Abstract
Background
Ginsenosides, which have strong biological activities, can be divided into polar or less-polar ginsenosides.
Methods
This study evaluated the phytochemical diversity of the saponins in Panax ginseng (PG) root, American ginseng (AG) root, and Panax notoginseng (NG) root; the stem-leaves from Panax ginseng (SPG) root, American ginseng (SAG) root, and Panax notoginseng (SNG) root as well as the saponins obtained following heating and acidification [transformed Panax ginseng (TPG), transformed American ginseng (TAG), transformed Panax notoginseng (TNG), transformed stem-leaves from Panax ginseng (TSPG), transformed stem-leaves from American ginseng (TSAG), and transformed stem-leaves from Panax notoginseng (TSNG)]. The diversity was determined through the simultaneous quantification of the 16 major ginsenosides.
Results
The content of ginsenosides in NG was found to be higher than those in AG and PG, and the content in SPG was greater than those in SNG and SAG. After transformation, the contents of polar ginsenosides in the raw saponins decreased, and contents of less-polar compounds increased. TNG had the highest levels of ginsenosides, which is consistent with the transformation of ginseng root. The contents of saponins in the stem-leaves were higher than those in the roots. The transformation rate of SNG was higher than those of the other samples, and the loss ratios of total ginsenosides from NG (6%) and SNG (4%) were the lowest among the tested materials. In addition to the conversion temperature, time, and pH, the crude protein content also affects the conversion to rare saponins. The proteins in Panax notoginseng allowed the highest conversion rate.
Conclusion
Thus, the industrial preparation of less-polar ginsenosides from SNG is more efficient and cheaper.
Keywords: acid transformation, less-polar ginsenosides, root ginsenosides, stem-leaf ginsenosides
Abbreviations: PG, Panax ginseng; AG, American ginseng; NG, Panax notoginseng; SPG, the stem-leaves from Panax ginseng; SAG, the stem-leaves from American ginseng; SNG, the stem-leaves from Panax notoginseng; TPG, transformed Panax ginseng; TAG, transformed American ginseng; TNG, transformed Panax notoginseng; TSPG, transformed stem-leaves from Panax ginseng; TSAG, transformed stem-leaves from American ginseng; TSNG, transformed stem-leaves from Panax notoginseng
1. Introduction
Members of the genus Panax, which includes three of the most important ginseng botanicals, American ginseng (Panax quinquefolius), Asian ginseng (Panax ginseng) and notoginseng (Panax notoginseng), are commonly used as adaptogens throughout the world [1]. Generally, the saponins in ginseng, termed ginsenosides, are thought to be pharmacologically active components. Ginsenosides are distributed in every part of Panax botanicals, such as their roots, fibrous roots, rhizomes, stems, leaves, flowers and fruit [[2], [3], [4]]. Moreover, the content of ginsenosides in the stem-leaves (3–6%) of Panax is higher than it in the roots (1–3%) [[4], [5], [6], [7]]. The saponin structure and content in ginseng stem-leaves and ginseng root are similar [8]. However, ginseng roots can be harvested every 4–6 years (2–3 years for notoginseng), but the stem-leaves can be harvested every year. Approximately 20.0 Mt of Asian ginseng stem-leaves, 7.7 Mt of American ginseng, and 3.3 Mt of notoginseng stem-leaves can be produced in China annually. Although stem-leaf ginseng has been classified as a medicine and food material in China, most ginseng is used for forage or discarded in the local environment.
Ginseng saponins can be divided into polar ginsenosides and less-polar ginsenosides [9]. Due to the lower natural abundances, less-polar ginsenosides are also known as rare saponins (include Rg3, Rg5, Rg6, Rk1, and Rh2) [9]. Because less-polar ginsenosides are easier to absorb and easily bind to cell membrane systems, some reports have confirmed that less-polar ginsenosides have stronger biological activities than their polar counterparts [[10], [11], [12], [13]]. Therefore, various physical and biological reactions, including via procedure involving microwave irradiation, vinegar, enzymes, endophytes and human intestinal bacteria, have been developed to transform saponins into less-polar ginsenosides [[14], [15], [16], [17], [18]]. Although the enzymatic reactions were selective, they were not suitable for industrial applications because of their poor productivity and high cost. Physical transformations involving heating (steaming and microwave) in a reactor are suitable for industrial applications [19,20].
Thermal and acidic treatment can promote the conversion of polar saponins to less-polar saponins compared to any single method [21]. Most of the current research focuses on changes in activity before and after saponin conversion, while ignoring the study of conversion materials. At present, the root of Panax is used to produce less-polar ginsenosides, but the stems and leaves of ginseng are rarely used. To screen the transformations of the different species and parts of Panax, we systematically analyzed the ginsenoside contents of these three herbs upon heating and treatment with acid, compared their loss rates and conversion rates, and explored the effects of the crude protein contents on saponin conversion. The data from this study could indicate the best raw materials for obtaining less-polar ginsenosides.
2. Materials and methods
2.1. Materials
The roots and stem-leaves of notoginseng [ Panax notoginseng (NG), stem-leaves from Panax notoginseng (SNG)] and the crude root saponins of notoginseng were purchased from Yunnan Kingtide Notoginseng Industry Co., Ltd., a subsidiary of Kunming Pharmaceutical Group Corporation, Ltd (Kunming, China). The roots and stem-leaves of American ginseng [American ginseng (AG), stem-leaves from American ginseng (SAG) and likewise the roots and stem-leaves of Panax ginseng (PG, SPG), and the crude root saponins of American ginseng and Panax ginseng were obtained from Jilin Hongju Biotechnology Co., Ltd. (Jilin, China). Acetonitrile and methanol were obtained from Merck (Darmstadt, Germany). Reference ginsenosides (R1, Re, Rg1, Rb1, Rc, Rb2, Rd, and Rh2 (>98% purity)) were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Ginsenosides Rg6, F4, Rk3, Rh4, 20(R)-Rg3, 20(S)-Rg3, Rk2, Rh3 and Rg5 (>95% purity) were isolated in our laboratory, and their structures were confirmed by hydrogen-1 (1H) and carbon-13 (13C) NMR spectroscopy and MS.
2.2. Sample preparation
The dry roots and stem-leaves of Panax were pulverized with a shredder and sifted through 60 mesh screens. The fine powders were mixed with 0.1% formic acid and heated in an MLS-3750 autoclave (SANYO, Osaka, Japan) at 140 °C for 2 h to obtain the heat-transformed saponins (TNG, TSNG, TAG, TSAG, TPG, and TSPG) [21]. The transformed saponins were dried in a blast drier at 60 °C for 2 h. A 20-fold volume of methanol was added to the heat-transformed saponins (v/w), and the mixtures were subjected to ultrasonic extraction for 40 min. The saponin extracts were stored at −20 °C until analysis.
2.3. Analysis of ginsenoside
HPLC analyses of the ginsenosides were performed on a SHIMADZU Prominence LC-20A HPLC instrument equipped with a UV detector (Shimadzu Corporation, Kyoto, Japan). Separations were performed on a YMC-Pack ODS-AM column (4.6 mm × 250 mm, YMC Co., Ltd., Kyoto, Japan). The UV detector wavelength was set at 202 nm. The mobile phase consisted of water (A) and acetonitrile (B). A previously reported gradient was used in the present study with slight modifications [22]. The flow rate was set to 1 mL/min, and the injection volumes were 60 μL and 20 μL for the root samples and stem-leaf samples, respectively.
2.4. Preparation of ginsenosides standard curves
The preparation of the ginsenoside standard curves and the recovery tests were performed by using a previously reported method [22,23]. Other standards, Rk2 (1.02 mg) and Rh3 (1.12 mg), were mixed with 70% (v/v) ethanol (1 mL). The spiked samples were analyzed in triplicate.
2.5. Loss ratio and conversion rate
The loss rate is the change in the total saponin content in the ginseng before and after transformation. The loss rates were calculated by using the following formula:
| Loss ratio%= (1- transformed saponins/total saponins) × 100% |
The conversion rate of the rare saponins indicates the percentage of ginseng rare saponins of the total ginsenosides remaining after the transformation reaction. The conversion rate was calculated by using the following formula:
| Conversion rate%= (less polar ginsenosides/total saponins) × 100% |
2.6. Determination of the total nitrogen and pH
The total nitrogen contents of the six samples were evaluated by the Kjeldahl method [24]. The pH of each sample was measured by using a pH meter.
2.7. Steaming of neutral root ginsenosides with crude protein
The ginsenosides from the roots of PG, AG and NG (5 mg) were dissolved in 4 mL of distilled water and mixed with 30, 40, 50, 60, 70, 80, and 90 μL of 1 mg/mL protein solution (6% to 18% protein). Formic acid (5 μL) was added to each sample, and then each sample was brought up to 5 mL with distilled water. The samples were steamed at 140 °C for 2 h using the same method as steam P. ginseng. R1 and Re were also steamed with proteins (6% to 18% protein in each sample) using the same method. When the heat processing was completed, the samples were freeze-dried. All the samples were re-dissolved in methanol and diluted to the desired concentration for HPLC analysis of the ginsenosides. The solutions were stored at 4 °C.
2.8. Statistical analysis
Data are presented as the mean ± standard deviation (SD) of at least three replicates, and the data were analyzed by SPSS software (IBM SPSS Statistics 17.0). Significance was determined at a p value of < 0.05 or < 0.01 by analysis of variance (ANOVA) followed by Duncan's multiple comparison tests.
3. Results and discussion
3.1. Ginsenoside profiles of the raw ginsenosides from the roots
The ginsenosides in Panax ginseng (PG), Panax notoginseng (NG), and American ginseng (AG) were identified by comparison with authentic standards and shown in Fig. 1A–C. The contents of the 16 most abundant ginsenoside monomers are presented in Table 1. The total contents of the ginsenosides were approximately 2.91 ± 0.23 mg/g in PG, 14.45 ± 1.02 mg/g in AG and 19.71 ± 0.93 mg/g in NG. PG contained three major types of polar ginsenosides, namely, Rb1 (0.53 ± 0.01 mg/g), Re/Rg1 (2.21 ± 0.18 mg/g) and Rb2 (0.36 ± 0.01 mg/g), and these species accounted for the majority of the total ginsenoside content. The kinds of ginsenosides identified were consistent with previous reports [25,26]; however, the contents were lower than those reported for white ginseng roots. These ginseng roots were harvested earlier in the season, which is likely responsible for this deviation [27,28]. Rb1 (7.87 ± 0.49 mg/g), Re/Rg1 (4.56 ± 0.37 mg/g), Rd (1.18 ± 0.12 mg/g) and Rb2 (0.84 ± 0.08 mg/g) were identified as the main components of American ginseng. There are five main components in NG: R1 (1.17 ± 0.08 mg/g), Re/Rg1 (8.34 ± 0.53 mg/g), Rb1 (7.6 ± 0.37 mg/g), Rd (1.17 ± 0.05 mg/g) and Rb2 (0.72 ± 0.01 mg/mg).
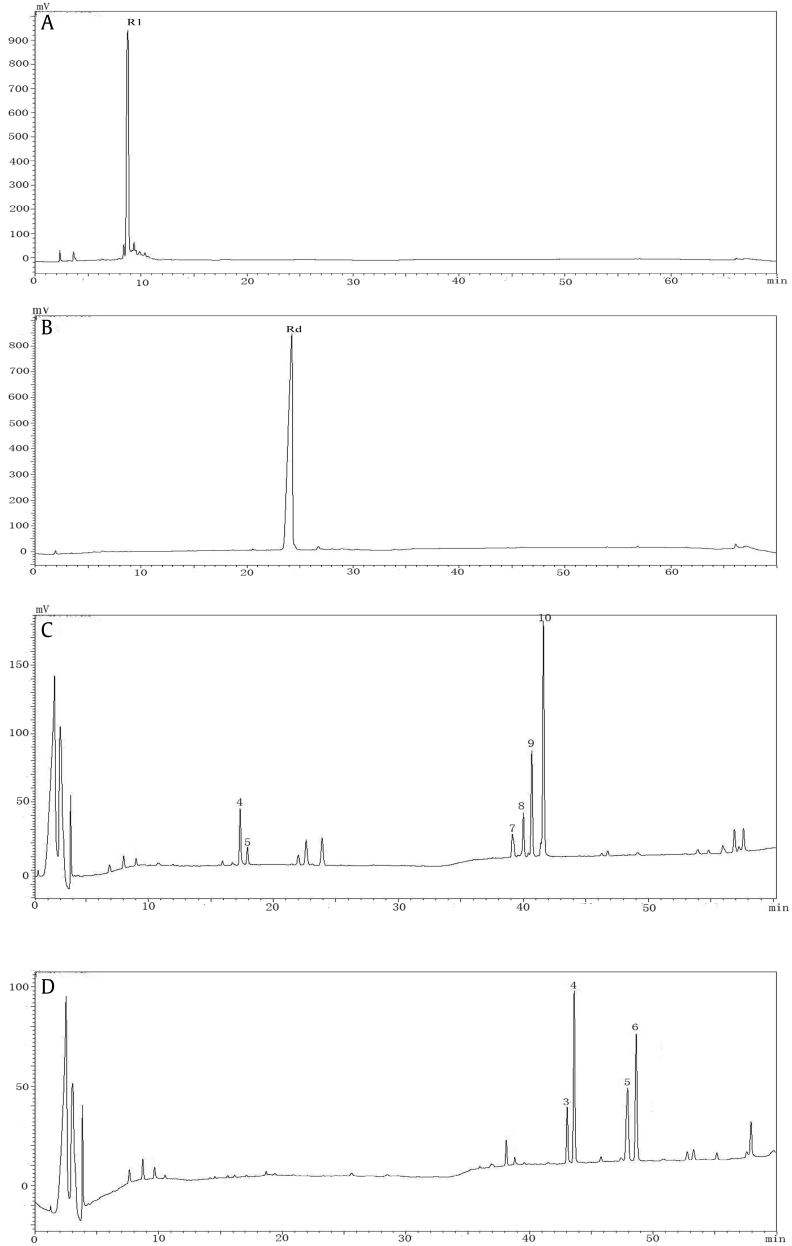
Fig. 1.
Chromatogram of Panax ginseng ginsenosides (PG, A), American ginseng saponins (AG, C), Notoginseng saponins (NG, B), and steamed ginseng at 130 °C for 4 h with 1% acid, transformed Panax ginseng ginsenosides (TPG, D), transformed American ginseng saponins (TAG, F), Notoginseng saponins (TNG, E); 1.R1 2. Rg1/Re 3.Rb1 4.Rb2 5. Rc 6. Rd 7. Rg6 8. F4 9. Rg3(S) 10. Rg3(R) 11. Rh4 12.Rk3 13. Rk1 14. Rg5 15. Rk2 16. Rh3.
Table 1.
Analytical characteristics of ginsenosides from roots (mg/g)
| Peak | RT | Calibration curve | R | PG | TPG | AG | TAG | NG | STNG |
|---|---|---|---|---|---|---|---|---|---|
| R1 | 9.013 | y = 659660x-29851 | 0.9973 | n.d | n.d | n.d | n.d | 1.17 ± 0.08 | n.d |
| Re(Rg1) | 10.387 | y = 481999x+93235 | 0.9985 | 2.21 ± 0.18 | n.d | 4.56 ± 0.37 | n.d | 8.34 ± 0.53 | n.d |
| Rb1 | 19.875 | y = 175257x+15971 | 0.9997 | 0.53 ± 0.01 | n.d | 7.87 ± 0.49 | 1.22 ± 0.09 | 7.6 ± 0.37 | n.d |
| Rb2 | 20.102 | y = 108262x+11323 | 0.9933 | 0.36 ± 0.01 | n.d | 0.84 ± 0.08 | n.d | 0.72 ± 0.01 | n.d |
| Rc | 20.645 | y = 172455x-32414 | 0.9945 | n.d | n.d | n.d | n.d | 0.12 ± 0.01 | 0.12 ± 0.01 |
| Rd | 24.543 | y = 271969x+427367 | 0.9837 | n.d | n.d | 1.18 ± 0.12 | 0.31 ± 0.01 | 1.17 ± 0.05 | 1.14 ± 0.03 |
| Rg6 | 38.751 | y = 719696x+188528 | 0.9982 | n.d | 0.18 ± 0.01 | n.d | 0.68 ± 0.01 | n.d | 0.86 ± 0.01 |
| F4 | 39.378 | y = 1E+06x-660748 | 0.9863 | n.d | 0.29 ± 0.01 | n.d | 0.55 ± 0.01 | n.d | 0.57 ± 0.01 |
| Rg3(S) | 41.631 | y = 177999x+62300 | 0.9926 | n.d | 0.15 ± 0.01 | n.d | 0.3 ± 0.01 | n.d | 2.68 ± 0.21 |
| Rg3(R) | 42.105 | y = 218319x-56590 | 0.9893 | n.d | 0.15 ± 0.01 | n.d | 1.06 ± 0.03 | n.d | 3.25 ± 0.32 |
| Rh4 | 42.168 | y = 774675x+146497 | 0.9978 | n.d | n.d | n.d | 0.45 ± 0.01 | n.d | 0.78 ± 0.02 |
| Rk3 | 43.168 | y = 774675x+146497 | 0.9978 | n.d | n.d | n.d | 0.21 ± 0.01 | n.d | 0.32 ± 0.02 |
| Rk1 | 44.734 | y = 73597x+34029 | 0.9944 | n.d | n.d | n.d | 1.38 ± 0.04 | n.d | n.d |
| Rg5 | 45.895 | y = 73597x+34029 | 0.9944 | n.d | n.d | n.d | n.d | n.d | 3.42 ± 0.37 |
| Rk2 | 48.011 | y = 218319x-56590 | 0.9893 | n.d | 0.12 ± 0.01 | n.d | 2.31 ± 0.12 | n.d | 2.78 ± 0.18 |
| Rh3 | 48.653 | y = 218319x-56590 | 0.9893 | n.d | 0.15 ± 0.01 | n.d | 2.72 ± 0.11 | n.d | 2.53 ±0.41 |
| total | 2.91 ± 0.23 | 1.16 ± 0.02 | 14.45 ± 1.02 | 10.98 ± 0.68 | 19.71 ± 0.93 | 18.43 ± 0.12 |
Consistent with the literature [25], the ratio of Rb1 (0.20 ± 0.02 mg/g) to Rg1 (0.08 ± 0.01 mg/g) was 2.5 in PG but 10.5 in AG. According to a previous report, the Rb1/Rg1 ratio in PG is usually between 1 and 3, while Rb1/Rg1 values of approximately 10 or greater are characteristic of AG [29]. With the popularity of mass spectrometry, some trace markers such as Rf, p-F11 and Rs1 can also be used for differentiating PG from PQ [30]. Notoginsenoside R1 was only observed in NG.
3.2. Ginsenoside profiles of the three transformed ginseng roots
Typical HPLC chromatograms of the transformed Panax ginseng roots (TPG), notoginseng roots (TNG), and transformed American ginseng root (TAG) are shown in Fig. 2D–F, respectively, and the 16 most abundant ginsenosides in the transformed ginseng roots are summarized in Table 1. The optimized transformation method involved a temperature, time, and concentration of formic acid of 120 °C, 4 h and 0.01%, respectively [21]. The total ginsenoside contents were approximately 1.16 ± 0.02 mg/g in steamed PG, 10.98 ± 0.68 mg/g in steamed AG, and 18.43 ± 0.12 mg/g in steamed NG. Treatment with steam and acid degrades the natural ginsenosides via deglycosylation and dehydration and causes the ginsenoside content to change dramatically between the natural and transformed ginseng extracts. The results shown in Fig. 2 and Table 1 indicate that the contents of the most abundant ginsenosides in raw ginseng, such as Rg1, Re, Rb1, Rb2, and Rd, significantly decreased, while the contents of less-polar ginsenosides were significantly (p < 0.05) greater in the transformed saponins.
Fig. 2.
Chromatogram of stem-leaves from Panax ginseng ginsenosides (SPG, A), American ginseng saponins (SAG, C), Notoginseng saponins (SNG, B) and transformed stem-leaves from Panax ginseng ginsenosides (STPG, D), American ginseng saponins (STAG, F), Notoginseng saponins (STNG, E), 1.R1 2. Rg1/Re 3.Rb1 4.Rb2 5. Rd 6. unknown 7. Rg6 8. F4 9. Rg3(S) 10. Rg3(R) 11. Rk1 12. Rg5 13. Rk2 14. Rh3.
Protopanaxatriol ginsenoside Re can be hydrolyzed to the ginsenosides Rk3, Rh4, Rg2, Rg6, and F4. Protopanaxadiol ginsenosides Rb2 and Rd can be converted into ginsenoside Rg3. Ginsenoside Rg3 can be converted into ginsenosides Rk1 and Rg5 by dehydration [29]. Overall, with the exception of PG, the content of transformed saponins accounted for more than 50% of the total ginsenoside content.
3.3. Ginsenoside profiles of raw ginsenosides in stem-leaves
As shown in Fig. 2, under chromatographic conditions, a total of 14 peaks in the HPLC chromatograms of the three stem-leaf samples were tentatively assigned. These saponins were identified by comparing their retention times with those of the peaks of the standard compounds. The fourteen ginsenosides from the six samples of stem-leaves of Panax are summarized in Table 2. Based on the identified saponins, SPG (22.56 ± 1.23 mg/g) had the highest content of total ginsenosides, whereas SAG (11.32 ± 0.81 mg/g) and SNG (10.68 ± 0.97 mg/g) had the lowest contents. SPG contained four major ginsenosides, namely, Rg1/Re (14.73 ± 0.84 mg/g), Rb1 (2.45 ± 0.13 mg/g), Rb2 (2.34 ± 0.11 mg/g), and Rd (3.04 ± 0.17 mg/g). Rg1/Re (5.66 ± 0.27 mg/g), Rb2 (2.54 ± 0.11 mg/g) and Rd (1.73 ± 0.07 mg/g) were less abundant in SAG than they were in SPG, and notoginsenoside R1 (1.59 ± 0.03 mg/g) was only detected in SNG. The major ginsenosides, including Rb1, Rd and Rb2, in the roots were all detected in the extracts of stem-leaves as well.
Table 2.
Analytical characteristics of ginsenosides from stem-leaves.(mg/g)
| No | RT | Ginsenoside | SPG | TSPG | SAG | TSAG | SNG | TSNG |
|---|---|---|---|---|---|---|---|---|
| 1 | 9.012 | R1 | n.d | n.d | n.d | n.d | 1.59 ± 0.03 | n.d |
| 2 | 10.176 | Re(Rg1) | 14.73 ± 0.84 | n.d | 5.66 ± 0.27 | n.d | n.d | n.d |
| 3 | 19.831 | Rb1 | 2.45 ± 0.13 | n.d | n.d | n.d | 3.35 ± 0.11 | n.d |
| 4 | 20.156 | Rb2 | 2.34 ± 0.11 | 0.42 ± 0.01 | 2.54 ± 0.11 | 0.12 ± 0.01 | 5.49 ± 0.25 | n.d |
| 5 | 24.714 | Rd | 3.04 ± 0.17 | 0.35 ± 0.01 | 1.73 ± 0.07 | 0.11 ± 0.01 | n.d | n.d |
| 6 | 37.639 | unknown | n.d | 3.45 ± 0.27 | n.d | 0.95 ± 0.02 | n.d | n.d |
| 7 | 38.275 | Rg6 | n.d | 3.00 ± 0.19 | n.d | 0.82 ± 0.02 | n.d | 0.87 ± 0.01 |
| 8 | 39.252 | F4 | n.d | 4.51 ± 0.32 | n.d | 0.91 ± 0.02 | n.d | 0.96 ± 0.01 |
| 9 | 40.687 | Rg3(S) | n.d | 1.7 ± 0.08 | n.d | 1.08 ± 0.03 | n.d | 1.59 ± 0.04 |
| 10 | 41.26 | Rg3(R) | n.d | 1.00 ± 0.04 | n.d | 1 ± 0.03 | n.d | 0.8 ± 0.01 |
| 11 | 44.539 | Rk1 | n.d | 0.97 ± 0.02 | n.d | n.d | n.d | 1.13 ± 0.03 |
| 12 | 45.141 | Rg5 | n.d | 0.84 ± 0.01 | n.d | n.d | n.d | 0.11 ± 0.01 |
| 13 | 46.864 | Rk2 | n.d | 3.12 ± 0.21 | n.d | 2.57 ± 0.11 | n.d | 2.5 ± 0.18 |
| 14 | 47.634 | Rh3 | n.d | 3.02 ± 0.18 | 1.37 ± 0.06 | 2.89 ± 0.12 | n.d | 2.86 ± 0.17 |
| total | 22.56 ± 1.23 | 22.01 ± 1.21 | 11.32 ± 0.81 | 10.43 ± 0.98 | 10.68 ± 0.97 | 10.14 ± 0.80 |
Consistent with previous reports, the total amount of ginsenosides in ginseng stem-leaves was higher than that in roots [31]. In fact, Panax species have been cultivated for many years throughout the world, including in China, America, Canada and South Korea, and different species have different contents and types of active substances, especially in their stem-leaves [27,28]. Because of their possession of high contents of ginsenosides, stem-leaves are an important resource for obtaining these compounds.
3.4. Ginsenoside profiles of the three samples of transformed Panax stem-leaves
As shown in Fig. 2D–F, the HPLC profiles of the three samples of Panax stem-leaves were distinctly different from the chromatograms shown in Fig. 2A–C. Similar to the changes observed in the ginseng root samples, the contents of ginsenosides R1, Re/Rg1, Rb1, Rb2 and Rd decreased significantly, while the contents of less-polar ginsenosides were significantly (p < 0.05) higher in the transformed saponins. This phenomenon indicates that the transformations of ginsenosides in the roots and stem-leaves may be similar.
The total contents of ginsenosides were approximately 22.01 ± 1.21 mg/g in TSPG, 10.43 ± 0.98 mg/g in TSAG and 10.14 ± 0.81 mg/g in TSNG. Ginsenosides Rg6, F4, Rh4, Rg3(S), Rg3(R), Rk1, Rg5, Rk2, and Rh3 were the major saponins, and their contents in TSPG were 3.00 ± 0.19 mg/g, 4.51 ± 0.32 mg/g, 1.7 ± 0.08 mg/g, 1.00 ± 0.04 mg/g, 0.97 ± 0.02 mg/g, 0.84 ± 0.01 mg/g, 3.12 ± 0.21 mg/g, and 3.02 ± 0.18 mg/g, respectively. TSAG had higher contents of ginsenosides Rk1, Rg5, Rk2 and Rh3 than Rg6 and F4. Notably, SNG also contained more of the less-polar ginsenosides F4, Rg6, Rg3(S), Rg3(R), Rk1, Rg5, Rk2 and Rh3 after thermal degradation. Many studies have confirmed that the components in ginseng roots and stems-leaves are essentially the same even if their contents were different [28]. However, there is almost no research on the transformations of stem-leaf saponins from Panax species. The major ginsenosides in SAG and PG have been successfully degraded into less-polar ginsenosides by heating [22,32]. Consistent with the transformations observed for SAG, SPG and SNG can also be converted into rare saponins.
3.5. Degradation mechanism
The mechanism by which the major ginsenosides are degraded into less polar ginsenosides was then investigated. The HPLC chromatograms of degraded ginsenoside standards R1 and Rd are shown in Fig. 3(A-D) and Fig. 4. Consistent with a previous report [21,32], protopanaxatriol-type ginsenoside R1 was dehydrated to form Rg2 under heating and acid treatment and further dehydrated to form F4/Rg6 and then degraded into Rk3/Rh4. Protopanaxatriol-type ginsenoside Rd was hydrolyzed by one molecule of water and sugar into Rg3(S/R), which was hydrolyzed to ginsenoside Rg5/Rk1 and eventually degraded into Rk2/Rh3.
Fig. 3.
Chromatogram of reference substance ginsenoside-R1(A) and ginsenoside-Rd, and transformed ginsenoside-R1(C) and transformed ginsenoside-Rd (D); 1. F4 2. Rg6 3. Rk1 4. Rg5 5. Rk2 6. Rh3.
Fig. 4.
Hydrolysis processes from ginsenoside-R1 and Rd to less-polar ginsenosides. A. Hydrolysis process of ginsenoside-R1to Rg2,F4 and Rg6; B. Hydrolysis process of ginsenoside-Rd to Rg3, Rk1 and Rh5.
Unlike what has been reported previously [33], the vast majority of ginsenoside Rd is converted into Rh2, and this polar ginsenoside can be converted into specific less-polar ginsenosides, including Rg3, which can be derived from Rd or Rc. In chemical transformations, each type of ginsenoside is degraded into 2–4 byproducts, leading to lower yields of the target products than can be achieved with biotransformations [34,35]. The principal reason for this difference was that thermal conversion is affected by various factors, such as time, temperature, and pH [21].
In the kinetic study of the conversion of ginsenoside Rb1 to Rg3, the temperature is inversely proportional to the heating time. The conversion conditions for most ginsenosides range from 90 °C to 160 °C, with corresponding heating times ranging from 0.5 h to 30 h[36], [37], [38]. Within 130 °C, the conversion rate and extraction rate increased with increasing temperature, but the crude saponin content decreased slightly above 130 °C [32]. Ginsenosides are difficult to degrade under heating in neutral conditions, and hydrolysis reactions can be initiated under acidic or basic conditions [39,40]. The reason why ginsenosides in natural plants are degraded under heating conditions is that they contain amino acids, proteins, and organic acids [38,41]. Meanwhile, the conversion results were only related to pH other than certain kind of acids [42]. Therefore, in order to promote the degradation of ginsenosides, organic acids, amino acids, and inorganic acids are added to maintain the pH level of about 5 [21,40,43].
Although the conversion process is affected by many factors, scientists are still trying to systematically optimize these factors to increase the yield of target compounds. The yield of Rg5 in ginseng extract can be significantly increased through heating at 160 °C for 30 min [44]. In addition, 25% acetic acid hydrolyzes saponins in stems and leaves from Panax notoginseng can significantly increase the yield of the desirable compound notoginsenoside Ft145. These researches provide perfect theoretical support for the industrial production of target ginsenosides.
3.6. Yields of less-polar ginsenosides
The yields of less-polar ginsenosides, as well as the conversion rates and saponin loss rates, are shown in Table 3. The conversion rates from the roots of Panax species were significantly different; the conversion rate of SNG was the highest (conversion rate of 95%), and the conversion rate of PG was the lowest (40%). Notably, the saponin conversion rates in the stem-leaves of Panax species were higher than 80%. The conversion rates of the various samples from high to low were in the order SNG > NG > SAG > SPG > AG > PG. The loss rates of the various samples from high to low were in the order PG > AG > SAG > NG > SNG > SPG. However, the ginsenoside standards were not completely degraded into less-polar ginsenosides. The differences in conversion may be due to the high contents of organic acids in the stem-leaves, as these acids promote the conversion of saponins [29,39]. Under optimal conditions, the biotransformation rate of the C–K in Panax ginseng is 66.34% [46]. Unfortunately, although rare saponins have important antibacterial activities, the concentration of saponins in the fermentation broth is very low, making this method unsuitable for industrial production [22,23]. Although the conversion rate of degrading Rb1 to C–K is as high as 90%, it may take more than 2 weeks to culture the fungi and transforming materials, which takes longer time than thermal conversion [47].
Table 3.
Transformation rate and loss ratio of less polar ginsenosides from Panax
| Sample | Total saponins mg/g | Transformed saponins mg/g | Less polar ginsenosides mg/g | Loss ratio % | Conversion rate % |
|---|---|---|---|---|---|
| PG | 2.91 ± 0.23 | 1.16 ± 0.02 | 1.16 ± 0.02 | 60 | 40 |
| AG | 14.45 ± 1.02 | 10.98 ± 0.68 | 9.75 ± 0.54 | 24 | 65 |
| NG | 19.71 ± 0.93 | 18.43 ± 0.12 | 17.29 ± 0.68 | 6 | 88 |
| SPG | 22.56 ± 1.23 | 22.01 ± 1.21 | 18.32 ± 1.15 | 3 | 82 |
| SAG | 11.32 ± 0.81 | 10.43 ± 0.98 | 9.73 ± 0.76 | 8 | 86 |
| SNG | 10.68 ± 0.97 | 10.14 ± 0.80 | 10.14 ± 0.80 | 5 | 95 |
| R1 | 1.2 | 1.1 | 0.82 | 32 | 68 |
| Rd | 1.1 | 1 | 0.93 | 16 | 84 |
In fresh ginseng roots, in addition to neutral saponins, there are also a large number of acidic saponins, which are chemically unstable and difficult to prepare, and thus are easily ignored by researchers [39]. Because the conversion of some ginsenosides are low, the detector cannot measure, which may be one of the reasons for the high rate of ginsenosides loss. Most studies have shown that after a temperature of more than 130 °C, the content of saponin is reduced, mainly because acidic saponins may be noncovalently bonded to a part of amino acids and protein compounds to form precipitates [48,49]. In order to explore the effect of ginseng protein on the loss rate of saponin, the verification of the action of crude saponin and protein was carried out.
3.7. Total nitrogen and pH
The total nitrogen and pH of the Panax samples are shown in Fig. 5(a and b). As shown in Fig. 5(a), the pH of each sample was approximately 5.2, and there were no significant differences among the pH values primarily because 1% acetic acid was added before the conversion. The steam temperature, time, and pH have remarkable impacts on the ginsenoside conversion from fresh ginseng to rare ginseng [19,39,50]. During the conversion process, these parameters are fixed, but variations in these parameters can cause significant differences. Notably, there is a significant difference in the crude protein contents of these samples. The protein contents from high to low were in the order PG (15.67% ± 0.16) > SPG (12.78% ± 0.74) > SNG (10.63% ± 0.13) > SAG (8.31% ± 0.13) > NG (8.13% ± 0.1) > AG (6.83% ± 0.53). The protein content is related to the conversion rate to less-polar ginsenosides.
Fig. 5.
The histogram of the protein content (a), pH (b) in six ginseng samples; the saponin conversion rate of PG, AG, NG(c).
3.8. Steaming of neutral root ginsenosides with protein
The conversion rates of the various neutral root ginsenosides with protein are shown in Fig. 5(c). As the concentration of protein increased, the conversion rate of PG first increased from 70% to 93.5% (with 10% added protein) and then decreased to 72% (with 16% added protein). Consistent with the results of PG, the conversion rate of AG increased from 78.2% to 94% and then decreased to 72.5%. The decomposition of a small amount of protein into amino acids promotes the degradation of the saponins [51,52]. The presence of amino acids, especially aspartate, increases the conversion of ginsenosides. When the protein content exceeds 14%, heating may denature the protein, and steric hindrance will limit hydrolysis of the saponins. Interestingly, the conversion rate of NG remained stable at approximately 92% regardless of the protein content. This consistency is mainly because Panax notoginseng contains a single type of ginsenoside, and the protein has little effect on the hydrolysis of that saponin. Thermal degradation sites are not selective and prone to chiral structures, resulting in the inability to produce a single type of compound [11,13,18]. However, system optimization of temperature, time and pH can significantly increase the yield of target compounds [44,45].
4. Conclusions
In conclusion, the present study compares different ginseng species, both raw and transformed samples, through the simultaneous quantification of 18 major ginsenosides using HPLC-UV analysis. The profiles of unique, less-polar ginsenosides were evaluated after steaming and acid treatment. Because leaf material is accessible throughout the entire lifespan of the plant, using leaf-derived ginsenosides instead of root-derived ginsenosides would be advantageous for preparing rare ginseng saponins from Panax species.
Despite the low specificity of this chemical transformation, the overall conversion rate to less-polar ginsenosides is considerable. In addition, thermal degradation leads to the generation of isomers, which complicate subsequent purification steps but provide more research objects for scientists. Some scientists have also been working hard to systematically optimize the conversion parameters to obtain the desirable products. Limited to the diversity of natural plant saponins, even in biotransformation, the diversity of ginsenosides in raw materials is not suitable for the acquisition of single saponin type.
The presence of protein affects the conversion of the saponins because the protein can be hydrolyzed into amino acids, which promote saponin degradation, and the protein can be denatured, which inhibits saponin hydrolysis. However, the effect of protein on Panax notoginseng is limited.
Taken together, thermal conversion is still a simple and effective method for obtaining rare ginseng saponins. Stems-leaves are a very economical alternative to ginseng roots. Our next focus is to reduce the formation of byproducts and increase the yield of target products.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This work was supported by the Doctoral research start-up funds of project No.ZR2019PH043 supported by Shandong Provincial Natural Science Foundation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jgr.2020.01.003.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Baeg I.H., So S.H. The world ginseng market and the ginseng (Korea) J. Ginseng Res. 2013;37:1–7. doi: 10.5142/jgr.2013.37.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wan J.B., Zhang Q.W., Hong S.J., Li P., Li S.P., Wang Y.T. Chemical investigation of saponins in different parts of Panax notoginseng by pressurized liquid extraction and liquid chromatography-electrospray ionization-tandem mass spectrometry. Molecules. 2012;17:5836–5853. doi: 10.3390/molecules17055836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang C.Z., Wu J.A., Mcentee E., Yuan C.S. Saponins composition in American ginseng leaf and berry assayed by high-performance liquid chromatography. J. Agric. Food. Chem. 2006;54:2261–2266. doi: 10.1021/jf052993w. [DOI] [PubMed] [Google Scholar]
- 4.Lee J.W., Choi B.R., Kim Y.C., Choi D.J., Lee Y.S., Kim G.S., Baek N.I., Kim S.Y., Lee D.Y. Comprehensive profiling and quantification of ginsenosides in the root, stem, leaf, and berry of Panax ginseng by UPLC-QTOF/MS. Molecules. 2017;22:2147–2159. doi: 10.3390/molecules22122147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang J.R., Yau L.F., Gao W.N., Liu Y., Yick P.W., Liu L., Jiang Z.H. Quantitative comparison and metabolite profiling of saponins in different parts of the root of Panax notoginseng. J. Agric. Food Chem. 2014;62:9024–9034. doi: 10.1021/jf502214x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xi M.F., Liu C.M., Li F.M., Zhou-Fang P.U., Liu Y.X., Zhao W.X. Determination of content of total saponins in Panpax Notoginseng roots and leaves by aqueous two phase system. J. Jilin Med. Coll. 2010;31:24–27. [Google Scholar]
- 7.Qu C.L., Bai Y.P., Jin X.Q., Wang Y.T., Zhang K., You J.Y. Study on ginsenosides in different parts and ages of Panax quinquefolius L. Food Chem. 2009;115:340–346. [Google Scholar]
- 8.Li F.L. Pharmacology effect of ginsenosides from stems and leaves of Panax ginseng were same like ginsenosides from roots. Guizhou Agrl. Sci. 2013;41:54–57. [Google Scholar]
- 9.Kwon S.W., Han S.B., Park I.H., Kim J.M., Park M.K., Park J.H. Liquid chromatographic determination of less polar ginsenosides in processed ginseng. J. Chromatogr. A. 2001;921:335–339. doi: 10.1016/s0021-9673(01)00869-x. [DOI] [PubMed] [Google Scholar]
- 10.Le T.H.V., Lee S.Y., Lee G.J., Nguyen N.K., Nguyen M.D. Effects of steaming on saponin compositions and anti-proliferative activity of Vietnamese ginseng. J. Ginseng Res. 2015;35(3):274–278. doi: 10.1016/j.jgr.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim S.J., Kim A.K. Anti-breast cancer activity of fine black ginseng (Panax ginseng, Meyer) and ginsenoside Rg5. J. Ginseng Res. 2015;39:125–134. doi: 10.1016/j.jgr.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee M.R., Yun B.S., Sung C.K. Comparative study of white and steamed black Panax ginseng, P. quinquefolium, and P. notoginseng on cholinesterase inhibitory and antioxidative activity. J. Ginseng Res. 2012;36:93–101. doi: 10.5142/jgr.2012.36.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duan Z., Deng J., Dong Y., Zhu C., Li W., Fan D. Anticancer effects of ginsenoside Rk3 on non-small cell lung cancer cells: in vitro and in vivo. Food Funct. 2017 doi: 10.1039/c7fo00385d. [DOI] [PubMed] [Google Scholar]
- 14.Kim S.J., Kim J.D., Ko S.K. Changes in ginsenoside composition of ginseng berry extracts after a microwave and vinegar process. J. Ginseng Res. 2013;37:269–272. doi: 10.5142/jgr.2013.37.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo S.L., Dang L.Z., Li J.F., Zou C.G., Zhang K.Q., Li G.H. Biotransformation of saponins by endophytes isolated from Panax notoginseng. Chem. Biodivers. 2013;10:2021–2031. doi: 10.1002/cbdv.201300005. [DOI] [PubMed] [Google Scholar]
- 16.Du J., Cui C.H., Park S.C., Kim J.K., Yu H.S., Jin F.X., Sun C., Kim S.C., Im W.T. Identification and characterization of a ginsenoside-transforming β-glucosidase from Pseudonocardia sp. Gsoil 1536 and its application for enhanced production of minor ginsenoside Rg2(S) PLOS One. 2014;9 doi: 10.1371/journal.pone.0096914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huq M.A., Siraj F.M., Kim Y., Yang D. Enzymatic transformation of ginseng leaf saponin by recombinant β-glucosidase (bgp1) and its efficacy in an adipocyte cell line. Biotechnol. Appl. Bioc. 2015;63:532–538. doi: 10.1002/bab.1400. [DOI] [PubMed] [Google Scholar]
- 18.Wan J.Y., Liu P., Wang H.Y., Qi L.W., Wang C.Z., Li P., Yuan C.S. Biotransformation and metabolic profile of American ginseng saponins with human intestinal microflora by liquid chromatography quadrupole time-of-flight mass spectrometry. J. Chromatogr. A. 2013;1286:83–92. doi: 10.1016/j.chroma.2013.02.053. [DOI] [PubMed] [Google Scholar]
- 19.Xu X.F., Gao Y., Xu S.Y., Liu H., Xue X., Zhang Y., Zhang H., Liu M.N., Xiong H., Lin R.C. Remarkable impact of steam temperature on ginsenosides transformation from fresh ginseng to red ginseng. J. Ginseng Res. 2018;42:277–287. doi: 10.1016/j.jgr.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu F., Ma N., Xia F.B., Li P., He C., Wu Z. Preparative separation of minor saponins from Panax notoginseng leaves using biotransformation, macroporous resins and preparative high-performance liquid chromatography. J. Ginseng Res. 2017 doi: 10.1016/j.jgr.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quan K., Liu Q., Wan J.Y., Zhao Y.J., Guo R.Z., Alolga R.N., Li P., Qi L.W. Rapid preparation of rare ginsenosides by acid transformation and their structure-activity relationships against cancer cells. Sci. Rep. 2015;5:85–98. doi: 10.1038/srep08598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xue P., Yang X., Sun X., Ren G.X. Antifungal activity and mechanism of heat-transformed ginsenosides from notoginseng against Epidermophyton floccosum, Trichophyton rubrum, and Trichophyton mentagrophyte. Rsc. Adv. 2017;7:10939–10946. [Google Scholar]
- 23.Xue P., Yao Y., Yang X.S., Feng J., Ren G.X. Improved antimicrobial effect of ginseng extract by heat transformation. J. Ginseng Res. 2017;41:180–186. doi: 10.1016/j.jgr.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamizake N.K.K., Gonçalves M.M., Zaia C.T.B.V., Zaia D.A.M. Determination of total proteins in cow milk powder samples: a comparative study between the Kjeldahl method and spectrophotometric methods. J. Food Compost. Anal. 2003;16:507–516. [Google Scholar]
- 25.Sun S., Qi L.W., Du G.J., Mehendale S.R., Wang C.Z., Yuan C.S. Red notoginseng: higher ginsenoside content and stronger anticancer potential than Asian and American ginseng. Food Chem. 2011;125:1299–1305. doi: 10.1016/j.foodchem.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiu Y., Li X., Sun X.L., Xiao D., Miao R., Zhao H.X., Liu S.Y. Simultaneous determination and difference evaluation of 14 ginsenosides in Panax ginseng, roots cultivated in different areas and ages by HPLC-MRM/MS combined with multivariate statistical analysis. J. Ginseng Res. 2017 doi: 10.1016/j.jgr.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bai H., Wang S., Liu J., Gao D., Cai Z. Localization of ginsenosides in panax ginseng with different age by matrix-assisted laser-desorption/ionization time-of-flight mass spectrometry imaging. J Chromatography. B. 2015;1026:263–271. doi: 10.1016/j.jchromb.2015.09.024. [DOI] [PubMed] [Google Scholar]
- 28.Wang S., Bai H., Cai Z., Gao D., Liu H. Maldi imaging for the localization of saponins in root tissues and rapid differentiation of three panax herbs. Electrophoresis. 2016;37:1956–1966. doi: 10.1002/elps.201600027. [DOI] [PubMed] [Google Scholar]
- 29.Yang W., Qiao X., Li K., Fan J., Bo T., Guo D.A., Ye M. Identification and differentiation of panax ginseng, panax quinquefolium, and panax notoginseng by monitoring multiple diagnostic chemical markers. Acta Pharm. Sin. B. 2016;6:568–575. doi: 10.1016/j.apsb.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jang G.Y., Min Y.K., Lee Y.J. Influence of organic acids and heat treatment on ginsenoside conversion. J. Ginseng Res. 2018 doi: 10.1016/j.jgr.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang C.X., Bao J.C., Li X.G., Zheng X.G. HPLC determination of the amount of ginsenosides in different part of Panax ginseng C.A.Mey. and P. quinquefolius L. and P. notoginseng (burk) F.H.Che. Chin J Pharm Anal. 2005;25:1190–1194. [Google Scholar]
- 32.Hwang C.R., Lee S.H., Jang G.Y., Hwang I.G., Kim H.Y., Woo K.S., Lee J., Jeon H.S. Changes in ginsenoside compositions and antioxidant activities of hydroponic-cultured ginseng roots and leaves with heating temperature. J. Ginseng Res. 2014;38:180–186. doi: 10.1016/j.jgr.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Popovich D.G., Kitts D.D. Generation of ginsenosides Rg3 and Rh2 from North American ginseng. Phytochemistry. 2004;65:337–344. doi: 10.1016/j.phytochem.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 34.Ma L.Y., Zhou Q.L., Yang X.B., Wang H.P., Yang X.W. Metabolism of 20(S)-Ginsenoside Rg2 by rat liver microsomes: bioactivation to SIRT1-activating metabolites. Molecules. 2016;21:757–772. doi: 10.3390/molecules21060757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim J.K., Cui C.H., Yoon M.H., Kim S.C., Im W.T. Bioconversion of major ginsenosides Rg1, to minor ginsenoside F1, using novel recombinant ginsenoside hydrolyzing glycosidase cloned from Sanguibacter keddieii, and enzyme characterization. J. Biotechnol. 2012;161:294–301. doi: 10.1016/j.jbiotec.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 36.Jo S.K., Kim I.S., Yoon K.S., Yoon H.H., Yoo H.H. Preparation of ginsenosides Rg3, Rk1, and Rg5-selectively enriched ginsengs by a simple steaming process. Eur. Food Res. Technol. 2015;240:251–256. [Google Scholar]
- 37.Vo H.T., Cho J.Y., Choi Y.E., Choi Y.S., Jeong Y.H. Kinetic study for the optimization of ginsenoside Rg3 production by heat treatment of ginsenoside Rb1. J. Ginseng Res. 2015;39:304–313. doi: 10.1016/j.jgr.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Z., Wen X., Wang C.Z., Li W., Huang W.H., Xia J., Ruan C.C., Yuan C.S. Remarkable impact of amino acids on ginsenoside transformation from fresh ginseng to red ginseng. J. Ginseng Res. 2019 doi: 10.1016/j.jgr.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Z., Xia J., Wang C.Z., Zhang J.Q., Ruan C.C., Sun G.Z., Yuan C.S. Remarkable impact of acidic ginsenosides and organic acids on ginsenoside transformation from fresh ginseng to red ginseng. J. Agric. Food Chem. 2016;64:5389–5399. doi: 10.1021/acs.jafc.6b00963. [DOI] [PubMed] [Google Scholar]
- 40.Yi J.H., Kim M.Y., Kim Y.C., Jeong W.S., Bae D.W., Hur J.M., Jun M. Change of ginsenoside composition in red ginseng processed with citric acid. Food. Sci. Biotech. 2010;19:647–653. [Google Scholar]
- 41.Chung I.M., Lim J.J., Ahn M.S., Jeong H.N., An T.J., Kim S.H. Comparative phenolic compound profiles and antioxidative activity of the fruit, leaves, and roots of Korean ginseng (Panax ginseng Meyer) according to cultivation years. J. Ginseng Res. 2016;40:68–75. doi: 10.1016/j.jgr.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang L., Zhou Q.L., Yang X.W. Determination of the transformation of ginsenosides in Ginseng Radix et Rhizoma during decoction with water using ultra-fast liquid chromatography coupled with tandem mass spectrometry. J. Sep. Sci. 2018;41:1039–1049. doi: 10.1002/jssc.201701228. [DOI] [PubMed] [Google Scholar]
- 43.Cho H.T., Kim J.H., Lee J.H., Kim Y.J. Effects of Panax ginseng extracts prepared at different steaming times on thermogenesis in rats. J. Ginseng Res. 2017;41:347–352. doi: 10.1016/j.jgr.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ryu J., Lee H.W., Yoon J., Seo B., Kwon D.E., Shin U.M., Lee Y.W. Effect of hydrothermal processing on ginseng extract. J. Ginseng Res. 2017;41:572–577. doi: 10.1016/j.jgr.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang R.F., Li J., Hu H.J., Li J., Yang Y.B., Yang L., Wang Z.T. Chemical transformation and target preparation of saponins in stems and leaves of Panax Notoginseng. J. Ginseng Res. 2018;42:270–276. doi: 10.1016/j.jgr.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cui L., Wu S.Q., Zhao C.A., Yin C.R. Microbial conversion of major ginsenosides in ginseng total saponins by Platycodon grandiflorum endophytes. J. Ginseng Res. 2015;40:366–374. doi: 10.1016/j.jgr.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Upadhyaya J., Kim M.J., Kim Y.H., Ko S.R., Park H.W., Kim M.K. Enzymatic formation of compound-k from ginsenoside rb1 by enzyme preparation from cultured mycelia of Armillaria mellea. J. Ginseng Res. 2016;40:105–112. doi: 10.1016/j.jgr.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qu C.L., Zhang H.Q., Zhang H.R., Bai Y.P., Wen H. Studies on fragmentation pathways of amino acids and their interactions with ginsenoside Rb3 by mass spectrometry. Chem. J. Chin. U. 2008;29:1721–1726. [Google Scholar]
- 49.Li F., Li Q., Song D., Liu P.P., Lu J.C. Effect of different drying methods on ginsenosides in flower of panax ginseng and panax quinquefolius. Chin. Tradit. Herb. Drugs. 2015;46:2937–2942. [Google Scholar]
- 50.Quan K., Liu Q., Wan J.Y., Zhao Y.J., Guo R.Z., Alolga R.N., Li P., Qi L.W. Rapid preparation of rare ginsenosides by acid transformation and their structure-activity relationships against cancer cells. Sci. Rep. 2015;5:8598. doi: 10.1038/srep08598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xia J., Zhang J.Q., Yuan C.C., Sun G.Z., Liu Z. Preparation of rare ginsenoside by trans -formation of ginsenoside Re catalyzed transformation of ginsenoside Re catalyzed with aspartic acid. Chin. Tradit. Herbal Drugs. 2016;19:3389–3394. [Google Scholar]
- 52.Liu Z., Xia J., Li W., Zhang J., Sun G., Ruan C.C. Degradation of protopanaxadiol-type ginsenosides with aspartic acid and antioxidant activity of Maillard reaction products. Food Chem. 2017;39:20–26. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.