Abstract
Background:
Increased visceral adipose tissue (VAT) precedes development of insulin resistance and dyslipidemia in adults. The associations of abdominal adiposity derived from dual-energy X-ray absorptiometry (DXA), including VAT, subcutaneous abdominal adipose tissue (SAAT), and total abdominal adipose tissue (TAAT) with cardio-metabolic risk in adolescents are understudied.
Objectives:
We examined the cross-sectional associations of DXA-measured abdominal adiposity with cardio-metabolic risk and related markers in early adolescence (mean [SD] age 13.0 [0.7] years).
Methods:
We collected data from 740 adolescents (374 girls and 366 boys) in Project Viva, a U.S. pre-birth cohort. We used DXA estimates of VAT, SAAT and TAAT area. We conducted overall and sex-stratified linear regression models, adjusting for age, sex (in overall models), race/ethnicity, puberty score, and body mass index (BMI) z-score.
Results:
Mean BMI z-score was 0.59 (1.28). After adjustment, greater VAT (per 1 standard deviation score) was associated with higher metabolic risk z-score (β 0.14 units; 95% CI 0.08, 0.20) and its related components, higher log high‐sensitivity C‐reactive protein (β 0.51 mg/L; 0.36, 0.66) and log leptin (β 0.36 ng/mL; 0.27, 0.44), and lower log adiponectin (β −0.08 ug/mL; −0.13, −0.02). SAAT and TAAT showed similar associations as VAT with comparable or greater effect sizes.
Conclusion:
In early adolescence, DXA-measured VAT, SAAT and TAAT are associated with cardio-metabolic risk and related markers, independent of current BMI. Among two adolescents with the same BMI, there is an associated higher cardio-metabolic risk in the adolescent with greater DXA-measured abdominal adiposity.
Keywords: epidemiology, adolescence, obesity, adiposity, metabolism
Introduction
Visceral adipose tissue (VAT) is associated with health risks, including metabolic dysregulation and cardiovascular disease.1,2 VAT is considered a pathogenic fat depot, and increased VAT has been shown to precede development of insulin resistance and atherogenic dyslipidemia in adults.3,4 The associations of VAT, and abdominal adiposity in general, with cardio-metabolic risk in adolescents are not as well investigated.
There are few population-based studies measuring both VAT and subcutaneous abdominal adipose tissue (SAAT) in relation to cardio-metabolic risk factors in adolescents. In addition, studies in children and adolescents have shown contrasting results in the associations of VAT with blood pressure, lipid tests, and other metabolic risk markers.5–10 Traditional anthropometry such as BMI, waist circumference, and suprailiac skin fold thickness have shown stronger correlation with SAAT than with VAT in adolescents.11 Better understanding of how and which adipose tissue compartments portend risk of cardio-metabolic disease is warranted.
DXA-measured VAT, or DXA-VAT, has been validated against VAT measured by computed tomography (CT) in adults and children.12,13 Whereas traditional anthropometry including waist circumference and BMI are indirect adiposity measures that are unable to distinguish between fat mass and lean mass, DXA-VAT is a direct adiposity measure focused on a specific abdominal region.14,15 Further, DXA is less costly and emits less radiation than CT, and thus it is feasible in large pediatric cohort studies. There are limited studies of DXA-VAT and its association with cardio-metabolic risk in children and adolescents. In adults, DXA-VAT has been demonstrated to be a valuable clinical marker of cardio-metabolic risk, defined by blood pressure, fasting glucose, triglycerides and HDL-C.16,17 Given the gap in literature specifically on DXA-measured VAT, SAAT and total abdominal adipose tissue (TAAT) in adolescents, we focused on these variables and their association with cardio-metabolic risk and related markers.
The aims of this cross-sectional study were to examine the associations of DXA-measured VAT, SAAT and TAAT with cardio-metabolic risk and related markers, and further to examine sex-specific associations in a large multi-ethnic cohort evaluated in early adolescence. We hypothesized higher VAT would be associated with greater cardio-metabolic risk and that these associations would be stronger than SAAT or TAAT associations. In addition, we hypothesized that VAT associations in boys would be stronger in comparison to girls, as males generally have more VAT than females do through later childhood and adulthood.
Methods
Participants
Project Viva is an on-going, longitudinal pre-birth cohort. We recruited women between 1999 and 2002 during prenatal visits at Atrius Harvard Vanguard Medical Associates, a large multispecialty group practice in eastern Massachusetts. We have previously published details about the full cohort, recruitment and follow up of participants.18 Study policies and protocols are available at https://www.hms.harvard.edu/viva/protocol-policies.html. The institutional review board of Harvard Pilgrim Health Care Institute approved the study protocols. All mothers provided written informed consent for themselves and their children, and the adolescents provided verbal assent.
There were 2128 original live-born singletons in Project Viva, of which 1684 remained eligible for the early teen visit and 1038 completed in-person study visits. At that visit, 741 teens completed DXA analysis, and 1 was excluded due to poor body positioning. Mothers of the 740 included participants vs. 1388 excluded participants were more likely to have a college degree or beyond (69% vs. 62%) and a higher proportion (64% vs. 59%) with reported household income >$70,000/year (see Supplementary Table S2). Included and excluded participants were similarly non-White (38% vs. 36%, respectively). Included participants had higher mean (SD) BMI z-score of 0.59 (1.28) compared to excluded participants with BMI z-score of 0.35 (1.13).
Measurements
Trained research assistants completed anthropometric and blood pressure measurement using standardized techniques. During in-person visits, we measured weight (TBF-300A scale, Tanita, Arlington Heights, IL), height (calibrated stadiometer, Shorr Productions, Olney, MD), and waist circumference right above the iliac crest using a Gulick II measuring tape (Performance Health, Warrenville, IL) while standing. We measured whole-body fat percentage using bioelectrical impedance analysis (BIA, Tanita TBF-300A scale).
We measured body composition by DXA scans (Hologic model Discovery A, Bedford, MA). We performed quality control on DXA scans per manufacturer instructions, and we used the same DXA machine for all participants. A single trained research assistant verified all scans and defined body regions for analysis. Intra-rater reliability on duplicate measurements was high (r=0.99). We used DXA Hologic Apex software 4.0 to obtain whole-body fat percentage, trunk fat percentage, SAAT area and TAAT area. As previously published, Hologic software estimates VAT area by subtracting SAAT area from TAAT area in a region at approximately the 4th/5th lumbar vertebrae, with the bottom edge placed at 1 cm above the iliac crest.19 Trunk fat percentage is estimated from a larger area encompassing the thoracic and abdominal regions (see Supplementary Figure S1).
A phlebotomist performed 8-hour fasting blood tests from the antecubital vein in amenable participants at the in-person early teen visits (n=579). There were 534 participants with DXA scans and at least one blood biomarker measured as part of our outcomes of interest. Alanine aminotransferase (ALT) and plasma fasting insulin were analyzed with the Roche Modular system, and fasting glucose was enzymatically measured with Roche Diagnostics reagents. We estimated insulin resistance using the homeostatic model assessment of insulin resistance (HOMA-IR), calculated by (glucose in mg/dL x insulin in μU/mL) / 405. We measured triglycerides (TG) and high-density lipoprotein cholesterol (HDL-C) enzymatically. Metabolic risk z-score was derived from age and sex-specific external z scores for waist circumference, systolic blood pressure (SBP), HDL-C (scaled inversely), TG and HOMA-IR. We standardized SBP as z-scores adjusted for age, sex and height based on National Heart, Lung and Blood Institute references. We assessed plasma leptin and adiponectin concentrations with radioimmunoassay (Linco Research, St Charles, MO, USA). We measured high‐sensitivity C‐reactive protein (hsCRP) via an immunoturbidimetric high-sensitivity assay on a Hitachi 911 analyzer (Roche Diagnostics, Indianapolis, IN, USA).20 We excluded biologically improbable values.
Covariates
During childhood and early teen visits we used questionnaires and interviews to obtain information about the child’s age, race/ethnicity, and puberty status. Parents reported on the pubertal phenotype of their children based on the appearance of body hair and breast development for girls; and body hair, facial hair and voice deepening for boys. We calculated and standardized BMI z-scores using WHO growth charts (https://www.who.int/childgrowth/standards/bmi_for_age/en/).21
Statistical Analyses
We used independent group t-tests to describe differences in DXA-measured abdominal adiposity by sex (2-tailed) with a threshold for significance of p<0.05. We calculated Spearman’s correlation coefficients between DXA-measured abdominal adiposity and other adiposity measurements. We log transformed variables including HDL-C, TG, HOMA-IR, ALT, hsCRP, leptin and adiponectin given lack of normal distribution. We also transformed VAT, SAAT and TAAT areas to internal, sex-specific standard deviation scores (SDS) to allow comparison between adipose tissue compartments. The regression coefficients (β) were summarized as the effect of 1 SDS increase in VAT, SAAT or TAAT on metabolic risk z-score and related markers. We conducted linear regression for the overall cohort, starting with an unadjusted model (Model 1). We then adjusted for age, sex, race/ethnicity, and puberty score (Model 2), and additionally for BMI z-score (Model 3). We determined covariates based on a priori knowledge of determinants of cardio-metabolic risk. We kept all a priori selected covariates in the adjusted models as correlation analysis confirmed the variables to be associated (p<0.05) with at least one cardio-metabolic risk or related biomarkers. We repeated the linear regressions separately in boys and girls as prior studies have suggested sex differences in abdominal adiposity and change in metabolic biomarkers in children and adolescents.20,22 We performed multiple imputation for all 2128 participants and then limited the analysis sample to only the participants with the exposure and at least one blood outcome. Since multiple imputation analysis yielded similar results, we presented the complete case analysis. Analyses were performed using Stata 16 software (StataCorp LP, College Station, Texas).
Results
We included data from 740 adolescents (374 girls and 366 boys). Mean (SD) age was 13.0 (0.7) years, BMI z-score was 0.59 (1.28), and VAT, SAAT and TAAT areas were 40.6 (23.4) cm2, 164.6 (121.9) cm2, and 205.2 (138.8) cm2, respectively. Maternal and participant characteristics, anthropometric and clinical information are outlined in Table 1. The race/ethnicity composition of the adolescents was 62% white, 18% black, 4% Hispanic, 3% Asian, and 13% other, which also included participants identifying with more than one race/ethnicity. Spearman’s correlation coefficients for VAT, SAAT and TAAT with other measures of adiposity are shown in Table 2 (all p≤0.0001). VAT was correlated with waist circumference r=0.74. SAAT was strongly correlated (r≥0.90) with TAAT, trunk percent fat, total percent fat, and BIA percent fat. TAAT was strongly correlated (r≥0.90) with trunk percent fat, total percent fat, and BIA percent fat. There were significant differences in VAT, SAAT and TAAT areas in boys and girls. Mean (SD) VAT area was higher among boys (47.6 cm2 [21.0]) than girls (33.7 cm2 [23.6]), p<0.0001. Boys had lower mean (SD) SAAT area (122.8 cm2 [114.7]) than girls (205.5 cm2 [114.9]), p<0.0001. In addition, boys had lower mean (SD) TAAT area (170.3 cm2 [132.8]) than girls (239.3 cm2 [136.3]), p<0.0001.
Table 1.
Participant characteristics overall and according to sex among 740 adolescents and their mothers in Project Viva
| Overall | Girls | Boys | |
|---|---|---|---|
| n=740 | n=374 (51%) | n=366 (49%) | |
| Mean (SD) or N (%) | |||
| Maternal characteristics | |||
| Age at enrollment, years | 32.2 (5.4) | 32.4 (5.0) | 31.9 (5.7) |
| Pre-pregnancy BMI, kg/m2 | 25.0 (5.3) | 24.9 (5.5) | 25.0 (5.1) |
| Pregnancy weight gain, kg | 15.5 (5.4) | 15.1 (5.5) | 16.0 (5.3) |
| Pregnancy smoking status, % | |||
| Never | 524 (71) | 268 (72) | 256 (70) |
| Former | 148 (20) | 74 (20) | 74 (20) |
| During pregnancy | 66 (9) | 30 (8) | 36 (10) |
| College degree or beyond, % | |||
| No | 231 (31) | 104 (28) | 127 (35) |
| Yes | 507 (69) | 269 (72) | 238 (65) |
| Married or cohabitating, % | |||
| No | 68 (9) | 35 (9) | 33 (9) |
| Yes | 670 (91) | 338 (91) | 332 (91) |
| Household income >$70,000/year, % | |||
| No | 241 (36) | 125 (36) | 116 (36) |
| Yes | 431 (64) | 223 (64) | 208 (64) |
| Child characteristics | |||
| Race/ethnicity, % | |||
| White | 462 (62) | 244 (65) | 218 (60) |
| Black | 131 (18) | 59 (16) | 72 (20) |
| Hispanic | 33 (4) | 17 (5) | 16 (4) |
| Asian | 20 (3) | 9 (2) | 11 (3) |
| Other | 93 (13) | 44 (12) | 49 (13) |
| Early teen characteristics | |||
| Age, years | 13.0 (0.7) | 13.0 (0.7) | 12.9 (0.6) |
| Puberty score, points | 2.5 (0.9) | 3.0 (0.6) | 2.0 (0.8) |
| VAT area, cm2 | 40.6 (23.4) | 33.7 (23.6) | 47.6 (21.0) |
| SAAT area, cm2 | 164.6 (121.9) | 205.5 (114.9) | 122.8 (114.7) |
| TAAT area, cm2 | 205.2 (138.8) | 239.3 (136.3) | 170.3 (132.8) |
| BMI, kg/m2 | 21.0 (4.7) | 21.1 (4.8) | 20.9 (4.6) |
| WHO BMI z-score | 0.59 (1.28) | 0.51 (1.28) | 0.68 (1.29) |
| Waist circumference, cm | 73.1 (12.1) | 73.0 (11.5) | 73.3 (12.7) |
| BIA % fat | 22.2 (10.4) | 26.1 (9.6) | 18.2 (9.6) |
| DXA total % fat | 28.5 (7.6) | 30.3 (6.6) | 26.7 (8.0) |
| DXA trunk % fat | 24.5 (8.3) | 26.2 (7.8) | 22.9 (8.6) |
| Metabolic risk z-score | −0.15 (0.46) | −0.19 (0.44) | −0.11 (0.48) |
| SBP z-score | −0.19 (0.80) | −0.26 (0.84) | −0.11 (0.75) |
| HDL-C, mg/dL | 55.7 (13.3) | 55.3 (12.5) | 56.1 (13.8) |
| TG, mg/dL | 69.3 (30.9) | 71.3 (31.1) | 67.5 (30.8) |
| HOMA-IR, units | 3.22 (2.37) | 3.56 (2.44) | 2.91 (2.27) |
| ALT, U/L | 19.1 (9.6) | 17.5 (7.2) | 20.5 (11.2) |
| hsCRP, mg/L | 1.00 (2.37) | 1.06 (2.57) | 0.94 (2.18) |
| Leptin, ng/mL | 12.2 (14.3) | 15.8 (15.8) | 8.9 (12.1) |
| Adiponectin, ug/mL | 6.15 (2.69) | 6.55 (2.72) | 5.79 (2.61) |
VAT, visceral adipose tissue; SAAT, subcutaneous abdominal adipose tissue; TAAT, total abdominal adipose tissue; BMI, body mass index; WC, waist circumference; BIA, bioelectrical impedance analysis; DXA, dual-energy X-ray absorptiometry; SBP, systolic blood pressure; HDL-C, high-density lipoprotein cholesterol; TG, triglycerides; HOMA-IR, homeostatic model assessment of insulin resistance; ALT, alanine aminotransferase; hsCRP, high‐sensitivity C‐reactive protein.
Table 2.
Spearman’s correlation coefficients between DXA-measured adiposity and other adiposity measures
| VAT area | SAAT area | TAAT area | BMI | WC | BIA % fat | DXA total % fat | DXA trunk % fat | |
|---|---|---|---|---|---|---|---|---|
| VAT area | 1.00 | |||||||
| 740 | ||||||||
| SAAT area | 0.43 | 1.00 | ||||||
| 740 | 740 | |||||||
| TAAT area | 0.54 | 0.99 | 1.00 | |||||
| 740 | 740 | 740 | ||||||
| BMI | 0.70 | 0.74 | 0.79 | 1.00 | ||||
| 740 | 740 | 740 | 740 | |||||
| WC | 0.74 | 0.75 | 0.81 | 0.89 | 1.00 | |||
| 740 | 740 | 740 | 740 | 740 | ||||
| BIA % fat | 0.47 | 0.90 | 0.91 | 0.80 | 0.77 | 1.00 | ||
| 738 | 738 | 738 | 738 | 738 | 738 | |||
| DXA total % fat | 0.57 | 0.91 | 0.93 | 0.72 | 0.71 | 0.85 | 1.00 | |
| 740 | 740 | 740 | 740 | 740 | 738 | 740 | ||
| DXA trunk % fat | 0.61 | 0.92 | 0.94 | 0.73 | 0.74 | 0.85 | 0.98 | 1.00 |
| 740 | 740 | 740 | 740 | 740 | 738 | 740 | 740 |
Number of observations
Significant correlation for all values at p<0.0001. VAT, visceral adipose tissue; TAAT, total abdominal adipose tissue; SAAT, subcutaneous abdominal adipose tissue; BMI, body mass index; WC, waist circumference; BIA, bioelectrical impedance analysis; DXA, dual-energy X-ray absorptiometry.
Overall, in unadjusted and adjusted models for age, sex, race/ethnicity, puberty score and BMI z-score (Model 3), greater DXA-measured VAT, SAAT and TAAT (per 1 sex-specific SDS) were associated with higher metabolic risk z-score. The effect estimates were attenuated in adjusted models, though they remained significant in Model 3 (VAT β 0.14 units; 95% CI 0.08, 0.20 vs. SAAT β 0.21 units; 0.16, 0.27 vs. TAAT β 0.23 units; 0.17, 0.29), and are presented in Figure 1.
Figure 1.
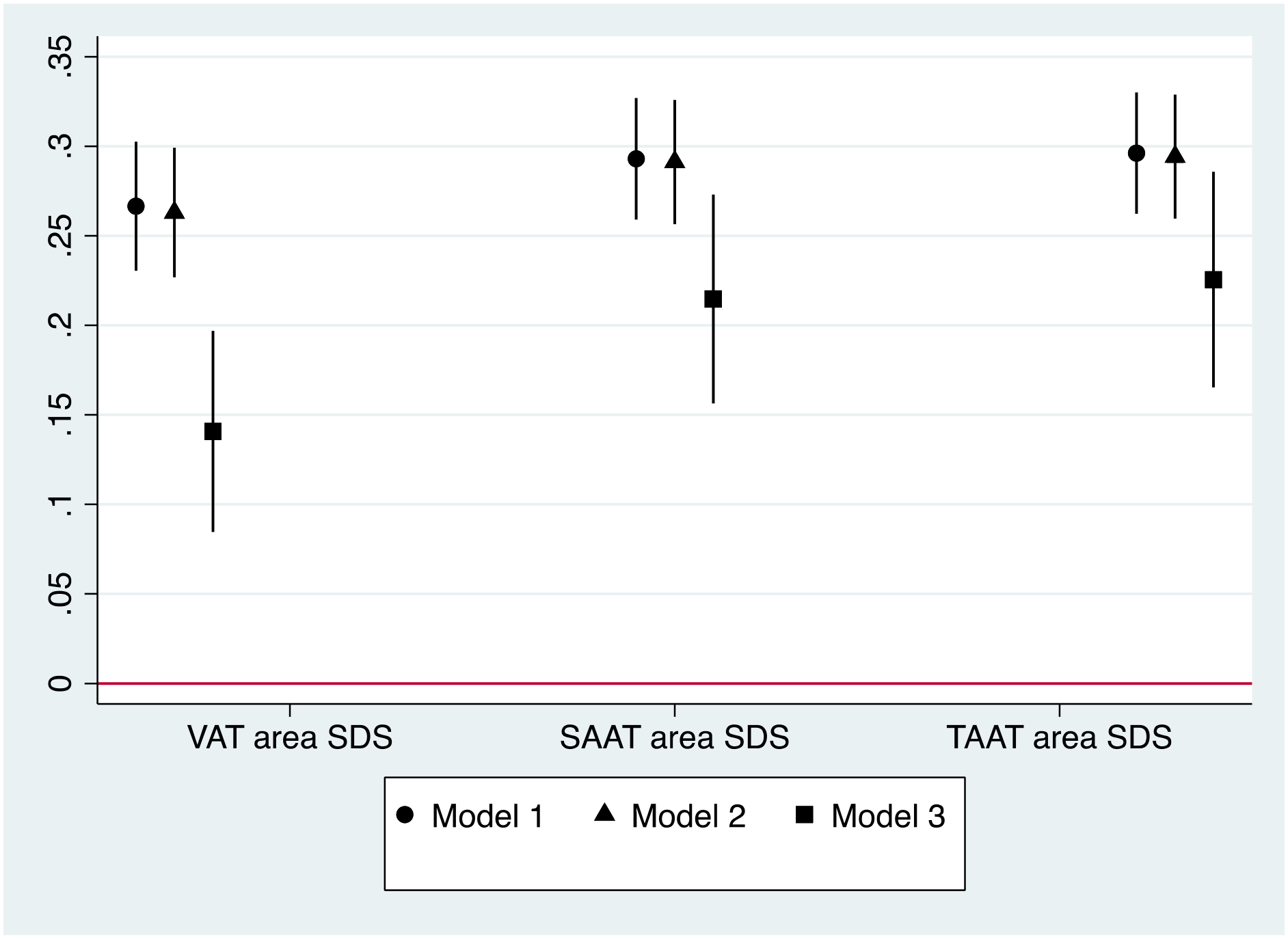
Cross-sectional associations of visceral adipose tissue (VAT), subcutaneous abdominal adipose tissue (SAAT), and total abdominal adipose tissue (TAAT) sex-specific standard deviation score (SDS) with metabolic risk z-score, β (95%CI)
Model 1. Unadjusted
Model 2. Adjusted for age, sex, race/ethnicity, puberty score
Model 3. Additionally adjusted for BMI z-score
The patterns of association of VAT, SAAT and TAAT with metabolic risk z-score, individual components of metabolic risk (SBP, HDL-C scaled inversely, TG and HOMA-IR) as well as related biomarkers for unadjusted and adjusted models are described in Table 3. In assessing the individual components that comprise metabolic risk z-score, greater VAT, SAAT and TAAT were not associated with higher SBP z-score, as 95% CIs crossed the null in all analyses. Among the other components of metabolic risk z-score, greater VAT, SAAT and TAAT were associated with higher log TG and log HOMA-IR in unadjusted and adjusted models. After adjustment for covariates in Model 3, while VAT did not show a significant association with log HDL-C, greater SAAT and TAAT were similarly associated with lower log HDL-C. In general, adjustment for age, sex, race/ethnicity, and puberty score (Model 2), had slight effects on effect estimates. Of the covariates, BMI z-score had the greatest impact on effect estimates, with the difference shown by comparing Models 2 and 3. Even so, after additional adjustment for BMI z-score, effect estimates were either comparable or mostly attenuated (see Table 3). Overall, the significant associations of SAAT and TAAT with metabolic risk z-score were greater than the VAT associations with metabolic risk z-score. We were unable to compare the regression coefficients of the individual components of metabolic risk z-score with one another given the outcome variables had different units.
Table 3.
Cross-sectional associations of visceral adipose tissue (VAT), subcutaneous abdominal adipose tissue (SAAT), and total abdominal adipose tissue (TAAT) sex-specific standard deviation score (SDS) with cardio-metabolic risk and related markers; overall and sex-interaction p-values
| All β (95% CI) | Sex interaction p value | ||||||
|---|---|---|---|---|---|---|---|
| N Obs | Model 1 | N Obs | Model 2 | Model 3 | Model 2 | Model 3 | |
| VAT area SDS | |||||||
| Metabolic risk z-score | 482 | 0.27 (0.23, 0.30) | 479 | 0.26 (0.23, 0.30) | 0.14 (0.08, 0.20) | 0.21 | 0.45 |
| SBP z-score | 734 | 0.05 (−0.01, 0.11) | 731 | 0.03 (−0.03, 0.09) | −0.04 (−0.14, 0.06) | 0.30 | 0.35 |
| Log HDL-C, mg/dL | 534 | −0.06 (−0.08, −0.04) | 531 | −0.06 (−0.08, −0.04) | −0.02 (−0.05, 0.01) | 0.29 | 0.47 |
| Log TG, mg/dL | 534 | 0.09 (0.05, 0.12) | 531 | 0.09 (0.06, 0.13) | 0.06 (0.01, 0.12) | 0.86 | 0.75 |
| Log HOMA-IR, units | 489 | 0.29 (0.24, 0.34) | 486 | 0.27 (0.22, 0.32) | 0.14 (0.06, 0.22) | 0.41 | 0.63 |
| Log ALT, U/L | 532 | 0.02 (−0.01, 0.05) | 529 | 0.03 (0.00, 0.06) | 0.01 (−0.03, 0.06) | 0.17 | 0.19 |
| Log hsCRP, mg/L | 516 | 0.65 (0.55, 0.74) | 513 | 0.65 (0.56, 0.74) | 0.51 (0.36, 0.66) | 0.53 | 0.36 |
| Log leptin, ng/mL | 532 | 0.75 (0.68, 0.82) | 529 | 0.74 (0.68, 0.80) | 0.36 (0.27, 0.44) | <0.0001 | 0.0002 |
| Log adiponectin, ug/mL | 534 | −0.12 (−0.16, −0.09) | 531 | −0.12 (−0.16, −0.08) | −0.08 (−0.13, −0.02) | 0.82 | 0.98 |
| SAAT area SDS | |||||||
| Metabolic risk z-score | 482 | 0.29 (0.26, 0.33) | 479 | 0.29 (0.26, 0.33) | 0.21 (0.16, 0.27) | 0.03 | 0.05 |
| SBP z-score | 734 | 0.04 (−0.02, 0.09) | 731 | 0.02 (−0.04, 0.08) | −0.09 (−0.19, 0.01) | 0.41 | 0.39 |
| Log HDL-C, mg/dL | 534 | −0.08 (−0.10, −0.06) | 531 | −0.08 (−0.10, −0.06) | −0.07 (−0.10, −0.04) | 0.01 | 0.01 |
| Log TG, mg/dL | 534 | 0.10 (0.07, 0.14) | 531 | 0.11 (0.08, 0.15) | 0.12 (0.06, 0.18) | 0.25 | 0.24 |
| Log HOMA-IR, units | 489 | 0.30 (0.25, 0.35) | 486 | 0.28 (0.23, 0.33) | 0.16 (0.08, 0.24) | 0.26 | 0.36 |
| Log ALT, U/L | 532 | 0.02 (−0.01, 0.05) | 529 | 0.04 (0.01, 0.07) | 0.03 (−0.02, 0.09) | 0.15 | 0.15 |
| Log hsCRP, mg/L | 516 | 0.71 (0.63, 0.80) | 513 | 0.72 (0.63, 0.81) | 0.72 (0.57, 0.88) | 0.97 | 0.97 |
| Log leptin, ng/mL | 532 | 0.82 (0.76, 0.88) | 529 | 0.82 (0.76, 0.87) | 0.54 (0.46, 0.63) | <0.0001 | <0.0001 |
| Log adiponectin, ug/mL | 534 | −0.13 (−0.17, −0.10) | 531 | −0.12 (−0.16, −0.09) | −0.08 (−0.14, −0.02) | 0.63 | 0.69 |
| TAAT area SDS | |||||||
| Metabolic risk z-score | 482 | 0.30 (0.26, 0.33) | 479 | 0.29 (0.26, 0.33) | 0.23 (0.17, 0.29) | 0.03 | 0.05 |
| SBP z-score | 734 | 0.04 (−0.02, 0.10) | 731 | 0.02 (−0.04, 0.08) | −0.09 (−0.19, 0.01) | 0.38 | 0.39 |
| Log HDL-C, mg/dL | 534 | −0.08 (−0.10, −0.06) | 531 | −0.08 (−0.10, −0.06) | −0.07 (−0.10, −0.03) | 0.01 | 0.02 |
| Log TG, mg/dL | 534 | 0.10 (0.07, 0.14) | 531 | 0.11 (0.08, 0.15) | 0.12 (0.06, 0.19) | 0.32 | 0.30 |
| Log HOMA-IR, units | 489 | 0.31 (0.26, 0.36) | 486 | 0.29 (0.24, 0.33) | 0.18 (0.09, 0.26) | 0.24 | 0.35 |
| Log ALT, U/L | 532 | 0.02 (−0.01, 0.05) | 529 | 0.04 (0.01, 0.07) | 0.03 (−0.02, 0.09) | 0.14 | 0.14 |
| Log hsCRP, mg/L | 516 | 0.72 (0.63, 0.80) | 513 | 0.73 (0.64, 0.82) | 0.76 (0.60, 0.92) | 0.99 | 0.96 |
| Log leptin, ng/mL | 532 | 0.83 (0.77, 0.89) | 529 | 0.82 (0.77, 0.88) | 0.57 (0.48, 0.66) | <0.0001 | <0.0001 |
| Log adiponectin, ug/mL | 534 | −0.14 (−0.17, −0.10) | 531 | −0.13 (−0.16, −0.09) | −0.09 (−0.15, −0.03) | 0.63 | 0.70 |
N Obs, number of observations (same in Models 2 and 3)
Model 1. Unadjusted
Model 2. Adjusted for age, sex, race/ethnicity, puberty score
Model 3. Additionally adjusted for BMI z-score
VAT, visceral adipose tissue; SAAT, subcutaneous abdominal adipose tissue; TAAT, total abdominal adipose tissue; SDS, standardized deviation score; SBP, systolic blood pressure; HDL-C, high-density lipoprotein cholesterol; TG, triglycerides; HOMA-IR, homeostatic model assessment of insulin resistance; ALT, alanine aminotransferase; hsCRP, high‐sensitivity C‐reactive protein.
Additionally, greater VAT, SAAT and TAAT were associated with higher log hsCRP and higher log leptin in all models and remained significant in the fully adjusted model (VAT β 0.51 mg/L; 0.36, 0.66 vs. SAAT β 0.72 mg/L; 0.57, 0.88 vs. TAAT β 0.76 mg/L; 0.60, 0.92 for log hsCRP, and VAT β 0.36 ng/mL; 0.27, 0.44 vs. SAAT β 0.54 ng/mL; 0.46, 0.63 vs. TAAT β 0.54 ng/mL; 0.46, 0.63, for log leptin, respectively). There was an inverse association of greater VAT, SAAT and TAAT with log adiponectin (VAT β −0.08 ug/mL; −0.13, −0.02 vs. SAAT β −0.08 ug/mL; −0.14, −0.02 vs. TAAT β −0.09 ug/mL; −0.15, −0.03). There were no significant associations of VAT, SAAT or TAAT with log ALT in overall participants in the fully adjusted model.
There was not strong evidence of effect modification by sex, except in a few associations. In the association of VAT, SAAT and TAAT with log leptin (sex interaction p<0.0001), we observed boys had a stronger positive association than girls (Supplementary Table S1). Effect modification by sex was present in the association of SAAT and TAAT with log HDL-C (sex interaction p=0.01 and p=0.02, respectively) with a greater inverse association of SAAT and TAAT with log HDL-C in boys than in girls.
Discussion
In this cross-sectional study of early adolescents, VAT was associated with metabolic risk z-score and some related markers, independent of current BMI. In addition to greater metabolic risk z-score, higher VAT was associated with adipocyte dysfunction (higher leptin, lower adiponectin) and higher CRP. Further, all associations of VAT with cardio-metabolic risk and related markers were captured, and effect sizes were similar or greater, in the analyses of SAAT and TAAT. We did not observe strong evidence of effect modification by sex, except in a few individual components.
The associations of VAT, SAAT and TAAT with specific cardio-metabolic risk markers and not others among these adolescents may be related to the progression of development of cardio-metabolic disease. VAT is hypothesized to be a metabolically active tissue, which secretes inflammatory markers, adipocytokines, and growth factors, among other vasoactive substances.1 Insulin resistance, adipokine dysfunction and chronic inflammation may precede development of hepatic steatosis and hypertension. Low grade chronic inflammation, circulating inflammatory cytokines leading to insulin resistance, and elevated free fatty acids followed by fatty infiltration of the liver, contribute to the progression of non-alcoholic fatty liver disease.23 Increased free fatty acids may also increase hepatic TG synthesis and very low-density lipoprotein cholesterol secretion, which are associated with lower HDL-C concentrations.24 Hypoadiponectinemia in youth has been shown to be associated with insulin resistance and VAT, while leptin has shown conflicting results in its association with VAT and SAAT, as well as sex differences.9,11,25
Our findings of sex differences in abdominal adipose tissue compartments are consistent with published studies indicating boys generally have more VAT than girls in later childhood and adolescence.22,26 Studies among prepubertal children have shown no significant sex differences in intra-abdominal adipose tissue area (i.e. VAT), or in growth of different fat compartments.27,28 Given our cohort had entered puberty, the sex differences observed may be related to the patterns of fat deposition during puberty. In puberty, whereas boys tend to deposit more fat in the abdomen in an android pattern, girls develop more total body fat overall and in a gynoid distribution.29
The addition of the covariates of child sex, race/ethnicity, and puberty score to the regression analyses did not vastly impact regression coefficients or 95% CIs. The effect sizes of the associations of DXA-measured abdominal adiposity with cardio-metabolic risk and related markers were comparable or reduced by including BMI z-score in the models. This was expected knowing that BMI is associated with cardio-metabolic risk. We included BMI z-score in the model to address our question of whether DXA-measured abdominal adiposity, independent of BMI, is associated with cardio-metabolic risk and additional related biomarkers. Our findings suggest among two adolescents with the same BMI, in the adolescent with greater DXA-measured abdominal adiposity, there is an associated higher cardio-metabolic risk.
The lack of strong evidence of effect modification by sex on VAT, SAAT and TAAT associations with cardio-metabolic risk may be related to smaller subgroup sample sizes, or differences that are not yet apparent in this age group, or simply absent. In a cross-sectional study of 324 adolescents in Canada, intra-abdominal fat measured by magnetic resonance imaging (MRI), was similarly associated with metabolic syndrome and CRP in both sexes, but affected blood pressure adversely only in males.9 The same study also found significant sex interaction in the association of VAT with leptin, with adolescents with high intra-abdominal fat demonstrating higher leptin levels, though the association was significantly stronger in females than in males. In our study, the few significant effect modifications by sex in SAAT and TAAT with log HDL-C as well as VAT, SAAT and TAAT with log leptin demonstrated slightly greater effect estimates in boys than in girls. A prior study found that leptin correlated more strongly with percent fat mass than BMI or waist circumference, particularly in boys.30 It may be that HDL-C and leptin are associated more broadly with overall percent body fat or mediated differently by unclear mechanisms by sex. While the overall analysis showed the sex-interaction for VAT, SAAT and TAAT with log ALT was not significant (p>0.10 for all), in the sex-stratified analysis, greater SAAT and TAAT were associated with higher log ALT in girls, while the association was not significant in boys. A prior study of older adolescents found the severity of hepatic steatosis was associated with SAAT for males and females, and with increased VAT in males alone.31 Given contrasting prior studies and lack of strong evidence of effect modification by sex, we were unable to draw strong conclusions based on these slight differences.
Prior studies have reported cross-sectional associations between VAT and markers of cardio-metabolic health in adolescents. A cross-sectional study in China found DXA-measured visceral fat mass (kg) and BMI were major independent determinants of a metabolically unhealthy nonobese phenotype in children and adolescents.32 While the study included a large cohort, the ages ranged vastly from 6–18 years, and all participants belonged to single ethnicity. Further, that study did not account for pubertal stage in analyses. In the Bosch study conducted in the U.S., DXA-VAT was associated with triglycerides, diastolic blood pressure, HOMA-IR and fasting insulin, while no associations were observed between DXA-VAT and systolic blood pressure or fasting glucose.13 Additionally, the Penn State Children Cohort Study found that VAT and SAAT were each associated with greater continuous metabolic syndrome score, with SAAT also having a slightly higher effect estimate compared to VAT after adjusting for age, race, gender and BMI percentile, while the association with mean arterial pressure was less pronounced.33 Our findings of DXA-VAT association with metabolic risk z-score, TG and HOMA-IR were similar to the findings above. However, the previous studies conducted in the U.S. had smaller adolescent cohorts (n<400) and did not report TAAT associations, thus we are unable to comment if our TAAT findings were comparable.13,33 Higher VAT, SAAT and TAAT were not significantly associated with SBP z-score in unadjusted or adjusted models. The effect estimate appeared to have flipped direction once adjusting for BMI z-score. However, this subtle finding may be less relevant as 95% CIs crossed the null in all models. Prior literature has shown inconsistent results in the association of VAT with blood pressure.9,13 Studies in animal models and humans have suggested subcutaneous fat, potentially via superficial subcutaneous fat, may serve a protective role in some aspects of cardio-metabolic risk.34–36 This protective effect may be playing a role in the flipped effect estimate for SAAT and TAAT with SBP z-score, though there was not a significant association.
While we hypothesized VAT would be more strongly associated with cardio-metabolic risk and related markers, deep subcutaneous fat may be involved and contributing to the greater associations of SAAT and TAAT with cardio-metabolic risk. Deep subcutaneous fat, and not superficial subcutaneous fat, has been shown to be strongly related to insulin resistance in adults.34 In adolescents with obesity, increases in deep subcutaneous fat tissue may generate an adipocyte milieu that promotes development of insulin resistance and hepatic steatosis.37,38 In addition, in this healthy group of adolescents there is overall less VAT compared to SAAT and TAAT. There may be greater associations of SAAT and TAAT with cardio-metabolic risk related to overall abdominal adiposity, and in youth their “pathogenic” adiposity may have not yet deposited into viscera.
Alternatively, VAT associations may have been less specific than hypothesized due to the imaging technique. Studies of our cohort previously assessed central adiposity using DXA-measured whole body fat and trunk fat. These measures are less discriminatory than VAT, SAAT and TAAT, which are limited to a specific abdominal region. Studies have shown mixed results in the correlation between CT-VAT and DXA trunk percentage fat; while some have found no significant correlations, others have shown higher correlations (r=0.6–0.8).39–41 Additionally, DXA has been found to underestimate trunk fat particularly in subjects with higher weight, and thus may not be as helpful a surrogate for VAT in subjects with obesity.42 Using DXA-VAT has inherent limitations as it analyzes a restricted abdominal region from a non-axial perspective, unlike in CT or MRI. DXA-TAAT may be a better proxy of abdominal adiposity as DXA-VAT may introduce estimate error, especially as VAT is calculated from estimated TAAT minus SAAT. Last, DXA currently is unable to differentiate superficial versus deep subcutaneous fat.
Our U.S. cohort drawn from the greater Boston area is relatively sizeable and more diverse compared to prior population-based studies in adolescents. We used a technique that was reproducible, less costly and with low radiation burden, compared to CT, to measure VAT, SAAT and TAAT. The limitations of this cross-sectional study include its inability to infer temporality or causal relationships between abdominal adiposity and cardio-metabolic health. Though we had a large dataset, the sex-stratified subgroups were smaller and thus our sex-stratified models were interpreted carefully given lack of sex-interactions, multiple analyses, and the possibility of type 1 error. The participants in our cohort were generally healthy, young adolescents who did not have much VAT, which may have been insufficient to detect associations. Further, we were limited by missing data and 27% of participants did not provide blood samples.
Conclusion
Our study suggests that DXA-measures of VAT, SAAT and TAAT are associated with cardio-metabolic risk and related markers in early adolescence. DXA-TAAT may better capture metabolic risk, beyond clinical anthropometry such as BMI. In other words, given two adolescents with the same BMI, higher abdominal adiposity assessed by DXA in one adolescent is associated with higher cardio-metabolic risk. Earlier and enhanced identification of adolescents at risk for cardio-metabolic disease enables prompt intervention. Further studies investigating the effect of longitudinal changes in abdominal adiposity on cardio-metabolic risk in adolescence are warranted and forthcoming.
Supplementary Material
Acknowledgements
We thank the participants and staff of Project Viva.
Funding
This work was supported by the US National Institutes of Health (grants R01 HD 034568 and UG3 OD023286). AJW was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (2 T32 DK007477-36).
Footnotes
Conflicts of Interest Statement
We have no conflicts of interest to disclose.
References
- 1.Fox CSM, Massaro JM, Hoffmann UM, et al. Abdominal Visceral and Subcutaneous Adipose Tissue Compartments: Association With Metabolic Risk Factors in the Framingham Heart Study. Circulation. 2007;116(1):39–48. doi: 10.1161/CIRCULATIONAHA.106.675355 [DOI] [PubMed] [Google Scholar]
- 2.Goodpaster BH, Krishnaswami S, Resnick H, et al. Association Between Regional Adipose Tissue Distribution and Both Type 2 Diabetes and Impaired Glucose Tolerance in Elderly Men and Women. Diabetes Care. 2003;26(2):372–379. doi: 10.2337/diacare.26.2.372 [DOI] [PubMed] [Google Scholar]
- 3.Boyko EJ, Fujimoto WY, Leonetti DL, Newell-Morris L. Visceral adiposity and risk of type 2 diabetes: a prospective study among Japanese Americans. Diabetes Care. 2000;23(4):465–471. doi: 10.2337/diacare.23.4.465 [DOI] [PubMed] [Google Scholar]
- 4.Hwang Y-C, Fujimoto WY, Hayashi T, Kahn SE, Leonetti DL, Boyko EJ. Increased Visceral Adipose Tissue Is an Independent Predictor for Future Development of Atherogenic Dyslipidemia. J Clin Endocrinol Metab. 2016;101(2):678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Owens S, Gutin B, Ferguson M, Allison J, Karp W, Le N-A. Visceral adipose tissue and cardiovascular risk factors in obese children. J Pediatr. 1998;133(1):41–45. [DOI] [PubMed] [Google Scholar]
- 6.Yanovski JA, Yanovski SZ, Filmer KM, et al. Differences in body composition of black and white girls. Am J Clin Nutr. 1996;64(6):833–839. doi: 10.1093/ajcn/64.6.833 [DOI] [PubMed] [Google Scholar]
- 7.Gower BA, Nagy TR, Goran MI. Visceral fat, insulin sensitivity, and lipids in prepubertal children. Diabetes. 1999;48(8):1515–1521. doi: 10.2337/diabetes.48.8.1515 [DOI] [PubMed] [Google Scholar]
- 8.Bacha F, Saad R, Gungor N, Janosky J, Arslanian SA. Obesity, Regional Fat Distribution, and Syndrome X in Obese Black Versus White Adolescents: Race Differential in Diabetogenic and Atherogenic Risk Factors. J Clin Endocrinol Metab. 2003;88(6):2534–2540. doi: 10.1210/jc.2002-021267 [DOI] [PubMed] [Google Scholar]
- 9.Syme C, Abrahamowicz M, Leonard GT, et al. Intra-abdominal Adiposity and Individual Components of the Metabolic Syndrome in Adolescence: Sex Differences and Underlying Mechanisms. Arch Pediatr Adolesc Med. 2008;162(5):453–461. doi: 10.1001/archpedi.162.5.453 [DOI] [PubMed] [Google Scholar]
- 10.Ali O, Cerjak D, Kent JW, James R, Blangero J, Zhang Y. Obesity, central adiposity and cardiometabolic risk factors in children and adolescents: a family-based study. Pediatr Obes. 2014;9(3):e58–e62. doi: 10.1111/j.2047-6310.2014.218.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tinggaard J, Hagen CP, Christensen AN, et al. Anthropometry, DXA, and leptin reflect subcutaneous but not visceral abdominal adipose tissue on MRI in 197 healthy adolescents. Pediatr Res. 2017;82(4):620–628. [DOI] [PubMed] [Google Scholar]
- 12.Kaul S, Rothney MP, Peters DM, et al. Dual-Energy X-Ray Absorptiometry for Quantification of Visceral Fat. Obesity. 2012;20(6):1313–1318. doi: 10.1038/oby.2011.393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bosch TA, Dengel DR, Kelly AS, Sinaiko AR, Moran A, Steinberger J. Visceral adipose tissue measured by DXA correlates with measurement by CT and is associated with cardiometabolic risk factors in children. Pediatr Obes. 2014;10(3):172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui Z, Truesdale KP, Cai J, Koontz MB. Anthropometric indices as measures of body fat assessed by DXA in relation to cardiovascular risk factors in children and adolescents: NHANES 1999–2004. Int J Body Compos Res. 2015;11(3–4):85–96. [PMC free article] [PubMed] [Google Scholar]
- 15.Hetherington-Rauth M, Bea JW, Lee VR, et al. Comparison of direct measures of adiposity with indirect measures for assessing cardiometabolic risk factors in preadolescent girls. Nutr J. 2017;16(1):15. doi: 10.1186/s12937-017-0236-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katzmarzyk PT, Greenway FL, Heymsfield SB, Bouchard C. Clinical utility and reproducibility of visceral adipose tissue measurements derived from dual-energy X-ray absorptiometry in white and African American adults. Obesity. 2013;21(11):2221–2224. doi: 10.1002/oby.20519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rothney MP, Catapano AL, Xia J, et al. Abdominal visceral fat measurement using dual-energy X-ray: Association with cardiometabolic risk factors. Obesity. 2013;21(9):1798–1802. doi: 10.1002/oby.20223 [DOI] [PubMed] [Google Scholar]
- 18.Oken E, Baccarelli AA, Gold DR, et al. Cohort profile: project viva. Int J Epidemiol. 2015;44(1):37–48. doi: 10.1093/ije/dyu008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bredella MA, Gill CM, Keating LK, et al. Assessment of abdominal fat compartments using DXA in premenopausal women from anorexia nervosa to morbid obesity. Obesity. 2013;21(12):2458–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perng W, Rifas-Shiman SL, Hivert M-F, Chavarro JE, Sordillo J, Oken E. Metabolic trajectories across early adolescence: differences by sex, weight, pubertal status and race/ethnicity. Ann Hum Biol. 2019;46(3):205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Child growth standards: BMI-for-age. Accessed February 5, 2020 https://www.who.int/childgrowth/standards/bmi_for_age/en/
- 22.Staiano AE, Broyles ST, Gupta AK, Katzmarzyk PT. Ethnic and sex differences in visceral, subcutaneous, and total body fat in children and adolescents. Obesity. 2013;21(6):1251–1255. doi: 10.1002/oby.20210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tarantino G, Savastano S, Colao A. Hepatic steatosis, low-grade chronic inflammation and hormone/growth factor/adipokine imbalance. World J Gastroenterol. 2010;16(38):4773–4783. doi: 10.3748/wjg.v16.i38.4773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caprio S, Hyman LD, McCarthy S, Lange R, Bronson M, Tamborlane WV. Fat distribution and cardiovascular risk factors in obese adolescent girls: importance of the intraabdominal fat depot. Am J Clin Nutr. 1996;64(1):12–17. doi: 10.1093/ajcn/64.1.12 [DOI] [PubMed] [Google Scholar]
- 25.Bacha F, Saad R, Gungor N, Arslanian SA. Adiponectin in Youth: Relationship to visceral adiposity, insulin sensitivity, and β-cell function. Diabetes Care. 2004;27(2):547–552. doi: 10.2337/diacare.27.2.547 [DOI] [PubMed] [Google Scholar]
- 26.Staiano AE, Katzmarzyk PT. Ethnic and sex differences in body fat and visceral and subcutaneous adiposity in children and adolescents. International Journal of Obesity. 2012;36:1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goran MI, Nagy TR, Treuth MS, et al. Visceral fat in white and African American prepubertal children. Am J Clin Nutr. 1997;65(6):1703–1708. doi: 10.1093/ajcn/65.6.1703 [DOI] [PubMed] [Google Scholar]
- 28.Huang TT-K, Johnson MS, Figueroa‐Colon R, Dwyer JH, Goran MI. Growth of Visceral Fat, Subcutaneous Abdominal Fat, and Total Body Fat in Children. Obesity Research. 2001;9(5):283–289. doi: 10.1038/oby.2001.35 [DOI] [PubMed] [Google Scholar]
- 29.Loomba-Albrecht L, Styne D. Effect of puberty on body composition. - PubMed - NCBI. Curr Opin Endocrinol Diabetes Obes. 16(1):10–15. doi: 10.1097/med.0b013e328320d54c [DOI] [PubMed] [Google Scholar]
- 30.Schoppen S, Riestra P, García-Anguita A, et al. Leptin and adiponectin levels in pubertal children: relationship with anthropometric variables and body composition. Clinical Chemistry and Laboratory Medicine. 2010;48(5):707–711. doi: 10.1515/CCLM.2010.142 [DOI] [PubMed] [Google Scholar]
- 31.Ayonrinde OT, Olynyk JK, Beilin LJ, et al. Gender-specific differences in adipose distribution and adipocytokines influence adolescent nonalcoholic fatty liver disease. Hepatology. 2011;53(3):800–809. doi: 10.1002/hep.24097 [DOI] [PubMed] [Google Scholar]
- 32.Ding WQ, Liu JT, Shang YX, et al. DXA-measured visceral fat mass and lean body mass reflect abnormal metabolic phenotypes among some obese and nonobese Chinese children and adolescents. Nutr Metab Cardiovasc Dis. 2018;28:618–628. [DOI] [PubMed] [Google Scholar]
- 33.He F, Rodriguez-Colon S, Fernandez-Mendoza J, et al. Abdominal Obesity and Metabolic Syndrome Burden in Adolescents-Penn State Children Cohort Study. J Clin Densitom. 2015;18(1):30–36. doi: 10.1016/j.jocd.2014.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelley DE, Thaete FL, Troost F, Huwe T, Goodpaster BH. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am J Physiol Endocrinol Metab. 2000;278(5):E941–E948. doi: 10.1152/ajpendo.2000.278.5.E941 [DOI] [PubMed] [Google Scholar]
- 35.Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial Effects of Subcutaneous Fat Transplantation on Metabolism. Cell Metab. 2008;7(5):410–420. doi: 10.1016/j.cmet.2008.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Porter SA, Massaro JM, Hoffmann U, Vasan RS, O’Donnel CJ, Fox CS. Abdominal Subcutaneous Adipose Tissue: A Protective Fat Depot? Diabetes Care. 2009;32(6):1068–1075. doi: 10.2337/dc08-2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burgert TS, Taksali SE, Dziura J, et al. Alanine Aminotransferase Levels and Fatty Liver in Childhood Obesity: Associations with Insulin Resistance, Adiponectin, and Visceral Fat. J Clin Endocrinol Metab. 2006;91(11):4287–4294. doi: 10.1210/jc.2006-1010 [DOI] [PubMed] [Google Scholar]
- 38.Burgert TS, Duran EJ, Goldberg‐Gell R, et al. Short-term metabolic and cardiovascular effects of metformin in markedly obese adolescents with normal glucose tolerance. Pediatr Diabetes. 2008;9(6):567–576. doi: 10.1111/j.1399-5448.2008.00434.x [DOI] [PubMed] [Google Scholar]
- 39.Hill AM, LaForgia J, Coates AM, Buckley JD, Howe PRC. Estimating Abdominal Adipose Tissue with DXA and Anthropometry. Obesity. 2007;15(2):504–510. doi: 10.1038/oby.2007.629 [DOI] [PubMed] [Google Scholar]
- 40.Ball SD, Swan P. Accuracy of estimating intra-abdominal fat in obese women. J Exerc Physiol Online. 2003;6(4):1–7. [Google Scholar]
- 41.Shuster A, Patlas M, Pinthus JH, Mourtzakis M. The clinical importance of visceral adiposity: a critical review of methods for visceral adipose tissue analysis. Br J Radiol. 2012;85(1009):1–10. doi: 10.1259/bjr/38447238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bredella MA, Ghomi RH, Thomas BJ, et al. Comparison of DXA and CT in the Assessment of Body Composition in Premenopausal Women With Obesity and Anorexia Nervosa. Obesity. 2010;18(11):2227–2233. doi: 10.1038/oby.2010.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


