Summary
Background:
Risk-based screening for type 2 diabetes (T2D) in youth with overweight/obesity is recommended, but rates remain low in practice. Identification of factors impacting provider ordering and patient completion of testing may guide strategies to improve screening.
Objective:
To evaluate predictors of hemoglobin A1c (A1c)-based T2D screening in pediatric primary care.
Methods:
This retrospective cohort study included 10 to 18 year-old patients with overweight/obesity (body mass index [BMI] Z-score ≥1.04) followed in a large academic-affiliated pediatric primary care network, 2009 to 2018. Percentages of patients with ordered and completed A1c were determined, and multivariable Cox proportional hazards regression was used to evaluate independent predictors of screening.
Results:
34 927 (48.0% female; 52.5% with BMI Z-score ≥1.64) youth followed for a median of 3.0 years were included. 21% (7457) of patients had screening ordered and 14% (4966) completed screening during follow-up. In multivariable regression, after controlling for race/ethnicity, BMI, family history of diabetes and age, males were significantly less likely to have ordered screening, but were equally or more likely to complete screening if ordered.
Conclusions:
Male adolescents were less likely to undergo A1c-based T2D screening due to differential ordering practices. The source of this differential practice should be pursued to avoid under-recognition of cardiometabolic risk in at-risk male youth.
Keywords: adolescent, body mass index, glycated hemoglobin a, obesity, overweight
1 ∣. INTRODUCTION
Youth-onset type 2 diabetes (T2D) has been rising by 5% annually in the United States1 and is associated with cardiometabolic complications.2 Since 2000, the American diabetes association has recommended screening in youth age ≥10 years who have overweight/obesity (body mass index [BMI] ≥85% for age/sex) with ≥2 additional risk factors (family history; native American, African American, Latino, Asian and Pacific Islander race/ethnicity; signs/conditions associated with insulin resistance: acanthosis nigricans, polycystic ovary syndrome [PCOS], dyslipidemia, hypertension and small-for-gestational-age).3 T2D is more common in female than male adolescents,1,4,5 but guidelines have not recommended sex-based screening.
Although early treatment is associated with durable metabolic control,6 more than half of at-risk adolescents are not screened.7,8 Previous work demonstrated that T2D screening is appropriately completed more often in youth with T2D family history and those with higher BMI percentile, but also more often among females.9 The origin of these differences, whether due to provider ordering practice or to patient follow-through, has not been explored. To inform screening implementation, we investigated factors associated with ordered and completed T2D screening in youth who had overweight or obesity across a large, diverse pediatric primary care network.
2 ∣. METHODS
2.1 ∣. Study population
This retrospective cohort study included youth ages 10 to 18 years without known diabetes mellitus (International classification of diseases, ninth Revision, clinical modification (ICD-9-CM) [249.x, 250.x]; ICD-10-CM [E08.x-E.11x., E13.x]) who had ≥1 visit in the children's hospital of Philadelphia [CHOP] primary care network January 2009 to August 2018. Only visits at which a patient had overweight/obesity (BMI ≥85%, or BMI Z-score ≥1.04 based on sex-/age-specific United States centers for disease control references10,11) were included. The CHOP institutional review board approved the study.
2.2 ∣. Statistical analysis
Although T2D may also be diagnosed using plasma glucose,12 only A1c (commonly used by pediatricians9 before endorsement in 201013) was investigated to ensure testing was for diabetes identification. The percent of patients in whom A1c was ordered or completed, and the percent found to have pre-diabetes-range (5.7%-6.4% [39-46 mmol/mol]) or diabetes-range (≥6.5% [48 mmol/mol]) A1c were reported by sex and weight category (overweight: BMI Z-score 1.04-1.63 vs obese: BMI Z-score ≥1.64).14 Continuous data, all right-skewed, were summarized using median and interquartile range (IQR). Pairwise comparisons were made using non-parametric Kruskal-Wallis test by ranks with Dunn test and Bonferroni adjustment for multiple comparisons. Categorical variables were summarized using proportions and distributions compared using chi.2
Predictors of ordered and completed tests were identified using multivariable multiple failure Cox proportional hazards regression with the Andersen-Gill formulation. Patients were censored after one diabetes-range A1c or at end of follow-up (age 19, or last visit). Covariates included BMI-Z, sex, age, year and race/ethnicity (Non-Hispanic White [NHW], Non-Hispanic Black [NHB], Hispanic, Asian/Native Hawaiian/Pacific Islander/American Indian/Alaskan Native [A/NH/PI/AI/AN], and “other” including unreported), and whether a phlebotomy laboratory was present onsite (completion models). The proportional hazards assumption was assessed graphically. In sensitivity analysis, patients with PCOS (ICD-9-CM 256.4, ICD-10-CM E28.2), which only impacts females and could contribute to sex-based discrepancies in screening, were excluded.
Two-sided P values <.05 were considered statistically significant. Analyses were performed using Stata 14 (StataCorp LP, College Station, TX).
3 ∣. RESULTS
3.1 ∣. Cohort characteristics
The cohort consisted of 34 927 (48% female) patients who had overweight/obesity (Table 1) followed for a median (IQR) of 3.0 (1.1-4.9) years. Patients with obesity were younger at baseline, with longer follow-up and more visits, had higher proportion of documented diabetes family history, and were more often non-NHW (P < .0001 for each). Follow-up was slightly longer for females. Diabetes family history was more commonly recorded for females than males with obesity (10.4% vs 7.8%, P < .0001).
TABLE 1.
Cohort Characteristics, by Weight status and sex
| Overweighta |
Obesea |
||||||
|---|---|---|---|---|---|---|---|
| Females | Males | P (F v M) | Females | Males | |||
| 8294 | 8289 | 8453 | 9891 | 5P (across all groups) |
|||
| n (% of cohort) | 23.7% | 23.7% | 24.2% | 28.3% | p (F v M) | ||
| BMI-Z during follow-up (median, IQR) | 1.23 (1.14-1.35) | 1.25 (1.15-1.37) | .04 | 1.85 (1.64-2.15) | 1.86 (1.64-2.16) | 0.2 | <.001 |
| Age (Years) at start of follow-up (median, IQR) | 11.9 (10.7-13.7) | 11.6 (10.6-13.6) | .003 | 10.9 (10.4-11.7) | 10.9 (10.4-11.8) | 0.2 | <.001 |
| Family history of diabetes, n (%) | 383 (4.6%) | 303 (3.7%) | .08 | 881 (10.4%) | 776 (7.8%) | < 0.001 | <.001 |
| Number of visits (median, IQR) | 2 (1–3) | 2 (1-3) | <.001 | 4 (3-5) | 4 (3-5) | 0.3 | <.001 |
| Years of follow-up (median, IQR) | 1.44 (0.00-3.39) | 1.21 (0.00-3.10) | <.001 | 4.16 (2.55-5.79) | 4.05 (2.44-5.66) | < 0.001 | <.001 |
| PCOS, n (% of females) | 37 (0.4%) | 154 (1.8%) | <.001 | ||||
| Race/ethnicity, n (%) | |||||||
| NHW | 4754 | 5039 | .2 | 3570 | 5063 | 0.05 | <.001 |
| 57.3% | 60.8% | 42.2% | 51.2% | ||||
| NHB | 2247 | 2008 | 3662 | 3246 | |||
| 27.1% | 24.2% | 43.3% | 32.8% | ||||
| Hispanic | 405 | 327 | 518 | 593 | |||
| 4.9% | 3.9% | 6.1% | 6.0% | ||||
| Asian/Native Hawaiian/Pacific Islander/American Indian/Alaskan Native | 192 | 216 | 143 | 220 | |||
| 2.3% | 2.6% | 1.7% | 2.2% | ||||
| Other | 696 | 699 | 560 | 769 | |||
| 8.4% | 8.4% | 6.6% | 7.8% | ||||
Overweight: BMI-Z 1.04 to 1.63 at all visits during follow-up; obese: BMI-Z ≥1.64 at least once during follow-up, otherwise 1.04 to 1.63.
Note: P values determined by Kruskal-Wallis test by ranks with Dunn test and Bonferroni correction for multiple comparisons.
Abbreviations: IQR, interquartile range; NHA, non-Hispanic white; NHB, non-Hispanic black; PCOS, polycystic ovary syndrome.
3.2 ∣. Screening and identification of pre-diabetes or T2D
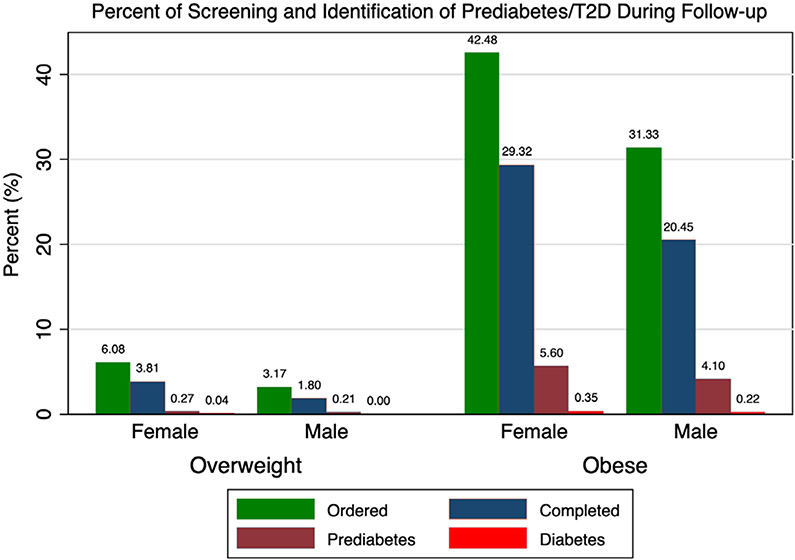
Overall, 21% (7457) of patients had screening ordered and 14% (4966) completed screening. Screening differed by sex, with females undergoing ordered and completed screening 30% (youth with obesity) to 70% (youth with overweight) more often than males (P < .0001 for each; Figure 1). No sex difference was found in pre-diabetes identification among patients with overweight who completed screening or among the cohort overall (completed: 7.0% of females vs 11.4% of males, P = 0.1; overall: 0.3% of females vs 0.2% of males, P = .4). Among patients with obesity, no sex difference in prediabetes prevalence was found among those who completed screening (females: 19.3% vs males: 20.2%, P = .5), but pre-diabetes was identified in more females than males with obesity overall (5.6% vs 4.1%, P < .0001). T2D prevalence did not differ by sex among those who completed testing (females: 1.2% vs males: 1.1%, P = .7) or overall among patients with obesity (females: 0.35% vs males: 0.22%, P = .09).
FIGURE 1.
Percent of cohort with ordered A1c, completed A1c, pre-diabetes-range A1c (5.7-6.4% [39-46 mmol/mol]), and diabetes-range A1c (≥ 6.5% [48 mmol/mol]) during follow-up. Within each weight group, females were more likely to have ordered and completed screening than males (P < .0001 for each). Pre-diabetes was identified in more females than males among patients with obesity (P < .0001) but not overweight (P = .4). Percent of patients with identified T2D did not differ by sex in either weight group (P > .05 for each comparison)
3.3 ∣. Predictors of ordering and completion of ordered screening
3.3.1 ∣. Ordered screening
Among patients with overweight status, ordered screening was more likely in NHB (HR 1.5, 95% CI 1.3-1.8) and Hispanic (HR 1.7, 95% CI 1.3-1.8) youth, higher BMI-Z (per 1.0 increase, HR 50.1, 95% CI 33.6-74.8), documented diabetes family history (HR 1.7, 95% CI 1.3-2.3), and more recent years (HR 1.2, 95% CI 1.1-1.2). Ordered screening was less likely among males (HR 0.6, 95% CI 0.5-0.7) but did not differ by age.
Among patients with obesity, ordered screening was again more likely among non-NHW youth (NHB HR 1.2, 95% CI 1.1-1.2; Hispanic HR 1.2, 95% CI 1.1-1.4; A/NH/PI/AI/AN HR 1.3, 95% CI 1.2-1.6; “other” HR 1.1, 95% CI 1.0-1.3) and higher BMI-Z (HR 4.7, 95% CI 4.4-4.9). Again, males (HR 0.7, 95% CI 0.6-0.7) were less likely to have ordered screening. Older patients (HR 0.51, 95% CI 0.49-0.53) were less likely to have screening ordered. Family history and year were not significant. Exclusion of patients with PCOS did not significantly alter hazard ratio estimates or significance.
3.3.2 ∣. Completed screening
Among patients with overweight status who had screening ordered, completion was more likely among youth with higher BMI-Z (HR 2.2, 95% CI 1.2-4.0) and those attending practices with onsite phlebotomy (HR 5.4, 95% CI 4.1-7.0) and less likely among older youth (HR 0.94, 95% CI 0.91-0.98) and in more recent years (HR 0.93, 95% CI 0.89-0.98). Sex, race/ethnicity and family history did not predict completion.
In patients with obesity, completion was more likely among males (HR 1.05, 95% CI 1.00-1.11), Hispanic youth (HR 1.2, 95% CI 1.1-1.3), and youth attending practices with onsite phlebotomy (HR 2.8, 95% CI 2.6-2.9). Completion was less likely with increasing age (HR 0.87, 95% CI 0.86-0.89), increasing BMI-Z (HR 0.88, 95% CI 0.82-0.95), and in more recent years (HR 0.94, 95% CI 0.93-0.95). Family history was not significant. Exclusion of patients with PCOS did not significantly alter hazard ratio estimates or significance.
4 ∣. DISCUSSION
Although T2D is more common in female than male adolescents,1,4,5 the reverse sex pattern is recognized for pre-diabetes, a condition also associated with cardiometabolic risk.15 Additionally, T2D is more common among adult men than women.16 Despite the absence of sex-based screening recommendations, a previous study found that completion of T2D screening was greater among female than male adolescents.9 Our findings confirmed this observation and identified its origin: provider-level differences in screen-ordering, even after adjustment for T2D risk factors.
Due to the asymptomatic nature of pre-diabetes and early T2D, screening is necessary to identify at-risk patients. The impact of surveillance is evident when our cohort's identified A1c-defined pre-diabetes prevalence (obese: 4.8%, overweight: 0.2%) is compared to that from a study by Andes et al using data from the national health and nutrition examination study, in which the entire cohort underwent screening (obese: 8%, overweight: 4.2%).15 As T2D disproportionately impacts racial- and ethnic-minority youth17 and our proportion of NHB and Hispanic youth was nearly identical to that of Andes et al (37% vs 34%),15 our lower identified prevalence was likely due to under-recognition. Furthermore, assuming males truly have a higher pre-diabetes risk,15 our finding of equal or lower pre-diabetes prevalence in males raises the possibility that male youth at highest risk were missed.
The identified sex-based differences in ordering were not explained by documented PCOS. Differential ordering may be related to provider knowledge of higher T2D prevalence in females; however, screening guidelines do not include sex as a criterion. Providers may also order more obesity-related screening in girls in an effort to garner more lifestyle change buy-in, which may be more challenging for families of females given greater parental underestimation of overweight in girls than boys.18 Qualitative studies are needed to explore this possibility.
Limitations related to the retrospective nature of our study should be considered. We assessed only A1c-based screening and were unable to determine the overall screening rate. Due to differential follow-up, screening rates for patients with overweight vs obesity cannot be directly compared. Family history may have been ascertained differently depending on the provider's level of concern for diabetes. Finally, differences in exam findings that may prompt screening, such as acanthosis nigricans, were not possible to assess due to limitations in the automated electronic medical record extraction required due to the large cohort.
In conclusion, despite no sex-based risk stratification in pediatric T2D screening guidelines, male youth were less likely to have A1c-based diabetes screening ordered and ultimately had lower screening rates. The source of this differential practice should be pursued to avoid under-recognition of cardiometabolic risk in at-risk male youth.
ACKNOWLEDGEMENTS
The authors thank Joanna Abaraoha, BS, for her assistance with data collection.
Funding information
Cystic Fibrosis Foundation, Grant/Award Numbers: PROMISE-OGTT18K0, RUBEN16A0; Eunice Kennedy Shriver National Institute of Child Health and Human Development, Grant/Award Numbers: R01HD074559, R01HD091185, UH3HD087979; Juvenile Diabetes Research Foundation United States of America; M-Diabetes Center of Excellence; National Institute of Diabetes and Digestive and Kidney Diseases, Grant/Award Numbers: 2R56 DK97830-06, 5K12DK094723-08, R01DK110749, R01DK115648, R01DK120886
Footnotes
CONFLICT OF INTEREST
No conflict of interest was declared.
REFERENCES
- 1.Mayer-Davis EJ, Lawrence JM, Dabelea D, et al. Incidence trends of type 1 and type 2 diabetes among youths, 2002-2012. N Engl J Med. 2017;376(15):1419–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Copeland KC, Zeitler P, Geffner M, et al. Characteristics of adolescents and youth with recent-onset type 2 diabetes: the TODAY cohort at baseline. J Clin Endocrinol Metab. 2011;96(1):159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Diabetes Association. Type 2 diabetes in children and adolescents. Diabetes Care. 2000;23(3):381–389. [DOI] [PubMed] [Google Scholar]
- 4.Haines L, Wan KC, Lynn R, Barrett TG, Shield JP. Rising incidence of type 2 diabetes in children in the U.K. Diabetes Care. 2007;30(5): 1097–1101. [DOI] [PubMed] [Google Scholar]
- 5.Fagot-Campagna A, Pettitt DJ, Engelgau MM, et al. Type 2 diabetes among north American children and adolescents: an epidemiologic review and a public health perspective. J Pediatr. 2000;136(5): 664–672. [DOI] [PubMed] [Google Scholar]
- 6.Bacha F, Cheng P, Gal RL, et al. Initial presentation of type 2 diabetes in adolescents predicts durability of successful treatment with metformin Monotherapy: insights from the pediatric diabetes consortium T2D registry. Horm Res Paediatr. 2018;89(1):47–55. [DOI] [PubMed] [Google Scholar]
- 7.Love-Osborne KA, Sheeder J, Svircev A, Chan C, Zeitler P, Nadeau KJ. Use of glycosylated hemoglobin increases diabetes screening for at-risk adolescents in primary care settings. Pediatr Diabetes. 2013;14(7): 512–518. [DOI] [PubMed] [Google Scholar]
- 8.Hannon TS, Dugan TM, Saha CK, McKee SJ, Downs SM, Carroll AE. Effectiveness of computer automation for the diagnosis and management of childhood Type 2 diabetes: a randomized clinical trial. JAMA Pediatr. 2017;171(4):327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anand SG, Mehta SD, Adams WG. Diabetes mellitus screening in pediatric primary care. Pediatrics. 2006;118(5):1888–1895. [DOI] [PubMed] [Google Scholar]
- 10.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat. 2002; 246(11):1–190. [PubMed] [Google Scholar]
- 11.Percentile Data Files with LMS Values. 2009. https://www.cdc.gov/growthcharts/percentile_data_files.htm.
- 12.American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical Care in diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S13–S28. [DOI] [PubMed] [Google Scholar]
- 13.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.USPSTF, Barton M. Screening for obesity in children and adolescents: US preventive services task force recommendation statement. Pediatrics. 2010;125(2):361–367. [DOI] [PubMed] [Google Scholar]
- 15.Andes LJ, Cheng YJ, Rolka DB, Gregg EW, Imperatore G. Prevalence of Prediabetes among adolescents and young adults in the United States 2005-2016. JAMA Pediatr. 2019;174(2):e194498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. [DOI] [PubMed] [Google Scholar]
- 17.Dabelea D, Mayer-Davis EJ, Saydah S, et al. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311(17):1778–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De La OA, Jordan KC, Ortiz K, et al. Do parents accurately perceive their child's weight status? J Pediatr Health Care. 2009;23(4):216–221. [DOI] [PubMed] [Google Scholar]