Abstract
AIM
To compare the visual outcomes of children with small (≤3 mm) posterior polar cataracts (PPC) and posterior lenticonus who had cataract extraction surgery with the visual outcomes of those who were managed conservatively.
METHODS
Children who initially had small PPC and posterior lenticonus who were followed up over 1-year period were retrospective reviewed in the study. Patients receiving surgery were compared with those receiving conservative therapy. The axial length, keratometry, refraction, best-corrected visual acuity (BCVA), and strabismus measurements were recorded. Lens morphology, i.e., the location, size, and depth of the cataract lesion, was measured with a Scheimpflug imaging system. To help control for baseline differences in the groups, patients were matched with controls by propensity score methodology.
RESULTS
The study evaluated 60 patients (30 in the surgery group and 30 in the conservative therapy group) after matching by propensity score. Patients who underwent cataract surgery showed greater BCVA improvements (0.36±0.24 logMAR) than patients who were treated without surgery (0.22±0.26 logMAR; P=0.036). Surgery was effective in patients with a rear projection length (RPL) less than 1.0 mm and a pretreatment BCVA worse than 0.52 logMAR.
CONCLUSION
Children with small PPC and posterior lenticonus who undergo cataract surgery experience greater BCVA improvements than those managed conservatively. Certain patients presenting with a RPL less than 1.0 mm and a pretreatment BCVA of 0.52 logMAR or worse may benefit from surgery.
Keywords: posterior polar cataracts, posterior lenticonus, visual outcomes, Pentacam
INTRODUCTION
A posterior polar cataract (PPC), including lenticonus, is usually unilateral and is a malformation of the lens[1]–[4]. Cataract surgery is the suggested treatment for children who have a lens opacity larger than 3 mm[5]–[6]. Generally it is thought that young children with posterior lens opacities or lenticonus smaller than 3 mm do not need surgery, and these patients often experience visual loss. Visual impairment may be caused by an opacity that blocks the visual axis, refractive error, posterior oil droplet lesion-induced optical distortion, or amblyopia[7]–[11]. This study aimed to compare visual acuity improvement in children with small PPCs or posterior lenticonus who were treated with or without surgery. We determined whether any factors associated with greater visual improvement existed in those who underwent surgical treatment.
We performed a retrospective study to assess the visual outcomes of children with a small PPC and posterior lenticonus who underwent cataract surgery or received nonsurgical treatment.
SUBJECTS AND METHODS
Ethical Approval
This study was approved by the Human Research Ethics Committee of the Zhongshan Ophthalmic Center. The study was performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from the patients.
Patient Population
Children with a small posterior lenticonus or lens cataract (≤3 mm) were included in this study. Children with fundus disease, inflammation, trauma history, Mittendorf's dot, systemic or topical steroid use, or cataracts caused by other reasons, such as radiation, were excluded. All cases attended follow-up sessions at least one year. A final best-corrected visual acuity (BCVA) measurement was necessary to determine the visual outcome; therefore, children who were too young to undergo a Snellen visual test were excluded from this study (inclusion range of age: 3-18y). For children with bilateral PPCs, one eye was randomly excluded from the analysis by selecting right and left eyes in an alternating fashion from the randomly ordered sample. Patients with surgical complications were also excluded from the analysis including one patient with a posterior lenticonus that had a posterior capsule tear with an intraocular lens (IOL; Sensar® AR40) implanted in the sulcus and 4 cases with a transient increase in postoperative intraocular pressure.
The following information was gathered from the children's records: age of first visit, age of detection, associated ocular and systemic disease, age of surgery, type and size of cataract, location of cataract, increase in cataract size, refractive status, anisometropia, type of treatment, strabismus, keratometery, axial length, and BCVA before and after treatment.
Propensity Score Matching
Because of the substantial differences in baseline characteristics between the treatment groups, propensity score methodology was used to identify comparable patients treated with each strategy. Propensity scores were calculated using Logistic regression modeling, including the following variables that were considered as determinant factors in selecting the methods for operative strategies: age of first visit, age of detection, sex, involved eye, type of cataract, size, and location of the lesion, whether the lesion progress, pretreatment BCVA, baseline and variation of axial length, keratometery, anisometropia, and strabismus. We matched propensity scores one-to-one using the nearest neighbor methods without replacement, using a 0.15 caliper width. Thus, a total of 60 patients (30 patients in the surgery group and 30 matched controls in the conservative group) were included in the final analyses.
Lens Morphometry
The diameter and rear projection length (RPL) of the lens abnormality were measured in millimeters (mm) using a Pentacam HR (Oculus, Inc., Wetzlar, Germany) after the pupil was fully dilated (tropicamide 5 mg/mL, every 10min, three times).
Twenty-five single-slit images of the anterior segment were captured in 2s with a rotating Pentacam camera from 0° to 360° after mydriasis. Eye movements automatically corrected as the imaging proceeded. The Pentacam system can provide a 3-dimensional scan of the anterior segment of the eye, including a sharply focused image of the whole lens, by combining the Scheimpflug video-photography system and a monochromatic slit-light source[12]–[13].
Pentacam software could also be used to analyze the overall three-dimensional lens volume by measuring the backward scatter. We selected the Scheimpflug image of the target eye for measurements, those whose Pentacam image quality was not displayed as “ok” were excluded. As shown in Figure 1, the maximum diameter of the lesion (MDL) and RPL were manually calculated using Pentacam. The MDL was defined as the greatest distance between the margins of both sides of the posterior lesion in all Scheimpflug images. The perpendicular distance from the center of the lens posterior surface to the focal protrusion apex defined the RPL. The same technician recorded the average of 3 measurements for each examination and operated the Pentacam.
Figure 1. Scheimpflug image of the horizontal cross-section of the lens.
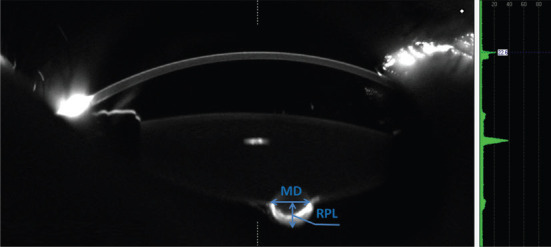
MDL was defined as the peak distance between the margins of both sides of the posterior lesion in all Scheimpflug image. The RPL was defined as the perpendicular distance from the center of the lens posterior surface to the focal protrusion apex.
Diagnostic Methods
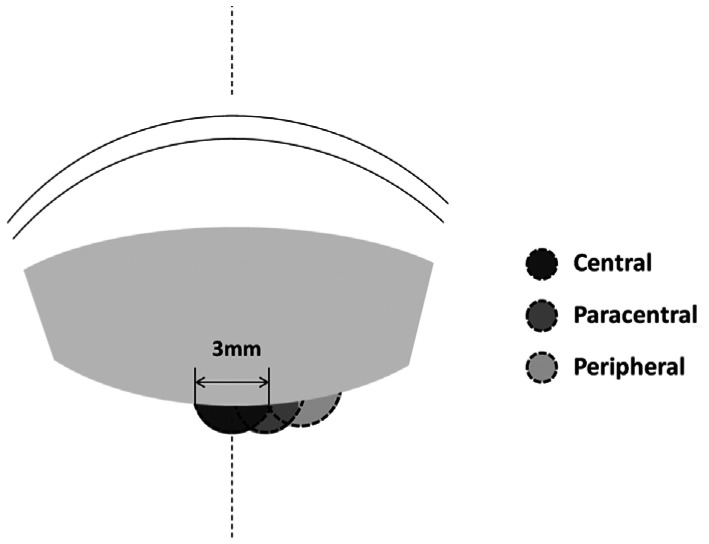
We classified the posterior cataracts into 2 types: posterior lenticonus (with or without opacity) and PPC. The locations of the cataracts were divided into peripheral, paracentral, and central. If the cataract was not centered on the visual axis and was located within 3 mm of the center of the lens, then the cataract was classified as paracentral. Cataracts that were located more than 3 mm from the center of the lens were considered peripheral[10] (Figures 2 and 3). Progression of the cataract was defined as an increase in size, as measured by the Pentacam. A difference in spherical equivalent refraction (SER) of 2 diopters or more was used to define anisometropia.
Figure 2. Classification patterns of the different cataract locations.
Figure 3. Typical Scheimpflug images of the different locations of a PPC and posterior lenticonus.
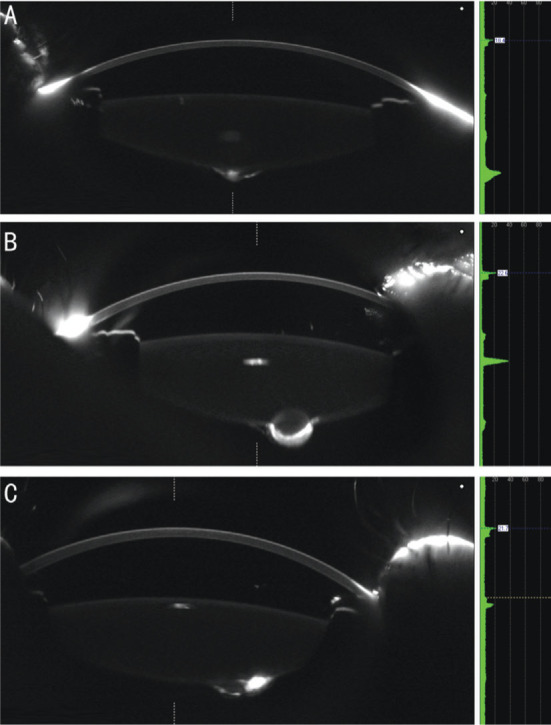
A: Central; B: Paracentral; C: Peripheral.
BCVA was measured using a Snellen chart and was recorded in logMAR units. Amblyopia was defined as a difference in BCVA of more than 0.2 logMAR units[14].
Amblyopia Treatment
Treatments for amblyopia included refractive correction, with or without patching, and atropine eye drops according to the Amblyopia Preferred Practice Pattern[14]–[16]. The patch was required for half of the waking time for children in both groups[17]–[19]. Amblyopia therapy was also needed during the perioperative period in the surgery group. This treatment was sometimes combined with mydriasis of the affected eye and refractive correction prior to surgery (bifocal spectacles or contact lens). The duration of eye patching was decreased according to the degrees of visual improvement after the operation, and the duration of wearing the eye patch was ultimately reduced to half an hour when the patient's vision was near or at the median viewing distance. The treatment period was determined to be the duration until the peak BCVA level was reached or until the treatment was discontinued.
Surgical Technique
A total of forty-six patients underwent cataract surgery. The indication for surgery was amblyopia treatment failure (BCVA worse than 0.6 logMAR) or an increase in cataract size.
Cataract surgery with IOL implantation was performed by an experienced cataract specialist (Chen WR). A standard scleral tunnel incision was executed. An anterior continuous curvilinear capsulotomy was completed. The cortex and nucleus were removed using an irrigation/aspiration device. A central posterior capsulotomy was performed manually or with a vitrectomy instrument in combination with a limited anterior vitrectomy. Hydrophobic acrylic or polymethylmethacrylate IOLs were implanted.
IOL Determination
The axial length and keratometry parameters were measured with an IOLMaster 700 (Carl Zeiss Meditec AG, Jena, Germany) in all patients. The Holladay I IOL power calculation formula was used to determine the power of the IOL. As previously reported, the target postoperative refraction was chosen based on factors including the patient's age, compliance to amblyopia therapy, and laterality of the cataract. Hyperopia was prearranged as the target to compensate for a myopic shift to ultimately achieve mild myopia or emmetropia in adulthood.
BCVA
We analyzed the final visual outcomes and visual acuity improvements for all of the patients to identify the factors associated with better visual improvements in patients who underwent cataract surgery. Patients with a final BCVA better than 0.3 logMAR were declared as having a good visual outcome[10].
Statistical Analysis
Chi-square tests, independent sample t-tests, Fisher's exact test, and nonparametric Mann-Whitney U tests were performed for comparative analyses. Propensity scores matching was performed using SPSS Windows version 22.0 (SPSS Inc., Chicago, IL, USA). Multivariable analysis was carried out to assess which of the studied variables were associated with greater visual improvements in children with small PPCs and posterior lenticonus. SPSS was used for data analysis. P values less than 0.05 indicated statistical significance.
RESULTS
Clinical Parameters
A total of 126 patients were included in this study. The mean follow-up time was 2.5y (range 12-90mo). The average follow-up period for patients treated with conservative treatment was 2.7y and that for patients who underwent surgery was 2.3y (P=0.073).
Table 1 shows the clinical characteristics of the patients in both groups. No statistically significant difference existed between the groups in terms of sex, age, and types of cataract. The size of the lesion in children who underwent surgery (2.14±0.95 mm) was somewhat larger than that in children treated without surgery (1.79±0.82 mm; P=0.035). The cataracts were progressive in seventeen eyes (13.5%).
Table 1. Clinical characteristics of the studied patients.
| Parameters | Amblyopia treated without surgery (n=80) | Cataract surgery and amblyopia treatment (n=46) | P |
| Sex (male) | 43 (53.8) | 25 (54.3) | 0.948 |
| Age of detection (mo) | 31.5±34.8 | 26.0±24.0 | 0.344 |
| Age of first visit (mo) | 61.3±25.6 | 60.6±27.5 | 0.878 |
| Age of last visit (mo) | 83.4±27.9 | 79.0±25.4 | 0.382 |
| Age of surgery (mo) | - | 66.1±28.5 | - |
| Patch treatment time (h/d) | 5.2±1.2 | 5.4±1.1 | 0.456 |
| Involved eye (OD) | 49 (61.3) | 21 (45.7) | 0.090 |
| Lens characteristics | |||
| Type | |||
| Posterior lenticonus | 21 (22.5) | 11 (21.7) | 0.921 |
| Posterior polar cataract | 59 (77.5) | 35 (78.3) | |
| Location | |||
| Central | 39 (48.8) | 28 (60.9) | 0.390 |
| Paracentral | 25 (31.3) | 12 (26.1) | |
| Peripheral | 16 (20.0) | 6 (13.0) | |
| Mean initial size (mm) | 1.79±0.82 | 2.14±0.95 | 0.035a |
| Mean final size (mm) | 1.85±0.88 | 2.26±0.97 | 0.016a |
| Rear projection length (mm) | 0.80±0.43 | 1.00±0.64 | 0.314 |
| Progression of cataract | 7 (8.8) | 10 (21.7) | 0.040a |
| Abnormalities in posterior capsule/anterior hyaloid | 3 (3.8) | 1 (2.2) | 0.536 |
| Axial length (mm) | |||
| Baseline | 21.04±1.17 | 21.63±2.32 | 0.065 |
| Last visit | 21.68±1.23 | 22.48±2.36 | 0.014a |
| Change | 0.64±0.60 | 0.85±0.66 | 0.062 |
| Difference (fellow-treated) | 0.61±0.54 | 0.70±0.62 | 0.400 |
| Keratometery (D) | |||
| K1 | 42.09±2.15 | 43.01±1.87 | 0.017a |
| K2 | 43.99±2.14 | 44.53±2.02 | 0.164 |
| IOL power (D) | - | 23.9±6.4 | - |
| Predicted postop. refraction | - | 1.00 (0, 2.13) | - |
| Actual postop. refraction | - | 1.25 (0.25, 2.56) | - |
| Prediction error | - | -0.06±0.55 | - |
| Absolute value of prediction error | - | 0.43±0.34 | - |
| Anisometropia | 19 (23.8) | 5 (10.9) | 0.076 |
| Strabismus | 20 (25.0) | 7 (15.2) | 0.198 |
Data are mean±standard deviation and median (first quartile, third quartile); aP<0.05.
n (%)
Strabismus was found in 27 patients: 17 patients had exotropia, 8 had esotropia, and 2 had dissociated vertical deviation.
Anisometropia was observed in 19% (n=24) of the children. The majority of the patients with anisometropia had hyperopia (22 cases) and 2 patients had myopia.
All of the patients developed amblyopia and were prescribed glasses or contact lenses prescribed in combination with an eye patch.
Comparison of Groups Matched by Propensity Analysis
The differences of baseline and clinical characteristics between the two groups were adjusted when the patients were matched using the propensity scores. As shown in Table 2, similar proportions of lesion progression were found in two matched groups (P=0.149). In addition, the size of the lesion was also similar (P=0.558). There were no other statistically significant differences between the two groups (P>0.05).
Table 2. Patient characteristics of surgery therapy and conservative therapy groups following matching by propensity analysis.
| Parameters | Amblyopia treated without surgery (n=30) | Cataract surgery and amblyopia treatment (n=30) | P |
| Sex (male) | 15 (50) | 14 (46.7) | 0.500 |
| Age of detection (mo) | 36.03±35.42 | 25.17±23.46 | 0.167 |
| Age of first visit (mo) | 61.30±28.64 | 64.97±26.94 | 0.611 |
| Age of last visit (mo) | 94.00±28.72 | 83.80±26.84 | 0.161 |
| Age of surgery (mo) | 67.50±30.42 | - | |
| Patch time (mo) | 5.4±1.3 | 5.3±1.2 | 0.758 |
| Involved eye (OD) | 13 (43.3) | 17 (56.7) | 0.219 |
| Lens characteristics | |||
| Type | |||
| Posterior lenticonus | 11 (36.7) | 5 (16.7) | 0.072 |
| Posterior polar cataract | 19 (63.3) | 25 (83.3) | |
| Location | |||
| Central | 18 (60) | 21 (70) | 0.118 |
| Paracentral | 11 (36.7) | 5 (16.7) | |
| Peripheral | 1 (3.3) | 4 (13.3) | |
| Mean initial size (mm) | 2.83±0.89 | 2.97±0.86 | 0.558 |
| Mean final size (mm) | 2.90±0.95 | 3.08±0.86 | 0.437 |
| Rear projection length (mm) | 0.54±0.50 | 0.40±0.63 | 0.336 |
| Progression of cataract | 3 (10) | 7 (23.3) | 0.149 |
| Abnormalities in posterior capsule/anterior hyaloid | 0 | 0 | - |
| Axial length (mm) | - | ||
| Baseline | 21.29±1.27 | 21.19±1.93 | 0.819 |
| Last visit | 22.04±1.30 | 22.07±1.77 | 0.941 |
| Change | 0.76±0.74 | 0.88±0.60 | 0.483 |
| Difference (fellow-treated) | 0.73±050 | 0.87±0.57 | 0.297 |
| Keratometery (D) | |||
| K1 | 42.86±1.72 | 43.26±1.74 | 0.374 |
| K2 | 44.66±1.87 | 44.93±1.88 | 0.572 |
| IOL power (D) | 24.60±5.62 | ||
| Predicted postop. refraction | 1.00 (0, 2.00) | - | |
| Actual postop. refraction | 1.25 (0.25, 2.56) | - | |
| Prediction error | -0.06±0.63 | ||
| Absolute value of prediction error | 0.48±0.40 | ||
| Anisometropia | 4 (13.3) | 4 (13.3) | 0.647 |
| Strabismus | 5 (16.7) | 7 (23.3) | 0.374 |
Data are mean±standard deviation and median (first quartile, third quartile).
n (%)
Visual Improvements
On presentation, the baseline BCVA of patients included in the final analyses was 0.61±0.36 (logMAR) in the conservative group and 0.68±0.41 (logMAR) in the surgery group after propensity scores matching. The pre- and post-treatment visual acuity results for patients treated with or without cataract surgery are shown in Table 3. The ages of these children at surgery ranged from 36 to 133mo (67.50±30.42mo). All patients were treated for amblyopia prior to surgery. In the surgery group, the final BCVA before cataract surgery and after amblyopia treatment was considered the preoperative BCVA.
Table 3. BCVA change and final visual outcomes of patients treated with and without cataract surgery following matching by propensity analysis.
| Parameters | Amblyopia treated without surgery (n=30) | Cataract surgery and amblyopia treatment (n=30) | P |
| BCVA before treatment | |||
| Mean±SD (range) | 0.61±0.36 (0.15-1.30) | 0.68±0.41 (0.22-2.00) | 0.434 |
| BCVA after treatment | |||
| Mean±SD (range) | 0.38±0.32 (0.10-1.52) | 0.32±0.34 (0-1.40) | 0.473 |
| >0.3 logMAR (0.5 Snellen) | 18 (60%) | 21 (70%) | 0.294 |
| BCVA improvement after treatment | |||
| Mean±SD (range) | 0.22±0.26 (-0.22 to 1.09) | 0.36±0.24 (0-1.00) | 0.036a |
The numbers are shown in logMAR units. aP<0.05.
Good visual outcomes with BCVA better than 0.30 (logMAR) were achieved in 21 cases (70%). Children who had cataract surgery showed greater BCVA improvements (0.36±0.24 logMAR) than those who did not undergo surgery (0.22±0.26 logMAR; P=0.036), however the preoperative visual acuity was relatively worse than conservative group and though it was not statistically significant (Table 3).
Multivariable analysis showed that the RPL of the lesion (P=0.045), pretreatment BCVA (P<0.001) and observation time (P=0.013) were correlated with visual improvement. The location of the lens anomaly was also correlated with BCVA improvement (central P=0.013, paracentral P=0.046). Patients with posterior lenticonus achieved less BCVA improvement than patients with PPC (P=0.001). Patients with strabismus (P=0.040) was found negatively related to the BCVA improvement. On the other hand, age of detection and age of first visit were not associated with visual improvements (P=0.121 and P=0.647, respectively). The diameter of the cataract (P=0.686) were also not correlated with BCVA improvement (Table 4).
Table 4. Multivariable analysis of the factors influencing BCVA improvement in surgery and conservative therapy groups following matching by propensity analysis.
| Influencing factors | Standardized β | Wald χ2 | P |
| Treatment (surgery) | 0.181 | 13.641 | <0.001a |
| Sex (female) | -0.025 | 0.276 | 0.599 |
| Eye (OS) | 0.087 | 2.846 | 0.092 |
| Lens characteristics | |||
| Type | |||
| Posterior lenticonus | -0.208 | 10.338 | 0.001a |
| Posterior polar cataract | - | - | - |
| Location | |||
| Central | 0.213 | 6.226 | 0.013a |
| Paracentral | 0.176 | 3.991 | 0.046a |
| Peripheral | - | - | - |
| Mean initial size | -0.151 | 0.164 | 0.686 |
| Mean final size | 0.142 | 0.150 | 0.698 |
| Rear projection length | 0.104 | 4.037 | 0.045a |
| Progression of cataract | 0 | 0 | 0.998 |
| Axial length (mm) | |||
| Baseline | 0.081 | 0.003 | 0.960 |
| Last visit | -0.051 | 0.001 | 0.974 |
| Change | 0.172 | 0.012 | 0.914 |
| Keratometery (D) | |||
| K1 | -0.005 | 0.035 | 0.851 |
| K2 | -0.012 | 0.206 | 0.650 |
| BCVA before treatment (logMAR) | |||
| >0.52 (<0.3 snellen) | 0.496 | 17.751 | <0.001a |
| 0.30-0.52 (0.3-0.5 snellen) | 0.238 | 4.651 | 0.031a |
| 0.10-0.30 (0.5-0.8 snellen) | - | - | - |
| Age of detection | 0.001 | 2.399 | 0.121 |
| Age of first visit | 0.000 | 0.210 | 0.647 |
| Observation time | 0.005 | 6.143 | 0.013a |
| Strabismus | -0.132 | 4.205 | 0.040a |
| Anisometropia | -0.050 | 0.408 | 0.523 |
BCVA: Best-corrected visual acuity. aP<0.05.
n=60
Further analysis showed that surgery was effective in patients with an RPL less than 1.0 mm (P=0.007) and a pretreatment BCVA worse than 0.52 logMAR (P=0.002). Patients with progression of cataract (P=0.029) and smaller flat K value (P=0.020) were found negatively related to the BCVA improvement (Table 5).
Table 5. Multivariable analysis of the factors influencing BCVA after surgery following matching by propensity analysis.
| Influencing factors | Standardized β | Wald χ2 | P |
| Sex (female) | 0.071 | 0.981 | 0.322 |
| Eye (OS) | 0.042 | 0.398 | 0.528 |
| Lens characteristics | |||
| Type | |||
| Posterior lenticonus | -0.126 | 1.212 | 0.271 |
| Posterior polar cataract | |||
| Location | |||
| Central | 0.108 | 0.989 | 0.320 |
| Paracentral | 0.023 | 0.043 | 0.836 |
| Peripheral | - | - | - |
| Mean initial size (mm) | 0.085; | 3.592 | 0.058 |
| Mean final size (mm) | 0 | ||
| Rear projection length (mm) | |||
| 0 | |||
| 0-1.0 | 0.306 | 7.389 | 0.007a |
| ≥1.0 | -0.130 | 0.450 | 0.502 |
| Progression of cataract | -0.234 | 4.757 | 0.029a |
| Axial length (mm) | |||
| Baseline | -0.527 | 0.116 | 0.734 |
| Last visit | 0.715 | 0.210 | 0.647 |
| Change | -0.627 | 0.166 | 0.684 |
| Keratometery (D) | |||
| K1 | 0.144 | 5.404 | 0.020a |
| K2 | -0.039 | 2.177 | 0.140 |
| IOL power | 0.054 | 3.110 | 0.078 |
| Absolute value of prediction error | -0.012 | 0.014 | 0.906 |
| BCVA before treatment | |||
| >0.52 (<0.3 snellen) | 0.626 | 9.601 | 0.002a |
| 0.30-0.52 (0.3-0.5 snellern) | 0.294 | 2.814 | 0.093 |
| 0.10-0.30 (0.5-0.8 snellen) | |||
| Age of surgery | 0.003 | 2.613 | 0.106 |
| Observation time before surgery | 0.008 | 2.204 | 0.138 |
| Strabismus | 0.046 | 0.112 | 0.738 |
| Anisometropia | -0.252 | 1.854 | 0.173 |
BCVA: Best-corrected visual acuity. aP<0.05.
n=30
DISCUSSION
In this study of small PPCs and posterior lenticonus, we analyzed and compared the visual outcomes of 30 patients who underwent cataract surgery and 30 patients who did not undergo surgery and were instead treated by conservative management. The results of our study showed that some patients achieved better visual improvement with cataract surgery than with conservative management.
The visual outcomes in both groups were good. In 65% of all cases, good visual acuity was achieved by the final follow-up. The mean postoperative BCVA was 0.35 logMAR, and these results are comparable to those of previous studies. Travi et al[10] found that the mean final BCVA was 0.40±0.23 logMAR (range 0-1.30 logMAR) in patients with small posterior lens opacities. Hosal et al[20] showed that 42.1% of the monocular posterior lenticonus cataract patients included in the study achieved a visual acuity of 0.3 logMAR or better, 36.8% (7 of 19) had 0.4 logMAR to 0.6 logMAR vision, and 21.1% (4 of 19) had less than or equal to 0.7 logMAR vision.
For the conservative group, 18 eyes (60.0%) had a final visual acuity better than 0.3 logMAR and 21 surgically-treated eyes (70.0%) had a BCVA of 0.3 logMAR or better. Our study showed that children who were treated with cataract surgery achieved greater visual acuity improvements than those treated with conservative management. These results are consistent with those of previous studies. In a study with a small sample size, 6 children who underwent cataract surgery had greater BCVA improvements than those who did not undergo surgery[10]. Cheng et al[7] found that children who underwent surgery attained a postoperative BCVA in the 0 to 0.3 logMAR range, and seven eyes (18%) had a BCVA of 0.4 logMAR to 0.7 logMAR. A recent study also showed that there were more than 51% of patients who had cataract extraction and IOL implantation for posterior lenticonus achieved a final BCVA of 0.5 (0.3 logMAR) or better[21].
However, our study showed that age at the time of surgery was not associated with greater visual improvements. This indicated that the timing of the cataract surgery does not depend on the age of the patient. In a case series reported by Schroeder[22], it was shown that the grade of pupillary obstruction caused by PPCs is an influencing factor for the timing of surgery.
Further analysis in this study suggested that the RPL of the lesion was a predictive factor for the final degree of visual improvement. In the current study, the depth of the lesion was measured with a Pentacam, which creates a three-dimensional, precise view of the lens. Pentacam uses digitally acquired data to measure the parameters of the lens. The multivariable analysis showed that surgery was effective in patients with RPL less than 1.0 mm.
The pathogenesis of the lenticonus is believed to involve an inherent weakness of a defined portion of the posterior lens capsule[23]. A longer projection of the lesion causes amblyopia in early age because of the refraction variations caused by the lesion in the visual axis. On the other hand, our study also showed that the keratometric values (flat K) associated with visual improvement after surgery. The irregularity of the cornea may be due to the compensation for the astigmatism caused by the abnormal shape of the lens during sensitive period of visual development. Therefore, the less irregularity of the cornea showed to be associated with greater visual improvement. Additionally, the normal intralenticular pressure bulges the cortex posteriorly within the area of the weakened capsule, which further stretches the weak capsule region and causes the posterior lens surface to bulge and expand[24]. Since the lenticonus bulges further with increasing age, the posterior lenticonus is progressive. The current study showed that progression of cataract was negatively relative to the visual improvement. The cataract forms later, which obscures vision and deteriorates as the amblyopia develops, finally could lead to strabismus. Our study proved that patients with strabismus had a less visual improvement.
Our study also showed that a preoperative BCVA worse than 0.52 logMAR was predictive of postoperative visual improvements in children who undergo surgery. The mean preoperative BCVA was 0.68±0.41 logMAR in the current series; it was 1.15 logMAR as according to Travi[10] and 0.4 logMAR according to Crouch and Parks[25]. However, previous studies on treatments for amblyopia have suggested that an initially poor BCVA often predicts poor visual outcomes[15]. Our study suggested that children with poor BCVA had a greater likelihood of visual improvements than did those with good pretreatment BCVA. Preoperatively, poor visual acuity may be associated with the amblyogenic characteristics of a PPC and long posterior lenticonus and the visual improvements may arise from a cleaning of the visual axis and immediately provision of optic correction without encountering an irregular shape caused by a lens malformation.
There are several limitations to our study. Due to the issues relating to retrospective collection and analysis of data, several measures of patient outcome were either not collected or not available for inclusion in this study. These include reading rate, accuracy, fluency, and comprehension, which have been shown to be critical measures of the potential utility of the affected eye. In addition, we did not randomize the patients in our study and our results may have been influenced by this selection bias. The propensity score methodology was used to help adjust for treatment selection bias. We were, however, able to determine which patients would benefit from surgery based on the clinical parameters at the initial presentation and precise measurements of the lens morphology using the newly developed Pentacam instrument.
In conclusion, the best treatment plan for patients with a small posterior lenticonus and PPC are controversial, and amblyopia is often associated with children with cataracts. After subject matching by propensity, we observed patients in two groups both achieved better BCVA after receiving amplyopia treatment. In the surgical group, patient with a BCVA of 0.52 logMAR or worse and an RPL greater less than 1.0 mm showed to have a greater BCVA improvement. The Pantacam device may be a useful tool for determining the RPL of the lesion, but this clinically important hypothesis should be tested in a randomized prospective trial.
Acknowledgments
Foundations: Supported by the National Natural Science Foundation of China (No.81970813; No.81770967); the National Key R&D Program of China (No.2018YFC0116500); the Natural Science Foundation of Guangdong Province, China (No.2018A030313635), Science and Technology Program of Guangzhou, China (No.201904010062).
Conflicts of Interest: Chen H, None; Chen W, None; Wu XH, None; Lin ZL, None; Chen JJ, None; Li XY, None; Chen WR, None; Lin HT, None.
REFERENCES
- 1.Howitt D, Hornblass A. Posterior lenticonus. Am J Ophthalmol. 1968;66(6):1133–1136. doi: 10.1016/0002-9394(68)90823-4. [DOI] [PubMed] [Google Scholar]
- 2.Khalil M, Saheb N. Posterior lenticonus. Ophthalmology. 1984;91(11):1429–1430,43A. doi: 10.1016/s0161-6420(84)34132-x. [DOI] [PubMed] [Google Scholar]
- 3.Foster GJL, Ayers B, Fram N, Hoffman RS, Khandewal S, Ogawa G, MacDonald SM, Snyder ME, Vasavada A. Phacoemulsification of posterior polar cataracts. J Cataract Refract Surg. 2019;45(2):228–235. doi: 10.1016/j.jcrs.2018.09.029. [DOI] [PubMed] [Google Scholar]
- 4.Pujari A, Yadav S, Sharma N, Khokhar S, Sinha R, Agarwal T, Titiyal JS, Sharma P. Study 1: Evaluation of the signs of deficient posterior capsule in posterior polar cataracts using anterior segment optical coherence tomography. J Cataract Refract Surg. 2020;46(9):1260–1265. doi: 10.1097/j.jcrs.0000000000000246. [DOI] [PubMed] [Google Scholar]
- 5.Parks MM. Visual results in aphakic children. Am J Ophthalmol. 1982;94(4):441–449. doi: 10.1016/0002-9394(82)90237-9. [DOI] [PubMed] [Google Scholar]
- 6.Vasavada AR, Vasavada VA. Managing the posterior polar cataract: an update. Indian J Ophthalmol. 2017;65(12):1350–1358. doi: 10.4103/ijo.IJO_707_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng KP, Hiles DA, Biglan AW, Pettapiece MC. Management of posterior lenticonus. J Pediatr Ophthalmol Strabismus. 1991;28(3):143–149. discussion 150. doi: 10.3928/0191-3913-19910501-07. [DOI] [PubMed] [Google Scholar]
- 8.Kushner BJ. Functional amblyopia associated with organic ocular disease. Am J Ophthalmol. 1981;91(1):39–45. doi: 10.1016/0002-9394(81)90346-9. [DOI] [PubMed] [Google Scholar]
- 9.Bradford GM, Kutschke PJ, Scott WE. Results of amblyopia therapy in eyes with unilateral structural abnormalities. Ophthalmology. 1992;99(10):1616–1621. doi: 10.1016/s0161-6420(92)31758-0. [DOI] [PubMed] [Google Scholar]
- 10.Travi GM, Schnall BM, Lehman SS, Kelly CJ, Hug D, Hirakata VN, Calhoun JH. Visual outcome and success of amblyopia treatment in unilateral small posterior lens opacities and lenticonus initially treated nonsurgically. J Am Assoc Pediatr Ophthalmol Strabismus. 2005;9(5):449–454. doi: 10.1016/j.jaapos.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 11.de Jong B, van der Meulen IJ, Lapid-Gortzak R, van den Berg TJ. Straylight in posterior polar cataract. J Cataract Refract Surg. 2019;45(1):72–75. doi: 10.1016/j.jcrs.2018.08.035. [DOI] [PubMed] [Google Scholar]
- 12.Kirkwood BJ, Hendicott PL, Read SA, Pesudovs K. Repeatability and validity of lens densitometry measured with Scheimpflug imaging. J Cataract Refract Surg. 2009;35(7):1210–1215. doi: 10.1016/j.jcrs.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 13.Bayrak G, Özdamar Erol Y, Kazanci B. An objective evaluation of crystalline lens density using Scheimpflug lens densitometry in different uveitis entities. Int Ophthalmol. 2020;40(8):2031–2040. doi: 10.1007/s10792-020-01379-4. [DOI] [PubMed] [Google Scholar]
- 14.Wallace DK, Repka MX, Lee KA, Melia M, Christiansen SP, Morse CL, Sprunger DT, American Academy of Pediatric Ophthalmology/Strabismus Preferred Practice Pattern Pediatric Ophthalmology Panel. Amblyopia preferred practice pattern®. Ophthalmology. 2018;125(1):105–142. [Google Scholar]
- 15.Repka MX, Kraker RT, Holmes JM, Summers AI, Glaser SR, Barnhardt CN, Tien DR, Pediatric Eye Disease Investigator Group Atropine vs patching for treatment of moderate amblyopia: follow-up at 15 years of age of a randomized clinical trial. JAMA Ophthalmol. 2014;132(7):799–805. doi: 10.1001/jamaophthalmol.2014.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levi DM. Rethinking amblyopia 2020. Vision Res. 2020;176:118–129. doi: 10.1016/j.visres.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown SM, Archer S, Del Monte MA. Stereopsis and binocular vision after surgery for unilateral infantile cataract. J AAPOS. 1999;3(2):109–113. doi: 10.1016/s1091-8531(99)70080-7. [DOI] [PubMed] [Google Scholar]
- 18.Jeffrey BG, Birch EE, Stager DR, Jr, Stager DR, Sr, Weakley DR., Jr Early binocular visual experience may improve binocular sensory outcomes in children after surgery for congenital unilateral cataract. J AAPOS. 2001;5(4):209–216. doi: 10.1067/mpa.2001.115591. [DOI] [PubMed] [Google Scholar]
- 19.Lloyd IC, Dowler JG, Kriss A, Speedwell L, Thompson DA, Russell-Eggitt I, Taylor D. Modulation of amblyopia therapy following early surgery for unilateral congenital cataracts. Br J Ophthalmol. 1995;79(9):802–806. doi: 10.1136/bjo.79.9.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hosal BM, Biglan AW, Elhan AH. High levels of binocular function are achievable after removal of monocular cataracts in children before 8 years of age. Ophthalmology. 2000;107(9):1647–1655. doi: 10.1016/s0161-6420(00)00226-8. [DOI] [PubMed] [Google Scholar]
- 21.Lee BJ, Kim JH, Yu YS. Surgical outcomes after intraocular lens implantation for posterior lenticonus-related cataract according to preoperative lens status. J Cataract Refract Surg. 2014;40(2):217–223. doi: 10.1016/j.jcrs.2013.07.048. [DOI] [PubMed] [Google Scholar]
- 22.Schroeder HW. The management of posterior polar cataract: the role of patching and grading. Strabismus. 2005;13(4):153–156. doi: 10.1080/09273970500387753. [DOI] [PubMed] [Google Scholar]
- 23.Ganesh S, Brar S, Chopra K. Jellyfish sign for intraoperative identification of posterior lenticonus. Int Ophthalmol. 2017;37(5):1239–1241. doi: 10.1007/s10792-016-0386-1. [DOI] [PubMed] [Google Scholar]
- 24.Amaya L, Taylor D, Russell-Eggitt I, Nischal KK, Lengyel D. The morphology and natural history of childhood cataracts. Surv Ophthalmol. 2003;48(2):125–144. doi: 10.1016/s0039-6257(02)00462-9. [DOI] [PubMed] [Google Scholar]
- 25.Crouch ER, Jr, Parks MM. Management of posterior lenticonus complicated by unilateral cataract. Am J Ophthalmol. 1978;85(4):503–508. doi: 10.1016/s0002-9394(14)75248-1. [DOI] [PubMed] [Google Scholar]