Abstract
Background
Most clinical studies of immune responses activated by Korean Red Ginseng (KRG) have been conducted exclusively in patients. However, there is still a lack of clinical research on immune-boosting benefits of KRG for healthy persons. This study aims to confirm how KRG boosts the immune system of healthy subjects.
Methods
A total of 100 healthy adult subjects were randomly divided into two groups that took either a 2 g KRG tablet or a placebo per day for 8 weeks. The primary efficacy evaluation variables included changes in T cells, B cells, and white blood cells (WBCs) before and after eight weeks of KRG ingestion. Cytokines (TNF-α, INF-γ, IL-2 and IL-4), WBC differential count, and incidence of colds were measured in the secondary efficacy evaluation variables. Safety evaluation variables were used to identify changes in laboratory test results that incorporated adverse reactions, vital signs, hematological tests, blood chemistry tests, and urinalysis.
Results
Compared to the placebo group, the KRG intake group showed a significant increase in the number of T cells (CD3) and its subtypes (CD4 and CD8), B cells, and the WBC count before and after eight weeks of the intake. There were no clinically significant adverse reactions or other notable results in the safety evaluation factors observed.
Conclusion
This study has proven through its eight-week intake test and subsequent analysis that KRG boosts the immune system through an increase in T cells, B cells, and WBCs, and that it is safe according to the study's safety evaluation.
Keywords: Immuno-enhancement, Korean Red Ginseng, T cell, B cell, White blood cell
1. Introduction
Modern society's awareness of immune diseases as serious health concerns have developed with the advances in modern health sciences [1]. As long as the human body is healthy, the immune system continuously fights to prevent viruses and bacteria from invading the body [1,2]. An immune function refers to the body's ability to defend against foreign bodies, and its decline weakens the body's natural healing ability as people are constantly exposed to the threat of reduced immunity due to the stresses of daily life. Several studies have shown that malnutrition is directly related to the deterioration of immune functions and that people should take a long-term approach to immunity improvement [3].
Natural foods come up in the discourse around enhancing bodily functions, one of which is ginseng. Ginseng is a plant in the family Araliaceae and the genus Panax, with the scientific name of Panax ginseng Meyer. Panax ginseng is notable for its genus name Panax, which comes from the word Panacea, meaning “cure-all.” Red ginseng (Korean Red Ginseng) has been recognized by the Korean Ministry of Food and Drug Safety for its six functions: improving immunity, improving fatigue, improving blood circulation (by preventing blood platelet aggregation), enhancing memory, antioxidation, and improving the health of post-menopausal women [4]. Ginseng has various types, namely Korean ginseng (Panax ginseng Meyer, Korea), American ginseng (Panax quinquefolium L, US and Canada), South China ginseng (Panax notoginseng, China), and Japanese ginseng (Panax japonicus Meyer, Japan) [5]. Panax ginseng (P. ginseng) has been called “mysterious medicine” for thousands of years and has been traditionally used for health improvement, mainly in Korea, China, and Japan [6]. According to the conventional manufacturing method of ginseng, P. ginseng is largely divided into three types: fresh ginseng, Korean Red Ginseng (KRG), and white ginseng. Of these, fresh ginseng refers to the raw ginseng harvested from the field that contains 70 to 80% of moisture. Therefore, it is susceptible to decay or damage during distribution and requires special storage facilities or packaging for long-term storage.
Red ginseng is only manufactured in Korea, so it has been named Korean Red Ginseng. KRG generally refers to unpeeled fresh ginseng cooked by steaming and drying. Its color ranges from light yellowish-brown to light reddish-brown. White ginseng is dried without peeling and cooking by sunlight, hot air, or other methods; its color ranges from white to light yellow [7,8]. P. ginseng contains various nutritional components, including glycosides-containing saponin, nitrogen-containing compound proteins, amino acids, nucleic acids, alkaloids, and fat-soluble fatty acids, essential oils, polyacetylenes, phenolic compounds, phytosterols, terpenoids, saccharides, including monosaccharides oligosaccharides, polysaccharides, pectic substances, vitamins (thiamine [B1], riboflavin [B2], cobalamin [B12], niacin, biotin, pantothenic acid, and folic acid), and minerals (manganese, copper, vanadium, cobalt, germanium, phosphorus, aluminum, and nickel) [4]. In particular, KRG undergoes secondary component conversion during the heat treatment process, producing new components (e.g. ginsenosides Rg2, Rh1, Rg3, and arginine-fructose-glucose) that are not found in fresh ginseng or white ginseng [[9], [10], [11], [12], [13], [14]].
To date, KRG's immunity activation has been studied in vitro and in vivo on components like ginsenoside, saponin fraction, and red ginseng acidic polysaccharide (RGAP). KRG primarily activates macrophage and natural killer cells (NK cells), which are responsible for primary innate immunity to protect the body against non-specific infections and harmful substances. Its secondary property is increasing the activity and number of T cells and B cells responsible for acquired immunity by acting on cellular and humoral immunity and modulating cytokine and other activities to enhance specific immune responses [4]. KRG is known to promote intraperitoneal macrophage activity [[15], [16], [17], [18], [19]], dendritic cell (DC) activity [20,21], and NK cell activity [22,23]. KRG promotes antibody production and T cell proliferation [[24], [25], [26]] and alleviates allergies and inflammatory diseases caused by an imbalance between Th1 and Th2 cell activity by regulating their activities [27,28]. KRG also regulates the immune response by controlling the activity and number of Treg cells [29,30].
KRG was found to have beneficial effects for subjects with acute respiratory diseases in clinical studies, including antiviral properties that specifically alleviate influenza [[31], [32], [33], [34], [35], [36]]. KRG also significantly reduced the incidence of contracting a cold in one study [37] and reduced the frequency of contracting acute respiratory diseases in another [38]. Clinical studies have confirmed that KRG had a positive effect on immune cell count and cytokine in cancer patients [[39], [40], [41]]. However, there is still a lack of clinical research regarding changes in the immune cells of healthy adult subjects who ingest KRG. In this light, the present study sought to examine the changes in the immune cells of healthy subjects after ingesting KRG.
2. Methods
2.1. Participants
The present study was conducted with the approval of the Institutional Review Board of Semyung University Korean Medicine Hospital (IRB no. SMJOH-2018-12-08). Volunteers who participated in the clinical study signed the consent form and were chosen according to the study's selection and exclusion criteria. A total of 100 subjects were randomly assigned between the test and placebo group (50 subjects each). On the one hand, the selection criteria were: (1) male and females aged between 40 and 65, (2) those with WBC level below 6.0 × 10/uL, (3) those who had an upper respiratory infection within a month of participation in the study (including patients with colds), and (4) those who volunteered to participate in the study and signed the consent form. On the other hand, the exclusion criteria were: (1) those who have experienced adverse reactions like hypersensitive reactions and allergies upon taking dietary supplements and medicines, including ginseng, (2) those with autoimmune diseases (multiple sclerosis, lupus, and rheumatoid arthritis), (3) those with a history of malignant tumor within 5 years, (4) those who were prescribed an immunosuppressive drug within 6 months of the first visit, (5) those who have been taking dietary supplements and herbal medicine that may affect the improvement of immune disease within 1 month of the first visit, (6) those who have a history of thromboembolic disorders, cerebrovascular disorders, and severe cardiovascular disorders within 1 year of the first visit, (7) those with liver function defects (aspartate aminotransferase and alanine aminotransferase values 3 times higher than normal value) and kidney function defects (Creatinine 2 mg/dL or higher), resistant hypertension (systolic blood pressure 160 or diastolic blood pressure 100 mmHg or higher after drug administration), uncontrolled diabetes (HbA1c 7.0% or higher after drug administration or fasting blood sugar of 160 mg/dL or higher), and patients taking thrombolysis (heparin and warfarin), (8) those with drug or alcohol addiction, (9) those who continued to carry out high-strength exercises within 3 months of the first visit (more than 10 hours of exercise), (10) those who have hypersensitivity to test food or food ingredients, (11) those who participated in other clinical tests within 1 month of the first visit.
2.2. Test methods
At the first screening visit, the researcher explained the purpose, process, and method of the study to the subjects before receiving their written consent. Subjects were each given a screening number in the order in which the consent form was signed, and the subjects' demographic information, medical history, and medication information were examined. The first visit was conducted within two weeks after screening. Test substances were packaged according to a randomized schedule made by the SAS 9.4 program and were prescribed to subjects in the order of the first visit. After taking the test substance for four weeks from the first visit, the second visit was conducted, where the test substance was newly prescribed after returning the remaining test substance and evaluating substance compliance. On the third visit, an immunity self-evaluation was conducted after returning the remaining test substance and evaluating substance compliance. Upon each visit, physical examination and vital signs were measured, and changes in concomitant medications and adverse reactions were investigated. On the first and fourth visits, laboratory blood tests and immunity self-evaluation were performed.
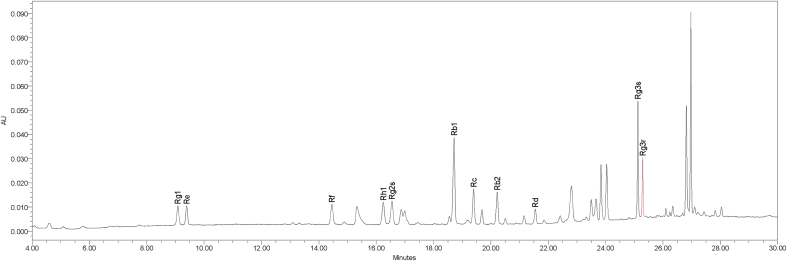
During the eight weeks, the test group and the control group (placebo) were directed to take two tablets twice a day. The test group was given 500 mg KRG tablets, while the control group was given 500 mg placebo (cellulose) tablets. The daily dose of dietary supplement suggested by the Ministry of Food and Drug Safety is “3 to 80 mg/day for the sum of ginsenosides Rg1, Rb1, and Rg3” [42]. The KRG dose used in this study was 2 g of KRG tablet/day, containing ginsenoside Rb1 (8.03 mg/g), Rc (3.29 mg/g), Rb2 (2.80 mg/g), Rg3 (2.50 mg/g), Rf (1.47 mg/g), Re (1.29 mg/g), Rg1 (1.18 mg/g), and Rd (1.0 mg/g) (Fig. 1).
Fig. 1.
High-performance liquid chromatogram of ginsenosides detected from a Korean Red Ginseng tablet.
The KRG formulation was prepared with 6-year-old P. ginseng steamed according to the International Organization for Standardization ISO 19610:2017. KRG tablets were prepared by dehydrating KRG extracts (3 g of KRG extracts per 2 g tablet) until they were light brown. Placebo tablets contained corn starch and cellulose with KRG flavoring and color.
2.3. Evaluation variables
Tests were conducted before and after the 8 weeks that the participants ingested the test substance to evaluate the efficacy of KRG for improving immune functions. The primary efficacy evaluation variables included changes in T cells, B cells, and WBCs before and after 8 weeks of ingesting KRG. Meanwhile, the secondary efficacy evaluation variables included WBC differential count and incidence of colds. Safety evaluation variables included changes in laboratory test results, like adverse reactions, vital signs, hematological tests, blood chemistry tests, and urinalysis.
2.4. Data and statistical analysis
The researchers determined whether there is a statistical difference between the test group and the placebo group in terms of demographic by calculating the continuous data through average, standard deviation. These values were then compared for the two groups using a t-test. The frequency distributions of categorical variables among groups were compared by Chi-square test. The SAS 9.4 program was used for analysis, and a p-value of less than 0.05 was defined as statistically significant. Meanwhile, the efficacy analysis of this clinical test analyzed the test substances in subjects who fully completed the administration. In addition, the safety set was randomized and analyzed for all subjects who took at least one test substance.
3. Results
3.1. Study population
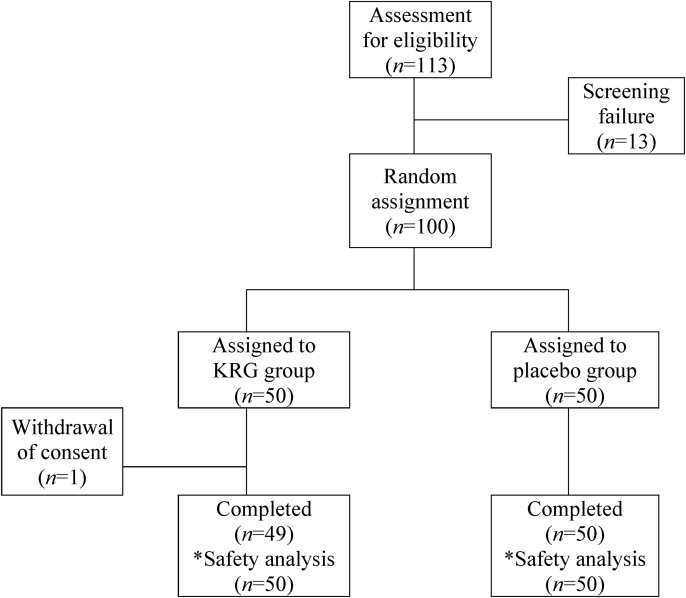
Fig. 2 shows the participants' dispositions. After the screening period, the 100 subjects who volunteered in this clinical trial were randomized into the KRG group (test group) and the placebo group (control group) (n = 50 for each group). In the test group, 1 subject withdrew from the test after the retraction of consent after the fourth visit, resulting in the final number of 99 subjects who completed the test. The safety assessment included 100 subjects who ingested clinical dietary supplements at least once (Fig. 2). The demographic and baseline characteristics did not differ between the KRG and placebo groups (Table 1).
Fig. 2.
Study design for evaluating the Immuno-enhancement properties and safety of test substance administration for 8 weeks.
Table 1.
Baseline characteristics of study participants
| Characteristics | KRG group (n = 50) | Placebo group (n = 50) | p-value | |
|---|---|---|---|---|
| Sex | Male, n (%) | 3 (6.00%) | 7 (14.00%) | 0.1824∗ |
| Female, n (%) | 47 (94.00%) | 43 (86.00%) | ||
| Age | Mean ± SD | 50.12 ± 6.43 | 50.38 ± 5.97 | 0.8344† |
| Height (cm) | Mean ± SD | 158.32 ± 5.98 | 160.87 ± 7.29 | 0.0587† |
| Weight (kg) | Mean ± SD | 58.75 ± 6.72 | 62.59 ± 11.35 | 0.0422† |
KRG, Korean Red Ginseng.
Analyzed by Chi-square test.
Analyzed by two-sample t-test.
3.2. Primary outcome
Changes in the total number of T cells, a subtype of T cells (helper T cells, cytotoxic T cells), B cells, and WBCs after the intake of the test substance for 8 weeks are shown in Table 2. There was no statistically significant difference in the total T cell number at the baseline. After 8 weeks, the number of T cells significantly increased to 78.38 ± 10.65% in the test group compared to 73.81 ± 8.31% in the control group (p = 0.0191). In addition, changes in the T cell (from baseline to 8 weeks) significantly increased in the test group compared to the control group (p = 0.0348).
Table 2.
Immune cell distribution changes between baseline and 8 weeks of test substance administration
| Cell (%) | KRG group (n = 49) |
Placebo group (n = 50) |
p-value‡ | |||
|---|---|---|---|---|---|---|
| Mean ± SD | p-value∗ | Mean ± SD | p-value† | |||
| Total T cell (CD3) | W0 | 76.86 ± 8.33 | 75.76 ± 6.45 | 0.0348‡ | ||
| W8 | 78.38 ± 10.65 | 0.2178 | 73.81 ± 8.31 | 0.0752 | ||
| W8–W0 | 1.52 ± 8.53 | −1.95 ± 7.57 | ||||
| Helper T cell (CD3+CD4+) | W0 | 48.86 ± 8.73 | 48.05 ± 8.42 | 0.0381‡ | ||
| W8 | 49.95 ± 11.48 | 0.3507 | 45.80 ± 7.71 | 0.0444† | ||
| W8–W0 | 1.09 ± 8.11 | −2.25 ± 7.72 | ||||
| Cytotoxic T cell (CD3+CD8+) | W0 | 25.65 ± 9.04 | 25.72 ± 10.82 | 0.0498‡ | ||
| W8 | 28.64 ± 12.64 | 0.0680 | 23.39 ± 10.88 | 0.2808 | ||
| W8–W0 | 2.93 ± 11.09 | −2.33 ± 15.08 | ||||
| B cell (CD19) | W0 | 7.85 ± 3.63 | 8.03 ± 3.34 | 0.0061‡ | ||
| W8 | 9.92 ± 5.65 | 0.0004∗ | 8.16 ± 2.99 | 0.7605 | ||
| W8–W0 | 2.07 ± 3.79 | 0.13 ± 3.05 | ||||
| WBC ( × 108/μL) | W0 | 4.67 ± 0.88 | 4.96 ± 0.79 | 0.0490‡ | ||
| W8 | 5.10 ± 1.22 | 0.0179∗ | 4.96 ± 0.91 | 0.9635 | ||
| W8–W0 | 0.43 ± 1.23 | 0.01 ± 0.86 | ||||
KRG, Korean Red Ginseng; SD, standard deviation; WBC, white blood cell; W8, 8th week; W0, Baseline.
Analyzed by Paired t-test (compared within treatment group).
Analyzed by Paired t-test (compared within control group).
Analyzed by Paired t-test (compared between groups: baseline–8th week).
The distribution and the amount of change in helper T cells (CD3+CD4+ T cells) after the administration of the test substances for 8 weeks are shown in Table 2. In the baseline, there was no statistically significant difference between the test group and the control group. However, after 8 weeks, the measurement increased significantly in the test group compared to the control group (p = 0.0370). Changes in CD4+ T cells (from baseline to 8 weeks) was 1.09 ± 8.11% in the test group and −2.25 ± 7.72% in the control group, showing a statistically significant difference between the two groups (p = 0.0381).
The distribution of and changes in cytotoxic T cells (CD3 + CD8 + T cells) at baseline and after 8 weeks of administration are shown in Table 2. Cytotoxic T cells did not differ significantly between the two groups at the baseline. After the subjects' intake of the test substance for 8 weeks, the measurement was increased in the test group to 28.64 ± 12.64% by 2.99 ± 11.19% from the baseline, while it decreased in the control group by −2.33 ± 15.08% from the baseline to 23.39 ± 10.88%. Meanwhile, the cytotoxic cell distribution (p = 0.0290) and the amount of change (p = 0.0498) significantly increased in the test group after 8 weeks.
The measurement of the distribution of B cells (CD 19) and the amount of change in the subjects' blood after taking the test substance for 8 weeks are shown in Table 2. The B cell distribution of the test group significantly increased by 2.07 ± 3.79% to 9.92 ± 5.65% from a baseline of 7.85 ± 3.63% after taking KRG for 8 weeks (p = 0.0004). The B cell distribution of the control group was 8.03 ± 3.34% for the baseline and 8.16 ± 2.99% after 8 weeks, indicating an increase of 0.13 ± 3.05% after ingesting KRG (p = 0.7605). Compared to the baseline, the B cell distribution also changed after 8 weeks and significantly increased in the test group compared to the control group (p = 0.0061).
The measurement of the amount of change in WBC count (103/μl) after taking the test substance for 8 weeks are shown in Table 2. At the baseline, there was no statistically significant difference between the groups. After 8 weeks, the test group showed 5.10 ± 1.22, and the control group 4.96 ± 0.91, with no statistically significant difference between both groups. From baseline to 8 weeks afterward, WBCs significantly increased in the test group compared to the control group (p = 0.0490).
3.3. Second outcome
The amount of change in cytokines, WBC differential count, and the incidence of cold was measured as secondary efficacy evaluation variables. Table 3 shows the changes in cytokines at the baseline and after taking the test substance for 8 weeks. A change in tumor necrosis factor (TNF)-α, a type of cytokine, was measured at 0.27 ± 19.84 pg/mL in the test group and −2.02 ± 3.69 pg/mL in the control group, with no statistically significant difference between both groups (p-value = 0.4261). A change in interferon (INF)-γ was measured at −2.76 ± 27.43 IU/mL in the test group and −4.36 ± 15.53 IU/mL in the control group, with no statistically significant difference between both groups (p-value = 0.7229). On the one hand, there was no statistically significant difference in IL-2 change at baseline and after 8 weeks between both groups (p-value = 0.7367). On the other hand, there was no significant difference in the amount of IL-4 between the test group and the control group.
Table 3.
Cytokine level changes between baseline and 8 weeks of test substance administration
| KRG group (n = 49) |
Placebo group (n = 50) |
p-value‡ | ||||
|---|---|---|---|---|---|---|
| Mean ± SD | p-value∗ | Mean ± SD | p-value† | |||
| TNF-α (pg/mL) | W0 | 10.99 ± 11.19 | 8.75 ± 2.36 | 0.4261 | ||
| W8 | 11.44 ± 15.46 | 0.9268 | 6.73 ± 3.22 | 0.0003† | ||
| W8–W0 | 0.27 ± 19.84 | −2.02 ± 3.69 | ||||
| INF-γ (IU/mL) | W0 | 19.60 ± 15.25 | 17.88 ± 14.17 | 0.7229 | ||
| W8 | 17.06 ± 21.36 | 0.4932 | 13.52 ± 5.80 | 0.0526 | ||
| W8–W0 | −2.76 ± 27.43 | −4.36 ± 15.53 | ||||
| IL-2 (IU/mL) | W0 | 25.81 ± 15.41 | 22.91 ± 7.46 | 0.7367 | ||
| W8 | 21.66 ± 11.25 | 0.0927 | 19.45 ± 8.28 | 0.0270† | ||
| W8–W0 | −4.47 ± 17.83 | −3.47 ± 10.72 | ||||
| IL-4 (IU/mL) | W0 | 17.52 ± 19.28 | 12.68 ± 5.85 | 0.2162 | ||
| W8 | 11.58 ± 9.30 | 0.0232∗ | 9.95 ± 6.61 | 0.0389† | ||
| W8–W0 | −6.43 ± 18.76 | −2.73 ± 9.11 | ||||
KRG, Korean Red Ginseng; SD, standard deviation; TNF, tumor necrosis factor; IFN, interferon; IL, Interleukin; W8, 8th week; W0, Baseline.
Analyzed by Paired t-test (compared within treatment group).
Analyzed by Paired t-test (compared within control group).
Analyzed by Paired t-test (compared between groups: baseline–8th week).
The study also recorded a change in the WBC differential count after the intake of the test substance for 8 weeks (data not shown). The neutrophil count increase in the test group was 2.13 ± 10.36% and 0.03 ± 9.19% in the control group, showing no statistically significant difference between the two groups (p-value = 0.2885). Changes in the lymphocyte count in the test group were −1.63 ± 8.91% and 0.92 ± 8.87% in the control group (no statistically significant difference; p-value = 0.1562). There were also changes in the monocyte count in the test group with −0.24 ± 2.14% and −0.49 ± 2.04% in the control group (no statistically significant difference; p-value = 0.5583). Meanwhile, the Eosinophile count change in the test group was −0.28 ± 1.41% and −0.41 ± 1.49% in the control group (no statistically significant difference; p-value = 0.6415). The researchers also recorded a Basophile change in the test group with 0.03 ± 0.59% and −0.05 ± 0.60% in the control group (no statistically significant difference; p-value = 0.5454).
The measurements of the incidence of colds during the test period. During the 8-week test period, there were 11 cases of colds in the test group and 10 in the control group, showing a higher incidence rate in the test group. However, there was no statistically significant difference between both groups (data not shown).
3.4. Safety evaluation
Safety evaluations, which included adverse reactions and all data from subjects, have been randomly assigned. The clinical test's 100 subjects, as well as 100 patients, were supplied with dietary supplements at least once. Out of a total number of 100 subjects in the safety group, there were 42 cases of adverse reactions that appeared during the test period, where a patient can have more than 1 case of adverse reaction. Out of the 50 patients in the test group, 30% (15/50 patients) had 20 cases, while 38 (19/50 patients) in the control group had 22 cases, showing no statistically significant difference between them (p-value = 0.3984).
Among the laboratory hematological tests (Table 4), the researchers found a significant difference in creatinine levels between the control group and the test group after measuring the change at the end of the study and comparing them to the measurements during screening. The researchers determined that the values were within the normal range. The test group showed statistically significant levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST). Meanwhile, the control group showed statistically significant levels of ALT, AST, creatinine, and alkaline phosphatase (ALP) (Table 4). However, these values were all within the normal range.
Table 4.
Hematological test result changes between baseline and 8 weeks of test substance administration
| KRG group (n = 50) |
Placebo group (n = 50) |
p-value‡ | ||||
|---|---|---|---|---|---|---|
| Mean ± SD | p-value∗ | Mean ± SD | p-value† | |||
| RBC ( × 106/㎕) | W0 | 4.32 ± 0.34 | 4.35 ± 0.40 | 0.8695 | ||
| W8 | 4.32 ± 0.31 | 0.9935 | 4.34 ± 0.44 | 0.8201 | ||
| W8–W0 | 0.00 ± 0.17 | −0.01 ± 0.17 | ||||
| Hemoglobin (g/dL) | W0 | 13.41 ± 0.98 | 13.69 ± 1.20 | 0.9846 | ||
| W8 | 13.45 ± 0.91 | 0.5788 | 13.74 ± 1.33 | 0.4934 | ||
| W8-W0 | 0.04 ± 0.56 | 0.05 ± 0.47 | ||||
| Hematocrit (%) | W0 | 40.58 ± 2.77 | 41.27 ± 3.44 | 0.7155 | ||
| W8 | 40.93 ± 2.74 | 0.1620 | 41.50 ± 3.86 | 0.3500 | ||
| W8–W0 | 0.35 ± 1.75 | 0.23 ± 1.69 | ||||
| Platelet ( × 108/μL) | W0 | 247.32 ± 58.05 | 242.58 ± 56.53 | 0.6142 | ||
| W8 | 242.96 ± 54.18 | 0.2608 | 240.96 ± 53.30 | 0.6742 | ||
| W8–W0 | −4.36 ± 27.10 | −1.62 ± 27.08 | ||||
| ALT (IU/L) | W0 | 19.56 ± 10.42 | 22.78 ± 10.41 | 0.5400 | ||
| W8 | 16.98 ± 5.59 | 0.0377∗ | 19.06 ± 7.11 | 0.0109† | ||
| W8–W0 | −2.58 ± 8.54 | −3.72 ± 9.94 | ||||
| AST (IU/L) | W0 | 24.04 ± 5.83 | 24.84 ± 7.30 | 0.5280 | ||
| W8 | 22.74 ± 4.96 | 0.0356∗ | 22.80 ± 4.88 | 0.0471† | ||
| W8–W0 | −1.30 ± 4.25 | −2.04 ± 7.08 | ||||
| Total cholesterol (mg/dL) | W0 | 216.54 ± 50.66 | 211.80 ± 35.60 | 0.5689 | ||
| W8 | 215.40 ± 43.75 | 0.7500 | 207.46 ± 38.10 | 0.3202 | ||
| W8–W0 | −1.14 ± 25.16 | −4.34 ± 30.56 | ||||
| Glucose (mg/dL) | W0 | 92.94 ± 10.75 | 95.52 ± 14.81 | 0.2205 | ||
| W8 | 93.84 ± 13.23 | 0.6406 | 92.90 ± 13.00 | 0.2216 | ||
| W8–W0 | 0.90 ± 13.55 | −2.62 ± 14.97 | ||||
| Blood urea nitrogen (mg/dL) | W0 | 13.67 ± 3.53 | 14.34 ± 3.81 | 0.3289 | ||
| W8 | 12.90 ± 3.15 | 0.1373 | 14.31 ± 3.64 | 0.9506 | ||
| W8–W0 | −0.76 ± 3.58 | −0.03 ± 3.86 | ||||
| Triglyceride (mg/dL) | W0 | 118.26 ± 55.94 | 140.64 ± 86.15 | 0.3077 | ||
| W8 | 118.52 ± 60.13 | 0.9712 | 128.18 ± 92.02 | 0.2246 | ||
| W8–W0 | 0.26 ± 50.64 | −12.46 ± 71.64 | ||||
| Creatinine (mg/dL) | W0 | 0.65 ± 0.10 | 0.67 ± 0.15 | 0.0214 | ||
| W8 | 0.64 ± 0.10 | 0.4404 | 0.70 ± 0.15 | 0.0258† | ||
| W8–W0 | −0.01 ± 0.06 | 0.03 ± 0.09 | ||||
| ALP (IU/L) | W0 | 77.74 ± 21.20 | 79.86 ± 23.63 | 0.4970 | ||
| W8 | 76.38 ± 21.51 | 0.2933 | 77.32 ± 21.08 | 0.0341† | ||
| W8–W0 | −1.36 ± 9.05 | −2.54 ± 8.24 | ||||
| T-protein | W0 | 7.55 ± 0.39 | 7.45 ± 0.32 | 0.8005 | ||
| W8 | 7.59 ± 0.37 | 0.3840 | 7.48 ± 0.34 | 0.5859 | ||
| W8–W0 | 0.04 ± 0.32 | 0.02 ± 0.31 | ||||
| r-glutamyl transferase | W0 | 20.64 ± 17.87 | 23.82 ± 20.41 | 0.9032 | ||
| W8 | 18.82 ± 12.92 | 0.2089 | 21.78 ± 18.37 | 0.0696 | ||
| W8–W0 | −1.82 ± 10.11 | −2.04 ± 7.77 | ||||
| Urine pH | W0 | 6.25 ± 0.90 | 6.14 ± 0.76 | 0.4588 | ||
| W8 | 6.31 ± 0.89 | 0.6846 | 6.36 ± 0.88 | 0.1681 | ||
| W8–W0 | 0.06 ± 1.04 | 0.22 ± 1.11 | ||||
| Urine specific gravity | W0 | 1.02 ± 0.01 | 1.02 ± 0.01 | 0.5071 | ||
| W8 | 1.02 ± 0.01 | 0.1327 | 1.02 ± 0.01 | 0.6945 | ||
| W8–W0 | 0.00 ± 0.01 | 0.00 ± 0.01 | ||||
KRG, Korean Red Ginseng; SD, standard deviation; RBC, red blood cell; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; W8, 8th week; W0, Baseline.
Analyzed by Paired t-test (compared within treatment group).
Analyzed by Paired t-test (compared within control group).
Analyzed by Paired t-test (compared between groups: baseline–8th week).
For vital signs (systolic and diastolic blood pressure, pulse, and body temperature) measured during each visit, there was no statistically significant change between the screening and the end of the study (Table 5).
Table 5.
Vital sign changes between baseline and 8 weeks of test substance administration
| KRG group (n = 50) |
Placebo group (n = 50) |
p-value‡ |
|||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | p-value∗ | Mean ± SD | p-value† | ||||
| Blood pressure (mmHg) | Systolic | W0 | 116.44 ± 10.70 | 119.32 ± 10.82 | 0.7679 | ||
| W8 | 116.33 ± 10.70 | 0.7733 | 118.36 ± 11.36 | 0.4944 | |||
| W8–W0 | −0.39 ± 9.37 | −0.96 ± 9.86 | |||||
| Diastolic | W0 | 72.74 ± 8.46 | 74.08 ± 8.06 | 0.1559 | |||
| W8 | 72.63 ± 9.76 | 1.0000 | 76.44 ± 9.03 | 0.0569 | |||
| W8–W0 | 0.00 ± 7.84 | 2.36 ± 8.56 | |||||
| Pulse (pulse/min) | W0 | 75.64 ± 10.61 | 76.52 ± 9.60 | 0.6861 | |||
| W8 | 74.69 ± 9.55 | 0.5767 | 74.64 ± 8.96 | 0.2497 | |||
| W8–W0 | −0.94 ± 11.69 | −1.88 ± 11.41 | |||||
| Body temperature (°C) | W0 | 36.62 ± 0.20 | 36.55 ± 0.21 | 0.5892 | |||
| W8 | 36.61 ± 0.23 | 0.8581 | 36.58 ± 0.25 | 0.5859 | |||
| W8–W0 | −0.01 ± 0.24 | 0.02 ± 0.31 | |||||
KRG, Korean Red Ginseng; SD, standard deviation; W8, 8th week; W0, Baseline.
Analyzed by Paired t-test (compared within treatment group).
Analyzed by Paired t-test (compared within control group).
Analyzed by Paired t-test (compared between groups: baseline–8th week).
4. Discussion
KRG is known to stimulate immunity by increasing T cell and B cell proliferation and activity, as well as WBC count. This immunoactivity of red ginseng has been demonstrated in many in vitro and in vivo clinical studies [4]. The oral administration of RGAP (a well-known immunoactivity ingredient in KRG) in mice led to a significant increase in LPS-stimulated B cell proliferation and concanavalin A-induced splenic T cell proliferation compared to the control group [43]. Kenarova B et al [44] showed that ginsenoside Rg1 enhances immunity by increasing the number of spleen plaque-forming cells, the titers of sera hemagglutinins, and the number of antigen-reactive T-cells when administered intraperitoneally and intravenously to mice. Their study has also shown that Rg1 increases the number of T-helper cells in relation to the total number of T-cells and natural killer activation of splenocyte [44]. Saba et al [45] confirmed that the number of T cells and B cells in the spleen and thymus significantly increased when KRG extract was orally administered to mice with reduced immunity. Also, KRG was reportedly effective in enhancing immunity by significantly increasing the number of WBCs in blood [45]. Most studies that examined the immunity enhancement effect of KRG measured immune cells and cytokines in patients [[37], [38], [39],46,47]. In patients with human immunodeficiency virus type 1 (HIV), the decrease in CD4+ T cell count was delayed by the concomitant administration of a therapeutic drug and KRG, or the long-term administration of KRG alone, and an immunity enhancement effect of maintaining a soluble CD8 level was also observed [46,47]. Suh et al conducted studies on patients who underwent surgery for digestive system cancer and divided them into groups, one that ingested KRG and one that ingested placebos. They then measured the number of immune cells and cytokines in both groups. The KRG group showed an increase in CD4+ and CD8+ T cell count, B cell count, WBC count, and blood IL-2 content—which improves immune function after cancer surgery—compared to the placebo group with a decrease in IL-10 [39,40].
In the present study, the KRG group showed significantly increased T cell (total T cell, helper T cell, and cytotoxic T cell), B cell, and WBC levels compared to the placebo group. These results confirmed that red ginseng increases immunity not only for cancer patients but also for healthy subjects with reduced immunity. KRG is known to regulate the body's immune response by regulating the secretion of cytokines, which mediate the immune response [4]. One study reported the efficacy of Rb1 in treating asthma, which measured the cytokine content in the bronchoalveolar lavage fluid after the administration of Rb1 to asthma-induced animals. The study observed a decrease in the IL-4 level and an increase in the IFN-γ level [48]. In another animal study, ginsenoside Rd inhibited transplant rejection by inhibiting cytokines IL-2, IL-12, TNF-α, and IFN-γ [49]. In mice, ginsenosides Rb1 and Rb2 inhibited the expression of TNF-α and other cytokines, and reduced infarction volume [[50], [51]]. Park et al demonstrated that KRG is effective for atopic dermatitis by reducing the TNF-α level in an atopy animal model [52]. Another study reported that KRG significantly reduced inflammatory cytokines IL-2, IL-10, IL-12, TNF-α, and IFN-γ in children after making a full recovery from cancer [53].
In the present clinical study, there was no significant change in TNF-α, INF-γ, IL-2, and IL-4 levels in the test group (KRG intake) compared to the control group due to large individual differences in cytokine levels. Although KRG may not significantly change cytokine levels in a healthy body, it may play a role in maintaining homeostasis in the body by regulating cytokine secretion in various disease conditions.
Saba et al showed that lymphocyte levels significantly increased after the oral administration of KRG extract to mice with reduced immunity compared to the control group, even when their neutrophil levels did not change [45]. However, Suh et al reported that cancer patients had no changes in lymphocyte and monocyte count after taking red ginseng [39]. Like Suh et al‘s study, the present study's results were also no significant differences in neutrophil, lymphocyte, monocyte, eosinophil, and basophil levels in the KRG group compared to the control group.
Recently, a KRG intake safety study was conducted in 1,000 healthy adult subjects. The results showed that KRG tablets are safe when consumed 2 g a day for 24 weeks [54]. In the present clinical study, there were no clinically significant adverse reactions due to the administration of KRG, and there were no clinically significant results in other safety evaluation factors. The present study determined how KRG affects immune functions by evaluating the changes in the healthy subjects' total T cell, helper T cell, cytotoxic T cell, and B cell and WBC levels before and after the 8-week period where subjects ingested KRG. The present study, on the other hand, is meaningful in that it confirmed immunity enhancement in healthy subjects who were administered with KRG. These results show that KRG increases the number of immune cells, especially T cells, B cells, and WBC, to help improve immunity when consumed by healthy adults with slightly downregulated immunity.
In summary, Korean Red Ginseng is a dietary supplement validated for safety, and it can be an excellent immunopotentiator for people who want to improve their immune systems.
Authorship contributions
Sun Hee Hyun: Conception and design of the study, Analysis and/or interpretation of data, Drafting the manuscript. Ha-Young Ahn: Conception and design of the study, Acquisition of data. Hyeong-Jun Kim: Conception and design of the study, Acquisition of data. Sung Won Kim: Analysis and/or interpretation of data, Drafting the manuscript. Seung-Ho So: Conception and design of the study, Analysis and/or interpretation of data. Gyo In: Revising the manuscript critically for important intellectual content. Chae-Kyu Park: Revising the manuscript critically for important intellectual content. Chang-Kyun Han: Analysis and/or interpretation of data, Drafting the manuscript, Revising the manuscript critically for important intellectual content.
Funding
The authors have no funding to declare.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
All persons who have made substantial contributions to the work reported in the manuscript (e.g., technical help, writing and editing assistance, general support), but who do not meet the criteria for authorship, are named in the Acknowledgments and have given us their written permission to be named. If we have not included an Acknowledgments, then that indicates that we have not received substantial contributions from non-authors.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jgr.2020.08.003.
Contributor Information
Sun Hee Hyun, Email: shhyun@kgc.co.kr.
Ha-Young Ahn, Email: freyja21@daum.net.
Hyeong-Jun Kim, Email: khjoongy@hanmail.net.
Sung Won Kim, Email: loveginseng@kgc.co.kr.
Seung-Ho So, Email: biopro@kgc.co.kr.
Gyo In, Email: 20109042@kgc.co.kr.
Chae-Kyu Park, Email: ckpark@kgc.co.kr.
Chang-Kyun Han, Email: ckhan@kgc.co.kr.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Abo T., Kawamura T., Watanabe H. Immunologic states of autoimmune diseases. Immunol Res. 2005;33:23–34. doi: 10.1385/IR:33:1:023. [DOI] [PubMed] [Google Scholar]
- 2.Kang S.W., Min H.Y. Ginseng, the “Immunity Boost”: the effects of Panax ginseng on immune system. J Ginseng Res. 2012;36:354–368. doi: 10.5142/jgr.2012.36.4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Childs C.E., Calder P.C., Miles E.A. Diet and immune function. Nutrients. 2019;11:1933. doi: 10.3390/nu11081933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.So S.H., Lee J.W., Kim Y.S., Hyun S.H., Han C.K. Red ginseng monograph. J Ginseng Res. 2018;42:549–561. doi: 10.1016/j.jgr.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hyun S.H., Kim S.W., Seo H.W., Youn S.H., Kyung J.S., Lee Y.Y., In G., Han C.K. Physiological and pharmacological features of the non-saponin components in Korean red ginseng. J Ginseng Res. 2020;44(4):527–537. doi: 10.1016/j.jgr.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baeg I.H., So S.H. The world ginseng market and the ginseng (Korea) J Ginseng Res. 2013;37:1–7. doi: 10.5142/jgr.2013.37.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y., Choi H.K., Brinckmann J.A., Jiang X., Huang L. Chemical analysis of Panax quinquefolius (North American ginseng): a review. J Chromatogr A. 2015;1426:1–15. doi: 10.1016/j.chroma.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y.C., Li G., Jiang C., Yang B., Yang H.J., Xu H.Y., Huang L.Q. Tissue-specific distribution of ginsenosides in different aged ginseng and antioxidant activity of ginseng leaf. Molecules. 2014;19:17381–17399. doi: 10.3390/molecules191117381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shin B.K., Kwon S.W., Park J.H. Chemical diversity of ginseng saponins from Panax ginseng. J Ginseng Res. 2015;39:287–298. doi: 10.1016/j.jgr.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiao L., Zhang X., Wang M., Li B., Liu Z., Liu S. Chemical and antihyperglycemic activity changes of ginseng pectin induced by heat processing. Carbohydr Polym. 2014;114:567–573. doi: 10.1016/j.carbpol.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 11.Lee S.M., Bae B.S., Park H.W., Ahn N.G., Cho B.G., Cho Y.L., Kwak Y.S. Characterization of Korean red ginseng (Panax ginseng Meyer): history, preparation method, and chemical composition. J Ginseng Res. 2015;39(4):384–391. doi: 10.1016/j.jgr.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang C.Z., Anderson S., Du W., He T.C., Yuan C.S. Red ginseng and cancer treatment. Chin J Nat Med. 2016;14:7–16. doi: 10.3724/SP.J.1009.2016.00007. [DOI] [PubMed] [Google Scholar]
- 13.Kim G.N., Lee J.S., Song J.H., Oh C.H., Kwon Y.I., Jang H.D. Heat processing decreases Amadori products and increases total phenolic content and antioxidant activity of Korean Red ginseng. J Med Food. 2010;13:1478–1484. doi: 10.1089/jmf.2010.1076. https://www.sciencedirect.com/science/article/pii/S1875536416300048 Available from: [DOI] [PubMed] [Google Scholar]
- 14.Matsuura Y., Zheng Y., Takaku T., Kameda K., Okuda H. Isolation and physiological activities of new amino acid derivatives from Korean Red ginseng. Korean J Ginseng Sci. 1994;18:204–211. https://www.wakan-iyaku.gr.jp/kensaku/2006/06/21/7576/ Available from: [Google Scholar]
- 15.Kim K.H., Jang S.A., Kim K.S., Park S.K., Park H.J., Lee S.J., Pyo S.K., Sohn E.H. Effects of non-saponin red ginseng components (NSRG) on functions of macrophages isolated from young and aged mice. J Ginseng Res. 2009;33:177–182. doi: 10.5142/JGR.2009.33.3.177. [DOI] [Google Scholar]
- 16.Park K.M., Kim Y.S., Jeong T.C., Joe C.O., Shin H.J., Lee Y.H., Nam K.Y., Pakr J.D. Nitric oxide is involved in the immunomodulating activities of acidic polysaccharide from Panax ginseng. Planta Med. 2001;67:122–126. doi: 10.1055/s-2001-11508. [DOI] [PubMed] [Google Scholar]
- 17.Choi H.S., Kim K.H., Sohn E.W., Park J.D., Kim B.O., Moon E.Y., Rhee D.K., Pyo S.K. Red ginseng acidic polysaccharide (RGAP) in combination with IFN results in enhanced macrophage function through activation of the NF-kappaB pathway. Biosci Biotechnol Biochem. 2008;72:1817–1825. doi: 10.1271/bbb.80085. [DOI] [PubMed] [Google Scholar]
- 18.Byeon S.E., Lee J., Kim J.H., Yang W.S., Kwak Y.S., Kim S.Y., Choung E.S. Molecular mechanism of macrophage activation by Red ginseng acidic polysaccharide from Korean Red ginseng. Mediators Inflamm. 2012;2012:732860. doi: 10.1155/2012/732860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y., Liu Y., Zhang X.Y., Xu L.H., Quyang D.Y., Liu K.P., Pan H., He J., He X.H. Ginsenoside Rg1 regulates innate immune responses in macrophages through differentially modulating the NF-κB and PI3K/Akt/mTOR pathways. Int Immunopharmacol. 2014;23:77–84. doi: 10.1016/j.intimp.2014.07.028. [DOI] [PubMed] [Google Scholar]
- 20.Kim D.S., Park J.E., Seo K.I., Ko S.R., Do J.H., Yee S.T. Effects of Red ginseng extract on the activation of dendritic cells. J Ginseng Res. 2006;30:117–127. doi: 10.5142/JGR.2006.30.3.117. [DOI] [Google Scholar]
- 21.Zhang W., Cho S.Y., Xiang G., Min K.J., Jin J.O. Ginseng berry extract promotes maturation of mouse dendritic cells. PLoS One. 2015;10 doi: 10.1371/journal.pone.0130926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Im J.K., Cho I.Y., Min K.Y., Lee H.Y., Kim S.J., Park Y.J., Lew J.H., Lee B.J., Kim S.W., Joo I.W. Comparative study of natural killer cell activity after Red ginseng medication on rat. J Int Korean Med. 2008;29:1075–1082. https://www.jikm.or.kr/journal/view.php?number=1384 Available from: [Google Scholar]
- 23.Kim Y.S., Park K.M., Shin H.J., Song K.S., Nam K.Y., Park J.D. Anticancer activities of Red ginseng acidic polysaccharide by activation of macrophages and natural killer cells. Yakhak Hoeji. 2002;46:113–119. http://www.yakhak.org/journal/view.html?uid=2010&sort=book_Seq&scale=&key=year&keyword=&s_v=46&s_n=2&pn=vol&year=2002&vmd=Full Available from: [Google Scholar]
- 24.Lee B.J., Heo H., Oh S.C., Lew J.H. Comparison study of Korean and Chinese ginsengs on the regulation of lymphocyte proliferation and cytokine production. J Ginseng Res. 2008;32:250–256. doi: 10.5142/JGR.2008.32.3.250. [DOI] [Google Scholar]
- 25.Jang S.K., Kim J.H., Chung Y.S., Ahn D.C., Kang M.J., Lee D.G., Kim S.H. An experimental study on the effect of immunopotential and the anticancer effect of Red ginseng extract. J Ginseng Res. 1994;18:151–159. [Google Scholar]
- 26.Lee H.Y., Lee H. Stimulatory effect of Korean Red ginseng extract on the proliferation and cellular activity of lymphocytes. Korean J Ginseng Sci. 1998;22:60–65. [Google Scholar]
- 27.Sumiyoshi M., Sakanaka M., Kimura Y. Effects of Red ginseng extract on allergic reactions to food in Balb/c mice. J Ethnopharmacol. 2010;132:206–212. doi: 10.1016/j.jep.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 28.Jung J.H., Kang I.G., Kim D.Y., Hwang Y.J., Kim S.T. The effect of Korean Red ginseng on allergic inflammation in a murine model of allergic rhinitis. J Ginseng Res. 2013;37:167–175. doi: 10.5142/jgr.2013.37.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heo S.B., Lim S.W., Jhun J.Y., Cho M.L., Chung B.H., Yang C.W. Immunological benefits by ginseng through reciprocal regulation of Th17 and Treg cells during cyclosporine-induced immunosuppression. J Ginseng Res. 2016;40:18–27. doi: 10.1016/j.jgr.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee J.S., Hwang H.S., Ko E.J., Lee Y.N., Kwon Y.M., Kim M.C., Kang S.M. Immunomodulatory activity of red ginseng against Influenza A virus infection. Nutrients. 2014;6:517–529. doi: 10.3390/nu6020517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoo D.G., Kim M.C., Park M.K., Song J.M., Quan F.S., Park K.M., Cho Y.K., Kang S.M. Protective effect of Korean Red ginseng extract on the infections by H1N1 and H3N2 influenza viruses in mice. J Med Food. 2012;15:855–862. doi: 10.1089/jmf.2012.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim J.Y., Kim H.J., Kim H.J. Effect of oral administration of Korean Red ginseng on influenza A (H1N1) virus infection. J Ginseng Res. 2011;35:104–110. doi: 10.5142/jgr.2011.35.1.104. [DOI] [Google Scholar]
- 33.Xu M.L., Kim H.J., Choi Y.R., Kim H.J. Intake of Korean Red ginseng extract and saponin enhances the protection conferred by vaccination with inactivated influenza A virus. J Ginseng Res. 2012;3:396–402. doi: 10.5142/jgr.2012.36.4.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park E.H., Yum J., Ku K.B., Kim H.M., Kang Y.M., Kim J.C., Kim J.A., Kang Y.K., Seo S.H. Red Ginseng-containing diet helps to protect mice and ferrets from the lethal infection by highly pathogenic H5N1 influenza virus. J Ginseng Res. 2014;38:40–46. doi: 10.1016/j.jgr.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee J.S., Lee Y.N., Lee Y.T., Hwang H.S., Kim K.H., Ko E.J., Kim M.C., Kang S.M. Ginseng protects against respiratory syncytial virus by modulating multiple immune cells and inhibiting viral replication. Nutrients. 2015;7:1021–1036. doi: 10.3390/nu7021021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee J.S., Ko E.J., Hwang H.S., Lee Y.N., Kwon Y.M., Kim M.C., Kang S.M. Antiviral activity of ginseng extract against respiratory syncytial virus infection. Int J Mol Med. 2014;34:183–190. doi: 10.3892/ijmm.2014.1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaneko H., Nakanishi K. Proof of the mysterious efficacy of ginseng: basic and clinical trials: clinical effects of medical ginseng, Korean Red ginseng: specifically, its anti-stress action for prevention of disease. J Pharmacol Sci. 2004;95:158–162. doi: 10.1254/jphs.fmj04001x5. [DOI] [PubMed] [Google Scholar]
- 38.Lee C.S., Lee J.H., Oh M., Choi K.M., Jeong M.R., Park J.D., Kwon D.Y., Ha K.C., Park E.O., Lee N.R. Preventive effect of Korean Red ginseng for acute respiratory illness: a randomized and double-blind clinical trial. J Korean Med Sci. 2012;27:1472–1478. doi: 10.3346/jkms.2012.27.12.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suh S.O., Jeung C.H., Cho M.Y., Soon G.S. The effect of Red ginseng for postoperative immune response in gastrointestinal carcinoma. J Ginseng Res. 1998;22:32–42. http://www.koreascience.or.kr/article/JAKO199811919374402.page Available from: [Google Scholar]
- 40.Suh S.O., Kim J., Cho M.Y. Prospective study for Korean Red ginseng extract as an immune modulator following a curative gastric resection in patients with advanced gastric cancer. J Ginseng Res. 2004;28:104–110. doi: 10.5142/JGR.2004.28.2.104. [DOI] [Google Scholar]
- 41.Boo Y.J., Park J.M., Kim J., Suh S.O. Prospective study for Korean Red ginseng extract as an immune modulator following a curative surgery in patients with advanced colon cancer. J Ginseng Res. 2007;31:54–59. doi: 10.5142/JGR.2007.31.1.054. [DOI] [Google Scholar]
- 42.Korean Food and Drug Administration . English ed. 2002. The Korean herbal pharmacopoeia. Korea. [Google Scholar]
- 43.Du X.F., Jiang C.Z., Wu C.F., Won E.K., Choung S.Y. Synergistic immunostimulating activity of pidotimod and red ginseng acidic polysaccharide against cyclophosphamide-induced immunosuppression. Arch Pharm Res. 2008;31:1153–1159. doi: 10.1007/s12272-001-1282-6. [DOI] [PubMed] [Google Scholar]
- 44.Kenarova B., Neychev H., Hadjiivanova C., Petkov V.D. Immunomodulating activity of ginsenoside Rg1 from Panax ginseng. Jpn J Pharmacol. 1990;54:447–454. doi: 10.1254/jjp.54.447. [DOI] [PubMed] [Google Scholar]
- 45.Saba E., Lee Y.Y., Kim M., Kim S.H., Hong S.B., Rhee M.H. A comparative study on immune-stimulatory and antioxidant activities of various types of ginseng extracts in murine and rodent models. J Ginseng Res. 2018;42:577–584. doi: 10.1016/j.jgr.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cho Y.K., Sung H., Lee H.J., Joo C.H., Cho G.J. Long-term intake of Korean Red ginseng in HIV-1-infected patients: development of resistance mutation to zidovudine is delayed. Int Immunopharmacol. 2001;1:1295–1305. doi: 10.1016/s1567-5769(01)00061-3. [DOI] [PubMed] [Google Scholar]
- 47.Sung H., Kang S.M., Lee M.S., Kim T.G., Cho Y.K. Korean Red ginseng slows depletion of CD4 T cells in human immunodeficiency virus type 1-infected patients. Clin Diagn Lab Immunol. 2005;12:497–501. doi: 10.1128/CDLI.12.4.497-501.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen T., Xiao L., Zhu L., Ma S., Yan T., Ji H. Anti-asthmatic effects of ginsenoside Rb1 in a mouse model of allergic asthma through relegating Th1/Th2. Inflammation. 2015;38:1814–1822. doi: 10.1007/s10753-015-0159-4. [DOI] [PubMed] [Google Scholar]
- 49.Wang L., Zhang Y., Chen J., Li S., Wang Y., Hu L., Wang L., Wu Y. Immunosuppressive effects of ginsenoside-Rd on skin allograft rejection in rats. J Surg Res. 2012;176:267–274. doi: 10.1016/j.jss.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 50.Wu C.F., Bi X.L., Yang J.Y., Zhan J.Y., Dong Y.X., Wang J.H., Wang J.M., Zhang R., Li X. Differential effects of ginsenosides on NO and TNF-alpha production by LPS-activated N9 microglia. Int Immunopharmacol. 2007;7:313–320. doi: 10.1016/j.intimp.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 51.Smolinski A.T., Pestka J.J. Modulation of lipopolysaccharide-induced proinflammatory cytokine production in vitro and in vivo by the herbal constituents apigenin (chamomile), ginsenoside Rb(1) (ginseng) and parthenolide (feverfew) Food Chem Toxicol. 2003;41:1381–1390. doi: 10.1016/s0278-6915(03)00146-7. [DOI] [PubMed] [Google Scholar]
- 52.Park J.H., Ahn E.K., Ko H.J., Lee J.Y., Hwang S.M., Ko S.M., Oh J.S. Korean Red Ginseng water extract alleviates atopic dermatitis-like inflammatory responses by negative regulation of mitogen-activated protein kinase signaling pathway in vivo. Biomed Pharmacother. 2019;117:109066. doi: 10.1016/j.biopha.2019.109066. [DOI] [PubMed] [Google Scholar]
- 53.Lee J.M., Hah J.O., Kim H.S. The effect of red ginseng extract on inflammatory cytokines after chemotherapy in children. J Ginseng Res. 2012;36:383–390. doi: 10.5142/jgr.2012.36.4.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Song S.W., Kim H.N., Shim J.Y., Yoo B.Y., Kim D.H., Lee S.H., Park J.S., Kim M.J., Yoo J.H., Cho B.L. Safety and tolerability of Korean Red Ginseng in healthy adults: a multicenter, double-blind, randomized, placebo-controlled trial. J Ginseng Res. 2018;42:571–576. doi: 10.1016/j.jgr.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.