Abstract
ACL injuries place the knee at risk for post-traumatic osteoarthritis (PTOA) despite surgical ACL reconstruction (ACLR). One parameter thought to affect PTOA risk is the initial graft tension. This randomized controlled trial (RCT) was designed to compare outcomes between two graft tensioning protocols that bracket the range commonly used. At 7-years post-surgery, we determined that most outcomes between the two tension groups were not significantly different, that they were inferior to an uninjured matched control group, and that PTOA was progressing in the both groups relative to controls. The trial database was also leveraged to gain insight into mechanisms of PTOA following ACL injury. We determined that the inflammatory response at the time of injury undermines one of the joint’s lubricating mechanisms. We learned that patients continue to protect their surgical knee 5 years post-injury compared to controls during a jump-pivot activity. We also established that pre-surgical knee function and mental health were correlated with symptomatic PTOA, that there were specific anatomical factors associated with poor outcomes, and that there were no changes in outcomes due to tunnel widening in patients receiving hamstring tendon autografts. We also validated an MRI technique to non-invasively assess graft strength. In conclusion, the RCT determined that initial graft tensioning does not have a major influence on 7-year outcomes. Therefore, surgeons can reconstruct the ACL using a graft tensioning protocol that is within the window of the two graft tensioning techniques evaluated in this RCT.
Keywords: ACL, reconstruction, outcomes, osteoarthritis
INTRODUCTION
ACL injured patients typically exhibit a functional deficit and are at greater risk for early post-traumatic osteoarthritis (PTOA).1 Although the mechanisms behind the onset of PTOA in the ACL reconstructed (ACLR) population remain controversial, it is argued that altered joint contact mechanics plays a roll.2–4 Thus, surgical variables, such as the “initial graft tension” applied at the time of surgery to restore normal joint contact mechanics,5; 6 could potentially reduce PTOA risk. Recent evidence suggests that the inflammatory response after injury may also negatively impact joint health.7; 8 As the articular cartilage is challenged biologically after injury, the negative mechanical consequences of over- or under-constraining the joint at the time of graft fixation could be exacerbated.
To better understand PTOA development in the ACLR knee and the impact of initial graft tension on surgical outcomes, a prospective randomized controlled trial (RCT) was performed [NCT00434837].9–11 The primary objective of the “Tension Trial” was to compare clinical, functional, patient-reported and PTOA-related imaging outcome measures 7 years post-surgery between two common “laxity based” graft tensioning protocols. The RCT was performed in patients with acute isolated ACL injuries. A matched uninjured control group was also recruited.10
We also leveraged the RCT cohort to explore additional questions related to outcomes following ACL injury: 1) To determine if preoperative patient-reported outcome measures were associated with the development of a symptomatic knee;12 2) To examine associations between key anatomical features of the knee and clinically relevant ACLR outcomes;13 3) To evaluate the progression of tibial tunnel widening following ACLR;14 4) To study the effect of ACL injury on lubricin concentrations in synovial fluid (SF) and its association with cartilage health;8 5) To compare kinetic/kinematic measurements of the ACLR and control subjects during a jump-cut maneuver;15 and 6) To determine if MRI derived parameters of graft volume and signal intensity, which have been used to non-invasively predict the biomechanical properties of the graft in an animal model,16 correlate with clinical, functional, and patient-reported outcome measures in ACLR patients.17
METHODS
The RCT was IRB approved. Male and female patients (15 to 50 years old) with a unilateral ACL injury, and were candidates for autograft ACLR were prospectively enrolled.10 Patients with concomitant injuries to the menisci or other ligaments, a previous knee injury, or evidence of arthritis were excluded. Of the 202 eligible patients, 90 were randomized between the two tension groups (low-tension, n=46; high-tension, n=44), and 18 (20%) were lost to follow-up at 7 years.9 Sixty subjects, who were matched by age, sex, race, and activity level, were also recruited to serve as an uninjured control group. Group demographics are provided in Table 1.
Table 1.
Subject demographics for the tension cohorts and matched control group.
| Low-tension group | High-tension group | Control group | |
|---|---|---|---|
| Age in years, mean±95%CI | 24±2.7 | 23±2.1 | 25±1.6 |
| Weight in Kg, mean±95%CI | 73±5.0 | 69±4.9 | 72±3.6 |
| Days to surgery, mean±95%CI | 114±23.2 | 105±20.0 | - |
| Sex, n (%) | |||
| Males | 24 (52.2) | 18 (40.9) | 34 (56.7) |
| Females | 22 (47.8) | 26 (59.1) | 26 (43.3) |
| Graft type, n (%) | |||
| B-PT-B graft | 31 (67.4) | 27 (61.4) | - |
| Hamstring graft | 15 (32.6) | 17 (38.6) | - |
| Meniscal lesions at Sx*, n (%) | |||
| Medial, # (%) | 11 (23.9) | 11 (25.0) | - |
| Lateral, # (%) | 8 (17.4) | 7 (15.9) | - |
| Chondral lesions at Sx**, n (%) | |||
| Medial, # (%) | 3 (6.5) | 1 (2.2) | - |
| Lateral, # (%) | 5 (10.9) | 9 (20.4) | - |
Minor debridement or repair
Grade II or less, no treatment necessary
Patients were reconstructed either with a bone-patellar tendon-bone (BTB) or a four-stranded semitendinosus/gracilis (HS) autograft.10 For those patients receiving the “low-tension” assignment, grafts were tensioned to restore normal anteroposterior (AP) laxity (firm tensioning with the knee at 0° of flexion).6; 10 For those receiving the “high-tension” assignment, grafts were tensioned to over-constrain AP laxity by 2 mm (firm tensioning with the knee at 30° of flexion).6; 10 AP knee laxity was checked intra-operatively with an arthrometer (KT-1000S; MEDmetrics Inc) and compared to the contralateral knee. If the targeted AP knee laxity value was not achieved, the tensioning procedure was repeated.
Outcome measures were assessed pre-operatively and at 1, 3, 5 and 7-years post-surgery. The clinical outcomes included AP knee laxity (KT-1000 Knee Arthrometer),18 and the 2000 IKDC Knee Examination Score.19 Subsequent ipsilateral or contralateral knee injuries were recorded at each follow-up visit. The functional outcomes included the 1-leg hop test.20 The patient-reported outcomes included the Knee Osteoarthritis Outcome Score (KOOS)21 and the SF-36v2.22 The composite KOOS model developed by Englund et al was used to identify subjects with symptomatic PTOA.23; 24 Activity was assessed using the Tegner activity scale.25
Structural changes related to PTOA were evaluated using imaging outcomes pre- and post-operatively. Medial minimum joint space widths (mJSW) were measured from standardized radiographs using a posterior-anterior view of the semi-flexed knee.26 The overall condition of the joint was scored pre- and post-surgery using the modified Osteoarthritis Research Society International (OARSI) radiographic atlas.27 PTOA was also assessed using the Whole Organ Magnetic Imaging Score (WORMS).28
To address the primary aim of the study, mixed linear models were used to evaluate differences among treatment groups with respect to temporal changes in the outcome variables. Chi-square tests were used to compare the frequency of subsequent injuries, the distributions of IKDC scores, and the percent of subjects within each group in which the composite KOOS model23; 24 indicated the presence of symptomatic PTOA. For each control subject, a knee was randomly selected to represent the reconstructed knee. Although graft type was not randomized, a subsequent analysis was performed to explore the effects of graft type and tension on outcomes.11 Several retrospective analyses (ancillary studies) were also performed using the Tension Trial database.8; 12–15; 17
RESULTS
Randomized Controlled Trial
In general, the clinical, functional and patient-reported outcomes for the two graft tension groups were inferior to those of the control group at 7 years.9 There were no significant differences in AP knee laxity between the two tension groups over time (p=.83; Fig. 1). However, the high-tension group was significantly greater than the control group (p=.03), while the low-tension group approached significance (p=.08). The 1-leg hop distances (Fig. 1) for the two tension groups were significantly less than the control group (p<.02), though there was no difference between tension groups (p=.59). There were no significant differences in the subsequent knee injury rates (i.e., injury to either knee) between the low-tension (17%) and high-tension (23%) groups (p=.53; Table 2). However, the rate for the high-tension group (p=.02) was significantly greater than the control group (7% knee injury rate) while the low-tension group was not (p=.08). The IKDC examination score distributions were significantly worse for the two tension groups relative to the control group (p<.01). There was a trend indicating that the IKDC examination score distribution of the high-tension group was better than that of the low-tension group (p=.08). Three of the 5 KOOS subscores for the high-tension group were not significantly different from the control group (KOOS-sport, p=.15, Fig. 1; KOOS-ADL, p=.29; KOOS-pain, p=.18), while these subscores were different in the low-tension group (p<.01, p=.06, p=.02, respectively). Both tension groups were significantly lower than the control group for the KOOS-symptoms (p=.03) and the KOOS-QOL (p<.01) subscores. Eight patients in the low-tension group, 5 patients in the high-tension group, and 1 subject in the control group had combined KOOS scores indicative of symptomatic PTOA.23; 24 Similarly, 4 of the 8 SF-36 health domains22 (bodily pain, general health, physical function, and social functioning) for the low-tension group scored significantly lower than the control group and there was a strong trend (p<.06) indicating that two of the scores (bodily pain, social functioning) were lower in the low-tension group compared to the high-tension group. While neither tension group fully restored clinical, functional, or patient-reported outcomes at 3 or 7 years,9; 10 the 7-year results show that the two procedures are beginning to diverge in several of the outcome measures, emphasizing the need to acquire longer-term data.
Figure 1.
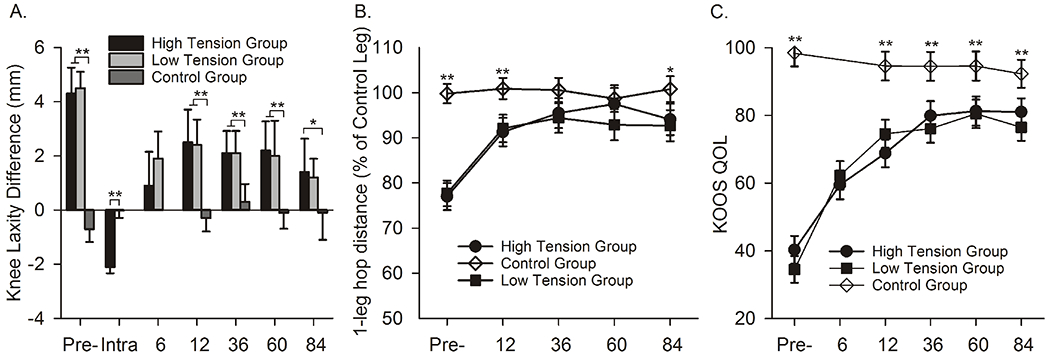
Examples of a clinical (KT-1000), functional (1-legged hop) and one of the patient-reported outcomes (KOOS-sport) at 7-year follow-up.9 Error bars represent the 95% confidence intervals. Significant differences are highlighted with * (p<.05) or ** (P<.01).
Table 2.
Subsequent knee injuries for the two tension cohorts and the control groups over the 7-year follow-up. Note that one of the failures Sx knee failures in the High-tension group also had a meniscal injury. Sx=surgical knee; Contra=contralateral knee.
| Subsequent Knee Injuries | Low-tension group | High-tension group | Control group |
|---|---|---|---|
| ACL graft (Sx) | 2 | 3 | 1 |
| ACL (Contra) | 2 | 3 | |
| Meniscus (Sx) | 2 | 4 | 1 |
| Meniscus (Contra) | 1 | 0 | |
| Other | 0 | 0 | 1 |
The imaging outcomes suggest that PTOA is evident 7 years post-surgery.9 There was a trend indicating that the mean mJSWs between the high- and low-tension groups were different (p=.08; Fig. 2). The mean mJSW for the high-tension group increased significantly relative to the control group (p<.01), while that of the low-tension group did not (p=.22).9 At 7 years, the mean OARSI radiographic scores were higher for the ACLR groups relative to the control group (p<.02); however, there was no difference between tension groups (Fig. 2).9 The mean WORMS for the low-tension group were greater than the control group (p<.01) while there was no difference between the high-tension group and control group (p=.17; Fig. 2).9 There was a trend that the mean WORMS for the low-tension group were worse than the high-tension cohort (p=.08).9 While there are discrepancies between the imaging measures of PTOA at 7 years, it is clear changes related to PTOA development occurred relative to the control group. Long-term follow-up is required to reconcile these findings.
Figure 2.
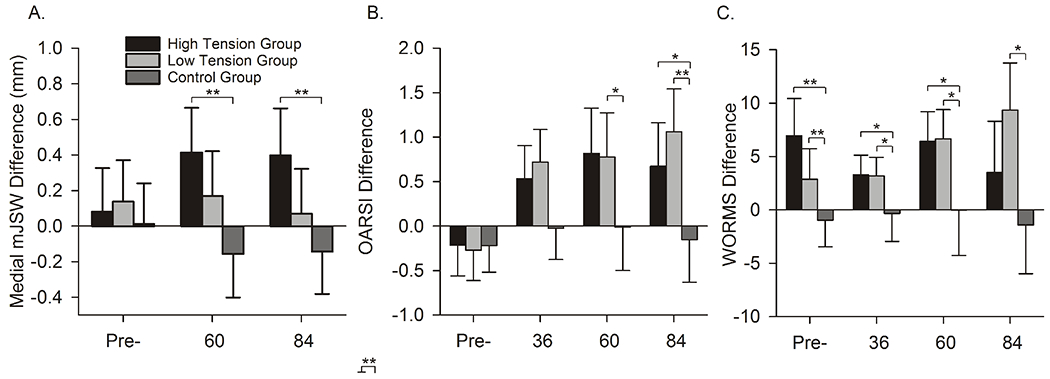
The mean differences (±95% CIs) between the surgical limb and the contralateral control knee for each group over 7 years for mJSW, OARSI radiographic, WORM scores. A positive mJSW indicates that the thickness of the cartilage in the surgical knee is greater than that of the contralateral knee. Higher OARSI radiographic or WORM scores indicated greater damage.9 Significant differences are highlighted with * (p<.05) or ** (P<.01).
In the subsequent analysis evaluating the effects of graft type and tensioning on outcomes,11 it was determined that patients reconstructed with HS autografts under the high-tension condition had better SF-36 scores (bodily pain, social functioning, and mental health scores; p<.014) than those HS patients reconstructed under low-tension, though there were no differences in any other outcome measures. Likewise, no differences in any outcome measures were found between the two graft tension conditions in those patients receiving BTB autografts.
Ancillary Studies
I. Preoperative Sports Performance and Depression are Associated with the Presence of Symptomatic PTOA at 7 years Following ACLR
We determined that the pre-operative KOOS-sports and the SF-36 mental health scores were predictive of who would present with a symptomatic knee at 7-year follow-up using stepwise logistic modeling.12 ACLR patients were divided into symptomatic and asymptomatic groups based on the previously defined KOOS-pain score ≤72.24 Demographic variables and preoperative KOOS and SF-36 scores were compared between these groups. Radiographic and MRI data were used to evaluate differences in mJSW,26 OARSI radiographic score,27 and the WORMS28 between groups. Multivariate analysis was performed to identify potential predictors of pain at 7-year follow-up. Seven of the 72 patients available at follow-up formed the symptomatic group. No differences were found between groups with respect to demographic variables or intraoperative findings. Lower preoperative KOOS sports (p<.01) and SF-36 mental health (p=.03) scores were associated with a painful knee at 7 years, with increased odds of 82% and 68% per 10-unit decrease in pre-operative scores, respectively (Table 3). The WORMS at 7 years showed evidence of osteoarthritic changes in the symptomatic group (p=.047), and there was a trend that the OARSI radiographic score (p=.051) suggested greater damage in the symptomatic group compared to the asymptomatic group (Table 4). These data demonstrate that the rate of symptomatic PTOA is relatively low at 7 years in patients with isolated ACL injuries treated with ACLR, and that pre-operative mental and physical performance may underly the prediction of outcomes.
Table 3.
Significant findings from multivariate analysis assuming a 10-point change in the preoperative score.12
| Baseline Measure | Odds Ratio | 95% CI | p-value |
|---|---|---|---|
| KOOS Sports | 1.821 | 1.106-3.003 | .005 |
| SF36 MH | 1.681 | 1.025-2.762 | .025 |
KOOS = Knee Osteoarthritis Outcome Score; Sports = Sports & Recreation; MH = General Mental Health.
Table 4.
Mean±SD for the imaging outcomes between the patients categorized as symptomatic compared to the asymptomatic patients using the KOOS-pain score at 7 years. WORM Score difference (Sx–Ctl), OARSI Radiographic Score difference (Sx–Ctl) and mJSW difference (Sx–Ctl, mm). Sx=surgical knee; Ctl=contralateral control knee. *indicates a significant difference between groups.12
| Outcome Measure | Symptomatic Patients (Mean±SD) | Asymptomatic Patients (Mean±SD) | p-value |
|---|---|---|---|
| WORM Score* | 13.7±22.2 | 5.8±14.5 | .047 |
| OARSI Xray Score | 2.8±5.0 | 0.8±1.9 | .051 |
| mJSW (mm) | −0.32±0.68 | −0.03±0.182 | .487 |
OARSI = Osteoarthritis Research Society International; WORM = Whole Organ MRI; mJSW = medial joint space width.
II. Anatomical Features of the Tibial Plateau Predict 7 Year Outcomes of ACLR
We also established that increasing slopes in the coronal and sagittal planes along with decreasing concavity of the medial tibial plateau led to less favorable outcomes 7 years after ACLR surgery.13 Multiple anatomical indices including tibial plateau morphology (posterior slope and concavity),29–32 tibial eminence size,33 and femoral inter-condylar notch size30; 34 have been associated with increased risk of injury or reinjury. However, reinjury does not occur in many patients. Therefore, it is important to understand if these anatomical features influence the outcomes of ACLR in patients who do not have a reinjury. We hypothesized that decreased femoral notch width, increased posterior and coronal slopes, and decreased concavity of the tibial plateau were associated with inferior outcomes 7 years after ACLR surgery. Prospectively collected data from 44 patients who did not have a subsequent graft or contralateral ACL failure within 7 years after unilateral ACLR were reviewed (23.7±9.2 years; 64% women). Notch width (post-surgery), posterior slopes of the medial and lateral tibial plateau, maximum depth of the medial tibial plateau, and coronal tibial slope were measured from MR images (Fig. 3). Anatomical predictors of side-to-side differences in AP knee laxity,18 KOOS scores,21 mJSW26 and side-to-side differences in OARSI radiographic score,27 measured at 7 years, were identified using stepwise regression modeling. We found that increased posterior slope of the lateral tibial plateau was correlated with increased side-to-side difference in AP knee laxity, increased side-to-side difference in OARSI radiographic score, and decreased KOOS subscores (R2>.10, p<.05) (Table 5). Increased posterior slope of the medial tibial plateau was associated with a higher side-to-side difference in OARSI radiographic score and lower KOOS subscores (R2>.11, p<.03). Increased coronal tibial slope was associated with lower KOOS subscores (R2>.11, p<.03). Decreased medial tibial depth was associated with increased AP knee laxity as well as decreased KOOS-QOL and symptoms subscores (R2>.12, p<.03). Post-surgical notch width was not a significant predictor for any of the surgical outcomes. These results highlight the importance of knee anatomy on outcomes of ACLR.13
Figure 3.
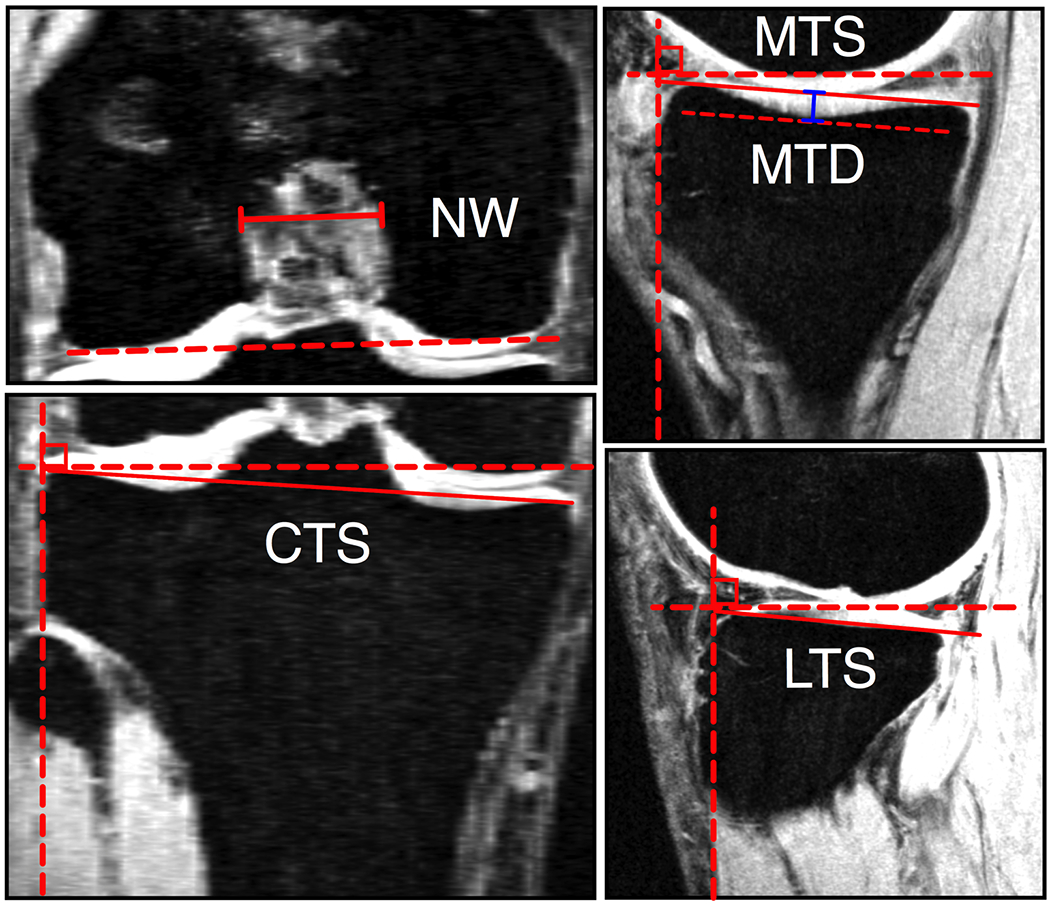
Measurement techniques used to quantify notch width (NW), coronal tibial slope (CTS), medial tibial slope (MTS), medial tibial depth (MTD) and lateral tibial slope (LTS).13
Table 5.
Stepwise regression analysis indicating significant predictors of surgical outcomes in a multivariate model with the demographic variables included. Notch width and mJSW were not considered as they were not significant in the univariate analyses. LTS = lateral tibial slope; MTS = medial tibial slope; CTS = coronal tibial slope; MTD = medial tibial depth; Int = intercept.13
| Outcome | Int | Sex (male) | Graft Type (Hamstring) | LTS | MTS | CTS | MTD | Model R2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | |||
| aAP Laxity | 5.5 | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | −1.9 (−3.2, 0.6) | .006 | .19 |
| KOOS-QoL | 70.8 | −13.5 (−25.7, −1.3) | .034 | --- | --- | --- | --- | −4.5 (−7.6, 1.4) | .002 | --- | --- | 15.1 (6.1, 24.2) | .009 | .42 |
| KOOS-Sports | 109. 7 | −10.4 (−20.0, −0.9) | .034 | 11.9 (2.5, 21.4) | .015 | --- | --- | −3.5 (−5.9, −1.1) | .006 | −4.2 (−6.4, −2.1) | <001 | --- | --- | .54 |
| KOOS-ADL | 110.4 | −5.9 (−11.2, −0.5) | .007 | 6.9 (1.5, 12.3) | .045 | −1.4 (−2.6, −0.3) | .016 | --- | --- | −2.55 (−3.7, −1.4) | <.001 | --- | --- | .54 |
| KOOS-Sympt | 68.9 | --- | --- | 13.8 (5.1, 22.6) | .014 | −2.7 (−4.6, −0.9) | .006 | --- | --- | --- | --- | 12.3 (5.9, 18.6) | <001 | .45 |
| aKOOS-Pain | 119.7 | −11.1 (−21.2, −1.0) | .035 | 13.0 (2.8, 23.2) | .015 | −2.7 (−4.9, −0.6) | .016 | --- | --- | −4.8 (−7.0, −2.6) | <.001 | --- | --- | .54 |
| aOARSI Score | −1.8 | --- | --- | --- | --- | 0.4 (0.1, 0.7) | .017 | --- | --- | --- | --- | --- | --- | .15 |
III. Tibial Tunnel Widening Following ACLR: A Retrospective 7 Year Study Evaluating the Effects of Initial Graft Tensioning and Graft Selection
We found that patients who underwent ACLR with HS autografts experienced significant tunnel widening over a 7-year period, which was not seen in patients with BTB autografts.14 Tunnel widening is an accepted phenomenon following ACLR, though there is disagreement about its clinical ramifications and underlying mechanisms.35–37 The objective was to evaluate the effects of initial graft tension on tibial widening and clinical outcomes for both autografts. Tunnel widening was assessed via radiographs at the 1, 3, and 7-year follow-up visits. Patient-reported outcomes were also compared. The mean±95% confidence intervals of the initial diameters for the BTB autografts were 10.3±0.5mm (low-tension) and 10.2±0.6mm (high-tension) with final diameters of 10.8±0.6mm (low-tension) and 9.9±0.6mm (high-tension). The initial diameters for HS autografts were 8.1±0.9mm (low-tension) and 8.4±0.7mm (high-tension) with final diameters of 11.5±1.1mm (low-tension) and 11.1±0.9mm (high-tension). For subjects with HS autografts, mean tunnel diameters significantly changed over time (p <.001); no significant changes were observed in BTB autografts (p=.29) (Fig. 4). No differences in patient-reported outcomes were found between tension groups or graft types. In conclusion, patients who underwent ACLR with HS autograft exhibited tibial tunnel widening over 7 years, while those with BTB autografts did not.
Figure 4.
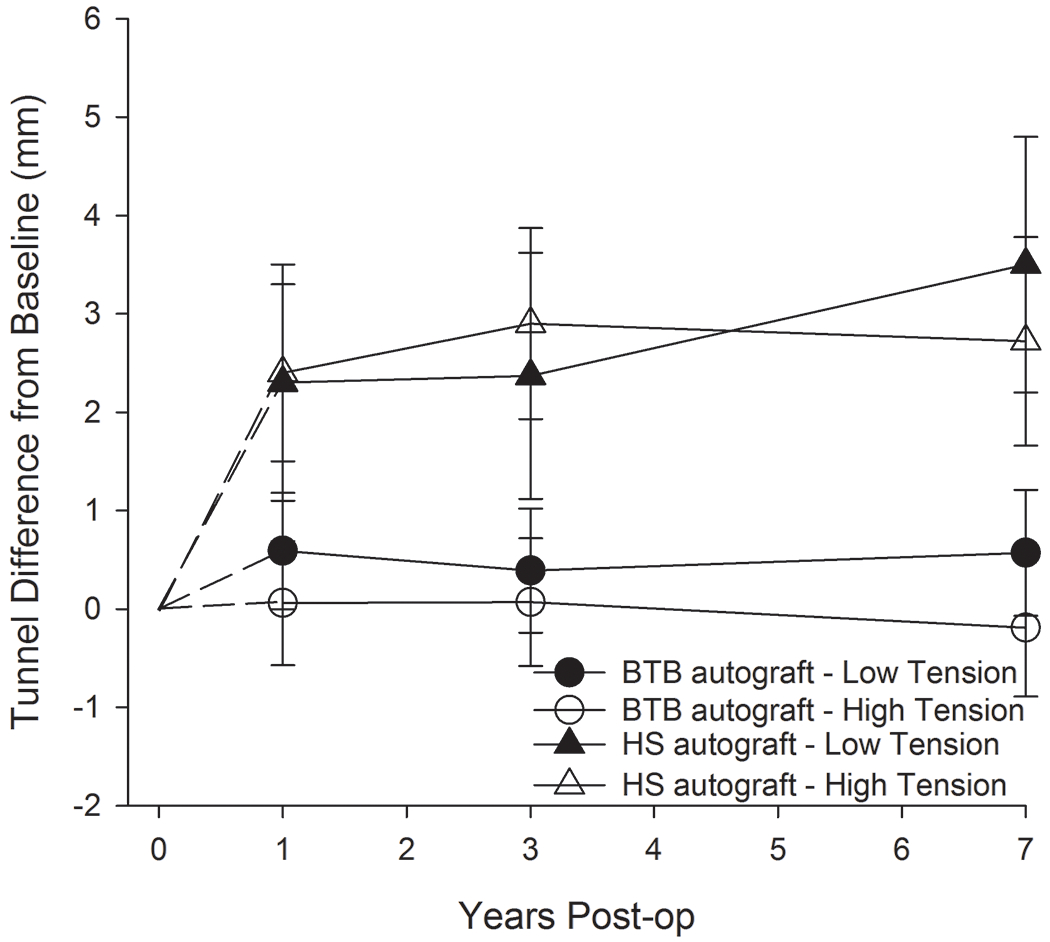
Mean changes in tunnel widening relative to time of surgery (0). The error bars represent the 95% confidence intervals. BTB = bone-tendon-bone; HS = hamstring tendon.14
IV. Decreased Lubricin Concentrations and Markers of Joint Inflammation in Synovial Fluids from Patients with ACL Injury
Using SF samples taken at the time of surgery, we determined that the concentrations of inflammatory cytokines increased while that of lubricin decreased post-injury and remained decreased 12 months post-injury.8 Lubricin is an important molecule that serves as a boundary lubricant for articular cartilage. A decrease in lubricin has been shown in animal models to increase the coefficient of friction of the articular surface, which in turn promotes PTOA.38 The objective was to evaluate the effects of ACL injury on lubricin concentrations in SF and its correlation with time post-injury, inflammatory cytokines, and SF proteoglycan content. SF samples were obtained from both knees of 30 Tension Trial patients 32-364 days post-injury. Lubricin and inflammatory cytokines (IL-1, TNF, and IL-6) were measured in SF taken from the injured and contralateral (uninjured) joints by enzyme-linked immunosorbent assay. Sulfated glycosaminoglycan (sGAG) levels in the SF were measured by Alcian blue binding assay. We determined that SF lubricin concentrations were significantly (p<.001) reduced soon after ACL injury compared to the contralateral joint (Fig. 5). Within 12 months, the lubricin concentration in the injured knee (p<.001) approached that of the contralateral knee. TNF levels showed a significant negative relationship with lubricin levels. There were no detectable cytokines in the SF of contralateral joints. Concentrations of sGAG were significantly (p<.001) higher in the SF from injured knees compared with the contralateral joints, suggesting altered cartilage metabolism. The decrease in SF lubricin concentration following ACL injury could place the joint at an increased risk of wear-induced PTOA.
Figure 5.
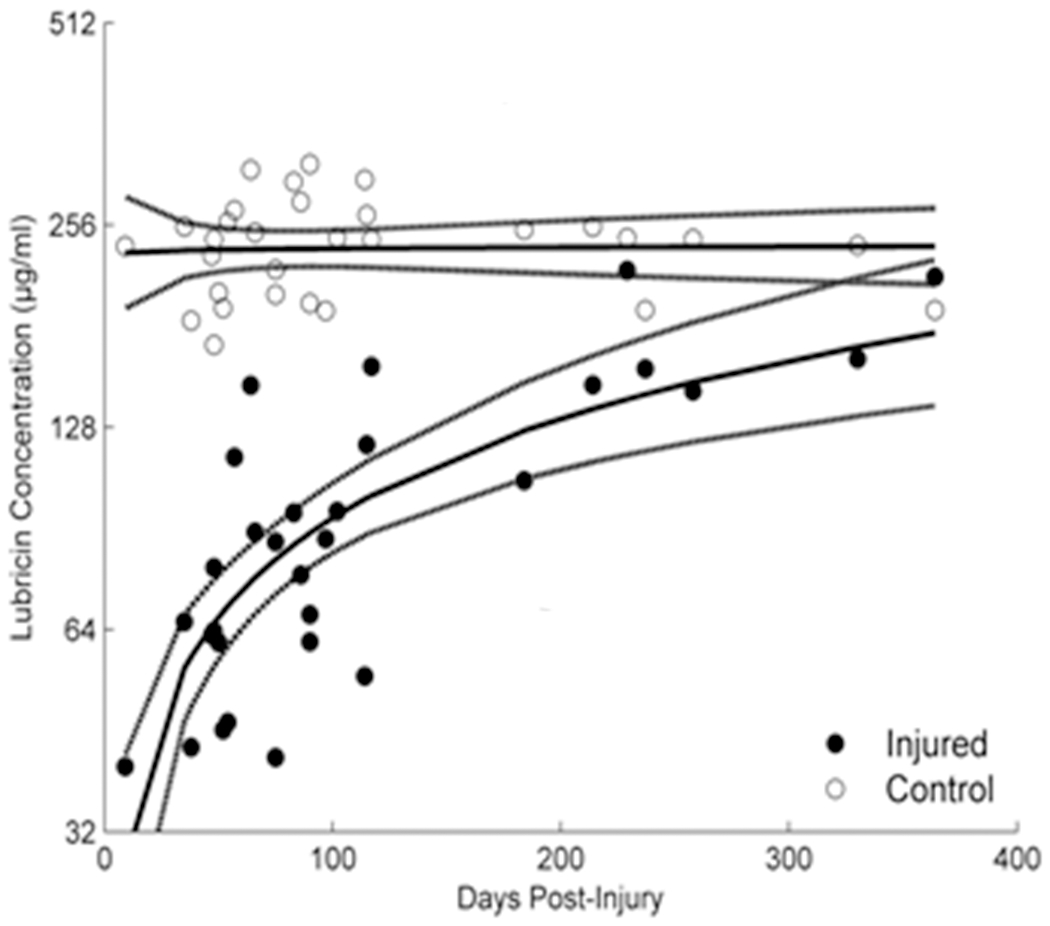
The lubricin synovial fluid concentrations in the ACL injured joint are reduced immediately after injury and trend back to normal with time.8
V. Knee Biomechanics during a Jump-Cut Maneuver: Effects of Sex and ACLR
Using biplanar videoradiography to measure knee joint kinematics during a jump-cut task, it was determined that women land with a stiffer knee resulting in a larger peak vertical ground reaction force and increased rate of anterior tibial translation, and that the ACLR subjects reduce the peak vertical ground reaction force compared to control subjects 5 years post-surgery.39 Given the unexplained greater risk of non-contact ACL injury in female subjects, differences between sex and ACLR status in kinematic/kinetic factors during sport activities may point to root causes for re-injury and PTOA. We investigated differences in joint kinematics and kinetics of the index knee of 10 ACLR (4 males) and 10 control subjects (5 males) performing a jump-cut maneuver. Ground reaction forces were measured with a force plate. 3D knee kinematics were determined using biplanar videoradiography. Female control subjects landed with a larger peak vertical ground reaction force (p<.001) compared to male control subjects (Fig. 6A). ACL intact control subjects (Fig. 6A) landed with a larger peak vertical ground reaction force (p≤.036) compared to ACLR subjects (Fig. 6B). Regardless of ACLR status, female subjects underwent less knee flexion angle excursion (p=.002) and increased average rate of anterior tibial translation (0.05±0.01%/millisecond; p=.037) after contact compared to male subjects. Furthermore, ACLR subjects had a lower rate of anterior tibial translation (Fig. 6D) compared to control subjects (0.05±0.01%/millisecond; p=.035) 5 years post-surgery (Fig. 6C). Differences in muscle activity about the knee, which could potentially explain graft failure risk and sex bias, were also found.40 These data suggest that there are kinetic and kinematic differences between males and females and that ACLR patients modify their functional activities in an effort to protect the reconstructed joint even after 5 years.
Figure 6.
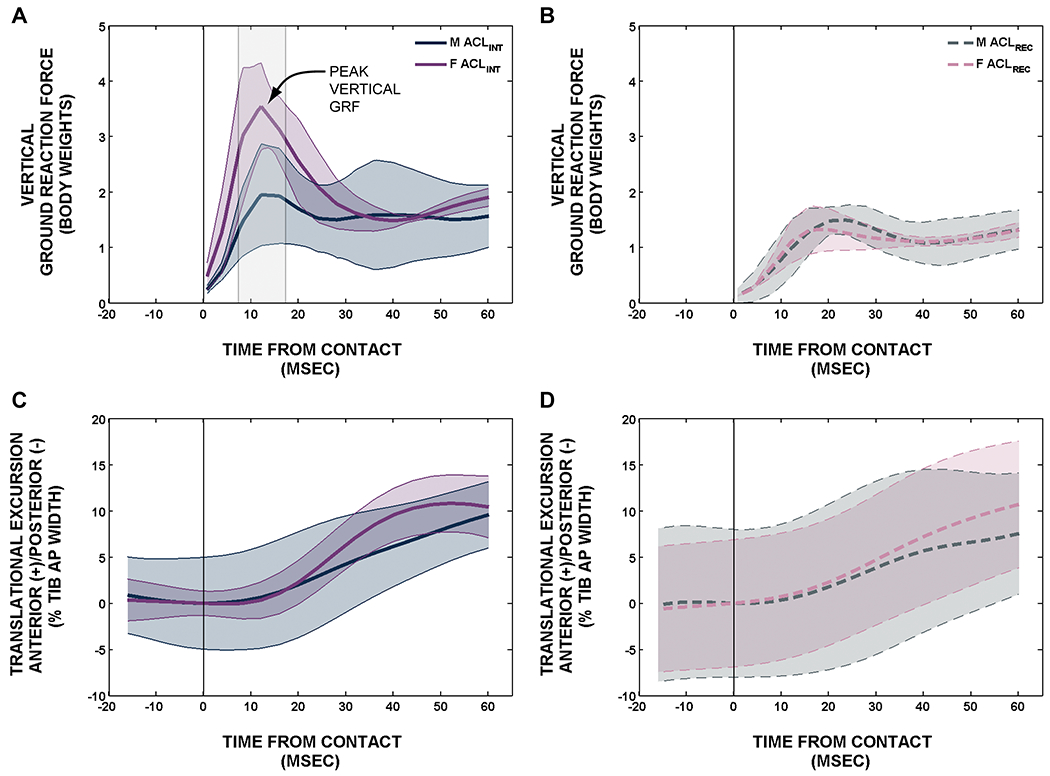
Differences in the ground reaction forces and anterior translation between males and females in control subjects (ACLINT) knees (A&B) and patients with ACLR (ACLREC) knees (C&D).15
VI. MRI Volume and Signal Intensity of the ACL Graft Predicts Clinical, Functional and Patient Reported Outcome Measures Following ACLR
Using Tension Trial patients, we determined that the MRI parameters (graft volume & median signal intensity) are associated with traditional outcome measures in patients at 5-year follow-up.17 Commonly used outcomes for clinical trials do not directly measure the biomechanical changes in the graft that occur with healing. However, MRI provides a non-invasive means to assess the integrity of the healing graft.16 The objective was to determine if the MRI-derived parameters of normalized graft volume and signal intensity, which have been used to predict the biomechanical (i.e., structural) properties of the graft in animal models,16 correlate with commonly used clinical (AP knee laxity), functional (1-leg hop) and patient-reported outcome measures (KOOS) in patients 5-years after ACLR. All ACLR subjects underwent an AP knee laxity exam, a 1-legged hop test for distance and a KOOS questionnaire at the 5-year follow-up visits. Three-dimensional T1-weighted MR images were collected.9 Both the normalized volume and median signal intensity of the healing graft were determined to be predictors in a multiple regression linear model. Graft volume combined with median signal intensity in a multiple linear regression model predicted results for the 1-legged hop test (R2=.62, p=.003), KOOS-QOL (R2=.49, p=.012), KOOS-sport (R2=.37, p=.048), KOOS-pain (R2=.46, p=.017) and KOOS symptoms (R2=.45, p=.021) at 5 years. The multiple linear regression model for AP knee laxity at 5-year follow-up approached significance (R2=.36, p=.088). Results from this study suggest that MRI could enhance clinical evaluation of graft healing and could potentially aid researchers in determining the appropriate timing for athletes to return to sport.
DISCUSSION
The Tension Trial determined that there were minimal differences in the clinical, functional, and patient-reported outcomes between the two ACLR initial graft tension groups, and that most of the outcomes for both groups were inferior to those of the control group at 7-years. Therefore, one may conclude that tensioning the graft within the boundaries that were tested would be adequate. However, several trends have emerged in the 7-year follow-up data worth noting. The IKDC examination scores good to excellent in 96% of the patients in the high-tension group relative to 78% in the low-tension group at 7-year follow-up. (p=.08). The Tegner activity score for the high-tension group was 1 point greater (p=.06). The KOOS-sports subscore for the high-tension group was 8 points higher than the low-tension group (p=.04), and the SF-36 subscores for bodily pain and social functioning were also greater (p<.06). We also found that the high-tension condition in the patients receiving HS autografts had better SF-36 subscores (i.e., bodily pain, social functioning, mental health) than those receiving the low-tension condition. These trends suggest that the high-tension group may be doing better than the low-tension group in the long-term.
The imaging outcomes suggest that knee PTOA is emerging 7 years post-surgery in both surgical groups, though trends suggest that there may be less PTOA in the high-tension group. The minimum JSW of the medial compartment, an indicator of cartilage thickness, was greater in the high-tension group compared to the low-tension group (p=.08). Likewise, the WORM score for the low-tension group was worse than the high-tension group (p=.08). The WORM scores were primarily driven by the cartilage signal and osteophyte formation. While not significant (p=.23), the OARSI radiographic score was also worse in the low-tension group and was driven primarily by osteophytes. Due to the relatively slow progression of PTOA following ACLR, more time may be needed to see the long-term implications of these trends.
While the RCT was designed to evaluate the effects of initial graft tension, we leveraged the cohort to ask additional questions related to long-term outcomes. We found that patients who were physically less able to perform sporting maneuvers (lower KOOS-sports) pre-operatively were at higher risk for symptomatic PTOA at 7-year follow-up.12 In addition, patients who were more depressed (lower SF-36 mental health score), were also at higher risk. These data suggest that the patients presenting with symptomatic PTOA at 7 years may be determined, in part, before surgery.
In a separate analysis, we looked at the anatomical factors previously linked to ACL injury risk to see how these factors affected other ACLR outcomes.13 We determined that knee laxity, KOOS and the OARSI x-ray score 7 years after ACLR were influenced by the slopes of the tibial plateau in the coronal and sagittal planes and the maximum depth of the medial tibial plateau. Again, these data suggest that the patients presenting with PTOA at 7 years may be determined, in part, prior to surgery.
We leveraged the cohort to perform additional studies into mechanisms of joint damage following ACL injury. We showed that lubricin levels decrease following joint injury due to inflammatory cytokine release, and that it takes approximately 1 year for the lubricin levels to return to normal.8 As lubricin contributes to the lubricating mechanism of the joint, it is possible that short-term lubricin depletion could influence long-term cartilage health. Our supplementary tunnel widening study determined that tunnel widening around the hamstring tendon graft had minimal impact on joint arthrosis.14 Comparisons between the kinematics/kinetics between the ACLR patients and control subjects at 5 years also show that the joint loading of the surgically treated knees are different and may therefore contribute to PTOA.15 Finally, we leveraged the Tension Trial database to help validate a non-invasive means to assess graft strength.16 We found that the MRI-associated values related to the strength of the Tension Trial patients’ grafts were related to clinical, functional, and patient-reported outcomes.17 Thus, this study set the stage for future development and optimization of the MRI method to relate graft integrity and PTOA.41 Therefore, the impact of the RCT database extends beyond the original objective of the RCT.
There are many advantages associated with the RCT. It addressed an important clinical question as previous RCTs evaluating initial graft tension were contradictory,42; 43 under-powered,42 and did not focus on cartilage health. The study design is a prospective, double-blind, randomized control trial (Level 1 study) using accepted imaging outcomes of PTOA in a patient population at risk for PTOA. The prospective database includes pre- and post-surgical assessments at 1, 3, 5, and 7 years,9 with 12- and 15-year follow-up assessments currently underway. The cohort also enabled us to look at the effects of ACL deficiency without confounding factors such as concomitant meniscal or chondral injuries, factors known to promote PTOA.1 While the 7-year data suggest that the differences between the initial graft tension groups were minimal, we recruited a control group so that the outcomes of the ACLR cohort could be compared to them. This is important for a trial focusing on joint arthrosis as the contralateral knee is also affected.44; 45 Thus, inclusion of the independent control group was critical.
There are several limitations. Participants who dropped out may have introduced bias. The patient dropout rate at 7-years was 20%, not insignificant, but considered acceptable for a Level 1 study. Radiographic assessment of the joint space narrowing is not a sensitive measure to detect changes in the early stages of OA. While joint space narrowing was minimal at 7 years, the mean OARSI radiographic score and WORMS, which focus on the response of the whole joint, were different than controls.9 It is likely that joint space narrowing will follow given enough time. Graft type was not randomized which may introduce a selection bias. We attempted to control confounding variables by only including patients who were candidates for autograft, using the same instrumentation across surgeons, and standardizing the same postsurgical rehabilitation program. An additional limitation affecting the ancillary studies was that they were retrospective assessments as the questions were asked after data collection began. However, all ancillary analyses were performed with the investigators blinded to the subject and the procedure. Nonetheless, these ancillary studies provide insight into factors that affect outcomes and can serve as a platform for designing future prospective trials.
In conclusion, minimal differences between the two graft tensioning techniques, which bracket the extremes that would be acceptable to any surgeon, were found at 7 years. Furthermore, most of the outcome measures were not restored with ACLR at 7 years. We also found that the ACLR patients presenting with poor outcomes at 7 years, have specific anatomical features and pre-operative functional and psychosocial deficits that may place them at greater risk for PTOA.
Figure 7.
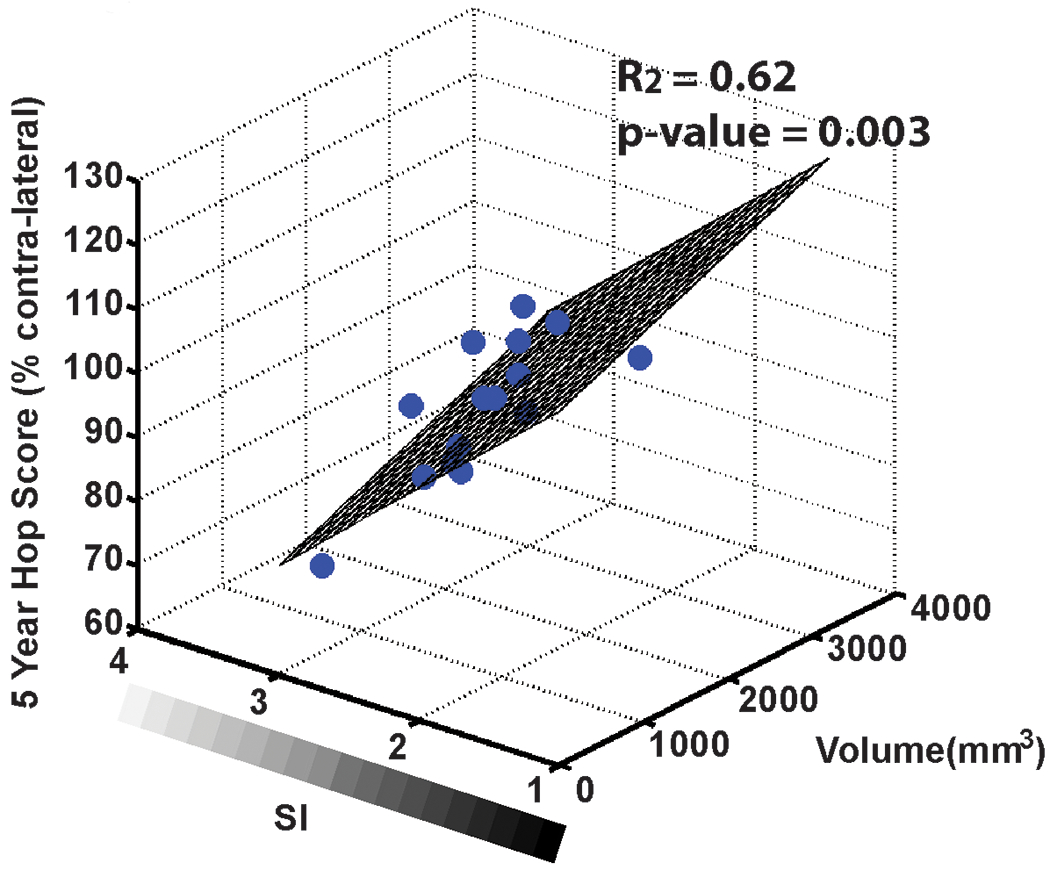
The patient prediction planes for hop score as a function of graft volume and median signal intensity (SI) at 5-year follow-up. Patients whose grafts had a higher volume and lower SI had higher functional hop scores.17
ACKNOWLEDGEMENTS
The project was made possible because of the dedicated work of the study team: Matthew A. Akelman, MD; Alison M. (Biercevicz) Chambers, PhD; Jillian E Beveridge, PhD; Steven F. DeFroda, MD; Cynthia Chrostek, BS; Orianna Duncan, MA; Meggin Q. Costa, BS; Khaled A. Elsaid, PhD; Stacy Faiola, PT, CSCS; Arlene Garcia BS; Holly C. Gil, MD; Gregory D. Jay, MD, PhD; Naga Padmini Karamchedu, MS; Ata M Kiapour, PhD; Martha M. Murray, MD; Heidi L. Oksendahl, MSPT; Brett D. Owens, MD; J. Kristopher Ware, MD; Melany A. Westwell, PT.
The research reported in this publication was supported by the National Institutes of Health (R01-AR047910, R01-AR047910S1, R01-AR065462, and R01-AR074973), the Lucy Lippitt Endowment, the RIH Orthopaedic Foundation, and the Boston Children's Hospital Orthopaedic Surgery Foundation.
Footnotes
DISCLOSURE
Dr. Fleming is a co-founder of Miach Orthopaedics, Inc, a start-up company aimed at translating a new ACL repair strategy to clinical use. He also served as a consultant for New York R&D Center for Translational Medicine and Therapeutics, Inc (2017). He received travel support to attend an academic conference from Smith & Nephew (2017). He also receives a stipend for his role as an associate editor for the American Journal of Sports Medicine (2013-present), and royalties from Springer Publishing for a book on the ACL (2013-present). None of these potential conflicts are directly related to the content of this manuscript.
REFERENCES
- 1.Ajuied A, Wong F, Smith C, et al. 2014. Anterior cruciate ligament injury and radiologic progression of knee osteoarthritis: A systematic review and meta-analysis. Am J Sports Med 42:2242–2252. [DOI] [PubMed] [Google Scholar]
- 2.Beveridge JE, Heard BJ, Shrive NG, et al. 2013. Tibiofemoral centroid velocity correlates more consistently with cartilage damage than does contact path length in two ovine models of stifle injury. J Orthop Res 31:1745–1756. [DOI] [PubMed] [Google Scholar]
- 3.Scanlan SF, Chaudhari AM, Dyrby CO, et al. 2010. Differences in tibial rotation during walking in ACL reconstructed and healthy contralateral knees. J Biomech 43:1817–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wellsandt E, Gardinier ES, Manal K, et al. 2016. Decreased knee joint loading associated with early knee osteoarthritis after anterior cruciate ligament injury. Am J Sports Med 44:143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brady MF, Fleming BC, Bradley MP, et al. 2007. Effects of initial graft tension on the tibiofemoral compressive forces and joint position following ACL reconstruction. Am J Sports Med 35:395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fleming BC, Brady MF, Bradley MP, et al. 2008. Tibiofemoral compression force differences using laxity- and force-based initial graft tensioning techniques in the anterior cruciate ligament-reconstructed cadaveric knee. Arthroscopy 24:1052–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haslauer CM, Elsaid KA, Fleming BC, et al. 2013. Loss of extracellular matrix from articular cartilage is mediated by the synovium and ligament after anterior cruciate ligament injury. Osteoarthritis Cartilage 21:1950–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elsaid KA, Fleming BC, Oksendahl HL, et al. 2008. Decreased lubricin concentrations and markers of joint inflammation in synovial fluids from patients with anterior cruciate ligament injury. Arthritis Rheum 58:1707–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akelman MR, Fadale PD, Hulstyn MJ, et al. 2016. Effect of matching or overconstraining knee laxity during anterior cruciate ligament reconstruction on knee osteoarthritis and clinical outcomes: A randomized controlled trial with 84-month follow-up. Am J Sports Med 44:1660–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleming BC, Fadale PD, Hulstyn MJ, et al. 2013. The effect of initial graft tension after anterior cruciate ligament reconstruction: A randomized clinical trial with 36-month follow-up. Am J Sports Med 41:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeFroda SF, Karamchedu NP, Budacki R, et al. 2020. Evaluation of graft tensioning effects in anterior cruciate ligament reconstruction between hamstring and bone-patellar tendon bone autografts. J Knee Surg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ware JK, Owens BD, Akelman MR, et al. 2018. Preoperative KOOS and SF-36 scores are associated with the development of symptomatic knee osteoarthritis at 7 years after anterior cruciate ligament reconstruction. Am J Sports Med 46:869–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiapour AM, Yang DS, Badger GJ, et al. 2019. Anatomical features of the tibial plateau predict outcomes of ACL reconstruction within 7 years after surgery. Am J Sports Med 47:303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeFroda SF, Karamchedu NP, Owens BD, et al. 2018. Tibial tunnel widening following anterior cruciate ligament reconstruction: A retrospective seven-year study evaluating the effects of initial graft tensioning and graft selection. The Knee 25:1107–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miranda DL, Fadale PD, Hulstyn MJ, et al. 2013. Knee biomechanics during a jump-cut maneuver: Effects of sex and ACL surgery. Med Sci Sports Exerc 45:942–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biercevicz AM, Miranda DL, Machan JT, et al. 2013. In situ, noninvasive, T2*-weighted MRI-derived parameters predict ex vivo structural properties of an anterior cruciate ligament reconstruction or bioenhanced primary repair in a porcine model. Am J Sports Med 41:560–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biercevicz AM, Akelman MR, Fadale PD, et al. 2015. MRI volume and signal intensity of ACL graft predict clinical, functional, and patient-oriented outcome measures after ACL reconstruction. Am J Sports Med 43:693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daniel DM, Stone ML, Sachs R, et al. 1985. Instrumented Measurement of anterior knee laxity in patients with acute anterior cruciate ligament disruption. Am J Sports Med 13:401–407. [DOI] [PubMed] [Google Scholar]
- 19.Irrgang JJ, Ho H, Harner CD, et al. 1998. Use of the International Knee Documentation Committee Guidelines to assess outcome following anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 6:107–114. [DOI] [PubMed] [Google Scholar]
- 20.Reinke EK, Spindler KP, Lorring D, et al. 2011. Hop tests correlate with IKDC and KOOS at minimum of 2 years after primary ACL reconstruction. Knee Surg Sports Traumatol Arthrosc 19:1806–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roos EM, Roos HP, Lohmander LS, et al. 1998. Knee Injury and Osteoarthritis Outcome Score (KOOS)--development of a self-administered outcome measure. J Orthop Sports Phys Ther 28:88–96. [DOI] [PubMed] [Google Scholar]
- 22.Ware JE, Sherbourne CD. 1992. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 30:473–483. [PubMed] [Google Scholar]
- 23.Englund M, Roos EM, Lohmander LS. 2003. Impact of type of meniscal tear on radiographic and symptomatic knee osteoarthritis: A sixteen-year followup of meniscectomy with matched controls. Arthritis Rheum 48:2178–2187. [DOI] [PubMed] [Google Scholar]
- 24.Wasserstein D, Huston LJ, Nwosu S, et al. 2015. KOOS pain as a marker for significant knee pain two and six years after primary ACL reconstruction: a Multicenter Orthopaedic Outcomes Network (MOON) prospective longitudinal cohort study. Osteoarthritis Cartilage 23:1674–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tegner Y, Lysholm J. 1985. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Rel Res 198:43–49. [PubMed] [Google Scholar]
- 26.Jones MH, Spindler KP, Fleming BC, et al. 2015. Meniscus treatment and age associated with narrower radiographic joint space width 2 - 3 years after ACL reconstruction: Data from the MOON onsite cohort. Osteoarthritis Cartilage 23:581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altman RD, Gold GE. 2007. Atlas of individual radiographic features in osteoarthritis, revised. Osteoarthritis Cartilage 15:A1–A56. [DOI] [PubMed] [Google Scholar]
- 28.Peterfy CG, Guermazi A, Zaim S, et al. 2004. Whole-organ magnetic resonance imaging score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage 12:177–190. [DOI] [PubMed] [Google Scholar]
- 29.Beynnon BD, Vacek PM, Sturnick DR, et al. 2014. Geometric profile of the tibial plateau cartilage surface is associated with the risk of non-contact anterior cruciate ligament injury. J Orthop Res 32:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levins JG, Sturnick DR, Argentieri EC, et al. 2016. Geometric risk factors associated with noncontact anterior cruciate ligament graft rupture. Am J Sports Med 44:2537–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salmon LJ, Heath E, Akrawi H, et al. 2018. 20-Year Outcomes of Anterior Cruciate Ligament Reconstruction With Hamstring Tendon Autograft: The Catastrophic Effect of Age and Posterior Tibial Slope. Am J Sports Med 46:531–543. [DOI] [PubMed] [Google Scholar]
- 32.Christensen JJ, Krych AJ, Engasser WM, et al. 2015. Lateral tibial posterior slope is increased in patients with early graft failure after anterior cruciate ligament reconstruction. Am J Sports Med 43:2510–2514. [DOI] [PubMed] [Google Scholar]
- 33.Sturnick DR, Argentieri EC, Vacek PM, et al. 2014. A decreased volume of the medial tibial spine is associated with an increased risk of suffering an anterior cruciate ligament injury for males but not females. J Orthop Res 32:1451–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whitney DC, Sturnick DR, Vacek PM, et al. 2014. Relationship between the risk of suffering a first-time noncontact ACL injury and geometry of the femoral notch and ACL: A prospective cohort study with a nested case-control analysis. Am J Sports Med 42:1796–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baumfeld JA, Diduch DR, Rubino LJ, et al. 2008. Tunnel widening following anterior cruciate ligament reconstruction using hamstring autograft: a comparison between double cross-pin and suspensory graft fixation. Knee Surg Sports Traumatol Arthrosc 16:1108–1113. [DOI] [PubMed] [Google Scholar]
- 36.Sabat D, Kundu K, Arora S, et al. 2011. Tunnel widening after anterior cruciate ligament reconstruction: a prospective randomized computed tomography--based study comparing 2 different femoral fixation methods for hamstring graft. Arthroscopy 27:776–783. [DOI] [PubMed] [Google Scholar]
- 37.Lind M, Feller J, Webster KE. 2009. Bone tunnel widening after anterior cruciate ligament reconstruction using EndoButton or EndoButton Continuous Loop. Arthroscopy 25:1275–1280. [DOI] [PubMed] [Google Scholar]
- 38.Teeple E, Elsaid KA, Fleming BC, et al. 2008. Coefficients of friction and cartilage damage in the guinea pig knee. J Orthop Res 26:231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miranda DL, Rainbow MJ, Crisco JJ, et al. 2013. Kinematic differences between optical motion capture and biplanar videoradiography during a jump-cut maneuver. J Biomech 46:567–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coats-Thomas MS, Miranda DL, Badger GJ, et al. 2013. Effects of ACL reconstruction surgery on muscle activity of the lower limb during a jump-cut maneuver in males and females. J Orthop Res 31:1890–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beveridge JE, Machan JT, Walsh EG, et al. 2018. Magnetic resonance measurements of tissue quantity and quality using T2 * relaxometry predict temporal changes in the biomechanical properties of the healing ACL. J Orthop Res 36:1701–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arneja S, McConkey MO, Mulpuri K, et al. 2009. Graft tensioning in anterior cruciate ligament reconstruction: a systematic review of randomized controlled trials. Arthroscopy 25:200–207. [DOI] [PubMed] [Google Scholar]
- 43.Kirwan GW, Bourke MG, Chipchase L, et al. 2013. Initial graft tension and the effect on postoperative patient functional outcomes in anterior cruciate ligament reconstruction. Arthroscopy 29:934–941. [DOI] [PubMed] [Google Scholar]
- 44.Murray MM, Fleming BC. 2013. Use of a bioactive scaffold to stimulate anterior cruciate ligament healing also minimizes posttraumatic osteoarthritis after surgery. Am J Sports Med 41:1762–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumar D, Su F, Wu D, et al. 2018. Frontal plane knee mechanics and early cartilage degeneration in people with anterior cruciate ligament reconstruction: A longitudinal study. Am J Sports Med 46:378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]


