Abstract
AIM
To observe changes in the content of matrix metalloproteinases (MMPs) in the corneal stroma after corneal cross-linking (CXL) in rabbits, and further explore the corneal pathophysiological process after CXL.
METHODS
Forty-two rabbits (42 eyes) were randomly divided into seven groups. One group served as the control group, while the other six groups were treated with CXL. The concentrations of MMPs in corneal stroma were evaluated through parallel reaction monitoring at baseline and 3, 7, 15, 30, 90, and 180d after treatment.
RESULTS
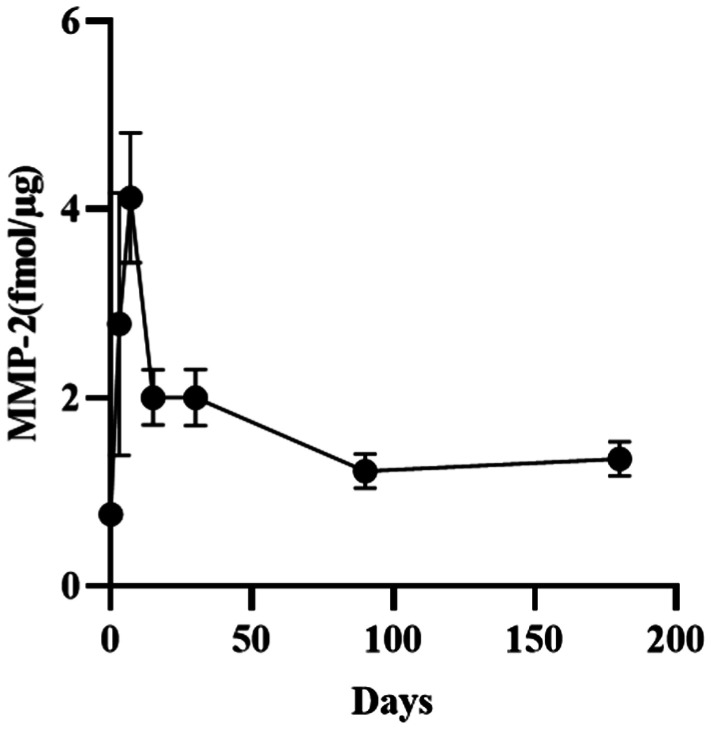
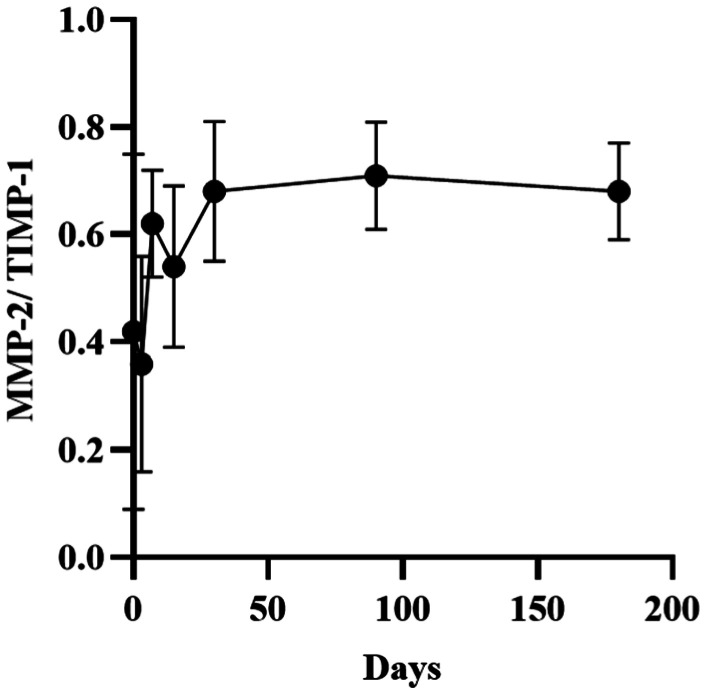
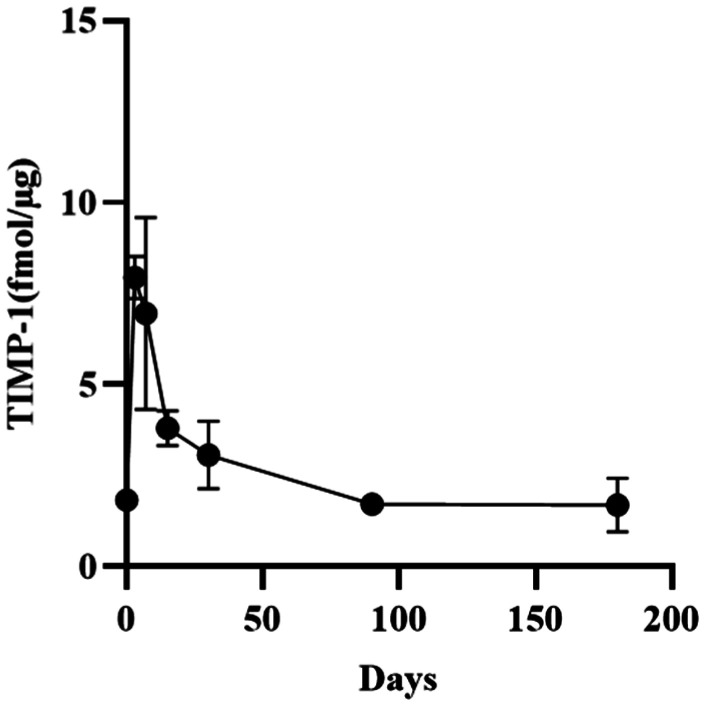
The levels of MMP-2 in the corneal stroma of rabbits were 0.76±0.07, 2.78±1.39, 4.12±0.69, 2.00±0.29, 2.00±0.30, 1.22±0.18, and 1.35±0.18 (10−9 mol/g) at baseline and 3, 7, 15, 30, 90, and 180d after treatment, respectively. The contents of tissue inhibitor of metalloproteinase-1 (TIMP-1) were 1.83±0.26, 7.94±0.58, 6.95±2.64, 3.81±0.48, 3.07±0.92, 1.72±0.19, and 1.69±0.74 (10−9 mol/g), respectively. The ratios of MMP-2/TIMP-1 were 0.42±0.33, 0.36±0.20, 0.62±0.10, 0.54±0.15, 0.68±0.13, 0.71±0.10, and 0.68±0.09, respectively. After CXL, the expression of MMP-2 and TIMP-1 in the rabbit corneal stroma was initially increased and subsequently decreased. The levels of MMP-2 remained higher than those recorded at baseline 180d after treatment, but it was not statistically significant. The levels of TIMP-1 returned to baseline levels at 90d after treatment. The ratio of MMP-2/TIMP-1 started to rise from 7d after CXL. It was significantly higher than that calculated at baseline 30-180d after CXL. The results for MMP-1, -3, -7, -9, -13, and TIMP-2 were negative.
CONCLUSION
CXL can lead to changes in the content of MMP-2 and TIMP-1 in the rabbit corneal stroma. The ratio of MMP-2/TIMP-1 remains higher versus baseline, indicating that MMP-2 is involved in the corneal pathophysiological process after CXL.
Keywords: corneal cross-linking, keratoconus, rabbit, riboflavin, matrix metalloproteinase
INTRODUCTION
Keratoconus (KC) is an early-onset, progressive and often bilateral eye disease characterized by thinning and weakening of the cornea. The cornea gradually bulges outward and finally becomes cone-shaped in patients with advanced disease[1]. During this process, keratocyte density decreases, the number of lamellae is reduced, and fibroblasts degrade in the corneal stroma. Additionally, an irregular distribution of corneal collagen fibrillar mass, and changes in the gross organization of the fibrillar lamellae, particularly around the apex of the corneal cone, were revealed in keratoconic corneas. Changes in the structure of the corneal extracellular matrix and the decrease in the number of corneal collagen lamellae cause thinning of the corneal stroma in KC. Moreover, structural abnormalities were reported in the central part of Bowman's layer in KC. Breaks in corneal Bowman's layer are usually filled with collagen derived from the corneal stroma[2]. KC is probably a multifactorial eye condition; several processes, including biochemical nature, can promote its development. Corneal thinning in KC may be ascribed to degradation of the extracellular matrix components and loss of keratocytes.
Corneal tissue contains matrix metalloproteinases (MMPs), which participate in corneal epithelial regeneration and neovascularization[3]. MMPs, also termed matrixins, are zinc-dependent endopeptidases and cell membrane-bound proteinases with the ability to degrade some extracellular molecules and an array of bioactive molecules. MMPs make an important impact on wound healing and tissue remodeling[3]. MMPs are important components of the normal cornea, and are minimally expressed. Under usual physiological conditions, they take part in maintaining homeostasis. It was reported that MMPs may make important impacts on the degradation associated with KC[1]. Several early studies[1],[4] revealed that the levels or activities of MMPs (e.g., MMP-1, -2, -3, -7, -9, -13) were increased in KC or patient tears. MMPs can be inhibited by tissue inhibitors of matrix metalloproteinase (TIMP), which contain TIMP-1, -2, -3 and -4. Kenney et al[5] detected that the levels of TIMP-1 in KC were reduced. Other studies[6]–[7] showed levels of TIMP-1 and TIMP-2 increased in advanced KC.
Corneal cross-linking (CXL) was first reported by Wollensak et al[8] and is presently considered a standard treatment for progressive KC. CXL aims to treat the underlying pathology of KC; its purpose is to effectively stiffen the cornea by enhancing its tensile strength[9], increase corneal enzymatic resistance[10] and subsequently arrest or retard the progression of KC, or even reverse KC in some cases[11]. It was reported that CXL could trigger damage and repair of corneal epithelial cells, apoptosis, proliferation and migration of keratocytes, and aggregation of neutrophils[12]. MMPs are secreted by corneal epithelial cells, keratocytes, and neutrophils in the human cornea[13]. Therefore, we hypothesized that CXL may have an effect on the synthesis and secretion of MMPs in the cornea. Thus far, limited research has been performed in this area. This experiment estimated the effects of CXL on the levels of MMP-1, -2, -3, -7, -9, -13, TIMP-1 and TIMP-2 in the corneal stroma of rabbits.
MATERIALS AND METHODS
Ethical Approval
All the operations protocol completed in our study were supported by the Ethics Committee of the Sixth Medical Center of Chinese PLA General Hospital and conformed to the Association for Research in Vision and Ophthalmology statement for the Use of Animals.
Animals
Forty-two New Zealand white rabbits (the Sixth Medical Center of Chinese PLA General Hospital's animal room, Beijing, China) were fed the same diet and sacrificed using an intravenous overdose of pentobarbital with a live weight of 2.5±0.25 kg. Thirty-six rabbits were treated with ultraviolet A/riboflavin CXL in one eye, and six rabbits were randomly killed at 3, 7, 15, 30, 90, and 180d after CXL, respectively. Another six rabbits served as normal controls without any intervention.
Ultraviolet A/Riboflavin CXL Procedure
The animals were anesthetized with intravenous injection of 3 mL/kg of 2% pentobarbital sodium (Sigma-Aldrich Trading Co., Shanghai, China). Before the cross-linking treatment, the corneas were anesthetized by a local anesthetic including 0.4% oxybuprocaine hydrochloride (Benoxil, Santen Pharmaceutical Co, Osaka, Japan). A 9.0-mm central corneal region was lightly marked using a surgical trephine, and the corneal epithelium was mechanically scraped with a blunt hockey knife. The CXL process was completed based on the technique reported by Xia et al[14]. Prior to irradiation with ultraviolet A, a 0.1% riboflavin solution containing 10 mg of riboflavin 5-phosphate (Sigma-Aldrich Trading Co.) which was dissolved in 10 mL of 20% dextran-T-500 solution (Sigma-Aldrich Trading Co.) was applied onto the cornea every 5min until confirming the corneal intrastromal riboflavin through slit-lamp microscopy. Then, the corneas were irradiated for 30min using ultraviolet A (370 nm, 3 mW/cm2) with a radiation device (UV-X Corneal Cross-linking System, Zurich, Switzerland). Riboflavin solution was administered to the corneas every 5min throughout the procedure. Eye drops (dexamethasone/tobramycin; Alcon Laboratories Inc.) were applied to the eyes after CXL.
Preparation of Corneal Samples
At 3, 7, 15, 30, 90, and 180d after the CXL procedure, the rabbits in the experimental and control groups were sacrificed with an intravenous overdose of pentobarbital sodium. Their 9.0-mm central corneal buttons were trephined. The corneal epithelium and endothelium of the buttons were mechanically removed using a blunt hockey knife. Subsequently, the buttons were put in centrifuge tubes and stored at -80°C.
Protein Extraction and Quality Detection
Corneal samples were cut with tissue scissors, mixed with proper radioimmunoprecipitation assay lysis buffer and phenylmethanesulfonyl fluoride, oscillated on MP FastPrep-24 5G crusher (MP Biomedicals LLC Corp., CA, USA, ) four times, lysed on ice for 30min, and centrifuged at 4°C (12 000 rpm, 15min) to gain the supernatant. Protein concentration was estimated with the bicinchoninic acid assay, and sodium dodecyl sulfate-polyacrylamide gel electrophoresis was performed.
Reduction, Alkylation, and Enzymolysis
For all samples, 150 µg of protein (volume: 150 µL) was lessened with tris (2-carboxyethyl) phosphine (10 mmol/L, 60min, 37°C) and alkylated with iodoacetamide (40 mmol/L, 40min, room temperature) under darkness. Pre-cooled acetone (acetone/sample volume ratio=6:1) was added to each protein sample, and all the samples were precipitated at -20°C for 4h. After centrifugation (10 000 g, 20min) the precipitate was dissolved with 150 µL of 100 mmol/L triethylamine bicarbonate. Proteins were trypsinized throughout the night at 37°C at a trypsin/protein ratio of 1:25 (weight/weight). The resulting peptide mixture was lyophilized throughout the night and peptides were desalted as reported by Sprung et al[15].
Parallel Reaction Monitoring Analysis
Experiments were completed using a Q Exactive mass spectrometer (Thermo, USA) together with Easy-nLC 1200 (Thermo). Peptide blends were divided by C18-reversed phase chromatography via an Easy column (75 µm×25 cm, Thermo) and the analytical separation was processed for 90min with a linear gradient of acetonitrile/formic acid 80%/0.1% (Solvent A) and acetonitrile/formic acid 2%/0.1% (Solvent B) at a flow rate of 300 nL/min. The gradient procedure was processed as follows: 5% B at 1min, rising to 23% B at 41min, 29% B at 51min, quickly rising to 100% B within 8min, and maintaining 100% B for 6min before resuming the original condition of 5% B. The column was re-equilibrated to 5% B for 20min after every procedure. Every sample was analyzed with a multiplexed parallel reaction monitoring (PRM) process based on a planned inclusion list, which included the target precursor ions demonstrating the standard peptides. The full-scan event was gathered at m/z 350-1300, the automatic gain control target at 3e6, an Orbitrap resolution of 70 000, and the maximum fill time at 20ms. Every full scan was followed by 20 PRM scans at a resolution of 17 500 with an isolation window: the maximum fill time of 100ms, an automatic gain control value of 5e5, 2.0 m/z, and a standardized collision energy of 28 in a higher-energy c-trap dissociation cell.
PRM data analysis and integration were completed with the Skyline v3.5 Software (AB Sciex Corp., WA, USA). Routine evaluation of instrument and chromatographic function was completed with a quality control standard including all artificial peptides, which was processed at a concentration of 20 fmol/µL in 0.1% formic acid. All samples were injected thrice; the peak area of each target peptide was obtained with Skyline, peak picking was artificially checked and corrected according to the retention time, mass accuracy, transitions, and tandem mass spectrometry. At least three transitions for every peptide were obtained from the PRM data. Each peptide was quantified by calculating the peak area under the curve of each transition. The abundance of peptides was standardized according to the total ion current obtained from the full-scan acquisition for each process. Proteins were quantified by calculating the abundances of the selected peptides. Precise quantitation of protein was needed to match that of a artificial standard peptide.
Statistical Analysis
Statistical analyses were completed with the SPSS Statistics version 22.0 software (IBM Corp., Armonk, NY, USA). The differences in MMP-2 and TIMP-1 at each time point were compared using the non-parametric repeated measurement test i.e. Friedman test. The values are described as the mean±standard deviation and P<0.05 denoted statistically significant differences.
RESULTS
Our results showed that CXL induced changes of MMP-2 and TIMP-1 levels in the rabbit corneas which are shown in Table 1. Notably, MMP-1, -3, -7, -9, -13, and TIMP-2 were not found in rabbit corneal stroma after CXL.
Table 1. Changes in the levels of MMP-2 and TIMP-1 in rabbit corneal stroma after CXL.
| Variables | Day 0 | Day 3 | Day 7 | Day 15 | Day 30 | Day 90 | Day 180 | χ2 | P |
| MMP-2 | 0.76±0.07 | 2.78±1.39a | 4.12±0.69a | 2.00±0.29a | 2.00±0.30a | 1.22±0.18 | 1.35±0.18 | 33.14 | 0.000 |
| TIMP-1 | 1.83±0.26 | 7.94±0.58a | 6.95±2.64a | 3.81±0.48a | 3.07±0.92 | 1.72±0.19 | 1.69±0.74 | 31.36 | 0.000 |
| MMP-2/TIMP-1 | 0.42±0.33 | 0.36±0.20 | 0.62±0.10 | 0.54±0.15 | 0.68±0.13a | 0.71±0.10a | 0.68±0.09a | 17.29 | 0.008 |
MMP: Matrix metalloproteinase; TIMP: Tissue inhibitors of matrix metalloproteinase; CXL: Corneal cross-linking; Day 0: Before CXL. aP<0.05 vs baseline.
10−9 mol/g
Tables 1 and 2 showed that the levels of MMP-2 and TIMP-1 in the rabbit corneal stroma initially increased and subsequently decreased after CXL, while the ratio of MMP-2/TIMP-1 continued to increase. The preoperative levels of MMP-2 were 0.76±0.07 fmol/µg, continued to rise, reached a peak value (4.12±0.69 fmol/µg) within 7d after CXL, and subsequently began to decline. The levels of MMP-2 were in the platform stage 3-30d after CXL, without significant change. The levels of MMP-2 90d after CXL were significantly lower than those recorded 30d after CXL, and were in the platform stage 90-180d after CXL, without significant change. Notably, the levels 180d after CXL remained higher than the preoperative levels, but it was not statistically significant.
Table 2. Paired comparison of MMP-2 and TIMP-1 content in rabbit corneal stroma at different time points after CXL.
| Variables | Day 0-3 | Day 0-7 | Day 0-15 | Day 0-30 | Day 0-90 | Day 0-180 | Day 3-7 | Day 3-30 | Day 3-90 | Day 3-180 | Day 7-90 | Day 7-180 | Day 30-90 |
| MMP-2 | 0.001 | 0.000 | 0.001 | 0.002 | b | b | b | b | 0.016 | b | 0.000 | 0.001 | 0.033 |
| TIMP-1 | 0.000 | 0.001 | 0.033 | b | b | b | b | 0.023 | 0.000 | 0.001 | 0.000 | 0.005 | b |
| MMP-2/TIMP-1 | b | b | b | 0.011 | 0.008 | 0.023 | 0.033 | 0.005 | 0.003 | 0.011 | b | b | b |
MMP: Matrix metalloproteinase; TIMP: Tissue inhibitors of matrix metalloproteinase; CXL: Corneal cross-linking; Day 0: Before CXL. bP>0.05. Statistical results of paired comparison with P>0.05 were not listed in the table.
P values
The levels of TIMP-1 prior to operation were 1.83±0.26 fmol/µg, and peaked at 3d after operation (7.94±0.58 fmol/µg) higher than the baseline level significantly. The levels gradually declined from 3 to 180d after CXL, until returned to preoperative levels. Of note, there was no significant change from 3 to 15d after CXL. There was no statistical difference compared with the baseline level 30-180d after surgery.
The ratio of MMP-2/TIMP-1 was 0.42±0.33 prior to surgery and began to rise from 7d after CXL. It was significantly higher than that calculated at baseline 30d (0.68±0.13) after surgery. However, there were no significant changes observed from 30 to 180d after CXL.
Figures 1 to 3 show the changing trends of MMP-2, TIMP-1 and MMP-2/TIMP-1 in the form of line graphs respectively. Figure 4 shows the process of CXL.
Figure 1. Change in the levels of MMP-2 in rabbit corneal stroma after CXL.
Figure 3. Change in the levels of MMP-2/TIMP-1 in rabbit corneal stroma after CXL.
Figure 4. CXL process in the rabbit eye.
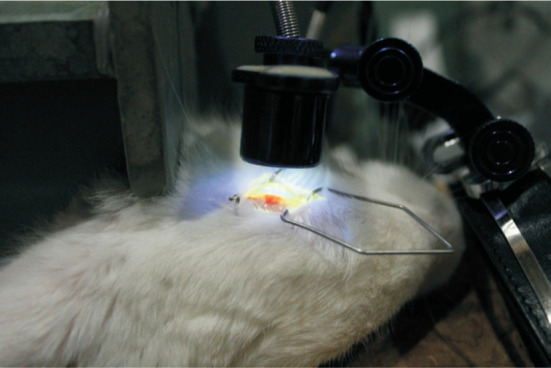
Figure 2. Change in the levels of TIMP-1 in rabbit corneal stroma after CXL.
DISCUSSION
In this experiment, PRM technology was adopted to quantitatively analyze the changes in the levels of MMPs in rabbit corneal stroma within 180d after CXL. The results displayed that the contents of MMP-2 and TIMP-1 in the corneal stroma of rabbits initially increased and subsequently decreased. At 180d after CXL, the contents of MMP-2 in the corneal stroma remained higher than the preoperative levels, but it was not statistically significant. In contrast, the levels of TIMP-1 had returned to the preoperative levels at 90d after the operation. The MMP-2/TIMP-1 ratio within 180d after CXL continued to rise and was significantly higher than that calculated at baseline from 30-180d after CXL.
There are relatively few reports on the effects of CXL on MMPs. Kolozsvári et al[16] studied changes in the concentrations of MMP-9, -13, and TIMP-1 in the tears of KC patients after CXL. At day 4 after CXL, the contents of MMP-13 were significantly reduced compared with the pre-CXL concentrations, whereas the concentrations of TIMP-1 and MMP-9 did not change significantly. At 38d, 6, or 12mo after CXL, there were no significant changes in the concentrations of MMP-9, -13, and TIMP-1 compared with baseline. However, changes were observed following the classification of pre-CXL concentrations of MMPs into high or low subgroups and individual analysis. In the low-concentration pre-CXL subgroup, the contents of MMP-9, -13 and TIMP-1 significantly increased at 6mo; only those of MMP-13 elevated at 12mo. In the high-concentration pre-CXL subgroup, there were no changes after 6mo in the contents of MMP-9, -13, and TIMP-1. At 12mo, the levels of MMP-9 and -13 showed a significant decreasing trend. In this study, the affected eyes were irritated, tear secretion was increased, and the medium concentration was diluted 4d after CXL. The results of the low- and high-concentration pre-CXL subgroups were different, which may be attributed to the different pathological stages of KC. The mediators synthesized and secreted during the process of KC may differ at different pathological stages, and the degree of response to CXL may also vary. Smith et al[7] showed that optimally maintained clear KC stromal cells created more MMP-2 than normal corneal stromal cells, but not TIMP. However, the contents of MMP-2 and TIMP-1 increased in scarred KC stromal cells.
Balasubramanian et al[17] studied the differences in protease concentration and proteolytic activity in tears among a normal control group, KC group, and 3-6mo post-CXL group. The contents of MMP-1, -3, -7, and -13 were significantly increased in KC patients compared with those observed in the control group. The difference in protease concentrations detected between the CXL group, KC, and normal control group were not significant. It didn't show significant differences in the expression of MMP-2, -8, -9, and TIMP-1, -2 among the three groups. The activity of MMPs in the tear film of the three groups was also examined. The activity of MMP-2 and -9 in patients with KC was significantly higher than that reported in normal subjects. However, the activity of MMP-2 and -9 in the CXL group was not significantly different compared with that noted in the KC or normal subject groups. Similar results were shown for the activity of MMP-1, -8, and -13. These MMPs were significantly more active in the KC group compared with the normal group. However, the differences were not significant when the CXL group was compared with the KC and normal groups. The maximum activity of MMPs in the tear film was observed in the KC group followed by the CXL group. In this study, the investigators did not compare the concentration and activity of MMPs in tears before and after CXL in the CXL group.
Both of the above studies examined the effect of CXL on MMPs in the tears of KC patients. However, the content of MMPs in tears may be affected by other factors. Mazzotta et al[4] found that the positive rate of MMP-9 in tears of KC patients with allergic conjunctivitis and/or allergic rhinitis was 81%, whereas it was only 4% in patients without allergic reactions. Sakimoto and Sawa[18] revealed that corneal epithelial defects may increase the levels of MMPs in the cornea and tear fluid. Mohamed-Noriega et al[19] reported that topical application of non-steroidal anti-inflammatory drugs induced an increase in the contents of MMPs in the tears and cornea. Balasubramanian et al[17] showed that the levels of MMP-13 in the tear film of KC patients were significantly increased, and decreased slightly 3-6mo after CXL (not statistically significant difference). Regarding MMP-9 and TIMP-1, there was no significant change in patients with KC or patients 3-6mo after CXL. The results of the study conducted by Balasubramanian et al[17] are inconsistent with those reported by Kolozsvári et al[16].
Mastropasqua et al[20] performed a quantitative analysis of MMP-1 in human cadaver eyes immediately after different protocols of CXL. The investigators observed a significantly higher expression of MMP-1 in iontophoresis-assisted epithelium-on CXL (10 mW/cm2, 9min) compared with untreated corneas. There was no difference observed between the control, standard epithelium-off CXL, and iontophoresis-assisted epithelium-on CXL (3 mW/cm2, 30min) groups. These results showed that different protocols of CXL had different effects on the pathophysiology of the cornea. CXL with iontophoresis and irradiation using 10 mW significantly induced corneal stromal fibroblasts and corneal remodeling to reestablish a normal cornea. We failed to quantitatively detect the expression of MMP-1 in the rabbit cornea before and within 180d after standard epithelium-off CXL. This finding is consistent with the above experimental results.
MMPs have been found at very low contents in normal tissues. Their expression is activated or upregulated during tissue remodeling and inflammation. The activity of MMPs can be highly inhibited by TIMPs to prevent uncontrolled and excessive tissue destruction[21]. Imbalance between TIMPs and MMPs leads to pathological conditions. In KC, an imbalance between TIMPs and MMPs may cause excessive degradation of the corneal tissue and thinning of the cornea. During the past 20y, more and more evidences have indicated a link between MMPs and KC[1].
MMP-2 is the most important MMP in corneal tissue. It is synthesized and secreted by corneal stromal cells, can hydrolyze type IV collagen, and attack type I collagen before the formation of newly synthesized fibrils. Studies[2],[21] showed that the contents and activity of MMP-2 were increased, whereas the mRNA contents of TIMP-1 were decreased in KC. Keratocyte cultures from KC corneas exhibited higher levels of MMP-2/TIMP-1 compared with cells of normal corneas[2]. Increased levels of MMP-2/TIMP-1 may be one of the causes of stroma degradation in KC. Our study found that the MMP-2/TIMP-1 ratio increased after CXL, which may be a negative factor for the development of KC. However, interestingly, Zhang et al[22] reported that cross-linked collagen type IV can resist degradation by MMP-9 and MMP-2, demonstrating that further impairment to the basement membrane of KC could be halted by CXL. Most cases of KC did not resolve after CXL. This may be attributed to the fact that the increase in the ability of corneal collagen to resist degradation by MMP-2 after CXL exceeds the increase in the MMP-2/TIMP-1 ratio. However, the increase in the MMP-2/TIMP-1 ratio may neutralize some long-term effects of CXL. Collagen fibers in KC are loosely arranged. Compared with the normal cornea, small changes in the levels and activity of protease in KC can affect the development of the condition. Following CXL, activated keratocytes in the corneal stroma can newly synthesize collagen, and this collagen is irregularly arranged. MMPs can degrade the irregularly arranged collagen, which is beneficial to corneal matrix remodeling.
Currently, there is still no animal model for KC. In this experiment, we used the normal rabbit cornea as the research object. After CXL, the pathophysiology of the rabbit cornea, especially change in the levels of MMPs, may not be completely consistent with that of KC. This is because keratocytes in KC have undergone the processes of apoptosis, proliferation, and migration, which can synthesize and secrete the extracellular matrix and MMPs. As shown by Kolozsvári et al[16], MMPs are synthesized and secreted differently at different pathological stages of KC, and the effect of CXL on MMPs may also differ. In addition, we only tested changes in the levels of MMPs within 6mo after CXL in the rabbit cornea. Six months after CXL, the levels of MMP-2/TIMP-1 remained higher than those observed at baseline. Further observation is warranted following the return of these levels to normal values. The small sample size is another weakness of this study.
Acknowledgments
Foundation: Supported by Beijing Municipal Science and Technology Commission (No.Z151100004015217).
Conflicts of Interest: Jia HZ, None; Pang X, None; Peng XJ, None.
REFERENCES
- 1.di Martino E, Ali M, Inglehearn CF. Matrix metalloproteinases in keratoconus – Too much of a good thing? Exp Eye Res. 2019;182:137–143. doi: 10.1016/j.exer.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 2.Wojcik KA, Blasiak J, Szaflik J, Szaflik JP. Role of biochemical factors in the pathogenesis of keratoconus. Acta Biochim Polonica. 2014;61(1):55–62. [PubMed] [Google Scholar]
- 3.Kalkanci A, Bilgihan K, Ozdemir HB, Yar Saglam AS, Karakurt F, Erdogan M. Corneal cross-linking has no effect on matrix metalloproteinase 9 and 13 levels during fungal keratitis on the early stage. Mycopathologia. 2018;183(2):329–336. doi: 10.1007/s11046-017-0207-8. [DOI] [PubMed] [Google Scholar]
- 4.Mazzotta C, Traversi C, Mellace P, Bagaglia SA, Zuccarini S, Mencucci R, Jacob S. Keratoconus progression in patients with allergy and elevated surface matrix metalloproteinase 9 point-of-care test. Eye Contact Lens. 2018;44(Suppl 2):S48–S53. doi: 10.1097/ICL.0000000000000432. [DOI] [PubMed] [Google Scholar]
- 5.Kenney MC, Chwa M, Atilano SR, Tran A, Carballo M, Saghizadeh M, Vasiliou V, Adachi W, Brown D. J. Increased levels of catalase and cathepsin V/L2 but decreased TIMP-1 in keratoconus corneas: evidence that oxidative stress plays a role in this disorder. Invest Ophthalmol Vis Sci. 2005;46(3):823–832. doi: 10.1167/iovs.04-0549. [DOI] [PubMed] [Google Scholar]
- 6.Kenney MC, Chwa M, Alba A, Saghizadeh M, Huang ZS, Brown DJ. Localization of TIMP-1, TIMP-2, TIMP-3, gelatinase A and gelatinase B in pathological human corneas. Curr Eye Res. 1998;17(3):238–246. doi: 10.1076/ceyr.17.3.238.5222. [DOI] [PubMed] [Google Scholar]
- 7.Smith VA, Matthews FJ, Majid MA, Cook SD. Keratoconus: Matrix metalloproteinase-2 activation and TIMP modulation. Biochim et Biophys Acta. 2006;1762(4):431–439. doi: 10.1016/j.bbadis.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135(5):620–627. doi: 10.1016/s0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
- 9.Mohammadpour M, Masoumi A, Mirghorbani M, Shahraki K, Hashemi H. Updates on corneal collagen cross-linking: indications, techniques and clinical outcomes. J Curr Ophthalmol. 2017;29(4):235–247. doi: 10.1016/j.joco.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aldahlawi NH, Hayes S, O'Brart DPS, O'Brart ND, Meek KM. An investigation into corneal enzymatic resistance following epithelium-off and epithelium-on corneal cross-linking protocols. Exp Eye Res. 2016;153:141–151. doi: 10.1016/j.exer.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Mohaimeed MM. Combined corneal CXL and photorefractive keratectomy for treatment of keratoconus: a review. Int J Ophthalmol. 2019;12(12):1929–1938. doi: 10.18240/ijo.2019.12.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wollensak G, Iomdina E, Dittert DD, Herbst H. Wound healing in the rabbit cornea after corneal collagen cross-linking with riboflavin and UVA. Cornea. 2007;26(5):600–605. doi: 10.1097/ICO.0b013e318041f073. [DOI] [PubMed] [Google Scholar]
- 13.Balasubramanian SA, Pye DC, Willcox MD. Effects of eye rubbing on the levels of protease, protease activity and cytokines in tears: relevance in keratoconus. Clin Exp Optom. 2013;96(2):214–218. doi: 10.1111/cxo.12038. [DOI] [PubMed] [Google Scholar]
- 14.Xia Y, Liu BC, Fan ZJ, Peng XJ. Corneal collagen fibril changes after ultraviolet A/riboflavin corneal crosslinking. Cornea. 2014;33(1):56–59. doi: 10.1097/ICO.0000000000000017. [DOI] [PubMed] [Google Scholar]
- 15.Sprung RW, Martinez MA, Carpenter KL, Ham AJL, Washington MK, Arteaga CL, Sanders ME, Liebler DC. Precision of multiple reaction monitoring mass spectrometry analysis of formalin-fixed, paraffin-embedded tissue. J Proteome Res. 2012;11(6):3498–3505. doi: 10.1021/pr300130t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kolozsvári BL, Berta A, Petrovski G, Miháltz K, Gogolák P, Rajnavölgyi E, Hassan Z, Széles P, Fodor M. Alterations of tear mediators in patients with keratoconus after corneal crosslinking associate with corneal changes. PLoS One. 2013;8(10):e76333. doi: 10.1371/journal.pone.0076333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balasubramanian SA, Mohan S, Pye DC, Willcox MDP. Proteases, proteolysis and inflammatory molecules in the tears of people with keratoconus. Acta Ophthalmol. 2012;90(4):e303–e309. doi: 10.1111/j.1755-3768.2011.02369.x. [DOI] [PubMed] [Google Scholar]
- 18.Sakimoto T, Sawa M. Metalloproteinases in corneal diseases. Cornea. 2012;31:S50–S56. doi: 10.1097/ICO.0b013e318269ccd0. [DOI] [PubMed] [Google Scholar]
- 19.Mohamed-Noriega K, Butrón-Valdez K, Vazquez-Galvan J, et al. Corneal melting after collagen cross-linking for keratoconus in a thin cornea of a diabetic patient treated with topical nepafenac: a case report with a literature review. Case Rep Ophthalmol. 2016;7(1):119–124. doi: 10.1159/000444437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mastropasqua L, Lanzini M, Curcio C, Calienno R, Mastropasqua R, Colasante M, Mastropasqua A, Nubile M. Structural modifications and tissue response after standard epi-off and iontophoretic corneal crosslinking with different irradiation procedures. Invest Ophthalmol Vis Sci. 2014;55(4):2526–2533. doi: 10.1167/iovs.13-13363. [DOI] [PubMed] [Google Scholar]
- 21.Wong TTL, Sethi C, Daniels JT, Limb GA, Murphy G, Khaw PT. Matrix metalloproteinases in disease and repair processes in the anterior segment. Surv Ophthalmol. 2002;47(3):239–256. doi: 10.1016/s0039-6257(02)00287-4. [DOI] [PubMed] [Google Scholar]
- 22.Zhang YT, Mao XL, Schwend T, Littlechild S, Conrad GW. Resistance of corneal RFUVA-cross-linked collagens and small leucine-rich proteoglycans to degradation by matrix metalloproteinases. Invest Ophthalmol Vis Sci. 2013;54(2):1014–1025. doi: 10.1167/iovs.12-11277. [DOI] [PMC free article] [PubMed] [Google Scholar]