Abstract
AIM
To verify the association between retinopathy, nephropathy, and periodontitis in type 2 diabetic (T2D) patients.
METHODS
Several electronic databases were available for our comprehensive search including China National Knowledge Infrastructure (CNKI), Chinese VIP Information (VIP), Wanfang, Web of Science, ScienceDirect and PubMed and were queried for relevant citations (updated to Mar. 2019). RevMan was utilized to perform Meta analysis and publication bias detection. After evaluation of the methodological quality of included studies, a fixed or random effect model was utilized to analyze data from included studies.
RESULTS
A total of eight articles were finally included in this Meta analysis. In all 3987 subjects, there were 1207 T2D patients accompanying with microvascular complications and 1734 patients with periodontitis as well. The Meta forest plot presented little heterogeneity of the eight studies (P<0.00001, I2=89%). The total effect demonstrated periodontitis was associated with overall microvascular complications (OR: 1.96, 95%CI: 1.67-2.30, Z=8.25, P<0.00001). Subgroup investigations among the studies in Asian (OR: 2.33, 95%CI: 1.91-2.85) and North American (OR: 1.42, 95%CI: 1.08-1.86) populations confirmed the existed association between retinopathy, nephropathy, and periodontitis. While the strength of such associations between periodontitis and diabetic microvascular complications were more obvious in the Asians than North Americans. All the results indicated that periodontitis was associated with diabetic retinopathy (OR: 3.77, 95%CI: 2.71-5.24), diabetic nephropathy (OR: 1.55, 95%CI: 1.24-1.94) in T2D patients.
CONCLUSION
The periodontitis is associated with diabetic retinopathy, diabetic nephropathy among T2D patients and further large sample size clinical trials are in need to confirm the findings.
Keywords: periodontitis, diabetic retinopathy, diabetic nephropathy, diabetes, Meta-analysis
INTRODUCTION
Diabetes mellitus (DM) is a kind of chronic disorder that could not be felt with serious symptoms at its early stage. DM was affecting over 217 million people worldwide in the last decade, and its prevalence is still increasing, and it is estimated that the prevalence of diabetes for all age-groups worldwide was estimated to be 4.4% by the year 2030[1]. The progress of DM-related microvascular dysfunctions are associated with cerebral and peripheral microvascular complications[2]–[3]. It's recognized that vascular complications of diabetes are the most serious manifestations of diabetes, of which retinopathy (DR) and diabetic nephropathy (DN) are two major contributors to end-stage blindness and renal disease, respectively[4].
Periodontitis, another chronic inflammatory disease has been reported to affect almost 50% of the general population globally[5]. Those individuals with chronic periodontitis could develop periodontal pockets, however, they might be unaware of their clinical periodontal status due to being asymptomatic and painless during the most process of its course[6]. The periodontitis was discovered to be associated with a variety of systemic diseases and conditions, which was in need of attention[7]–[10]. Similarly, such association was also echoed in DM[11]–[12]. Previous clinical investigations showed that the risk of periodontitis is greater in DM patients, and the disease course of diabetes might result in more severe tooth loss[13]. Moreover, the periodontitis has a significant impact on diabetes control, incidence and complications[14]–[15]. Recently, the risk of peripheral arterial disease was high in patients with periodontitis has recently been discosed[16] and the severity of periodontitis was found to be significantly associated with the number of microvascular complications too[17]. Several clinical investigations revealed that the periodontitis had certain association with DR in those DM patients[18]. And the similar findings were indicated in those DM patients with DN[19]. However, such association reported in each individual publication has not been verified in large population. Despite several summarized analyses have been conducted to prove the relationship between diabetes and periodontitis[20]–[22], similar summarized studies have not yet been performed to confirm the association between periodontitis and microvascular complications in type 2 diabetic (T2D) patients. Therefore, we conducted this Meta analysis to summarize the results of previous studies and investigate such potential association between periodontitis and microvascular complications in T2D patients in order to provide more reliable evidence for correlated clinical researches.
MATERIALS AND METHODS
Search Strategy
Several electronic databases were available for our comprehensive search including China National Knowledge Infrastructure (CNKI), Chinese VIP Information (VIP), Wanfang, Web of Science, ScienceDirect and PubMed and were queried for relevant citations (updated to Mar 2019). By virtue of the following search terms: “periodont*”, “alveolar bone loss”, “anodontia”, “edentulous” and “toothless”, “diabet*”, “diabetic retinopathy”, “fundus”, “DR”, “proliferative diabetic retinopathy”, “PDR”, “non-proliferative diabetic retinopathy”, “NPDR”, “microaneurysm*”, “neovascularization”, “cotton-wool spots”, “hard exudate*”, “soft exudate*”, “h*emorrahge”, “bleed*”, “degeneration” “blind*”, “diabetic nephropathy”, “DN”, “albuminuria”, “dialysis”, “serum creatinine”, “urea nitrogen”. Some of above terms were combined to generate a subset of citations that address the purpose of our investigation. The reference lists of relevant articles were later under prudent scrutiny in quest of potential qualified studies beyond the electronic searches.
Inclusion and Exclusion Criteria
The trials were included if they have satisfied the following criteria: 1) According to the updated CDC-AAP case definition for monitoring periodontitis in 2012, the number of periodontitis categories was expanded from 3 to 4, i.e., no, light, moderate, and severe periodontitis[23]. 2) Trials that included patients with DM which was comply with the standard of WHO (1985) or American Diabetes Association (ADA, 1997)[24]. 3) Diagnosis of DR was determined by the Guideline for DR Clinical Diagnosis and Treatment which was developed by the Eye Institute of Chinese Academic of Ophthalmology in 2014 and other guidelines[25]–[27]. 4) DN was diagnosed on the presence of proteinuria >0.5 g/24h, a stage referred to as overt nephropathy, clinical nephropathy, proteinuria, or macroalbuminuria[28]. 5) Adequate information about the occurrence of periodontitis was available. 6) The language was written in English or Chinese. The exclusion criteria were as follows: 1) The subjects of study were patients accompanied with other diseases affecting teeth, eye or kidney organs. 2) Insufficient data about DR, DN, and periodontitis. 3) The literature reporting language used failed to be English and Chinese.
Quality Assessment and Data Extraction
By virtue of the Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomized studies in Meta-analyses and RevMan 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark), the quality of the included studies was evaluated thoroughly. Using the “star system”, the study was judged from three broad perspectives: the choice of the study group; the comparability of each group; and the ascertainment of either the exposure or outcome of interest for case-control or cohort studies respectively[29]. On the basis of the NOS, each study will be scored as high quality for studies receiving at least 8 stars; medium quality for those awarded seven stars, or low quality if studies had fewer than seven stars[30]–[31]. Nothing is more crucial for a good quality case-control study than the adequate definition and representativeness of the case, the definition and selection of the controls, acceptable comparability of cases and controls on the basis of the design or analysis, low no-response rate, ascertainment of exposure, together with the same method of ascertainment for cases and controls given the pretty established scale. Three reviewers (Qi JY, Wei X, Yao JY) independently extracted data with a piloted extraction form, and reviewed all data prudently via identifying all studies by the other author (Wu HQ). The following items was extracted the from the reports: year of publication, inclusion or exclusion criteria, sample size, ages, sex, the number of T2D patients with microvascular complications, the number of patients with periodontitis, and other co-variables such as smoking, total cholesterol, hypertension, metabolic syndrome events etc. We screened abstracts of articles and obtain full articles of studies that met all predetermined.All the duplicated studies were excluded. Two reviewers (Qi JY, Wei X) estimated the quality of the included studies. If there were divergences, they would reach a consensus after a discussion with a third reviewer (Wu HQ).
Statistical Analysis
In pursuit of improving the accuracy of these tests, subgroup analyses were used to identify the test-related or other factors responsible for heterogeneity. Utilizing RevMan (version: 5.3) to perform Meta analysis, odds ratio (OR) and its 95% confidence interval (CI) were calculated for statistical analysis. In addition, heterogeneity was assessed using a Chi-square test and quantified by inconsistency index I2. Generally, inter-group heterogeneity was evaluated by I2 and heterogeneity. While the heterogeneity value was less than 0.1, pooled OR was estimated by either using a random effect model or using a fixed effect model. A two-sided P value which is less than 0.05 denotes statistical significance. What's more, funnel plots were utilized for investigating publication and other biases in this Meta analysis.
RESULTS
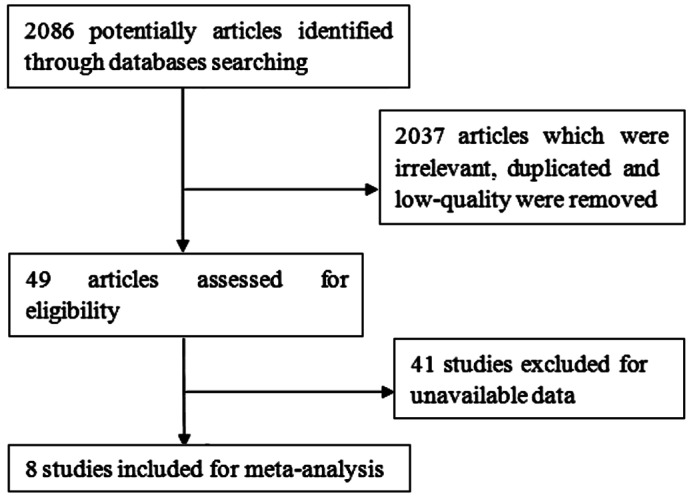
The literature search yielded 2086 references from PubMed, Web of Science, the CNKI, VIP, Wanfang databases, eight articles[17]–[19],[32]–[36] out of which were qualified for this inclusion finally according to the flowchart. Figure 1 presented the study selection process.
Figure 1. Flow chart of study selection.
Summary Characteristics of Included Studies
All included studies met inclusion and exclusion criteria. Eight studies from different regions including USA, Japan, Korea, Iran and China were finally included for this Meta analysis. In all 3987 subjects, there were 1207 T2D patients accompanying with microvascular complications and 1734 patients with periodontitis as well. The age of the included population ranged from 30 to 69y. Each study recruited both men and women in the case and control groups. However, only Ricardo et al[33] included the same number of male and female subjects. Four of articles were only concerned with the association between periodontitis and DR, and another three, Han et al[18], Sharma et al[32], Ricardo et al[33] referred to association between periodontitis and DN. While Nitta et al[17] recruited subjects with overall microvascular complications of diabetes. The current WHO body mass index (BMI) cut-off points of 23, 27.5, 32.5, and 37.5 kg/m2 were refered[37]. In the literatures we included in the study, the BMI of the people was over 22, indicating that some patients were overweight to different extent. Diabetes was defined as HbA1c≥6.5% or fasting plasma glucose (FPG) ≥7.0 mmol/L. Prediabetes was classified as HbA1c between 5.7% and 6.4%[38]. In our study, the values of HbA1c were more than 7%, showing that patients blood glucose level were not well controlled. In addition, studies have shown that baseline blood glucose HbA1c control has no significant effect on longitudinal changes in diabetic complications. A worsening or persistent failure of glycemic control is associated with an increased risk of increased complications of diabetes[13]. In these eight articles, Ricardo et al[33] studied 51.6% hypertensive patients. What's more, Sharma et al[32] involved nearly four fifths patient who drunk a lot in their study. The detailed description of the characteristics of included studies could be observed in Table 1.
Table 1. Characteristics of the included studies.
| Study | Region | Study design | Complication type | Age (y) | Sex (M/F) | BMI (kg/m2) | HbA1c | eGFR (mL/min/1.73 m) | Smoking (%) | Hypertension (%) | Exercises (%) | Drinking (%) |
| Gong 2010[35] | China | Case control | DR(+) | 56.95±12.66 | 1.67 | NA | 9.27±2.53 | NA | 25. 12 | NA | NA | NA |
| DR(-) | 49.85±10.62 | 2.73 | NA | 8.90±2.30 | NA | 26. 15 | NA | NA | NA | |||
| Han 2015[18] | Korea | Case control | DN | 58 | 1.11 | 25.3 | 7.3 | 90.4 | 31.63 | 58 | 13.71 | 10.79 |
| Sharma 2016[32] | USA | Case control | DN | 47 | 0.86 | 27.1 | NA | 100.3 | 25.37 | 23.08 | 79.26 | 81.74 |
| Ricardo 2015[33] | USA | Case control | DN | 41.5 | 1 | 26.4 | NA | NA | 51.6 | 20.4 | NA | NA |
| Song 2017[34] | Korea | Case control | DR(-) | 57±0.7 | 0.82 | 22.7±0.1 | 7.2±0.1 | 92.6 | 23.2 | 52.4 | 23.1 | 54.2 |
| DR(+) | 58.5±1.6 | 1.18 | 22.2±0.3 | 8.2±0.2 | 87.7 | 27.3 | 37.2 | 20 | 48.1 | |||
| Amiri 2014[19] | Iran | Case control | DR(-) | 55.69±8.49 | 0.42 | NA | NA | NA | NA | 28.17 | NA | NA |
| DR(+) | 56.97±10.46 | 0.46 | NA | NA | NA | NA | 26.87 | NA | NA | |||
| Nitta 2017[17] | Japan | Case control | DR/DN (+) | 55.1±9.3 | 1.72 | 24.0±3.9 | 8.7±1.8 | NA | 42.6 | NA | NA | NA |
| DR/DN (-) | 52.4±10.3 | 1.44 | 23.9±3.9 | 7.7±1.5 | NA | 38 | NA | NA | NA | |||
| Veena 2018[36] | India | Prospective cross-sectional study | DR(-) | 30-65 | 2.57 | NA | NA | NA | NA | NA | NA | NA |
| DR(+) |
NA: Not accessible; BMI: Body mass index; eGFR: Estimated glomerular filtration rate; HbA1c: Hemoglobin A1c.
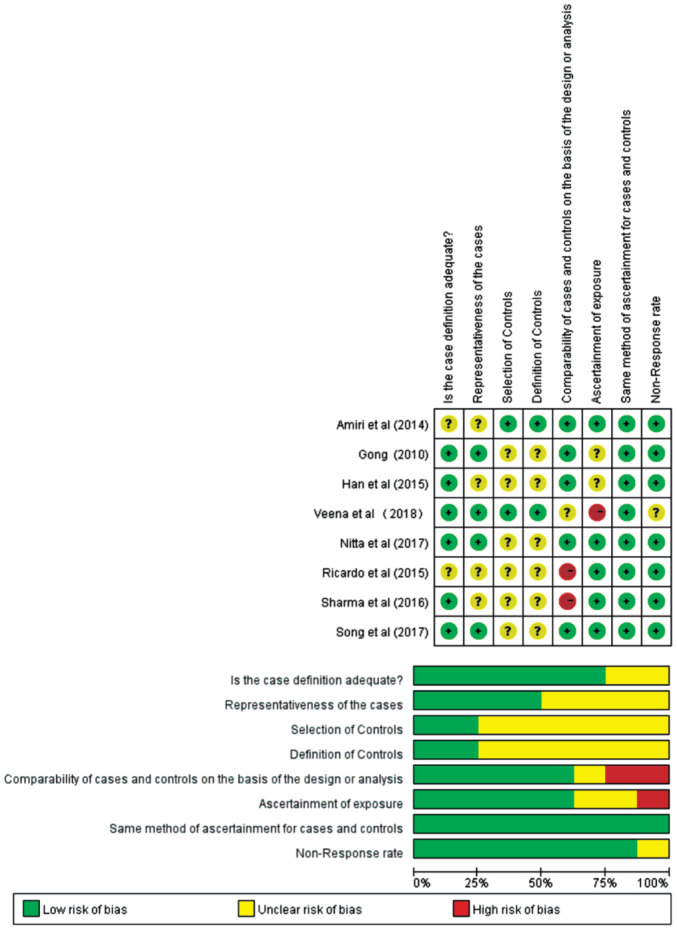
Quality of Included Studies
The methodological quality of the included studies was graphically demonstrated in Figure 2. The overall quality of the included studies was variable. The comparability of cases and controls on the basis of the design or analysis had introduced high bias in some studies whereas the no-response rate was fairly low in all studies. In most studies, the definition and selection of the controls were vaguely revealed, and the ascertainment of exposure, together with the same method of ascertainment for cases and controls were opaque in some studies as well.
Figure 2. The methodological quality of the included studies.
Association Between Periodontitis and DR or DN in T2D Patients
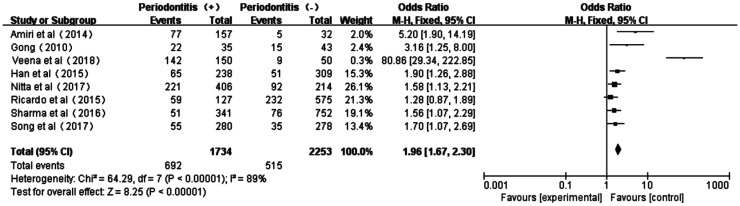
The Meta forest plot presented little heterogeneity of the eight studies[17]–[19],[32]–[36] (P<0.00001, I2=89%); and the total effect size OR and Z value were 1.96 (95%CI: 1.67-2.30) and 8.25 (P<0.00001), respectively, indicating that periodontitis was associated with overall microvascular complications (Figure 3).
Figure 3. Forest plot comparing DR/DN with non-DR/DN in diabetic patients with periodontitis and non-periodontitis.
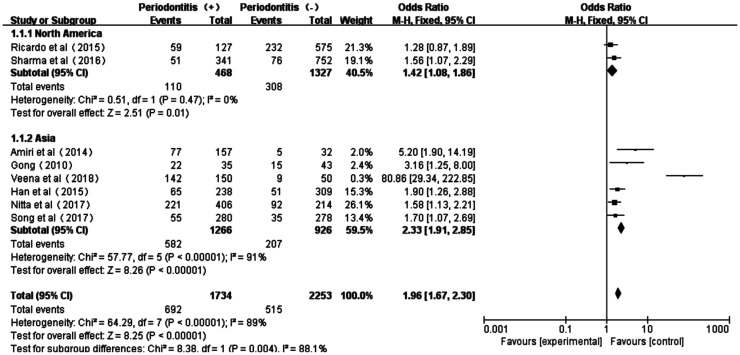
Moreover, the subgroup investigations among the studies in Asian and North American populations showed acceptable heterogeneity too (North American: P=0.47, I2=0; Asian: P<0.00001, I2=91%). And the subtotal effect size OR in North American (1.42, 95%CI: 1.08-1.86) and Asian populations (2.33, 95%CI: 1.91-2.85) all confirmed the existed association between periodontitis and diabetic microvascular complications. However, the strength of such association between periodontitis and DR or DN T2D patients was more obvious in Asians than that in North Americans (Figure 4).
Figure 4. Forest plot comparing DR/DN with non-DR/DN in subgroup Asian and North American diabetic populations.
Association Between Periodontitis and DR
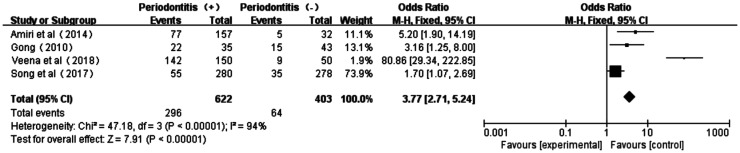
As shown from the forest plot of association between periodontitis and DR (Figure 5), there was a certain degree of heterogeneity (P<0.00001, I2=94%) in these four studies[19],[34]–[36]. The total effect size OR in this study was 3.77 (95%CI: 2.71-5.24), and the Z value was 7.91 (P<0.00001), indicating the association between periodontitis and DR (Figure 5).
Figure 5. Forest plot comparing DR with non-DR in diabetic patients with periodontitis and non-periodontitis.
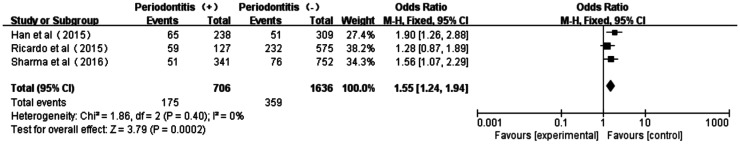
Association Between Periodontitis and DN
The forest plot of association between periodontitis and DN was shown in Figure 6. Three studies[18],[32]–[33] displayed no heterogeneity in patients with periodontitis and non-periodontitis (P=0.40, I2=0). The total effect size OR in this study was 1.55 (95%CI: 1.24-1.94) and the Z value was 3.79 (P=0.0002), indicating that periodontitis was associated to DN.
Figure 6. Forest plot comparing DN with non-DN in diabetic patients with periodontitis and non-periodontitis.
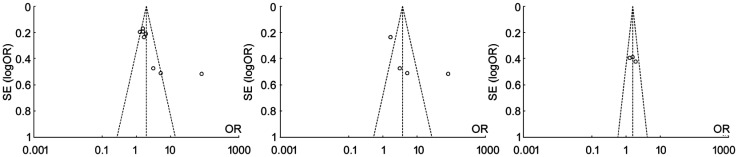
Publication Bias
Funnel plots of each group of Meta analysis were shown, and those funnel plots without outlines were due to the use of the random effect model (Figure 7).
Figure 7. Funnel plots between diabetic patients with periodontitis and non-periodontitis in different comparions.
A: Comparing DR/DN with non-DR/DN; B: Comparing DR with non-DR; C: Comparing DN with non-DN.
DISCUSSION
Diabetes has been validated as a significant risk factor for periodontitis, which risk is estimated to increase approximately three times in diabetic individuals compared with those non-diabetic individuals[38]. The possible reason is that diabetes could lead to inflammation in periodontal tissues. For instance, compared with those non-diabetic persons with periodontitis, levels of prostaglandin E2 (PGE2) and interleukin (IL)-1β in gingival crevicular fluid (GCF) were higher in diabetic patients with periodontitis[39]. It's found that DM patients with HbA1c>8% had a considerably higher GCF IL-1β level, and both HbA1c and glucose were considered as independent predictors of an elevated GCF IL-1β level[40]. The possible reason might be due to that hyperglycaemia was a cause of the activation of pathways that increase inflammation, oxidative stress and apotosis[41].
It is generally believed that the initial factor of periodontitis is a large number of bacteria in dental plaque, and periodontitis was recognized as an oral disease that could contribute to low-grade chronic inflammation, thus producing proinflammatory cytokines and promoting systemic inflammation[42]. In those patients with periodontitis, some inflammatory cytokine such as serum levels of IL-6 and C-reactive protein (CRP) were increasing, and the elevated levels of such systemic inflammatory biomarkers were believed to be connected with DM[43]. Tumor necrosis factor (TNF)-α and IL-6 are the main inducers of acute-phase proteins, including CRP, and both have been shown potentially contributing to insulin resistance[44]–[45]. Moreover, the serum levels of IL-6 and CRP could be used to predict future occurrence of DM[46], suggesting the possible mechanisms underlying the association between periodontities and DM in our findings. Interestingly, Casanova et al[42] reviewed the relevant articles and summarized the two way relationship between periodontitis and DM. Chronic periodontitis may enhance insulin resistance and increase the risk of multiple long-term complications of diabetes[47]. That is, the systemic inflammation associated with periodontitis might enhance the diabetic state and the resulted hyperglycemia further increase the risk of microvascular complications. The two of such microvascular complications, DR and DN, were investigated in our study. It confirmed that association between retinopathy, nephropathy, and periodontitis, and that the outcome of periodontal destruction is significantly more frequent and severe in subjects with DR. Improved control of hyperglycemia has been proven to reduce the risk of microvascular complications[48]. Therefore, simultaneous periodontal treatments should be implemented to improve glycemic control and assuage periodontitis in diabetes patients with periodontitis. In this way, it might also inhibit the development and progression of diabetic microvascular complications such as DR and DN.
As is prevalently perceived by all, Meta analysis is a comprehensive statistical method that has been under ever-growing utilization for combining and integrating data from a multitude of independent studies. However, the results of Meta analysis depend on the quality of primary researches included for further analysis, having implications on the summarized results. Even though a definite association between periodontitis status and the occurrence of diabetic microvascular complications has been established, this study cannot identify a cause-and-effect relationship given its case-control design. Furthermore, the limited populations of the studies and indefinite DM duration might limit the generalization of our conclusions.
Despite the above-mentioned weaknesses, this Meta analysis approves the association between the DR, DN and periodontitis in T2D patients. Moreover, it is in need of large-sample, well-conducted prospective clinical investigations to assure that association.
Acknowledgments
Foundations: Supported by the National Key R&D Program of China (No.2018YFC1314900; No.2018YFC1314902); Nantong “226 Project” and Excellent Key Teachers in the “Qing Lan Project” of Jiangsu Colleges and Universities [No.(2018)III-436].
Conflicts of Interest: Wu HQ, None; Wei X, None; Yao JY, None; Qi JY, None; Xie HM, None; Sang AM, None; Jiang K, None.
REFERENCES
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.van Sloten TT, Sedaghat S, Carnethon MR, Launer LJ, Stehouwer CDA. Cerebral microvascular complications of type 2 diabetes: stroke, cognitive dysfunction, and depression. Lancet Diabetes Endocrinol. 2020;8(4):325–336. doi: 10.1016/S2213-8587(19)30405-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sabanayagam C, Banu R, Chee ML, Lee R, Wang YX, Tan G, Jonas JB, Lamoureux EL, Cheng CY, Klein BEK, Mitchell P, Klein R, Cheung CMG, Wong TY. Incidence and progression of diabetic retinopathy: a systematic review. Lancet Diabetes Endocrinol. 2019;7(2):140–149. doi: 10.1016/S2213-8587(18)30128-1. [DOI] [PubMed] [Google Scholar]
- 4.Rask-Madsen C, King GL. Vascular complications of diabetes: mechanisms of injury and protective factors. Cell Metab. 2013;17(1):20–33. doi: 10.1016/j.cmet.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eke PI, Dye BA, Wei L, et al. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012;91(10):914–920. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- 6.Pitiphat W, Garcia RI, Douglass CW, Joshipura KJ. Validation of self-reported oral health measures. J Public Health Dent. 2002;62(2):122–128. doi: 10.1111/j.1752-7325.2002.tb03432.x. [DOI] [PubMed] [Google Scholar]
- 7.Jepsen S, Caton JG, Albandar JM, et al. Periodontal manifestations of systemic diseases and developmental and acquired conditions: Consensus report of workgroup 3 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol. 2018;89(Suppl 1):S237–S248. doi: 10.1002/JPER.17-0733. [DOI] [PubMed] [Google Scholar]
- 8.de Molon RS, Rossa C, Jr, Thurlings RM, Cirelli JA, Koenders MI. Linkage of periodontitis and rheumatoid arthritis: current evidence and potential biological interactions. Int J Mol Sci. 2019;20(18):E4541. doi: 10.3390/ijms20184541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ming Y, Hsu SW, Yen YY, Lan SJ. Association of oral health-related quality of life and Alzheimer disease: a systematic review. J Prosthet Dent. 2020;124(2):168–175. doi: 10.1016/j.prosdent.2019.08.015. [DOI] [PubMed] [Google Scholar]
- 10.D'Cruz L. Periodontitis-a silent risk that has become louder. Prim Dent J. 2020;8(4):62–66. doi: 10.1308/205016820828463780. [DOI] [PubMed] [Google Scholar]
- 11.Seppälä B, Seppälä M, Ainamo J. A longitudinal study on insulin-dependent diabetes mellitus and periodontal disease. J Clin Periodontol. 1993;20(3):161–165. doi: 10.1111/j.1600-051x.1993.tb00338.x. [DOI] [PubMed] [Google Scholar]
- 12.Soskolne WA, Klinger A. The relationship between periodontal diseases and diabetes: an overview. Ann Periodontol. 2001;6(1):91–98. doi: 10.1902/annals.2001.6.1.91. [DOI] [PubMed] [Google Scholar]
- 13.Shlossman M, Knowler WC, Pettitt DJ, Genco RJ. Type 2 diabetes mellitus and periodontal disease. J Am Dent Assoc. 1990;121(4):532–536. doi: 10.14219/jada.archive.1990.0211. [DOI] [PubMed] [Google Scholar]
- 14.Joshipura KJ, Muñoz-Torres FJ, Dye BA, Leroux BG, Ramírez-Vick M, Pérez CM. Longitudinal association between periodontitis and development of diabetes. Diabetes Res Clin Pract. 2018;141:284–293. doi: 10.1016/j.diabres.2018.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graziani F, Gennai S, Solini A, Petrini M. A systematic review and meta-analysis of epidemiologic observational evidence on the effect of periodontitis on diabetes an update of the EFP-AAP review. J Clin Periodontol. 2018;45(2):167–187. doi: 10.1111/jcpe.12837. [DOI] [PubMed] [Google Scholar]
- 16.Cho DH, Song IS, Choi J, Gwon JG. Risk of peripheral arterial disease in patients with periodontitis: a nationwide, population-based, matched cohort study. Atherosclerosis. 2020;297:96–101. doi: 10.1016/j.atherosclerosis.2020.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Nitta H, Katagiri S, Nagasawa T, et al. The number of microvascular complications is associated with an increased risk for severity of periodontitis in type 2 diabetes patients: Results of a multicenter hospital-based cross-sectional study. J Diabetes Investig. 2017;8(5):677–686. doi: 10.1111/jdi.12633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han K, Nam GE, Kim DH, Park JB, Ko Y, Roh YK, Cho KH, Park YG. Association of periodontitis with urinary albumin excretion in Korean adults with diabetes: the 2012 Korea National Health And Nutrition Examination Survey. Medicine (Baltimore) 2015;94(42):e1839. doi: 10.1097/MD.0000000000001839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amiri AA, Maboudi A, Bahar A, Farokhfar A, Daneshvar F, Khoshgoeian HR, Nasohi M, Khalilian A. Relationship between type 2 diabetic retinopathy and periodontal disease in Iranian adults. N Am J Med Sci. 2014;6(4):190. [PMC free article] [PubMed] [Google Scholar]
- 20.Chávarry NG, Vettore MV, Sansone C, Sheiham A. The relationship between diabetes mellitus and destructive periodontal disease: a meta-analysis. Oral Health Prev Dent. 2009;7(2):107–127. [PubMed] [Google Scholar]
- 21.Khader YS, Dauod AS, El-Qaderi SS, Alkafajei A, Batayha WQ. Periodontal status of diabetics compared with nondiabetics: a meta-analysis. J Diabetes Complications. 2006;20(1):59–68. doi: 10.1016/j.jdiacomp.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Mealey BL, Ocampo GL. Diabetes mellitus and periodontal disease. Periodontology. 2007;44(1):127–153. doi: 10.1111/j.1600-0757.2006.00193.x. [DOI] [PubMed] [Google Scholar]
- 23.Eke PI, Page RC, Wei L, Thornton-Evans G, Genco RJ. Update of the case definitions for population-based surveillance of periodontitis. J Periodontol. 2012;83(12):1449–1454. doi: 10.1902/jop.2012.110664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wahl PW, Savage PJ, Psaty BM, Orchard TJ, Robbins JA, Tracy RP. Diabetes in older adults: comparison of 1997 American Diabetes Association classification of diabetes mellitus with 1985 WHO classification. Lancet. 1998;352(9133):1012–1015. doi: 10.1016/S0140-6736(98)04055-0. [DOI] [PubMed] [Google Scholar]
- 25.Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, Lauria G, Malik RA, Spallone V, Vinik A, Bernardi L, Valensi P, Toronto Diabetic Neuropathy Expert Group Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33(10):2285–2293. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chinese Medical Association of Ophthalmology Fundopathic Group. Chinese diabetic retinopathy clinical diagnosis and treatment guidelines (2014) Chin J Ophthalmol. 2014;50(11):851–865. [Google Scholar]
- 27.Solomon SD, Chew E, Duh EJ, Sobrin L, Sun JK, VanderBeek BL, Wykoff CC, Gardner TW. Diabetic Retinopathy: A position statement by the American Diabetes Association. Diabetes Care. 2017;40(3):412–418. doi: 10.2337/dc16-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corcóstegui B, Durán S, González-Albarrán MO, Hernández C, Ruiz-Moreno JM, Salvador J, Udaondo P, Simó R. Update on diagnosis and treatment of diabetic retinopathy: a consensus Guideline of the Working Group of Ocular Health (Spanish Society of Diabetes and Spanish Vitreous and Retina Society) J Ophthalmol. 2017;2017:8234186. doi: 10.1155/2017/8234186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005;28(1):164–176. doi: 10.2337/diacare.28.1.164. [DOI] [PubMed] [Google Scholar]
- 30.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 31.Abariga SA, Whitcomb BW. Periodontitis and gestational diabetes mellitus: a systematic review and meta-analysis of observational studies. BMC Pregnancy Childbirth. 2016;16(1):344. doi: 10.1186/s12884-016-1145-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharma P, Dietrich T, Ferro CJ, Cockwell P, Chapple IL. Association between periodontitis and mortality in stages 3-5 chronic kidney disease: NHANES III and linked mortality study. J Clin Periodontol. 2016;43(2):104–113. doi: 10.1111/jcpe.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ricardo AC, Athavale A, Chen JS, Hampole H, Garside D, Marucha P, Lash JP. Periodontal disease, chronic kidney disease and mortality: results from the third National Health and Nutrition Examination Survey. BMC Nephrol. 2015;16:97. doi: 10.1186/s12882-015-0101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song SJ, Lee SS, Han K, Park JB. Periodontitis is associated with diabetic retinopathy in non-obese adults. Endocrine. 2017;56(1):82–89. doi: 10.1007/s12020-016-1215-z. [DOI] [PubMed] [Google Scholar]
- 35.Gong R. Research on the relationship between type-2 diabetic retinopathy and periodontal disease postgraduate. Urumqi: Xinjiang Medical University; 2010. [Google Scholar]
- 36.Veena HR, Natesh S, Patil SR. Association between diabetic retinopathy and chronic periodontitis-a cross-sectional study. Med Sci (Basel) 2018;6(4):E104. doi: 10.3390/medsci6040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 38.Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: framework and proposal of a new classification and case definition. J Periodontol. 2018;89(Suppl 1):S159–S172. doi: 10.1002/JPER.18-0006. [DOI] [PubMed] [Google Scholar]
- 39.Salvi GE, Carollo-Bittel B, Lang NP. Effects of diabetes mellitus on periodontal and peri-implant conditions: update on associations and risks. J Clin Periodontol. 2008;35(8 Suppl):398–409. doi: 10.1111/j.1600-051X.2008.01282.x. [DOI] [PubMed] [Google Scholar]
- 40.Salvi GE, Yalda B, Collins JG, Jones BH, Smith FW, Arnold RR, Offenbacher S. Inflammatory mediator response as a potential risk marker for periodontal diseases in insulin-dependent diabetes mellitus patients. J Periodontol. 1997;68(2):127–135. doi: 10.1902/jop.1997.68.2.127. [DOI] [PubMed] [Google Scholar]
- 41.Engebretson SP, Hey-Hadavi J, Ehrhardt FJ, Hsu D, Celenti RS, Grbic JT, Lamster IB. Gingival crevicular fluid levels of interleukin-1beta and glycemic control in patients with chronic periodontitis and type 2 diabetes. J Periodontol. 2004;75(9):1203–1208. doi: 10.1902/jop.2004.75.9.1203. [DOI] [PubMed] [Google Scholar]
- 42.Casanova L, Hughes FJ, Preshaw PM. Diabetes and periodontal disease: a two-way relationship. Br Dent J. 2014;217(8):433–437. doi: 10.1038/sj.bdj.2014.907. [DOI] [PubMed] [Google Scholar]
- 43.Birkedal-Hansen H. Role of cytokines and inflammatory mediators in tissue destruction. J Periodontal Res. 1993;28(6 Pt 2):500–510. doi: 10.1111/j.1600-0765.1993.tb02113.x. [DOI] [PubMed] [Google Scholar]
- 44.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25(1):4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 45.Hotamisligil GS. Molecular mechanisms of insulin resistance and the role of the adipocyte. Int J Obes Relat Metab Disord. 2000;24(Suppl 4):S23–S27. doi: 10.1038/sj.ijo.0801497. [DOI] [PubMed] [Google Scholar]
- 46.Rotter V, Nagaev I, Smith U. Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J Biol Chem. 2003;278(46):45777–45784. doi: 10.1074/jbc.M301977200. [DOI] [PubMed] [Google Scholar]
- 47.Nesto R. C-reactive protein, its role in inflammation, Type 2 diabetes and cardiovascular disease, and the effects of insulin-sensitizing treatment with thiazolidinediones. Diabet Med. 2004;21(8):810–817. doi: 10.1111/j.1464-5491.2004.01296.x. [DOI] [PubMed] [Google Scholar]
- 48.Ryan ME, Raja VS, Sussman SK. Periodontitis and diabetes mellitus: a complex relationship. In: Craig R, Kamer A., editors. A Clinician's Guide to Systemic Effects of Periodontal Diseases. Berlin, Heidelberg: Springer; 2016. pp. 19–37. [Google Scholar]