Abstract
Rationale:
The recent discovery of meningeal lymphatics in mammals is reshaping our understanding of fluid homeostasis and cellular waste management in the brain, but visualization and experimental analysis of these vessels is challenging in mammals. Although the optical clarity and experimental advantages of zebrafish have made this an essential model organism for studying lymphatic development, the existence of meningeal lymphatics has not yet been reported in this species.
Objective:
Examine the intracranial space of larval, juvenile, and adult zebrafish to determine whether and where intracranial lymphatic vessels are present.
Methods and Results:
Using high-resolution optical imaging of the meninges in living animals, we show that zebrafish possess a meningeal lymphatic network comparable to that found in mammals. We confirm that this network is separate from the blood vascular network and that it drains interstitial fluid from the brain. We document the developmental origins and growth of these vessels into a distinct network separated from the external lymphatics. Finally we show that these vessels contain immune cells and perform live imaging of immune cell trafficking and transmigration in meningeal lymphatics.
Conclusions:
This discovery establishes the zebrafish as a important new model for experimental analysis of meningeal lymphatic development, and opens up new avenues for probing meningeal lymphatic function in health and disease.
Subject Terms: Basic Science Research, Developmental Biology, Imaging, Vascular Biology
Keywords: brain, lymphatic capillary, lymphatics, zebrafish
Graphical Abstract
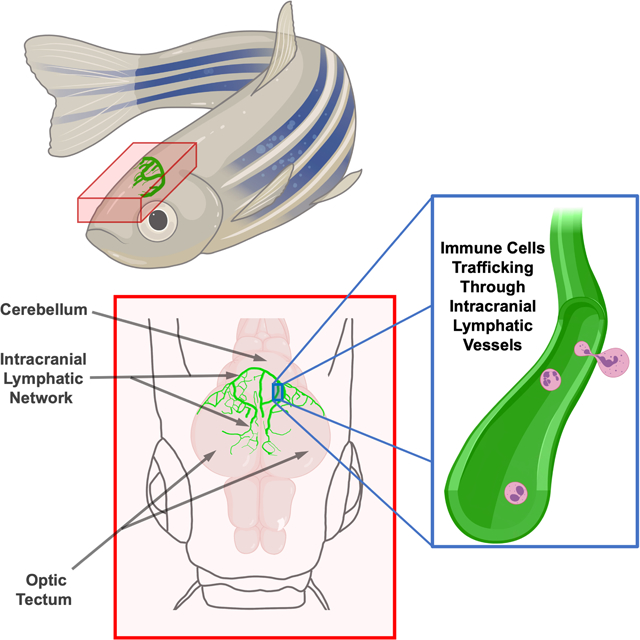
INTRODUCTION
The discovery of meningeal lymphatic vessels in mammals is changing our understanding of how cerebrospinal fluid and interstitial fluid are removed from the brain1, 2. Fluid and macromolecules are collected by lymphatic vessels closely associated with sagittal and transverse sinuses and are drained into the cervical lymph nodes2, and cerebrospinal fluid has been shown to drain through lymphatic vessels lining the base of the skull2, 3. In mice these lymphatic vessels first begin to develop just before birth, first observed around the foramen magnum (the opening at the base of the skull), and are observed next to blood vessels in the meninges at later stages4. This intracranial lymphatic network is believed to be a route for immune cells to cause neuroinflammation5. Additionally, the role of these lymphatic vessels in draining macromolecules, including amyloid-β, has been shown to be relevant to the development of Alzheimer’s Disease and improving intracranial lymphatic function may be a useful target for disease treatment6. Meningeal lymphatics have also been shown to be a promising new avenue for the treatment of glioblastoma. By increasing VEGF-C (Vascular endothelial growth factor C) levels in the brain, researchers were able to increase the amount of meningeal lymphatic vessels which improved immune surveillance allowing the mouse’s immune system to attack the brain tumor7. The meningeal lymphatic network has great physiological and clinical relevance but has only been described in mammalian research models. Although meningeal lymphatics have been imaged in humans and non-human primates using MRI scans8, the thickness and opacity of the mammalian skull makes high resolution live optical imaging of these vessels extremely challenging, requiring methods such as multi-photon excitation and skull thinning5.
The optical clarity of the zebrafish larva and a number of available transgenic lines for visualizing lymphatic vessels in living animals9–12 have made the zebrafish an excellent model for studying lymphatic development in vivo. Although fish may lack some of the more specialized features of mammalian lymphatics, use of the zebrafish model has led to important observations regarding the origins and assembly of lymphatic networks, and the genetic pathways that control this13–16, as well as extensive descriptions of larval and juvenile lymphatic networks9, 10. The importance of lymphatic vessels during heart regeneration in zebrafish has also been described17, 18. Close examination of the developing zebrafish brain has led to a thorough description of unusual perivascular lymphatic-related cells called “Fluorescent Granular Perithelial cells” (FGPs) aka “Mato cells”19, “meningeal mural Lymphatic Endothelial Cells” (muLECs)20, or “Brain Lymphatic Endothelial cells” (BLECs)21 with a macrophage-like morphology and a high capacity to take up macromolecules. However, while these cells have a gene expression profile very similar to that of lymphatic endothelial cells they do not form tubes under normal physiological conditions, and bona fide intracranial lymphatics have not been described in the zebrafish.
Here, we document a complex intracranial lymphatic vessel network in the juvenile and adult zebrafish comparable to that found in mammals, describe its development from the facial lymphatic vascular plexus, and demonstrate its function in brain fluid clearance and immune cell trafficking. This discovery establishes an important new model for experimental analysis of meningeal lymphatic development and function in health and disease.
METHODS
Data Availability.
All data and materials have been made publicly available through the Zebrafish International Resource Center (https://zebrafish.org) or by contacting the Weinstein Laboratory (https://www.nichd.nih.gov/research/atNICHD/Investigators/weinstein).
Fish Husbandry and Fish Strains.
Fish were housed in a large zebrafish dedicated recirculating aquaculture facility (4 separate 22,000L systems) in 6L and 1.8L tanks. Fry were fed rotifers and adults were fed Gemma Micro 300 (Skretting) once per day. Water quality parameters were routinely measured and appropriate measures were taken to maintain water quality stability (water quality data available upon request). The following transgenic fish lines were used for this study: Tg(mrc1a:eGFP)y2519, Tg(−5.2lyve1b:DsRed)nz10110, Tg(prox1aBAC:KalTA4–4xUAS-E1b:uncTagRFP)nim512, Tg(kdrl:mcherry)y20622, Tg(lyz:DsRed2)nz5023, Tg(Ola.Sp7:mCherry-Eco.NfsB)pd46 previously known as Tg(osterix:mCherry-NTRo)pd46 24. Most of the lines imaged were maintained and imaged in a casper (roy, nacre double mutant25) genetic background in order to increase clarity for visualization of the meninges by eliminating melanocyte and iridophore cell populations from the top of the head. This study was performed in an AAALAC accredited facility under an active research project overseen by the NICHD ACUC, Animal Study Proposal # 18–015.
Image Acquisition and Selection.
Confocal images of intracranial lymphatics were acquired using a Nikon Ti2 inverted microscope with Yokogawa CSU-W1 spinning disk confocal, Hamamatsu Orca Flash 4 v3 camera with the following Nikon objectives: 4X Air 0.2 N.A., 10X Air 0.45 N.A., 20X water immersion 0.95 N.A., and 25X silicone immersion 1.05 NA, 40X water immersion 1.15 NA, 60X water immersion 1.20 N.A. Stereo microscope pictures were taken using a Leica M205 microscope using MultiFocus focus stacking. The large size of the juvenile and adult zebrafish head often required tile acquisitions that were later stitched using Nikon Elements software. Approximately five fish were imaged for each data point per experiment. All images were compared, and the images selected for presentation were representative of all the images collected, including vascular and lymphatic complexity. Most of the fish in this study were imaged at juvenile stages of development before visual determination of male or female sexual development is possible, however when adult fish were imaged both male and female fish were selected for analysis.
Image Processing.
Images were processed using Nikon Elements and Photoshop. Tg(mrc1a:egfp)y251 expression is significantly stronger in FGPs than in intracranial lymphatics. To account for this brightness discrepancy, in some images where intracranial lymphatics are displayed as a different color than FGP’s, the brightness of FGP’s and intracranial lymphatics was adjusted separately to make the intracranial lymphatics easier to see. We determined which vessels were inside vs. outside of the skull by scrolling through z stack planes of images with either Tg(Ola.Sp7:mCherry-Eco.NfsB)pd46 expression or 405 nm autofluorescence of calcified skull bone. Unless otherwise specified, maximum intensity projections of confocal stacks are shown. Focus stacking of confocal images with DIC was done using Nikon Elements EDF (Extended Depth of Focus). 3D rotation videos and time-lapse videos were made using Nikon Elements and exported to Adobe Premiere Pro CC 2019. Adobe Premiere Pro CC 2019 and Adobe Photoshop CC 2019 were used to add labels and arrows to videos and to add coloring or pseudo-coloring. Schematics where made using Adobe Photoshop CC 2019 and Bio Render software.
Developmental Series.
Fish from 14 dpf to 37 dpf were imaged repeatedly by anesthetizing them in 168 mg/L tricaine in system water and mounting them in 0.8% low melting point agarose in a 35mm glass bottomed petri dish (MatTek # P35–1.5–14-C) with the top of their head touching the glass cover slip. After imaging (10–15 minutes) fish were freed from the agarose and revived by placing them back into water from the main aquarium system and applying gentle water flow over the fish using a transfer pipette. Revival sometimes took as long as 30 minutes.
Juvenile and Adult Intracranial Imaging.
Mounting fish older than 30 dpf in low melting point agarose can cause morbidity and death, possibly because of the agarose covering the adult gills. Instead, older fish were mounted by anesthetizing them in 126–168 mg/L tricaine in system water and then placed into a slit in a sponge (Jaece Identi-Plugs L800-D) moistened with tricaine water, cut into a rectangle to fit inside a single chamber imaging dish (Lab-TekII #155360). The sponge containing the fish is placed into the imaging dish with the head against the glass (fish upside down) and the chamber is filled with tricaine water. For longer term imaging (greater than one hour), fish were intubated using a modification of the methods described by C. Xu et al.26 adapted for inverted confocal microscopy (Online Fig. I E). Fish lengths are reported as standard length from the tip of the snout to the base of the tail.
Imaging the inside of the dissected zebrafish skull.
Fish were first euthanized by a 10 minute submersion in an ice bath. The skull was removed using two pairs of Dumont L5 forceps. The ventral side of the skull was removed, followed by the brain using L5 forceps. The dorsal side of the skull was then dipped in PBS to remove any debris. The dorsal side of the skull was then placed in a 35mm glass bottomed petri dish (MatTek # P35–1.5–14-C) in PBS with a circular cover slip placed on top to stabilize it and was imaged using a Nikon Ti2 inverted microscope with Yokogawa CSU-W1 spinning disk confocal, Hamamatsu Orca Flash 4 v3 camera with a 10X Air 0.45 N.A. objective. Area measurements (Online Fig. VI B,F–H) were calculated using Photoshop CC 2019.
Angiography and Lymphatic Drainage Injections.
Injections were performed as described by Yaniv et al.27. All injections were performed using a Drummond Nanoject II microinjector (Item# 3-000-204) or Drummond Nanoject III microinjector (Item# 3-000-207) with pulled glass capillary needles (Drummond item # 3-00-203-G/X). One or two injection boluses were given at each injection site with a volume setting of 36.8 nL. Angiography and lymphatic drainage assays were done using undiluted (2 uM) Qtracker™ 705 Vascular labels (Invitrogen cat# Q21061MP). Lymphatic drainage assays were also done using 10,000 MW Cascade Blue™ Dextran (Invitrogen Cat# D1976). Fish were held in a moistened sponge with a slit cut into it and viewed under a stereo microscope (Leica MZ 12) for the injections.
Alizarin Red Staining.
Alizarin red has been shown to be an effective bone stain in living zebrafish28. Adult zebrafish were bathed in 1.9 mM alizarin red bath (400 ul of Alizarin-Red Staining Solution (Sigma Aldrich TMS-008-C 40 mM) in 80 mL of system water) for 40 minutes. After the bath fish were given a one hour rinse in clean system water before imaging.
Imaging Neutrophils and Intracranial Lymphatics.
Living casper mutant Tg(mrc1a:egfp)y251, Tg(lyz:DsRed2)nz50 double-transgenic zebrafish were mounted and imaged as described in the Juvenile and Adult Intracranial Imaging section. The number of neutrophils seen in the cranial region was highly variable from fish to fish. Oxazalone has been used to induce an immune response in zebrafish29. By soaking adult zebrafish in a 0.0005% Oxazolone (4-Ethoxymethylene-2-phenyl-2-oxazolin-5-one, Sigma E0753–5G) solution in system water for 3 hours the number of neutrophils in the cranial region could be greatly increased. This technique was used on the fish shown in Fig. 7 B–D.
VEGF-C Intracranial Injections.
50 day old casper, Tg(mrc1a:egfp)y251, Tg(kdrl:mcherry y206 zebrafish were anesthetized in 126–168 mg/L tricaine in system water and placed in the slit of a moistened sponge under a stereo microscope. Using a Drummond Nanoject III microinjector (Item# 3-000-207) with pulled glass capillary needles (Drummond item # 3-00-203-G/X), 75 nl of either PBS, or 100ug/ml VEGF-C (Recombinant Human VEGF-C Protein, R&D Systems, Catalog # 9199-VC), with 10 percent Qtracker™ 705 Vascular labels (Invitrogen cat# Q21061MP) added as a tracer was injected through the dorsal skull. After injections, fish were imaged using the 10X objective on the Nikon Ti2 confocal described above. If Qdot705 labeling was distributed throughout the meninges around the cerebellum and optic tecta, the fish was revived and returned to the main zebrafish housing system for six days. Six days after the injections fish were re-anesthetized and imaged again. Lymphatic area was calculated by selecting the intracranial lymphatic vessels shown in the maximum intensity projection with photoshop and calculating the area. Blood vessel area was calculated using Nikon Elements General Analysis 3. The maximum intensity projection of the blood vessels was thresholded so that the meningeal blood vessels were selected and the area was calculated. The percent increase between PBS and VEGF-C vessel area was compared using a two tailed Student’s T-test assuming unequal variances.
Statistics.
When analyzing blood and lymphatic vessel area in PBS or VEGF-C injected animals, and lymphatic vessel area in older animals, low numbers and lack of previous literature references on these vessels made assessing normality difficult, so non-parametric tests were used. To compare blood and lymphatic vessel area in VEGF-C and PBS injected animals, a Mann-Whitney U test was used and raw p-values were Bonferroni-adjusted (applied to the two tests). To compare lymphatic vessel area in fish of different ages a (single) Kruskal-Wallis test was used. Significance was declared/claimed at a usual threshold of (adjusted) p-value < 0.05. Statistical analysis was performed using GraphPad Prism 8.
Please see the Major Resources Table in the Supplemental Materials.
RESULTS
Meningeal lymphatics in the adult zebrafish.
Historically, lymphatic vessels have been thought to be excluded from the central nervous system, but several recent reports have documented the presence of lymphatic vessels in the meninges surrounding the mouse brain, particularly adjacent to dural sinuses, and have demonstrated the important function of these vessels for draining cerebrospinal fluid (CSF), their strong association with immune cells, and their importance for immune surveillance of the brain1, 2. Since the zebrafish provides a superb model for in vivo analysis of vascular development and function30 and since it has a lymphatic vascular system comparable in most respects to that of mammals31, 32, we sought to determine whether zebrafish also possess intracranial lymphatics, and whether fish could provide a valuable model for live imaging of intracranial lymphatic development and function.
The brain can be visualized through the top of the skull in dorsal views of the heads of adult casper (roy, nacre double mutant) animals that lack melanin pigment except in their eyes (Fig. 1A). The large prominent optic tecta are readily visible in dorsal views of casper heads, as are the forebrain and cerebellum (Fig. 1A,B). Using a Tg(mrc1a:egfp)y251 zebrafish transgenic reporter line we recently developed for visualizing lymphatic vessels in vivo 9, confocal imaging of the dorsal heads of living (Online Fig. I A–C,E) casper, Tg(mrc1a:egfp)y251 transgenic adult animals reveals complex networks of superficial lymphatic vessels covering much of the surface of the head, especially over the forebrain area (Online Fig. I A–C). However, in addition to the superficial lymphatics outside the skull, we also observe an elaborate network of intracranial meningeal lymphatics immediately beneath the skull, particularly over the optic tecta and cerebellum (Fig. 1B,C, Online Fig. 1A,B,D).
Fig. 1. Intracranial lymphatic vessels in the adult zebrafish.
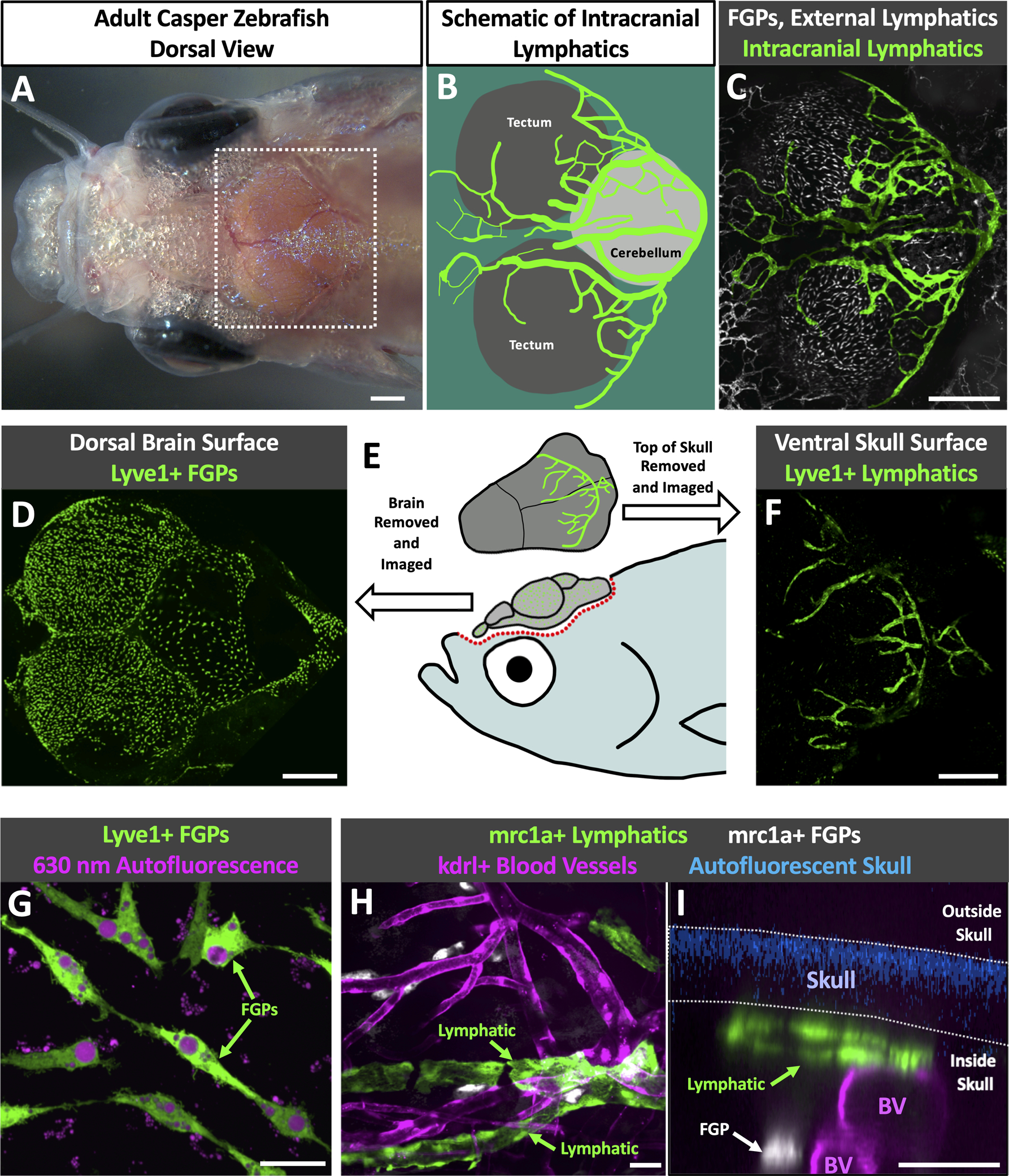
A. Dorsal view image of the head of an adult casper (roy, nacre double mutant) zebrafish. B. Schematic diagram of the boxed region in panel A, showing the optic tecta and cerebellum with a typical network of intracranial lymphatic vessels in a young adult zebrafish. C. Confocal image of the dorsal head of a Tg(mrc1a:egfp)y251, casper adult zebrafish, with mrc1a+FGPs and superficial lymphatics in grey and intracranial meningeal lymphatics pseudocolored green. D. Confocal image of the dorsal surface of a dissected brain from a Tg(−5.2lyve1b:DsRed)nz101, casper adult zebrafish, with lyve1+ FGPs but no lymphatic vessels. E. Schematic diagram of dissection of an adult zebrafish head for imaging the dorsal surface of the brain and the ventral surface of the skull. F. Confocal image of the ventral (inner) surface of a dissected brain from a Tg(−5.2lyve1b:DsRed)nz101, casper adult zebrafish, with lyve1+ lymphatic vessels but no FGPs. G. Higher magnification confocal image of the dorsal surface of a dissected brain removed from a Tg(−5.2lyve1b:DsRed)nz101, casper adult zebrafish, showing individual separated lyve1+ FGPs with characteristic large autofluorescent internal vacuoles. H. Higher magnification confocal image of the outer layer of the brain imaged through the skull of a living casper adult double transgenic zebrafish Tg(mrc1a:egfp)y251, Tg(kdrl:mcherry) y206, showing mrc1a+ lymphatic vessels and kdrl+ blood vessels. I. Higher magnification confocal image of the dorsal head of a Tg(mrc1a:egfp)y251, Tg(kdrl:mcherry) y206, casper adult zebrafish. This orthogonal view shows a cross section of an mrc1a+ lymphatic vessel immediately below the blue autofluorescent skull and an mrc1a+ FGP in a deeper layer immediately adjacent to a kdrl+ blood vessel. Unless otherwise noted all images are dorsal views, rostral to the left. Scale bars: 500 um (A,C,E,F) 25 um (G,H,I). (BV- blood vessels, roy- roy orbison, mrc1a-mannose receptor C, type 1a, eGFP- green fluorescent protein, Tg- transgenic, lyve1b- lymphatic vessel endothelial hyaluronic receptor 1b, FGP- fluorescent granular perithelial cells, kdrl-kinase insert domain receptor like)
These intracranial lymphatics are distinct and separate from mrc1a-positive “Fluorescent Granular Perithelial” cells (FGPs, aka “Mato Cells,” “MuLECs,” or “BLECs,”), non-tube forming meningeal perivascular cells we and others recently described that are molecularly very similar to lymphatic endothelial cells19–21. FGPs are found in the leptomeninges closely associated with the outer surface of the brain19, and they are readily observed on dissected brains removed from Tg(−5.2lyve1b:DsRed)nz101 transgenic adult animals (Fig. 1D,E). However, no lyve1+ lymphatic vessels are observed on the surface of dissected brains. In contrast, the inner surface of dissected skull caps removed from Tg(−5.2lyve1b:DsRed)nz101 transgenic adult animals completely lacks lyve1+ FGPs, and instead shows robust networks of lyve1+ lymphatic vessels (Fig. 1E,F). This is analogous to observations from mice, where intracranial lymphatics are found exclusively in the skull-associated meninges and not in the brain-associated leptomeninges1, 2. Closer examination of lyve1+ cells associated with the outer surface of the brain (Fig. 1D) and inner surface of the skull (Fig. 1F) confirms the distinct identities of the cells in these two locations. The outer surface of the dissected brain contains exclusively FGPs; individual, separated cells immediately adjacent to blood vessels with a macrophage-like morphology and abundant large autofluorescent intracellular vacuoles (Fig. 1G). The ventral surface of the skull cap has lyve1+ tubular vessels (Fig. 1F) but no FGPs. Orthogonal views of confocal images taken through the intact head of a casper, Tg(mrc1a:egfp)y251, Tg(kdrl:mcherry y206 double transgenic adult animal confirm that mrc1a+ lymphatic tubes are closely apposed to the skull while mrc1a+ FGPs lie immediately adjacent to kdrl+ blood vessels in a deeper layer (Fig. 1I, Online Video I). Endogenous autofluorescence of calcified bone under ultraviolet light33 allows us to determine which lymphatic vessels are located inside the skull and which are located outside the skull in adult zebrafish without the use of additional transgenics labeling bone, or bone stains (Online Fig. II, Online Video II, Fig 1I).
To confirm their lymphatic molecular identity, we carried out additional confocal imaging of intracranial lymphatic vessels in casper, Tg(mrc1a:egfp)y251, Tg(−5.2lyve1b:DsRed)nz101 (Fig. 2A–C) or casper, Tg(mrc1a:egfp)y251, Tg(prox1aBAC:KalTA4–4xUAS-E1b:uncTagRFP)nim5 (Fig. 2D–F) adult zebrafish to determine whether these different characteristic lymphatic transgenes co-localize in presumptive zebrafish intracranial lymphatics. mrc1a, lyve1, and prox1a are widely used markers for lymphatic vessels, and Tg(mrc1a:egfp)y251, Tg(−5.2lyve1b:DsRed)nz101, and Tg(prox1aBAC:KalTA4–4xUAS-E1b:uncTagRFP)nim5 transgenic lines have been developed using the promoters from each of these genes to drive fluorescent reporter expression for live imaging of lymphatic vessels in the zebrafish9, 10, 12. As expected, zebrafish intracranial lymphatic vessels co-express mrc1a and lyve1 (Fig. 2A–F) and co-express mrc1a and prox1a (Fig. 2G–I), showing they possess the molecular signature of lymphatic vessels. To further confirm that these vessels are bona fide lymphatic vessels and validate both their separation from the blood vessels of the cardiovascular system and their function in intracranial drainage, we carried out blood vessel labeling (via angiography, injection into the dorsal aorta; Fig. 3A) and lymphatic vessel labeling (via cranial intracisternal injection; Fig. 3B spinal intracisternal injection; Online Fig. III B–D ventral intracisternal injection through the jaw; Online Fig. III E–G) using Qdot705, blue dextran, or both together to label either blood vessels (Fig. 3D–F) or draining intracranial lymphatics (Fig. 3G–L, Online Fig. III C,D,F,G), respectively. Angiographic injection of Qdot705 into the trunk dorsal aorta leads to rapid labeling of the entire circulatory system including intracranial blood vessels without any labeling of adjacent mrc1a:egfp+ intracranial lymphatics (Fig. 3D–F). In contrast, intracisternal injection of Qdot705 and blue dextran leads to filling of the subarachnoid, and subdural spaces (Online Fig. IIIA) quickly followed by drainage through and labelling of nearby mrc1a:egfp+ intracranial lymphatics without labelling of adjacent blood vessels (Fig. 3G–L, Online Fig. III C,D,F,G Online Video III). FGPs have previously been described as having the ability to absorb substances injected into the brain of zebrafish19, 21 and we confirmed that intracisternally injected Qdot705 and blue dextran are also taken up by FGPs (Online Fig. IV C–F). Interestingly, we saw dextran labeling throughout the cell, while Qdot labeling appeared to be compartmentalized (Online Fig. IV F).
Fig. 2. Molecular validation of zebrafish intracranial lymphatics.
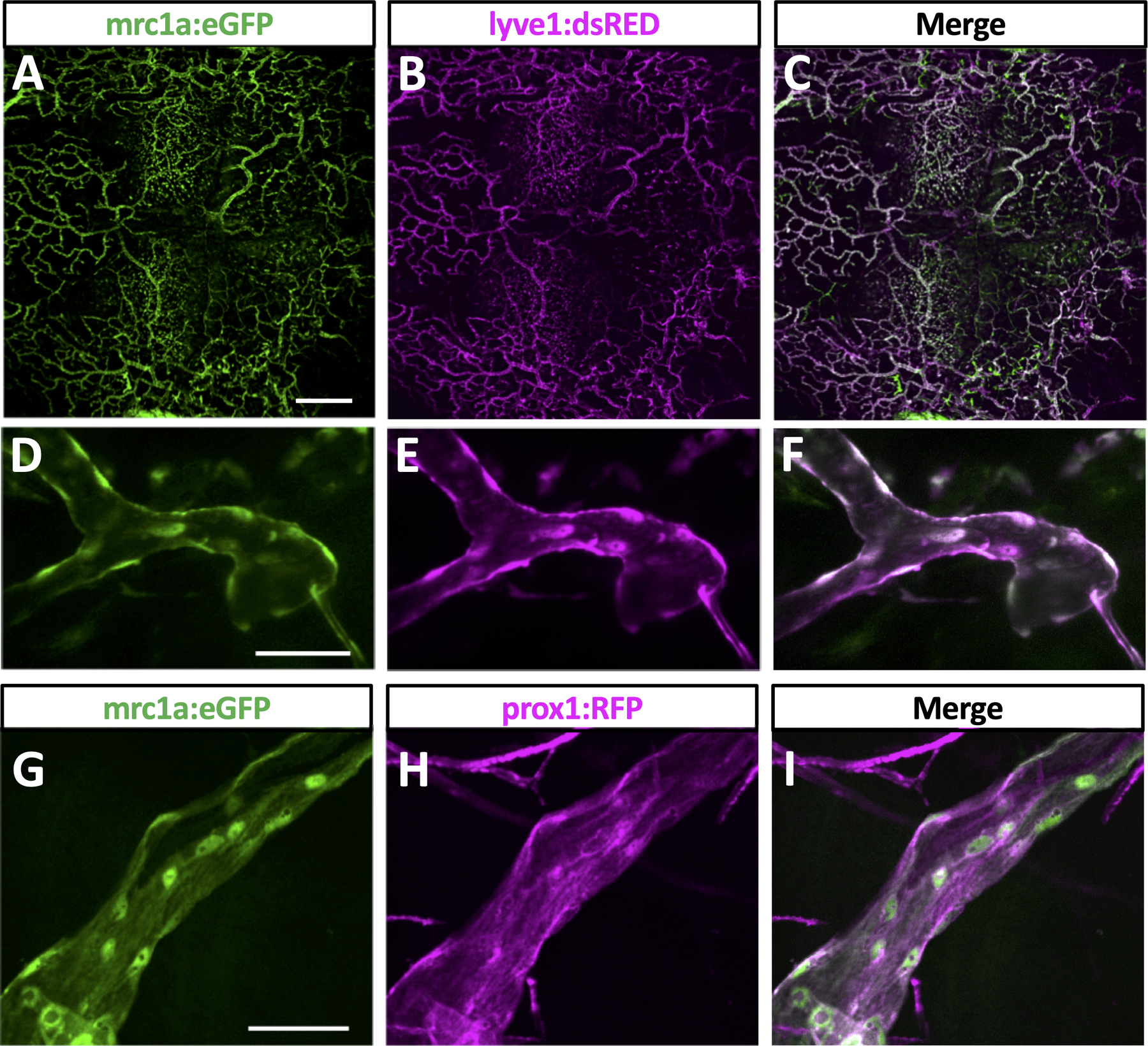
A-C. Confocal imaging overview intracranial and extracranial lymphatic vessel in the dorsal head of an adult casper, Tg(mrc1a:egfp)y251, Tg(−5.2lyve1b:DsRed)nz101 double transgenic zebrafish, showing mrc1a:egfp (A), lyve1b:dsred (B), and combined mrc1a:egfp and lyve1b:dsred (C) images. D-F. Confocal imaging of an intracranial lymphatic vessel in the dorsal head of an adult casper, Tg(mrc1a:egfp)y251, Tg(−5.2lyve1b:DsRed)nz101 double transgenic zebrafish, showing mrc1a:egfp (D), lyve1b:dsred (E), and combined mrc1a:egfp and lyve1b:dsred (F) images. D-F. Confocal imaging of an intracranial lymphatic vessel in the dorsal head of an adult casper, Tg(mrc1a:egfp)y251, Tg(prox1aBAC:KalTA4–4xUAS-E1b:uncTagRFP)nim5 double transgenic zebrafish, showing mrc1a:egfp (D), prox1:rfp (E), and combined mrc1a:egfp and prox1:rfp (F) images. Scale bars: 500 um (A), 50 um (D,G). (Tg-transgenic, mrc1a- mannose receptor C, type 1a, egfp-green fluorescent protein, lyve1b- lymphatic vessel endothelial hyaluronic receptor 1b, prox1- prospero homeobox 1a)
Fig. 3. Functional validation of zebrafish intracranial lymphatics.
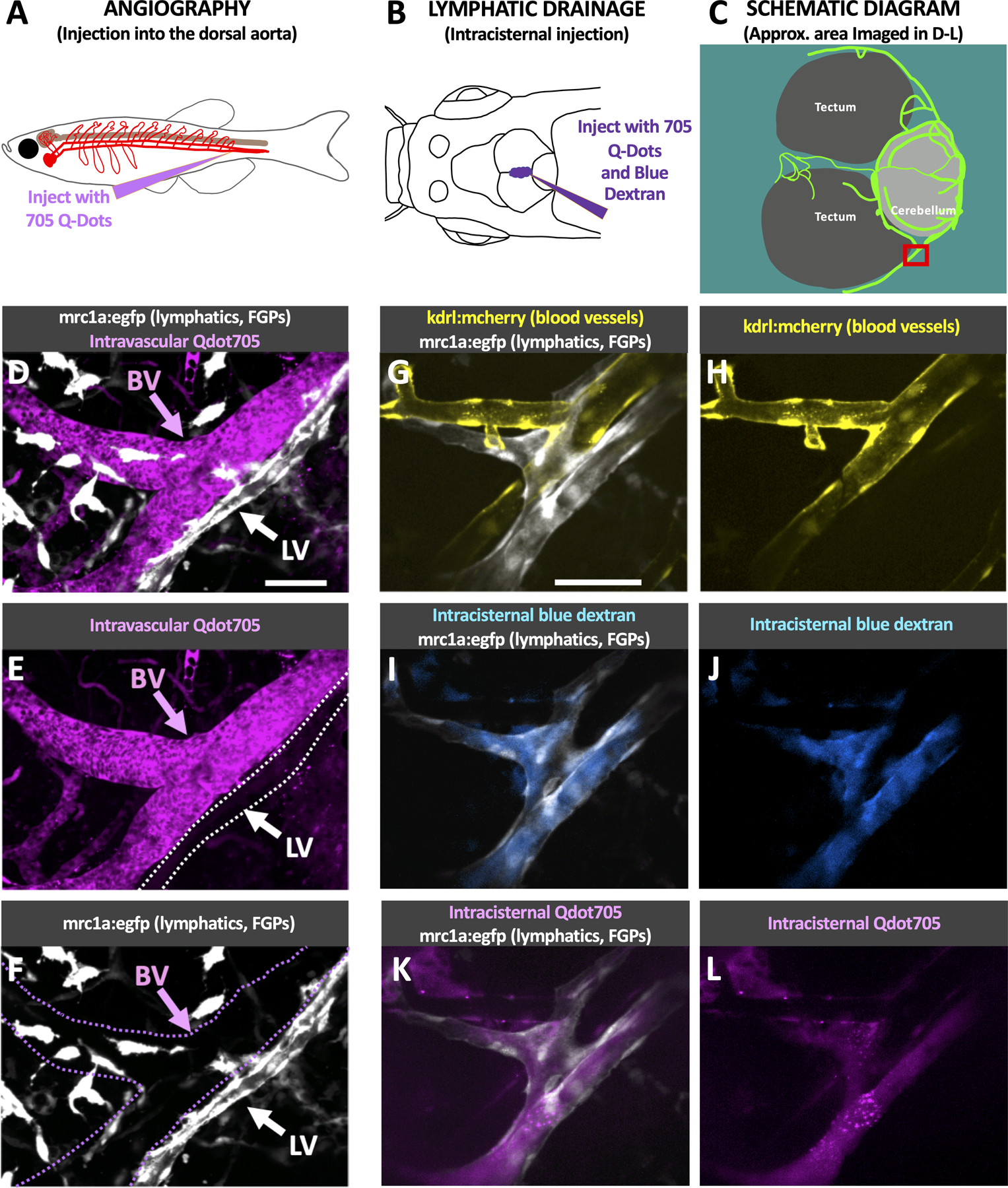
A. Schematic diagram of the intravascular injection procedure for filling blood vessels. B. Schematic diagram of the intracisternal injection procedure for filling intracranial lymphatics. C. Schematic diagram of the optic tecta, cerebellum, and intracranial lymphatics in the dorsal head of an adult casper mutant, Tg(mrc1a:egfp)y251 zebrafish. The red box notes the approximate area shown in the high-magnification images in panels D-L. D-F. Confocal images of intracranial blood vessels (BV) and lymphatic vessels (LV) in the dorsal head of a Tg(mrc1a:egfp)y251 adult fish injected intravascularly with Qdot705, showing mrc1a:egfp+ lymphatics and FGPs + Qdot705 (D), Qdot705 alone (E), or mrc1a:egfp+ alone (F). G-L. Confocal images of intracranial blood vessels (BV) and lymphatic vessels (LV) in the dorsal head of a Tg(mrc1a:egfp)y251, Tg(kdrl:mcherry) y206 adult fish injected intracranially with Qdot705 and blue dextran, showing mrc1a:egfp+ lymphatics and kdrl:mcherry+ blood vessels (G), kdrl:mcherry+ blood vessels (H), mrc1a:egfp+ lymphatics and blue dextran (I), blue dextran (J), mrc1a:egfp+ lymphatics and Qdot705 (K), Qdot705 (L). Scale bars: 50 um. See Online Video III for 3D renderings and real-time imaging of the vessels shown in panels G-L. (mrc1a- mannose receptor C, type 1a, FGPs- fluorescent granular perithelial cells, kdrl- kinase insert domain receptor like, BV- blood vessels, LV-lymphatic vessels)
Together these results demonstrate that zebrafish possess a network of intracranial meningeal lymphatic vessels comparable to those described in mammals1, 2.
Development of intracranial lymphatics in the zebrafish.
Having established the existence of intracranial meningeal lymphatics in the adult zebrafish, we sought to understand when and how this network of vessels develops by confocal imaging of the intact dorsal heads of casper Tg(mrc1a:egfp)y251 transgenic zebrafish. Meningeal lymphatics initially sprout from dorsal-medial branches of the bilateral facial lymphatic networks beginning at approximately 9–10 days post fertilization (dpf) or when they are approximately 4 mm in length (Fig. 4A,E,I, Online Video IV), initially growing rostrally and laterally along the caudal margin of the cerebellum and optic tecta (Fig. 4B,F,J, Online Video IV). These initial branches continue to develop into enlarged sacs and also grow medially eventually connecting across the midline at the caudal edge of the cerebellum (Fig. 4C,D,G,H,K,L, Online Video IV). The developing meningeal lymphatics are initially connected to the facial lymphatics from which they emerge on either side (Fig. 4A,E,I, Online Video IV), but at later stages of development they become disconnected from the facial lymphatics (Fig. 4B,C,F,G,J,K, Online Video IV) connecting instead to more dorsal superficial networks of lymphatic vessels over the cerebellum and hindbrain (Fig. 4D,H,L, Online Video IV). The facial lymphatics also connect to this dorsal superficial network at later stages so the developing intracranial lymphatics thus appear to be effectively (albeit indirectly) reconnected to the facial lymphatics in many cases, at least temporarily, although these connections are also lost over time. Although the timing is somewhat variable, the sequence of events in development of the primary intracranial network is quite stereotypical, as shown by imaging of numerous different animals from 14 dpf to 36 dpf (Online Fig. V). Although intracranial and extracranial vessels could be distinguished by careful examination of Z-stacks, to further verify and validate our identification of intracranial lymphatics we examined casper mutant, Tg(mrc1a:egfp)y251, Tg(Ola.Sp7:mCherry-Eco.NfsB)pd46 double transgenic zebrafish that label both lymphatic vessels in green and developing bone in red (Fig. 5).
Fig. 4. Initial development of intracranial lymphatics in larval zebrafish.
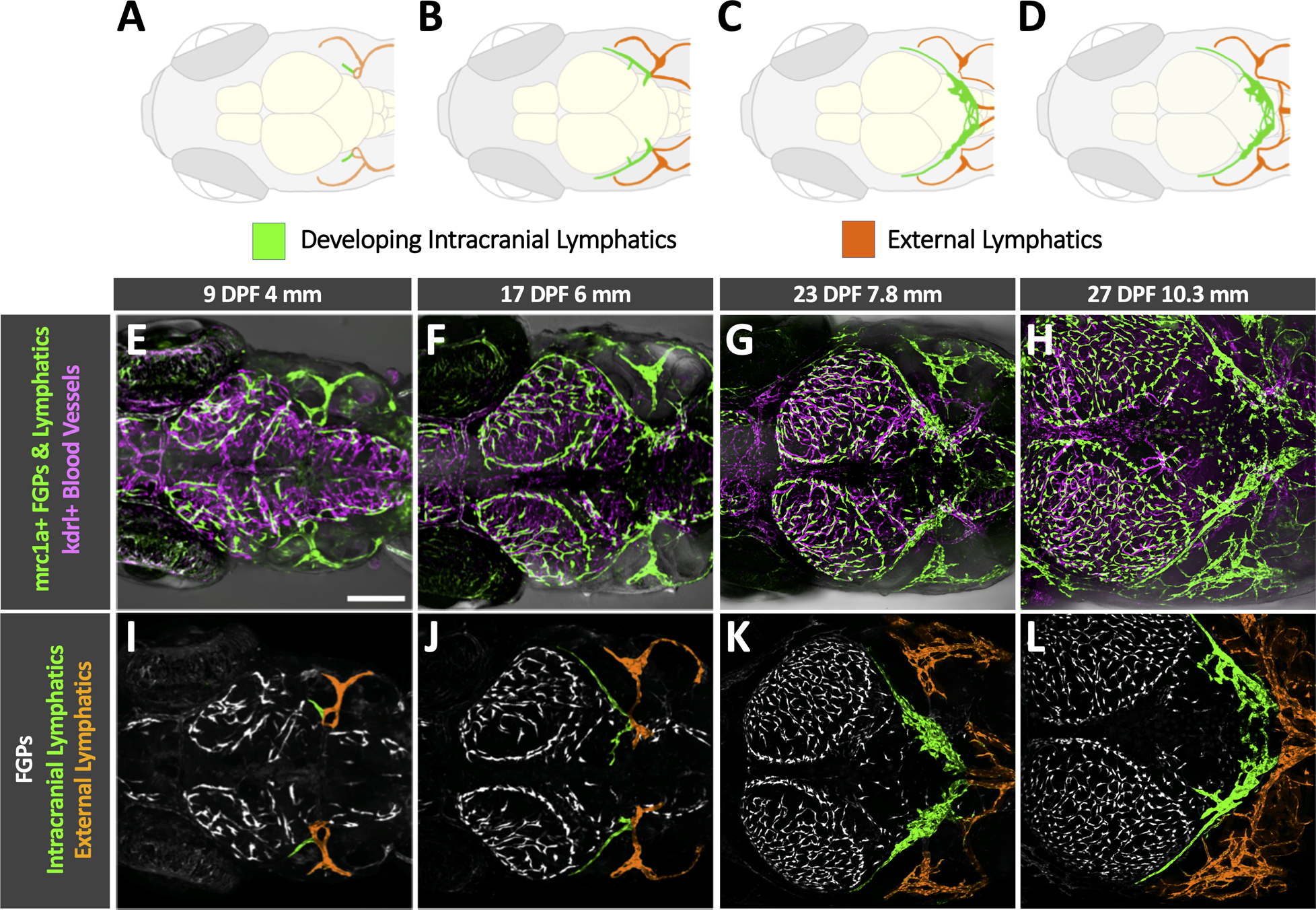
A-D. Schematic diagrams showing brain structures, developing intracranial lymphatics (green), and external lymphatics (orange) in the dorsal heads of 9 dpf/4 mm (A), 17 dpf/6 mm (B), 23 dpf/7.8 mm (C), and 27 dpf/10.3 mm (D) zebrafish. Each diagram illustrates features in the corresponding confocal micrograph below (panels E-H). OT, optic tecta; C, cerebellum. E-H. Confocal images of kdrl:mcherry positive blood vessels (magenta) and mrc1a:egfp positive lymphatic vessels and FGPs (green) in the dorsal heads of 9 dpf/4 mm (E), 17 dpf/6 mm (F), 23 dpf/7.8 mm (G), and 27 dpf/10.3 mm (H) Tg(mrc1a:egfp)y251, kdrl:mcherry) double-transgenic zebrafish. I-L. Colored versions of the same confocal image fields shown in panels E-H with only the mrc1a:egfp fluorescence channel visible, revealing FGPs (white, uncolored), external lymphatics (colored orange), and developing intracranial lymphatics (colored green). Scale bar: 250 um. a See Online Video IV for 3D renderings of the same image stacks shown in this figure. Intracranial and extracranial vessels were distinguished by careful examination of Z-stacks and by comparison to similar staged transgenic or stained animals marking developing bone (see Figure 5 for examples). Dpf- days post fertilization, mm- millimeters, mrc1a- mannose receptor C, type 1a, FGPS- fluorescent granular perithelial cells, kdrl-kinase insert domain receptor like)
Fig. 5. Intracranial lymphatic development and skull formation.
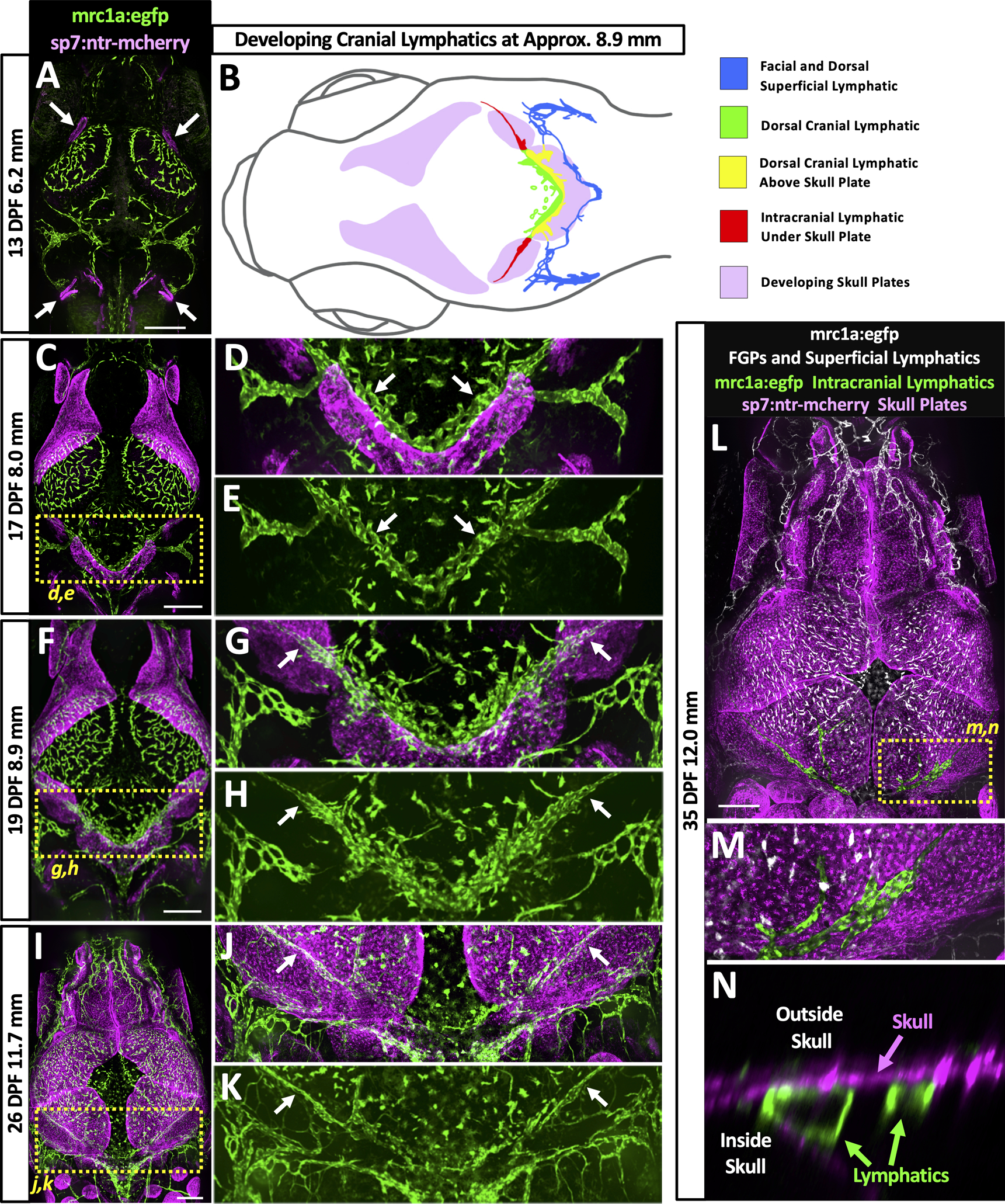
A,C,F,I,L. Overview confocal images of mrc1a:egfp+ lymphatic vessels and FGPs (green) and sp7:mcherry+ developing skull plates (magenta) in the dorsal heads of 13 dpf/6.2 mm (A), 17 dpf/8.0 mm (C), 19 dpf/8.9 mm (F), 26 dpf/11.7 mm (I), and 35 dpf/12.0 mm (L) Tg(mrc1a:egfp)y251, Tg(Ola.Sp7:mCherry-Eco.NfsB)pd46 double-transgenic zebrafish. Arrows in panel A note beginning of skull plate formation. Yellow dashed boxes in panels C, F, I, and L note the areas shown in the corresponding higher magnification images in the panels to the right (D,E,G,H,J,K) or below (M,N). B. Schematic diagram corresponding to the confocal image of a 19 dpf/8.9 mm Tg(mrc1a:egfp)y251, Tg(Ola.Sp7:mCherry-Eco.NfsB)pd46 double-transgenic zebrafish shown in panel F. Diagram shows developing skull plates (pink), superficial lymphatics (blue), still-uncovered intracranial lymphatic (green), portions of intracranial lymphatic network present above edge of growing skull plates (yellow), and portions of intracranial lymphatics already covered by the growing skull plates (red). D,G,J. Higher magnification confocal images of the yellow boxed regions in the panels to the left, showing mrc1a:egfp+ lymphatics (green) and sp7:mcherry+ developing skull plates (magenta). White arrows note lymphatics adjacent to (D) or under (G,J) skull plates. E,H,K. Higher magnification confocal images of the yellow boxed regions in the panels to the left, showing only mrc1a:egfp+ lymphatics (green). White arrows note lymphatics adjacent to (E) or under (H,K) skull plates. M. Higher magnification confocal image of the yellow boxed region in panel L, showing mrc1a:egfp+ meningeal lymphatics (green) below the nearly completed sp7:mcherry+ skull (magenta). N. Higher magnification orthogonal (side) view confocal image of the yellow boxed region in panel L, showing mrc1a:egfp+ meningeal lymphatic tubes (green) inside the skull below the sp7:mcherry+ skull (magenta). Scale bars: 250 um. (dpf- days post fertilization, mm- millimeters, mrc1a-mannose receptor C type 1a, GFP- green fluorescent protein, ntr-nitro-reductase, FGPs- fluorescent granular perithelial cells, sp7- sp7 transcription factor)
Although the initial development of the future intracranial lymphatics precedes skull formation, continued later development of intracranial lymphatics occurs concomitantly with growth and closure of the bony skull plates, with growth of the plates across the top of the head successively covering consecutive portions of the meningeal lymphatics. We used confocal imaging of the intact dorsal heads of casper, Tg(mrc1a:egfp)y251, Tg(Ola.Sp7:mCherry-Eco.NfsB)pd46 (previously known as Tg(osterix:mCherry-NTRo)pd46) double transgenic zebrafish to simultaneously image Mrc1a:egfp positive developing lymphatics and sp7:mCherry positive developing skull plate bone. Sp7+ skull plates begin to appear at approximately 13 dpf/6.2 mm at the lateral corners of the dorsal head (Fig. 5A, arrows). The plates grow continuously over approximately the first month of development, gradually closing the bone-free space at the top of the head and successively covering developing intracranial lymphatics (diagrammed in Fig. 5B). By approximately 17 dpf/8.0 mm the growing plates abut portions of the developing future intracranial lymphatics along the caudal margin of the cerebellum (Fig. 5C–E, arrows in D,E, Online Video V). As seen in a 19 dpf/8.9 mm animal, the growing plates of the optic tecta begin to cover portions of the developing intracranial lymphatics (Fig. 5B,F–H, arrows in G,H, Online Video VI). The mrc1a+ intracranial lymphatics are further covered by the growing sp7+ plates in a 26 dpf/11.7 mm animal (Fig. 5I–K, arrows in J,K), and are nearly completely covered by the fused skull plates in a 35 dpf/12.0 mm animal (Fig. 5L–N). Higher magnification images of intracranial lymphatics in the 35 dpf/12.0 mm animal confirm that mrc1a+ vessels are immediately below the sp7+ skull (Fig. 5M,N).
From approximately 3 weeks of development the initial relatively simple larval network of intracranial lymphatic vessels found along the caudal margin of the optic tecta and cerebellum (Fig. 4C,G,K) grows rostrally and elaborates into a much more complex juvenile and adult intracranial lymphatic vascular system, as visualized by confocal imaging of the intact dorsal heads of casper Tg(mrc1a:egfp)y251 transgenic zebrafish (Fig. 6A–D). In a 30 dpf/16 mm animal rostral projections are observed just beginning to emerge from the larval intracranial lymphatics (Fig. 6A), while in a slightly more developed 40 dpf/17 mm animal the rostral projections are extended branches curving around the cerebellum toward the dorsal midline (Fig. 6B). In a 41 dpf/19 mm animal the lymphatics form a network circumscribing and also beginning to cross the dorsal surface of the cerebellum, and also extending rostrally between the optic tecta on either side of the midline dural sinus (Fig. 6C). An even more elaborate network can be seen in a 45 dpf/24 mm animal, with intracranial vessels further extending across the optic tecta and elsewhere (Fig. 6D). Although the complete network of intracranial lymphatics become difficult to image through the skull after the first 1–1/2 months of development, the intracranial lymphatics in older animals appear very similar to the network observed in 1–1/2 month old animals, albeit with somewhat increasing complexity (data not shown). Higher magnification imaging of developing juvenile lymphatics suggests that the elaborate rostral lymphatics grow via both sprouting lymphangiogenesis and formation and fusion of locally developing small lymphatic cell clusters/sacs (Fig. 6E–H).
Fig. 6. Later development of intracranial lymphatics in juvenile zebrafish.
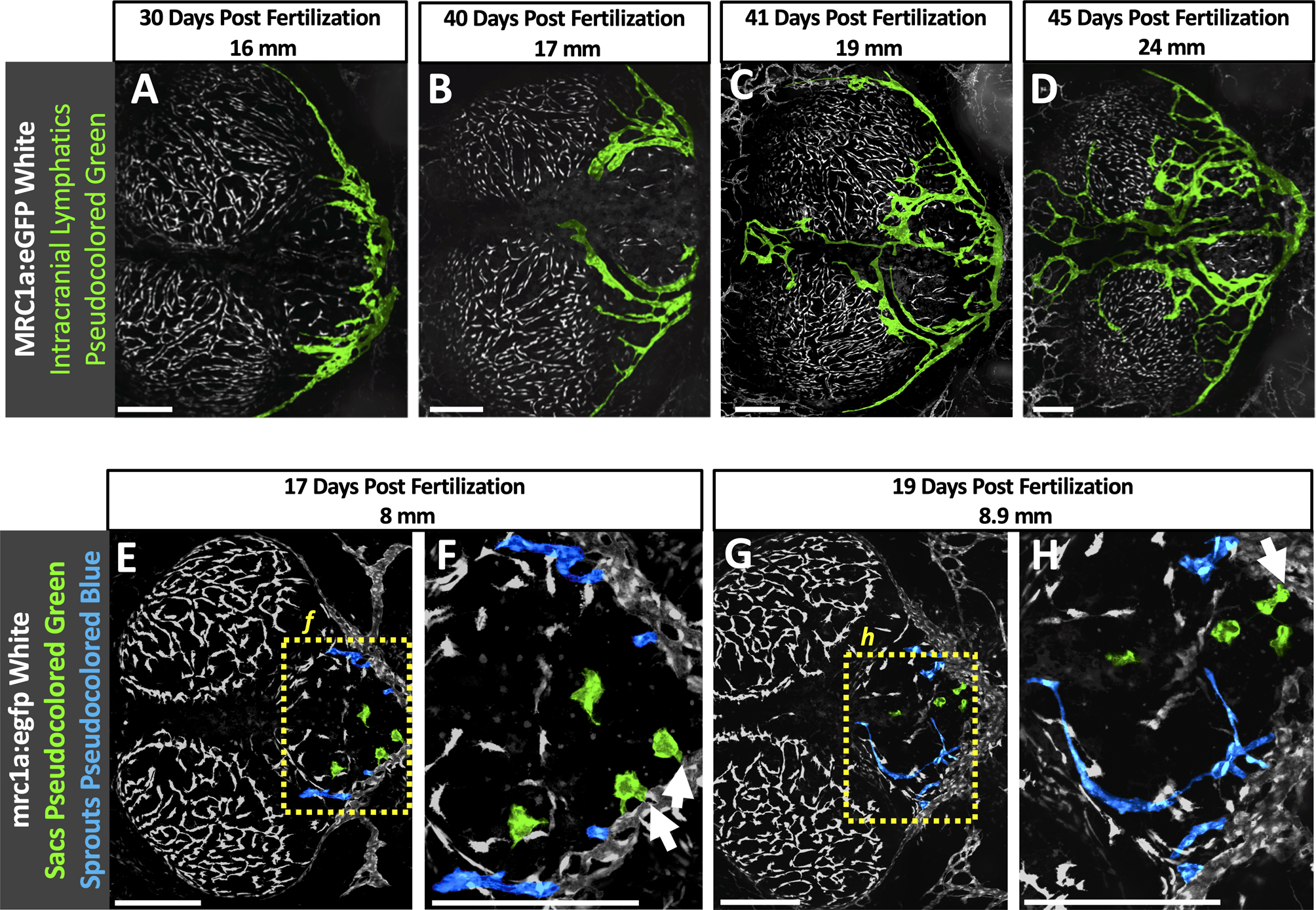
A-D. Colored confocal images of mrc1a:egfp+ FGPs (white, uncolored) and intracranial lymphatic vessels (colored green) in the dorsal heads of 30 dpf/16 mm (A), 40 dpf/17 mm (B), 41 dpf/19 mm (C), and 45 dpf/24 mm (D) Tg(mrc1a:egfp)y251 transgenic zebrafish. E-H. Colored confocal images of mrc1a:egfp+ FGPs and the primary intracranial lymphatic plexus (white, uncolored), sprouting intracranial lymphatic vessels (colored blue) and lymphatic sacs (colored green) in the dorsal head of a 15 dpf/6.7 mm Tg(mrc1a:egfp)y251 transgenic zebrafish (E), Higher magnification view of the boxed region in panel E, showing sprouting intracranial lymphatics (arrows) (F), and a 17 dpf/8 mm Tg(mrc1a:egfp)y251 transgenic zebrafish (G) with higher magnification view of the boxed region in panel G, showing presumptive lymphatic sacs fusing with the growing lymphatic vessels (arrows) (H). Scale Bars: 250 um. (dpf- days post fertilization, mm-millimeters, mrc1a- mannose receptor C type 1a, GFP- green fluorescent protein)
We continued to evaluate the intracranial lymphatic network throughout the lifespan of the zebrafish to determine if there were any major morphological changes to the network as the zebrafish age. We removed and imaged the inside if the skull of Tg(−5.2lyve1b:DsRed)nz101 zebrafish (Online Fig. VI A) at three different ages, 4.5 months (n = 6), 22 months (n = 4), and 36 months (n = 6) (Online Fig. VI C–E) and the lymphatic area in the imaging field was calculated (Online Fig. VI F–H). The average lymphatic area was compared between the three different age groups and no significant differences were found (Online Fig. VI B). This result differs from the regression of mouse intracranial lymphatic vessels seen with advanced age3.
Immune cell trafficking in meningeal lymphatic vessels.
In addition to draining interstitial fluid, lymphatic vessels have an important function in trafficking of immune cells. We carried out time-lapse confocal imaging of the intact dorsal heads of living casper Tg(mrc1a:egfp)y251, Tg(lyz:DsRed2)nz50 double-transgenic zebrafish to observe whether lyz:dsred+ neurotrophils could be seen transiting through and/or across the walls of mrc1a+ intracranial lymphatics. Live imaging of lymphatic vessels running along the boundary of the cerebellum and optic tectum inside the meninges of an adult casper double transgenic animal (Fig. 7A–C) reveals neutrophils moving slowly through the lymphatic vessels (Fig. 7D–F, Online Video VII), in contrast to the very rapid movement of neutrophils through nearby blood vessels (Online Video VII). In addition to moving through the lymphatics, neutrophils can also occasionally be observed transmigrating from the meningeal interstitial spaces through the lymphatic endothelial wall (Fig. 7G–K, Online Video VIII). Intracranial (Fig. 7L) and external superficial (Fig. 7M) lymphatic vessels both contain abundant numbers of neutrophils. These data suggest that zebrafish intracranial lymphatics play an important role in immune protection of the brain, as has been suggested for mammalian meningeal lymphatics.
Fig. 7. Immune cell trafficking in zebrafish intracranial lymphatics.
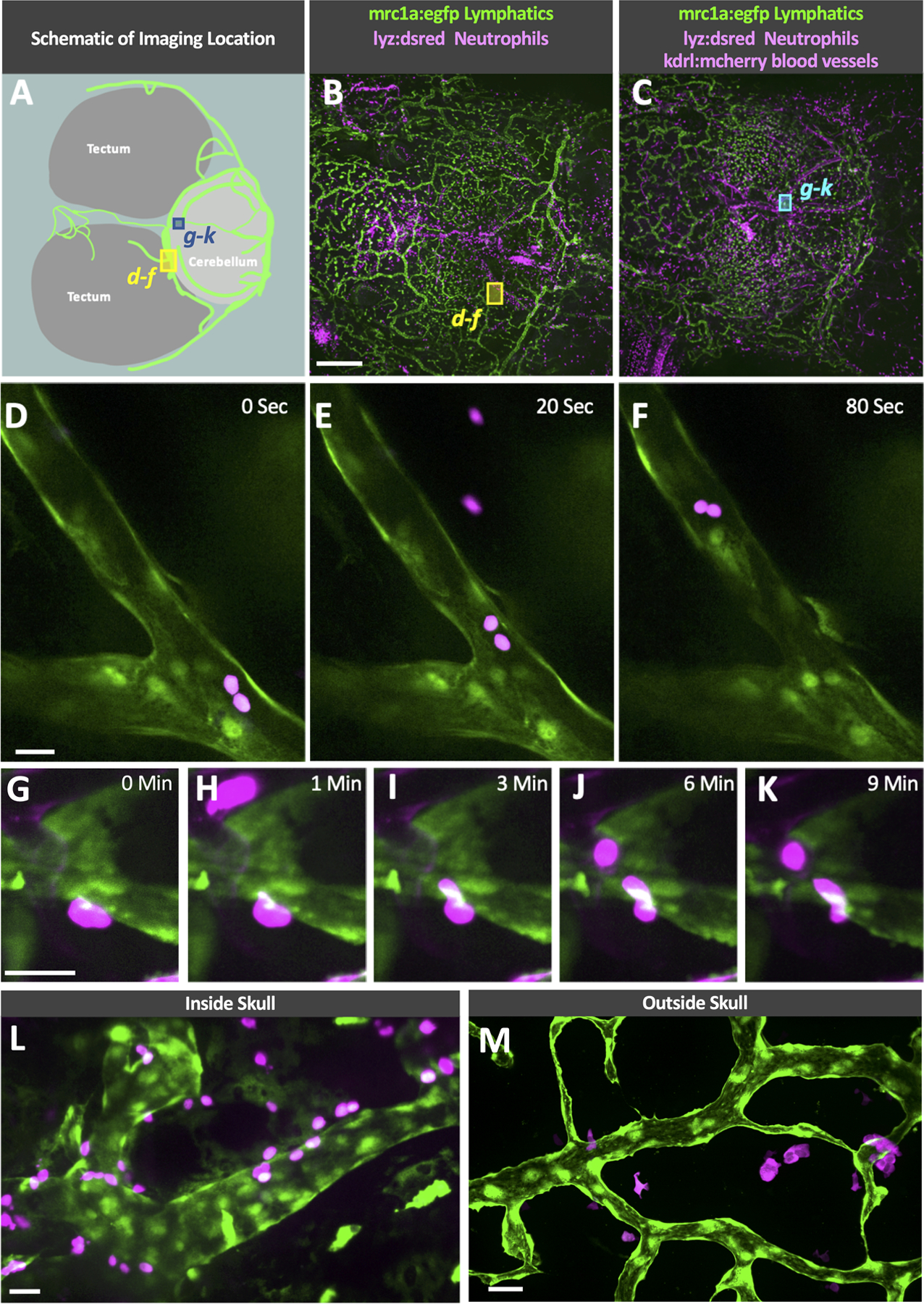
A. Schematic diagram of the optic tecta, cerebellum, and intracranial lymphatics in the dorsal head of an adult casper, Tg(mrc1a:egfp)y251 zebrafish. The blue and yellow boxes note the approximate area shown in the high-magnification images in panels D-F and G-K. B-C. Confocal overview images series through the skull of a living adult casper, Tg(mrc1a:egfp)y251, Tg(lyz:DsRed2)nz50 double transgenic zebrafish (B) and Tg(mrc1a:egfp)y251, Tg(lyz:DsRed2)nz50, Tg(kdrl:mcherry) y206 tripple transgenic zebrafish showing the imaging locations of D-F (yellow box) and G-K (blue box). D-F. Selected frames from a time-lapse confocal image series taken through the skull of a living adult casper, Tg(mrc1a:egfp)y251, Tg(lyz:DsRed2)nz50 double transgenic zebrafish showing neutrophils trafficking through an intracranial lymphatic vessel (see Online Video VII). G-K. Selected frames from a time-lapse confocal image series taken through the skull of a living adult casper, Tg(mrc1a:egfp)y251, Tg(lyz:DsRed2)nz50, Tg(kdrl:mcherry) y206 triple transgenic zebrafish showing a neutrophil transmigrating into an intracranial lymphatic vessel (see Online Video VIII). L. Image taken through the skull of a living adult casper, Tg(mrc1a:egfp)y251, Tg(lyz:DsRed2)nz50 double transgenic zebrafish showing neutrophils on the inside and outside of an intracranial lymphatic vessel. M. Image taken of superficial lymphatics on the dorsal head of a living adult casper, Tg(mrc1a:egfp)y251, Tg(lyz:DsRed2)nz50 double transgenic zebrafish showing neutrophils interacting with a lymphatic vessel outside of the skull. Scale bars: 500 um (B), 25 um (D, G, L, M). (mrc1a- mannose receptor C type 1a, eGFP- green fluorescent protein, lyz-lysozyme)
VEGF-C stimulates lymphangiogenesis in zebrafish intracranial lymphatic vessels.
The growth of mammalian intracranial lymphatic vessels is stimulated by the pro-lymphangiogenic ligand VEGF-C4, 7. To examine whether zebrafish intracranial lymphatic vessels are similarly responsive to VEGF-C, we injected 50 day old casper mutant, Tg(mrc1a:egfp)y251, Tg(kdrl:mcherry y206 double transgenic zebrafish through the dorsal skull (Fig. 8A) with either VEGF-C or PBS together with a 705Qdot tracer. Fish that showed tracer in the meninges (Fig. 8C) were returned to the main zebrafish system and re-imaged six days later. Images taken at the time of injection (Fig. 8C–E) were compared to images taken 6 days post injection (Fig. 8G–I) and the percent increase in dorsal intracranial blood vessel or lymphatic vessel area was measured (Fig. 8F,J). Only a small increase in blood vessels was noted in injected fish, and the increase was not significantly different whether fish were injected with PBS or VEGF-C. In contrast, intracranial lymphatic vessels of fish injected with VEGF-C showed a significantly higher percent area increase compared to the intracranial lymphatic vessels of fish injected with PBS (p = 0.034) (Fig. 8B).
Fig. 8. Injection of VEGF-C stimulates intracranial lymphatic growth in zebrafish.
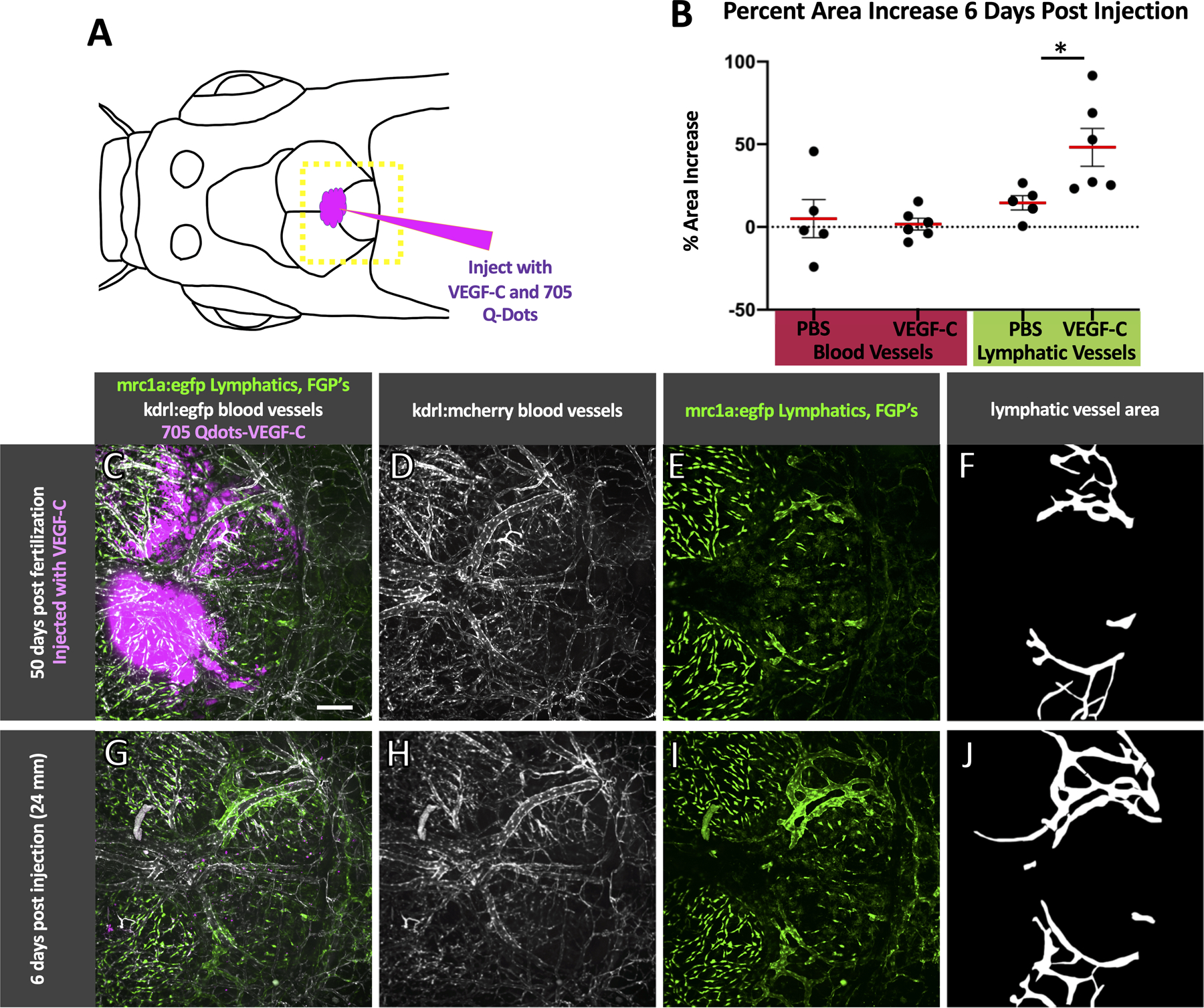
A. Schematic diagram of the intracranial injection of VEGF-C (n = 6) or PBS (n = 5) into 50 dpf casper, Tg(mrc1a:egfp)y251; Tg(kdrl:mcherry) y206 zebrafish (19–25 mm) with the approximate area imaged shown by the dashed yellow box. B. Strip plots with a red line showing the average percent increase in area of blood and lymphatic vessels from the time of injection to six days post injection with error bars showing the standard error of the mean. VEGF-C injected fish showed a significant increase in lymphatic area (Bonferroni-adjusted p = 0.034 Mann-Whitney U test, p < 0.05). C-E. Maximum projection confocal image of the dorsal head of a casper mutant, Tg(mrc1a:egfp)y251; Tg(kdrl:mcherry) y206 double transgenic animal at the time of VEGF-C/705Qdot injection with 705Qdots marking the VEGF-C injection site shown in magenta (C), blood vessels in white (C,D), and FGPs and lymphatics in green (C,E). G-I. The same fish as shown in C-E six days post-injection, showing an increase in lymphatic vessel growth,. 705Qdots shown in magenta (G), blood vessels in white (G,H), and FGPs and lymphatics in green (G,I). F,J. Area selections of lymphatic vessels based on E and I, used for percent area calculations in B. Scale bar: 200 um. (VEGF-C – vascular endothelial growth factor C, dpf- days post fertilization, mm- millimeters, mrc1a- mannose receptor C, type 1a, kdrl-kinase insert domain receptor like, FGPs- fluorescent granular perithelial cells)
DISCUSSION
In this study we use high-resolution confocal imaging of the meninges in living transgenic zebrafish to show that zebrafish possess a complex and robust meningeal lymphatic network comparable to that found in mammals. We use angiography and intracisternal lymphatic drainage assays to confirm that zebrafish meningeal lymphatics are distinct from the blood vascular network and that they drain interstitial fluid from the brain. We show that intracranial lymphatics initially emerge from the dorsal edge of the previously described facial lymphatic network10, first growing along the caudal edge of the cerebellum and optic tecta before forming a more complex network extending further rostrally. We also show that, like the meningeal lymphatics in mammals, these vessels contain immune cells migrating both through and in and out of them. The discovery of intracranial meningeal lymphatics in the zebrafish provides an important new model for experimental analysis of meningeal lymphatic development and function in health and disease.
The Italian anatomist Paolo Mascagni reported the existence of meningeal lymphatics in humans over 200 years ago34, but his early description was largely ignored and mammalian meningeal lymphatics were only “rediscovered” a few years ago1, 2. It is likely that meningeal lymphatics were overlooked for several centuries at least in part because most researchers were examining isolated brains, and mammalian intracranial lymphatics are found exclusively in the dural layer of the meninges that adheres tightly to the skull when the brain is removed1, 2. Although the dural meninges of the zebrafish have not yet been characterized, we show that, as in mammals, dissected zebrafish brains with the skull removed have no lymphatic vessels associated with them while the inner surface of the zebrafish skull has a complex lymphatic vascular network comparable to that observed in mammals, suggesting that zebrafish intracranial lymphatics may also reside within a dural meningeal layer (Fig 1). The elaborate branched network of zebrafish meningeal lymphatics also shares other features with mammalian meningeal lymphatics, including alignment along dural sinuses and drainage of CSF2, 3.
There are a number of likely reasons why zebrafish meningeal lymphatics were overlooked up to now, despite the availability of transgenic lines enabling their visualization. First, most zebrafish researchers study vascular development in embryonic or early larval animals (up to 5–7 days old), and meningeal lymphatics do not even begin to form until several days after this, relatively late in development (as in mammals). Second, the zebrafish skull is quite thin and zebrafish meningeal lymphatics develop immediately below the skull, making it relatively easy on casual examination to mistake intracranial lymphatics for the abundant superficial extracranial lymphatics found later in development and in adult fish. Third, as in mammals, most zebrafish researchers study brain-associated vessels by first removing the skull, which also removes the dural meninges, precluding observation of the associated dural meningeal lymphatics.
We and others recently reported unusual macrophage-like, highly phagocytic cells closely associated with blood vessels in the brain-associated leptomeninges, that despite their macrophage-like morphology display a transcriptional profile highly similar to lymphatic endothelial cells19–21. Although these perivascular cells (called FGPs, Mato cells, muLECs, or BLECs in different reports) have a lymphatic molecular identity, they do not form tubes, at least not under normal physiological conditions, and our findings show that these “lymphatic-like” cells are only present in the brain-associated meninges, unlike the bona fide lymphatic vascular network present only in the skull-associated meninges (Fig 1). Since FGPs and meningeal lymphatics are actually both external to the brain proper, and since FGPs in particular represent a distinct cell population from vessel-forming lymphatic endothelial cells in meningeal lymphatics, we would suggest that “brain lymphatic endothelial cells” (BLECs) represents a less appropriate designation for Mato cells/FGPs/muLECs. FGPs and meningeal lymphatics also appear to have very distinct functions- FGPs absorb extracellular materials and sequester them intracellularly (Online Fig. IV), while meningeal lymphatics drain extracellular fluid through an elaborate network of tubes and serve as a route for immune cell trafficking, although future studies may reveal functional interconnections between FGPs and meningeal lymphatics.
By imaging large numbers of animals at different stages of larval and juvenile development we have shown that zebrafish meningeal lymphatics originate from the facial lymphatic plexus prior to skull formation, disconnecting from the facial lymphatics as the skull plates continue to grow and fuse (Fig. 4,5, Online Fig. V). In mammals, the first meningeal lymphatic vessels appear at a reasonably comparable anatomical location at the foramen magnum, although in contrast to fish they first begin to appear after the skull has already developed4. Following initial zebrafish intracranial lymphatic network formation at the caudal margins of the cerebellum and optic tecta, further meningeal lymphatic development continues through rostral growth of new lymphatic vessels from this primary plexus. These new lymphatic vessels most often grow alongside major dural blood vessels (Fig. 6A–D), with growth apparently taking place both by lymphangiogenic sprouting from the preexisting plexus as well as by formation and incorporation of lymphatic cell clusters or sacs (Fig. 6E–H), as has been reported for growing meningeal lymphatics in mice4, 35. In mice the dorsal meningeal lymphatics grow along the transverse sinus, the confluence of sinuses, the superior sagittal sinus, and the rostral rhinal vein4. These sinuses have yet to be described in zebrafish, but the pattern of lymphatic vessel growth is similar though the brain regions are different in zebrafish. Zebrafish intracranial lymphatic vessels grow along meningeal blood vessels that surround the cerebellum and between the optic tecta, growing toward the telencepholon with some lymphatic vessels branching out over the cerebellum and tecta.
In mice, interstitial and cerebrospinal fluid drain through dorsal meningeal lymphatic vessels5 and basal meningeal lymphatic vessels3 emptying into the deep cervical node. Cerebrospinal fluid produced in the choroid plexus flows into the subarachnoid space where it is believed to be drained by arachnoid villi36 and the meningeal lymphatic network1, 2, 4, 3, 5, 6. In addition to draining cerebrospinal fluid and interstitial fluid, the meningeal lymphatic network also drains macromolecules, cellular waste products, and serves as a conduit for trafficking of immune cells37. Our studies suggest that zebrafish meningeal lymphatics also drain interstitial and cerebrospinal fluid and actively traffic immune cells, although due to the challenges of imaging very deep structures in juvenile and adult animals the precise drainage points for this network remain to be determined. At this point we cannot distinguish whether zebrafish intracranial lymphatics drain directly into blood vessels at or near the base of the skull or if they exit the skull and connect to other lymphatic vessels. The possibility that the dorsal meningeal lymphatic network of the zebrafish drains into an as-yet-undiscovered structure analogous to the deep cervical lymph node also cannot be ruled out.
VEGF-C is known to stimulate lymphatic growth in intracranial vessels in mammals4, 7 and we show that zebrafish intracranial lymphatic vessels are similarly sensitive to VEGF-C (Fig 8). In addition to confirming that zebrafish intracranial lymphatic vessels are similar to mammalian structures, we also show that zebrafish can tolerate intracranial injection using a standard injection tools common to most zebrafish laboratories, demonstrating the usefulness of the zebrafish as a model for screening the effect of intracranially injected drugs and compounds on the development and function of intracranial lymphatic vessels.
The existence of a meningeal lymphatic network in the zebrafish provides a unique and useful new model for visualization and for experimental and genetic manipulation of this critical vascular system. The thin skull of the zebrafish permits high resolution optical imaging of meningeal lymphatics in living adult animals, while the vast array of transgenic tools, mutants, and disease models available in the fish facilitates investigation of the development, anatomy, and function of this system. Although zebrafish provide many genetic and experimental advantages for studying meningeal lymphatics, they are phylogenetically further from humans than mice, have substantially different brain structure, and live in an aquatic vs. terrestrial environment, and it still remains to be seen how closely fish meningeal morphology and function will continue to resemble that of humans and other mammals. On the other hand, there are useful zebrafish models for a number of diseases in which meningeal function has been implicated. As noted in the introduction, exciting new research has suggested that intracranial lymphatics play important roles in and/or may provide an important gateway for treatment of a variety of brain pathologies including Alzheimer’s disease6, brain cancer7, 38, 39, multiple sclerosis5, and meningitis. Zebrafish models of meningitis have in fact recently been developed40, 41 and examining the role of meningeal lymphatic vessels in these models should prove to be a fruitful avenue of study. Overall, the ability to carry out high resolution optical imaging and experimental and genetic manipulation in the fish, and to maintain large number of fish and to obtain enormous numbers of their embryonic and larval progeny for analysis, make this a superb model to pursue large scale genetic and chemical screens, as well as other future studies aimed at understanding more about this important but heretofore elusive vascular system and how it functions in health and disease.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
Lymphatic vessels associated with the mammalian brain were first described in 2015
Intracranial lymphatic vessels play important roles in brain homeostasis and brain pathologies, and provide a potential new avenue for treatment of brain cancers
Due to their location inside the skull, intracranial lymphatics are difficult to visualize and study in most mammals
What New Information Does This Article Contribute?
Zebrafish, a genetically and experimentally accessible vertebrate model organism, have intracranial lymphatic vessels similar to those of mammals
Using optically clear transgenic zebrafish, we show that intracranial lymphatics can be imaged easily in living animals, providing an exciting new model for visualization and experimental analysis of these important vessels
Since the discovery of lymphatic vessels associated with the mammalian nervous system in 2015, a variety of studies have highlighted their important role in fluid balance within the nervous system, in neurodegenerative diseases, and their potential as a possible novel route for the treatment of brain tumors. Although mice provide an excellent model to study these vessels, the ability to perform high resolution live imaging is limited and difficult. Here we show that the zebrafish, a genetically and experimentally accessible model vertebrate, has intracranial lymphatic vessels comparable to those of mammals that can be easily imaged through adulthood. We describe the emergence and development of intracranial lymphatics in the fish, and document immune cell trafficking through and into these vessels. Combined with a plethora of available transgenic lines facilitating visualization of a wide range of different tissues and cell types, the optical clarity of the developing fish together with the ability of zebrafish to produce large numbers of offspring that can be easily treated with chemicals and drugs makes this an exciting new model to understand the role of intracranial lymphatic vessels in health and disease.
ACKNOWLEDGMENTS
The authors would like to thank members of the Weinstein laboratory for their critical comments on this manuscript. The Authors would also like to thank the Research Animal Branch of the Eunice Kennedy Shriver National Institute of Child Health and Human Development as well as the Charles River Staff for excellent animal care and husbandry. The authors are grateful to Dr. Gennady Margolin of the Bioinformatics and Scientific Programing Core, Eunice Kennedy Shriver National Institute of Child Health and Human Development, for assistance with statistical analysis.
SOURCES OF FUNDING
This work was supported by the intramural program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (ZIA-HD008915, ZIA-HD008808, and ZIA-HD001011, to BMW).
Nonstandard Abbreviations and Acronyms:
- VEGF-C
Vascular Endothelial Growth Factor C
- FGPs
Fluorescent Granular Perithelial cells
- muLECs
meningeal mural Lymphatic Endothelial Cells
- BLECs
Brain Lymphatic Endothelial cells
- CSF
cerebrospinal fluid
- mrc1a
mannose receptor, C type 1a
- lyve1b
lymphatic vessel endothelial hyaluronic receptor 1b
- dpf
Days Post Fertilization
Footnotes
Publisher's Disclaimer: This article is published in its accepted form. It has not been copyedited and has not appeared in an issue of the journal. Preparation for inclusion in an issue of Circulation Research involves copyediting, typesetting, proofreading, and author review, which may lead to differences between this accepted version of the manuscript and the final, published version.
DISCLOSURES
None.
SUPPLEMENTAL MATERIALS
Online Figures I-VI
Online Videos I-VIII
Major Resources Table
REFERENCES
- 1.Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, Alitalo K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212:991–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahn JH, Cho H, Kim JH, Kim SH, Ham JS, Park I, Suh SH, Hong SP, Song JH, Hong YK, et al. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature. 2019;572:62–66 [DOI] [PubMed] [Google Scholar]
- 4.Antila S, Karaman S, Nurmi H, Airavaara M, Voutilainen MH, Mathivet T, Chilov D, Li Z, Koppinen T, Park JH, et al. Development and plasticity of meningeal lymphatic vessels. J Exp Med. 2017;214:3645–3667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Louveau A, Herz J, Alme MN, Salvador AF, Dong MQ, Viar KE, Herod SG, Knopp J, Setliff JC, Lupi AL, et al. Cns lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat Neurosci. 2018;21:1380–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Da Mesquita S, Louveau A, Vaccari A, Smirnov I, Cornelison RC, Kingsmore KM, Contarino C, Onengut-Gumuscu S, Farber E, Raper D, et al. Functional aspects of meningeal lymphatics in ageing and alzheimer’s disease. Nature. 2018;560:185–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song E, Mao T, Dong H, Boisserand LSB, Antila S, Bosenberg M, Alitalo K, Thomas JL, Iwasaki A. Vegf-c-driven lymphatic drainage enables immunosurveillance of brain tumours. Nature. 2020;577:689–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Absinta M, Ha SK, Nair G, Sati P, Luciano NJ, Palisoc M, Louveau A, Zaghloul KA, Pittaluga S, Kipnis J, et al. Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by mri. Elife. 2017;6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jung HM, Castranova D, Swift MR, Pham VN, Venero Galanternik M, Isogai S, Butler MG, Mulligan TS, Weinstein BM. Development of the larval lymphatic system in zebrafish. Development. 2017;144:2070–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okuda KS, Astin JW, Misa JP, Flores MV, Crosier KE, Crosier PS. Lyve1 expression reveals novel lymphatic vessels and new mechanisms for lymphatic vessel development in zebrafish. Development. 2012;139:2381–2391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawson ND, Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol. 2002;248:307–318 [DOI] [PubMed] [Google Scholar]
- 12.van Impel A, Zhao Z, Hermkens DM, Roukens MG, Fischer JC, Peterson-Maduro J, Duckers H, Ober EA, Ingham PW, Schulte-Merker S. Divergence of zebrafish and mouse lymphatic cell fate specification pathways. Development. 2014;141:1228–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yaniv K, Isogai S, Castranova D, Dye L, Hitomi J, Weinstein BM. Imaging the developing lymphatic system using the zebrafish. Novartis Found Symp. 2007;283:139–148; discussion 148–151, 238–141 [DOI] [PubMed] [Google Scholar]
- 14.Kuchler AM, Gjini E, Peterson-Maduro J, Cancilla B, Wolburg H, Schulte-Merker S. Development of the zebrafish lymphatic system requires vegfc signaling. Curr Biol. 2006;16:1244–1248 [DOI] [PubMed] [Google Scholar]
- 15.Cha YR, Fujita M, Butler M, Isogai S, Kochhan E, Siekmann AF, Weinstein BM. Chemokine signaling directs trunk lymphatic network formation along the preexisting blood vasculature. Dev Cell. 2012;22:824–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicenboim J, Malkinson G, Lupo T, Asaf L, Sela Y, Mayseless O, Gibbs-Bar L, Senderovich N, Hashimshony T, Shin M, et al. Lymphatic vessels arise from specialized angioblasts within a venous niche. Nature. 2015;522:56–61 [DOI] [PubMed] [Google Scholar]
- 17.Harrison MR, Feng X, Mo G, Aguayo A, Villafuerte J, Yoshida T, Pearson CA, Schulte-Merker S, Lien CL. Late developing cardiac lymphatic vasculature supports adult zebrafish heart function and regeneration. Elife. 2019;8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gancz D, Raftrey BC, Perlmoter G, Marin-Juez R, Semo J, Matsuoka RL, Karra R, Raviv H, Moshe N, Addadi Y, et al. Distinct origins and molecular mechanisms contribute to lymphatic formation during cardiac growth and regeneration. Elife. 2019;8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Venero Galanternik M, Castranova D, Gore AV, Blewett NH, Jung HM, Stratman AN, Kirby MR, Iben J, Miller MF, Kawakami K, et al. A novel perivascular cell population in the zebrafish brain. Elife. 2017;6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bower NI, Koltowska K, Pichol-Thievend C, Virshup I, Paterson S, Lagendijk AK, Wang W, Lindsey BW, Bent SJ, Baek S, et al. Mural lymphatic endothelial cells regulate meningeal angiogenesis in the zebrafish. Nat Neurosci. 2017;20:774–783 [DOI] [PubMed] [Google Scholar]
- 21.van Lessen M, Shibata-Germanos S, van Impel A, Hawkins TA, Rihel J, Schulte-Merker S. Intracellular uptake of macromolecules by brain lymphatic endothelial cells during zebrafish embryonic development. Elife. 2017;6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gore AV, Swift MR, Cha YR, Lo B, McKinney MC, Li W, Castranova D, Davis A, Mukouyama YS, Weinstein BM. Rspo1/wnt signaling promotes angiogenesis via vegfc/vegfr3. Development. 2011;138:4875–4886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall C, Flores MV, Storm T, Crosier K, Crosier P. The zebrafish lysozyme c promoter drives myeloid-specific expression in transgenic fish. BMC Dev Biol. 2007;7:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh SP, Holdway JE, Poss KD. Regeneration of amputated zebrafish fin rays from de novo osteoblasts. Dev Cell. 2012;22:879–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White RM, Sessa A, Burke C, Bowman T, LeBlanc J, Ceol C, Bourque C, Dovey M, Goessling W, Burns CE, et al. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell. 2008;2:183–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu C, Volkery S, Siekmann AF. Intubation-based anesthesia for long-term time-lapse imaging of adult zebrafish. Nat Protoc. 2015;10:2064–2073 [DOI] [PubMed] [Google Scholar]
- 27.Yaniv K, Isogai S, Castranova D, Dye L, Hitomi J, Weinstein BM. Live imaging of lymphatic development in the zebrafish. Nat Med. 2006;12:711–716 [DOI] [PubMed] [Google Scholar]
- 28.Bensimon-Brito A, Cardeira J, Dionisio G, Huysseune A, Cancela ML, Witten PE. Revisiting in vivo staining with alizarin red s--a valuable approach to analyse zebrafish skeletal mineralization during development and regeneration. BMC Dev Biol. 2016;16:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brugman S, Liu KY, Lindenbergh-Kortleve D, Samsom JN, Furuta GT, Renshaw SA, Willemsen R, Nieuwenhuis EE. Oxazolone-induced enterocolitis in zebrafish depends on the composition of the intestinal microbiota. Gastroenterology. 2009;137:1757–1767 e1751 [DOI] [PubMed] [Google Scholar]
- 30.Jung HM, Isogai S, Kamei M, Castranova D, Gore AV, Weinstein BM. Imaging blood vessels and lymphatic vessels in the zebrafish. Methods Cell Biol. 2016;133:69–103 [DOI] [PubMed] [Google Scholar]
- 31.Padberg Y, Schulte-Merker S, van Impel A. The lymphatic vasculature revisited-new developments in the zebrafish. Methods Cell Biol. 2017;138:221–238 [DOI] [PubMed] [Google Scholar]
- 32.Venero Galanternik M, Stratman AN, Jung HM, Butler MG, Weinstein BM. Building the drains: The lymphatic vasculature in health and disease. Wiley Interdiscip Rev Dev Biol. 2016;5:689–710 [DOI] [PubMed] [Google Scholar]
- 33.Prentice AI. Autofluorescence of bone tissues. J Clin Pathol. 1967;20:717–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sandrone S, Moreno-Zambrano D, Kipnis J, van Gijn J. A (delayed) history of the brain lymphatic system. Nat Med. 2019;25:538–540 [DOI] [PubMed] [Google Scholar]
- 35.Izen RM, Yamazaki T, Nishinaka-Arai Y, Hong YK, Mukouyama YS. Postnatal development of lymphatic vasculature in the brain meninges. Dev Dyn. 2018;247:741–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weed LH. Studies on cerebro-spinal fluid. No. Iii : The pathways of escape from the subarachnoid spaces with particular reference to the arachnoid villi. J Med Res. 1914;31:51–91 [PMC free article] [PubMed] [Google Scholar]
- 37.Papadopoulos Z, Herz J, Kipnis J. Meningeal lymphatics: From anatomy to central nervous system immune surveillance. J Immunol. 2020;204:286–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV. Blood-brain barrier: From physiology to disease and back. Physiol Rev. 2019;99:21–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu X, Deng Q, Ma L, Li Q, Chen Y, Liao Y, Zhou F, Zhang C, Shao L, Feng J, et al. Meningeal lymphatic vessels regulate brain tumor drainage and immunity. Cell Res. 2020;30:229–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patterson H, Saralahti A, Parikka M, Dramsi S, Trieu-Cuot P, Poyart C, Rounioja S, Ramet M. Adult zebrafish model of bacterial meningitis in streptococcus agalactiae infection. Dev Comp Immunol. 2012;38:447–455 [DOI] [PubMed] [Google Scholar]
- 41.Jim KK, Engelen-Lee J, van der Sar AM, Bitter W, Brouwer MC, van der Ende A, Veening JW, van de Beek D, Vandenbroucke-Grauls CM. Infection of zebrafish embryos with live fluorescent streptococcus pneumoniae as a real-time pneumococcal meningitis model. J Neuroinflammation. 2016;13:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and materials have been made publicly available through the Zebrafish International Resource Center (https://zebrafish.org) or by contacting the Weinstein Laboratory (https://www.nichd.nih.gov/research/atNICHD/Investigators/weinstein).


