Abstract
COVID-19 patients typically present with lower airway disease, although involvement of other organ systems is usually the rule. Hematological manifestations such as thrombocytopenia and reduced lymphocyte and eosinophil numbers are highly prevalent in COVID-19 and have prognostic significance. Few data, however, are available about the prevalence and significance of anemia in COVID-19. In an observational study, we investigated the prevalence, pathogenesis and clinical significance of anemia among 206 patients with COVID-19 at the time of their hospitalization in an Internal Medicine unit. The prevalence of anemia was 61% in COVID-19, compared with 45% in a control group of 71 patients with clinical and laboratory findings suggestive of COVID-19, but nasopharyngeal swab tests negative for SARS-CoV-2 RNA (p = 0.022). Mortality was higher in SARS-CoV-2 positive patients. In COVID-19, females had lower hemoglobin concentration than males and a higher prevalence of moderate/severe anemia (25% versus 13%, p = 0.032). In most cases, anemia was mild and due to inflammation, sometimes associated with iron and/or vitamin deficiencies. Determinants of hemoglobin concentration included: erythrocyte sedimentation rate, serum cholinesterase, ferritin and protein concentrations and number of chronic diseases affecting each patient. Hemoglobin concentration was not related to overall survival that was, on the contrary, influenced by red blood cell distribution width, age, lactate dehydrogenase and the ratio of arterial partial oxygen pressure to inspired oxygen fraction. In conclusion, our results highlight anemia as a common manifestation in COVID-19. Although anemia does not directly influence mortality, it usually affects elderly, frail patients and can negatively influence their quality of life.
Keywords: COVID-19, Anemia, Oxygen partial pressure/oxygen concentration, Red blood cell distribution width, Anemia of inflammation
Introduction
Hematological abnormalities, such as thrombocytopenia, reduced numbers of peripheral blood lymphocytes and eosinophils with an increased polymorphonuclear-to-lymphocyte ratio are common features of novel coronavirus disease 2019 (COVID-19), especially in more severe cases [1–7]. To date, no reports specifically addressed the investigation of anemia in COVID-19, with determination of its prevalence, pathogenesis and prognostic significance. The results from published case series are often conflicting, with some papers reporting similar hemoglobin (Hb) concentrations in patients who survived and those who died because of SARS-CoV-2 infection [1], or in intensive care unit (ICU) compared with non-ICU patients [5], whereas others reported lower Hb levels in patients with more severe disease [8]. Recent case reports described the association of COVID-19 with autoimmune hemolytic anemia (AHA), including one case of cold agglutinin disease AHA [9], but cases of AHA in COVID-19 are probably uncommon, and it is still unknown if AHA prevalence is higher in COVID-19 than in the general population [10, 11].
On the other hand, systemic inflammation is the rule in COVID-19; in some cases, it progresses to a secondary hemophagocytic lymphohistiocytosis-like condition, characterized by hyperinflammation, endothelial cell damage with systemic impairment of microcirculation and angiogenesis, and acute respiratory distress syndrome (ARDS) that, for many patients, represents the final cause of death [12–16]. Inflammation profoundly affects erythropoiesis through different mechanisms, partly sustained by abnormal iron metabolism mediated by interleukin (IL)-6 overproduction and partly due to pro-inflammatory cytokines, such as interferon-γ, IL-1, IL-33 and tumor necrosis factor (TNF)-α [17, 18]. The latter exert inhibitory effects on erythroid progenitor and precursor cells and may reduce erythrocyte lifespan [19–23]. These perturbations frequently lead to the development of anemia of inflammation, the second most common form of anemia worldwide and, probably, the most common among hospitalized patients in industrialized countries. Given the importance of inflammatory processes associated with COVID-19 and their role in the pathogenesis of anemia, we investigated the prevalence of anemia, with its clinical and biologic correlates, in COVID-19 patients at the time of hospitalization in the Internal Medicine unit of our Institution.
Patients and methods
Study design
In this observational study, we analyzed data from 206 patients hospitalized in the Internal Medicine Unit of our Institution with a laboratory-confirmed diagnosis of COVID-19 between March 1, 2020, and April 11, 2020. For comparison, 71 patients admitted to the same Unit with a clinical picture (fever, respiratory failure), chest x-ray imaging (bilateral interstitial pneumonia) and laboratory findings (increased serum concentrations of lactate dehydrogenase (LDH), C-reactive protein, D-dimer and ferritin with lymphopenia and eosinopenia) strongly suggestive of COVID-19, but with a minimum of two nasopharyngeal swab tests negative for SARS-CoV-2 RNA, were also investigated as controls. The study was approved by the local ethics committee and performed in the respect of the Helsinki declaration. Routine clinical data were collected in an anonymized format; as such, the study is exempt from the need to take specific written informed consent.
Patient investigations
Most patients presented to the Emergency Department because of persistent fever and/or shortness of breath, often associated with respiratory failure. Based on admission laboratory tests, we determined the prevalence and pathogenesis of anemia and its relationship with outcome and other clinical and laboratory findings. The patients were classified as anemic if Hb < 130 g/L in males and < 120 g/L in females; mild anemia was defined by Hb ≥ 95 g/L, moderate anemia by 80 ≤ Hb < 95 g/L, severe anemia by Hb < 80 g/L [24]. Iron deficiency anemia was diagnosed if serum ferritin < 30 mcg/L; with serum ferritin ≥ 30 mcg/L but ≤ 100 mcg/L and transferrin saturation < 20% the diagnosis was iron deficiency with inflammation; a serum ferritin > 100 mcg/L with transferrin saturation < 20% defined anemia of inflammation [17, 25]. The glomerular filtration rate was estimated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [26]. Determination of the individual patient burden of disease before the index hospitalization was based on the number of different chronic conditions affecting each patient [27].
Statistical analysis
Data were analyzed by descriptive statistics and results reported as mean and SD or median and interquartile range (IQR), depending on each variable value distribution; differences between groups were tested by the Student’s t-test or the Mann–Whitney U test. Categorical variables are presented as proportions and were compared by the Chi-square test. Correlations were analyzed by either the Pearson or the Spearman correlation coefficients and by multiple regression in case of multivariate analysis. Survival data are shown as Kaplan–Meier Survival Plots and were analyzed by the Cox proportional hazards model. All tests were two-sided; differences and correlations were considered significant if p < 0.050.
Results
General features of study patients
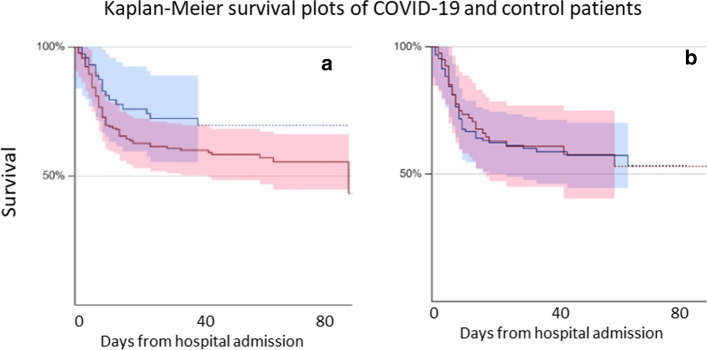
Table 1 shows the main demographic and laboratory parameters of the patients investigated in the present study, comparing subjects with laboratory-confirmed COVID-19 and those with negative nasopharyngeal swab test. At hospital admission, the group with a confirmed diagnosis of COVID-19 was characterized by lower Hb concentration, a higher prevalence of overall anemia and moderate/severe anemia (OR 1.88, 95% CI 1.09–3.24, for overall anemia and 4.16, 95% CI 1.39–15.68, for moderate/severe anemia), reduced platelet counts and more compromised renal function (Table 1). Inflammation and erythropoiesis-associated indices were not significantly different between the two groups. After a median follow-up of 22 days (IQR 8–49 days), mortality was lower for SARS-CoV-2 RNA negative patients than for those with a confirmed COVID-19 diagnosis (OR 0.52, 95% CI 0.29–0.94, p = 0.0332; Fig. 1a).
Table 1.
Demographic and general features of the study patients
| Characteristics | All patients (N = 277) | SARS-CoV-2 positive patients (N = 206) | Controls, SARS-CoV-2 negative (N = 71) | p value |
|---|---|---|---|---|
| Age, years (SD) | 71 (15) | 71 (14) | 71 (14) | 0.610 |
| Gender | 0.204 | |||
|
Male, n (%) Female, n (%) |
171 (62) 105 (38) |
133 (65) 72 (35) |
40 (56) 31 (44) |
|
| Died or transferred to the ICU, n (%) | 114 (41) | 91 (44) | 24 (34) | 0.162 |
| Hospitalization days, n (IQR) | 11 (7–21) | 13 (7–22) | 9 (7–13) | 0.061 |
| Hemoglobin, g/L (SD) | 120 (22) | 118 (23) | 126 (18) | 0.008 |
| Anemic patients, n (%) | 157 (57) | 125 (61) | 32 (45) | 0.022 |
| Moderate/severe anemia, n (%) | 38 (14) | 35 (17) | 3 (4) | 0.007 |
| Hematocrit, % (SD) | 35.9 (6.0) | 35.3 (6.3) | 37.6 (4.7) | 0.006 |
| MCV, fL (SD) | 90.3 (7.6) | 90.9 (7.3) | 88.8 (8.4) | 0.434 |
| RDW, % (SD) | 15.1 (2.4) | 15.2 (2.4) | 14.9 (2.0 | 0.369 |
| Platelet count, × 109/L (SD) | 216 (105) | 206 (94) | 244 (129) | 0.008 |
| White blood cell count, × 109/L (IQR) | 6.7 (4.9–9.4) | 6.5 (4.6–9.2) | 7.8 (5.8–10.6) | 0.015 |
| Lymphocyte count, × 109/L (IQR) | 0.7 (0.5–1.0) | 0.7 (0.5–1.0) | 0.8 (0.6–1.0) | 0.139 |
| Ferritin, mcg/L (IQR) | 635 (255–1338) | 640 (304–1338) | 390 (144–1265) | 0.4654 |
| Transferrin saturation, % (IQR) | 12 (8–20) | 12 (7–21) | 12 (8–16) | 0.8965 |
| VitaminB12, mcg/L (IQR) | 539 (309–858) | 520 (309–845) | 582 (378–891) | 0.6965 |
| Folate, mg/L (IQR) | 6.1 (3.8–11.2) | 6.4 (3.8–12.0) | 5.1 (3.2–8.6) | 0.3222 |
| Reticulocyte index, % (IQR) | 0.37 (0.27–0.54) | |||
| hs-CRP, mg/dL (IQR) | 11.47 (6.16–16.33) | 11.50 (6.07–16.78) | 10.73 (7.03–15.75) | 0.7566* |
| ESR, mm/hour (IQR) | 77 (46–99) | 77 (47–102) | 75 (34–91) | 0.2340 |
| PCTI, ng/ml (IQR) | 0.24 (0.09–0.71) | 0.26 (0.09–0.81) | 0.18 (0.08–0.49) | 0.3320* |
| eGFR, mL/1.73 m2/min (IQR) | 77 (46–94) | 72 (44–92) | 82 (55–99) | 0.0488 |
hs-CRP, high sensitivity C-reactive protein; ESR erythrocyte sedimentation rate; PCTI, procalcitonin; eGFR estimated glomerular filtration rate; bold p values represent statistically significant differences
Fig. 1.
Kaplan–Meier survival curves of (a) SARS-CoV-2 positive (red line) and control SARS-CoV-2 negative (blue line) patients and (b) of anemic (red line) and non-anemic (blue line) SARS-CoV-2 positive patients. The difference between SARS-CoV-2 positive and SARS-CoV-2 negative patients is significant (p = 0.033)
COVID-19 and anemia
Subsequent analysis was restricted to patients with laboratory-confirmed COVID-19. The global prevalence of anemia was 61% and females had lower Hb concentrations than males (112 ± 22 g/L vs 122 ± 22 g/L, p < 0.001), although the proportion of subjects with anemia was not different between sexes (Table 2). Anemia was mild (Hb ≥ 95 g/L) in 91 out of 126 anemic subjects (72%), and moderate/severe anemia was more prevalent among females (18 of 72 females versus 17 of 134 males, OR 2.294, 95% CI 1.098–4.795, p = 0.032); age did not influence the severity of anemia (data not shown). Figure 2 shows the pathogenesis of anemia in the current series of patients. In 93% of cases, serum ferritin concentration was above 100 mcg/L (> 300 mcg/L in 75% of patients), usually in association with a transferrin saturation below 20% and a reticulocyte count inadequate for the degree of anemia (reticulocyte index < 2% in all but one patient); all together, these observations suggest that most cases of anemia were due to inflammation. Only one subject had serum ferritin < 30 mcg/L, indicative of absolute iron deficiency as the main mechanism of anemia, whereas 7% of anemic patients had 30 ≤ serum ferritin ≤ 100 mcg/L, with transferrin saturation < 20%, expression of iron deficiency associated with inflammation.
Table 2.
Clinical and laboratory features of anemic and non-anemic SARS-CoV-2 positive patients
| Characteristics | All patients (N = 206) | Anemic patients (N = 126) | Non-anemic patients (N = 80) | p value |
|---|---|---|---|---|
| Age, years (SD) | 71 (14) | 71 (16) | 72 (15) | 0.833 |
| Gender | ||||
|
Male, N (%) Female, N (%) |
134 (65) 72 (35) |
82 (65) 44 (35) |
52 (65) 28 (35) |
1.000 |
| Hospitalization days, N (IQR) | 13 (7–22) | 13 (7–25) | 12 (7–19) | 0.562 |
| Hemoglobin, g/L (SD) | 118 (23) | 105 (16) | 139 (13) | < 0.001 |
| MCV, fL (SD) | 90.9 (7.3) | 90.7 (8.5) | 91.0 (4.8) | 0.233 |
| RDW, % (SD) | 15.2 (2.4) | 15.9 (2.8) | 14.2 (1.3) | < 0.001 |
| Reticulocyte index (available for 43 anemic patients), % (IQR) | 0.37 (0.27–0.54) | |||
| Ferritin, mcg/L (IQR) | 640 (304–1338) | 635 (213–1338) | 693 (437–1279) | 0.222 |
| Transferrin saturation, % (IQR) | 12 (8–21) | 11 (7–19) | 18 (12–25) | 0.624 |
| Platelet count, × 109/L (IQR) | 197 (136–251) | 204 (136–250) | 193 (137–250) | 0.887 |
| White blood cell count, × 109/L (IQR) | 6.5 (4.6–9.2) | 6.4 (4.6–8.7) | 6.5 (4.6–10.2) | 0.780 |
| Lymphocyte count, × 109/L (IQR) | 0.7 (0.5–1.0) | 0.7 (0.5–0.9) | 0.7 (0.5–1.0) | 0.492 |
| eGFR, ml/1.73 m2/min (IQR) | 60 (32–89) | 49 (26–87) | 77 (45–90) | 0.039 |
| hs-CRP, mg/dL (IQR) | 11.50 (6.07–16.78) | 11.72 (5.45–17.66) | 11.20 (6.52–15.39) | 0.849 |
| ESR, mm/h (IQR) | 77 (47–102) | 92 (68–107) | 44 (34–59) | < 0.001 |
| Cholinesterase, U/L (SD) | 6201 (2187) | 5698 (2169) | 6970 (1995) | < 0.001 |
| LDH, U/L (SD) | 381 (146) | 371 (138) | 400 (153) | 0.0265 |
| Serum proteins, g/L (IQR) | 63 (58–68) | 61 (57–66) | 66 (62–68) | < 0.001 |
| Albumin, g/L (IQR) | 29 (26–32) | 28 (25–31) | 30 (28–33) | 0.002 |
| Number of chronic diseases, N (IQR) | 2.0 (1.0–3.0) | 2.0 (1.0–3.5) | 1.0 (1.0–2.0) | < 0.0001 |
| One-month mortality, N (%) | 78 (38) | 49 (39) | 29 (36) | 0.769 |
hs-CRP, high sensitivity C-reactive protein; ESR, erythrocyte sedimentation rate; eGFR, estimated glomerular filtration rate; significant differences are shown in bold
Fig. 2.
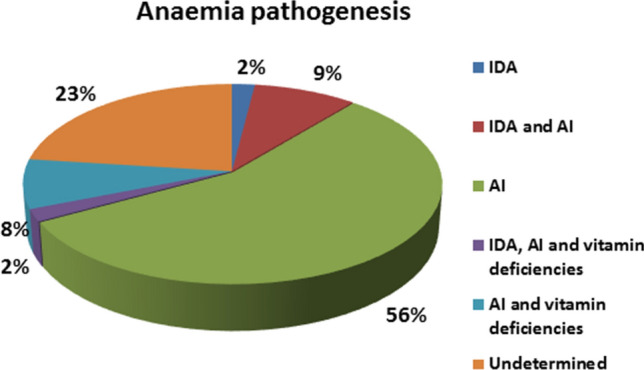
Causes of anemia in 126 anemic patients with laboratory-confirmed COVID-19. Serum concentrations of vitamin B12 and folate were available only for a subset of patients (N = 57); the prevalence of vitamin deficiencies can, therefore, be underestimated
Of 57 patients with anemia for whom serum concentrations of vitamin B12 and folate were available, 13 had vitamin deficiencies; of these, 11 had an associated anemia of inflammation (transferrin saturation < 20% with serum ferritin > 100 mcg/L) and one had a combination of iron deficiency anemia and anemia of inflammation.
Patients with anemia had a higher number of comorbidities (Table 2), whereas the proportion of patients on anticoagulant or antiplatelet treatment at hospital admission was not different between the two groups (53 of 126 anemic compared with 38 of 80 non-anemic patients, p = 0.474). Several laboratory parameters correlated with Hb (Table 3); on multivariate analysis, however, only erythrocyte sedimentation rate, total serum protein concentration, cholinesterase, the logarithm of serum ferritin and the number of chronic diseases affecting each patient preserved significant correlations (multiple regression coefficient R = 0.792). No association was found between anemia or Hb concentration and the neutrophil-to-lymphocyte or the platelet-to-lymphocyte ratios (data not shown).
Table 3.
Correlations of Hb with demographic and laboratory parameters in SARS-CoV-2 positive patients
| Parameter | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| Correlation coefficient | p value | Partial correlation coefficient | p value | |
| Age | − 0.015 | 0.830 | – | – |
| Gender | 0.210 | 0.003 | – | – |
| Ferritin* | 0.209 | 0.040 | 0.360 | 0.028 |
| hs-CRP* | 0.134 | 0.058 | – | – |
| ESR | − 0.570 | < 0.001 | − 0.406 | 0.013 |
| AST | 0.229 | 0.001 | – | – |
| ALT | 0.194 | 0.006 | – | – |
| Cholinesterase | 0.279 | < 0.001 | 0.344 | 0.037 |
| LDH | 0.250 | < 0.001** | – | – |
| Serum proteins | 0.305 | < 0.001 | 0.380 | 0.020 |
| eGFR | 0.178 | 0.006** | – | – |
| Number of chronic diseases | 0.366 | < 0.001 | − 0.328 | − 0.047 |
| PO2/FiO2 | − 0.198 | 0.039 | – | – |
*Analysis was performed using the logarithms of serum ferritin and hs-CRP; **indicate Spearman rank order correlations; the other correlations were determined using the Pearson correlation coefficient
Anemia had no influence on overall survival (Fig. 1b shows the Kaplan–Meier survival curves for anemic and non-anemic patients) and other outcome measures including transfer to the ICU and/or death during hospitalization and death within one month from hospital admission. Cox proportional hazards survival regression, on the contrary, showed that the red blood cell distribution width (RDW), together with age, LDH and the ratio of partial oxygen pressure to fraction of inspired oxygen (PaO2/FiO2) in arterial blood, were independent predictors of mortality (Table 4). The only clinical outcome showing a significant inverse correlation with Hb was the length of hospital stay for females surviving to discharge (r = − 0.393, p = 0.010 in females vs. r = − 0.001, p = 0.987 in males).
Table 4.
Cox proportional hazards survival regression
| Covariates | Risk ratio | 95% CI | p value |
|---|---|---|---|
| RDW | 1.1853 | 1.0436–1.3463 | 0.0089 |
| Age | 1.0639 | 1.0317–1.0971 | 0.0001 |
| P/F | 0.9957 | 0.9924–0.9991 | 0.0128 |
| LDH | 1.0026 | 1.0005–1.0046 | 0.0162 |
Covariates significantly associated with mortality in SARS-CoV-2 RNA positive patients;
P/F, oxygen partial pressure/oxygen concentration
Within 1 week from hospital admission, Hb further decreased a mean of 7 g/L in SARS-Cov-2 positive patients, the reduction being more pronounced in non-anemic compared with anemic patients (11 g/L, vs. 4 g/L, p = 0.008).
Discussion
At hospital admission in the Internal Medicine unit of our Institution, anemia affected 61% of COVID-19 patients, compared with a 45% prevalence observed in control subjects with similar clinical and laboratory features, but negative COVID-19 nasopharyngeal swabs, who were admitted during the same period. Most cases of COVID-19-associated anemia were due to inflammation, as suggested by normal/high serum ferritin concentrations combined with reduced transferrin saturation and by increased inflammatory indices such as erythrocyte sedimentation rate and high sensitivity C-reactive protein in the large majority of cases. Low transferrin saturation and a reticulocyte index < 2.0 suggest that functional iron deficiency, due to macrophage iron retention, and inadequate bone marrow response to anemia were major factors contributing to the development of anemia.
COVID-19 is commonly associated with a coagulopathy that has been suggested to represent a combination of low-grade disseminated intravascular coagulation and localized pulmonary thrombotic microangiopathy and might contribute to anemia through intravascular hemolysis [28]. In our series, however, the absence of any correlation of Hb with bilirubin, the weak positive correlation with LDH (that lost significance on multivariate analysis), together with a low reticulocyte index and a low prevalence of thrombocytopenia (platelet count < 100 × 109/L was present in 18 out of 206 SARS-CoV-2 positive patients) rule out a role for hemolysis and/or thrombotic microangiopathy as contributing factors in most cases of COVID-19-associated anemia.
Iron and vitamin deficiencies, either isolated or associated with inflammation, were detected in less than 10% of COVID-19 patients with anemia; however, our definition of iron deficiency with or without inflammation, based on serum ferritin concentration ≤ 100 mcg/L and transferrin saturation < 20%, may be too restrictive for COVID-19 patients in whom serum ferritin is often markedly increased due to “hyperinflammation”, thus leading to underestimation of iron deficiency prevalence. At hospital admission, no cases of anemia due to bleeding were observed although, as previously reported, five patients from the present series subsequently developed severe anemization from peptic ulcer bleeding while on thromboprophylaxis with fractionated heparin [29].
Of the hematologic parameters included in complete blood counts, only RDW had prognostic significance, increased RDW representing an independent risk factor for mortality. Similar observations have been reported in COVID-19 by Wang et al. [8] and Foy et al. [30], who found that higher RDW values were associated with more severe COVID-19 and increasing mortality rates, in heart disease, sepsis and in critically ill patients [31–36]. Although the underlying mechanisms relating elevated RDW with critical disease and mortality are unclear, it has been suggested that systemic inflammation, oxydative stress, renal dysfunction and malnutrition represent common pathogenetic mechanisms leading to increased RDW and to a more severe course of the underlying disease [33]; all these factors are common features of severe COVID-19.
The patients described in the present study are older than typical COVID-19 subjects investigated in other studies, mean age being 71 years compared with medians of 41–65 years in previously published series of hospitalized COVID-19 patients [1–7, 37]. As a consequence, our results may overestimate the prevalence of anemia in COVID-19 at hospitalization. Older age is a well-known risk factor for anemia; the prevalence of elderly anemia is up to 12% in subjects 65 years or older living in the community and 47% in nursing home residents and is often characterized by low-grade inflammation [38–44]. Exacerbation of inflammation by the cytokine storm which occurs during the SARS-CoV-2 infection can explain the high prevalence of anemia found in our study, although we could not show any influence of age on Hb concentration. Older age, in addition, is a risk factor for a more severe course of SARS-CoV-2 infection [45, 46] and probably accounts for the high mortality rate reported in this study (38% at one month from hospital admission).
In conclusion, our data show that anemia is a common and persistent finding in COVID-19 during hospitalization outside of the ICU. Given the impact that anemia has on quality of life [44, 47], the problem cannot be overlooked and the pathogenesis of anemia should be investigated and treatment instituted whenever possible. Given high costs, risk of side effects and shortage of blood supply, a problem that has become more serious during the COVID-19 pandemic, red blood cell transfusions in COVID-19 should be used according to effective blood management strategies and efforts must be directed to reduce anemia prevalence and severity [48]. Since iron deficiency anemia can be effectively treated without red blood cell transfusions, stringent and accurate criteria defining iron deficiency and iron-restricted erythropoiesis in COVID-19, especially during the “hyperinflammatory” phase of the disease, must be established. Hopefully, together with emerging treatment strategies [49], the correct diagnosis and effective treatment of iron deficiency will reduce the clinical burden of anemia in COVID-19.
Acknowledgements
Internal Medicine Covid-19 Collaborators: Giampiera Bertolino, Silvia Codega, Filippo Costanzo, Roberto Cresci, Giuseppe Derosa, Michele Di Stefano, Francesco Falaschi, Carmine Iadarola, Elisabetta Lovati, Pietro Carlo Lucotti, Alessandra Martignoni, Caterina Mengoli, Emanuela Miceli, Amedeo Mugellini,Chiara Muggia, Patrizia Noris, Elisabetta Pagani, Ilaria Palumbo, Alessandro Pecci, Tiziano Perrone, Carla Pieresca, Paola Stefania Preti, Maria Concetta Russo, Carmelo Sgarlata, Luisa Siciliani, Andrea Staniscia, Francesca Torello Vjera, Giovanna Achilli, Andrea Agostinelli,Valentina Antoci, Francesco Banfi, Irene Benedetti, Michele Brattoli, Ginevra Cambiè, Roberta Canta, Sara Cococcia, Federico Conca, Mariangela Delliponti, Virginia Del Rio, Francesco Di Terlizzi, Anna Fiengo, Tommaso Forni, Giulia Freddi, Chiara Frigerio, Alessandra Fusco, Margherita Gabba, Matteo Garolfi, Giulia Gori, Giacomo Grandi, Paolo Grimaldi, Alice Lampugnani, Federica Lepore, Gianluca Lettieri, Jacopo Mambella, Chiara Mercanti, Alba Nardone, Luca Pace, Lucia Padovini, Lavinia Pitotti, Margherita Reduzzi, Giovanni Rigano, Giorgio Rotola, Umberto Sabatini, Lucia Salvi, Giovanni Santacroce, Jessica Savioli, Simone Soriano, Carmine Spataro, Debora Stefani.
The work was supported by Fondazione IRCCS Policlinico San Matteo, Pavia, Italy. The investigators are grateful to the patients who participated in this study and acknowledge the contribution of the healthcare professionals who faced the COVID-19 epidemic at the Internal Medicine Unit of San Matteo Hospital Foundation in Pavia, Italy.
Author contributions
All Authors have contributed to the design of the study, acquisition, analysis, and interpretation of data. Gaetano Bergamaschi, Federica Borrelli de Andreis and Antonio Di Sabatino wrote the manuscript. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
The Authors declare that they have no conflict of interest.
Footnotes
The original online version of this article was revised: The co-authorship of the members of “Internal Medicine Covid-19 Collaborators” was inadvertently not clearly indicated and the given name and family name of the second author was incorrectly tagged in the xml data, The correct given name is Federica and family name is Borrelli de Andreis.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
3/17/2021
A Correction to this paper has been published: 10.1007/s10238-021-00699-8
Contributor Information
Gaetano Bergamaschi, Email: n.bergamaschi@smatteo.pv.it.
the Internal Medicine Covid-19 Collaborators:
Giampiera Bertolino, Silvia Codega, Filippo Costanzo, Roberto Cresci, Giuseppe Derosa, Michele Di Stefano, Francesco Falaschi, Carmine Iadarola, Elisabetta Lovati, Pietro Carlo Lucotti, Alessandra Martignoni, Caterina Mengoli, Emanuela Miceli, Amedeo Mugellini, Chiara Muggia, Patrizia Noris, Elisabetta Pagani, Ilaria Palumbo, Alessandro Pecci, Tiziano Perrone, Carla Pieresca, Paola Stefania Preti, Maria Concetta Russo, Carmelo Sgarlata, Luisa Siciliani, Andrea Staniscia, Francesca Torello Vjera, Giovanna Achilli, Andrea Agostinelli, Valentina Antoci, Francesco Banfi, Irene Benedetti, Michele Brattoli, Ginevra Cambiè, Roberta Canta, Sara Cococcia, Federico Conca, Mariangela Delliponti, Virginia Del Rio, Francesco Di Terlizzi, Anna Fiengo, Tommaso Forni, Giulia Freddi, Chiara Frigerio, Alessandra Fusco, Margherita Gabba, Matteo Garolfi, Giulia Gori, Giacomo Grandi, Paolo Grimaldi, Alice Lampugnani, Federica Lepore, Gianluca Lettieri, Jacopo Mambella, Chiara Mercanti, Alba Nardone, Luca Pace, Lucia Padovini, Lavinia Pitotti, Margherita Reduzzi, Giovanni Rigano, Giorgio Rotola, Umberto Sabatini, Lucia Salvi, Giovanni Santacroce, Jessica Savioli, Simone Soriano, Carmine Spataro, and Debora Stefani
References
- 1.Zhou M, Qi J, Li X, et al. The proportion of patients with thrombocytopenia in three human-susceptible coronavirus infections: a systematic review and meta-analysis. Br J Haematol. 2020;189:438–441. doi: 10.1111/bjh.16655. [DOI] [PubMed] [Google Scholar]
- 2.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan China [published online ahead print, 2020 Feb 7] JAMA. 2020;323:1061–9. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun DW, Zhang D, Tian RH, et al. The underlying changes and predicting role of peripheral blood inflammatory cells in severe COVID-19 patients: a sentinel? Clin Chim Acta. 2020;508:122–129. doi: 10.1016/j.cca.2020.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China [published correction appears in Lancet. 2020 Jan 30] Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheng L, Wang X, Tang N, Meng F, Huang L, Li D. Clinical characteristics of moderate and severe cases with COVID-19 in Wuhan, China: a retrospective study. Clin Exp Med [published online ahead print, 2020 Sep 19] 2020 doi: 10.1007/s10238-020-00662-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gavriatopoulou M, Korompoki E, Fotiou D, et al. Organ-specific manifestations of COVID-19 infection. Clin Exp Med. 2020;20:493–506. doi: 10.1007/s10238-020-00648-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang C, Deng R, Gou L, et al. Preliminary study to identify severe from moderate cases of COVID-19 using combined hematology parameters. Ann Transl Med. 2020;8:593. doi: 10.21037/atm-20-3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zagorski E, Pawar T, Rahimian S, Forman D. Cold agglutinin autoimmune haemolytic anaemia associated with novel coronavirus (COVID-19) [published online ahead of print, 2020 May 27] Br J Haematol. 2020 doi: 10.1111/bjh.16892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lazarian G, Quinquenel A, Bellal M, et al. Autoimmune haemolytic anaemia associated with COVID-19 infection. Br J Haematol. 2020;190:29–31. doi: 10.1111/bjh.16794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopez C, Kim J, Pandey A, Huang T, DeLoughery TG. Simultaneous onset of COVID-19 and autoimmune haemolytic anaemia. Br J Haematol. 2020;190:31–32. doi: 10.1111/bjh.16786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hariri L, Hardin CC. Covid-19, angiogenesis, and ARDS endotypes. N Engl J Med. 2020;383:182–183. doi: 10.1056/NEJMe2018629. [DOI] [PubMed] [Google Scholar]
- 14.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;39:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goshua G, Pine AB, Meizlish ML, et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7:e575–e582. doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 18.Ganz T. Anemia of inflammation. N Engl J Med. 2019;381:1148–1157. doi: 10.1056/NEJMra1804281. [DOI] [PubMed] [Google Scholar]
- 19.Orsini M, Chateauvieux S, Rhim J, et al. Sphingolipid-mediated inflammatory signaling leading to autophagy inhibition converts erythropoiesis to myelopoiesis in human hematopoietic stem/progenitor cells. Cell Death Differ. 2019;26:1796–1812. doi: 10.1038/s41418-018-0245-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Libregts SF, Gutiérrez L, de Bruin AM, et al. Chronic IFN-γ production in mice induces anemia by reducing erythrocyte life span and inhibiting erythropoiesis through an IRF-1/PU.1 axis. Blood. 2011;118:2578–88. doi: 10.1182/blood-2010-10-315218. [DOI] [PubMed] [Google Scholar]
- 21.Means RT, Jr, Dessypris EN, Krantz SB. Inhibition of human erythroid colony-forming units by interleukin-1 is mediated by gamma interferon. J Cell Physiol. 1992;150:59–64. doi: 10.1002/jcp.1041500109. [DOI] [PubMed] [Google Scholar]
- 22.Swann JW, Koneva LA, Regan-Komito D, Sansom SN, Powrie F, Griseri T. IL-33 promotes anemia during chronic inflammation by inhibiting differentiation of erythroid progenitors. J Exp Med. 2020;217:e20200164. doi: 10.1084/jem.20200164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zoller EE, Lykens JE, Terrell CE, et al. Hemophagocytosis causes a consumptive anemia of inflammation. J Exp Med. 2011;208:1203–1214. doi: 10.1084/jem.20102538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and mineral nutrition information system. World health organization; Geneva, Switzerland: 2011. WHO/NMH/NHD/MNM/11.1.
- 25.Bergamaschi G, Di Sabatino A, Albertini R, et al. Prevalence and pathogenesis of anemia in inflammatory bowel disease. Influence of anti-tumor necrosis factor-alpha treatment. Haematologica. 2010;95:199–205. doi: 10.3324/haematol.2009.009985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate [published correction appears in Ann Intern Med. 2011 Sep 20;155(6):408] Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedman B, Jiang HJ, Elixhauser A, Segal A. Hospital inpatient costs for adults with multiple chronic conditions. Med Care Res Rev. 2006;63:327–346. doi: 10.1177/1077558706287042. [DOI] [PubMed] [Google Scholar]
- 28.Levi M, Thachil J, Iba T, Levy JH. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7:e438–e40d. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melazzini F, Lenti MV, Mauro A, De Grazia F, Di Sabatino A. Peptic ulcer disease as a common cause of bleeding in patients with coronavirus disease 2019. Am J Gastroenterol. 2020;115:1139–1140. doi: 10.14309/ajg.0000000000000710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foy BH, Carlson JCT, Reinertsen E, et al. Association of red blood cell distribution width with mortality risk in hospitalized adults with SARS-CoV-2 infection. JAMA Netw Open. 2020;3:e2022058. doi: 10.1001/jamanetworkopen.2020.22058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pascual-Figal DA, Bonaque JC, Redondo B, et al. Red blood cell distribution width predicts long-term outcome regardless of anaemia status in acute heart failure patients. Eur J Heart Fail. 2009;11:840–846. doi: 10.1093/eurjhf/hfp109. [DOI] [PubMed] [Google Scholar]
- 32.Sangoi MB, Da Silva SH, da Silva JE, Moresco RN. Relation between red blood cell distribution width and mortality after acute myocardial infarction. Int J Cardiol. 2011;146:278–280. doi: 10.1016/j.ijcard.2010.10.084. [DOI] [PubMed] [Google Scholar]
- 33.Kim CH, Park JT, Kim EJ, et al. An increase in red blood cell distribution width from baseline predicts mortality in patients with severe sepsis or septic shock. Crit Care. 2013;17(6):R282. doi: 10.1186/cc13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bazick HS, Chang D, Mahadevappa K, Gibbons FK, Christopher KB. Red cell distribution width and all-cause mortality in critically ill patients. Crit Care Med. 2011;39:1913–1921. doi: 10.1097/CCM.0b013e31821b85c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang F, Pan W, Pan S, Ge J, Wang S, Chen M. Red cell distribution width as a novel predictor of mortality in ICU patients. Ann Med. 2011;43:40–46. doi: 10.3109/07853890.2010.521766. [DOI] [PubMed] [Google Scholar]
- 36.Hunziker S, Celi LA, Lee J, Howell MD. Red cell distribution width improves the simplified acute physiology score for risk prediction in unselected critically ill patients. Crit Care. 2012;16:R89. doi: 10.1186/cc11351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colaneri M, Bogliolo L, Valsecchi P, et al. Tocilizumab for treatment of severe COVID-19 patients: preliminary results from SMAtteo COvid19 REgistry (SMACORE) Microorganisms. 2020;8(5):695. doi: 10.3390/microorganisms8050695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104:2263–2268. doi: 10.1182/blood-2004-05-1812. [DOI] [PubMed] [Google Scholar]
- 39.Tettamanti M, Lucca U, Gandini F, et al. Prevalence, incidence and types of mild anemia in the elderly: the "Health and Anemia" population-based study. Haematologica. 2010;95:1849–1856. doi: 10.3324/haematol.2010.023101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bach V, Schruckmayer G, Sam I, Kemmler G, Stauder R. Prevalence and possible causes of anemia in the elderly: a cross-sectional analysis of a large European university hospital cohort. Clin Interv Aging. 2014;9:1187–1196. doi: 10.2147/CIA.S61125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Artz AS, Xue QL, Wickrema A, et al. Unexplained anaemia in the elderly is characterised by features of low grade inflammation. Br J Haematol. 2014;167:286–289. doi: 10.1111/bjh.12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferrucci L, Semba RD, Guralnik JM, et al. Proinflammatory state, hepcidin, and anemia in older persons. Blood. 2010;115:3810–3816. doi: 10.1182/blood-2009-02-201087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.den Elzen WP, de Craen AJ, Wiegerinck ET, Westendorp RG, Swinkels DW, Gussekloo J. Plasma hepcidin levels and anemia in old age. The Leiden 85-Plus Study. Haematologica. 2013;98:448–54. doi: 10.3324/haematol.2012.068825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wouters HJCM, van der Klauw MM, de Witte T, et al. Association of anemia with health-related quality of life and survival: a large population-based cohort study. Haematologica. 2019;104:468–476. doi: 10.3324/haematol.2018.195552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tabata S, Imai K, Kawano S, et al. Clinical characteristics of COVID-19 in 104 people with SARS-CoV-2 infection on the Diamond Princess cruise ship: a retrospective analysis [published online ahead print, 2020 Jun 12] Lancet Infect Dis. 2020;20:1043–50. doi: 10.1016/S1473-3099(20)30482-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hung IF, Cheng VC, Li X, et al. SARS-CoV-2 shedding and seroconversion among passengers quarantined after disembarking a cruise ship: a case series [published online ahead of print, 2020 Jun 12] Lancet Infect Dis. 2020;S1473–3099(20):30364–9. doi: 10.1016/S1473-3099(20)30364-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stoltzfus RJ. Iron deficiency: global prevalence and consequences. Food Nutr Bull. 2003;24(4 Suppl):S99–S103. doi: 10.1177/15648265030244S106. [DOI] [PubMed] [Google Scholar]
- 48.Baron DM, Franchini M, Goobie SM, et al. Patient blood management during the COVID-19 pandemic: a narrative review. Anaesthesia. 2020;75:1105–1113. doi: 10.1111/anae.15095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gavriatopoulou M, Ntanasis-Stathopoulos I, Korompoki E, et al. Emerging treatment strategies for COVID-19 infection. Clin Exp Med. 2020 doi: 10.1007/s10238-020-00671-y. [DOI] [PMC free article] [PubMed] [Google Scholar]