Abstract
Colonic intramuscular interstitial cells of Cajal (ICC-IM) are associated with cholinergic varicosities, suggesting a role in mediating excitatory neurotransmission. Ca2+ release in ICC-IM activates Ano1, a Ca2+-activated Cl− conductance, causing tissue depolarization and increased smooth muscle excitability. We employed Ca2+ imaging of colonic ICC-IM in situ, using mice expressing GCaMP6f in ICC to evaluate ICC-IM responses to excitatory neurotransmission. Expression of muscarinic type 2, 3 (M2, M3) and NK1 receptors were enriched in ICC-IM. NK1 receptor agonists had minimal effects on ICC-IM, whereas neostigmine and carbachol increased Ca2+ transients. These effects were reversed by DAU 5884 (M3 receptor antagonist) but not AF-DX 116 (M2 receptor antagonist). Electrical field stimulation (EFS) in the presence of L-NNA and MRS 2500 enhanced ICC-IM Ca2+ transients. Responses were blocked by atropine or DAU 5884, but not AF-DX 116. ICC-IM responses to EFS were ablated by inhibiting Ca2+ stores with cyclopiazonic acid and reduced by inhibiting Ca2+ influx via Orai channels. Contractions induced by EFS were reduced by an Ano1 channel antagonist, abolished by DAU 5884 and unaffected by AF-DX 116. Colonic ICC-IM receive excitatory inputs from cholinergic neurons via M3 receptor activation. Enhancing ICC-IM Ca2+ release and Ano1 activation contributes to excitatory responses of colonic muscles.
Keywords: optogenetics, Ca2+ imaging, c-Kit, Ca2+ stores, cholinergic, SIP syncytium, gastrointestinal motility
Introduction
Coordinated smooth muscle cell (SMC) contractions that underlie gastrointestinal (GI) motility patterns, such as peristalsis, segmentation and tone, result from the integrated behaviors of SMCs and non-contractile interstitial cells and further regulation by enteric motor neurons and hormones (1–3). Two types of interstitial cells, identified by antibodies to c-Kit (interstitial cells of Cajal; ICC) or platelet-derived-growth-factor-receptor-alpha (PDGFRα+ cells) are linked to SMCs via gap junctions, forming an electrical syncytium known as the SIP syncytium (i.e. SMCs, ICC, PDGFRα+ cells (1)). Electrophysiological responses developing in any of the cells of the SIP syncytium can conduct and modulate the behaviors of the other cells. Thus, the integrated output of the SIP syncytium defines what has traditionally been known as myogenic regulation of motility. Enteric motor neurons innervate cells of the SIP syncytium, and each type of cell exhibits specific patterns of receptor and effector expression that facilitate specific components of neural regulation. Thus, functional innervation of GI muscles involves cell-specific transduction of the multiple neurotransmitters released by enteric motor neurons, and integration of these responses in the SIP syncytium to generate the complex regional motor patters of GI motility.
Several different classes of ICC exist in the GI tract. In the colon, at least four discrete populations have been described by morphological studies: i) an interconnected network of ICC at the submucosal border (ICC-SM) that provides pacemaker signals to the circular muscle (CM) layer (4–6), ii) spindle shaped intra-muscular ICC (ICC-IM) that have no apparent pacemaker role but may transduce neural inputs and regulate the excitability of colonic SMCs (6–12), iii) a network of ICC within the plane of the myenteric plexus (ICC-MY) between the CM and longitudinal layers (LM) that also provides pacemaker activity and possibly responses to neural inputs (5, 13, 14), and iv) a network of ICC along the serosal surface of the LM (ICC-SS that provides pacemaker input to the LM and responds to nitrergic stimulation (15–18). Colonic ICC-IM, like other ICC-IM throughout the GI tract, are thought to transduce neural inputs from enteric motor neurons to SMCs. This hypothesis was originally based on the close anatomical associations of ICC-IM with the varicosities of excitatory and inhibitory motor neurons in the esophagus (19); stomach (20–24), small intestine (25, 26), colon (12) and internal anal sphincter (27–29). ICC-IM express signaling molecules and receptors required for binding and transducing enteric neurotransmitters and form synaptic-like contacts with enteric nerve endings (12, 19, 20, 22, 24, 30). Many studies have demonstrated that mutant animal models either lacking ICC-IM or with impaired ICC-IM function, exhibit compromised post-junctional neuronal responses (20, 28, 29, 31–40).
Ca2+ release from intracellular stores is fundamental to the functions of ICC (1, 41). Ca2+ release, sustained by Ca2+ influx (42) couples to activation of Ano1, a Ca2+-activated Cl− channel expressed selectively in ICC (43, 44), and generation of spontaneous transients inward currents (STICs) and spontaneous transient depolarizations (STDs) in ICC (41, 45, 46). STICs conduct to the other cells of the SIP syncytium, causing a net depolarizing influence which, in general, increases the excitability of SMCs (1, 10, 11, 15, 47, 48).
The colon contains a large population of ICC-IM in the CM layer (9, 18, 49). These cells are highly active in situ, exhibiting spontaneous Ca2+ release events from intracellular stores via inositol triphosphate (IP3) receptors (10). Ongoing repetitive Ca2+ release is sustained by the Ca2+ gradient across the plasma membrane and Ca2+ influx through store-operated Ca2+ entry (SOCE) (10). Ca2+ signaling in colonic ICC-IM and activation of Ano1 channels leads to substantial tonic depolarization of colonic SMCs (10, 11). Colonic ICC-IM are closely associated with excitatory motor neurons, suggesting that they could play a role in mediating excitatory inputs (12). However, direct innervation and the role of ICC-IM in mediating excitatory/propulsive signals in the colon has been difficult to demonstrate conclusively. This is because mutant animal models (e.g. W/Wv mice or WS/WS rats) have only partial reductions in colonic ICC (23, 40) and no definitive loss-of-function with regard to the role of ICC-IM in neurotransmission.
In the present study, we hypothesized that the close associations between excitatory nerve varicosities and ICC-IM in the proximal colon (12) would make ICC-IM targets for excitatory innervation. We evaluated neurotransmitter receptor expression in sorted ICC-IM to determine whether these cells express the necessary post-junctional apparatus to bind and transduce excitatory neurotransmitter signals. We used mice expressing the genetically-encoded Ca2+ indicator GCaMP6f in an ICC-specific manner and fluorescence videomicroscopy to characterize responses of ICC-IM to excitatory stimulation in situ. Contractile experiments were performed to determine whether Ano1 channels, expressed exclusively in ICC, are involved in mediating whole tissue post-junctional excitatory responses.
Methods
Animal ethics & approval
All animals used and the protocols performed for this study were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All procedures were approved by the Institutional Animal Use and Care Committee at the University of Nevada, Reno.
Animals
Floxed GCaMP6f mice, Ai95 (RCL-GCaMP6f)-D (GCaMP6ffl/fl mice), and their wild-type siblings (C57BL/6) were acquired from the Jackson Laboratory (Bar Harbor, MN, USA). c-Kit+/Cre-ERT2 (Kit-Cre mice) were gifted from Dr. Dieter Saur of the Technical University Munich, Germany. Mice that expressed an ICC specific fluorescent reporter for cellular identification (Kit+/copGFP mice) were generated and bred in house (50).
Tamoxifen preparation and administration
GCaMP6ffl/fl mice were crossed with Kit-Cre mice, as outlined above, and the resulting offspring are referred to as Kit-Cre-GCaMP6f. Kit-Cre-GCaMP6f mice were injected at 6-8 weeks of age with tamoxifen to activate Cre Recombinase and GCaMP6f. Tamoxifen (Sigma T5648; 80mg) was dissolved in 800 μL of ethanol (Pharmco-Aaper 200 Proof - Absolute, Anhydrous) by vortexing. 3.2 ml of Safflower (generic) was added to create final solutions of 20 mg/ml, and sonicated for 30 minutes before injection.
Mice were injected (intraperitoneal injection; IP) with 0.1 ml of tamoxifen solution (2 mg tamoxifen) for three consecutive days. Mice were used in imaging experiments 10 days after the first injection. Expression of GCaMP6f was confirmed by genotyping and imaging. Using this Cre Recombinase, we have previously found that ~87.5% of ICC successfully express GCaMP (48). Mice were anaesthetized by inhalation with isoflurane (Baxter, Deerfield, IL, USA) and killed by cervical dislocation before removing tissues for experimentation.
Tissue preparation
After sacrifice, abdomens of mice were opened and colons were removed and placed in fresh Krebs-Ringer bicarbonate (KRB) solution. The proximal portion of the colon was opened along the mesenteric border and cleaned of intra-luminal contents by washing with KRB solution. The tunica muscularis was dissected free of mucosal and submucosal layers and retained for experiments.
Calcium imaging
Colonic muscles were pinned with the CM layer facing upward to the bottom of a 60 mm dish coated with Sylgard (Dow Corning, Midland, MI) and perfused with KRB solution (37°C) for 1 hr before experiments were begun. Ca2+ imaging was performed on ICC-IM in situ with an Eclipse E600FN microscope (Nikon Inc., Melville, NY, USA) equipped with a 40-60x 1.0 CFI Fluor lens (Nikon instruments INC, NY, USA). GCaMP6f was excited at 488 nm (T.I.L.L. Polychrome IV, Grafelfing, Germany). Using this acquisition configuration, the pixel size was 0.225 μm. Image sequences of Ca2+ transients in ICC-IM were collected at 33 fps with TILLvisION software (T.I.L.L. Photonics GmbH, Grafelfing, Germany). Movement artefacts were stabilized digitally with custom made Volumetry software (10, 51–53) prior to analysis of Ca2+ transients. For experiments involving pharmacological treatments, control image sequences were collected for 20-30 sec, and then KRB solution containing the drug concentration to be tested was perfused into the bath for 12-15 mins before another 20-30 sec period of imaging was performed.
Analysis of Ca2+ transients
Ca2+ transients in ICC-IM were imaged and analyzed as described previously (10, 52). Briefly, movies of Ca2+ events were converted to a stack of TIFF (tagged image file format) images and imported into custom software (Volumetry G8c, GW Hennig) for initial processing. Whole-cell ROIs were created to generate spatio-temporal maps (STMs) of Ca2+ transients in individual cells within a field of view (FOV). These STMs were imported as TIFF files into Image J (version1.52a, National Institutes of Health, MD, USA, http://rsbweb.nih.gov/ij) for post hoc analysis. Basal fluorescence was acquired from regions of cells that displayed the most uniform and least intense fluorescence (F0). Then fluorescence values throughout the rest of the cell were divided by the F0 value to calibrate the STM for the amplitudes of Ca2+ transients as F/F0. Ca2+ event amplitude, duration and spread were then calculated from the STM. Ca2+ transient frequency was expressed as the number of events fired per cell per minute (min−1). The amplitude of Ca2+ transients was expressed as ΔF/F0, the duration of Ca2+ transients was expressed as full duration at half maximum amplitude (FDHM) and the spatial spread of Ca2+ transients was expressed as μm of cell propagated per Ca2+ transient.
Contractile experiments
CM muscle strips from the proximal colon of wildtype mice (5-8mm in length and ~2mm in diameter) were cut parallel to the CM axis and attached to a stable mount and to a Gould strain gauge and immersed in jacketed 15 ml tissue baths. The CM muscles were continuously oxygenated and maintained in KRB solution at 37°C. Colon muscles were stretched to an initial tension of 5 mN, and experiments began after 1 hr of equilibration. Isometric contractions were recorded using AcqKnowledge software (3.9.1; Biopac Systems, Goleta, CA) and the area under the curve (AUC) of neurally induced excitatory responses was calculated using pClamp 9.0 as the mean integrated tension, measured as mN/s (mN.s).
Nerve stimulation
Responses to electrical field stimulation (EFS) of intrinsic nerves were recorded in Ca2+ imaging experiments and in contractile experiments, as previously described (15). EFS was delivered by two parallel platinum electrodes placed on either side of colon muscle sheets (imaging) or strips (contractile experiments). EFS was applied by a Grass stimulator (S48; Quincy MA, USA) at 0.3ms duration, 100 V and train durations, as provided in the text and figures. In Ca2+ imaging experiments, EFS was performed at 10 Hz for 10 s. We found that single pulses did elicit consistent responses in ICC-IM and when observed consisted of very brief increases in Ca2+ transient firing. For the purposes of this study, we performed 10 s long trains of stimulation at 10 Hz to elicit sustained excitatory responses to more reliably compare between control conditions and when different neuroeffector pathways or Ca2+ release / influx pathways were inhibited.
Cell isolation and Fluorescence Activated Cell Sorting (FACS)
ICC-IM were isolated from the CM layer of proximal colons from Kit+/copGFP mice as previously described (10). After removing the mucosa and submucosa from the muscles, bundles of CM were peeled free from the submucosal aspect of the muscle sheet. ICC along the submucosal surface (ICC-SM) were removed from the muscle sheet along with the submucosa, and ICC-MY, lying within the plane of the myenteric plexus, were not disturbed. The muscle bundles peeled from CM therefore provided a relatively uncontaminated source of ICC-IM. The CM bundles were incubated in Ca2+-free Hanks solution for 30 min and then enzymatically dispersed. Kit+/copGFP cells were sorted and collected by FACS using a Becton-Dickinson FACSAria II with an excitation laser (488 nm) and emission filter (530/30 nm). Sorting of ICC-IM was accomplished with a 130-μm nozzle at a sheath pressure of 12 psi and sort rate of 1,000 to 3,000 events/s. Live cells, first gated on exclusion of Hoechst 33258 viability indicator, were subsequently gated on GFP fluorescence intensity. Enriched populations of ICC-IM were analyzed for expression of transcript for ICC-specific markers (Kit, Ano1), and other cellular markers (Myh11, Cd45, Pdgfra, Uch11) as described previously (10) to verify purity of ICC yields. Sorted populations of ICC-IM showed minimal expression of Myh11, Cd45, Pdgfra, Uch11 and enrichment in expression of Ano1 and Kit.
RNA extraction and quantitative PCR
Total RNA was isolated from sorted ICC-IM and total CM colon cells before sorting using an illustra RNAspin Mini RNA Isolation Kit (GE Healthcare), and First-strand cDNA was synthesized using SuperScript III (Invitrogen, Carlsbad, CA), according to the manufacturer’s instructions. The PCR primers used and their GenBank accession numbers are listed in Table 1. Using GoTaq DNA Polymerase (Promega, Madison, WI), PCR products were analyzed on 2% agarose gels and visualized by ethidium bromide. Quantitative PCR (qPCR) was performed with the same primers as PCR using SYBR green chemistry on the 7500 HT Real-time PCR System (Applied Biosystems) and analyzed as previously described (10, 47). Individual sorts and subsequent qPCR analysis were performed on 4 animals and gene expression is illustrated as expression relative to that of Gadph, mean ± standard deviation (S.D.).
Table 1:
Summary table of cholinergic and neurokinin receptor primer sequences
| Gene | Sequence | GenBank Accession Number |
|---|---|---|
| mGapdh-F | AGACGGCCGCATCTTCTT | NM_008084 |
| mGapdh-R | TTCACACCGACCTTCACCAT | |
| mChrm2-F | GGTGTCTCCCAGTCTAGTGCAAGG | NM_203491 |
| mChrm2-R | ATGTCTGCCTAGAGTTGTCATCTTTGGA | |
| mChrm3-F | TGTGGCCAGCAATGCTTCTGTCATGA | NM_033269 |
| mChrm3-R | CCACAGGACAAAGGAGATGACCCAAG | |
| mTacr1-F | GTGGTGAACTTCACCTACGCAGTC | NM_009313 |
| mTacr1-R | GCCATGTATGCTTCAAAGGCCACAG | |
| mTacr2-F | CCATCGCCGCTGACAGGTACA | NM_009314 |
| mTacr2-R | GGCCCCCTGGTCCACAGTGA |
Drugs and solutions
Tissues used for imaging and electrophysiological experiments were perfused with KRB containing (mmol/L): NaCl, 120.35; KCl, 5.9; NaHCO3, 15.5; NaH2PO4, 1.2; MgCl2, 1.2; CaCl2, 2.5; and glucose, 11.5. KRB was bubbled with a mixture of 97% O2 – 3% CO2 and warmed to 37 ± 0.2 °C. Atropine, neostigmine, Nω-nitro-L-arginine (L-NNA) and carbachol (CCh) were purchased from Sigma-Aldrich (St Louis, MO, USA). Tetrodotoxin (TTX), Ani9, cyclopiazonic acid (CPA), RP 67580, Substance P, GR 73632, AF-DX 116, DAU 5884, MEN 10376 and MRS 2500 were purchased from Tocris Bioscience (Ellisville, Missouri, USA). GSK 7975A was purchased from Aobious. All drugs were dissolved as recommended by the manufacturers and then diluted to the desired concentrations with KRB solution.
Statistics
Unless otherwise stated in the text, data is represented as mean ± standard deviation (S.D.). In summary graphs, mean data is represented by coloured columns with individual data points within the dataset plotted as black circles. Statistical analysis was performed using paired student’s t-tests (comparing two paired groups) or a one-way ANOVA with a Tukey post hoc test (comparing three or more paired groups) as appropriate. In all statistical analyses, P<0.05 was taken as significant. Probabilities < 0.05 are represented by a single asterisk (*), probabilities < 0.01 are represented by two asterisks (**), probabilities < 0.001 are represented by three asterisks (***) and probabilities < 0.0001 are represented by four asterisks (****). When describing data throughout the text, “n” refers to the number of animals used in that dataset while “c” refers to the numbers of cells or colonic muscle strips analyzed in that same data set.
Results
Expression of tachykinin and muscarinic receptors in colonic ICC-IM
The primary excitatory neuroeffector response of proximal colon smooth muscle to nerve stimulation is mediated by acetylcholine (ACh) which acts through post junctional muscarinic type 2 (M2) and/or type 3 (M3) receptors (54–59)), although some studies have also reported additional excitatory responses due to tachykinins mediated by NK1 or NK2 receptors (60, 61). Quantitative PCR (qPCR) was performed to analyze relative levels of gene transcripts for NK1 (Tacr1), NK2 (Tacr2), M2 (Chrm2) and M3 receptors (Chrm3) expressed in ICC-IM from the proximal colon of Kit+/copGFP mice (see Methods and (10)). ICC-IM showed enrichment of Tacr1 in comparison to unsorted colonic cells from the same region (0.014 ± 0.0015 in ICC-IM vs. 0.004 ± 0.0004 in unsorted cells, relative expression to Gapdh, n=4). Tacr2 transcripts were detected in unsorted colon cells, but expression of this gene was unresolved in ICC-IM. Transcripts for both M2 and M3 receptors were enriched in ICC-IM. While minor enrichment of Chrm2 (0.045 ± 0.002 in ICC-IM vs. 0.031 ± 0.002; expression relative to Gapdh, n=4) was observed in ICC-IM cells, far greater enrichment of Chrm3 was observed in ICC-IM (i.e. 8 fold increase vs. unsorted cells; 0.08 ± 0.01 in ICC-IM vs. 0.01 ± 0.0004 in unsorted cells; expression relative to Gapdh, n=4).
Effect of tachykinins on Ca2+ transients in ICC-IM
Based on the expression data, we investigated whether NK1 receptor agonists and antagonists modulate Ca2+ transients in ICC-IM. Spontaneous Ca2+ transients were observed in ICC-IM in situ, as imaged with 40x or 60x objectives (see Methods and (10)). Multiple cells within fields of view (FOV) displayed very active Ca2+ transient activity. Spatio-temporal maps (STMs) were generated to illustrate and quantify Ca2+ transients in ICC-IM, as described previously (10, 52). Fig. 1A shows STMs of spontaneous Ca2+ transients in colonic ICC-IM under control conditions and during exposure to an NK1 receptor antagonist, RP 67580 (1 μM). RP 67580 had insignificant effects on the frequency (P=0.87), amplitude (P=0.87), duration (P=0.43) or spatial spread (P=0.36) of Ca2+ transients (Fig. 1B, paired t tests, c=14, n=5). We also tested the effects of two NK1 receptor agonists, substance P (1 μM, Fig. 1C–D) and GR 73632 (1 μM, Fig. 1E–F), on Ca2+ transients in ICC-IM. Muscles were pre-incubated with TTX (1 μM) in these experiments to reduced contamination from ganglionic activation of neurokinin receptors. NK1 receptor agonists had minor effects on ICC-IM, yielding insignificant effects on Ca2+ transient frequency (Sub P, P=0.14; GR 7362, P=0.62, paired t tests), amplitude (Sub P, P=0.18; GR 7362, P=0.23, paired t tests) and spatial spread (Sub P, P=0.83; GR 7362, P=0.58, paired t tests). However, both agonists yielded small but significant increases in the duration of Ca2+ transients. Substance P increased Ca2+ transients from 155.9 ± 23.25 ms to 190.6 ± 59.8 ms (Fig. 1D, P=0.036, paired t test, c=14, n=5) and GR 73632 increased Ca2+ transient duration from 170 ± 21.5 ms to 223.1 ± 62.8 ms (Fig. 1F, P=0.024, paired t test, c=11, n=4).
Fig. 1: Effect of NK1 receptor antagonist and agonists on Ca2+ transients in ICC-IM.
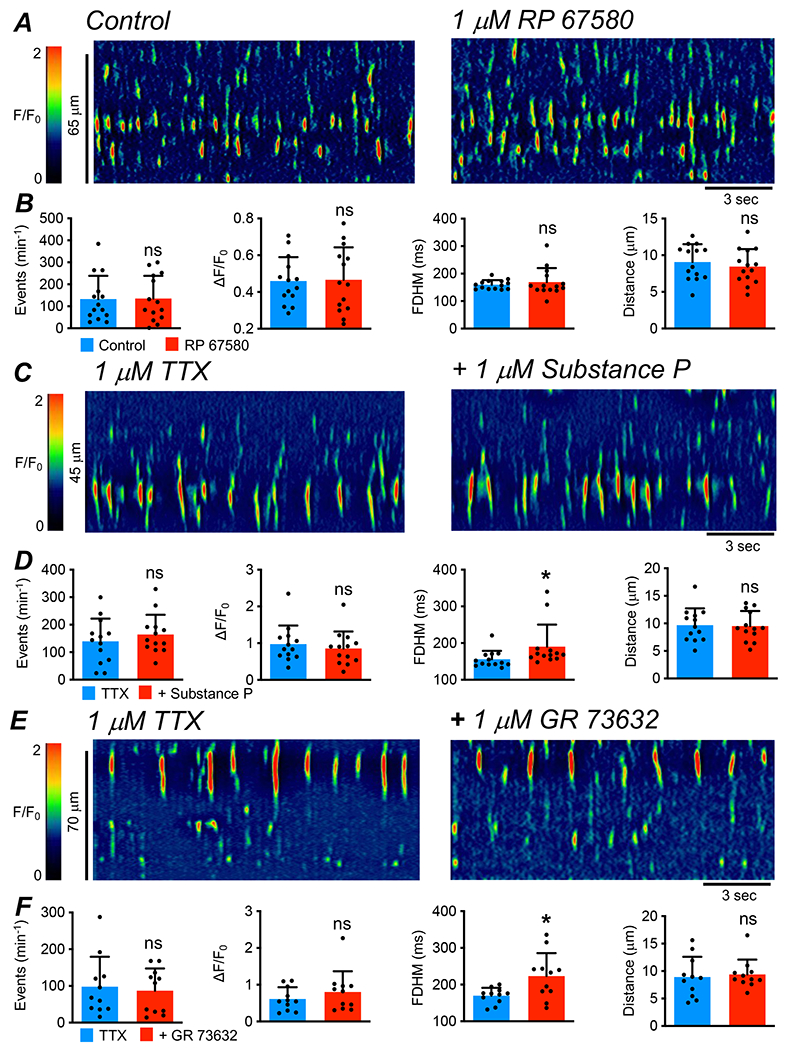
A STMs showing the effect of the NK1 receptor antagonist RP 67580 (1 μM) on Ca2+ transients in ICC-IM. B Summary data of the effects of RP 67580 on Ca2+ transient frequency, amplitude, duration and spatial spread, c=14, n=5. C STMs showing the effects of NK1 receptor agonist, Substance P (1 μM), on Ca2+ transients in ICC-IM in the presence of tetrodotoxin (TTX, 1 μM). D Summary data of the effect of Substance P on Ca2+ transient frequency, amplitude, duration and spatial spread, c=13, n=5. E STMs showing the effects of the NK1 receptor agonist, GR 73632 (1 μM), on Ca2+ transients in ICC-IM in the presence of TTX (1 μM). F Summary data of the effects of GR 73632 on Ca2+ transient frequency, amplitude, duration and spatial spread, c=11, n=4.
Effect of muscarinic agonists on basal ICC-IM Ca2+ transients
The effects of muscarinic agonists and antagonists on basal Ca2+ transient activity were also assessed. Atropine (1 μM, Fig. 2A) had no effect on the frequency (P=0.13), amplitude (P=0.13), duration (P=0.1) or spatial spread (P=0.52) of ICC-IM Ca2+ transients (Fig. 2B, paired t tests, c=12, n=4), suggesting at first glance that there was no basal excitatory drive on ICC-IM. However, the lack of effect of atropine did not appear to be due to a lack of release of ACh, because the acetylcholinesterase (AChE) inhibitor, neostigmine (3 μM, Fig. 2C), dramatically increased Ca2+ transients (e.g. firing frequency more than doubled), increasing from 105.2 ± 94.6 min−1 to 172.6 ± 104.1 min−1 (Fig. 2D, P=0.0006, paired t test, c=18, n=5). Neostigmine did not affect Ca2+ transient amplitude (P=0.061), duration (P=0.08) or spatial spread (P=0.098), c=18, n=5 (Fig. 2D, paired t tests). Carbachol (CCh, 1 μM, Fig. 2E), tested after pre-treatment with TTX, did not affect Ca2+ transient amplitude (P=0.11), duration (P=0.27) or spatial spread (P=0.48, paired t tests), but greatly increased Ca2+ transient frequency, more than doubling firing frequency from 217.1 ± 150.6 min−1 to 441 ± 177.8 min−1 (Fig. 2F, P=0.003, paired t test, c=12, n=4).
Fig. 2: Effect of muscarinic receptor antagonist and agonists on Ca2+ transients in ICC-IM.
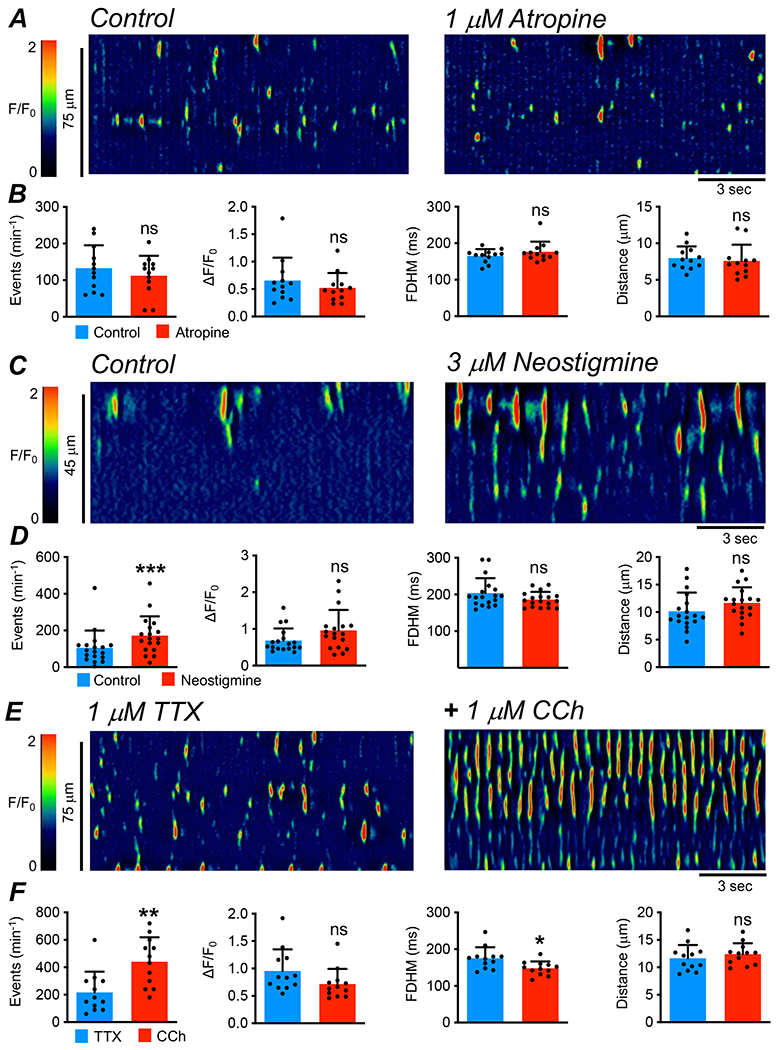
A STMs showing the effects of the muscarinic receptor antagonist, atropine (1 μM), on Ca2+ transients in ICC-IM. B Summary data of the effects of atropine on Ca2+ transient frequency, amplitude, duration and spatial spread, c=12, n=4. C STMs showing the effects of the acetylcholinesterase inhibitor, neostigmine (3 μM), on Ca2+ transients in ICC-IM. D Summary data of the effects of neostigmine on ICC-IM Ca2+ transient frequency, amplitude, duration and spatial spread, c=18, n=5. E STMs showing the effects of carbachol (CCh, 1 μM) on Ca2+ transients in ICC-IM in the presence of tetrodotoxin (TTX, 1 μM). F Summary data showing the effects of CCh on Ca2+ transient frequency, amplitude, duration and spatial spread, c=12, n=4.
Relative contributions of M2 and M3 receptors to cholinergic responses on basal ICC-IM activity
Based on the transcript data, ICC-IM express both M2 and M3 receptors. We evaluated the relative contributions of each receptor subtype in mediating muscarinic excitatory effects on Ca2+ transients in ICC-IM. Fig. 3A shows STMs in which muscles were pre-incubated with TTX (1 μM), and CCh (1 μM) was added to enhance Ca2+ transient firing. Addition of an M2 antagonist, AF-DX 116 (0.1 μM, 10 min incubation), had no significant effects on Ca2+ transients stimulated by CCh (Fig. 3A–B, P>0.05, One Way ANOVA, Tukey post-test, c=10, n=4). However, further addition of an M3 antagonist, DAU 5884 (0.1 μM; 10 min incubation) in the continued presence of AF-DX 116, inhibited responses to CCh (Fig. 3A). The frequency of Ca2+ transients was 204 ± 38.5 min−1 in the presence of CCh, and after addition of AF-DX 116 this was not changed significantly to 184.5 ± 95.9 min−1 (Fig. 3B, P=0.39, One Way ANOVA, Tukey post-test, c=10, n=4). DAU 5884 reduced the frequency of Ca2+ transients to 51 ± 72.1 min−1 (Fig. 3B, P=0.0007, One Way ANOVA, Tukey post-test, c=10, n=4). Similarly, AF-DX 116 did not affect Ca2+ transient amplitude in the presence of CCh (0.7 ± 0.35 ΔF/F0 vs. 0.8 ± 0.19 ΔF/F0, Fig. 3B, P=0.71, One Way ANOVA, Tukey post-test, c=10, n=4), but DAU 5884 reduced this value to 0.46 ± 0.32 ΔF/F0 (Fig. 3B, P=0.025, One Way ANOVA, Tukey post-test, c=10, n=4). AF-DX 116 had no effect on the the spatial spread of Ca2+ transients in CCh 10.6 ± 2.3 to 8.8 ± 1.5 μm (Fig. 3B, P=0.054, c=10, One Way ANOVA, Tukey post-test, n=4), but this was reduced to 4.9 ± 2.8 μm by DAU 5884 (Fig. 3B, P=0.003, One Way ANOVA, Tukey post-test, c=10, n=4).
Fig. 3: Excitatory effects of CCh and neostigmine on ICC-IM are mediated by M3 receptors.
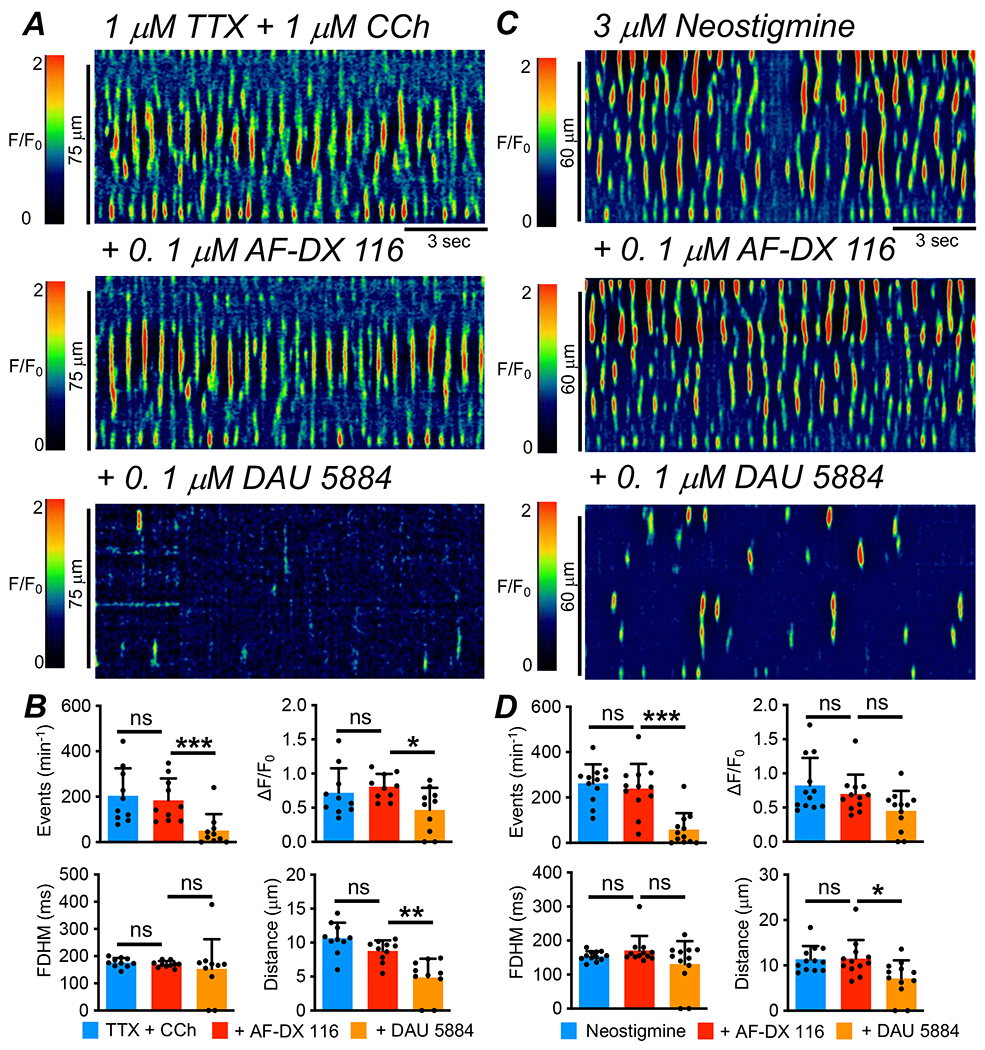
A STMs showing the effect of an M2 receptor antagonist, AF-DX 116 (0.1 μM), and an M3 receptor antagonist, DAU 5884 (0.1 μM), on the excitatory effects of CCh (1 μM) on Ca2+ transients in ICC-IM in the presence of TTX (1 μM). B Summary data showing effects of AF-DX 116 and DAU 5884 on Ca2+ transient frequency, amplitude, duration and spatial spread in the presence of CCh and TTX, c=10, n=4. C STMs showing the effects AF-DX 116 (0.1 μM) and DAU 5884 (0.1 μM) on the excitatory effects of neostigmine (3 μM) on Ca2+ transients in ICC-IM. D Summary data showing the effects of AF-DX 116 and DAU 5884 on Ca2+ transient frequency, amplitude, duration and spatial spread in the presence of neostigmine, c=12, n=3.
When ICC-IM Ca2+ transients were enhanced with neostigmine (3 μM, Fig. 3C), a similar pattern was observed where an M2 receptor antagonist had no effect, while a specific M3 receptor antagonist significantly reduced Ca2+ transients. In the presence of neostigmine, ICC-IM Ca2+ transient frequency was 262 ± 83.8 min−1 and this was unchanged by AF-DX 116 (239.8 ± 108.6 min−1, Fig. 3D, P=0.29, One Way ANOVA, Tukey post-test, c=12, n=3) but was significantly reduced by DAU 5884 to 58.8 ± 72.35 min−1 (Fig. 3D, P=0.001, One Way ANOVA, Tukey post-test, c=12, n=3). The spread of ICC-IM Ca2+ transients in the presence of neostigmine was unaffected by AF-DX 116 (11.4 ± 2.9 μm vs. 11.5 ± 4.1 μm, Fig. 3D, P=0.99, One Way ANOVA, Tukey post-test, c=12, n=3) but was significantly reduced to 7.1 ± 4 μm by DAU 5884 (Fig. 3D, P=0.013, One Way ANOVA, Tukey post-test, c=12, n=3).
Effect of excitatory electrical field stimulation on ICC-IM Ca2+ transients
Electrical field stimulation (EFS) was performed in the presence of Nω-nitro-L-arginine (L-NNA, 100 μM) and a P2Y1 receptor antagonist, MRS 2500 (1 μM) to reduce contamination from the release of inhibitory neurotransmitters that would also be likely to occur with EFS. The STM in Fig. 4A shows the effects of EFS (10 Hz; 10 sec trains) on Ca2+ transients in ICC-IM. Ca2+ transients occurred spontaneously during pre-EFS periods, and these were greatly enhanced during EFS. A large amplitude, global Ca2+ transient fired at the onset of EFS (Fig. 4A) and this was followed by a period of enhanced firing from individual Ca2+ transient release sites. The excitatory response stopped abruptly after cessation of EFS, perhaps revealing the high capacity for local neurotransmitter metabolism indicated by the effects of neostigmine (Fig. 2C–D). The excitatory response to EFS is also illustrated by plotting F/F0 values (Fig. 4B) for the 2 firing sites indicated by the asterisks in Fig. 4A and construction of 3D plots of the STM (Fig. 4A) in Fig. 4C. EFS (10 Hz, 10 sec) in the presence of L-NNA and MRS 2500, nearly doubled the firing frequency of ICC-IM Ca2+ transients from 86.1 ± 61.9 to 158.3 ± 86.1 min−1 (Fig. 4D, P<0.0001, paired t test, c=60, n=12). The amplitude of ICC-IM Ca2+ transients was increased during EFS from 0.4 ± 0.2 to 0.7 ± 0.3 ΔF/F0 (Fig. 4D, P<0.0001, paired t test, c=60, n=12). Ca2+ transient duration was increased from 164.5 ± 29 ms to 178.7 ± 35.3 ms during EFS (Fig. 4D, P=0.0008, paired t test, c=60, n=12) and Ca2+ transient spatial spread increased during EFS from 11.1 ± 4.3 to 19.5 ± 10.8 μm (Fig. 4D, P<0.0001, paired t test, c=60, n=12).
Fig. 4: Ca2+ transients in ICC-IM are increased by excitatory innervation.
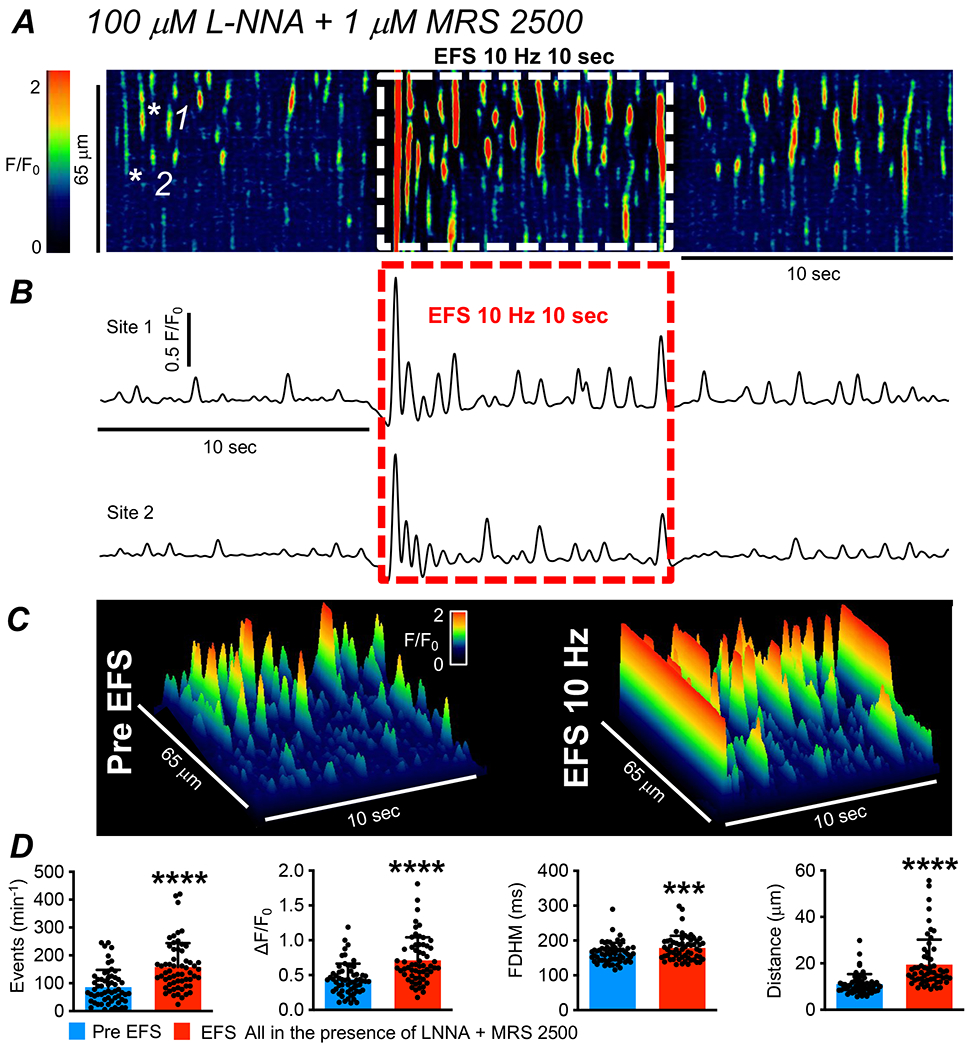
A STM of Ca2+ transients in ICC-IM showing the effects of EFS (10 Hz, 10 sec). These experiments were performed in the presence of an nNOS synthase inhibitor, L-NNA (100 μM), and a purinergic receptor (P2Y1R) inhibitor, MRS 2500 (1 μM), to block input from inhibitory neurons. B Plot Ca2+ transients at firing sites highlighted by the white asterisks in the STM in panel A. The period of EFS is indicated by the dashed red box. C 3-D plots derived from the STM in A showing the effects of EFS in the presence of L-NNA and MRS 2500. D Summarized effects of excitatory EFS on Ca2+ transient frequency, amplitude, duration and spread (c=60, n=12).
It was also noted that excitatory EFS did not lead to coupling or entrainment of Ca2+ transients in adjacent ICC-IM within colonic muscles. This conclusion is demonstrated in Fig. 5, which shows STMs of adjacent ICC-IM in the same FOV (Fig. 5A). Ca2+ transients in these cells were plotted individually as STMs that were thresholded and colour coded to be either a uniform green or red colour (Fig. 5B). Both cells responded to excitatory EFS (Fig. 5B), however when STMs from both cells were merged (Fig. 5C), it can be seen that apart from the initial, global Ca2+ transient at the onset of EFS, no subsequent entrainment or coordination of Ca2+ transient firing occurred during the remaining period of stimulation (Fig. 5C). It was also noted that not every ICC-IM within a given FOV responded to excitatory EFS. Fig. 6A shows several ICC-IM within a FOV, and 2 cells are highlighted. The summated Ca2+ activity within the highlighted cells is plotted as traces in Fig. 6B, with the onset of excitatory EFS shown by the dashed black box. These traces show that cell 1 responded to excitatory EFS, but cell 2 did not. This is also shown in the STMs of the two cells shown in Fig. 6C, with cell 1 exhibiting the increase in Ca2+ transient frequency, amplitude, duration and spatial spread already described, but cell 2 in the same FOV showed no change in Ca2+ activity during EFS. On average, 60 ± 4.2 % of ICC-IM within a FOV showed marked increases in Ca2+ transient activity during excitatory EFS (n=12).
Fig. 5: Excitatory EFS does not entrain the activity of ICC-IM.
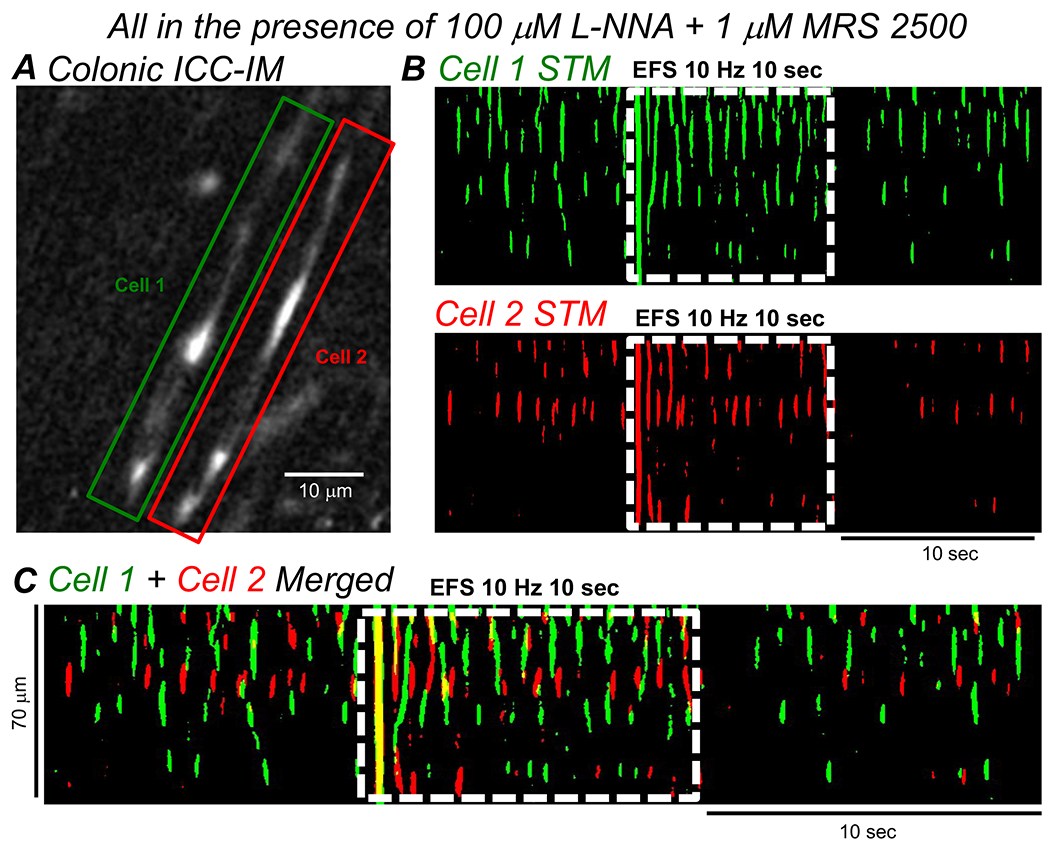
A Image of two adjacent ICC-IM in the CM layer of the proximal colon of a Kit-Cre-GCaMP6f mouse. The cells are highlighted by Green (Cell 1) and Red (Cell 2) boxes. B STMs of the Ca2+ transient activity in the two highlighted cells from A, with their Ca2+ activity thresholded to a uniform Green (Cell 1) or Red color (Cell 2). Period of excitatory EFS (10Hz, 10 sec, LNNA (100 μM) + MRS 2500 (1 μM)) is indicated by the dashed white box. C Merged STM of the colored STMs in B. Period of excitatory EFS (10Hz, 10 sec, LNNA (100 μM) + MRS 2500 (1 μM)) is shown by the dashed white box.
Fig. 6: Excitatory EFS does not yield post-junctional responses in all ICC-IM.
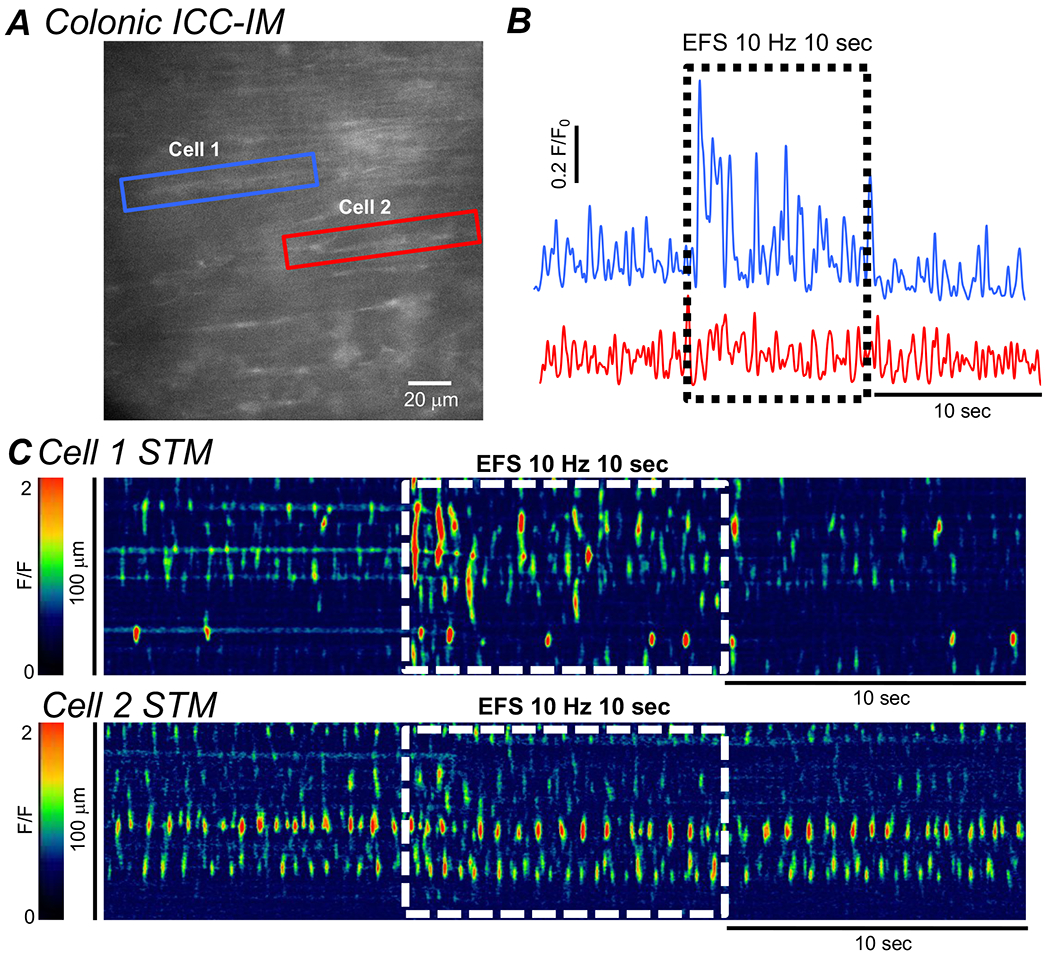
A Image of two adjacent ICC-IM in the CM layer of the proximal colon of a Kit-Cre-GCaMP6f mouse. Cells are highlighted by Blue (Cell 1) or Red (Cell 2) boxes. B Plot profiles of the summated Ca2+ transient activating in the two-highlighted ICC-IM in A, Period of excitatory EFS (10Hz, 10 sec, L-NNA (100 μM) + MRS 2500 (1 μM)) is shown by the dashed black box. C STMs of the Ca2+ transient activity in Cell 1 and Cell 2 in A. Period of excitatory EFS (10Hz, 10 sec, L-NNA (100 μM) + MRS 2500 (1 μM)) is shown by the dashed white boxes.
Neural pathways responsible for the excitatory responses to EFS
The excitatory response of ICC-IM Ca2+ transients to EFS was unaffected by a specific NK1 receptor antagonist (Fig. 7A). Pre-exposure of muscles to RP 67580 (1 μM) did not affect the frequency, amplitude, duration or spatial spread of Ca2+ transients during EFS. For example, the firing frequency of Ca2+ transients before EFS was 36 ± 34.15 min−1 and this was increased to 96 ± 43.5 min−1 during EFS (Fig. 7B, P=0.013, One Way ANOVA, Tukey post-test, c=11, n=5). The frequency during EFS remained unchanged after pre-exposure to RP 67580 (97.6 ± 50.6 min−1, Fig. 7B, P=0.97, One Way ANOVA, Tukey post-test, c=11, n=5).
Fig. 7: Excitatory EFS responses in ICC-IM were unaffected by NK1 receptor antagonist.
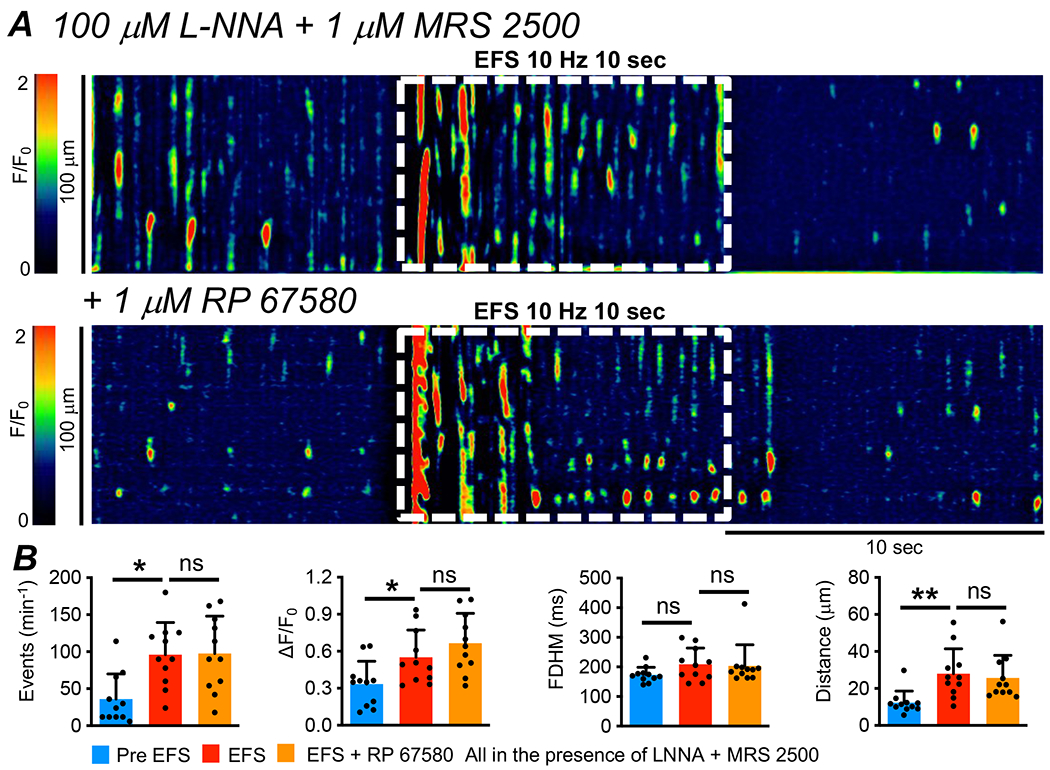
A STM of Ca2+ transients in ICC-IM showing the effects of excitatory EFS (10Hz, 10 sec, L-NNA (100 μM) + MRS 2500 (1 μM)) before and after application of RP 67580 (1 μM). B Summarized effects of RP 67580 on Ca2+ transient frequency, amplitude, duration and spread during EFS (c=11, n=5).
In contrast to the lack of effect of an NK1 receptor antagonist, atropine (1 μM) inhibited the excitatory effects of EFS on Ca2+ transients (Fig. 8A). EFS under control conditions increased the frequency of Ca2+ transients from 100.4 ± 51.1 min−1 to 170.7 ± 59 min−1 (Fig. 8B, P=0.0003, One Way ANOVA, Tukey post-test, c=11, n=5), but the frequency of Ca2+ transients was 87.8 ± 65 min−1 in the presence of atropine (Fig. 8B, P=0.0003, One Way ANOVA, Tukey post-test, c=11, n=5). Before EFS, the amplitude of ICC-IM Ca2+ transients was 0.4 ± 0.1 ΔF/F0. Amplitude increased to 0.6 ± 0.3 ΔF/F0 during EFS (Fig. 8B, P=0.007, One Way ANOVA, Tukey post-test, c=11, n=5), but this effect was reduced to 0.25 ± 0.1 ΔF/F0 by pre-treatment with atropine (Fig. 8B, P=0.014, One Way ANOVA, Tukey post-test, c=11, n=5). Similarly, the spatial spread of Ca2+ transients increased from 10.5 ± 4.2 μm to 15.2 ± 2.9 μm by EFS (Fig. 8B, P=0.002, One Way ANOVA, Tukey post-test, c=11, n=5). This was reduced to 10 ± 4.35 μm by atropine (Fig. 8B, P=0.0045, One Way ANOVA, Tukey post-test, c=11, n=5). Atropine did not affect the duration of Ca2+ transients during EFS (Fig. 8B, P=0.36, One Way ANOVA, Tukey post-test, c=11, n=5).
Fig. 8: Muscarinic receptors mediate excitatory neural responses in ICC-IM.
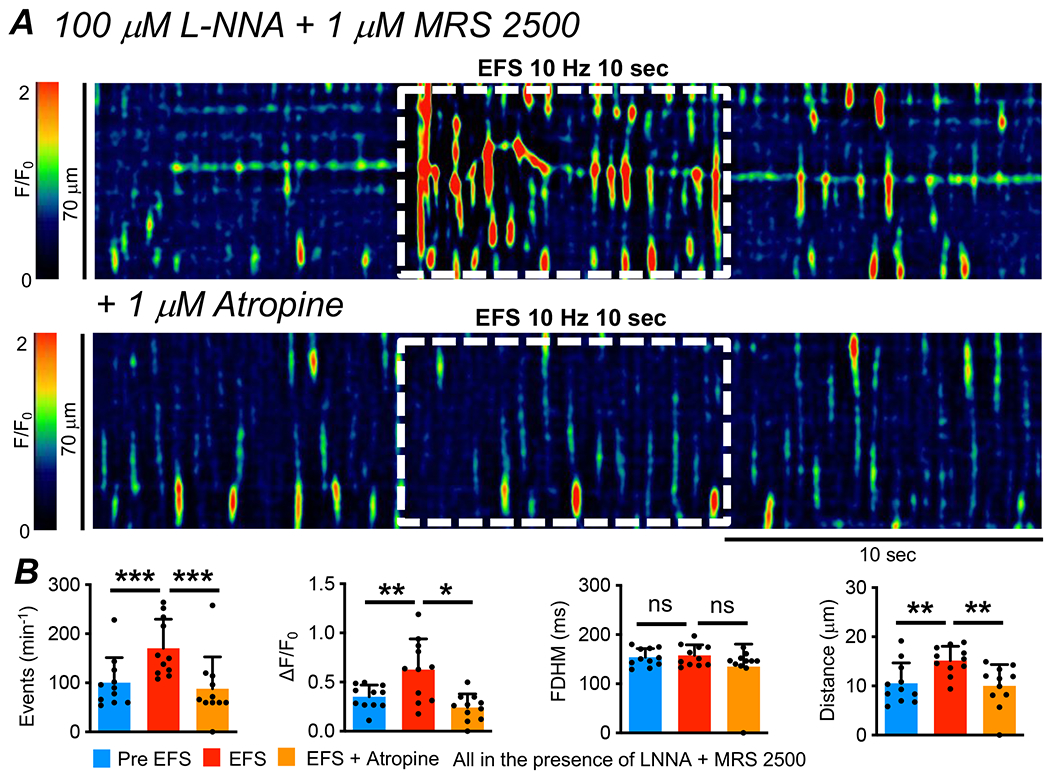
A STM of Ca2+ transients in ICC-IM showing the effects of excitatory EFS (10Hz, 10 sec, L-NNA (100 μM) + MRS 2500 (1 μM)) before and after application of atropine (1 μM). B Summarized effects of atropine on Ca2+ transient frequency, amplitude, duration and spread during EFS in the presence of L-NNA and MRS 2500 (c=11, n=5).
The muscarinic receptors responsible for the excitatory effects of EFS on Ca2+ transients in ICC-IM were also investigated. The increase in Ca2+ transients during EFS was unaffected by AF-DX 116 (0.1 μM, Fig. 9A) and blocked by DAU 5884 (0.1 μM, Fig. 9A). Under excitatory stimulation, the firing frequency of Ca2+ transients was 126 ± 40.4 min−1, and this was unchanged by AF-DX 116 (124.6 ± 53.7 min−1, Fig. 9B, P=0.98, One Way ANOVA, Tukey post-test, c=16, n=6). Excitatory responses were reduced significantly to a frequency pf 55.5 ± 50.65 min−1 by DAU 5884 (Fig. 9B, P<0.001, One Way ANOVA, Tukey post-test, c=16, n=6). The amplitude of Ca2+ transients during EFS was 0.7 ± 0.3 ΔF/F0 and remained unchanged in the presence of AF-DX 116 (0.7 ± 0.2 ΔF/F0, Fig. 9B, P=0.93, One Way ANOVA, Tukey post-test, c=16, n=6). DAU 5884 reduced the amplitude of Ca2+ transients during EFS to 0.4 ± 0.2 ΔF/F0 (Fig. 9B, P=0.0007, One Way ANOVA, Tukey post-test, c=16, n=6). AF-DX 116 had no effect on spatial spread of Ca2+ transients during EFS (Fig. 9B, P=0.71, One Way ANOVA, Tukey post-test, c=16, n=6), however DAU 5884 reduced spatial spread from 16.1 ± 6.6 μm to 10.4 ± 7.2 μm (Fig. 9B, P=0.0009, One Way ANOVA, Tukey post-test, c=16, n=6).
Fig. 9: M3 receptors mediate excitatory responses to EFS in ICC-IM.
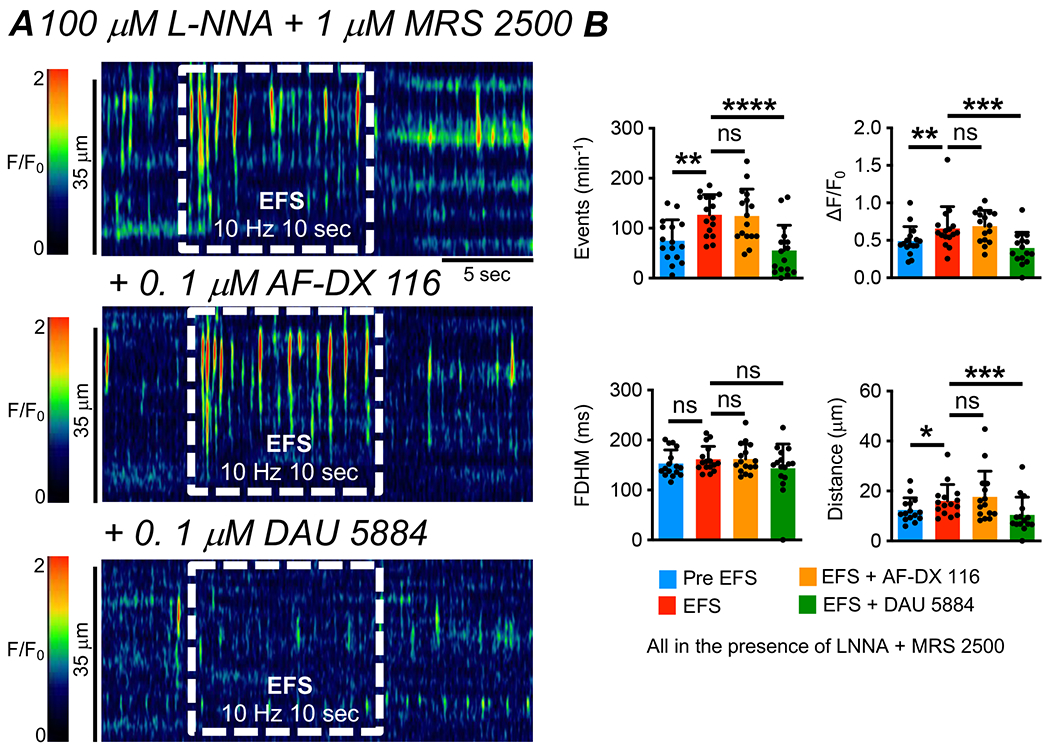
A STM of Ca2+ transients in ICC-IM showing the effects of excitatory EFS (10Hz, 10 sec, L-NNA (100 μM) + MRS 2500 (1 μM)) before and after application of AF-DX 116 (0.1 μM) and DAU 5884 (0.1 μM) B Summarized effects of AF-DX 116 and DAU 5884 on Ca2+ transient frequency, amplitude, duration and spread during EFS in the presence of L-NNA and MRS 2500 (c=16, n=6).
Contribution of Ca2+ release and SOCE to excitatory responses in ICC-IM
Ca2+ transient firing in ICC is due to Ca2+ release from endoplasmic reticulum (ER) stores and sustained by SOCE via Ca2+ influx through Orai channels (10, 42, 62). We tested how these Ca2+ handling mechanisms contributed to excitatory neural responses of ICC-IM. As above, EFS increased Ca2+ transients in the presence of L-NNA and MRS 2500, however when ER Ca2+ stores were disrupted with the sarcoplasmic/endoplasmic Ca2+-ATPase (SERCA) pump inhibitor cyclopiazonic acid (CPA, 10 μM, 15 min incubation), basal Ca2+ transients were abolished and excitatory EFS responses were reduced significantly (Fig. 10). For example, Ca2+ transient firing frequency was 203.5 ± 114.4 min−1 in response to excitatory EFS, and this was reduced to 6.5 ± 4.2 min−1 when CPA was present. (Fig. 10A, P=0.0005, One Way ANOVA, Tukey post-test, c=11, n=4). Excitatory EFS usually induced only a single Ca2+ transient at the onset of the stimulation after exposure to CPA (Fig. 10). This initial transient likely represented unloading of the remaining Ca2+ in ER stores, as subsequent stimulation in the continued presence of CPA failed to yield any response (not shown).
Fig. 10: Ca2+ release contribute to excitatory responses evoked by EFS in ICC-IM.
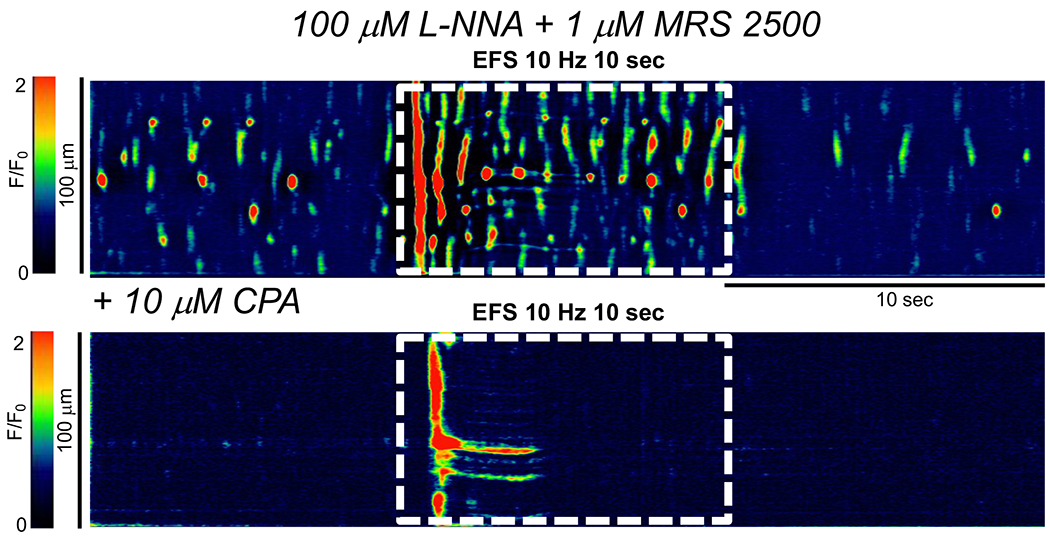
STM of Ca2+ transients in ICC-IM showing the effects of excitatory EFS (10Hz, 10 sec, L-NNA (100 μM) + MRS 2500 (1 μM)) before and after application of the SERCA pump inhibitor, cyclopiazonic acid (CPA, 10 μM, c=11, n=4).
A selective antagonist of Orai channels, GSK 7975A, was used to reduce SOCE in ICC-IM (Fig. 11A). The STMs and traces (of the activity of the Ca2+ transient firing site indicated by the white asterisk in the STM of Fig. 11A) in Fig. 11A–B show that GSK 7975A (10 μM) attenuated excitatory responses of ICC-IM to EFS. While the initial 1-2 sec of responses to EFS looked similar to those before GSK 7975A, the excitatory response was not sustained and ran down during 10 sec of EFS. This resulted in a significant reduction in the overall firing frequency of Ca2+ transients from 208.5 ± 108.8 min−1 during EFS to 124.9 ± 70.6 min−1 during EFS in the presence of GSK 7975A (Fig. 11C, P=0.005, One Way ANOVA, Tukey post-test, c=11, n=5). The duration of ICC-IM Ca2+ transients during EFS was also reduced by GSK 7975A from 184.4 ± 21.4 ms to 168.4 ± 21.05 ms (Fig. 11C, P<0.0055, One Way ANOVA, Tukey post-test, c=11, n=5).
Fig. 11: SOCE sustains excitatory responses evoked by EFS in ICC-IM.

A STM of Ca2+ transients in ICC-IM showing the effects of excitatory EFS (10Hz, 10 sec, LNNA + MRS 2500) before and after application of the Orai Ca2+ channel inhibitor, GSK 7975A (10 μM). B Plot of the Ca2+ transient firing site indicted by the white asterisk in panel A, before and after addition of GSK 7975A. C Summarized effects of GSK 7975A on Ca2+ transient frequency, amplitude, duration and spread during EFS in the presence of L-NNA and MRS 2500 (c=11, n=5).
Role of ICC in excitatory contractions of colonic muscles
Ca2+ transients in ICC-IM are likely coupled to the activation of Ano1 channels and generation of spontaneous transient inward currents (STICs) (41). Due to electrical coupling between cells in the SIP syncytium, STICs generated in thousands of ICC-IM would be expected to produce a depolarizing trend in SMCs that would tend to enhance contractile responses (10). This hypothesis was tested by examining the effects of antagonists of Ano1 channels, that are expressed exclusively in ICC, on colonic contractions induced by excitatory EFS.
Contractions were recorded from CM strips from the proximal colon (see Methods). The muscles were pre-treated with L-NNA (100 μM) and MRS 2500 (1 μM) to remove inhibitory effects from nitric oxide and purines released from enteric inhibitory neurons. Preliminary experiments showed that in the absence of these antagonists an inhibitory effect on contractions predominated. EFS was applied for 10 sec (1, 3, 5, 10 and 20 Hz). Each frequency of EFS produced sustained contraction (Fig. 12A–B). EFS was repeated after addition of Ani9 (1 μM), an Ano1 antagonist (63), and this agent caused a reduction in the contractile responses (Fig. 12A–B). Ani9 reduced the area under the curve (AUC) of EFS induced contraction from 2683 ± 1638 mN.s to 1137 ± 1132 mN.s at 1 Hz (Fig. 12B, P=0.014, One Way ANOVA, Tukey post-test, 12 strips, n=5), 3884 ± 2567 mN.s to 1758 ± 1692 mN.s at 3 Hz (Fig. 12B, P=0.012, One Way ANOVA, Tukey post-test, 12 strips, n=5), 4828 ± 3212 mN.s to 1960 ± 1838 mN.s at 5 Hz (Fig. 12B, P=0.0087, One Way ANOVA, Tukey post-test, 12 strips, n=5), 5917 ± 3740 mN.s to 2512 ± 2203 mN.s at 10 Hz (Fig. 12B, P=0.0096, One Way ANOVA, Tukey post-test, 12 strips, n=5) and 6951 ± 5251 mN.s to 3347 ± 3338 mN.s at 20 Hz (Fig. 12B, P=0.032, One Way ANOVA, Tukey post-test, 12 strips, n=5).
Fig. 12: Contractions in proximal colon evoked by EFS are due to activation of M3 receptors.
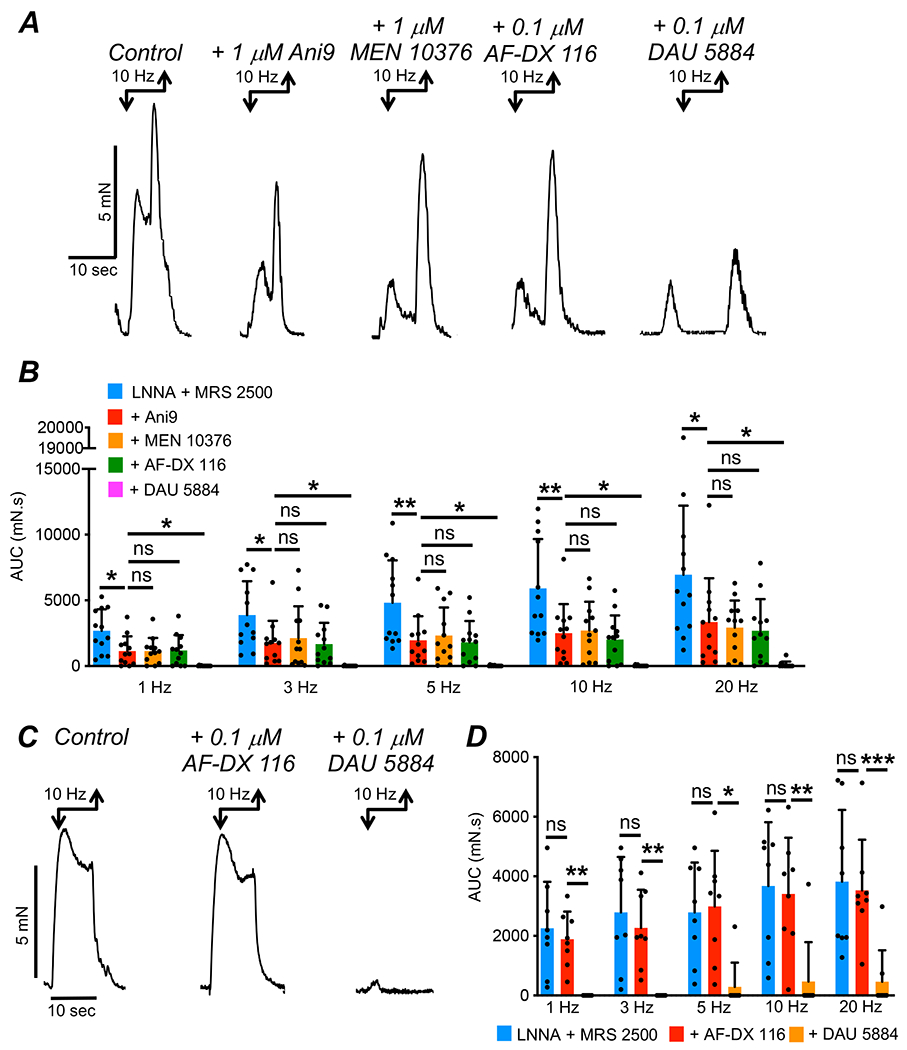
A Isometric tension recording of CM strips of proximal colon from wildtype mice. EFS (10 Hz; 10 sec) evoked contractions in the presence of L-NNA (100 μM) and MRS 2500 (1 μM) before and after addition of Ani 9 (1 μM) and subsequent addition of MEN 10376 (1 μM), AF-DX 116 (0.1 μM) and DAU 5884 (0.1 μM). B Summary data showing the effects of Ani9, MEN 10376, AF-DX 116 and DAU 5884 on the area under the curve (AUC) of contractions evoked by EFS (c=12, n=5). C Contractions of CM strips of proximal colon from wildtype mice. EFS (10 Hz; 10 sec) evoked contractions in the presence of L-NNA (100 μM) and MRS 2500 (1 μM) before and after addition of AF-DX 116 (0.1 μM) and subsequent addition of DAU 5884 (0.1 μM). D Summary data showing the effects of sequential addition of AF-DX 116 and DAU 5884 on the area under the curve (AUC) of contractions evoked by EFS (c=8, n=4).
These data show that at all frequencies of EFS, contractile responses were inhibited by Ani9. We hypothesized that the residual contraction could be a result of either an incomplete block of Ano1 channels with Ani9 or an excitatory response in SMCs due to EFS induced activation of NK2 receptors or M2 receptors in these cells. We tested this latter hypothesis by inhibiting Ano1 channels with Ani9 (1 μM), and then challenging the remaining contraction with applications of a specific NK2 receptor antagonist (MEN 10376, 1 μM) and subsequently AF-DX 116 (0.1 μM). As shown in Fig. 12A–B, Ani9 reduced EFS evoked contractions in the presence of L-NNA and MRS 2500 by ~ 50% at all frequencies and subsequent addition of MEN 10376 or AF-DX 116 had no effect on the residual contraction (Fig. 12B, P>0.05, c=12, n=5). However, addition of DAU 5884 (0.1 μM) to inhibit M3 receptors led to a near abolition of all responses across all frequencies tested (Fig. 12B, P<0.05, c=12, n=5). Similarly, in the absence of Ani9, AF-DX 116 (0.1 μM) had no effect on excitatory colonic responses evoked by EFS (Fig. 12C–D, P>0.05, 8 strips, n=4), whereas DAU 5884 (0.1 μM) almost abolished all contractions in response to EFS (Fig. 12C–D, P<0.05, 8 strips, n=4).
Discussion
ICC-IM in the colon are closely associated with varicosities of enteric motor neurons, including those of excitatory motor neurons (12, 22). This morphological observation suggested that ICC-IM may play a role in mediating excitatory neural responses in colonic SMCs and therefore be an important functional element in generating propulsive motility and normal colonic transit. Having found that expression of Ano1 channels and dynamic Ca2+ handling mechanisms are important for the functions of other classes of ICC (10, 11, 15, 41, 42, 47, 48, 51, 64, 65), we investigated the hypothesis that regulation of Ca2+ transients may be a mechanism for mediation of excitatory motor effects in colonic muscles. Mice expressing GCaMP6f selectively in ICC, were used to allow imaging of ICC-IM in situ, so neural responses transduced by ICC-IM in intact muscles could be monitored directly. ICC-IM fire Ca2+ transients spontaneously (10), and the effects of the 2 major neurotransmitter pathways known to be responsible for excitatory neural responses in the tunica muscularis of colonic muscles were evaluated. Receptors mediating responses to muscarinic (M2 & M3) and neurokinergic (NK1) neurotransmitters were expressed by ICC-IM, however only M3 receptors were coupled to the regulation Ca2+ transients. Post-junctional responses to M2 and NK1 receptor agonists were not resolved by our methods. Ca2+ transients enhanced by M3 receptor activation resulted from Ca2+ release from ER stores, and these responses were maintained by SERCA pumps operating in concert with SOCE. Inhibition of either of these mechanisms inhibited post-junctional responses of ICC-IM to muscarinic stimulation. Ca2+ release in ICC-IM couples to activation of Ano1 and exerts a depolarizing influence on the CM (10). Increasing Ca2+ transients through excitatory neural inputs would tend to increase activation of Ano1 channels, further depolarize muscles and increase excitation-contraction coupling. This hypothesis was confirmed by demonstrating significant reductions in contractile responses to EFS by an Ano1 channel antagonist, which would reduce cholinergic electrophysiological responses in ICC-IM and thus excitation-contraction coupling in SMCs.
The role of ICC in excitatory neurotransmission has been studied in other regions of the GI tract, typically using mutant animals with genetic defects prohibiting normal development of ICC or causing levels of impairment of ICC functions (20, 28, 29, 31–40). These approaches provided significant conclusions in tissues, such as the gastric fundus, in which only a single population of ICC is manifest and lesions in ICC are relatively extensive. However, it has been much more difficult to study the role of specific classes of ICC in the colon, as there are a minimum of 4 anatomical classes of these cells, and incomplete loss of ICC has been reported in colons of the mutant animals used elsewhere (23, 40). Optogenetics coupled with digital imaging provided a powerful means of directly monitoring the activities of ICC-IM because the sensor, GCaMP6f, was engineered to be expressed selectively in ICC, and the specific morphology and anatomical locations of ICC-IM allowed unequivocal imaging of ICC-IM in situ. Thus, it was possible, leaving the natural associations between motor neurons and post-junctional cells intact, to characterize the innervation and responsiveness of ICC-IM to excitatory motor input in situ.
We found that isolated and sorted ICC-IM expressed gene transcripts for neurokinin (NK1) and muscarinic (M2, M3) receptors, but responses to only M3 receptors, in terms of regulation of Ca2+ transients, were resolved in ICC-IM. Atropine and a selective M3 receptor antagonist blocked the effects of excitatory EFS, but M2 and NK1 receptor antagonists had no effect. Enhancement in Ca2+ transient firing during excitatory EFS was due to increased Ca2+ release from intracellular ER stores and sustained throughout the stimulus by SOCE via Orai channels. Ca2+ signaling in ICC-IM is coupled to activation of Ano1 channels (10, 41), and an Ano1 antagonist significantly reduced the contractile force of colonic contractions induced by excitatory EFS.
The findings from the current study, which strongly support dominance of cholinergic excitatory innervation of colonic ICC-IM, contrast with a previous investigation of another class of ICC, ICC-DMP in the small intestine, that have been typically considered to be a type of ICC-IM (7, 10). ICC-DMP are also closely associated with nerve varicosities in the region of the deep muscular plexus (25, 66–69), and these cells fire stochastic Ca2+ transients in a manner similar to the activity of colonic ICC-IM (48). Colonic ICC-IM and small intestinal ICC-DMP express NK1 receptors, and ICC-DMP exhibited intense excitatory responses to NK1 agonists such as substance P and GR 73632 (64). Release of neurokinins from enteric nerves by EFS activates and causes internalization of NK1 receptors in ICC-DMP, and these cells are in close association with nerve varicosities immunoreactive for substance P (22, 61, 67). ICC-IM in the colon displayed only minor effects to NK1 agonists. Basal Ca2+ transients in ICC-DMP are reliant on tonic release of tachykinins and ACh, as activity was depressed by antagonists of NK1 receptors and atropine (64). However, these antagonists had no effect on basal Ca2+ transients in colonic ICC-IM. Stimulation of ICC-DMP with EFS generated excitatory responses that were largely inhibited by NK1 receptor antagonists (64), but NK1 receptor antagonists had no effect on excitatory responses to EFS in colonic ICC-IM. ICC-DMP displayed excitatory responses to muscarinic agonists, such as CCh, but atropine had only minor effects on responses to EFS in comparison to NK1 receptor antagonists (64). As shown in the current study, excitatory responses in colonic ICC-IM were completely blocked by atropine or a selective M3 antagonist. The different effector pathways could be due to differential expression of NK1 receptors in the two ICC subtypes. ICC-DMP express NK1 receptors much robustly than colonic ICC-IM, and neurokinin immunopositive varicosities are in close association with ICC-DMP and more these varicosities are concentrated in the deep muscular plexus of the small intestine (70). These discrepancies highlight important distinctions between the pathways responsible for post-junctional responses in 2 classes of ICC previously assumed to have similar phenotypes and functions. Obviously, at the level of intact muscles, post-junctional responses are likely to be more complex and other cells of the SIP syncytium may respond to neurotransmitters and contribute excitation-contraction coupling.
Inhibiting Ano1 channels with Ani9, led to a ~50% reduction in excitatory contractile responses in colonic muscles. Remaining contractions were unaffected by MEN 10376 or AF-DX 116, suggesting that the residual responses were not due to activation of NK2 or M2 receptors in SMCs. Instead, it is likely that Ani9 at 1 μM does not completely block Ano1 channels in ICC. We chose to use Ani9 as the Ano1 antagonist in this study because it has been identified as one of the most potent and selective inhibitors of Ano1 (IC50=77 nM in single cells, but endogenous channels in human nasal epithelial cells were somewhat less sensitive; (63)). Higher concentrations of Ani9 or other inhibitors of Ano1 (IC50s > 18 fold greater than Ani9; (63)) were avoided because we showed previously that concentrations of 3 μM Ani9 and other inhibitors have non-specific effects on Cav1.2 channels in colonic SMCs (11). Block of Cav1.2 channels would also have the effect of inhibiting colonic contraction, confounding the interpretation of the results. Non-specific effects on voltage-dependent Ca2+ conductances were not resolved with Ani9 at 1 μM. Thus, the inability of Ani9 to completely inhibit contractions induced by EFS may be due to achieving a non-total block of Ano1 channels. Of course, it is also possible that the residual contraction after Ani9 is due to other responses activated via M3 receptors in cells of the SIP syncytium.
An interesting observation in this study was that atropine had no effect on basal Ca2+ transients in ICC-IM, however an inhibitor of AChE (neostigmine) caused dramatic enhancement in Ca2+ transients through muscarinic-dependent mechanism. Taken together, these observations suggest that tonic release of ACh occurs in colonic muscles under the conditions of our experiments, however the activity of AChEs is sufficient to rapidly metabolize basal neurotransmitter release and prohibit post-junctional effects in ICC-IM. Similar conclusions were made in studies of gastric muscles, where atropine had no effect on basal pacemaker activity (also generated by ICC) but positive chronotropic effects due to binding of muscarinic receptors were observed when muscles were treated with neostigmine (71, 72). So active are AChEs in the gastric fundus that ACh released during nerve stimulation appears to be confined to an extracellular volume close to varicosities. This restricted volume likely represents the synapse-like associations between nerve terminals and ICC-IM (about 20 nm), because ACh released from neurons activates different post-junctional mechanisms than muscarinic agonists applied to the solution bathing the muscles (73, 74). AChE is expressed by enteric motor neurons (72). Thus, high rates of substrate metabolism may occur in excluded volumes near the sites of neurotransmitter release. Basal release of ACh also appears to occur in the small intestine, as atropine significantly reduced Ca2+ transients in ICC with the deep muscular plexus (ICC-DMP; (64)). Whether the differences in the responsiveness of colonic ICC-IM and ICC-DMP to basal ACh release are due to differences in the rate or amount of ACh released from cholinergic nerve terminals, differences in the expression or activities of AChE in the two organs or differences in degree of contacts between nerve varicosities and ICC-DMP are unanswered questions.
The major pattern of mechanical activity that underlies the fecal pellet propulsion appears to involve repetitive activation of cholinergic neurons generating cholinergic excitatory junction potentials (79). Colonic ICC-IM expressed both M2 and M3 receptors, but cholinergic input to colonic ICC-IM occurred predominantly through M3 receptors. The excitatory effects of neostigmine and CCh on basal ICC-IM Ca2+ transients were unaffected by a selective antagonist of M2 receptors but significantly reduced and nearly abolished by an M3 receptor antagonist. Excitatory responses to EFS were also unaffected by the M2 receptor antagonist but blocked completely by the M3 receptor antagonist. M3 receptor gene transcripts are plentiful in ICC-IM, showing an 8-fold enrichment over unsorted cells from the CM layer. M2 and M3 receptors are linked to Gi/o and Gq-coupled receptors, respectively (55, 56, 58, 75). Based on this coupling, it is easy to understand how binding of M3 receptors could enhance Ca2+ transients, as Gq-coupled receptors lead to the activation of phospholipase Cβ (PLCβ), cleavage of phosphatidylinositol 4,5-bisphosphate (PIP2) to generate IP3, and increased Ca2+ release from ER stores via IP3 receptors (76, 77). This was supported by the current study, as excitatory cholinergic responses were almost abolished by disrupting ER Ca2+ stores by blocking the SERCA pump. Furthermore, an Orai channel antagonist, which would reduce Ca2+ influx in ICC-IM via SOCE, reduced the ability of excitatory neural responses to be sustained during stimulation. While SOCE has been demonstrated to mediate the pacemaker activity of ICC-MY in the small intestine (42, 62) and basal Ca2+ activity of ICC-IM and ICC-SS in the colon (10, 15), this is the first study demonstrating that this pathway is required to sustain neural responses in ICC. Binding of M2 receptors and coupling via Gi/o would tend to depress generation of cAMP (78). This could conceivably enhance Ca2+ transients in ICC-IM, as we have noted a net inhibitory effect of cAMP-dependent agonists on Ca2+ transients in ICC (unpublished). However, this mechanism might only be recruited during periods of time in which there is significant adenylate cyclase activity. At present the role of M2 receptors in cholinergic excitatory responses in colonic muscles is unknown.
This study provides evidence that colonic ICC-IM respond to cholinergic agonists and excitatory nerve stimulation. Contractile studies demonstrated that activation of a signaling pathway in ICC-IM (i.e. increased Ca2+ release and activation of Ano1 channels) is linked to whole muscle mechanical responses because an Ano1 antagonist reduced excitatory responses to EFS. However, a goal for future studies should be to extend the observations in the present study by direct, simultaneous imaging of responses in ICC-IM and colonic SMC to cholinergic nerve stimulation. This will require dual imaging experiments using simultaneous excitations of both GCaMP expressed in ICC-IM and either an RCaMP Ca2+ indicator (emits red fluorescence under appropriate excitation) expressed in SMC or a traditional, membrane permeable red-fluorescence Ca2+ indicator dye (Rhod-2, Cal 590). Such experiments are currently problematic due to tissue movements. In the current study, we bathed colonic muscles in a low dose of the Cav1.2 channel antagonist, nicardipine (100 nM), to reduce Ca2+ influx into and contraction of SMC (nicardipine at this low dose does not affect ICC-IM (10)). It was necessary to limit movement so Ca2+ transients in ICC-IM could be monitored at high magnifications (40-60x). Without movement stabilization recordings of the type collected in the current study would be impossible. Resolving of excitatory Ca2+ signaling originating in ICC-IM and spreading to SMCs will necessitate omission of a CaV1.2 antagonist because excitatory responses linked to depolarization of SMCs elicited by activation of Ano1 currents in ICC-IM are at least partially due to Ca2+ influx via CaV1.2 channels. We are currently searching for an effective way to uncouple contractile responses downstream from activation of CaV1.2 and Ca2+ entry in SMCs. A recent study of the mouse proximal colon described distinct rhythmic electrical patterns, one that was myogenic (poorly synchronized and coordinated over large distances) and another that was neurogenic, spatially and temporally coordinated and abolished by hexamethonium (80). The extent to which the neurogenic pattern is dependent upon the excitatory responses we have characterized in ICC-IM and how these responses are integrated with responses of other cells in the SIP syncytium to generate colonic motility patterns are important questions for future investigation.
In summary, this study demonstrates that ICC-IM in the proximal colon receive excitatory neural input from enteric excitatory motor neurons. Cholinergic activation of M3/Gq-coupled receptors increased Ca2+ release from ER in ICC-IM via the Gq, PLC - IP3 pathway. Increased Ca2+ release during cholinergic stimulation leads to activation of Ano1 channels in ICC-IM, and the resulting inward currents conduct to SMCs via gap junctions (Fig. 13). Thus, as part of the integrative functions of the SIP syncytium, the mechanisms identified in ICC-IM in this study are likely to influence colon SMC excitability and colonic motility patterns. This more detailed understanding of excitatory mechanisms in colonic muscles may provide new ideas for the development of pro-kinetic drugs to treat constipation.
Fig. 13: Cholinergic neurotransmission involving regulation of Ca2+ transients in ICC-IM.
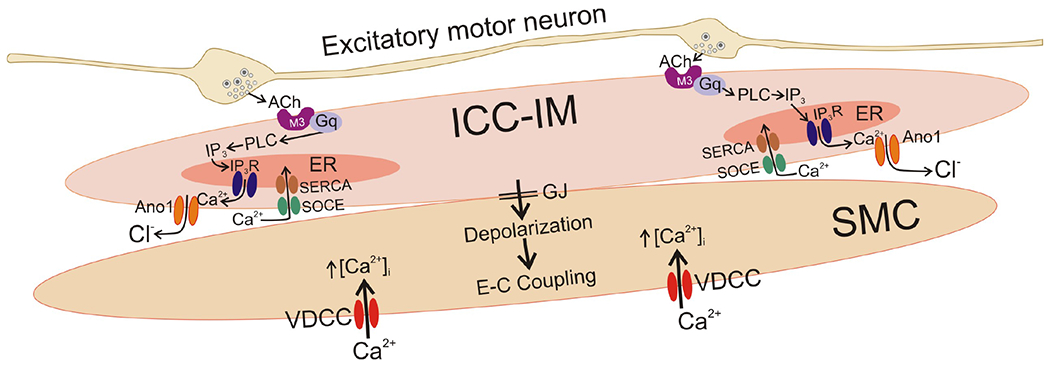
The neuromuscular apparatus in colonic muscles includes excitatory and inhibitory motor neurons (inhibitory neuron not shown) and 3 types of post-junctional cells, known as the SIP syncytium (PDGFRα+ cells not shown in this figure for simplicity). Excitatory motor neurons make close, synaptic-like contacts with ICC-IM in the proximal colon. The present study supports the following transduction and effector mechanisms: ACh release from varicosities binds to M3 receptors expressed by ICC-IM. These receptors are coupled through Gq to activate PLCβ and increase production of IP3. IP3 activates release of Ca2+ from IP3R1 receptors in the ER. The resulting localized Ca2+ transients, that occur from multiple firing sites in ICC-IM, activate Ano1 chloride channels, resulting in Cl− efflux (inward current) and depolarization of ICC-IM. Depolarizing currents conduct to SMCs via gap junctions (GJ) that couple these cells into the SIP syncytium. Depolarization of SMCs increases the open probability of voltage-dependent Ca2+ channels (VDCC), predominantly Cav1.2 in colonic SMCs, and Ca2+ entry through these channels initiates excitation-contraction coupling. Maintenance of Ca2+ transients, and therefore post-junctional excitatory responses in colonic muscles, requires refilling of ER stores. This is accomplished by store-operated Ca2+ entry (SOCE), mediated by Orai channels, and active uptake of Ca2+ into the ER by SERCA. In this study we showed that an Ano1 (Ani9) antagonist inhibited whole-muscle contractile responses to excitatory EFS. These results, indicate a significant role for ICC-IM in transduction of excitatory neural inputs in the proximal colon. These findings also provide an explanation of why reduced ICC in the colon is linked to slow transit constipation (see (81–84)).
Acknowledgements:
The authors would like to thank Nancy Horowitz for maintenance and breeding of mice, Lauren Peri for qPCR analysis and Dr. Caroline Cobine for the use of the contractile apparatus.
Funding: This project was supported by R01 DK-120759 from the NIDDK.
Non-standard abbreviations
- CM
Circular muscle layer
- EFS
Electrical field stimulation
- ER
Endoplasmic reticulum
- FOV
Field of view
- IAS
Internal anal sphincter
- ICC
Interstitial cell of Cajal
- ICC-IM
Intramuscular interstitial cell of Cajal
- ICC-MY
Myenteric interstitial cell of Cajal
- ICC-SM
Submucosal interstitial cell of Cajal
- ICC-SS
Subserosal interstitial cell of Cajal
- IP3R
Inositol-triphosphate receptor
- LM
Longitudinal muscle layer
- PDGFRα+ Cell
Platelet derived growth factor receptor alpha positive interstitial cell
- ROI
Region of interest
- SERCA
Sarcoplasmic/endoplasmic reticulum Ca2+-ATPase pump
- SIP
Cellular syncytium of smooth muscle cells, interstitial cells of Cajal and platelet derived growth factor receptor alpha positive interstitial cells
- SMC
Smooth muscle cell
- SOCE
Store operated Ca2+ entry
Footnotes
Competing interests: None
All authors read and approved the manuscript before submission. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved
References
- 1.Sanders KM, Ward SM, and Koh SD (2014) Interstitial cells: Regulators of smooth muscle function. Physiological Reviews 94, 859–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanders KM, Koh SD, Ro S, and Ward SM (2012) Regulation of gastrointestinal motility-insights from smooth muscle biology. Nat Rev Gastroenterol Hepatol 9, 633–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spencer NJ, and Hu H (2020) Enteric nervous system: sensory transduction, neural circuits and gastrointestinal motility. Nat Rev Gastroenterol Hepatol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith TK, Reed JB, and Sanders KM (1987) Origin and propagation of electrical slow waves in circular muscle of canine proximal colon. American Journal of Physiology - Cell Physiology 252, C215–224 [DOI] [PubMed] [Google Scholar]
- 5.Smith TK, Reed JB, and Sanders KM (1987) Interaction of two electrical pacemakers in muscularis of canine proximal colon. American Journal of Physiology-Cell Physiology 252, C290–C299 [DOI] [PubMed] [Google Scholar]
- 6.Ishikawa K, and Komuro T (1996) Characterization of the interstitial cells associated with the submuscular plexus of the guinea-pig colon. Anatomy and Embryology 194, 49–55 [DOI] [PubMed] [Google Scholar]
- 7.Sanders KM (1996) A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Vol. 111 pp. 492–515 [DOI] [PubMed] [Google Scholar]
- 8.Komuro T (1999) Comparative morphology of interstitial cells of Cajal: ultrastructural characterization. Microscopy research and technique 47, 267–285 [DOI] [PubMed] [Google Scholar]
- 9.Komuro T (2006) Structure and organization of interstitial cells of Cajal in the gastrointestinal tract. The Journal of Physiology 576, 653–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drumm BT, Hwang SJ, Baker SA, Ward SM, and Sanders KM (2019) Ca2+ signalling behaviours of intramuscular interstitial cells of Cajal in the murine colon. Journal of Physiology 597, 3587–3617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drumm BT, Rembetski BE, Baker SA, and Sanders KM (2019) Tonic inhibition of murine proximal colon is due to nitrergic suppression of Ca 2+ signaling in interstitial cells of Cajal. Scientific Reports 9, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang XY, Sanders KM, and Ward SM (2000) Relationship between interstitial cells of Cajal and enteric motor neurons in the murine proximal colon. Cell and Tissue Research 302, 331–342 [DOI] [PubMed] [Google Scholar]
- 13.Lee HT, Hennig GW, Park KJ, Bayguinov PO, Ward SM, Sanders KM, and Smith TK (2009) Heterogeneities in ICC Ca2+Activity Within Canine Large Intestine. Gastroenterology 136, 2226–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bayguinov PO, Hennig GW, and Smith TK (2010) Ca2+ imaging of activity in ICC-MY during local mucosal reflexes and the colonic migrating motor complex in the murine large intestine. The Journal of Physiology 588, 4453–4474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drumm BT, Rembetski BE, Messersmith K, Manierka MS, Baker SA, and Sanders KM (2020) Pacemaker function and neural responsiveness of subserosal interstitial cells of Cajal in the mouse colon. Journal of Physiology 598, 651–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aranishi H, Kunisawa Y, and Komuro T (2009) Characterization of interstitial cells of Cajal in the subserosal layer of the guinea-pig colon. Cell and Tissue Research 335, 323–329 [DOI] [PubMed] [Google Scholar]
- 17.Rumessen JJ, Vanderwinden JM, Hansen A, and Horn T (2013) Ultrastructure of interstitial cells in subserosa of human colon. Cells Tissues Organs 197, 322–332 [DOI] [PubMed] [Google Scholar]
- 18.Vanderwinden JM, Rumessen JJ, Bernex F, Schiffmann SN, and Panthier JJ (2000) Distribution and ultrastructure of interstitial cells of Cajal in the mouse colon, using antibodies to Kit and Kit(W-lacZ) mice. Cell and Tissue Research 302, 155–170 [DOI] [PubMed] [Google Scholar]
- 19.Daniel EE, and Posey-Daniel V (1984) Neuromuscular structures in opossum esophagus: role of interstitial cells of Cajal. Am J Physiol 246, G305--315 [DOI] [PubMed] [Google Scholar]
- 20.Burns AJ, Lomax AE, Torihashi S, Sanders KM, and Ward SM (1996) Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proceedings of the National Academy of Sciences of the United States of America 93, 12008–12013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sung TS, Hwang SJ, Koh SD, Bayguinov Y, Peri LE, Blair PJ, Webb TI, Pardo DM, Rock JR, Sanders KM, and Ward SM (2018) The cells and conductance mediating cholinergic neurotransmission in the murine proximal stomach. Journal of Physiology 596, 1549–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blair PJ, Bayguinov Y, Sanders KM, and Ward SM (2012) Relationship between enteric neurons and interstitial cells in the primate gastrointestinal tract. Neurogastroenterology and Motility 24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ward SM, and Sanders KM (2006) Involvement of intramuscular interstitial cells of Cajal in neuroeffector transmission in the gastrointestinal tract. The Journal of physiology 576, 675–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ward SM, Beckett EAH, Wang XY, Baker F, Khoyi M, and Sanders KM (2000) Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. Journal of Neuroscience 20, 1393–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ward SM, McLaren GJ, and Sanders KM (2006) Interstitial cells of Cajal in the deep muscular plexus mediate enteric motor neurotransmission in the mouse small intestine. Journal of Physiology 573, 147–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamamoto M (1977) Electron Microscopic Studies on the Innervation of the Smooth Muscle and the Interstitial Cell of Cajal in the Small Intestine of the Mouse and Bat. Archivum histologicum japonicum 40, 171–201 [DOI] [PubMed] [Google Scholar]
- 27.Cobine CA, Hennig GW, Kurahashi M, Sanders KM, Ward SM, and Keef KD (2011) Relationship between interstitial cells of Cajal, fibroblast-like cells and inhibitory motor nerves in the internal anal sphincter. Cell and Tissue Research 344, 17–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cobine CA, Sotherton AG, Peri LE, Sanders KM, Ward SM, and Keef KD (2014) Nitrergic neuromuscular transmission in the mouse internal anal sphincter is accomplished by multiple pathways and postjunctional effector cells. Am J Physiol Gastrointest Liver Physiol 307, G1057–G1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duffy AM, Cobine CA, and Keef KD (2012) Changes in neuromuscular transmission in the W/Wv mouse internal anal sphincter. Neurogastroenterology & Motility 24, e41–e55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beckett EAH, Takeda Y, Yanase H, Sanders KM, and Ward SM (2005) Synaptic specializations exist between enteric motor nerves and interstitial cells of cajal in the murine stomach. Journal of Comparative Neurology 493, 193–206 [DOI] [PubMed] [Google Scholar]
- 31.Beckett EAH, Horiguchi K, Khoyi M, Sanders KM, and Ward SM (2002) Loss of enteric motor neurotransmission in the gastric fundus of Sl/Sl(d) mice. The Journal of physiology 543, 871–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beckett EAH, Bayguinov YR, Sanders KM, Ward SM, and Hirst GDS (2004) Properties of unitary potentials generated by intramuscular interstitial cells of Cajal in the murine and guinea-pig gastric fundus. Journal of Physiology 559, 259–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beckett EAH, Sanders KM, and Ward SM (2017) Inhibitory responses mediated by vagal nerve stimulation are diminished in stomachs of mice with reduced intramuscular interstitial cells of Cajal. Scientific Reports 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Durnin L, Lees A, Manzoor S, Sasse KC, Sanders KM, and Mutafova-Yambolieva VN (2017) Loss of nitric oxide-mediated inhibition of purine neurotransmitter release in the colon in the absence of interstitial cells of Cajal. American Journal of Physiology - Gastrointestinal and Liver Physiology 313, G419–G433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Groneberg D, Lies B, König P, Jäger R, Seidler B, Klein S, Saur D, and Friebe A (2013) Cell-specific deletion of nitric oxide-sensitive guanylyl cyclase reveals a dual pathway for nitrergic neuromuscular transmission in the murine fundus. Gastroenterology 145, 188–196 [DOI] [PubMed] [Google Scholar]
- 36.Groneberg D, Zizer E, Lies B, Seidler B, Saur D, Wagner M, and Friebe A (2015) Dominant role of interstitial cells of Cajal in nitrergic relaxation of murine lower oesophageal sphincter. Journal of Physiology 593, 403–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lies B, Beck K, Keppler J, Saur D, Groneberg D, and Friebe A (2015) Nitrergic signalling via interstitial cells of Cajal regulates motor activity in murine colon. Journal of Physiology 593, 4589–4601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lies B, Gil V, Groneberg D, Seidler B, Saur D, Wischmeyer E, Jiménez M, and Friebe A (2014) Interstitial cells of Cajal mediate nitrergic inhibitory neurotransmission in the murine gastrointestinal tract. American Journal of Physiology - Gastrointestinal and Liver Physiology 307, G98–G106 [DOI] [PubMed] [Google Scholar]
- 39.Ward SM, Morris G, Reese L, Wang XY, and Sanders KM (1998) Interstitial cells of Cajal mediate enteric inhibitory neurotransmission in the lower esophageal and pyloric sphincters. Gastroenterology 115, 314–329 [DOI] [PubMed] [Google Scholar]
- 40.Sanders KM, Hwang SJ, and Ward SM (2010) Neuroeffector apparatus in gastrointestinal smooth muscle organs. The Journal of Physiology 588, 4621–4639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu MH, Sung TS, O’Driscoll K, Koh SD, and Sanders KM (2015) Intracellular Ca2+ release from endoplasmic reticulum regulates slow wave currents and pacemaker activity of interstitial cells of Cajal. American Journal of Physiology Cell Physiology 308, C608–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng H, Drumm BT, Earley S, Sung TS, Koh SD, and Sanders KM (2018) SOCE mediated by STIM and Orai is essential for pacemaker activity in the interstitial cells of cajal in the gastrointestinal tract. Science Signaling 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gomez-Pinilla PJ, Gibbons SJ, Bardsley MR, Lorincz A, Pozo MJ, Pasricha PJ, Van de Rijn M, West RB, Sarr MG, Kendrick ML, Cima RR, Dozois EJ, Larson DW, Ordog T, and Farrugia G (2009) Ano1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract. American journal of physiology. Gastrointestinal and liver physiology 296, G1370–G1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hwang SJ, Blair PJA, Britton FC, O’Driscoll KE, Hennig G, Bayguinov YR, Rock JR, Harfe BD, Sanders KM, and Ward SM (2009) Expression of anoctamin 1/TMEM16A by interstitial cells of Cajal is fundamental for slow wave activity in gastrointestinal muscles. The Journal of Physiology 587, 4887–4904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu MH, Kim TW, Ro S, Yan W, Ward SM, Koh SD, and Sanders KM (2009) A Ca2+-activated Cl− conductance in interstitial cells of Cajal linked to slow wave currents and pacemaker activity. The Journal of Physiology 587, 4905–4918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu MH, Sung IK, Zheng H, Sung TS, Britton FC, O’Driscoll K, Koh SD, and Sanders KM (2011) Muscarinic activation of Ca2+-activated Cl− current in interstitial cells of Cajal. The Journal of Physiology 589, 4565–4582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Drumm BT, Hennig GW, Battersby MJ, Cunningham EK, Sung TS, Ward SM, Sanders KM, and Baker SA (2017) Clustering of Ca2+ transients in interstitial cells of Cajal defines slow wave duration. The Journal of General Physiology 149, 703–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baker SA, Drumm BT, Saur D, Hennig GW, Ward SM, and Sanders KM (2016) Spontaneous Ca2+ transients in interstitial cells of Cajal located within the deep muscular plexus of the murine small intestine. The Journal of Physiology 594, 3317–3338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vanderwinden JM, Rumessen JJ, Liu H, Descamps D, De Laet H, and Vanderhaeghen JJ (1996) Interstitial cells of Cajal in human colon and in Hirschsprung’s disease. Gastroenterology 111, 901–910 [DOI] [PubMed] [Google Scholar]
- 50.Ro S, Chanjae P, Jin J, Zheng H, Blair P, Redelman D, Ward SM, Yan W, and Sanders KM (2010) A Model to Study the Phenotypic Changes of Interstitial Cells of Cajal in Gastrointestinal Diseases. Gastroenterology 138, 1068–1078. e1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baker SA, Drumm BT, Cobine CA, Keef KD, and Sanders KM (2018) Inhibitory neural regulation of the Ca2+ transients in intramuscular interstitial cells of cajal in the small intestine. Frontiers in Physiology 9, 1–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Drumm BT, Hennig GW, Baker SA, and Sanders KM (2019) Applications of spatio-temporal mapping and particle analysis techniques to quantify intracellular Ca 2+ signaling in situ. Journal of Visualized Experiments 2019, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Drumm BT, Sung TS, Zheng H, Baker SA, Koh SD, and Sanders KM (2018) The effects of mitochondrial inhibitors on Ca2+signalling and electrical conductances required for pacemaking in interstitial cells of Cajal in the mouse small intestine. Cell Calcium 72, 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang L, Horowitz B, and Buxton ILO (1991) Muscarinic receptors in canine colonic circular smooth muscle. I. Coexistence of M2 and M3 subtypes. Molecular Pharmacology 40, 943–951 [PubMed] [Google Scholar]
- 55.Ehlert FJ, Ostrom RS, and Sawyer GW (1997) Subtypes of the muscarinic receptor in smooth muscle. Vol. 61 pp. 1729–1740 [DOI] [PubMed] [Google Scholar]
- 56.Sawyer GW, and Ehlert FJ (1998) Contractile roles of the M2 and M3 muscarinic receptors in the guinea pig colon. Journal of Pharmacology and Experimental Therapeutics 284, 269–277 [PubMed] [Google Scholar]
- 57.Furness JB (2012) The enteric nervous system and neurogastroenterology. Vol. 9 pp. 286–294 [DOI] [PubMed] [Google Scholar]
- 58.Eglen RM, Hegde SS, and Watson N (1996) Muscarinic receptor subtypes and smooth muscle function. Vol. 48 pp. 531–565 [PubMed] [Google Scholar]
- 59.Iino S, and Nojyo Y (2006) Muscarinic M2 acetylcholine receptor distribution in the guinea-pig gastrointestinal tract. Neuroscience 138, 549–559 [DOI] [PubMed] [Google Scholar]
- 60.Kunze WAA, and Furness JB (1999) the Enteric Nervous System and Regulation of Intestinal Motility. Annual Review of Physiology 61, 117–142 [DOI] [PubMed] [Google Scholar]
- 61.Lavin ST, Southwell BR, Murphy R, Jenkinson KM, and Furness JB (1998) Activation of neurokinin 1 receptors on interstitial cells of Cajal of the guinea-pig small intestine by substance P. Histochemistry and Cell Biology 110, 263–271 [DOI] [PubMed] [Google Scholar]
- 62.Youm JB, Zheng H, Koh SD, and Sanders KM (2019) Na-K-2Cl Cotransporter and Store-Operated Ca2+ Entry in Pacemaking by Interstitial Cells of Cajal. Biophys J 117, 767–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seo Y, Lee HK, Park J, Jeon DK, Jo S, Jo M, and Namkung W (2016) Ani9, a novel potent small-molecule ANO1 inhibitor with negligible effect on ANO2. PLoS ONE 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baker SA, Drumm BT, Skowronek KE, Rembetski BE, Peri LE, Hennig GW, Perrino BA, and Sanders KM (2018) Excitatory neuronal responses of Ca2+ transients in interstitial cells of cajal in the small intestine. eNeuro 5, ENEURO.0080-0018.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zheng H, Drumm BT, Zhu MH, Xie Y, O’Driscoll KE, Baker SA, Perrino BA, Koh SD, and Sanders KM (2020) Na+/Ca2+ Exchange and Pacemaker Activity of Interstitial Cells of Cajal. Front Physiol 11, 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blair PJ, Bayguinov Y, Sanders KM, and Ward SM (2012) Interstitial cells in the primate gastrointestinal tract. Cell and Tissue Research 350, 199–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Iino S, Ward SM, and Sanders KM (2004) Interstitial cells of Cajal are functionally innervated by excitatory motor neurones in the murine intestine. Journal of Physiology 556, 521–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang XY, Sanders KM, and Ward SM (1999) Intimate relationship between interstitial cells of Cajal and enteric nerves in the guinea-pig small intestine. Cell and Tissue Research 295, 247–256 [DOI] [PubMed] [Google Scholar]
- 69.Rumessen JJ, Mikkelsen HB, and Thuneberg L (1992) Ultrastructure of interstitial cells of Cajal associated with deep muscular plexus of human small intestine. Gastroenterology 102, 56–68 [DOI] [PubMed] [Google Scholar]
- 70.Southwell BR, and Furness JB (2001) Immunohistochemical demonstration of the NK(1) tachykinin receptor on muscle and epithelia in guinea pig intestine. Gastroenterology 120, 1140–1151 [DOI] [PubMed] [Google Scholar]
- 71.Forrest AS, Ördög T, and Sanders KM (2006) Neural regulation of slow-wave frequency in the murine gastric antrum. American Journal of Physiology - Gastrointestinal and Liver Physiology 290, G488–G495 [DOI] [PubMed] [Google Scholar]
- 72.Worth AA, Forrest AS, Peri LE, Ward SM, Hennig GW, and Sanders KM (2015) Regulation of gastric electrical and mechanical activity by cholinesterases in mice. Journal of Neurogastroenterology and Motility 21, 200–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bhetwal BP, Sanders KM, An C, Trappanese DM, Moreland RS, and Perrino BA (2013) Ca2+ sensitization pathways accessed by cholinergic neurotransmission in the murine gastric fundus. The Journal of Physiology 591, 2971–2986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gao N, Chang AN, He W, Chen CP, Qiao YN, Zhu M, Kamm KE, and Stull JT (2016) Physiological signalling to myosin phosphatase targeting subunit-1 phosphorylation in ileal smooth muscle. Journal of Physiology 594, 3209–3225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Eglen RM (2012) Muscarinic receptors and gastrointestinal tract smooth muscle function. 68, 2573–2578 [DOI] [PubMed] [Google Scholar]
- 76.Berridge MJ, Lipp P, and Bootman M (1999) Calcium signalling. Current biology : CB 9, R157–159 [DOI] [PubMed] [Google Scholar]
- 77.Berridge MJ (2009) Inositol trisphosphate and calcium signalling mechanisms. Biochim Biophys Acta 1793, 933–940 [DOI] [PubMed] [Google Scholar]
- 78.Fryer AD, and Jacoby DB (1998) Muscarinic receptors and control of airway smooth muscle. American Journal of Respiratory and Critical Care Medicine 158, 154–160 [DOI] [PubMed] [Google Scholar]
- 79.Spencer NJ, Hibberd TJ, Travis L, Wiklendt L, Costa M, Hu H, Brookes SJ, Wattchow DA, Dinning PG, Keating DJ, and Sorensen J (2018) Identification of a rhythmic firing pattern in the enteric nervous system that generates rhythmic electrical activity in smooth muscle. The Journal of Neuroscience, 3489–3417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Spencer NJ, Travis L, Wiklendt L, Hibberd TJ, Costa M, Dinning P, and Hu H (2020) Diversity of neurogenic smooth muscle electrical rhythmicity in mouse proximal colon. Am J Physiol Gastrointest Liver Physiol 318, G244–G253 [DOI] [PubMed] [Google Scholar]
- 81.Kashyap P, Gomez-Pinilla PJ, Pozo MJ, Cima RR, Dozois EJ, Larson DW, Ordog T, Gibbons SJ, and Farrugia G (2011) Immunoreactivity for Ano1 detects depletion of Kit-positive interstitial cells of Cajal in patients with slow transit constipation. Neurogastroenterology and Motility 23, 760–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.He CL, Burgart L, Wang L, Pemberton J, YoungFadok T, Szurszewski J, and Farrugia G (2000) Decreased interstitial cell of Cajal volume in patients with slow-transit constipation. Gastroenterology 118, 14–21 [DOI] [PubMed] [Google Scholar]
- 83.Wedel T, Spiegler J, Soellner S, Roblick UJ, Schiedeck THK, Bruch HP, and Krammer HJ (2002) Enteric nerves and interstitial cells of Cajal are altered in patients with slow-transit constipation and megacolon. Gastroenterology 123, 1459–1467 [DOI] [PubMed] [Google Scholar]
- 84.Lyford GL, He CL, Soffer E, Hull TL, Strong SA, Senagore AJ, Burgart LJ, Young-Fadok T, Szurszewski JH, and Farrugia G (2002) Pan-colonic decrease in interstitial cells of Cajal in patients with slow transit constipation. Gut 51, 496–501 [DOI] [PMC free article] [PubMed] [Google Scholar]


