Abstract
Differences in the common aortic arch branching pattern arise from abnormal embryological development. Aberrant origin of the right subclavian artery is the most common of these anomalies. We report the case of a 47-year-old female with progressive dysphagia, found to have an aberrant right subclavian artery (ARSA) running posterior to the esophagus on computed tomography angiography. She was managed successfully with a hybrid procedure involving a right supraclavicular incision for ARSA ligation and subclavian to carotid transposition followed by endovascular closure of the ARSA origin.
Keywords: Cardiovascular abnormalities, Subclavian artery, Endovascular procedures
INTRODUCTION
An aberrant right subclavian artery (ARSA) is a variation in the anatomy of the aortic arch and is usually an incidental finding [1,2]. Most cases are asymptomatic; however, in some instances, it can present with dysphagia as it courses posterior to the esophagus causing compression, also known as dysphagia lusoria [3]. Nowadays, endovascular advances offer hybrid management with less invasive procedures. We describe a case of dysphagia lusoria treated successfully with a hybrid procedure. Informed consent was obtained from the patient for publication of this report, which does not release any personal information. The case report was approved by the ethics committee of the Centro Medico ABC (No. APABC-20-015).
CASE
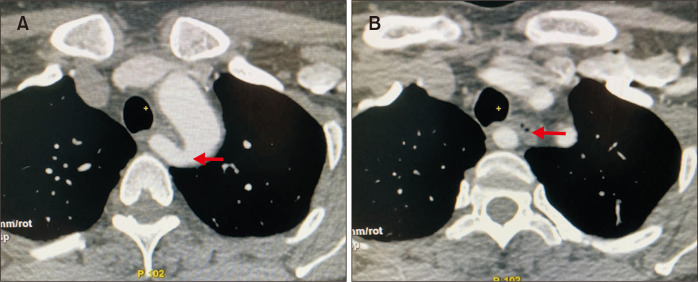
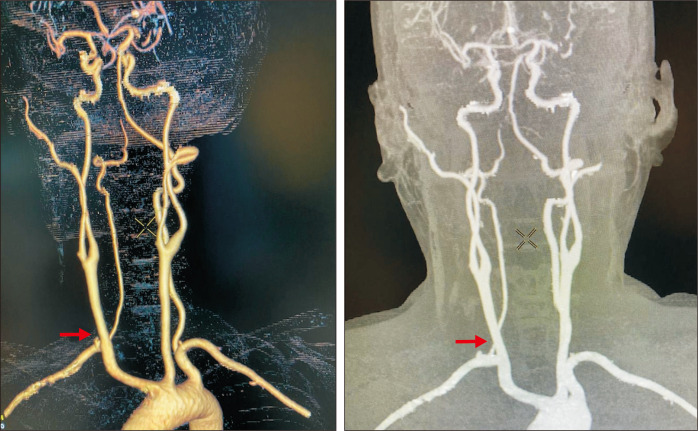
A 47-year-old female with an unremarkable past medical history presented with a 5-month history of progressive hoarseness and dysphagia. She was treated initially by an otorhinolaryngologist with non-steroidal anti-inflammatory drugs without symptomatic relief. Computed tomography (CT) angiography of the neck and chest demonstrated an ARSA running posterior to the esophagus without evident aneurysmal degeneration (Fig. 1-3).
Fig. 1.
Preoperative three-dimensional reconstruction of computed tomography scan showed an aberrant right subclavian artery (arrow).
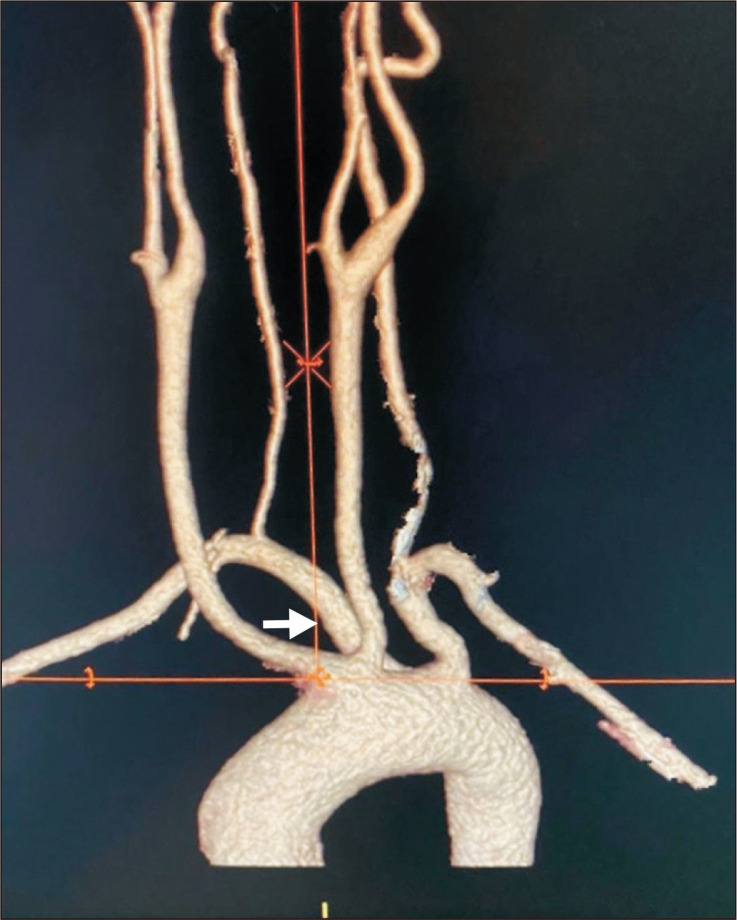
Fig. 2.
Posterior view of preoperative computed tomography scan showed aneurysmal degeneration at the origin (arrow head) of the aberrant right subclavian artery (arrow).
Fig. 3.
Axial view of computed tomography scan showed: (A) The origin of the aberrant right subclavian artery (arrow). (B) Posterior to the esophagus with esophageal compression (arrow).
A hybrid procedure was planned. The surgical approach involved a right supraclavicular incision (8 cm) with division and suture ligation of the proximal ARSA followed by a subclavian to carotid transposition with an end-to-side anastomosis and the right vertebral artery preservation.
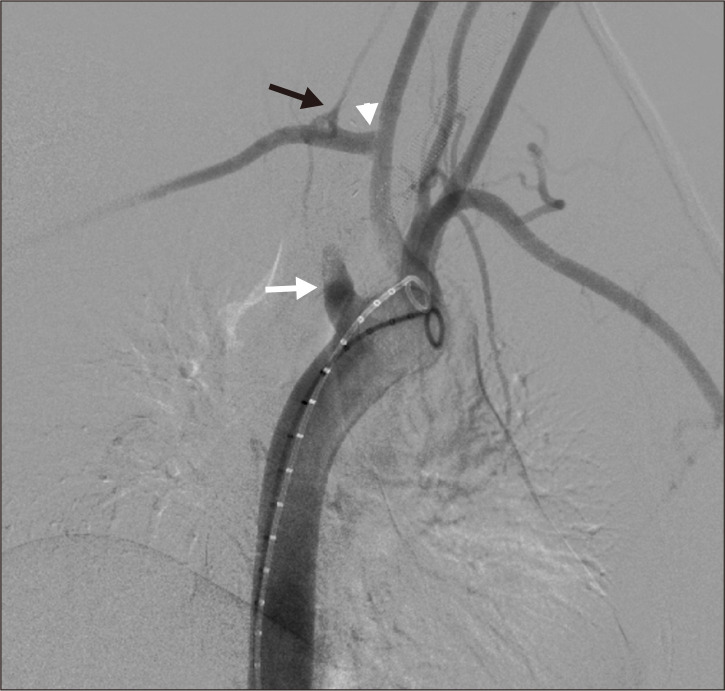
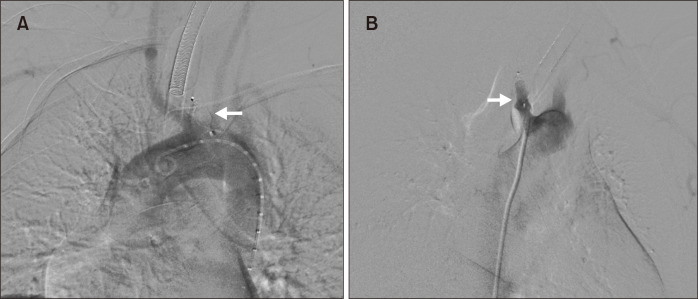
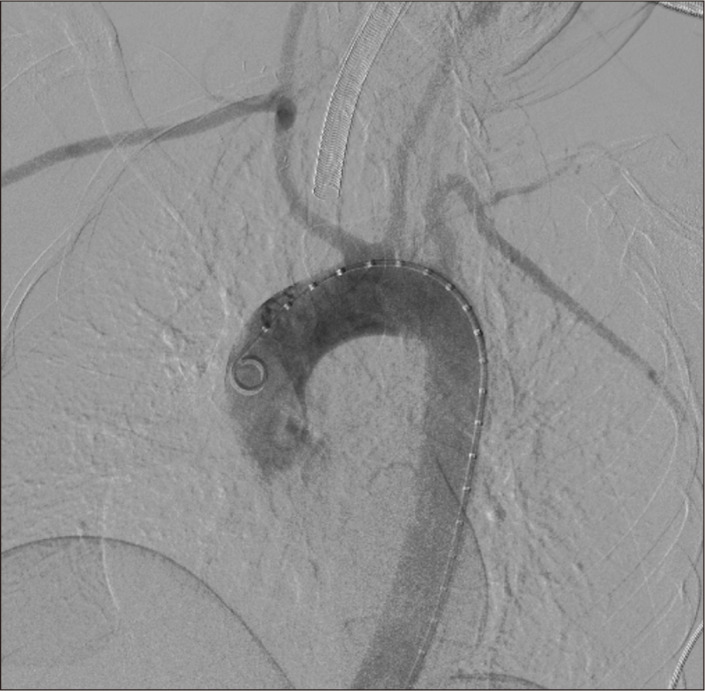
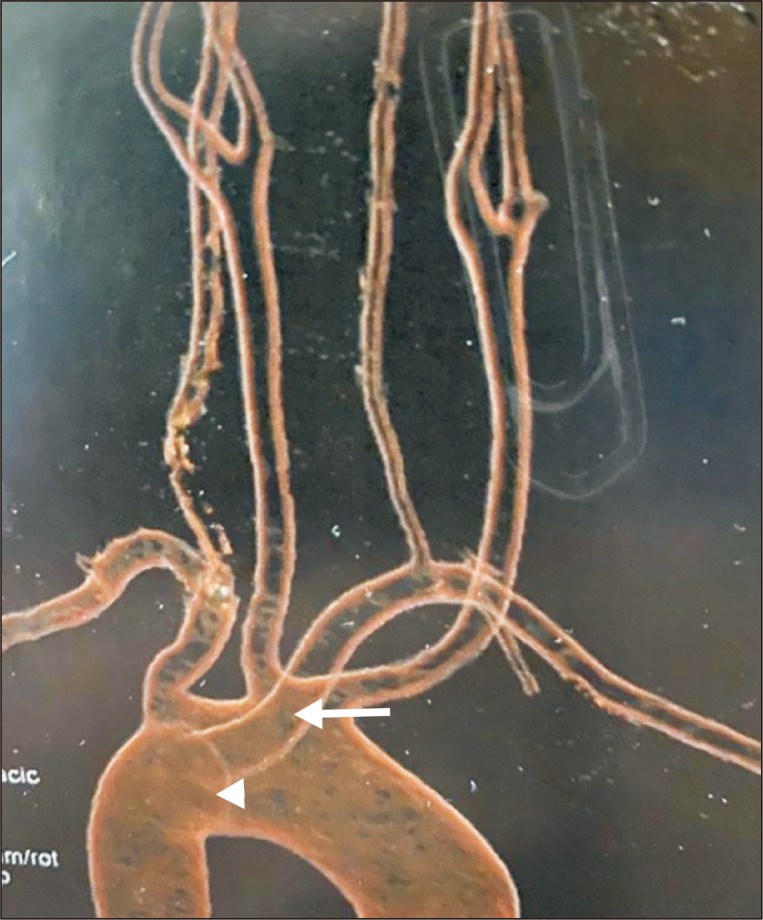
At the end of the open surgical approach, angiography was performed by percutaneously accessing the right common femoral artery (CFA) with a 6 Fr introducer sheath. The ARSA origin was found to have a dilatation (Kommerell’s diverticulum) (Fig. 4). An endovascular closure of the ARSA stump was carried out using a 12 mm×9 mm Amplatzer vascular plug (AGA Medical Corporation, Golden Valley, MN, USA) (Fig. 5). At the end of the procedure (Fig. 6), the CFA was closed with a 6-Fr percutaneous arterial closure device (Angio-Seal; St. Jude Medical, St. Paul, MN, USA).
Fig. 4.
Angiogram (right anterior oblique) showed the ab- errant right subclavian artery origin to have a dilatation (white arrow), subclavian to carotid transposition (arrow head), and vertebral artery preservation (black arrow).
Fig. 5.
Left anterior oblique view (A) and right anterior oblique view (B) of angiogram showed endovascular closure of the aberrant right subclavian artery stump with Amplatzer vascular plug (arrows).
Fig. 6.
Completion angiogram showed patent right subclavian to carotid transposition with absence of contrast material in the aberrant right subclavian artery origin.
No complications occurred during the procedures. The patient did well postoperatively with immediate resolution of dysphagia and was discharged on postoperative day 2 with aspirin prescribed at a dosage of 100 mg daily.
At the 6-month follow-up, the patient was asymptomatic and without dysphagia. CT angiography of the neck and chest showed a patent right subclavian to carotid transposition and no aneurysmal degeneration of the residual ARSA stump (Fig. 7).
Fig. 7.
Six-month follow-up computed tomography angiography of the neck and chest showed a patent right subclavian to carotid transposition and vertebral artery preservation (arrows).
DISCUSSION
ARSA is a common aortic arch abnormality with an incidence of 0.4% to 0.7% [1-3]. It occurs as a consequence of involution of the right fourth aortic arch and the connecting right dorsal aorta. The distal right dorsal aorta joins a persistent seventh intersegmental artery to form the right subclavian artery [4,5]. The most common course of ARSA is the one presented by our patient, posterior to the esophagus; it is observed in 80% of patients. It runs between the esophagus and trachea in 15% of cases and anterior to the trachea and esophagus in 15% of cases [6].
ARSA was first identified in autopsies by Hunauld in 1735 [5]. However, in 1761, Bayford described a patient with dysphagia due to ARSA anteriorly compressing the esophagus and named it “Dysphagia Lusoria” (Lusus naturae meaning dysphagia from freaks of nature) [7].
The involution of the right dorsal aorta can form a diverticulum at the origin of the ARSA (Kommerell´s diverticulum). The presence of this dilatation is reported in 3% to 8% of patients and may be associated with an increase in symptoms [8].
Although most patients remain asymptomatic throughout life, symptoms are most likely to present in the 4th decade of life [6,9]. The development of symptoms is related to tracheal and esophageal compression by arterial degeneration with age, related to atherosclerotic hardening, decreased elasticity, and aneurysmal degeneration. Our patient presented with progressive hoarseness and dysphagia. Other reported symptoms included chest pain, weight loss, shortness of breath, chronic cough, and upper extremity claudication [6,10].
The most common method for diagnosis is a CT scan. Previously, ARSA was diagnosed by endoscopy or upper gastrointestinal barium imaging [6]. We believe that a CT scan with vascular reconstruction is the best preoperative image to define the lesion and aids in planning the intervention.
Treatment is reserved for symptomatic patients or preventing complications such as aneurysmal dilatation [11]. For many years, the classical approach was a left thoracotomy with division and ligation of the ARSA [12].
Other procedures used were right thoracotomy, median sternotomy, and reimplantation of the ARSA to the ascending aorta or the right common carotid artery [13,14].
Over the last few years, the number of endovascular and hybrid techniques available for ARSA has increased; these have the advantage of treating symptomatic or aneurysmal ARSA without thoracotomy, thus, reduce the morbidity associated with this procedure [11].
Orvald et al. [15] reported the first hybrid approach of cervical incision and transposition of the subclavian artery to the right common carotid artery, which was later modified by Valentine et al. [16] who used only a right-sided supraclavicular incision.
Treatment by the division of the ARSA through a right supraclavicular incision and transposition of the subclavian artery to the carotid with no intervention for the ARSA stump could lead to persistent compression of the esophagus by the stump [9]. Endovascular occlusion of the proximal ARSA using an Amplatzer plug or a Zenith iliac plug (Cook Medical, Inc., Bloomington, IN, USA) has been described [17]. After endovascular occlusion, an open right supraclavicular approach for revascularization yielded good outcomes. Revascularization has been achieved by carotid-subclavian bypass with Dacron or carotid-subclavian transposition.
We opted for a hybrid procedure with right supraclavicular incision with division and oversewing of the proximal ARSA followed by a subclavian to carotid transposition with an end-to-side anastomosis and right vertebral artery preservation. The supraclavicular incision has low morbidity and allows for direct reconstruction of blood flow with vertebral artery preservation. We recommend obliteration of the stump of the ARSA, which can be safely achieved with an endovascular procedure, to avoid relapsing of the esophageal compression. However, endovascular devices have a risk of eroding the vessel wall and causing an esophageal fistula or dysphagia by direct compression after migration [18].
Other authors reported good outcomes with hybrid procedures using thoracic endovascular aortic repair (TEVAR) with graft exclusion of ARSA plus revascularization, which allows for better control of the aneurysmal degeneration of ARSA; however, it often requires coverage of the left subclavian artery orifice and bilateral subclavian to carotid transposition [4,19]. TEVAR may unnecessarily increase morbidity, and patients undergoing TEVAR or stump plugging will need periodic CT imaging for surveillance [4].
Other approaches include mediastinoscopy or thoracoscopy assisted ligation of ARSA [20].
Knowing the patient´s specific anatomy is critical to decide the best surgical approach, and the patient must be notified of the treatment options. Previous reports emphasized the need for open thoracotomy in the presence of aneurysmal degeneration of ARSA for ligating the origin and avoiding recurrence of symptoms [10,11]. Although over the last few years, hybrid ARSA repair procedure reports have increased, no standard therapy has been established. We report a successful hybrid procedure, which can be easily reproducible, has low morbidity, and results in complete resolution of symptoms and total reconstruction of blood flow to the upper extremity with preservation of spinal and vertebrobasilar circulation.
In conclusion, surgical options vary for the management of symptomatic ARSA. Ligation of ARSA with revascularization followed by endovascular occlusion of the stump yielded good results in our patient. Preoperative CT scan is crucial in the planning and prevention of intraoperative and postoperative events. Management of symptomatic patients should be tailored according to anatomical variations of the patient. More research with larger samples and longer follow-up periods are needed to determine the best surgical techniques.
Footnotes
CONFLICTS OF INTEREST
The authors have nothing to disclose.
AUTHOR CONTRIBUTIONS
Concept and design: ML, MG, FV. Analysis and interpretation: ML, CRC. Data collection: MG, FV. Writing the article: ML, MG, CRC. Critical revision of the article: CRC, SCM. Final approval of the article: ML, MG, FV, CRC, SCM. Obtained funding: none. Overall responsibility: ML, MG, FV, CRC, SCM.
REFERENCES
- 1.Jakanani GC, Adair W. Frequency of variations in aortic arch anatomy depicted on multidetector CT. Clin Radiol. 2010;65:481–487. doi: 10.1016/j.crad.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Popieluszko P, Henry BM, Sanna B, Hsieh WC, Saganiak K, Pękala PA, et al. A systematic review and meta-analysis of variations in branching patterns of the adult aortic arch. J Vasc Surg. 2018;68:298–306.e10. doi: 10.1016/j.jvs.2017.06.097. [DOI] [PubMed] [Google Scholar]
- 3.Bennett AL, Cock C, Heddle R, Morcom RK. Dysphagia lusoria: a late onset presentation. World J Gastroenterol. 2013;19:2433–2436. doi: 10.3748/wjg.v19.i15.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker AC, Atkins BZ, Clouse WD, Noll R, Sampson J, Williams T. Repair of aberrant right subclavian artery entirely via a supraclavicular approach. Ann Vasc Surg. 2014;28:489.e1–489.e4. doi: 10.1016/j.avsg.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Felson B, Cohen S, Courter SR, McGuire J. Anomalous right subclavian artery. Radiology. 1950;54:340–349. doi: 10.1148/54.3.340. [DOI] [PubMed] [Google Scholar]
- 6.Stone WM, Ricotta JJ, 2nd, Fowl RJ, Garg N, Bower TC, Money SR. Contemporary management of aberrant right subclavian arteries. Ann Vasc Surg. 2011;25:508–514. doi: 10.1016/j.avsg.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 7.Asherson N. David Bayford. His syndrome and sign of dysphagia lusoria. Ann R Coll Surg Engl. 1979;61:63–67. [PMC free article] [PubMed] [Google Scholar]
- 8.van Son JA, Konstantinov IE. Burckhard F. Kommerell and Kommerell's diverticulum. Tex Heart Inst J. 2002;29:109–112. [PMC free article] [PubMed] [Google Scholar]
- 9.Cobos-González E, Aragón-López JA, García Buen-Abad R, Rojas JA, Gutiérrez A. Hybrid treatment of dysphagia lusoria: right carotid to subclavian bypass and endovascular insertion of an Amplatzer II Vascular Plug. Rev Esp Enferm Dig. 2016;108:835–837. doi: 10.17235/reed.2016.4121/2015. [DOI] [PubMed] [Google Scholar]
- 10.Levitt B, Richter JE. Dysphagia lusoria: a comprehensive review. Dis Esophagus. 2007;20:455–460. doi: 10.1111/j.1442-2050.2007.00787.x. [DOI] [PubMed] [Google Scholar]
- 11.Jalaie H, Grommes J, Sailer A, Greiner A, Binnebösel M, Kalder J, et al. Treatment of symptomatic aberrant subclavian arteries. Eur J Vasc Endovasc Surg. 2014;48:521–526. doi: 10.1016/j.ejvs.2014.06.040. [DOI] [PubMed] [Google Scholar]
- 12.Gross RE. Surgical treatment for dysphagia lusoria. Ann Surg. 1946;124:532–534. doi: 10.1097/00000658-194609000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Bailey CP, Hirose T, Alba J. Re-establishment of the continuity of the anomalous right subclavian artery after operation for dysphagia lusoria. Angiology. 1965;16:509–513. doi: 10.1177/000331976501600901. [DOI] [PubMed] [Google Scholar]
- 14.Siderys H. A new operation for symptomatic aberrant right subclavian artery in the adult (dysphagia lusoria) J Thorac Cardiovasc Surg. 1969;57:269–272. doi: 10.1016/S0022-5223(19)42750-5. [DOI] [PubMed] [Google Scholar]
- 15.Orvald TO, Scheerer R, Jude JR. A single cervical approach to aberrant right subclavian artery. Surgery. 1972;71:227–230. [PubMed] [Google Scholar]
- 16.Valentine RJ, Carter DJ, Clagett GP. A modified extrathoracic approach to the treatment of dysphagia lusoria. J Vasc Surg. 1987;5:498–500. doi: 10.1016/0741-5214(87)90065-6. [DOI] [PubMed] [Google Scholar]
- 17.Morris ME, Benjamin M, Gardner GP, Nichols WK, Faizer R. The use of the Amplatzer plug to treat dysphagia lusoria caused by an aberrant right subclavian artery. Ann Vasc Surg. 2010;24:416.e5–416.e8. doi: 10.1016/j.avsg.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 18.Soo Hoo AJ, Rokkas CK, Rossi PJ. Migration of endovascular plug in hybrid repair of dysphagia lusoria. J Vasc Surg Cases Innov Tech. 2018;4:140–143. doi: 10.1016/j.jvscit.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tinelli G, Ferrer C, Giudice R, Ferraresi M, Pogany G, Cao P, et al. Long-term results of hybrid repair techniques for Kommerell's diverticulum. J Vasc Surg. 2020;72:1213–1221. doi: 10.1016/j.jvs.2019.11.052. [DOI] [PubMed] [Google Scholar]
- 20.Varetto G, Castagno C, Trevisan A, Quaglino S, Garneri P, Mossetti C, et al. Mediastinoscopy-assisted treatment of an aberrant right subclavian artery. Ann Vasc Surg. 2016;31:210.e9–210.e11. doi: 10.1016/j.avsg.2015.05.044. [DOI] [PubMed] [Google Scholar]