Abstract
The properties of the glucopolymer dextran are versatile and linked to its molecular size, structure, branching, and secondary structure. However, suited strategies to control and exploit the variable structures of dextrans are scarce. The aim of this study was to delineate structural and functional differences of dextrans, which were produced in buffers at different conditions using the native dextransucrase released by Liquorilactobacillus (L.) hordei TMW 1.1822. Rheological measurements revealed that dextran produced at pH 4.0 (MW = 1.1 * 108 Da) exhibited the properties of a viscoelastic fluid up to concentrations of 10% (w/v). By contrast, dextran produced at pH 5.5 (MW = 1.86 * 108 Da) was gel-forming already at 7.5% (w/v). As both dextrans exhibited comparable molecular structures, the molecular weight primarily influenced their rheological properties. The addition of maltose to the production assays caused the formation of the trisaccharide panose instead of dextran. Moreover, pre-cultures of L. hordei TMW 1.1822 grown without sucrose were substantial for recovery of higher dextran yields, since the cells stored the constitutively expressed dextransucrase intracellularly, until sucrose became available. These findings can be exploited for the controlled recovery of functionally diverse dextrans and oligosaccharides by the use of one dextransucrase type.
Keywords: Lactic acid bacteria, Dextransucrase, Dextran, Molecular weight, Rheology, Panose
Introduction
The utilization of microbial exopolysaccharides (EPSs) is of growing importance and commercial interest due to their great structural diversity and concomitant manifold properties. They can be used instead of synthetically produced additives in cosmetics and food products and may replace commonly used emulsifiers because of their water-binding capacity or rather hydrocolloid properties [1–5]. Furthermore, EPSs may play a future role in applications like tissue engineering, drug delivery systems, and other medical applications [6–10] or even fields like biofuel research [11], as they are usually involved in the formation of biofilms [12–19].
One of the most prominent EPSs is dextran, which is commercially exploited in fermented foods like sourdoughs, panettone, or fruit juices, and applied as blood plasma volume expander or as stationary phase of size-exclusion columns [6, 20–24]. It is composed of α-(1,6)-linked glucose monomers (backbone), which can be branched at positions O2, O3, or O4 depending on the catalytic domain of their producing dextransucrases (EC 2.4.1.5) [25]. These extracellular enzymes are exclusively expressed by lactic acid bacteria and belong to the family 70 of glycoside hydrolases according to the CAZy database. They use the energy of the glycosidic bond of sucrose for polymerization of the contained glucose moiety while fructose is continuously released [20, 26–30]. The size, structure, and yield of sucrose-derived EPSs like dextrans are multiply influenced by extrinsic factors, e.g., pH, temperature, or substrate concentration [31–38], but also by the genetic constitution and physiology of the bacterial producer strain [25, 39]. Moreover, if present, maltose can be involved in the dextransucrase (acceptor) reaction. This usually results in the formation of diverse short-chain gluco-oligosaccharides that exhibit a low degree of polymerization and are promising prebiotics [30, 40–43]. The extreme differences in possible chain lengths contribute to the variable functional properties of the produced dextrans and other sucrase-derived EPSs [33, 35, 44, 45] and can be controlled during production for the manufacture of tailor-made EPSs exhibiting the desired properties. By contrast, the structures and sizes of other commercially used plant-derived hydrocolloids such as cellulose or starch are comparably fixed, why they need to be synthetically modified to obtain varying hydrocolloid properties [46–49]. In a previous study, we were able to produce high molecular weight dextrans of different sizes via the application of different pH values upon the polymerization process using the dextransucrase natively released by Liquorilactobacillus hordei TMW 1.1822. As these dextrans appeared to differ regarding their thickening properties depending on their molecular size [50], we wanted to elucidate their basic structural and rheological differences. In this way, we wanted to get novel insights into the structure–function relationship of these dextrans, which are essential for their knowledge-based application as hydrocolloids. Moreover, the impact of maltose on dextran formation should be elucidated for possible further extension and exploitation of the product spectrum of this dextransucrase.
Material and Methods
Strain, Standard Media and Basic Growth Kinetics
Liquorilactobacillus hordei TMW 1.1822 was originally isolated from water kefir [51] and cultivated at 30 °C on modified MRS (mMRS) as described before [50, 52], containing 10 g/L peptone from casein, 5 g/L yeast extract, 5 g/L meat extract, 4 g/L K2HPO4, 2.6 g/L KH2HPO4, 3 g/L NH4Cl, 1 g/L Tween 80, 0.5 g/L cysteine-HCl, 0.2 g/L MgSO4·7H2O, 0.036 g/L MnSO4·H2O, and 15 g/L agar for solid media, and a pH of 6.2. For the determination of the growth kinetics depending on different sugar sources, TMW 1.1822 was cultivated in mMRS containing either glucose + fructose (each 10 g/L) or sucrose (19 g/L) for 24 h. In these experiments, the pH, colony-forming units (CFUs), and dextran yields were determined after 0, 4, 8, 14, 19, and 24 h. For verification of the strain identity and to exclude any contamination, matrix-assisted laser desorption–ionization time-of-flight mass spectrometry (MALDI-TOF-MS) was randomly performed and validated through our in-house database [53].
Production of High Molecular Weight Dextrans Exhibiting Different Sizes
Dextran production was performed according to Schmid et al. [50] and is briefly described. The mMRS broths (glucose + fructose each 10 g/L) were inoculated with 4% of 20-h pre-cultures with an adjusted OD590nm of 2.0, to obtain cells in the exponential growth phase after 18 h at 30 °C. The media supernatants were removed after centrifugation (10000×g, 10 min), followed by the addition of 0.1 M citrate-phosphate buffer containing 0.1 M sucrose with pH 4.0 or 5.5. After 3 h of static incubation at 30 °C, the cells were removed by centrifugation (10000×g, 10 min) and filtration (0.2-μm nylon filters, Phenomenex, Germany). Subsequently, dextran production with the dextransucrase containing supernatants in the respective buffers (pH 4.0 and 5.5) was performed for 24 h at 30 °C. Additionally, one intermediate condition was analyzed, where the cells were not removed, and therefore, uncontrolled pH decrease occurred due to the metabolic activity of the remaining cells (UC).
Dextran Formation After Pre-Cultivation in mMRS Broths Media Containing Sucrose
To study the influence of sucrose in pre-cultivation on the dextran formation (3.3; Fig. 3), the sugar compositions in mMRS were varied. Therefore, mMRS was either supplemented with 10 g/L glucose + 10 g/L fructose (glc + fru), 5 g/L glucose + 5 g/L fructose + 9.5 g/L sucrose (glc + fru + suc), or 19 g/L sucrose (suc). The respective sugar amounts were adjusted to obtain almost equal total sugar molarities (~ 0.05 M), respectively. These media were also inoculated with 4% of 20-h pre-cultures grown in mMRS (glc + fru) with an adjusted OD590nm of 2.0, to obtain cells in the exponential growth phase after 18 h. The cells were then incubated in a 0.1 M citrate-phosphate buffer pH 4.5 [50] containing 0.1 M sucrose, yielding native dextransucrase for subsequent enzymatic dextran formation, as stated in the “Production of High Molecular Weight Dextrans Exhibiting Different Sizes” section.
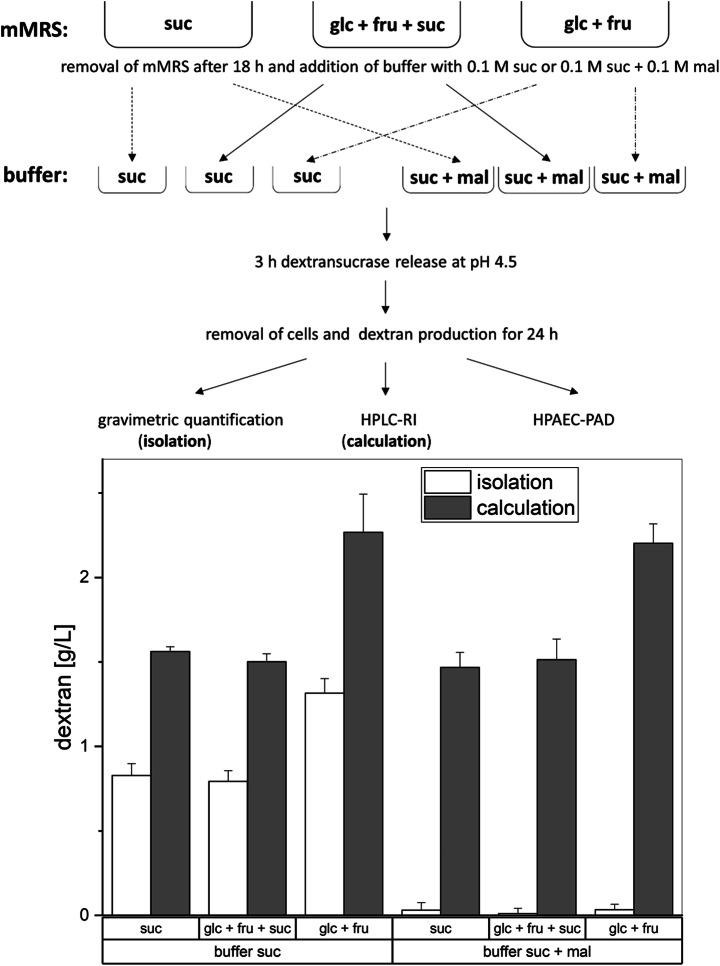
Fig. 3.
Top: overview of the experimental setups used to characterize extracellular dextran production depending on the presence of sucrose in the pre-cultivation and maltose during dextran formation. Cells were cultivated in medium containing solely sucrose (suc), glucose + fructose + sucrose (glc + fru + suc), and glucose + fructose (glc + fru) for 18 h; harvested; and re-dissolved in 0.1 M citrate-phosphate buffer pH 4.5. This buffer was supplemented with either 0.1 M sucrose (suc) or 0.1 M sucrose + 0.1 M maltose (suc + mal). After 3 h of incubation, the cells were removed by centrifugation and sterile filtration. The cell-free supernatants were incubated for 24 h, followed by dextran isolation, and HPLC-RI and HPAEC-PAD measurements. Bottom: dextran yields for the described experiment. The bars indicate the produced dextran amounts in g/L, which were determined either gravimetrically (isolation) or via the calculated transglycosylation activity and the resulting dextran amounts after 24 h (calculation). Data are expressed with mean ± SD of three biological replicates
Dextran Production in the Presence of Maltose and Sucrose
For the investigation of the influence of maltose on the products of the dextransucrase, the experimental setup depicted in Fig. 3 was carried out. For this purpose, we used the cells from the three different pre-cultivations, which are described in the “Dextran Formation After Pre-Cultivation in mMRS Broths Media Containing Sucrose” section. As described in the “Production of High Molecular Weight Dextrans Exhibiting Different Sizes” section, the supernatant was removed, and a 0.1 M citrate-phosphate buffer pH 4.5 [50] containing 0.1 M sucrose + 0.1 M maltose was added, followed by the dextran production for 24 h at 30 °C.
Dextran Production Using Dextransucrase Containing Cell Lysates
Additionally, cells obtained from mMRS with glucose + fructose (each 10 g/L) or sole sucrose (19 g/L) were recovered after 18 h of incubation by centrifugation and lysed with 10 μg/mL lysozyme at 37 °C overnight. The lysates were subsequently re-dissolved in 0.1 M citrate-phosphate buffer pH 4.5 containing 0.1 M sucrose and incubated for 24 h at 30 °C.
Dextran Isolation
Dextran isolation was performed as previously described [54] by applying an initial centrifugation step (10000×g, 30 mins) for removal of insoluble material, precipitation of water-soluble dextrans with 2 volumes of ethanol for 24 h at 4 °C, dialysis against ddH2O at 4 °C for 48 h (3500-Da cutoff; SERVAPOR, SERVA Electrophoresis GmbH, Germany), and applying a final lyophilization step.
Analysis of the Macromolecular Structure and Size of Dextrans by AF4-MALS-UV
To determine the molecular weights and the root mean square (rms) radii of the isolated dextrans, asymmetric flow field-flow fractionation (AF4) coupled with multi-angle laser light scattering (MALS) (Dawn Heleos II, Wyatt Technologies, Germany) analysis and UV detection (Dionex Ultimate 3000, Thermo Scientific, USA) was performed. The lyophilized dextrans were re-dissolved in 0.05 M NaNO3, which furthermore served as the eluent. A total of 100 μL of the respective samples with a concentration of 0.1 mg/mL was injected and separated in the long channel (LC) using a 10-kDa regenerated cellulose membrane, a detector flow rate of 1 mL/min, and a cross-flow gradient of 3 to 0.1 mL/min over 15 min. Subsequently, the cross-flow was kept constant at 0.1 mL/min for 15 min. The dextrans produced from sucrose at different pH [50] were characterized regarding their molecular size and structure. The MALS analysis was performed with all 17 detectors, and the chromatograms were further analyzed and evaluated with the ASTRA 6.1 software (Wyatt Technologies, Germany). The model berry 1 was most suited for evaluation of the dextrans (R2 > 0.90), whereby the rms radii were determined in the particle mode. In advance of the molecular mass calculation, the extinction coefficients of the dextrans were determined in 96-well microtiter plates at λ = 400 nm using concentrations of 0.5–2.5 mg/mL (SPECTROstar, BMG LABTECH) according to the Beer–Lambert law. Finally, the extinction coefficients were used for mass calculations using UV-based concentration detection of the separated dextran fractions at λ = 400 nm (Dionex Ultimate 3000, Thermo Scientific, USA).
Analysis of the Glycosidic Linkages of Dextrans by Methylation Analysis
Methylation analysis was carried out as described previously by Fels et al. [18]. Briefly, dextrans were methylated in DMSO by using powdered sodium hydroxide and methyl iodide. Methylated polysaccharides were extracted by using dichloromethane, dried and hydrolyzed by using 2 M trifluoroacetic acid (90 min, 121 °C). After evaporation of the acid, the partially methylated monosaccharides were reduced by using sodium borodeuteride. The reaction was terminated by using glacial acetic acid, and 1-methylimidazole and acetic anhydride were added for acetylation. The obtained partially methylated alditol acetates were identified by using GC-MS and semiquantitatively analyzed by using GC-FID with the previously described conditions (molar response factors according to Sweet et al. [55]). All analyses were performed in duplicate.
Characterization of the Rheological Properties of High Molecular Weight Dextrans
For determination of the basic rheological properties of the produced dextrans, flow curves were recorded and the capability of gel formation by the respective dextrans was studied using the rheometer Physica MCR 501 (Anton Paar, Austria). For the measurements of samples with lower viscosity (< 7.5% w/v), the concentric cylinder geometry CC 27-SS was used; for samples of higher viscosity, the cone–plate geometry CP 25-1 was used (Anton Paar, Austria). To re-dissolve the dextran in ddH2O, the samples were vortexed for 10 min, heated in a water bath at 55 °C for 45 min, and centrifuged (10000×g, 10 min) for removal of air bubbles. The measurements were performed at 20 °C and a shear rate ranging from 0.1 to 1000 s-1. To determine the linear viscoelastic (LVE) region, strain sweep tests were performed at 1 rad/s with stress ranging from 0.1 to 100%, followed by frequency sweep tests at angular frequencies of 0.1 to 100 rad/s and 1.0% strain. Concentrations of highest differences and interest were picked for visualization, resulting in 7.5, 5, 2.5, and 1% w/v for the rotational tests (concentric cylinder) and 7.5, 10, and 12.5% w/v for the strain/frequency sweep tests (cone–plate).
Detection of Mono- and Disaccharides by HPLC-RI
Mono- and disaccharides were quantified by high-performance liquid chromatography (HPLC) coupled to refractive index detection (ERC Refractomax 521, Thermo Fisher Scientific, USA). Sugar separations were performed on a Rezex RPM-Monosaccharide Pb2+ column (Phenomenex Ltd., Germany) with water as mobile phase at a constant flow rate of 0.6 mL/min at 85 °C and 20 μL of injection volume. For identification and quantification of the respective sugars, sugar standards were used and the calibration curves were generated with the ChromeleonTM software (version 6.8, Dionex, Germany).
Identification of Low Molecular Weight Oligosaccharides by HPAEC-PAD
For identification of short-chain gluco-oligosaccharides produced by dextransucrases in the presence of maltose (“Dextran Formation After Pre-Cultivation in mMRS Broths Media Containing Sucrose”), high-performance anion-exchange chromatography (HPAEC) with pulsed amperometric detection (PAD) (ICS5000, Thermo Fisher Scientific, USA) on a CarboPac PA20 column (Thermo Fisher Scientific, USA) was performed. The separation was accomplished at a flow rate of 0.5 mL/min and an isocratic elution with 150 mM NaOH (Merck Millipore, USA) for 80 min and final flushing step with 200 mM NaOH and 1 M sodium acetate (Merck Millipore, USA) for 20 min. For identification and quantification of oligosaccharides, external sugar standards were used (Carbosynth, Switzerland) and the calibration curves were generated with the ChromeleonTM software (version 6.8, Dionex, Germany).
Results
Molecular Size and Structure of High Molecular Weight Dextrans
The dextrans produced in buffer at pH 5.5 were of bigger size (Table 1, 94.3 ± 0.95 nm) and higher molecular weight (1.86 ± 0.02 × 108 Da) compared with dextrans produced at pH 4.0 (73.5 ± 0.7 nm and 1.09 ± 0.04 × 108) and the dextran UC produced at uncontrolled pH (77.3 ± 1.0 nm and 1.40 ± 0.02 × 108 Da). The polydispersity, which was calculated by MW/Mn and RW/Rn, was similar among the samples (Table 1). The hydrodynamic coefficients vg, which are obtained by double-logarithmic plotting of rms radii vs. molar masses over the entire polymer fraction [34], were in a comparable range of 0.74–0.79 (Table 1), indicating similar spatial (secondary) structures of the dextran molecules in aqueous solution as reported previously [5]. The molecular structure of the samples was further investigated by methylation analysis (Table 2) and confirmed the presence of dextrans with a 1,6-linked backbone (1,6-linked glucose), which is branched at position O3 (1,3,6-linked glucose) to a low extent. The portions of the glycosidic linkages only showed slight variations among the samples, which suggests a comparable molecular structure for the investigated polysaccharides.
Table 1.
Molecular weights, radii, distribution quotient, and slope of confirmation plot determined by AF4-MALS-UV for dextrans produced at pH 5.5, 4.0, and uncontrolled (UC)
| Mw (Da) | Mw/Mn | Rw (nm) | Rw/Rn | Slope of confirmation plot | |
|---|---|---|---|---|---|
| pH 4.0 | 1.09 ± 0.04 × 108 | 1.10 ± 0.01 | 73.5 ± 0.7 | 1.13 ± 0.02 | 0.74 ± 0.1 |
| pH 5.5 | 1.86 ± 0.02 × 108 | 1.09 ± 0.01 | 94.3 ± 0.9 | 1.11 ± 0.01 | 0.77 ± 0.2 |
| UC | 1.40 ± 0.02 × 108 | 1.08 ± 0.00 | 77.3 ± 1.0 | 1.12 ± 0.01 | 0.79 ± 0.2 |
Table 2.
Glycosidic linkage (mol %) of dextrans produced at pH 5.5, pH 4.0, and uncontrolled (UC) pH as determined by methylation analysis
| Glycosidic linkage | pH 5.5 | UC | pH 4.0 |
|---|---|---|---|
| t-Glcp | 5.0 ± 0.0 | 4.9 ± 0.0 | 5.0 ± 0.2 |
| 1,3-Glcp | 1.4 ± 0.0 | 1.5 ± 0.0 | 1.5 ± 0.0 |
| 1,6-Glcp | 90.9 ± 0.0 | 90.6 ± 0.0 | 90.8 ± 0.3 |
| 1,3,6-Glcp | 2.8 ± 0.1 | 3.0 ± 0.1 | 2.6 ± 0.1 |
Rheological Properties of the High Molecular Weight Dextrans
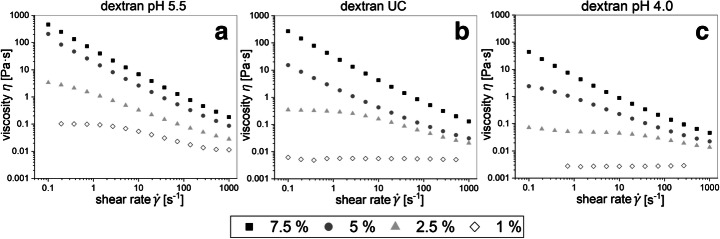
The high molecular weight dextrans described in the “Molecular Size and Structure of High Molecular Weight Dextrans” section were further analyzed regarding their flow and viscoelastic behavior. Figure 1 depicts the flow curves of the dextrans produced at pH 4.0 and 5.5 and of the dextran produced without pH control (UC). The dextran pH 5.5 showed shear thickening behavior for all tested specific concentrations 1, 2.5, 5, and 7.5% (w/v) (Fig. 1A) and the highest detected viscosity of around 460 Pa/s (shear rate 0.1 s-1). In contrast, dextran pH 4.0 (Fig. 1C) exhibited the comparatively lowest viscosities at equally applied specific concentrations and no distinct shear thinning behavior at concentrations below 5% (w/v), while the dextran UC (Fig. 1B) was in between dextrans pH 4.0 and 5.5 regarding determined viscosities at equal test conditions.
Fig. 1.
Concentration-dependent viscosity curves of dextrans produced at pH 5.5 (A), uncontrolled pH (UC) (B), and pH 4.0 (C)
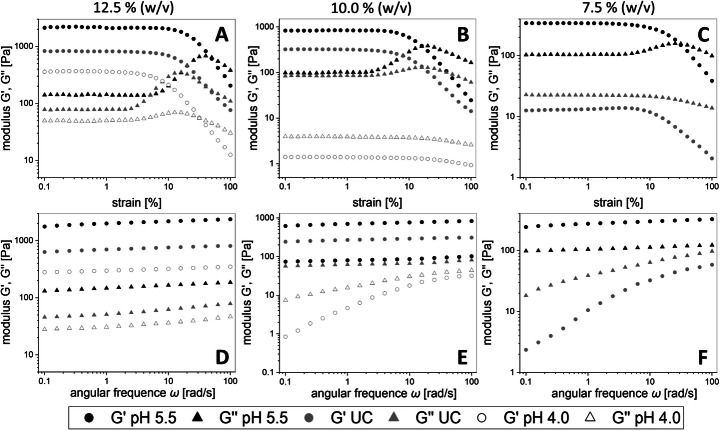
For the determination of the upper limits of the LVE region, amplitude sweep tests were performed. At concentrations of 12.5% (w/v), all samples showed a gel-like behavior with G″ (loss modulus) < G′ (storage modulus). The highest storage and loss moduli were measured for dextran pH 5.5 followed by dextran UC and dextran pH 4.0 (Fig. 1A). The loss of the gel-like behavior for dextrans pH 4.0 at a concentration of 10% w/v was evident due to no crossover of G′ and G″. A similar observation was made for dextran UC at a specific concentration of 7.5% (w/v) (Fig. 1B and C). Dextrans pH 4.0 could not be analyzed at 7.5% (w/v) due to its distinct fluid-like behavior.
In Fig. 2, the results of the frequency sweep tests performed at a constant strain of 1.0% are depicted. The highest shear storage moduli G′ and loss moduli G″ were determined for dextran pH 5.5, while G′ > G″ was measured at each concentration for this dextran in contrast to dextrans UC (Fig. 2F) and pH 4.0 (Fig. 2E).
Fig. 2.
Strain sweep (A–C) and frequency sweep (D–F) tests depicting storage (G′, circle) and loss (G″, triangle) moduli of dextrans produced at pH 5.5 (black), uncontrolled pH (UC, gray), and pH 4.0 (white). The measurements were performed by applying the specific concentrations 12.5% (A, D), 10% (B, E), and 7.5% (w/v) (C, F) of each dextran, respectively
Influence of Sucrose in the MRS Pre-Cultivation Medium on the Dextran Production in Buffers
The method to produce dextran under controlled pH (“Production of High Molecular Weight Dextrans Exhibiting Different Sizes”) [50] was slightly modified to study the influence of sucrose in the pre-cultivation medium on the produced dextran amounts in buffers (Fig. 3, “Dextran Formation After Pre-Cultivation in mMRS Broths Media Containing Sucrose”). The amounts of produced dextrans were determined by weighing after isolation and by calculation of the totally produced dextran amounts using the equation c (transglycosylated glucose) × 162.16 g/mol (molar mass of glucose in dextran). If sucrose was present in the mMRS pre-cultivation medium, the dextran yields obtained after production in buffers were considerably lower compared with those obtained without sucrose (Fig. 3, bottom). Furthermore, no significant differences in the produced dextran amounts were observed, if L. hordei TMW 1.1822 had been pre-cultivated in mMRS medium with sucrose as the sole sugar source or with glucose/fructose/sucrose as mixed sugar sources. Similar results were obtained, if lysates of L. hordei TMW 1.1822 harvested from mMRS ± sucrose were used for dextran production (− suc: 1.29 ± 0.014 g/L; + suc: 0.71 ± 0.056 g/L). The growth and acidification of L. hordei TMW 1.1822 in mMRS with either glucose/fructose or sucrose as the carbon source were highly similar within 24 h of cultivation (Fig. 4). In the presence of sucrose, up to 4.5 g/L dextran was additionally produced.
Fig. 4.
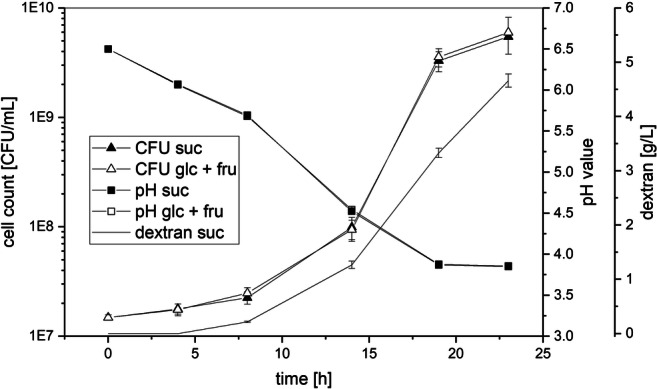
Monitoring of the colony-forming units (CFU), pH, and dextran formation of L. hordei TMW 1.1822 during cultivation in either mMRS medium containing glucose + fructose (glc + fru) or mMRS medium containing solely sucrose (suc) as respective carbohydrate sources. Dextran yields are only shown for mMRS with sucrose since no dextran formation took place without sucrose
Impact of Maltose on the Product Specificity of the Dextransucrase
To study the impact of maltose on the product specificity of the dextransucrase released by L. hordei TMW 1.1822, maltose (0.1 M) was added to the production buffer containing 0.1 M sucrose. The amounts of isolated dextran were drastically reduced in the presence of maltose to less than 0.05 g/L (Fig. 3, right part). However, the overall activity of the dextransucrase was in a similar range as compared with the corresponding experimental series performed without maltose (Fig. 3, left part). As the concentrations of free glucose and fructose were similar among the particularly comparable approaches (± maltose), the previously reported initial hydrolysis of sucrose prior to transglycosylation by these dextransucrases [50] occurred at all tested conditions. The low molecular weight oligosaccharides formed in the presence of maltose were analyzed by using HPAEC-PAD (Fig. 5). Besides the peaks corresponding to the sugars added to the production buffers (glucose 1, fructose 2, sucrose 3, maltose 4), an additional peak [6] was detected. As determined by the retention time, this peak corresponds to panose [6] in the standard mixture, eluting after isomaltotetraose [5] and before maltotriose [7]. The determined average concentration of panose was 15 mM, which is consistent with the maltose reduction after 24 h (16 mM) and the calculated glucose used for transglycosylation (15 mM). Peaks at later retention times than 80 min, which might refer to longer glucose oligosaccharides, were not detected.
Fig. 5.
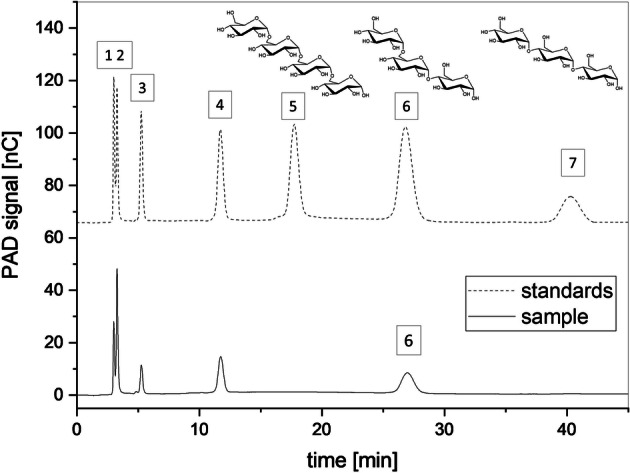
HPAEC-PAD chromatograms of the diluted supernatant from the production buffer containing sucrose + maltose and a standard solution containing the sugars glucose (1), fructose (2), sucrose (3), maltose (4), isomaltotetraose (5), panose (6), and maltotriose (7)
Discussion
Dextransucrases are widely abundant among lactic acid bacteria (LAB), while their primary and secondary structures are highly variable [25, 27, 56]. Consequently, dextrans differing in molecular size, structure, branching, and secondary structure can be produced using different dextransucrases [56–59]. However, variations in the size and molecular weight as well as the molecular structure are also influenceable by extracellular factors [36–38, 60]. As we showed in our previous study [50], the size of dextran from L. hordei TMW 1.1822 is strongly dependent on the extracellular pH value during the dextran polymerization process. These dextrans empirically showed distinct differences in their gelling properties at equally applied concentrations [50]. Thus, one aim of the present study was to investigate these differences in more detail using rheological measurements. As reported in previous studies about microbial levans [37, 45], the viscosity of these dextrans increased with rising molecular size despite comparable molecular structure and degree of branching (Table 2). Comparable thickening and gelling properties were reported for dextrans, which were linear and had a higher molecular weight than the dextrans investigated in the present study [58]. The AF4-MALS-UV analysis revealed only minor differences in their polydispersity and random coil–like secondary structure in aqueous solution (Table 1) [5]. These findings indicate that the chain length/molecular weight is the most decisive factor influencing the variations in the rheological behavior of this dextran and other uncharged, water-soluble EPSs [37, 45]. Longer dextran molecules applied at critical concentrations may have more contact points for establishment of more intermolecular hydrogen bonds and of a more stable physical network than shorter dextran molecules [45]. Consequently, EPS production needs to be optimized towards the desired molecule size in addition to the final EPS yield. As EPS-producing LAB are frequently used as starter cultures for improvement of the structural properties of foods [61, 62], a proper pH control during food fermentation may allow the controlled in situ production of, e.g., gelling or thickening dextrans. This approach could replace the use of synthetically modified thickeners and gelling agents, which have to be labeled on food packages in contrast to EPSs produced naturally from sucrose by food-grade LAB [47, 61, 63–65]. Moreover, the portfolio of commercially available dextrans recovered from Leuconostoc mesenteroides B-512F [23, 58] could be extended by further triggered glucans that exhibit other molecular structures, sizes, and concomitant properties.
The influence of other extrinsic factors besides the pH value on the dextran polymerization, such as the availability of sugars and the applied sugar concentrations as well as the temperature, was subject of several studies [30, 42, 43, 66, 67]. The presented approach for dextran production revealed that the concentration of dextran was reduced if the bacterium was in contact with sucrose prior to the dextran polymerization process (Fig. 3). Hence, the intracellular dextransucrase reservoir of L. hordei TMW 1.1822 [25, 50] appeared to be partially dissipated, although its growth with or without sucrose was comparable (Fig. 4). This finding was confirmed by determination of the amounts of dextran produced in buffer using the crude cell lysates of L. hordei recovered with or without sucrose in the mMRS pre-cultivation medium, respectively (3.3). One reasonable way to boost native dextran production in L. hordei TMW 1.1822 may thus be to optimize constitutive dextransucrase accumulation. The cells could then be used as source of native dextransucrases that can be released by sucrose in minimal growth media without the aim of using the cells as growing metabolic machines.
As described for some other dextransucrases [16, 30, 40, 66, 68], maltose was more favored as acceptor than sucrose or its glucose residue. However, the elongation to isolable high molecular weight dextran was drastically inhibited (Fig. 3, bottom), leading to the assumption that the formed product panose was no suited acceptor for further glucose moieties. Due to the metabolization by various probiotic bacteria [69, 70], panose is regarded as prebiotic carbon source, whose production could be optimized by simple addition of maltose in addition to sucrose. However, this reaction could compete with the production of high molecular weight dextran in some fermented plant-based foods, which intrinsically contain maltose as degradation product of starch [71–73].
Conclusion
This study reveals that the elongation activity of dextransucrases can be triggered by application of different production pH values or by addition of maltose. The properties of high molecular weight dextrans obtained at different pH values depend on the dextran concentration and especially on the molecular weight of the dextran molecules. These findings can be used for the controlled manufacture of functionally diverse products by the use of the dextransucrase of L. hordei TMW 1.1822 or other lactic acid bacteria.
Acknowledgments
We are indebted to Petra Först (Chair of Systems and Process Engineering, Technical University of Munich) for her kind support and introduction regarding the use of the rheometer.
Abbreviations
- AF4-MALS-UV
asymmetric flow field-flow fractionation coupled with multi-angle laser light scattering and UV detection
- EPS
exopolysaccharide
- fru
fructose
- glc
glucose
- HPAEC-PAD
high-performance anion-exchange chromatography with pulsed amperometric detection
- HPLC-RI
high-performance liquid chromatography with refractive index detection
- L.
Liquorilactobacillus
- mal
maltose
- MALDI-TOF-MS
matrix-assisted laser desorption–ionization time-of-flight mass spectrometry
- rms
root mean square
- suc
sucrose
- TMW
Technische Mikrobiologie Weihenstephan
shear rate
- η
viscosity
- G′
storage modulus
- G″
loss modulus
- ω
angular frequency
Authors’ Contributions
JS and FJ conceptualized the study. JS performed the main experimental work, evaluated the dataset, and wrote the main text of the manuscript. DW performed the linkage type analyses. JS, DW, RFV, and FJ were involved in proofreading of the manuscript.
Funding Information
Open Access funding provided by Projekt DEAL. Part of this study was supported by the German Federal Ministry for Economics and Energy via the German Federation of Industrial Research (AiF) and the Industrial Association of Food Technology and Packaging (IVLV) project number AiF 18749 N.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Compliance with Ethical Standards
Competing Interests
The authors declare that they have no competing interests.
Ethics Approval and Consent to Participate
Not applicable. The manuscript does not contain data collected from humans or animals.
Consent for Publication
Not applicable. The manuscript does not contain any individual person’s data.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McCurdy RD, Goff HD, Stanley DW, Stone AP. Rheological properties of dextran related to food applications. Food Hydrocolloids. 1994;8(6):609–623. doi: 10.1016/S0268-005X(09)80068-4. [DOI] [Google Scholar]
- 2.De Vuyst L, De Vin F, Vaningelgem F, Degeest B. Recent developments in the biosynthesis and applications of heteropolysaccharides from lactic acid bacteria. International Dairy Journal. 2001;11(9):687–707. doi: 10.1016/S0958-6946(01)00114-5. [DOI] [Google Scholar]
- 3.Rehm BHA. Bacterial polymers: biosynthesis, modifications and applications. Nature Reviews Microbiology. 2010;8(8):578–592. doi: 10.1038/nrmicro2354. [DOI] [PubMed] [Google Scholar]
- 4.Ryan PM, Ross RP, Fitzgerald GF, Caplice NM, Stanton C. Sugar-coated: exopolysaccharide producing lactic acid bacteria for food and human health applications. Food & Function. 2015;6(3):679–693. doi: 10.1039/C4FO00529E. [DOI] [PubMed] [Google Scholar]
- 5.Eckel VPL, Vogel RF, Jakob F. In situ production and characterization of cloud forming dextrans in fruit-juices. International Journal of Food Microbiology. 2019;306:108261. doi: 10.1016/j.ijfoodmicro.2019.108261. [DOI] [PubMed] [Google Scholar]
- 6.Vercueil A, Grocott MP, Mythen MG. Physiology, pharmacology, and rationale for colloid administration for the maintenance of effective hemodynamic stability in critically ill patients. Transfus Med Rev. 2005;19(2):93–109. doi: 10.1016/j.tmrv.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Smith AM, Moxon S, Morris GA. In: Wound Healing Biomaterials, vol. 2: 13 - Biopolymers as wound healing materials. Ågren MS, editor. Sawston: Woodhead Publishing; 2016. pp. 261–287. [Google Scholar]
- 8.Nair AV, Raman M, Doble M. In: Materials for Biomedical Engineering, vol. 1: Chapter 13 - Polysaccharide-based hydrogels for targeted drug delivery. Holban A-M, Grumezescu AM, editors. Amsterdam: Elsevier; 2019. pp. 343–382. [Google Scholar]
- 9.Rimondo S, Perale G, Rossi F. In: Functional Polysaccharides for Biomedical Applications, vol. 1: 6 - Polysaccharide-based scaffold for tissue-regeneration. Maiti S, Jana S, editors. Sawston: Woodhead Publishing; 2019. pp. 189–212. [Google Scholar]
- 10.Varghese SA, Rangappa SM, Siengchin S, Parameswaranpillai J. In: Hydrogels Based on Natural Polymers, vol. 1: Chapter 2 - Natural polymers and the hydrogels prepared from them. Chen Y, editor. Amsterdam: Elsevier; 2020. pp. 17–47. [Google Scholar]
- 11.Veerubhotla R, Varanasi JL, Das D. In: Progress and recent trends in microbial fuel cells, vol. 1: Chapter 12 - Biofilm formation within microbial fuel cells. Kundu PP, Dutta K, editors. Amsterdam: Elsevier; 2018. pp. 231–242. [Google Scholar]
- 12.Laue H, Schenk A, Li H, Lambertsen L, Neu TR, Molin S, Ullrich MS. Contribution of alginate and levan production to biofilm formation by Pseudomonas syringae. Microbiology. 2006;152(10):2909–2918. doi: 10.1099/mic.0.28875-0. [DOI] [PubMed] [Google Scholar]
- 13.Koczan JM, McGrath MJ, Zhao Y, Sundin GW. Contribution of Erwinia amylovora exopolysaccharides amylovoran and levan to biofilm formation: implications in pathogenicity. Phytopathology™. 2009;99:1237–1244. doi: 10.1094/PHYTO-99-11-1237. [DOI] [PubMed] [Google Scholar]
- 14.Zhu M, Ajdić D, Liu Y, Lynch D, Merritt J, Banas JA. Role of the Streptococcus mutans irvA gene in GbpC-independent, dextran-dependent aggregation and biofilm formation. Applied and Environmental Microbiology. 2009;75(22):7037–7043. doi: 10.1128/AEM.01015-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dogsa I, Brloznik M, Stopar D, Mandic-Mulec I. Exopolymer diversity and the role of levan in Bacillus subtilis biofilms. PLOS ONE. 2013;8(4):e62044. doi: 10.1371/journal.pone.0062044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leemhuis H, Pijning T, Dobruchowska JM, van Leeuwen SS, Kralj S, Dijkstra BW, Dijkhuizen L. Glucansucrases: three-dimensional structures, reactions, mechanism, α-glucan analysis and their implications in biotechnology and food applications. Journal of Biotechnology. 2013;163(2):250–272. doi: 10.1016/j.jbiotec.2012.06.037. [DOI] [PubMed] [Google Scholar]
- 17.Augimeri RV, Varley AJ, Strap JL. Establishing a role for bacterial cellulose in environmental interactions: lessons learned from diverse biofilm-producing Proteobacteria. Frontiers in Microbiology. 2015;6:1282–1282. doi: 10.3389/fmicb.2015.01282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fels L, Jakob F, Vogel RF, Wefers D. Structural characterization of the exopolysaccharides from water kefir. Carbohydrate Polymers. 2018;189:296–303. doi: 10.1016/j.carbpol.2018.02.037. [DOI] [PubMed] [Google Scholar]
- 19.Xu D, Fels L, Wefers D, Behr J, Jakob F, Vogel RF. Lactobacillus hordei dextrans induce Saccharomyces cerevisiae aggregation and network formation on hydrophilic surfaces. International Journal of Biological Macromolecules. 2018;115:236–242. doi: 10.1016/j.ijbiomac.2018.04.068. [DOI] [PubMed] [Google Scholar]
- 20.Monsan P, Bozonnet S, Albenne C, Joucla G, Willemot R-M, Remaud-Siméon M. Homopolysaccharides from lactic acid bacteria. International Dairy Journal. 2001;11(9):675–685. doi: 10.1016/S0958-6946(01)00113-3. [DOI] [Google Scholar]
- 21.Decock P, Cappelle S. Bread technology and sourdough technology. Trends in Food Science & Technology. 2005;16(1-3):113–120. doi: 10.1016/j.tifs.2004.04.012. [DOI] [Google Scholar]
- 22.Kothari D, Das D, Patel S, Goyal A. In: Polysaccharides: Bioactivity and Biotechnology, vol. 1: Dextran and food application. Ramawat KG, Mérillon J-M, editors. Cham: Springer International Publishing; 2015. pp. 735–752. [Google Scholar]
- 23.De Belder, A. N. (2003) Dextran. Handbook provided by Amersham Biosiences.
- 24.Porath J, Flodin PER. Gel filtration: a method for desalting and group separation. Nature. 1959;183(4676):1657–1659. doi: 10.1038/1831657a0. [DOI] [PubMed] [Google Scholar]
- 25.Bechtner J, Wefers D, Schmid J, Vogel RF, Jakob F. Identification and comparison of two closely related dextransucrases released by water kefir borne Lactobacillus hordei TMW 1.1822 and Lactobacillus nagelii TMW 1.1827. Microbiology. 2019;165(9):956–966. doi: 10.1099/mic.0.000825. [DOI] [PubMed] [Google Scholar]
- 26.Robyt JF, Kimble BK, Walseth TF. The mechanism of dextransucrase action: direction of dextran biosynthesis. Archives of Biochemistry and Biophysics. 1974;165(2):634–640. doi: 10.1016/0003-9861(74)90291-4. [DOI] [PubMed] [Google Scholar]
- 27.Sidebotham RL. Dextrans. Advances in Carbohydrate Chemistry and Biochemistry. 1974;30:371–444. doi: 10.1016/S0065-2318(08)60268-1. [DOI] [PubMed] [Google Scholar]
- 28.Luzio GA, Mayer RM. The hydrolysis of sucrose by dextransucrase. Carbohydrate Research. 1983;111(2):311–318. doi: 10.1016/0008-6215(83)88315-3. [DOI] [Google Scholar]
- 29.Mooser G, Iwaoka KR. Sucrose 6-.alpha.-D-glucosyltransferase from Streptococcus sobrinus: characterization of a glucosyl-enzyme complex. Biochemistry. 1989;28(2):443–449. doi: 10.1021/bi00428a006. [DOI] [PubMed] [Google Scholar]
- 30.Moulis C, Joucla G, Harrison D, Fabre E, Potocki-Veronese G, Monsan P, Remaud-Simeon M. Understanding the polymerization mechanism of glycoside-hydrolase family 70 glucansucrases. Journal of Biological Chemistry. 2006;281(42):31254–31267. doi: 10.1016/S0021-9258(19)84038-3. [DOI] [PubMed] [Google Scholar]
- 31.Otts DR, Day DF. Dextransucrase secretion in Leuconostoc mesenteroides depends on the presence of a transmembrane proton gradient. Journal of bacteriology. 1988;170(11):5006–5011. doi: 10.1128/JB.170.11.5006-5011.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim D, Robyt JF, Lee S-Y, Lee J-H, Kim Y-M. Dextran molecular size and degree of branching as a function of sucrose concentration, pH, and temperature of reaction of Leuconostoc mesenteroides B-512FMCM dextransucrase. Carbohydrate Research. 2003;338(11):1183–1189. doi: 10.1016/S0008-6215(03)00148-4. [DOI] [PubMed] [Google Scholar]
- 33.Sarwat F, Ul Qader SA, Aman A, Ahmed N. Production & characterization of a unique dextran from an indigenous Leuconostoc mesenteroides CMG713. International Journal of Biological Sciences. 2008;4:379–386. doi: 10.7150/ijbs.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jakob F, Pfaff A, Novoa-Carballal R, Rübsam H, Becker T, Vogel RF. Structural analysis of fructans produced by acetic acid bacteria reveals a relation to hydrocolloid function. Carbohydrate Polymers. 2013;92(2):1234–1242. doi: 10.1016/j.carbpol.2012.10.054. [DOI] [PubMed] [Google Scholar]
- 35.Ua-Arak T, Jakob F, Vogel RF. Fermentation pH modulates the size distributions and functional properties of Gluconobacter albidus TMW 2.1191 levan. Frontiers in Microbiology. 2017;8:807. doi: 10.3389/fmicb.2017.00807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prechtl RM, Wefers D, Jakob F, Vogel RF. Cold and salt stress modulate amount, molecular and macromolecular structure of a Lactobacillus sakei dextran. Food Hydrocolloids. 2018;82:73–81. doi: 10.1016/j.foodhyd.2018.04.003. [DOI] [Google Scholar]
- 37.Hundschell, C., Jakob, F. and Wagemans, A. (2019) Molecular weight dependent structure and polymer density of the exopolysaccharide levan. arXiv preprint arxiv.org/abs/1909.07737. [DOI] [PubMed]
- 38.Jakob F, Gebrande C, Bichler RM, Vogel RF. Insights into the pH-dependent, extracellular sucrose utilization and concomitant levan formation by Gluconobacter albidus TMW 2.1191. Antonie van Leeuwenhoek. 2020;113(7):863–873. doi: 10.1007/s10482-020-01397-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Notararigo S, Nácher-Vázquez M, Ibarburu I, Werning ML, de Palencia PF, Dueñas MT, Aznar R, López P, Prieto A. Comparative analysis of production and purification of homo- and hetero-polysaccharides produced by lactic acid bacteria. Carbohydrate Polymers. 2013;93(1):57–64. doi: 10.1016/j.carbpol.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 40.Koepsell HJ, Tsuchiya HM, Hellman NN, Kazenko A, Hoffman CA, Sharpe ES, Jackson RW. Enzymatic synthesis of dextran acceptor specificity and chain initiation. The Journal of Biological Chemistry. 1953;200:793–801. doi: 10.1016/S0021-9258(18)71427-0. [DOI] [PubMed] [Google Scholar]
- 41.Paul F, Oriol E, Auriol D, Monsan P. Acceptor reaction of a highly purified dextransucrase with maltose and oligosaccharides. Application to the synthesis of controlled-molecular-weight dextrans. Carbohydrate Research. 1986;149(2):433–441. doi: 10.1016/S0008-6215(00)90063-6. [DOI] [Google Scholar]
- 42.Böker M, Jördening H-J, Buchholz K. Kinetics of leucrose formation from sucrose by dextransucrase. Biotechnology and Bioengineering. 1994;43(9):856–864. doi: 10.1002/bit.260430904. [DOI] [PubMed] [Google Scholar]
- 43.Heincke K, Demuth B, Jördening H-J, Buchholz K. Kinetics of the dextransucrase acceptor reaction with maltose—experimental results and modeling. Enzyme and Microbial Technology. 1999;24(8-9):523–534. doi: 10.1016/S0141-0229(98)00150-1. [DOI] [Google Scholar]
- 44.Zarour K, Llamas MG, Prieto A, Rúas-Madiedo P, Dueñas MT, de Palencia PF, Aznar R, Kihal M, López P. Rheology and bioactivity of high molecular weight dextrans synthesised by lactic acid bacteria. Carbohydrate Polymers. 2017;174:646–657. doi: 10.1016/j.carbpol.2017.06.113. [DOI] [PubMed] [Google Scholar]
- 45.Hundschell CS, Braun A, Wefers D, Vogel RF, Jakob F. Size-dependent variability in flow and viscoelastic behavior of levan produced by Gluconobacter albidus TMW 2.1191. Foods. 2020;9:192. doi: 10.3390/foods9020192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murray JCF. In: Handbook of hydrocolloids vol. 2: 25 - Cellulosics. Phillips GO, Williams PA, editors. Sawston: Woodhead Publishing; 2009. pp. 710–723. [Google Scholar]
- 47.Saha D, Bhattacharya S. Hydrocolloids as thickening and gelling agents in food: a critical review. Journal of Food Science and Technology. 2010;47(6):587–597. doi: 10.1007/s13197-010-0162-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaur L, Singh J. In: Encyclopedia of food and health: Starch: modified starches. Caballero B, Finglas PM, Toldrá F, editors. Oxford: Academic Press; 2016. pp. 152–159. [Google Scholar]
- 49.Chen Y-F, Kaur L, Singh J. In: Starch in food vol. 2: Chapter 7 - Chemical modification of starch. Sjöö M, Nilsson L, editors. Sawston: Woodhead Publishing; 2018. pp. 283–321. [Google Scholar]
- 50.Schmid J, Bechtner J, Vogel RF, Jakob F. A systematic approach to study the pH-dependent release, functionality and product specificity of dextransucrases. Microbial Cell Factories. 2019;18(1):153. doi: 10.1186/s12934-019-1208-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gulitz A, Stadie J, Wenning M, Ehrmann MA, Vogel RF. The microbial diversity of water kefir. International Journal of Food Microbiology. 2011;151(3):284–288. doi: 10.1016/j.ijfoodmicro.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 52.Stolz P, Böcker G, Vogel RF, Hammes WP. Utilisation of maltose and glucose by lactobacilli isolated from sourdough. FEMS Microbiology Letters. 1993;109(2-3):237–242. doi: 10.1111/j.1574-6968.1993.tb06174.x. [DOI] [Google Scholar]
- 53.Kern CC, Vogel RF, Behr J. Differentiation of Lactobacillus brevis strains using matrix-assisted-laser-desorption-ionization-time-of-flight mass spectrometry with respect to their beer spoilage potential. Food Microbiology. 2014;40:18–24. doi: 10.1016/j.fm.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 54.Korakli M, Rossmann A, Gänzle MG, Vogel RF. Sucrose metabolism and exopolysaccharide production in wheat and rye sourdoughs by Lactobacillus sanfranciscensis. Journal of Agricultural and Food Chemistry. 2001;49(11):5194–5200. doi: 10.1021/jf0102517. [DOI] [PubMed] [Google Scholar]
- 55.Sweet DP, Shapiro RH, Albersheim P. Quantitative analysis by various g.l.c. response-factor theories for partially methylated and partially ethylated alditol acetates. Carbohydrate Research. 1975;40(2):217–225. doi: 10.1016/S0008-6215(00)82604-X. [DOI] [Google Scholar]
- 56.Naessens M, Cerdobbel A, Soetaert W, Vandamme EJ. Leuconostoc dextransucrase and dextran: production, properties and applications. Journal of Chemical Technology & Biotechnology. 2005;80(8):845–860. doi: 10.1002/jctb.1322. [DOI] [Google Scholar]
- 57.Netsopa S, Niamsanit S, Sakloetsakun D, Milintawisamai N. Characterization and rheological behavior of dextran from Weissella confusa. International Journal of Polymer Science. 2018;2018:1–10. doi: 10.1155/2018/5790526. [DOI] [Google Scholar]
- 58.Vuillemin M, Grimaud F, Claverie M, Rolland-Sabaté A, Garnier C, Lucas P, Monsan P, Dols-Lafargue M, Remaud-Siméon M, Moulis C. A dextran with unique rheological properties produced by the dextransucrase from Oenococcus kitaharae DSM 17330. Carbohydrate Polymers. 2018;179:10–18. doi: 10.1016/j.carbpol.2017.09.056. [DOI] [PubMed] [Google Scholar]
- 59.Sabatié J, Choplin L, Doublier JL, Arul J, Paul F, Monsan P. Rheology of native dextrans in relation to their primary structure. Carbohydrate Polymers. 1988;9(4):287–299. doi: 10.1016/0144-8617(88)90047-1. [DOI] [Google Scholar]
- 60.Sabatie J, Choplin L, Paul F, Monsan P. The effect of synthesis temperature on the rheological properties of native dextran. Biotechnology Letters. 1986;8(6):425–430. doi: 10.1007/BF01026747. [DOI] [Google Scholar]
- 61.Torino MI, Font de Valdez G, Mozzi F. Biopolymers from lactic acid bacteria. Novel applications in foods and beverages. Frontiers in Microbiology. 2015;6:834. doi: 10.3389/fmicb.2015.00834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oleksy M, Klewicka E. Exopolysaccharides produced by Lactobacillus sp.: biosynthesis and applications. Critical Reviews in Food Science and Nutrition. 2018;58(3):450–462. doi: 10.1080/10408398.2016.1187112. [DOI] [PubMed] [Google Scholar]
- 63.Milani J, Maleki G. In: Food Industrial Processes: Hydrocolloids in food industry. Valdez B, editor. Rijeka: IntechOpen; 2012. [Google Scholar]
- 64.Duboc P, Mollet B. Applications of exopolysaccharides in the dairy industry. International Dairy Journal. 2001;11(9):759–768. doi: 10.1016/S0958-6946(01)00119-4. [DOI] [Google Scholar]
- 65.Tieking M, Korakli M, Ehrmann MA, Gänzle MG, Vogel RF. In situ production of exopolysaccharides during sourdough fermentation by cereal and intestinal isolates of lactic acid bacteria. Applied and Environmental Microbiology. 2003;69(2):945–952. doi: 10.1128/AEM.69.2.945-952.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Robyt JF, Walseth TF. The mechanism of acceptor reactions of Leuconostoc mesenteroides B-512F dextransucrase. Carbohydrate Research. 1978;61(1):433–445. doi: 10.1016/S0008-6215(00)84503-6. [DOI] [PubMed] [Google Scholar]
- 67.Falconer DJ, Mukerjea R, Robyt JF. Biosynthesis of dextrans with different molecular weights by selecting the concentration of Leuconostoc mesenteroides B-512FMC dextransucrase, the sucrose concentration, and the temperature. Carbohydrate Research. 2011;346(2):280–284. doi: 10.1016/j.carres.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 68.Killey M, Dimler RJ, Cluskey JE. Preparation of panose by the action of NRRL B-512 Dextransucrase on a sucrose-maltose mixture2. Journal of the American Chemical Society. 1955;77(12):3315–3318. doi: 10.1021/ja01617a048. [DOI] [Google Scholar]
- 69.Mäkeläinen H, Hasselwander O, Rautonen N, Ouwehand AC. Panose, a new prebiotic candidate. Letters in Applied Microbiology. 2009;49(6):666–672. doi: 10.1111/j.1472-765X.2009.02698.x. [DOI] [PubMed] [Google Scholar]
- 70.Ejby M, Fredslund F, Andersen JM, Vujičić Žagar A, Henriksen JR, Andersen TL, Svensson B, Slotboom DJ, Abou Hachem M. An ATP binding cassette transporter mediates the uptake of α-(1,6)-linked dietary oligosaccharides in Bifidobacterium and correlates with competitive growth on these substrates. The Journal of Biological Chemistry. 2016;291(38):20220–20231. doi: 10.1074/jbc.M116.746529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sánchez-Mata MC, Peñuela-Teruel MJ, Cámara-Hurtado M, Díez-Marqués C, Torija-Isasa ME. Determination of mono-, di-, and oligosaccharides in legumes by high-performance liquid chromatography using an amino-bonded silica column. Journal of Agricultural and Food Chemistry. 1998;46(9):3648–3652. doi: 10.1021/jf980127w. [DOI] [Google Scholar]
- 72.Halford NG, Curtis TY, Muttucumaru N, Postles J, Mottram DS. Sugars in crop plants. Annals of Applied Biology. 2011;158(1):1–25. doi: 10.1111/j.1744-7348.2010.00443.x. [DOI] [Google Scholar]
- 73.Dramićanin AM, Andrić FL, Poštić DŽ, Momirović NM, Milojković-Opsenica DM. Sugar profiles as a promising tool in tracing differences between potato cultivation systems, botanical origin and climate conditions. Journal of Food Composition and Analysis. 2018;72:57–65. doi: 10.1016/j.jfca.2018.06.005. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.