Abstract
AIM
To explore the significance of corneal epithelial thickness analysis in diagnosing early keratoconus.
METHODS
There were 26 clinical keratoconus, 21 forme fruste keratoconus, 40 high corneal astigmatism (ΔK) and 40 low ΔK eyes involved in the study. Fourier-domain optical coherence tomography was used to measure the corneal epithelial thickness of four groups. The morphological features of topographic map and the thickness of corneal epithelial thinnest point were analyzed. The distribution curve of corneal epithelial thickness at 45°, 90°, and 135° axial directions that are through the pupil center was also analyzed. One-way ANOVA was performed to compare the data.
RESULTS
The topographic map of forme fruste keratoconus corneal epithelial thickness was uniformity shape; crater shape existed only in clinical keratoconus group; and central island shape mainly existed in high ΔK group. The thinnest point of corneal epithelial thickness of forme fruste keratoconus group was significantly lower than that of low ΔK group (P=0.022). The thickness of corneal epithelium in the forme fruste keratoconus at 90° was thinner than that in the low astigmatism group at -1, and -2 mm points (P-1 mm=0.015, P-2 mm=0.036).
CONCLUSION
The analysis of the thinnest point in forme fruste keratoconus corneal epithelium appears earlier than corneal epithelial remodeling. The topographic map of corneal epithelium in high ΔK eyes appears in central island shape, and can be used for the differential diagnosis of early keratoconus.
Keywords: forme fruste keratoconus, corneal epithelial thickness, optical coherence tomography, corneal astigmatism
INTRODUCTION
Keratoconus is a bilateral ectatic corneal disease and is more in the incidence in adolescence[1]. It manifests as progressive loss of vision and causes visual impairment, seriously affect patients' life and work. About 20% of patients may require corneal transplantation ultimately[2]. If keratoconus can be diagnosed early, patients will have the opportunity to undergo treatments, like corneal cross linking, which can reduce the need for future corneal transplantation[3]–[4]. Although the equipment and technology of keratorefractive surgery have been continuously improving in the past 30 years, corneal ectasia after refractive surgery is still an inevitable complication[5]–[7]. Post-refractive surgery corneal ectasia might be related to surgical stimulation that causes the “potential” keratoconus to enter the active stage[8]–[9]. Corneal topography is a reliable detection technique for clinical keratoconus[10]–[11]. There are many techniques can be used for the early detection of keratoconus. Scheimpflug camera can measure posterior corneal elevation for discriminating subclinical keratoconus[12]. Ocular Response Analyzer can obtain corneal biomechanical waveform parameters, which changes prior to corneal topography[13]–[14]. Since keratoconus is a congenital disease, next-generation sequencing can also be used for the early diagnosis of keratoconus[15]–[17]. VSX1[18], DOCK9[19], SOD1[18] and TGFBI[19] had been proven to be related to keratoconus. But for all this, misdiagnosis of keratoconus is not uncommon[1].
Forme fruste keratoconus is topographically normal-appearing eye of patients with keratoconus in the fellow eyes[20]. Corneal hysteresis and corneal resistance factors in forme fruste keratoconus eyes were lower than that of normal eyes[14],[21]. During the long-term follow-up period, about 20% of eyes with forme fruste keratoconus have progressed to clinical keratoconus within 6y[22]. Therefore, biological study of forme fruste keratoconus provides reference for the early diagnosis of keratoconus.
Corneal epithelial remodeling refers to the ability of corneal epithelium to repair irregular anterior corneal surface by compensatory thickening in a certain range when the corneal stroma changes[23]. During the early stages of keratoconus, corneal epithelial remodeling can mask the irregular changes of the stroma, resulting in no differences in the detection of topographic maps of the anterior surface[24]. Currently, the most commonly used clinical methods to measure corneal epithelial thickness included optical coherence tomography (OCT)[25], very-high frequency ultrasound[26]–[27], confocal microscopy[28] and so on. The Fourier-domain OCT (FD-OCT) has excellent repeatability and accuracy[29], avoiding the measurement error caused by corneal edema without anesthesia. Therefore, OCT might be considered as one of the most valuable techniques in the diagnosis of early keratoconus[1]. Hence, in this study, the thickness of corneal epithelium in the range of 9 mm diameter centered pupil center in forme fruste keratoconus, clinical keratoconus and corneal astigmatism (ΔK) eyes was analyzed by FD-OCT.
SUBJECT AND METHODS
Ethical Approval
This retrospective case-control study included the data collected before and was of low risk, and so the need for consent was waived by the Medical Ethics Committee of the First Affiliated Hospital of Soochow University [(2019) Ethical approval No.176]. This study was conducted in accordance with the ethical standards of the institutional and/or national research committee and the principles of Helsinki declaration.
Subjects
The study included 47 keratoconic patients (37 males, and 10 females) who were newly diagnosed in the Ophthalmology Department of the First Affiliated Hospital of Soochow University from January 2015 to December 2019 and 80 astigmatic patients (44 males, 36 females) who underwent treatment in the same period as controls. All patients had no rigid contact lens wear and did not wear soft contact lens during the previous 2wk. All patients do not have any history of corneal refractive surgery or corneal cross-linking surgery.
Diagnostic Criteria
The diagnostic criteria of clinical keratoconus group (A group) were as follows[30]: the patients' both eyes included a typical corneal topographical change (central or paracentral conical ectasia); and the slit lamp examination should include at least one of the following clinical signs: 1) Munson sign; 2) Fleischer ring; 3) Vogt striae; and 4) corneal conical ectasia or corneal stromal thinning. The diagnostic criteria of forme fruste keratoconus group (B group) were as follows: patients with no clinical signs of keratoconus with slit lamp microscope and no abnormalities were found by using corneal topography. The contralateral eye presented a typical corneal topography of keratoconus and clinical signs.
Inclusion criteria for high ΔK group (C group) were as follows: 1) both slit lamp examination and corneal topographic examination showed no corneal abnormalities; and 2) ΔK (steep K-flat K) of >2.0 D as examined by corneal topography. Inclusion criteria for low ΔK group (D group) were as follows: 1) both slit lamp examination and corneal topographic examination showed no corneal abnormalities; and 2) ΔK ≤2.0 D as examined by corneal topography.
Except for forme fruste keratoconic patients, all other patients were enrolled with the right eye.
Corneal Topography
The topographical maps of the corneal anterior surfaces were examined by TMS-4 corneal topographer (Tomy, Japan) and the examinations were performed by the same technician. The eye check has been done thrice, choosing the best pattern (X, Y, Z axes deviation is less than 0.3 mm) to obtain flat keratometry (Kf), steep keratometry (Ks), average keratometry (AveK), ΔK, corneal surface regularity index (SRI), and corneal surface asymmetry index (SAI).
Corneal Optical Coherence Tomography
The corneal epithelial thickness was measured by an FD-OCT system RTVue-100 (Optovue Inc., Fremont, CA, USA). The examination was carried out in a semi-dark environment, and no drugs that affect the tear dynamics were used 2h before the examination. This examination was done by the same technician. Scanning with PachymetryWide mode was done by instructing the patient to fix on the light spot, and obtaining data of corneal epithelial thickness within a circular area of a diameter of 9 mm centered on the pupil center. The results were expressed in a topographical map.
Topographic Map of Corneal Epithelial Thickness
The topographic map of corneal epithelial thickness was divided into 25 zones: a 2.0 mm diameter circle central zone centered on the pupil center, and 24 sector-shaped ring zones that are equally divided by 4 axial directions from 2.0 to 5.0 mm, 5.0 to 7.0 mm, and 7.0 to 9.0 mm annular areas. The average corneal epithelial thickness was read from the 25 zones per eye.
Morphology classification of corneal epithelial thickness topography (Figure 1): 1) uniformity shape: no obvious local thickening or thinning of corneal epithelium was observed, and the color was uniform; 2) crater shape: the corneal epithelium was thickened in the peripheral part centered on the thinnest point of the corneal epithelium. The crater shape was subdivided into: a) c font shape: the corneal epithelium is thickened in c-shape and centered on the thinnest point of the corneal epithelium; b) bracket shape: the corneal epithelium is thickened on both sides of the thinnest point of the corneal epithelium; and c) ring shape: the corneal epithelium is annularly thickened around the thinnest point of corneal epithelium; 3) irregular shape: the corneal epithelium is uneven in thickness and is colored in a disordered manner; 4) central island shape: the corneal epithelium is obviously thickened in the central region of the cornea.
Figure 1. Morphological classification of corneal epithelial thickness topography.
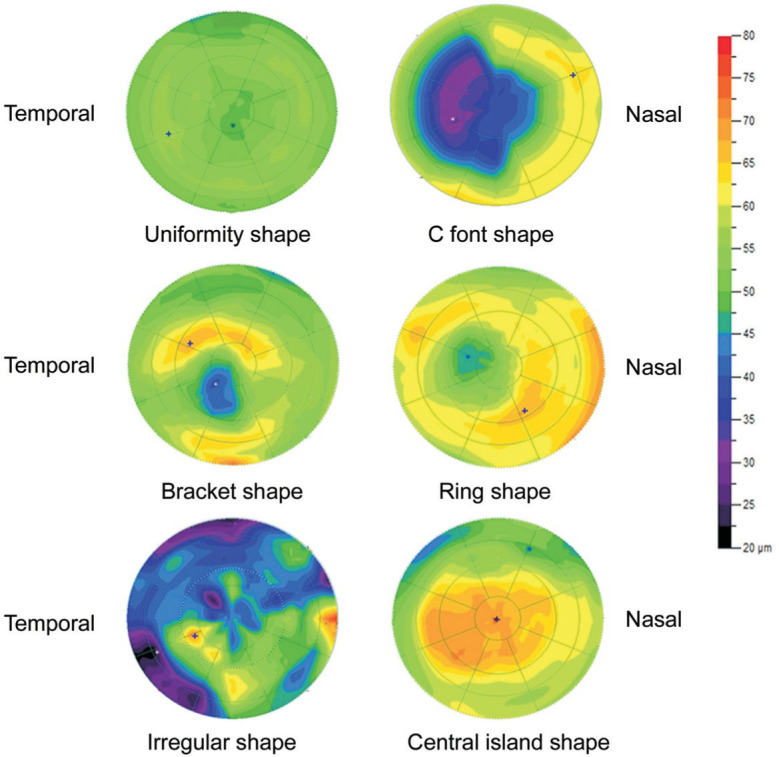
The cool-tone portion (cyan) indicated a thinner portion, and the warm-tone portion (orange-red) indicated a thicker portion.
Comparison of the Thickness of the Thinnest Point of the Corneal Epithelium
The FD-OCT system was used to obtain the thickness of the thinnest point of the corneal epithelium.
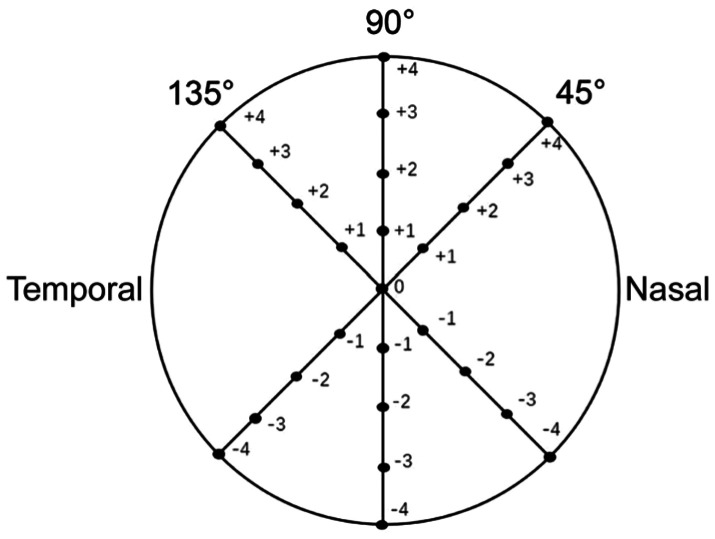
Distribution Curve of Corneal Epithelial Thickness in 45°, 90°, and 135° Axes
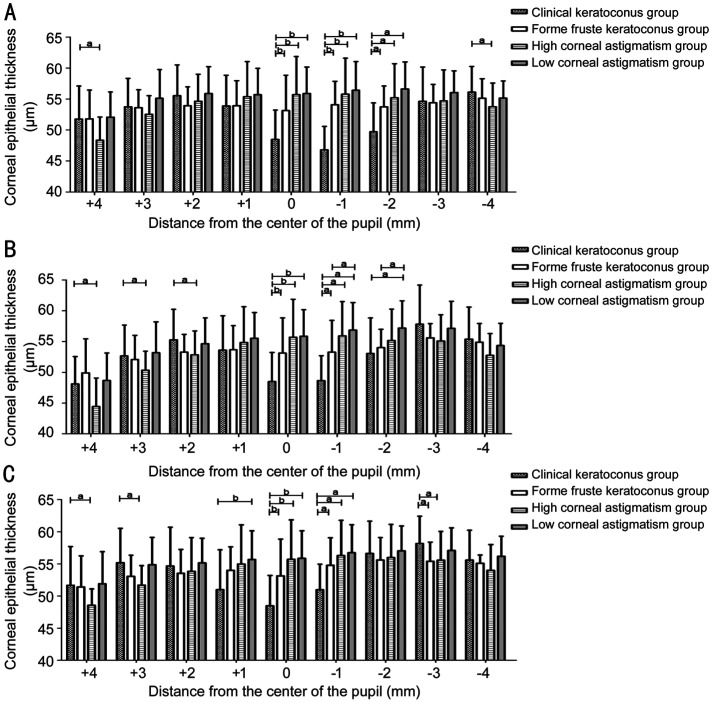
The 45°, 90°, and 135° axial direction measurement lines through the pupil center are taken to read the thickness of corneal epithelium at 1 mm intervals. The corneal epithelial thickness distribution curve of +4, +3, +2, +1, -1, -2, -3, and -4 mm data points at 45°, 90°, 135° axes were obtained from the clinical keratoconus group, forme fruste keratoconus group, high ΔK group, and low ΔK group, respectively (Figure 2). The differences of corneal epithelial thickness at the same data point from the three axes in the clinical keratoconus group, the forme fruste keratoconus group, the high ΔK group and the low ΔK group were compared and then analyzed.
Figure 2. Schematic representation of 45°, 90°, and 135° axial data points (unit: millimeter).
Statistical Analysis
Data analysis was performed with SPSS 17.0 statistical software (version 17.0, Chicago, Illinois, USA). One-way ANOVA was performed to compare the thinnest point in the corneal epithelial thickness of the four groups. If there is a statistically significant difference in one-way ANOVA, further LSD test was performed (P<0.05). For comparison of the thickness of corneal epithelium at the same data point in each axis in each group, the homogeneity test of variance was first performed. If the variance is uniform, then one-way ANOVA was used. If there was any significant difference, further LSD test was performed (statistically significant difference at P<0.05); and if the variance was not uniform, then Kruskal-Wallis test was done. Furthermore, Bonferroni correction was performed if there was a statistically significant difference (adjust test level α'=α/6=0.008).
According to the sample size estimation formula of completely random design one-way ANOVA, we set α=0.05 (two-side), β=0.10 (one-side). The mean of the thinnest point of thickness of corneal epithelium of the four groups was 42, 49, 48, and 52 µm, and standard deviation σ=5 µm. The final calculated sample size was 9 cases in each group, 36 cases in total. Under such sample size, the statistical power was about 90%. In our article, the sample size of the four groups is 26, 21, 40 and 40 cases. There is sufficient statistical power to detect the difference between the groups.
RESULTS
Patient Data
A total of 47 keratoconic patients involved in the study, 26 of them were clinical keratoconus and the other were forme fruste keratoconus patients. Forty high ΔK subjects and 40 low ΔK subjects were also involved in the study (Table 1).
Table 1. The general data of the subjects.
| Parameters | A group (clinical keratoconus group) | B group (forme fruste keratoconus group) | C group (high ΔK group) | D group (low ΔK group) |
| Eyes | 26 | 21 | 40 | 40 |
| Male:female | 20:6 | 17:4 | 24:16 | 20:20 |
| Mean age | 23.81±7.16 | 22.33±7.39 | 20.15±6.34 | 23.13±3.99 |
ΔK: Corneal astigmatism.
Compared with other groups, clinical keratoconus group showed significantly higher Kf, Ks, AveK, ΔK, SRI and SAI (all P=0.000). Compared with high ΔK group, forme fruste keratoconus group demonstrated significantly lower Ks, ΔK, and SRI (PKs=0.004, PΔK=0.000, PSRI=0.005). No statistically significant differences were found between high ΔK group and forme fruste keratoconus group in Kf, AveK, and SAI. Compared with low ΔK group, there was no statistically significant difference between forme fruste keratoconus group and low ΔK group in Kf, Ks, AveK, ΔK, SRI and SAI (Table 2).
Table 2. Comparison of corneal topography parameters of each group.
| Parameters | Clinical keratoconus group (n=26) | Forme fruste keratoconus group (n=21) | High ΔK group (n=40) | Low ΔK group (n=40) |
| Kf (D) | 45.37±3.54 | 42.89±1.28a | 43.02±1.37a | 42.38±0.99a |
| Ks (D) | 50.90±5.12 | 44.05±1.20a | 46.63±1.32a,b | 43.55±0.95a |
| AveK (D) | 48.11±4.06 | 43.68±1.34a | 44.79±1.22a | 42.97±0.94a |
| ΔK (D) | 5.31±3.11 | 1.14±0.62a | 3.66±0.96a,b | 1.17±0.44a |
| SRI | 0.94±0.56 | 0.15±0.13a | 0.45±0.26a,b | 0.12±0.13a |
| SAI | 2.42±1.76 | 0.48±0.39a | 0.42±0.19a | 0.28±0.11a |
Kf: Flat keratometry; Ks: Steep keratometry; AveK: Average keratometry; ΔK: Corneal astigmatism; SRI: Corneal surface regularity index; SAI: Corneal surface asymmetry index; D: Diopters. aP<0.05 vs clinical keratoconus group; bP<0.05 vs forme fruste keratoconus group.
Corneal Epithelial Thickness Analysis
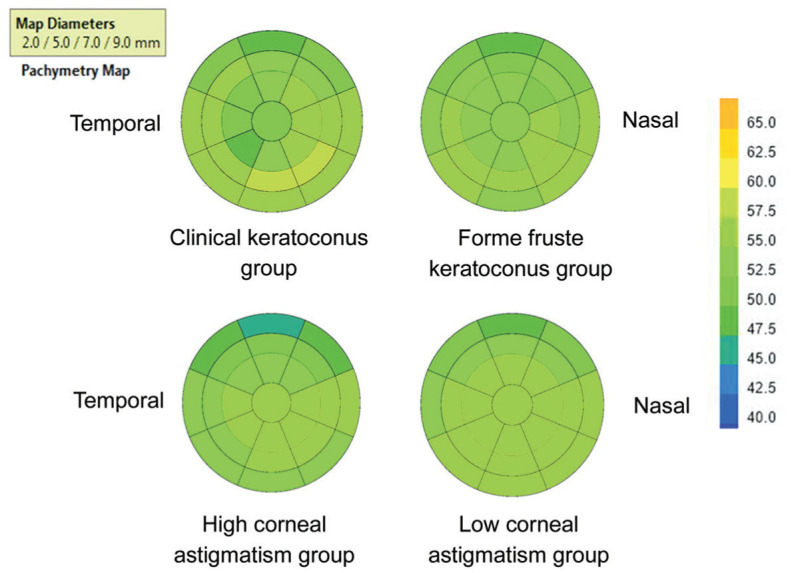
The overall thickness of forme fruste keratoconus group was thicker than that of clinical keratoconus group. Comparison of high ΔK group with low ΔK group showed that the forme fruste keratoconus group has thinner thickness and more uneven distribution (Figure 3).
Figure 3. Topographic map of corneal epithelial average thickness of each group with a diameter of 9 mm.
Morphology Classification of Corneal Epithelial Thickness Topography
1) Clinical keratoconus group: 25 eyes were of crater shape (96.15%), and 1 eye was of irregular shaped (3.85%). The crater shape was subdivided into: c font shape: 10 eyes (40%), bracket shape: 10 eyes (40%), and ring shape: 5 eyes (20%). 2) Forme fruste keratoconus group: 21 eyes were of uniformity shape (100%). 3) High ΔK group: 28 eyes were uniformity shape (70%), 12 eyes were of central island shape (30%). 4) Low ΔK group: 37 eyes were uniformity shape (92.5%), and 3 eyes were central island shape (7.5%).
The Thinnest Point Thickness of Corneal Epithelium
The thinnest point in the corneal epithelial thickness was an average of 42.81±5.63 µm in the clinical keratoconus group, 49.33±3.33 µm in the forme fruste keratoconus group, 48.78±3.35 µm in the high ΔK group and 52.37±3.98 µm in the low ΔK group. There were statistically significant differences between the groups (F=32.012, P=0.000). The thinnest point in the corneal epithelial thickness of clinical keratoconus group was significantly lower than that of the other groups (all P=0.000). The thinnest point in the corneal epithelial thickness of forme fruste keratoconus group was significantly lower than that of low ΔK group (P=0.022). There was no statistically significant difference between forme fruste keratoconus group and high ΔK group (P=0.668).
Comparison of Corneal Epithelial Thickness of Each Group at 45°, 90°, and 135° Axes
The corneal epithelial thickness of clinical keratoconus group at 0 mm point was significantly thinner than that of other groups (P<0.008).
Comparison of corneal epithelial thickness of the four groups at +4, +3, +2, +1, -1, -2, -3, and -4 mm points of three axes were as follows: 1) 45° axis: The corneal epithelial thickness of clinical keratoconus group was significantly thicker than that of high ΔK group at +4, and -4 mm points (P+4 mm=0.015, P-4 mm=0.042), and were significantly thinner than that of the other groups at -1, and -2 mm points (P-1 mm<0.008, P-2 mm<0.05). 2) 90° axis: The corneal epithelial thickness of clinical keratoconus group was significantly thicker than that of high ΔK group at +4, +3, and +2 mm points (P+4 mm=0.014, P+3 mm=0.022, P+2 mm= 0.010), and significantly thinner than that of other groups at -1 mm point (P<0.05), and thinner than that of low ΔK group at -2 mm point (P=0.000). The corneal epithelial thickness of forme fruste keratoconus group was significantly thinner than that of low ΔK group at -1, and -2 mm points (P-1 mm=0.015, P-2 mm=0.036). 3) 135° axis: The corneal epithelial thickness of clinical keratoconus group was significantly thicker than that of high ΔK group at +4, +3, and -3 mm points (P+4 mm=0.031, P+3 mm=0.000, P-3 mm=0.005), and was significantly thinner than that of low ΔK group at +1 mm point (P=0.000), and significantly thinner than that of other groups at -1 mm point (P<0.05), and significantly thicker than that of forme fruste group at -3 mm point (P=0.024). No statistically significant differences were observed between groups at other points (Figure 4).
Figure 4. Bar chart of corneal epithelial thickness of each group in three axes.
A: 45°; B: 90°; C: 135° axes. aP<0.05, bP<0.008.
DISCUSSION
Clinical keratoconus diagnosis mainly relies on the morphology of corneal anterior surface topography and typical slit-lamp findings, such as Fleischer ring, Vogt striae and corneal scar[1]. As the subclinical keratoconus stromal changes can be compensated by corneal epithelial remodeling to a certain extent[31], patients often do not have any typical clinical signs, and the changes in the anterior corneal surface can be masked[24]. In this study, there were no statistically significant differences in the parameters of the anterior surface topography between the forme fruste keratoconus group and the low ΔK group. They are even smaller than the high ΔK group with regards to the parameters of Ks, ΔK, and SRI. Therefore, anterior corneal topography is not considered sensitive to the diagnosis of early keratoconus, and may even lead to misdiagnosis of early keratoconus.
Li et al[32] evaluated the corneal epithelial pattern map in 6 mm diameter range found that epithelial thinning inferiorly in forme fruste keratoconus. Reinstein et al[24] found that the corneal epithelial thickness topography showed a similar epithelial doughnut pattern. On this basis, 21 cases of corneal epithelial thickness topography in forme fruste keratoconus in 9.0 mm diameter range were evaluated, and similar findings were observed in our study. The “crater shape” corneal epithelial thickness topography was observed only in the clinical keratoconus group, but no similar changes were found in the forme fruste keratoconus group and the ΔK group in our sthdy. The “crater shape” corneal epithelial thickness topography can be further subdivided into the c font shape, the bracket shape and the ring shape. Corneal epithelial remodeling is an important mechanism for the formation of the “crater shape” corneal epithelial thickness topography, and its initiating factors are controversial. One hypothesis is that epithelium thickening is to compensate for the thinning of the corneal stroma and is related to the rate of corneal stromal curvature change[33]. The epithelial thinning to compensate for relative peaks in stromal and thickening to compensate for relative troughs in stromal. The cornea surface tends to form a smooth shape that maintains its optical properties. This can explain the formation of the “crater shape” corneal epithelial thickness topography. Another hypothesis is that biomechanically unstable cornea drived epithelial remodeling, which was related to intraocular pressure variations, eye rubbing and blinking mechanism[34]. The cornea local biomechanical properties reduction results in tissue thinning as the weaker area strains[35]. Forme fruste keratoconus is usually considered as the mildest and the earliest form of keratoconus[36]. This study showed that all corneal epithelial thickness topographies of forme fruste keratoconus group were uniformity shape, and so the forme fruste keratoconus did not undergo corneal epithelial remodeling.
Corneal epithelial thickness topography in astigmatism group can be divided into uniformity shape and central island shape. The proportion of central island shape in high ΔK group is much higher than that in the low ΔK group. A study showed that the rate of corneal stromal curvature change drived epithelial remodeling[33]. Therefore, it the classification of corneal epithelial thickness topography was related to the rate of corneal stromal curvature change. In clinical keratoconus, different cone sizes lead to different rates of curvature changes, resulting in different types of epithelial thickening. The higher the ΔK is, the greater the rate of curvature change is. Therefore, the corneal epithelial thickness topography of high ΔK group was more of central island shape. A previous study has shown that epithelial irregularity should be considered as a possible contributing factor to astigmatism. Therefore, corneal epithelial thickness topography can be used to differentiate early keratoconus from high astigmatism[37].
The thinnest point of corneal epithelial thickness in the clinical keratoconus group was lower than that in the other groups, which was consistent with the clinical manifestations of keratoconus. The thinnest point thickness of corneal epithelium of forme fruste keratoconus group was significantly lower than that of the low ΔK group, but showed no statistical differences from that of the high ΔK group. Blinking action and force may be another mechanism of corneal epithelial remodeling[38]. Considering that the high ΔK patients had higher frequency of half-shutting eyes than the low ΔK patients, the eyelid force led to corneal epithelial remodeling, which was then manifested by the thinning in the upper corneal epithelium and thickening in the central corneal epithelium. So, more central island shape appeared in the high ΔK group. Under the circumstances, the corneal epithelial thinnest point of high ΔK group appears in upper corneal epithelium. In this study, central island shape accounted for 30% in the high ΔK group. This may be the cause of low thickness of the thinnest point of corneal epithelium in high ΔK group. Therefore, the thinnest point thickness of corneal epithelium alone is not enough to distinguish forme fruste keratoconus eye from high ΔK eye.
The thickness of corneal epithelium in the clinical keratoconus group and other groups was mainly manifested in the thinning of a 2.0 mm diameter circle central zone centered on the pupil center, especially in the temporal inferior area. This phenomenon in turn is associated with corneal epithelial thinning at the top of the cone. The epithelial thickness of clinical keratoconus group was significantly thicker than that of forme fruste keratoconus group at -3 mm point at 135° axial direction, and thicker than that of high ΔK group at -3 mm point at 135° axial direction. The “crater shape” thickening of keratoconus corneal epithelium is most prominent in the nasal inferior area. The epithelial thickness of forme fruste keratoconus group was significantly thinner than that of low ΔK group at -1, -2 mm points at 90° axial direction. This may be related to the thinning of corneal epithelial thinnest point, as it appears earlier than the corneal epithelial remodeling. The epithelial thickness of high ΔK group was significantly thinner than that of clinical keratoconus group at +4, -4 mm points at 45° axial direction and +4, +3, +2 mm points at 90° axial direction and +4, +3 mm points at 135° axial direction. This was consistent with the thinning of the peripheral corneal epithelium in the high ΔK group.
The phenomenon of epithelial remodeling, thinnest point thinning, thinnest point position change[38] existed in the keratoconus. Combined with our study, the corneal epithelial thickness topography of the forme fruste keratoconus group showed no changes, but the thinnest point thickness was significantly lower than that of the low ΔK group. This inferred that the phenomenon of the thinnest point thinning appears earlier than the corneal epithelial remodeling in the pathogenesis of keratoconus. Therefore, the phenomenon that the thinnest point thickness of corneal epithelium was lower than that of the normal remained significant in the diagnosis of early keratoconus.
In summary, the clinical keratoconus corneal epithelial thickening was unique and typical crater shape, and the thickening was obvious in the nasal inferior area. The phenomenon of the thinnest point thinning in forme fruste keratoconus corneal epithelium appeared earlier than the corneal epithelial remodeling. Central island shape of corneal epithelial topography can be found in patients with high ΔK, which subsequently can be used to differentiate early keratoconus from high astigmatism.
Acknowledgments
Authors' contributions: Zhang XF conceived and designed the study; Zhang XF, Yang XL, Wang Y, Luo BG and Xu Y were involved in data collection; Yang XL and Wang Y did statistical analyzed; Zhang XF, Yang XL and Wang Y interpreted of the data; Zhang XF and Yang XL drafted the manuscript; Zhang XF, Yang XL, Wang Y, Luo BG and Xu Y reviewed and approved the manuscript.
Conflicts of Interest: Yang XL, None; Wang Y, None; Luo BG, None; Xu Y, None; Zhang XF, None.
REFERENCES
- 1.Gomes JAP, Tan D, Rapuano CJ, Belin MW, Ambrósio R, Jr, Guell JL, Malecaze F, Nishida K, Sangwan VS. Global consensus on keratoconus and ectatic diseases. Cornea. 2015;34(4):359–369. doi: 10.1097/ICO.0000000000000408. [DOI] [PubMed] [Google Scholar]
- 2.Tuft SJ, Moodaley LC, Gregory WM, Davison CR, Buckley RJ. Prognostic factors for the progression of keratoconus. Ophthalmology. 1994;101(3):439–447. doi: 10.1016/s0161-6420(94)31313-3. [DOI] [PubMed] [Google Scholar]
- 3.Godefrooij DA, Gans R, Imhof SM, Wisse RPL. Nationwide reduction in the number of corneal transplantations for keratoconus following the implementation of cross-linking. Acta Ophthalmol. 2016;94(7):675–678. doi: 10.1111/aos.13095. [DOI] [PubMed] [Google Scholar]
- 4.Nicula C, Pop R, Rednik A, Nicula D. 10-year results of standard cross-linking in patients with progressive keratoconus in Romania. J Ophthalmol. 2019;2019:1–5. doi: 10.1155/2019/8285649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jarade EF, Chelala E, Arej N, El Rami H. Inducing fibrogenesis and new interfibrillary bonds in post-laser in situ keratomileusis keratectasia. J Cataract Refract Surg. 2018;44(9):1062–1065. doi: 10.1016/j.jcrs.2018.05.028. [DOI] [PubMed] [Google Scholar]
- 6.Ueki R, Maeda N, Fuchihata M, Asai T, Koh S, Fujimoto H, Uematsu M, Nishida K. Evaluation of corneal biomechanics in patients with keratectasia following LASIK using dynamic Scheimpflug analyzer. Jpn J Ophthalmol. 2018;62(4):443–450. doi: 10.1007/s10384-018-0594-5. [DOI] [PubMed] [Google Scholar]
- 7.Bohac M, Koncarevic M, Pasalic A, Biscevic A, Merlak M, Gabric N, Patel S. Incidence and clinical characteristics of post LASIK ectasia: a review of over 30, 000 LASIK cases. Semin Ophthalmol. 2018;33(7-8):869–877. doi: 10.1080/08820538.2018.1539183. [DOI] [PubMed] [Google Scholar]
- 8.Saad A, Binder PS, Gatinel D. Evaluation of the percentage tissue altered as a risk factor for developing post-laser in situ keratomileusis ectasia. J Cataract Refract Surg. 2017;43(7):946–951. doi: 10.1016/j.jcrs.2017.04.040. [DOI] [PubMed] [Google Scholar]
- 9.Santhiago MR, Giacomin NT, Smadja D, Bechara SJ. Ectasia risk factors in refractive surgery. Clin Ophthalmol. 2016;10:713–720. doi: 10.2147/OPTH.S51313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klyce SD. Computer-assisted corneal topography. High-resolution graphic presentation and analysis of keratoscopy. Invest Ophthalmol Vis Sci. 1984;25(12):1426–1435. [PubMed] [Google Scholar]
- 11.Rabinowitz YS, Garbus J, McDonnell PJ. Computer-assisted corneal topography in family members of patients with keratoconus. Arch Ophthalmol. 1990;108(3):365–371. doi: 10.1001/archopht.1990.01070050063032. [DOI] [PubMed] [Google Scholar]
- 12.Consejo A, Glawdecka K, Karnowski K, Solarski J, Rozema JJ, Wojtkowski M, Iskander DR. Corneal properties of keratoconus based on scheimpflug light intensity distribution. Invest Ophthalmol Vis Sci. 2019;60(8):3197–3203. doi: 10.1167/iovs.19-26963. [DOI] [PubMed] [Google Scholar]
- 13.Zarei-Ghanavati S, Ramirez-Miranda A, Yu F, Hamilton DR. Corneal deformation signal waveform analysis in keratoconic versus post-femtosecond laser in situ keratomileusis eyes after statistical correction for potentially confounding factors. J Cataract Refract Surg. 2012;38(4):607–614. doi: 10.1016/j.jcrs.2011.11.033. [DOI] [PubMed] [Google Scholar]
- 14.Luz A, Lopes B, Hallahan KM, Valbon B, Fontes B, Schor P, Dupps WJ, Jr, Ambrósio R., Jr Discriminant value of custom ocular response analyzer waveform derivatives in forme fruste keratoconus. Am J Ophthalmol. 2016;164:14–21. doi: 10.1016/j.ajo.2015.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu Y, Vitart V, Burdon KP, et al. Genome-wide association analyses identify multiple loci associated with central corneal thickness and keratoconus. Nat Genet. 2013;45(2):155–163. doi: 10.1038/ng.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burdon KP, MacGregor S, Bykhovskaya Y, et al. Association of polymorphisms in the hepatocyte growth factor gene promoter with keratoconus. Invest Ophthalmol Vis Sci. 2011;52(11):8514–8519. doi: 10.1167/iovs.11-8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li XH, Bykhovskaya Y, Haritunians T, Siscovick D, Aldave A, Szczotka-Flynn L, Iyengar SK, Rotter JI, Taylor KD, Rabinowitz YS. A genome-wide association study identifies a potential novel gene locus for keratoconus, one of the commonest causes for corneal transplantation in developed countries. Hum Mol Genet. 2012;21(2):421–429. doi: 10.1093/hmg/ddr460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joob B, Wiwanitkit V. VSX1 and SOD1 mutation screening in patients with keratoconus. J Ophthalmic Vis Res. 2018;13(2):212. doi: 10.4103/jovr.jovr_135_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karolak JA, Polakowski P, Szaflik J, Szaflik JP, Gajecka M. Molecular screening of keratoconus susceptibility sequence variants in VSX1, TGFBI, DOCK9, STK24, and IP05 genes in Polish patients and novel TGFBI variant identification. Ophthalmic Genet. 2016;37(1):37–43. doi: 10.3109/13816810.2014.926375. [DOI] [PubMed] [Google Scholar]
- 20.Klyce SD. Chasing the suspect: keratoconus. Br J Ophthalmol. 2009;93(7):845–847. doi: 10.1136/bjo.2008.147371. [DOI] [PubMed] [Google Scholar]
- 21.Schweitzer C, Roberts CJ, Mahmoud AM, Colin J, Maurice-Tison S, Kerautret J. Screening of forme fruste keratoconus with the ocular response analyzer. Invest Ophthalmol Vis Sci. 2010;51(5):2403–2410. doi: 10.1167/iovs.09-3689. [DOI] [PubMed] [Google Scholar]
- 22.Shirayama-Suzuki M, Amano S, Honda N, Usui T, Yamagami S, Oshika T. Longitudinal analysis of corneal topography in suspected keratoconus. Br J Ophthalmol. 2009;93(6):815–819. doi: 10.1136/bjo.2008.140012. [DOI] [PubMed] [Google Scholar]
- 23.Vogt A. Textbook and Atlas of Slit Lamp Microscopy of the Living Eye. Bonn: Springer; 1981. [Google Scholar]
- 24.Reinstein DZ, Archer TJ, Gobbe M. Corneal epithelial thickness profile in the diagnosis of keratoconus. J Refract Surg. 2009;25(7):604–610. doi: 10.3928/1081597X-20090610-06. [DOI] [PubMed] [Google Scholar]
- 25.Prakash G, Agarwal A, Jacob S, Kumar DA, Agarwal A, Banerjee R. Comparison of Fourier-domain and time-domain optical coherence tomography for assessment of corneal thickness and intersession repeatability. Am J Ophthalmol. 2009;148(2):282–290.e2. doi: 10.1016/j.ajo.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 26.Reinstein DZ, Gobbe M, Archer TJ, Silverman RH, Coleman DJ. Epithelial, stromal, and total corneal thickness in keratoconus: three-dimensional display with Artemis very-high frequency digital ultrasound. J Refract Surg. 2010;26(4):259–271. doi: 10.3928/1081597X-20100218-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reinstein DZ, Silverman RH, Sutton HFS, Coleman DJ. Very high-frequency ultrasound corneal analysis identifies anatomic correlates of optical complications of lamellar refractive surgery: anatomic diagnosis in lamellar surgery. Ophthalmology. 1999;106(3):474–482. doi: 10.1016/S0161-6420(99)90105-7. [DOI] [PubMed] [Google Scholar]
- 28.Li HF, Petroll WM, Møller-Pedersen T, Maurer JK, Cavanagh HD, Jester JV. Epithelial and corneal thickness measurements by in vivo confocal microscopy through focusing (CMTF) Curr Eye Res. 1997;16(3):214–221. doi: 10.1076/ceyr.16.3.214.15412. [DOI] [PubMed] [Google Scholar]
- 29.Ma XJ, Wang L, Koch DD. Repeatability of corneal epithelial thickness measurements using Fourier-domain optical coherence tomography in normal and post-LASIK eyes. Cornea. 2013;32(12):1544–1548. doi: 10.1097/ICO.0b013e3182a7f39d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maguire LJ, Bourne WM. Corneal topography of early keratoconus. Am J Ophthalmol. 1989;108(2):107–112. doi: 10.1016/0002-9394(89)90001-9. [DOI] [PubMed] [Google Scholar]
- 31.Silverman RH, Urs R, Roychoudhury A, Archer TJ, Gobbe M, Reinstein DZ. Epithelial remodeling as basis for machine-based identification of keratoconus. Invest Ophthalmol Vis Sci. 2014;55(3):1580–1587. doi: 10.1167/iovs.13-12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y, Chamberlain W, Tan O, Brass R, Weiss JL, Huang D. Subclinical keratoconus detection by pattern analysis of corneal and epithelial thickness maps with optical coherence tomography. J Cataract Refract Surg. 2016;42(2):284–295. doi: 10.1016/j.jcrs.2015.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reinstein DZ, Archer TJ, Gobbe M. Rate of change of curvature of the corneal stromal surface drives epithelial compensatory changes and remodeling. J Refract Surg. 2014;30(12):799–802. doi: 10.3928/1081597X-20141113-02. [DOI] [PubMed] [Google Scholar]
- 34.Kanellopoulos AJ, Aslanides IM, Asimellis G. Correlation between epithelial thickness in normal corneas, untreated ectatic corneas, and ectatic corneas previously treated with CXL; is overall epithelial thickness a very early ectasia prognostic factor? Clin Ophthalmol. 2012;6:789–800. doi: 10.2147/OPTH.S31524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scarcelli G, Besner S, Pineda R, Yun SH. Biomechanical characterization of keratoconus corneas ex vivo with Brillouin microscopy. Invest Ophthalmol Vis Sci. 2014;55(7):4490–4495. doi: 10.1167/iovs.14-14450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saad A, Gatinel D. Evaluation of total and corneal wavefront high order aberrations for the detection of forme fruste keratoconus. Invest Ophthalmol Vis Sci. 2012;53(6):2978–2992. doi: 10.1167/iovs.11-8803. [DOI] [PubMed] [Google Scholar]
- 37.El Wardani M, Hashemi K, Aliferis K, Kymionis G. Topographic changes simulating keratoconus in patients with irregular inferior epithelial thickening documented by anterior segment optical coherence tomography. Clin Ophthalmol. 2019;13:2103–2110. doi: 10.2147/OPTH.S208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y, Tan O, Brass R, Weiss JL, Huang D. Corneal epithelial thickness mapping by Fourier-domain optical coherence tomography in normal and keratoconic eyes. Ophthalmology. 2012;119(12):2425–2433. doi: 10.1016/j.ophtha.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]