Structured Abstract
Background:
We hypothesized that the ratio of positive lymph nodes to total assessed lymph nodes (LNR) is an indicator of cancer burden in esophageal adenocarcinoma and may identify patients who may most benefit from adjuvant chemotherapy (AC).
Objective:
The aim of this study was to discern whether there is a threshold LNR above which AC is associated with a survival benefit in this population.
Methods:
The 2004-2015 National Cancer Database was queried for patients who underwent upfront, complete resection of pT1-4N1-3M0 esophageal adenocarcinoma. The primary outcome, overall survival, was examined using multivariable Cox proportional hazards models employing an interaction term between LNR and AC.
Results:
A total of 1733 patients were included: 811 (47%) did not receive AC while 922 (53%) did. The median LNR was 20% (IQR 9-40). In a multivariable Cox model, the interaction term between LNR and receipt of AC was significant (p=0.01). A plot of the interaction demonstrated that AC was associated with improved survival beyond a LNR of about 10-12%. In a sensitivity analysis, the receipt of AC was not associated with improved survival in patients with LNR <12% ((hazard ratio [HR] 1.02; 95%CI 0.72-1.44) but was associated with improved survival in those with LNR ≥12% (HR 0.65; 95%CI 0.50-0.79).
Conclusions:
In this study of patients with upfront, complete resection of node-positive esophageal adenocarcinoma, AC was associated with improved survival for LNR ≥12%. LNR may be used as an adjunct in multidisciplinary decision-making about adjuvant therapies in this patient population.
Condensed Abstract
In this National Cancer Database study, we found that adjuvant chemotherapy was associated with improved survival in patients with pT1-4N1-3M0 esophageal adenocarcinoma with a ratio of positive lymph nodes to total number of examined nodes (LNR) exceeding 12%.
Introduction
The National Comprehensive Cancer Network (NCCN) recommends adjuvant chemotherapy (AC) with or without radiation for patients with pathologically node-positive esophageal adenocarcinoma following esophagectomy1. While AC is associated with improved survival in this population, it is only used in about half of patients who undergo upfront surgery2. The variability in guideline-concordant use of AC is attributable to many factors including morbidity and poor recovery from esophagectomy, type and volume of treatment centers, and disparities in age, race, insurance status, and income3-6. We sought to identify subpopulations of patients with node-positive esophageal cancer who least and most benefit from AC. The ratio of the number of positive lymph nodes to the total number of nodes examined during surgery (LNR) has been theorized as a measure of the nodal metastatic burden in patients with esophageal and other cancers7-24. We hypothesized that there exists a threshold LNR above which patients with node-positive esophageal adenocarcinoma are more likely to experience improved survival after AC and below which patients are less likely to experience a survival benefit. We used data from a large, national registry to investigate the relationship between LNR, receipt of AC, and survival in this patient population.
Methods
Data Source
This study was deemed exempt by the Duke University Institutional Review Board. The 2004-2015 National Cancer Database (NCDB) was used for this study. The NCDB is a collaborative effort of the American Cancer Society and the American College of Surgeons, and maintains prospectively collected data about 70% of malignancies diagnosed across 1500 centers in the United States and Puerto Rico annually25. Data are collected by certified, independent tumor registrars who employ standardized coding guidelines.
Cohort
The NCDB was queried for patients with pathologic T1-4N1-3M0 esophageal adenocarcinoma who had undergone esophagectomy with negative margins (Supplemental Figure 1). Patients were excluded from the study cohort for the following reasons: postoperative 90-day mortality; missing survival or adjuvant treatment information; missing data about intraoperative nodal assessment; receipt of neoadjuvant therapy; receipt of adjuvant therapy more than 180 days following surgery; death prior to receipt of adjuvant chemotherapy; or deemed medically unfit for therapy.
Analysis
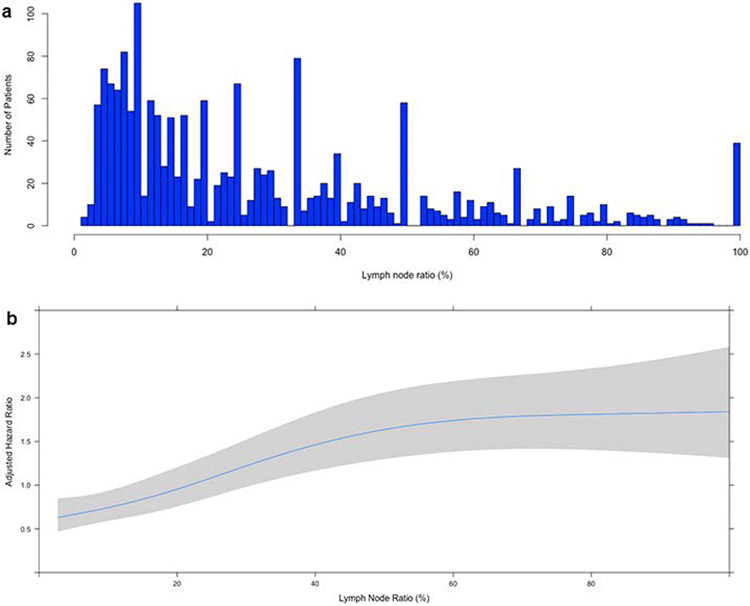
Patients were stratified by receipt of adjuvant chemotherapy (AC). Background characteristics were compared using the Wilcoxon rank sum and Pearson’s chi-squared tests for continuous and categorical variables, respectively. The primary outcome was overall survival (OS), computed from the time of surgery. Unadjusted survival was estimated using the Kaplan-Meier method. A multivariable Cox proportional hazards model was developed using variables selected a priori based on perceived prognostic significance from clinical experience and prior literature. Covariates included age, sex, race/ethnicity, year of diagnosis, Charlson-Deyo comorbidity score (CDS), insurance status, treatment at an academic center, pathologic tumor size, pathologic T status, tumor grade, annualized center surgical volume, unplanned 30-day postoperative readmission, postoperative length of stay, receipt of adjuvant radiation, receipt of adjuvant chemotherapy, and lymph node ratio. Lymph node ratio (LNR) was computed as the percentage of positive lymph nodes relative to all nodes examined during surgery (Figure 1a).
Figure 1.
(a) Histogram of lymph node ratio (X-axis) vs. number of patients (Y-axis) in the cohort. (b) A plot of lymph node ratio, modelled using restricted cubic splines, against adjusted hazard of mortality (Y-axis). The grey region represents the bounds of the 95% confidence interval
To test our hypothesis that the LNR mediates the relationship between receipt of AC and survival, we included in our primary Cox model an additional interaction term between LNR and AC (Supplemental Table 2). Three-way interactions between LNR, AC, and other variables were tested (Supplemental Table 1). The interaction between LNR and AC on adjusted survival was plotted to identify a threshold LNR below which AC was not associated with a significant survival difference and above which AC was associated with significant survival benefit. Variables with potentially non-linear relationships with survival, including age, tumor size, length of hospitalization, and LNR, were modelled using restricted cubic splines with three pre-specified knots26. The region in LNR where the survival curves of patients who did or did not receive AC started to diverge and where the confidence intervals were narrowest was determined to represent the approximate threshold.
Three additional analyses were performed. (1) To mitigate the imbalance in baseline characteristics between the two groups of patients, a 1:1 propensity score-matched analysis employing a ‘greedy’ nearest neighbor algorithm without replacement and a caliper width 0.1 of the standard deviation of the logit propensity score was used27. Patients were matched based on age, sex, race/ethnicity, year of diagnosis, CDS, insurance status, academic center treatment, pathologic T status, tumor size, grade, annualized center volume, unplanned 30-day readmission, and postoperative length of stay. Because patients who received AC were much more likely to also receive adjuvant radiation, radiation was not used for matching. Covariate balance was checked using standardized mean differences (Supplemental Table 3). A multivariable Cox proportional hazards model with the same covariates listed above was constructed, including an interaction term between LNR and AC to test the combined effect of LNR and AC on survival. This interaction was again plotted to identify a possible threshold LNR. (2) Based on the primary and propensity score-matched analyses, a sensitivity analysis was performed dividing the cohort into subgroups based on the threshold LNR observed (<12% or ≥12%). A multivariable Cox model including covariates described above but excluding an interaction term was performed in each subgroup to estimate the size of the effect contributed by AC. (3) To test the question of whether there is a threshold absolute number of positive nodes above which AC is associated with a survival benefit, we created a multivariable Cox model including the above covariates, the number of nodes examined, a two-way interaction between the number of nodes examined and the number of positive nodes, and a two-way interaction between the number of positive nodes and the receipt of adjuvant chemotherapy. This interaction was again plotted to identify a threshold number of positive nodes above which AC was associated with a survival benefit. Based on this threshold, a subgroup analysis was performed dividing the cohort based on the observed threshold number of positive nodes. Complete case analysis was used to handle missing data in regression. A p value less than 0.05 was considered significant. All analyses were performed using R version 3.5.1 (Vienna, Austria).
Results
Baseline Characteristics
A total of 1733 patients were included: 811 (47%) did not receive AC while 922 (53%) did. Patients who received AC were more likely to be younger, have fewer comorbidities, be privately insured, receive treatment at non-academic centers and lower volume centers, undergo adjuvant radiation, have a 30-day postoperative readmission, and have a shorter hospitalization after surgery (Table 1). There were no differences in pathologic T status, tumor size, number of nodes examined, and LNR between the two groups. In the overall cohort, the median number of nodes examined was 14 (interquartile range [IQR] 9-21) and the median LNR was 20% (IQR 9-40) (Figure 1a).
Table 1.
Background characteristics of study patients stratified receipt of adjuvant chemotherapy. Categorical variables are expressed as count (percentage) and continuous variables as median (interquartile range)
| Variable | No Adjuvant Chemotherapy (n=811) (%) |
Adjuvant Chemotherapy (n=922) (%) |
p-value |
|---|---|---|---|
| Age (median [IQR]) | 67(60-75) | 62(55-70) | <0.001 |
| Sex (female) | 115(14) | 103(11) | 0.07 |
| Race/ethnicity | 0.63 | ||
| White | 771(96) | 888(97) | |
| Black | 20(3) | 17(2) | |
| Other | 11(1) | 11(1) | |
| Year of diagnosis | 2007(2006-2010) | 2008(2005-2010) | 0.93 |
| Charlson-Deyo score | 0.002 | ||
| 0 | 533(66) | 657(71) | |
| 1 | 211(26) | 224(24) | |
| ≥2 | 67(8) | 41(4) | |
| Insurance | <0.001 | ||
| Private | 301(38) | 482(53) | |
| Government | 484(61) | 417(46) | |
| None | 12(1) | 10(1) | |
| Academic center | 454(57) | 404(44) | <0.001 |
| Pathologic T status | 0.24 | ||
| T1 | 178(22) | 173(19) | |
| T2 | 172(21) | 182(20) | |
| T3 | 446(55) | 549(60) | |
| T4 | 15(2) | 18(2) | |
| Tumor size (mm) | 38(25-50) | 38(25-55) | 0.49 |
| Grade | 0.923 | ||
| Low | 24(3) | 29(3) | |
| Moderate | 286(37) | 323(36) | |
| High | 462(60) | 542(61) | |
| Annualized center volume | 6(3-16) | 4(2-10) | <0.001 |
| Lymph node ratio (%) | 19(8-40) | 21(10-40) | 0.10 |
| Number of nodes examined | 14(9-20) | 15(10-21) | 0.12 |
| Number of nodes positive | 2(1-4) | 3(1-5) | 0.001 |
| Adjuvant radiation | 80(10) | 650(71) | <0.001 |
| Number of radiation treatments | 28(25-30) | 26(25-28) | N/A |
| Radiation dose (Gy) | 50.4(45.0-50.4) | 45.0(45.0-50.4) | N/A |
| Unplanned 30-day readmission | 70(9) | 50(5) | 0.01 |
| Postoperative length of stay | 11(8-18) | 9(7-13) | <0.001 |
Interaction between Lymph Node Ratio and Adjuvant Chemotherapy
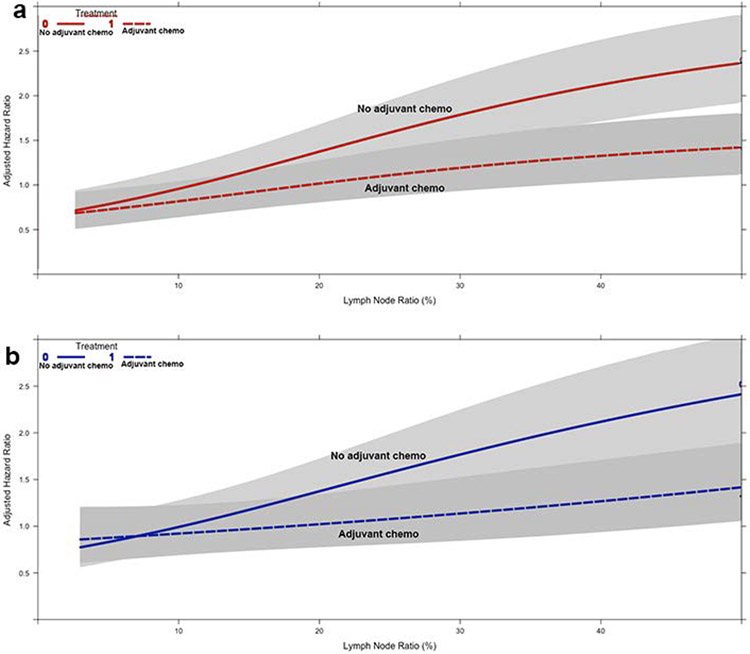
The unadjusted five-year OS was 19% (95% confidence interval [CI] 16-22) and 27% (95%CI 24-31) for patients who did not and did receive AC, respectively (Supplemental Figure 2a). An adjusted survival plot of LNR, modelled using restricted cubic splines, showed no obvious inflection point to represent a potential threshold (Figure 1b). In a multivariable Cox model, the interaction between LNR and receipt of AC was significant, suggesting that LNR influenced the relationship between AC and survival (Supplemental Table 2). No significant three-way interactions between LNR, AC, and other covariates including age, tumor size, center volume, pathologic T status, grade, adjuvant radiation, and number of nodes examined were found (Supplemental Table 1). A plot of the interaction between LNR and receipt of AC demonstrated that the survival curves for patients receiving and not receiving AC diverged beyond a LNR of about 10-12%, suggesting that patients were less likely to experience a survival benefit with receipt of AC below a LNR of about 12% and more likely to experience a survival benefit with an increasing LNR beyond 12% (Figure 2a).
Figure 2.
Graphs of the interaction between lymph node ratio (X-axis), receipt of adjuvant chemotherapy (curves), and adjusted hazard of mortality (Y-axis) in (a) patients in the overall cohort and (b) propensity score-matched subgroup. The grey regions represent the bounds of the 95% confidence interval
Additional Analyses
Propensity score-matching identified 456 pairs of patients with balanced background characteristics (Supplemental Table 3). Unadjusted five-year OS was 19% (95%CI 16-24) and 27% (95%CI 23-31) for patients who did not and did receive AC, respectively (Supplemental Figure 2b). A multivariable Cox model again demonstrated a significant interaction between LNR and receipt of AC (Supplemental Table 4). A plot of the interaction demonstrated significant divergence of the survival curves of patients who did or did not receive AC beyond a LNR of 10-12% (Figure 2b). A subsequent sensitivity analysis divided the overall cohort of patients into those with a LNR <12% and those with a LNR ≥12%. In 585 patients with a LNR <12%, the receipt of AC was not associated with improved survival compared to patients who did not receive AC in multivariable analysis (hazard ratio [HR] 1.02; 95%CI 0.72-1.44; p=0.93). In 1148 patients with a LNR ≥12%, the receipt of AC was associated with improved survival compared to no AC (HR 0.65; 95%CI 0.50-0.79; p<0.001).
In a separate analysis, the number of positive lymph nodes was found to have a significant interaction with the receipt of AC, after accounting for a separate interaction with the number of nodes examined in surgery (Supplemental Table 5). A plot of this interaction revealed that AC was associated with increasing survival benefit beyond two positive nodes on pathology (Supplemental Figure 3). In a subgroup analysis of 902 patients with ≤2 positive nodes, the receipt of AC was not associated with improved survival (HR 0.83; 95%CI 0.64-1.08; p=0.17). In an analysis of 831 patients with >2 positive nodes, the receipt of AC was associated with improved survival (HR 0.60; 95%CI 0.48-0.75; p<0.001).
Discussion
In this study, we identified a meaningful relationship between lymph node ratio, which is the number of positive lymph nodes relative to the total number of nodes examined, and the survival benefit associated with receipt of adjuvant chemotherapy in patients with completely resected, node-positive esophageal adenocarcinoma who did not receive neoadjuvant therapy. Adjuvant chemotherapy was not associated with a survival benefit in patients with a LNR of below 10-12% but was associated with significantly improved survival beyond a LNR of 12%. Our work suggests that LNR may be used an adjunct tool in multidisciplinary and shared decision-making about adjuvant chemotherapy in this patient population, especially since only about half of patients received guideline-concordant AC in this cohort.
While there is abundant literature about the prognostic significance of LNR in esophageal and other malignancies, our study is the first, to our knowledge, that specifically examines the association between LNR and the potential benefit of AC. Several observational studies using multinational and institutional datasets have demonstrated that an increasing LNR is associated with worse survival in patients with esophageal cancer, though these studies were limited by treatment of LNR as a categorical variable and multivariable modelling including related covariates like pathologic N status, number of examined nodes, and LNR7-19,28. None of these studies examined the interaction between LNR and adjuvant therapies in this patient population. Similar to these studies, we found that increasing LNR is associated with worse survival, likely reinforcing that the LNR is a measure of oncologic burden in patients following esophagectomy. However, the specific aim of this study was to understand if we could use LNR to stratify patients into subpopulations less or more likely to benefit from AC. Using multivariable modelling with interaction terms and sensitivity analyses including propensity score-matching, we found that patients with a LNR <10-12% were unlikely to experience a survival benefit with AC. The divergence of the survival curves of patients who did and did not receive AC beyond a LNR of about 15% also suggests that the survival benefit associated with AC increases at higher values of the LNR. AC is not readily offered to patients following esophagectomy even if they have nodal involvement. In addition to disparities in sex, race, insurance, and economic status, barriers to AC include the morbidity associated with esophagectomy, prolonged recovery from the operation, and toxicity associated with AC. In addition, there is no consensus in the literature that adjuvant chemotherapy is associated with improved survival in this patient population2,29. Our data suggest that LNR may be used to preferentially consider AC in some patients, in conjunction with the other factors traditionally used for planning of adjuvant therapies. Further, the methods described in this manuscript can be applied to other malignancies to evaluate the relationship between LNR and the potential benefit of adjuvant therapies.
An analysis in our study also revealed that the receipt of AC is associated with improved survival in patients with more than two positive lymph nodes on pathology. The absolute number of positive nodes may be an easy measure to utilize in multidisciplinary decision making about adjuvant chemotherapy for patients following esophagectomy. However, the model used in this analysis also accounted for a significant interaction between the number of positive nodes and the number of nodes assessed in surgery, suggesting the two variables are closely related in association with survival. In the context of this relationship, the LNR may be of greater utility because it incorporates both variables and also prompts clinicians to consider the number of nodes examined in surgery, which may itself be a measure of the quality of the operation.
Our study has important limitations. As a retrospective cohort study, the most significant limitation is likely selection bias. We do not know the reasons some patients were offered AC and others were not, even though we attempted to mitigate such bias by including in our multivariable models gross surrogate markers of poor postoperative recovery, propensity score-matched subgroup analyses, and exclusion from our study patients who were explicitly coded as having died or been deemed medically unfit for chemotherapy. We were also limited by the variables available in the NCDB. The NCDB does not catalogue specific postoperative complications, measures of frailty or malnutrition, the specific identity of adjuvant chemotherapy regimens, and disease-free survival. These variables could have confounded the results of our study. In addition, there are limitations associated with the use of LNR as a marker of disease burden. There is likely variability in pathologic identification of discrete nodes and the possibility of fragmentation of nodes during surgery for which we could not account. In addition, the absolute number of nodes examined has been described as a surrogate marker of an oncologically effective operation and an increasing number of nodes examined was associated with improved survival in our study as well (Supplemental Figure 4). We did test for a three-way interaction between the number of nodes sampled, LNR, and AC, and did not find a significant interaction to suggest that the absolute number of nodes may have influenced the relationship between LNR and the survival associated with AC. However, because most patients in this cohort had >10 nodes sampled, our findings are not generalizable to patients in whom fewer than 10 nodes were sampled. Clinicians should continue to employ national guidelines in performing lymphadenectomy during esophagectomy. Finally, because this is the first study to report the use of interaction term modelling to examine the relationship between LNR and the potential survival associated with adjuvant therapy, the external validity of our findings remains unclear and should be tested in other malignancies and datasets. Our data cannot be extrapolated to patients who receive neoadjuvant chemotherapy for node-positive esophageal adenocarcinoma; however, the benefit of adjuvant therapy remains unclear in this patient population and is rarely provided23,24.
In this study of patients who underwent upfront, complete resection of esophageal adenocarcinoma with pathologically node-positive disease, the use of adjuvant chemotherapy was not associated with a survival benefit in patients with a lymph node ratio <12%, while patients with a ratio above 12% experienced a significant survival benefit. Since adjuvant chemotherapy is only offered in about half of eligible patients, our data suggest that lymph node ratio may be used in a multidisciplinary setting to inform decisions about adjuvant therapy planning in this patient population. Our work can also be applied to other malignancies to examine the relationship between LNR and survival associated with adjuvant therapies.
Supplementary Material
Supplemental Figure 1. Scheme of patient selection for the study
Supplemental Figure 2. Kaplan-Meier survival curves for patients in the (a) overall cohort and (b) propensity score-matched subgroup, stratified by receipt of adjuvant chemotherapy. The shaded regions represent the bounds of the 95% confidence interval. The numbers at risk are provided beneath each graph. The p values represent the log-rank test
Supplemental Figure 3. Graph of the interaction between total number of positive nodes on pathology (X-axis), receipt of adjuvant chemotherapy (curves), and adjusted hazard of mortality (Y-axis) in patients in the overall cohort, after adjustment for a separate two-way interaction between the number of nodes sampled and number of positive nodes. The grey regions represent the bounds of the 95% confidence interval
Supplemental Figure 4. A plot of the number of nodes examined in surgery, modelled using restricted cubic splines, against the adjusted hazard of mortality (Y-axis). The grey region represents the bounds of the 95% confidence interval
Acknowledgments and Funding
The American College of Surgeons is in a Business Associate Agreement that includes a data use agreement with each of its Commission on Cancer accredited hospitals. The data used in the study are derived from a de-identified National Cancer Data Base file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology used or the conclusions drawn from these data by the investigators.
Drs. Raman and Farrow were supported by a National Institutes of Health T-32 grant 5T32CA093245 in surgical oncology. Dr. Jawitz was supported by a National Institutes of Health T-32 grant 5T32HL069749 in clinical research.
This work received no direct funding.
Footnotes
This paper was selected for oral presentation at the American Association of Thoracic Surgeons (AATS) annual meeting in New York City in April 2020, which has been cancelled.
The authors have no conflicts of interest to disclose.
References
- 1.National Comprehensive Cancer Network. Esophageal and Esophagogastric Junction Cancers (Version 1.2020). https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf. Accessed April 27, 2020.
- 2.Speicher PJ, Englum BR, Ganapathi AM, et al. Adjuvant chemotherapy is associated with improved survival after esophagectomy without induction therapy for node-positive adenocarcinoma. J Thorac Oncol. 2015;10(1):181–188. doi: 10.1097/JTO.0000000000000384 [DOI] [PubMed] [Google Scholar]
- 3.Revels SL, Morris AM, Reddy RM, Akateh C, Wong SL. Racial Disparities in Esophageal Cancer Outcomes. Ann Surg Oncol. 2013;20(4):1136–1141. doi: 10.1245/s10434-012-2807-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ikoma N, Cormier JN, Feig B, et al. Racial disparities in preoperative chemotherapy use in gastric cancer patients in the United States: Analysis of the National Cancer Data Base, 2006–2014. Cancer. 2018;124(5):998–1007. doi: 10.1002/cncr.31155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steyerberg EW, Neville B, Weeks JC, Earle CC. Referral patterns, treatment choices, and outcomes in locoregional esophageal cancer: a population-based analysis of elderly patients. J Clin Oncol. 2007;25(17):2389–2396. doi: 10.1200/JCO.2006.09.7931 [DOI] [PubMed] [Google Scholar]
- 6.Liu JH, Zingmond DS, McGory ML, et al. Disparities in the Utilization of High-Volume Hospitals for Complex Surgery. JAMA. 2006;296(16):1973–1980. doi: 10.1001/jama.296.16.1973 [DOI] [PubMed] [Google Scholar]
- 7.Liu YP, Ma L, Wang SJ, et al. Prognostic value of lymph node metastases and lymph node ratio in esophageal squamous cell carcinoma. Eur J Surg Oncol. 2010;36(2):155–159. doi: 10.1016/j.ejso.2009.09.005 [DOI] [PubMed] [Google Scholar]
- 8.Wilson M, Rosato EL, Chojnacki KA, et al. Prognostic significance of lymph node metastases and ratio in esophageal cancer. J Surg Res. 2008;146(1):11–15. doi: 10.1016/j.jss.2007.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tachibana M, Dhar DK, Kinugasa S, et al. Esophageal cancer with distant lymph node metastasis: prognostic significance of metastatic lymph node ratio. J Clin Gastroenterol. 2000;31(4):318–322. doi: 10.1097/00004836-200012000-00010 [DOI] [PubMed] [Google Scholar]
- 10.Greenstein AJ, Litle VR, Swanson SJ, Divino CM, Packer S, Wisnivesky JP. Prognostic significance of the number of lymph node metastases in esophageal cancer. J Am Coll Surg. 2008;206(2):239–246. doi: 10.1016/j.jamcollsurg.2007.09.003 [DOI] [PubMed] [Google Scholar]
- 11.Tan Z, Ma G, Yang H, Zhang L, Rong T, Lin P. Can lymph node ratio replace pn categories in the tumor-node-metastasis classification system for esophageal cancer? J Thorac Oncol. 2014;9(8):1214–1221. doi: 10.1097/JTO.0000000000000216 [DOI] [PubMed] [Google Scholar]
- 12.Bhamidipati CM, Stukenborg GJ, Thomas CJ, Lau CL, Kozower BD, Jones DR. Pathologic lymph node ratio is a predictor of survival in esophageal cancer. Ann Thorac Surg. 2012;94(5):1643–1651. doi: 10.1016/j.athoracsur.2012.03.078 [DOI] [PubMed] [Google Scholar]
- 13.He Z, Wu S, Li Q, Lin Q, Xu J. Use of the metastatic lymph node ratio to evaluate the prognosis of esophageal cancer patients with node metastasis following radical esophagectomy. PLoS ONE. 2013;8(9):e73446. doi: 10.1371/journal.pone.0073446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu W-H, Hsu P-K, Hsieh C-C, Huang C-S, Wu Y-C. The metastatic lymph node number and ratio are independent prognostic factors in esophageal cancer. J Gastrointest Surg. 2009;13(11):1913–1920. doi: 10.1007/s11605-009-0982-8 [DOI] [PubMed] [Google Scholar]
- 15.Wei C, Deng W-Y, Li N, et al. Lymph Node Ratio as an Alternative to the Number of Metastatic Lymph Nodes for the Prediction of Esophageal Carcinoma Patient Survival. Dig Dis Sci. 2015;60(9):2771–2776. doi: 10.1007/s10620-015-3681-1 [DOI] [PubMed] [Google Scholar]
- 16.Wang N, Jia Y, Wang J, et al. Prognostic significance of lymph node ratio in esophageal cancer. Tumour Biol. 2015;36(4):2335–2341. doi: 10.1007/s13277-014-2840-x [DOI] [PubMed] [Google Scholar]
- 17.Hou X, Wei J-C, Xu Y, et al. The positive lymph node ratio predicts long-term survival in patients with operable thoracic esophageal squamous cell carcinoma in China. Ann Surg Oncol. 2013;20(5):1653–1659. doi: 10.1245/s10434-012-2794-4 [DOI] [PubMed] [Google Scholar]
- 18.Zhao Y, Zhong S, Li Z, Zhu X, Wu F, Li Y. Pathologic lymph node ratio is a predictor of esophageal carcinoma patient survival: a literature-based pooled analysis. Oncotarget. 2017;8(37):62231–62239. doi: 10.18632/oncotarget.19258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lagergren J, Mattsson F, Zylstra J, et al. Extent of Lymphadenectomy and Prognosis After Esophageal Cancer Surgery. JAMA Surg. 2016;151(1):32–39. doi: 10.1001/jamasurg.2015.2611 [DOI] [PubMed] [Google Scholar]
- 20.Jabo B, Selleck MJ, Morgan JW, et al. Role of lymph node ratio in selection of adjuvant treatment (chemotherapy vs. chemoradiation) in patients with resected gastric cancer. J Gastrointest Oncol. 2018;9(4):708–717. doi: 10.21037/jgo.2018.05.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aoyama T, Yamamoto N, Kamiya M, et al. The Lymph Node Ratio Is an Independent Prognostic Factor in Pancreatic Cancer Patients Who Receive Curative Resection Followed by Adjuvant Chemotherapy. Anticancer Res. 2018;38(8):4877–4882. doi: 10.21873/anticanres.12801 [DOI] [PubMed] [Google Scholar]
- 22.Ooki A, Akagi K, Yatsuoka T, et al. Lymph Node Ratio as a Risk Factor for Recurrence After Adjuvant Chemotherapy in Stage III Colorectal Cancer. J Gastrointest Surg. 2017;21(5):867–878. doi: 10.1007/s11605-017-3382-5 [DOI] [PubMed] [Google Scholar]
- 23.Burt BM, Groth SS, Sada YH, et al. Utility of Adjuvant Chemotherapy After Neoadjuvant Chemoradiation and Esophagectomy for Esophageal Cancer. Ann Surg. 2017;266(2):297–304. doi: 10.1097/SLA.0000000000001954 [DOI] [PubMed] [Google Scholar]
- 24.Atay SM, Blum M, Sepesi B. Adjuvant chemotherapy following trimodality therapy for esophageal carcinoma—Is the evidence sufficient? Journal of Thoracic Disease. 2017;9(10):3626–3629. doi: 10.21037/jtd.2017.09.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15(3):683–690. doi: 10.1245/s10434-007-9747-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harrell FE. Multivariable Modeling Strategies. In: Harrell Frank E Jr, ed. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. Springer Series in Statistics. Cham: Springer International Publishing; 2015:63–102. doi: 10.1007/978-3-319-19425-7_4 [DOI] [Google Scholar]
- 27.MatchIt: Nonparametric Preprocessing for Parametric Causal Inference. https://gking.harvard.edu/matchit. Accessed April 18, 2019.
- 28.Li Y, Zhao W, Ni J, et al. Predicting the Value of Adjuvant Therapy in Esophageal Squamous Cell Carcinoma by Combining the Total Number of Examined Lymph Nodes with the Positive Lymph Node Ratio. Ann Surg Oncol. 2019;26(8):2367–2374. doi: 10.1245/s10434-019-07489-3 [DOI] [PubMed] [Google Scholar]
- 29.Ando N, Iizuka T, Kakegawa T, et al. A randomized trial of surgery with and without chemotherapy for localized squamous carcinoma of the thoracic esophagus: the Japan Clinical Oncology Group Study. J Thorac Cardiovasc Surg. 1997;114(2):205–209. doi: 10.1016/S0022-5223(97)70146-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Scheme of patient selection for the study
Supplemental Figure 2. Kaplan-Meier survival curves for patients in the (a) overall cohort and (b) propensity score-matched subgroup, stratified by receipt of adjuvant chemotherapy. The shaded regions represent the bounds of the 95% confidence interval. The numbers at risk are provided beneath each graph. The p values represent the log-rank test
Supplemental Figure 3. Graph of the interaction between total number of positive nodes on pathology (X-axis), receipt of adjuvant chemotherapy (curves), and adjusted hazard of mortality (Y-axis) in patients in the overall cohort, after adjustment for a separate two-way interaction between the number of nodes sampled and number of positive nodes. The grey regions represent the bounds of the 95% confidence interval
Supplemental Figure 4. A plot of the number of nodes examined in surgery, modelled using restricted cubic splines, against the adjusted hazard of mortality (Y-axis). The grey region represents the bounds of the 95% confidence interval