supported by an NHMRC Project Grant 1022922 (KLR) and ACT Health Private Practice Fund Major Grants in 2015 and 2016 (KLR). We thank the Australian Phenomics Facility for mouse care and genotyping.
References
- 1. Zhang Q, Davis JC, Lamborn IT et al Combined immunodeficiency associated with DOCK8 mutations. N Engl J Med 2009; 361: 2046–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Engelhardt KR, McGhee S, Winkler S et al Large deletions and point mutations involving the dedicator of cytokinesis 8 (DOCK8) in the autosomal‐recessive form of hyper‐IgE syndrome. J Allergy Clin Immunol 2009; 124: 1289–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aydin SE, Kilic SS, Aytekin C et al DOCK8 deficiency: clinical and immunological phenotype and treatment options ‐ a review of 136 patients. J Clin Immunol 2015; 35: 189–198. [DOI] [PubMed] [Google Scholar]
- 4. Randall KL, Chan SS, Ma CS et al DOCK8 deficiency impairs CD8 T cell survival and function in humans and mice. J Exp Med 2011; 208: 2305–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Randall KL, Lambe T, Johnson AL et al Dock8 mutations cripple B cell immunological synapses, germinal centers and long‐lived antibody production. Nat Immunol 2009; 10: 1283–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang Q, Dove CG, Hor JL et al DOCK8 regulates lymphocyte shape integrity for skin antiviral immunity. J Exp Med 2014; 211: 2549–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harada Y, Tanaka Y, Terasawa M et al DOCK8 is a Cdc42 activator critical for interstitial dendritic cell migration during immune responses. Blood 2012; 119: 4451–4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Su HC, Jing H, Angelus P, Freeman AF. Insights into immunity from clinical and basic science studies of DOCK8 immunodeficiency syndrome. Immunol Rev 2019; 287: 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Janssen E, Morbach H, Ullas S et al Dedicator of cytokinesis 8‐deficient patients have a breakdown in peripheral B‐cell tolerance and defective regulatory T cells. J Allergy Clin Immunol 2014; 134: 1365–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Janssen E, Kumari S, Tohme M et al DOCK8 enforces immunological tolerance by promoting IL‐2 signaling and immune synapse formation in Tregs. JCI Insight 2017; 2: e94298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Daley SR, Teh C, Hu DY, Strasser A, Gray DHD. Cell death and thymic tolerance. Immunol Rev 2017; 277: 9–20. [DOI] [PubMed] [Google Scholar]
- 12. Daley SR, Hu DY, Goodnow CC. Helios marks strongly autoreactive CD4+ T cells in two major waves of thymic deletion distinguished by induction of PD‐1 or NF‐κB. J Exp Med 2013; 210: 269–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lio CW, Hsieh CS. A two‐step process for thymic regulatory T cell development. Immunity 2008; 28: 100–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Singh AK, Eken A, Hagin D et al DOCK8 regulates fitness and function of regulatory T cells through modulation of IL‐2 signaling. JCI Insight 2017; 2: e94275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Janssen E, Tohme M, Hedayat M et al A DOCK8‐WIP‐WASp complex links T cell receptors to the actin cytoskeleton. J Clin Invest 2016; 126: 3837–3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wirasinha RC, Chan A, Yap JY et al Deletion of self‐reactive CCR7‐ thymocytes in the absence of MHC expression on thymic epithelial cells. Cell Death Differ 2019; 26: 2727–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ogilvy S, Metcalf D, Print CG, Bath ML, Harris AW, Adams JM. Constitutive Bcl‐2 expression throughout the hematopoietic compartment affects multiple lineages and enhances progenitor cell survival. Proc Natl Acad Sci USA 1999; 96: 14943–14948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hu DY, Yap JY, Wirasinha RC, Howard DR, Goodnow CC, Daley SR. A timeline demarcating two waves of clonal deletion and Foxp3 upregulation during thymocyte development. Immunol Cell Biol 2016; 94: 357–366. [DOI] [PubMed] [Google Scholar]
- 19. Hu DY, Wirasinha RC, Goodnow CC, Daley SR. IL‐2 prevents deletion of developing T‐regulatory cells in the thymus. Cell Death Differ 2017; 24: 1007–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Owen DL, Mahmud SA, Sjaastad LE et al Thymic regulatory T cells arise via two distinct developmental programs. Nat Immunol 2019; 20: 195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tai X, Erman B, Alag A et al Foxp3 transcription factor is proapoptotic and lethal to developing regulatory T cells unless counterbalanced by cytokine survival signals. Immunity 2013; 38: 1116–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thiault N, Darrigues J, Adoue V et al Peripheral regulatory T lymphocytes recirculating to the thymus suppress the development of their precursors. Nat Immunol 2015; 16: 628–634. [DOI] [PubMed] [Google Scholar]
- 23. Cowan JE, McCarthy NI, Anderson G. CCR7 Controls Thymus Recirculation, but Not Production and Emigration, of Foxp3+ T Cells. Cell Rep 2016; 14: 1041–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lesage S, Hartley SB, Akkaraju S, Wilson J, Townsend M, Goodnow CC. Failure to censor forbidden clones of CD4 T cells in autoimmune diabetes. J Exp Med 2002; 196: 1175–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yap JY, Wirasinha RC, Chan A, Howard DR, Goodnow CC, Daley SR. Indirect presentation in the thymus limits naive and regulatory T‐cell differentiation by promoting deletion of self‐reactive thymocytes. Immunology 2018; 154: 522–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Crawford G, Enders A, Gileadi U et al DOCK8 is critical for the survival and function of NKT cells. Blood 2013; 122: 2052–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lambe T, Crawford G, Johnson AL et al DOCK8 is essential for T‐cell survival and the maintenance of CD8+ T‐cell memory. Eur J Immunol 2011; 41: 3423–3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ueda Y, Katagiri K, Tomiyama T et al Mst1 regulates integrin‐dependent thymocyte trafficking and antigen recognition in the thymus. Nat Commun 2012; 3: 1098. [DOI] [PubMed] [Google Scholar]
- 29. Ho WY, Cooke MP, Goodnow CC, Davis MM. Resting and anergic B cells are defective in CD28‐dependent costimulation of naive CD4+ T cells. J Exp Med 1994; 179: 1539–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Akkaraju S, Ho WY, Leong D, Canaan K, Davis MM, Goodnow CC. A range of CD4 T cell tolerance: partial inactivation to organ‐specific antigen allows nondestructive thyroiditis or insulitis. Immunity 1997; 7: 255–271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1


