Abstract
BACKGROUND:
A pandemic of coronavirus disease (COVID-19) has been declared by the World Health Organization (WHO) and caring for critically ill patients is expected to be at the core of battling this disease. However, little is known regarding an early detection of patients at high risk of fatality.
METHODS:
This retrospective cohort study recruited consecutive adult patients admitted between February 8 and February 29, 2020, to the three intensive care units (ICUs) in a designated hospital for treating COVID-19 in Wuhan. The detailed clinical information and laboratory results for each patient were obtained. The primary outcome was in-hospital mortality. Potential predictors were analyzed for possible association with outcomes, and the predictive performance of indicators was assessed from the receiver operating characteristic (ROC) curve.
RESULTS:
A total of 121 critically ill patients were included in the study, and 28.9% (35/121) of them died in the hospital. The non-survivors were older and more likely to develop acute organ dysfunction, and had higher Sequential Organ Failure Assessment (SOFA) and quick SOFA (qSOFA) scores. Among the laboratory variables on admission, we identified 12 useful biomarkers for the prediction of in-hospital mortality, as suggested by area under the curve (AUC) above 0.80. The AUCs for three markers neutrophil-to-lymphocyte ratio (NLR), thyroid hormones free triiodothyronine (FT3), and ferritin were 0.857, 0.863, and 0.827, respectively. The combination of two easily accessed variables NLR and ferritin had comparable AUC with SOFA score for the prediction of in-hospital mortality (0.901 vs. 0.955, P=0.085).
CONCLUSIONS:
Acute organ dysfunction combined with older age is associated with fatal outcomes in COVID-19 patients. Circulating biomarkers could be used as powerful predictors for the in-hospital mortality.
Keywords: Coronavirus disease, Neutrophil-to-lymphocyte ratio, Mortality, Predictor
INTRODUCTION
The infection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is spreading worldwide.[1-5] A pandemic of coronavirus disease (COVID-19) has been declared by the World Health Organization (WHO) despite many restrictive measures that have been implemented globally.[6] As of March 15, 2020, more than 1,500,000 cases have been confirmed, and around 16,000 death occurred.[6] The estimated mortality of COVID-19 is 3%–4% based on the data collected. Although this may be an overestimate as more potential cases with mild symptoms haven’t been identified, the mortality of COVID-19 is considerably higher than that caused by influenza. The early recognition and treatment of patients at a high risk of developing serious illness are critical to improving the outcome of diseases caused by microbial invasion such as sepsis.[7] Some recent studies have reported the epidemiologic and clinical features of patients with COVID-19,[ 1,2,8] but little is known regarding the characteristics of critically ill patients and how to early detect patients who are at a high risk of death. Caring for critically ill patients will be a crucial part of battling COVID-19.
In this retrospective cohort study, we aim to investigate the clinical characteristics and laboratory findings of intensive care unit (ICU) patients with COVID-19 and to identify potential indicators for early recognition of patients with a high risk of death.
METHODS
Participants
This retrospective cohort study recruited consecutive adult patients (aged ≥18 years) admitted between February 8 to February 29, 2020, to the three ICUs of Zhongfaxincheng Campus of Tongji Hospital Affiliated to Huazhong University of Science and Technology in Wuhan, which is one of the designated hospitals to receive patients with confirmed SARS-CoV-2 infection by using a quantitative polymerase chain reaction test of throat swab samples or sputum samples according to the WHO guidance. Those who met the following criteria would be considered to be transferred to the ICU: (1) respiratory rate >30 breaths per minute; (2) SpO2 <93%; (3) PaO2/FiO2 <300 mmHg (1 mmHg=0.133 kPa); (4) in presence with respiratory failure; (5) in presence with shock; (6) with other conditions that need to be monitored in the ICU. These ICUs were in charged by the medical staff from the hospitals affiliated with Peking University. This study was approved by the Ethics Committee of Peking University People’s Hospital and was conducted in accordance with the Declaration of Helsinki.
Data collection
The detailed clinical information of each patient was obtained by physicians using a standard questionnaire after they were admitted to the ICU. Clinical information including demographic data, medical history, comorbidities, symptoms, signs, laboratory findings, chest computed tomographic (CT) scans, and treatments was recorded. We also collected each patient’s clinical characteristics, including the Sequential Organ Failure Assessment (SOFA) score and quick SOFA (qSOFA) score.
To identify useful biomarkers for predicting in-hospital death of these patients, we included laboratory variables measured on day one after ICU admission in the analysis.
Laboratory measurements
The complete blood count was measured by Sysmex XN-9000 automatic hematology analyzer (Sysmex, Japan). The neutrophil-to-lymphocyte ratio (NLR) was calculated by the absolute neutrophil count divided by the absolute lymphocyte count. Myohemoglobin (Myo), creatine kinase muscle brain isoenzyme (CK-MB), and high sensitive troponin I (hs-TNI) were measured by Abbott ARCHITECT i2000SR chemiluminescence immunoanalyzer (Abbott Laboratories, USA). Coagulation parameters were performed by Stago STA-R automatic blood coagulation analyzer (Stago, France). Alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TB), direct bilirubin (DB), albumin, blood urea nitrogen (BUN), creatinine, high sensitive C-reactive protein (hs-CRP), and ferritin were measured using Roche Cobas 8000 automatic biochemical analyzer (Roche, Switzerland). Thyroid hormones, cytokines interleukin-2 receptor (IL-2R), IL-1β, IL-6, IL-8, IL-10, tumor necrosis factor-α (TNF-α), and N-terminal pro-brain natriuretic peptide (NT-proBNP) were detected by Roche Cobas e602 electrochemical luminescence analyzer (Roche, Germany). Immunoglobulin A (IgA), IgG, IgM, and complement 3 (C3) and C4 were performed by IMAGE 8000.
Outcomes
The primary outcome was in-hospital mortality. Secondary outcomes included the incidence of acute respiratory distress syndrome (ARDS), acute kidney injury (AKI), heart failure, liver dysfunction, and coagulopathy. ARDS was defined according to the Berlin definition,[9] and AKI was diagnosed based on the Kidney Disease Improving Global Outcomes (KDIGO) criteria.[10] Heart failure was diagnosed according to the 2016 European Society of Cardiology guidelines.[11] Septic shock was defined by persisting hypotension requiring vasopressors to maintain a mean arterial pressure of 65 mmHg or higher and a serum lactate level greater than 2 mmol/L despite adequate volume resuscitation.[12]
Statistical analysis
Continuous variables were presented as median and interquartile range (IQR), and categorical variables were expressed as percentages. Baseline characteristics between the survivor and non-survivor groups were compared with the unpaired Student’s t-test or Mann-Whitney U-test for continuous variables and the Chi-square or Fisher’s exact test for categorical variables. Univariate logistic regression analyses were performed to examine the association between each predictor and in-hospital mortality separately. We also conducted a forward stepwise multivariate logistic regression to determine the independent predictors of ICU mortality. A criterion of P<0.05 for entry and P≥0.05 for removal was imposed in this procedure. Odds ratios (ORs) for continuous variables were described using standardized ORs, which were associated with a one standard deviation change in the variable. The receiver operating characteristic (ROC) curve was used to evaluate the performance of indicators to predict mortality. The curve represented a plot of sensitivity (se) vs. 1-specificity (sp). A ROC curve was also constructed for the combination of NLR and ferritin for predicting in-hospital mortality according to the Mackinnon and Mulligan’s weighted sum rule.[13] The differences between the area under the curve (AUC) (C-index) were tested by Hanley-McNeil methods.[14] A two-sided P-value of less than 0.05 was considered to indicate statistical significance. The analyses were performed with SPSS 25.0 software (SPSS Inc., Chicago, Illinois, USA) and MedCalc 18.11.3 software (MedCalc Software bvba, Ostend, Belgium).
RESULTS
Baseline characteristics
A total of 121 ICU patients were screened for eligibility during the study period. The median age was 66 (56–72) years, and 57.0% were male. Their SOFA and qSOFA scores were 1 (1–4) and 1 (1–1), respectively. A total of 35 (28.9%) patients died during their hospital stay, and 86 patients were discharged after recovery.
Compared with survivors, non-survivors were older and had worse organ function indicted by different parameters and higher Acute Physiology and Chronic Health Evaluation II (APACHE II), SOFA, and qSOFA score. More patients developed ARDS (97.1% vs. 5.8%), AKI (54.3% vs. 1.2%), septic shock (57.1% vs. 0%), heart failure (57.1% vs. 11.6%), liver dysfunction (20.0% vs. 3.5%), and coagulopathy (77.1% vs. 19.8%) in non-survivors than in survivors.
Of six different cytokines or their receptors, the level of circulating IL-2R was significantly higher in non-survivors than in survivors, while the differences in the levels of IL-6, IL-8, IL-10, and TNF-α between groups reached marginal statistic significance. There was no significant difference in the levels of IgA, IgG, IgM, C3, and C4 between survivors and non-survivors.
Predictors of in-hospital mortality
Univariate logistic regression analysis demonstrated that those who were older, and had higher SOFA and qSOFA scores had significantly greater hazard of in-hospital death. To detect potential biomarkers for the prediction of fatal outcomes of patients with COVID-19, all laboratory variables were also examined by univariate logistic regression analysis. We found a total of 28 variables were associated with in-hospital mortality (Table 1).
Table 1.
Performance of variables for predicting in-hospital mortality in COVID-19 patients*
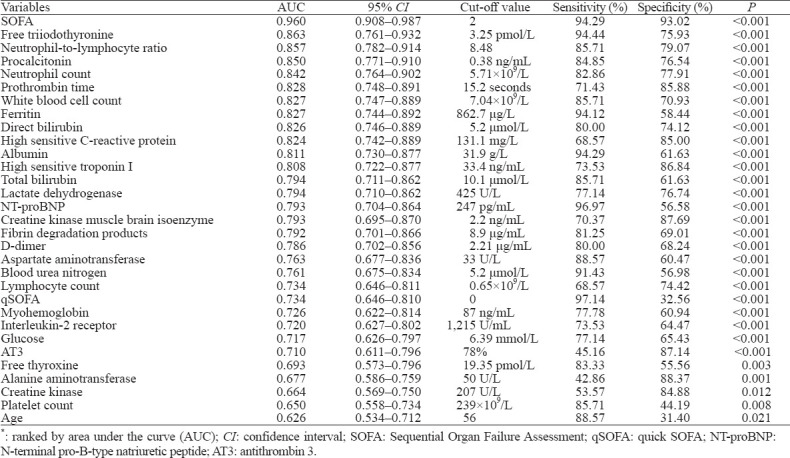
To evaluate the performance of these laboratory variables for the prediction of in-hospital mortality, ROC curves were constructed. In addition to the SOFA score, which had the highest discriminability to predict in-hospital death, we found 12 variables with the potential ability to early detect patients at a high risk of fatality, as suggested by AUC greater than 0.80. The D-dimer, which was reported as a risk factor of death in a previous study,[15] had a relatively lower ability to identify patients with a high risk of fatality than those variables, including other coagulation parameters prothrombin time (PT) and fibrinogen degradation product (FDP).
When each of these variables was included in a stepwise multiple logistic model in which in-hospital mortality was the dependent variable, all variables could independently predict the primary outcome.
Values of neutrophil/lymphocyte, triiodothyronine (T3), and ferritin in predicting in-hospital mortality
Among these 12 variables, some of them are unspecific markers of inflammation such as white blood cells, neutrophils, hs-CRP, and procalcitonin, while others represent the dysfunction of different organs, including direct bilirubin, albumin, PT, hs-TNI, and international normalized ratio of PT (INR-PT). We further evaluated biomarkers, including NLR, free triiodothyronine (FT3), and ferritin. The AUCs for NLR, FT3, and ferritin were 0.857, 0.863, and 0.827, and the optimal cut-off values were 8.48, 3.25 pmol/L, and 863 μg/L, respectively. The AUCs of these three biomarkers were lower than that of SOFA score. However, the combination of NLR and ferritin had a comparable AUC with SOFA score to predict the in-hospital mortality (0.901 vs. 0.955, P=0.085) (Figure 1).
Figure 1.
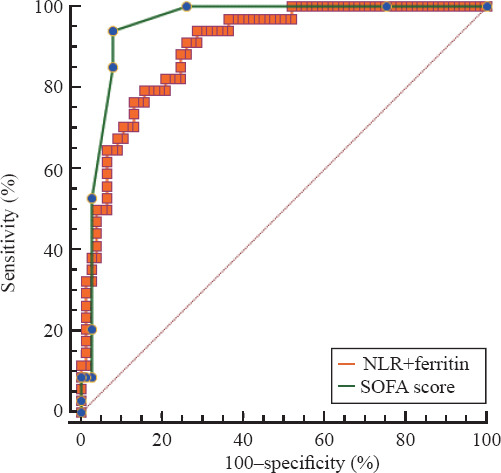
Receiver operating characteristic curve for the combination of neutrophil-to-lymphocyte ratio (NLR) and ferritin as compared with Sequential Organ Failure Assessment (SOFA) score in predicting in-hospital mortality in COVID-19 patients.
DISCUSSION
In this retrospective cohort study, we found that the infection of SARS-CoV-2 not only caused lung injury but also targeted other organs to induce multiple-organ dysfunction, including ARDS, AKI, and septic shock. These acute organ dysfunctions combined with older age were associated with fatal outcomes of these patients. In addition, this study investigated in detail the laboratory parameters and found biomarkers that can be used to identify COVID-19 patients at a high risk of death.
A previous study reported that COVID-19 patients with worse outcome tended to be older, had more underlying medical conditions, and were more likely to have organ dysfunction than those survived.[16] A later study, which confirmed previous findings, further demonstrated that age, D-dimer, and SOFA scores were independently associated with in-hospital mortality in critically ill patients with COVID-19.[15] Our current study extended these findings by investigating more biomarkers and their performance to predict a fatal outcome. We found biomarkers with moderate-to-high predictive performance ability for poor outcomes, including NLR, FT3, and ferritin. The calculation of SOFA scores, which is comprised of six variables, can be time-consuming. This might limit its application in clinical practice, especially when facing the heavy burdens on the health care system during the pandemic. Therefore, the current study provides useful information to quickly evaluate the patients at a high risk of death. Importantly, the combination of NLR and ferritin is as powerful as the SOFA score for the prediction of in-hospital mortality.
The NLR is an easily accessed indicator that combines both the changes in neutrophils and lymphocytes. NLR has been gaining growing attraction in many fields of medicine in the past decade.[17] It is more sensitive than neutrophils and lymphocytes alone to detect inflammation and predict the outcome of different diseases.[18-20] Under physiologic stress, its level can be increased by endogenous cortisol and catecholamines. Our study demonstrates that NLR has a potential ability to identify COVID-19 patients at a high risk of death, indicating that a higher inflammation and physiologic stress existed in non-survival patients with COVID-19.
Ferritin is a regulator of iron homeostasis.[21] Growing evidence showed that it can be served as a biomarker of inflammation.[22] Moreover, ferritin also has a pathogenic role in inflammatory diseases by directly modulating lymphocyte function.[23] During the acute phase of infection, macrophages and other cells secrete ferritin to suppress overactive inflammatory responses that cause so-called hyperferritinemia syndrome.[23,24] This process is believed to contribute to cytokine storm.[23] Our study found that the level of circulating ferritin was significantly higher in non-survivors than in survivors, and can be used as a useful biomarker to predict worse outcomes of patients with COVID-19. As a coincidence, a very recent study found that ferritin was a predictor of poor outcomes in patients with influenza infection.[25] In addition, some effective treatments of inflammatory diseases have been found to benefit from targeting ferritin.[23] The potential therapeutic target of ferritin is worthy of further study.
Changes in circulating hormone levels are a common phenomenon during critical illness.[26] Thyroid hormones play a key role in the maintenance of body growth by modulating metabolism and the immune system. Our previous study[27] and others demonstrated that reduced thyroid hormones were associated with the severity of the diseases and the outcomes of critically ill patients.These alterations of thyroid hormone levels are referred to as “euthyroid sick syndrome” or “nonthyroidal illness syndrome (NTIS)”.[28] This study found FT3 was significantly lower in non-survivors than in survivors, indicating that it reflected the severity of COVID-19. Indeed, FT3 is associated with the SOFA score and has a moderate-to-excellent ability to predict in-hospital mortality of patients with COVID-19. The reduced circulating FT3 is a predictor of in-hospital mortality, indicating that the infection of SARS-CoV-2 may suppress the production of FT3 or promote the clearance of it. The underlying mechanism of this is however unclear and needs to be investigated by further studies.
Cytokines play important roles in regulating the immune response to microbial infection. However, the unregulated release of cytokines, so-called cytokine storm, can lead to multiple organ injury and cause a fatal outcome.[29] Although the cytokine storm has drawn broad attention regarding its pathogenic role in COVID-19,[30] little is known about the alteration in the level of different cytokines during the process of this disease. A recent study reported that the level of pro-inflammatory cytokine IL-6 was significantly higher in non-survivors than in survivors. Patients with elevated IL-6 had a higher risk of death.[15] However, the performance of IL-6 for the prediction of fatality was not evaluated in this study. Moreover, other major pro- and anti-inflammatory cytokines were not investigated. The current study evaluated six different cytokines or their receptors and found that IL-2R was significantly higher in non-survivors than in survivors. The current study found, besides IL-6, pro-inflammatory cytokines and their receptors (IL-8, TNF-α, IL-2) as well as anti-inflammatory cytokines (IL-10) were also higher in non-survivor, indicating that a higher degree of both pro- and anti-inflammatory occurred in severer ill patients with SARS-CoV-2 infection. These cytokines and their signal pathways may serve as the potential therapeutic target for COVID-19, which requires further studies to explore.
This study has some limitations. First, this was a single-center study with 121 ICU patients recruited. The relationship between these indicators and in-hospital mortality may be changed if more patients from different centers were included. Second, we only collected the laboratory indicators at admission to ICU, and the dynamics of these indicators were unknown. However, only patients with life-threatening conditions can be transferred to the ICU, and therefore laboratory findings at day one only reflected the severity of these ICU patients at an early stage of the disease. Third, not every variable was available for all patients in our study. Thus the direct comparison of discriminability between some variables was not conducted as the number of available cases was limited. Fourth, some patients may have bacterial infection during the treatment which might affect the level of these biomarkers.
CONCLUSIONS
In this retrospective cohort study, we find three biomarkers to predict in-hospital death of patients with COVID-19, and provide important information for the risk stratification of these patients in future clinical practice.
ACKNOWLEDGMENTS
The authors would like to thank Ms. Jennifer Shing for English editing for this paper.
Footnotes
Funding: This work was supported by the National Key Research and Development Project of the Ministry of Science and Technology, China (2018YFC1313700) and “Gaoyuan” Project of Pudong Health and Family Planning Commission (PWYgy2018-6).
Ethical approval: This study was approved by the Ethics Committee of Peking University People’s Hospital.
Conflicts of interests: The authors declare that they have no competing interests.
Contributors: WG and LYR contributed equally to this work. FLW and TBW conceived, designed the study, analyzed the data and wrote the paper. WG, WBG, LYR, JHZ, ZD, and QGG contributed to data acquisition and analysis.
REFERENCES
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–33. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–9. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang D, Lin M, Wei L, Xie L, Zhu G, Cruz CS, et al. Epidemiologic and clinical characteristics of novel coronavirus infections involving 13 patients outside Wuhan, China. JAMA. 2020;323(11):1092–3. doi: 10.1001/jama.2020.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382(10):929–36. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allione A, Giamello JD, Bernardi S, Paglietta G, Cavalot GLM, Dutto LA, et al. Coronavirus disease 2019 (COVID-19) and prosthetic heart valve:An additional coagulative challenge. World J Emerg Med. 2020;11(4):258–9. doi: 10.5847/wjem.j.1920-8642.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO. Coronavirus disease 2019 (COVID-19) situation report –55, Mar, 2020. [Accessed March 16, 2020]. https: //www.who.int/docs/default-source/coronaviruse/situation-reports/20200315-sitrep-55-covid-19.pdf?sfvrsn=33daa5cb_6 .
- 7.Levy MM, Evans LE, Rhodes A. The surviving sepsis campaign bundle:2018 update. Intensive Care Med. 2018;44(6):925–8. doi: 10.1007/s00134-018-5085-0. [DOI] [PubMed] [Google Scholar]
- 8.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Force AD, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E. Acute respiratory distress syndrome. JAMA. 2012;307(23):2526–33. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 10.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–84. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 11.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;18(8):891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 12.Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A, et al. Assessment of clinical criteria for sepsis:for the third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):762–74. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mackinnon A, Mulligan R. Combining cognitive testing and informant report to increase accuracy in screening for dementia. Am J Psychiatry. 1998;155(11):1529–35. doi: 10.1176/ajp.155.11.1529. [DOI] [PubMed] [Google Scholar]
- 14.Hanley JA, McNeil BJ. A method of comparing the areas under ROC curves derived from same cases. Radiology. 1983;148(3):839–43. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 15.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–81. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faria SS, Fernandes Jr PC, Silva MJ, Lima VC, Fontes W, Freitas-Junior R, et al. The neutrophil-to-lymphocyte ratio:a narrative review. Ecancermedicalscience. 2016;10:702. doi: 10.3332/ecancer.2016.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors:a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 19.Bhat T, Teli S, Rijal J, Bhat H, Raza M, Khoueiry G, et al. Neutrophil to lymphocyte ratio and cardiovascular diseases: a review. Expert Rev Cardiovasc Ther. 2013;11(1):55–9. doi: 10.1586/erc.12.159. [DOI] [PubMed] [Google Scholar]
- 20.Hwang SY, Shin TG, Jo IJ, Jeon K, Suh GY, Lee TR, et al. Neutrophil-to-lymphocyte ratio as a prognostic marker in critically-ill septic patients. Am J Emerg Med. 2017;35(2):234–9. doi: 10.1016/j.ajem.2016.10.055. [DOI] [PubMed] [Google Scholar]
- 21.Knovich MA, Storey JA, Coffman LG, Torti SV, Torti FM. Ferritin for the clinician. Blood Rev. 2009;23(3):95–104. doi: 10.1016/j.blre.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kell DB, Pretorius E. Serum ferritin is an important inflammatory disease marker, as it is mainly a leakage product from damaged cells. Metallomics. 2014;6(4):748–73. doi: 10.1039/c3mt00347g. [DOI] [PubMed] [Google Scholar]
- 23.Rosário C, Zandman-Goddard G, Meyron-Holtz EG, D'Cruz DP, Shoenfeld Y. The hyperferritinemic syndrome:macrophage activation syndrome, Still's disease, septic shock and catastrophic antiphospholipid syndrome. BMC Med. 2013;11(1):185. doi: 10.1186/1741-7015-11-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen LA, Gutierrez L, Weiss A, Leichtmann-Bardoogo Y, Zhang DL, Crooks DR, et al. Serum ferritin is derived primarily from macrophages through a nonclassical secretory pathway. Blood. 2010;116(9):1574–84. doi: 10.1182/blood-2009-11-253815. [DOI] [PubMed] [Google Scholar]
- 25.Lalueza A, Ayuso B, Arrieta E, Trujillo H, Folgueira D, Cueto C, et al. Elevation of serum ferritin levels for predicting a poor outcome in hospitalized patients with influenza infection. Clin Microbiol Infect. 2020;26(11):1557.e9–1557.e15. doi: 10.1016/j.cmi.2020.02.018. [DOI] [PubMed] [Google Scholar]
- 26.van den Berghe G. The neuroendocrine response to stress is a dynamic process. Best Pract Res Clin Endocrinol Metab. 2001;15(4):405–19. doi: 10.1053/beem.2001.0160. [DOI] [PubMed] [Google Scholar]
- 27.Wang F, Pan W, Wang H, Wang S, Pan S, Ge J. Relationship between thyroid function and ICU mortality:a prospective observation study. Crit Care. 2012;16(1):R11. doi: 10.1186/cc11151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warner MH, Beckett GJ. Mechanisms behind the non-thyroidal illness syndrome:an update. J Endocrinol. 2010;205(1):1–3. doi: 10.1677/JOE-09-0412. [DOI] [PubMed] [Google Scholar]
- 29.Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76(1):16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mehta P, McAuley DF, Brown M, Sanchez M, Tattersall RS, Manson JJ. COVID-19:consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–4. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


