Abstract
Background
Short-latency intracortical inhibition (SICI) is extensively used to probe GABAergic inhibitory mechanisms in M1. Task-related changes in SICI are presumed to reflect changes in the central excitability of GABAergic pathways. Usually, the level of SICI is evaluated using a single intensity of conditioning stimulus so that inhibition can be compared in different brain states.
Objective
Here, we show that this approach may sometimes be inadequate since distinct conclusions can be drawn if a different CS intensity is used.
Methods
We measured SICI using a range of CS intensities at rest and during a warned simple reaction time task.
Conclusions
Our results show that SICI changes that occurred during the task could be either larger or smaller than at rest depending on the intensity of the CS. These findings indicate that careful interpretation of results are needed when a single intensity of CS is used to measure task-related physiological changes.
Keywords: Transcranial magnetic stimulation, Short intra-cortical inhibition, Movement preparation, Preparatory inhibition
Abbreviations: Reaction time, RT; warned simple reaction time task, wSRTT; electromyogram, EMG; first dorsal interosseous, FDI; transcranial magnetic stimulation, TMS; test stimulus, TS; conditioning stimulus, CS; warning stimulus, WS; imperative stimulus, IS; motor cortex, M1; motor evoked potential, MEP; short interval intra-cortical inhibition, SICI
Highlights
-
•
We measured SICI using several conditioning stimulus (CS) intensities at rest and during a warned simple reaction time task.
-
•
Pre-movement SICI changes can be either larger or smaller than at rest depending on the intensity of the CS used.
-
•
Careful interpretation of SICI is needed when a single intensity of CS is used to measure task-related physiological changes.
Introduction
Short-latency intracortical inhibition (SICI) is a paired-pulse transcranial magnetic stimulation (TMS) technique in which a low intensity conditioning stimulus (CS) is used to suppress the response evoked by a higher intensity test stimulus [1]. However, the CS has two separate actions: at very low intensities it appears to have purely inhibitory effects that are usually ascribed to GABAergic inhibition [2]. At higher intensities, a facilitation is also recruited that overlies the inhibition leading to a “U”-shaped recruitment curve [3].
SICI has been examined in neurological conditions as well as in healthy individuals during the performance of different tasks in order to infer changes in the excitability of GABAergic inhibitory pathways in motor cortex [4,5]. But in the majority of cases, SICI has been tested using only a single intensity of CS. This can lead to erroneous conclusions: for example, some studies report less SICI in Parkinson’s disease [6,7], but this may be due to the fact that facilitation from the CS appears at lower intensities than normal, giving the impression of less inhibition [8]. This can be avoided if testing is limited to very low CS intensities that are subthreshold for recruitment of any facilitatory effects. However, the disadvantage of that approach is that very low CS intensities recruit only a small proportion of the total pool of available inhibitory neurones, and it is possible that they do not give an accurate representation of the behaviour of the whole GABAergic system. Effectively, there is a sampling problem.
Here we illustrate the importance of using a range of CS intensities to probe SICI in different mental states by comparing SICI measured at rest with SICI at different times during a warned simple reaction time task (wSRTT). Previous studies have usually reported that as the time for movement approaches, SICI becomes less effective. This observation has been interpreted as evidence of a release of resting cortical inhibition during action preparation [9]. Here we show that different conclusions can be drawn depending on the intensity of the CS used to measure SICI.
Methods
Twenty-four right-handed individuals were recruited and gave written informed consent prior to a single-experimental session (13 female; 18–45 years). All procedures were performed in accordance with the Declaration of Helsinki and approved by University College London ethics committee.
Participants sat in front of a computer screen with their pronated right-hand relaxed on a pillow. EMG was acquired from the right first dorsal interosseous (FDI) muscle. Signals were amplified, band-pass filtered (20–2000 Hz, Digitimer D360, Digitimer Ltd, UK) digitised at 5 kHz and stored using a Power1401 DAQ controlled with Signal6.2 (CED, UK). TMS was delivered with a 70 mm figure-of-eight coil (Magstim, UK) over the M1-FDI “hot-spot” eliciting motor evoked potentials (MEPs). We used a wSRTT programmed in Matlab (Mathworks, MA, USA) to probe corticospinal excitability and SICI changes during movement preparation (task description in Fig. 1A).
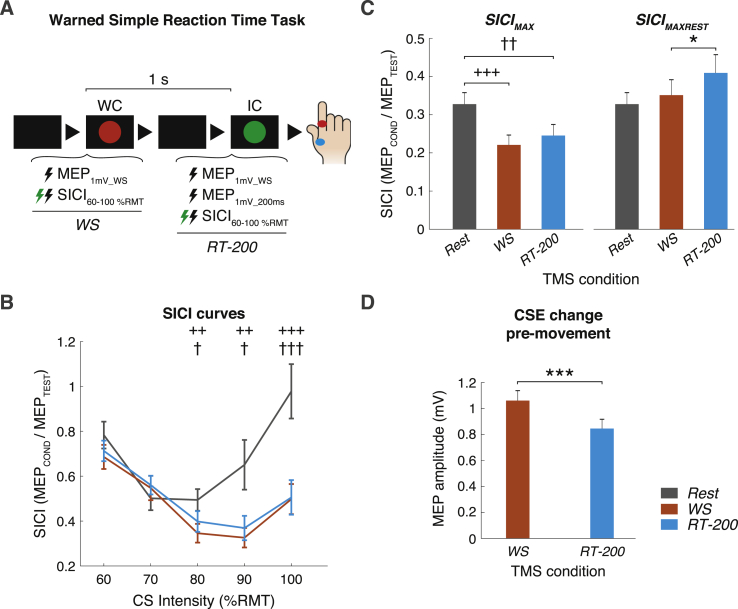
Fig. 1.
Measurements of MEP and SICI at two time points in preparation for movements in a wSRTT (WS and RT-200) and at Rest. A) Scheme of the wSRTT. Each trial started with a black screen (2s), after which a red circle was presented (warning cue, WC) followed 1s later by a green circle (imperative cue, IC). Participants were asked to stay fully relaxed during the periods between WC and IC and to react to seeing the IC by performing ballistic right index finger flexions. B) SICI Curves. SICI conditioning stimulus curves recorded at each state. The y-axis displays the SICI ratio (conditioned/unconditioned MEP amplitudes) where values below 1 correspond to inhibition and values above 1 are facilitation. The x-axis shows CS intensities. When compared to Rest, we found that WS and RT-200 states were associated with stronger SICI with 80, 90 and 100% conditioning stimulus intensities. C) SICIMAXand SICIMAXREST. The y-axis depicts the SICI ratio and x-axis represents the different states. When considering the maximum value of SICI measured for each state (SICIMAX), WS and RT-200 showed significantly greater SICIMAX than Rest. On the other contrary, when solely considering the CS intensity eliciting the strongest SICI at Rest (SICIMAXREST), SICIMAXREST at RT-200 was reduced in comparison to WS. D) Preparatory inhibition. The y-axis depicts the average MEP amplitude (mV) recorded at WS and RT-200. We found evidence for significant MEP suppression as participants prepared to move. Symbols: *, + and † refer to the paired comparisons WS-RT-200, WS-Rest and RT-200-Rest. Significance levels are expressed with */+/†, P < 0.05; **/++/††, P < 0.01; ***/+++/†††, P < 0.001. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article).
SICI was assessed with ∼1 mV TMS test stimuli (TS) given alone compared to ones preceded (interstimulus interval 2.5 ms) by a CS at 5 different intensities (60%, 70%, 80%, 90% and 100% of the RMT). We refer to this as a SICI recruitment curve. SICI was probed at rest (Rest) and at two time points during the wSRTT: at the warning stimulus (WS) and at 200 ms before the average reaction time (RT) of each subject (RT-200). Since corticospinal excitability may change between these three states, TS was adjusted in each case to obtain MEPs of around 1 mV. This led to three TS intensities: 1mVREST, 1mVWS and 1 mV200 ms.
Sessions began with wSRTT familiarization, followed by 20 additional trials used to estimate the participants’ RT, defined as the average time when the rectified and smoothed (5 ms sliding windows) EMG exceeded five times the amplitude at rest [10]. We then measured RMT and the three TS intensities (1mVREST, 1mVWS and 1 mV200 ms). SICI was first assessed at Rest, with 15 MEPs being obtained with each CS. The next step was to use the wSRTT to assess SICI at WS and RT-200. Participants performed 5 blocks of 70 trials each of the wSRTT. A randomly selected condition was probed on each trial: 90 trials had no TMS; 20 trials were obtained for each CS (including TS given alone) and each brain state (WS or RT-200); finally, 20 single TMS pulses at 1mVWS intensity were given alone at RT-200, which allowed us to compare corticospinal excitability changes between WS and RT-200 using the same TMS intensity.
SICI was measured as the ratio between conditioned and unconditioned MEPs. We used each individual’s pre-TMS segments (200 ms) to estimate an EMG noise threshold. This was set to ± 1SD of the baseline peak-to-peak EMG activity. We removed trials in which the EMG prior to TMS exceeded this threshold and trials in which MEP amplitudes were below it. Supplementary Tables 1 and 2 show the number of valid trials per condition and their noise levels. Valid trials were then used to compare SICI levels between the STATES (Rest, WS, RT-200). We ran three comparisons: i) SICI curves comparison: 2-way repeated measures ANOVA (rmANOVA) with factors STATE and CS-intensity (five levels: 60–100%); ii) maximum SICI (SICIMAX) comparison: 1-way rmANOVA with factor STATE to test for differences in the maximum SICI in each state (selecting in each participant and for each STATE the CS resulting in the maximum SICI); and iii) maximum SICI rest (SICIMAXREST) comparison: 1-way ANOVA with factor STATE to compare SICI levels obtained with the CS that produced the maximum SICI at Rest in each participant. Note that SICIMAXREST comparison is the one used in some previous publications to examine SICI at different times during movement preparation [9,11]. Finally, we used a t-test to compare MEP amplitudes at WS and RT-200 in order to verify the presence of preparatory inhibition as reported previously in a similar task [12,13].
Results
SICI curves comparison revealed a strong main effect of STATE (F[2,46] = 16.580; p < 0.001) and a STATE x CS-intensity interaction (F[8,184] = 7.018; p = 0.002). Higher CS intensities at WS and RT-200 evoked more SICI than at rest while there was no difference at lower intensities (Fig. 1B; paired comparisons in Supplementary Table 3). The curve of SICI recruitment at Rest is very similar to that reported in previous papers [3] in which the gradual reduction in SICI at CS ≥ 80%RMT is usually ascribed to the fact that these CS intensities likely recruit some minimal corticospinal activity. This increases spinal excitability and reduces the apparent amount of SICI, eventually producing facilitation. This effect seems absent during the task at WS and RT-200. There are two possible explanations for this. One possibility is that corticospinal excitability is lower at these time points than at Rest. This would imply that, at WS and RT-200, CS evokes less corticospinal activity and, as a consequence, a less effective short-interval facilitation effect is superimposed on SICI effect [14]. However, this seems unlikely, particularly at WS: 1mVREST and 1mVWS intensities are comparable, suggesting no change in corticospinal excitability. Indeed, other studies have reported higher, rather than lower, corticospinal excitability during the performance of a task compared with rest [15]. The second possibility is that SICI is more effective during the task, overcoming the increased spinal excitability produced by the CS. This leads to a different conclusion to that in the literature, by indicating that at these time points the total pool of neurones available to suppress MEPs is more excitable than at rest.
Maximum SICI comparison led to similar results. STATE had a significant effect on SICIMAX (F[2,46] = 11.623, p < 0.001). SICIMAX at Rest was significantly smaller than at WS (p < 0.001) and at RT-200 (p = 0.006), while no significant differences were found in SICIMAX between WS and RT-200 (p = 0.216; Fig. 1C-left). Interestingly, results changed when examining SICI using only a fixed CS intensity across states: the rmANOVA run to compare SICIMAXREST revealed no significant effect of STATE and subsequent paired comparisons indicated that, if anything, the only significant difference was between WS and RT-200 (p = 0.025; Fig. 1C-right), in line with previous work suggesting that SICI becomes less effective as the time to move approaches.
Finally, single-pulse TMS using 1mVWS intensity at RT-200 resulted in significantly smaller MEP amplitudes than at WS (t = 3.916, p = 0.001), confirming that corticospinal excitability decreased during movement preparation (Fig. 1D) [16].
Discussion
We conclude, in agreement with some previous literature [8,17,18], that testing SICI using a wide range of CS intensities provides a more nuanced interpretation of possible GABAergic changes in M1 than testing with a single CS intensity. In the present data, testing with low CS intensities suggests that SICI is the same at rest and during task performance. However, when we examined larger proportions of the total inhibitory pool by using higher CS intensities, there seemed to be an increase of SICI during task performance and movement preparation compared with rest. It is possible that some of this could be caused by a reduced short-interval intracortical inhibition (SICF), which superimposes on SICI, particularly at higher intensities of CS [14]. However, this seems unlikely since corticospinal excitability to single pulse TMS was unchanged at that time compared with rest. We conclude that sampling a small portion of the total inhibitory pool using a single intensity of CS may not give a representative picture of the behaviour of the whole pool.
Finally, it should be noted that we studied SICI changes during the early phase of movement preparation (i.e., ∼200 ms before movement onsets), rather than immediately prior to the onset of EMG as others have done previously (e.g. [5]). Thus we cannot draw any definite conclusions about how SICI may change during later stages of motor generation as a function of the intensity of the CS used. Our point is only that it would be useful to use a range of CS intensities when examining SICI in any task.
Funding
JI was supported in part by Grant No. #H2020-MSCA-IF-2015-700512 from the European Commission (European Union). JI and JCR were supported by the Biotechnology and Biological Sciences Research Council (BBSRC) (UK; Grant No. BB/N016793/1). DAS and JCR were supported by the Medical Research Council (UK; MR/P006671/1).
Declaration of competing interest
Authors declare no conflicts of interest.
Acknowledgments
We gratefully acknowledge the technical assistance of Paul Hammond.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.brs.2019.11.002.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Rothwell J.C., Day B.L., Thompson P.D., Kujirai T. Short latency intracortical inhibition: one of the most popular tools in human motor neurophysiology. J Physiol. 2009;587:11–12. doi: 10.1113/jphysiol.2008.162461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ziemann U., Reis J., Schwenkreis P., Rosanova M., Strafella A., Badawy R. TMS and drugs revisited 2014. Clin Neurophysiol. 2015;126:1847–1868. doi: 10.1016/j.clinph.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 3.Kujirai T., Caramia M., Rothwell J.C., Day B.L., Thompson P.D., Ferbert A. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Talelli P., Greenwood R.G., Rothwell J.C. Exploring Theta Burst Stimulation as an intervention to improve motor recovery in chronic stroke. Clin Neurophysiol. 2007;118:333–342. doi: 10.1016/j.clinph.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 5.Reynolds C., Ashby P. Inhibition in the human motor cortex is reduced just before a voluntary contraction. Neurology. 1999;53:730–735. doi: 10.1212/wnl.53.4.730. [DOI] [PubMed] [Google Scholar]
- 6.Ridding M.C., Rothwell J.C., Inzelberg R. Changes in excitability of motor cortical circuitry in patients with Parkinson’s disease. Ann Neurol. 1995;37:181–188. doi: 10.1002/ana.410370208. [DOI] [PubMed] [Google Scholar]
- 7.Berardelli A., Abbruzzese G., Chen R., Orth M., Ridding M., Stinear C. Consensus paper on short-interval intracortical inhibition and other transcranial magnetic stimulation intracortical paradigms in movement disorders. Brain Stimul. 2008;1:183–191. doi: 10.1016/j.brs.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 8.MacKinnon C.D., Gilley E.A., Weis-McNulty A., Simuni T. Pathways mediating abnormal intracortical inhibition in Parkinson’s disease. Ann Neurol. 2005;58:516–524. doi: 10.1002/ana.20599. [DOI] [PubMed] [Google Scholar]
- 9.Dupont-Hadwen J., Bestmann S., Stagg C.J. Motor training modulates intracortical inhibitory dynamics in motor cortex during movement preparation. Brain Stimul. 2019;12:300–308. doi: 10.1016/j.brs.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu L., Rocchi L., Hannah R., Xu G., Rothwell JCJJC, Ibáñez J. Corticospinal excitability modulation by pairing peripheral nerve stimulation with cortical states of movement initiation. J Physiol. 2019 doi: 10.1113/JP278536. in press. [DOI] [PubMed] [Google Scholar]
- 11.Duque J., Ivry R.B. Role of corticospinal suppression during motor preparation. Cerebr Cortex. 2009;19:2013–2024. doi: 10.1093/cercor/bhn230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hannah R., Cavanagh X.S.E., Tremblay S., Simeoni S., Rothwell J.C. Selective suppression of local interneuron circuits in human motor cortex contributes to movement preparation. J Neurosci. 2018;38 doi: 10.1523/JNEUROSCI.2869-17.2017. 2869–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duque J., Greenhouse I., Labruna L., Ivry R.B. Physiological markers of motor inhibition during human behavior. Trends Neurosci. 2017;40:219–236. doi: 10.1016/j.tins.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peurala S.H., Müller-dahlhaus J.F.M., Arai N., Ziemann U. Interference of short-interval intracortical inhibition (SICI) and short-interval intracortical facilitation (SICF) Clin Neurophysiol. 2008;119:2291–2297. doi: 10.1016/j.clinph.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 15.Hannah R., Rocchi L., Rothwell J.C. Observing without acting: a balance of excitation and suppression in the human corticospinal pathway? Front Neurosci. 2018;12:1–10. doi: 10.3389/fnins.2018.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ibáñez J., Hannah R., Rocchi L., Rothwell J. Premovement suppression of corticospinal excitability may be a necessary part of movement preparation. Cerebr Cortex. 2019 doi: 10.1101/470153. Preprint at bioRxiv, In press. [DOI] [PubMed] [Google Scholar]
- 17.Mackinnon C.D., Rothwell J.C. Unraveling the mysteries of motor cortical function in Parkinson disease. Neurology. 2013;80:1726–1727. doi: 10.1212/WNL.0b013e3182918d4b. [DOI] [PubMed] [Google Scholar]
- 18.Bütefisch C.M., Weßling M., Netz J., Seitz R.J., Hömberg V. Relationship between interhemispheric inhibition and motor cortex excitability in subacute stroke patients. Neurorehabilitation Neural Repair. 2008;22:4–21. doi: 10.1177/1545968307301769. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.