Abstract
Background
Korean Red Ginseng (KRG) is a natural product with antiinflammatory and anticarcinogenic effects. We have previously reported that the endocrine-disrupting compound bisphenol A (BPA)-induced cyclooxygenase-2 (COX-2) via nuclear translocation of nuclear factor-kappa B (NF-κB) and activation of mitogen-activated protein kinase and promoted the migration of A549. Here, in this study, we assessed the protective effect of KRG on the BPA-induced reactive oxygen species (ROS) and expression of COX-2 and matrix metalloproteinase-9 (MMP-9) in A549 cells.
Methods
The effects of KRG on the upregulation of ROS production and COX-2 and MMP-9 expression by BPA were evaluated by fluorescence-activated cell sorting (FACs) analysis, quantitative reverse transcription polymerase chain reaction, and western blotting. Antimigration ability by KRG was evaluated by migration assay in A549 cells.
Results
KRG significantly suppressed the BPA-induced COX-2, the activity of NF-κB, the production of ROS, and the migration of A549 cells. These effects led to the downregulation of the expression of MMP-9.
Conclusions
Overall, our results suggest that KRG exerts an antiinflammatory effect on BPA-treated A549 cells via the suppression of ROS and downregulation of NF-κB activation and COX-2 expression which leads to a decrease in cellular migration and MMP-9 expression. These results provide a new possible therapeutic application of KRG to protect BPA-induced possible inflammatory disorders.
Keywords: Bisphenol A, Cyclooxygenase-2, Korean Red Ginseng, Matrix metalloproteinase-9, Reactive oxygen species
1. Introduction
Bisphenol A (BPA) is an endocrine-disrupting chemical (EDC) that is used to manufacture polycarbonate plastics and epoxy resins for many consumer products, including plastic cups, food storage containers, and beverage cans [1,2]. BPA exposure causes reproductive disorders, such as precocious puberty, prostate developmental abnormalities, decreased sperm production, and reproductive organ malignancies [3,4]. The cellular effects of BPA are mediated by binding to estrogen receptors (ERs) alpha and beta [5]. Disrupted ER signaling can cause reproductive disorders and inflammatory dysregulation [6,7]. In an endometriosis animal model, ER hyperstimulation caused enhanced cell proliferation and vascularization and increased neuron innervation and the inflammatory responses [8]. Hyperestrogenic stimulation and inflammation are linked by a feed-forward loop [9]. There is accumulating evidence of cross-talk between ER signaling and the inflammasome; therefore, an ER agonistic ligand-like BPA very likely affects inflammation [[10], [11], [12]]. BPA exposure stimulates interferon signaling and activates inflammasome activity, leading to the deterioration of autoimmune diseases such as systemic lupus erythematosus [13]. Studies have examined the effects of BPA on hormone-dependent and other tumors [14]. BPA produces inflammation and reactive oxygen species (ROS) in human lung cancer cells and promotes the development of pulmonary inflammatory diseases [15,16]. Previously, we showed that BPA induces cyclooxygenase-2 (COX-2) and the migration in A549 human lung cancer cells [15]. Although precautions to minimize exposure to environmental chemicals are useful, remedies that will alleviate possible adverse effects of these environmental chemicals are important.
Ginseng has been used in traditional medicine for thousands of years. Ginseng is divided into two species: Panax ginseng (Korean ginseng) and Panax quinquefolius (American ginseng) [17]. The word Panax means “all healing” and it comes from the traditional belief that ginseng can cure all illnesses [18]. However, the two ginseng species have different ginsenoside contents and some compounds are unique to Korean ginseng, such as ginsenoside Rf [19,20]. When Korean ginseng is steamed and dried, its ginsenoside and polysaccharide components change [21]. The resulting product, called Korean Red Ginseng (KRG), has obtained approval of the Korean Food and Drug Administration and is reported to improve immunity, relieve fatigue, improve the blood circulation, have antioxidative effects, and improve the symptoms of menopause in women [17]. With these activities, KRG is therapeutically effective against various diseases [18,22]. Many studies have shown that KRG targets oxidative stress by inhibiting ROS formation and blocking inflammation, making it effective in various inflammatory diseases, such as atherosclerosis, coronary artery dysfunction, cancer, and neurodegenerative diseases [[23], [24], [25], [26]]. We hypothesized that KRG could protect against the inflammation-related phenomena caused by BPA that we observed previously. In this study, we investigated whether KRG prevents BPA-induced COX-2 expression and cellular migration in A549 cells.
2. Materials and methods
2.1. Experimental design
We have previously found that COX-2 and cell migration are increased by BPA in human lung adenocarcinoma A549 cells [15]. Therefore, we evaluated the protective effect of KRG against BPA-induced COX-2 in A549 cells. First, to ensure that the increased COX-2 by BPA is suppressed by KRG, two concentrations of KRG were pretreated for 1 h and BPA was processed and incubated for 24 h to check COX-2 protein and mRNA expression. And the amount of ROS generation was measured by FACs analysis to determine whether KRG inhibits BPA-induced ROS. To determine whether COX-2 and matrix metalloproteinase-9 (MMP-9) increased by BPA increased dependent on ROS, we examined the protein and mRNA expression of COX-2 and MMP-9 by treating with N-acetyl-L-cysteine (NAC), a ROS inhibitor. In addition, the effect of KRG on BPA-induced cellular migration was analyzed by using transwell migration assay in A549 cells.
2.2. Materials (reagents and antibodies)
BPA, 2′,7′-dichlorofluorescin diacetate (DCF-DA), NAC, dimethyl sulfoxide, thiazolyl blue tetrazolium bromide (MTT), celecoxib and Anti-β-actin were purchased from Sigma-Aldrich (St. Louis, MO, USA). Roswell Park Memorial Institute 1640 Medium and TOM™ Transfection Optimized Medium were purchased from WelGENE Inc. (Daegu, Korea). Trizol reagent, fetal calf serum, and antibiotic-antimycotic were purchased from GIBCO Invitrogen (Grand Island, NY, USA). Polyethylenimine was purchased from Polyscience (Warrington, PA, USA). Enhanced chemiluminescence was obtained from Amersham Pharmacia Biotech (Buckinghamshire, UK). Anti-COX-2 was used from Cayman Chemical Co. (Ann Arbor, MI, USA). Anti–nuclear factor-kappa B (NF-κB) p65 and lamin B were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-MMP-9 (Matrix Metalloproteinase-9) was used from Cell Signaling Technology (Beverly, MA, USA).
2.3. Preparation of KRG
KRG was manufactured and kindly provided by the Korea Ginseng Corporation (KGC, Daejeon, Korea) [27]. The analysis of KRG was based on the reported method [28]. The phytochemical characteristics of KRG with standard ginsenosides were confirmed by high-performance liquid chromatography (HPLC) analysis (Rb1, 7.34 mg/g; Rb2, 2.54 mg/g; Rc, 3.41mg/g; Rd, 0.97 mg/g; Re, 1.64 mg/g; Rf, 1.26 mg/g; Rg1, 1.21 mg/g; Rg2 (s), 1.36 mg/g; Rg3(s), 2.21 mg/g; Rg3(r), 1.17mg/g and Rh1, 1.56 mg/g).
2.4. Cell culture conditions
Human adenocarcinoma A549 cells were maintained in Roswell Park Memorial Institute 1640 Medium containing 10% FCS, 100 units/mL of penicillin, 100 μg/mL of streptomycin and 250 ng/mL of Fungizone® (amphotericin B) mixed antibiotic-antimycotic. The cells were cultured at 37°C in the humidified 5% CO2 atmosphere.
2.5. Luciferase reporter assay
The NF-κB luciferase was constructed using the enhanced luciferase reporter gene pELAM promotor. A549 cells were transiently transfected with plasmid using polyethylenimine reagent and Tom medium. Luciferase assay was performed according to a previously reported method [29].
2.6. Reverse transcription polymerase chain reaction
Total RNA was extracted using the trizol reagent, and the first-strand cDNA was synthesized according to a previously reported method [30]. Quantitative real-time polymerase chain reaction was performed with StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) using a AccuPower® GreenStar™ qPCR Pre Mix (Bioneer Corporation, Daejeon, Korea) according to the manufacturer's instructions [31]. The primers used were MMP-9: 5′-ACTTTGACAGCGACAAGAAGTG-3′, 5′-GGCACTGAGGAATGATCTAAGC-3′. The primers of COX-2 and β-actin were described previously [15]. The relative expression was calculated and standardized by the expression of β-actin taken from the same sample using the comparative cycle threshold (Ct).
2.7. Western blot analysis
Protein isolation of whole cells and western blot analysis were performed according to previously reported methods [32]. The blots were incubated for overnight or 1 h with primary antibodies (NF-κB-p65, Lamin B, MMP-9, COX-2, and β-actin) diluted to 1:1000 or 1:5000 in Tris Buffered Saline with Tween 20 (TBST). The blots were washed and incubated with secondary antibody diluted to 1:5000 with skim milk for 1 h. The bands were detected the using enhanced chemiluminescence. Quantity One analysis software (Bio-Rad, Hercules, CA, USA) was used to quantify the strength of the band.
2.8. Extraction of nuclear and cytoplasmic proteins
Cytoplasmic and nuclear proteins of A549 cells were detached using the nuclear and cytoplasmic protein extraction kits (Abcam, Cambridge, UK) according to the manufacturer's instructions. Fractions were analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE) and western blot with specific antibodies.
2.9. Cell migration assays
As previously described, the migration assay was performed using transwell inserts (Corning Inc., NY, USA) that have 6.5 mm polycarbonate membranes with 8.0 µm pores [27]. After 24 h of incubation under BPA or KRG or celecoxib, the migrated cells were fixed and stained with crystal violet.
2.10. MTT assays
A549 cells were seeded in a 96-well plate at a density of 5000 cells/well. Next day, A549 cells were treated with the BPA or KRG into the culture media and incubated for 24 h. MTT assays were performed as reported previously [33].
2.11. Measurement of reactive oxygen species production
The levels of intracellular ROS were measured with a FACSCalibur flow cytometer using the BD CellQuest Pro software (BD Biosciences, San Jose, CA, USA) according to previously reported methods [15]. FlowJo software (TreeStar, Inc., Ashland, OR, USA) was used to analyze data.
2.12. Statistical analysis
Data were described as the means ± standard deviation. We compared each group using one-way analysis of variance and Tukey's multiple-comparison posttest using GraphPad Prism software (GraphPad Software Inc., La Jolla, CA, USA). Differences between groups were considered significant at a P value of less than 0.05.
3. Results
3.1. KRG suppresses BPA-induced COX-2 expression
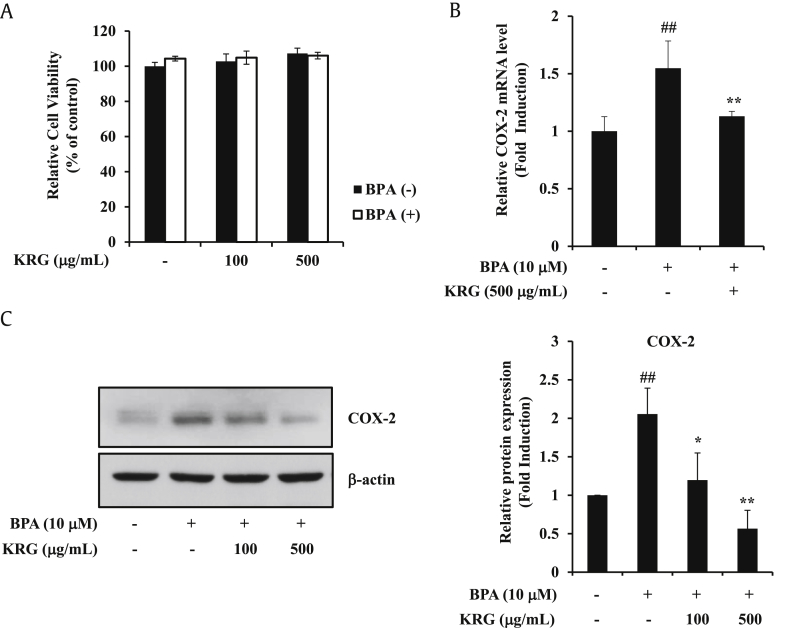
Inflammation is closely associated with cancer progression, and release of inflammatory molecules is known to induce the cancer progression and metastasis [34]. Among various inflammatory mediators, COX-2 is a major factor in inflammation [35]. Previously, we showed that BPA upregulates COX-2 expression in A549 cells [15]. We also investigated whether KRG inhibited BPA-induced COX-2 expression. KRG did not affect cell viability at concentrations of 100–500 μg/mL under BPA treatment (Fig. 1A). Therefore, this concentration was selected as the treatment condition in the following experiment. KRG at a dose of 500 μg/mL efficiently blocked BPA-induced COX-2 mRNA levels (Fig. 1B). KRG also significantly suppressed BPA-induced COX-2 protein (Fig. 1C). These results indicate that KRG exerts an inhibitory effect on COX-2 expression by BPA in A549 cells.
Fig. 1.
Effects of KRG on BPA-induced COX-2 expression. (A) A549 cells were preincubated with KRG for 1h and treated with BPA. After 24 h incubation, cell viability was measured by MTT assay. (B) A549 cells were pretreated with KRG (500 μg/mL) for 1 h and treated with BPA for 24 h. The levels of COX-2 mRNA were determined by qRT-PCR. (C) A549 cells were treated as described in (A). COX-2 and β-actin were evaluated by western blot analysis. ##p < 0.01, CON vs. BPA; *p < 0.05 and **p < 0.01, BPA vs. BPA + KRG.
KRG, Korean Red Ginseng; BPA, bisphenol A; MTT, thiazolyl blue tetrazolium bromide; qRT-PCR, quantitative reverse transcription polymerase chain reaction; COX-2, cyclooxygenase-2.
3.2. KRG suppresses BPA-induced NF-κB promoter activity and NF-κB nuclear translocation
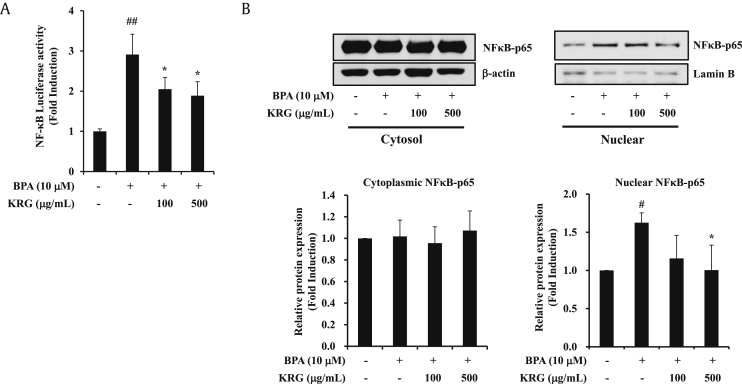
NF-κB is a transcriptional regulator of the expression of various genes, including COX-2, which is involved in cell proliferation and inflammatory responses [36]. Previously, we confirmed that BPA upregulates NF-κB activity in A549 cells [15]. Next, we examined whether the increase in NF-κB activity induced by BPA was reduced by KRG treatment. KRG inhibited the BPA-induced NF-κB promoter activity at concentrations of 100 and 500 μg/mL (Fig. 2A). The activation of NF-κB is initiated by translocation of the NF-κB p65 subunit from the cytoplasm into the nucleus [37]. Nuclear p65 acts as a transcription factor that causes the expression of inflammatory genes, such as COX-2 [38]. Treatment with BPA (10 μM) increased the nuclear expression of p65 protein. KRG (500 μg/mL) significantly attenuated BPA-induced translocation of NF-κB p65 into the nucleus (Fig. 2B). These results demonstrate that KRG blocked the BPA-induced NF-κB promoter activity and NF-κB nucleus translocation.
Fig. 2.
Effects of KRG on BPA-induced NF-κB activation. (A) A549 cells were transfected with the NF-κB luciferase reporter gene. The next day, A549 cells were pretreated with KRG for 1 h and then treated with BPA for 24 h. Luciferase activities of NF-κB were measured. (B) A549 cells were treated as described in (A). The cytoplasmic and nuclear protein extracts were isolated with different lysis buffers. NF-κB p65, lamin B, and β-actin were evaluated by western blot analysis. #p < 0.05 and ##p < 0.01, CON vs. BPA; *p < 0.05, BPA vs. BPA + KRG.
NF-κB, nuclear factor-kappa B; KRG, Korean Red Ginseng; BPA, bisphenol A.
3.3. KRG inhibits BPA-induced cell migration
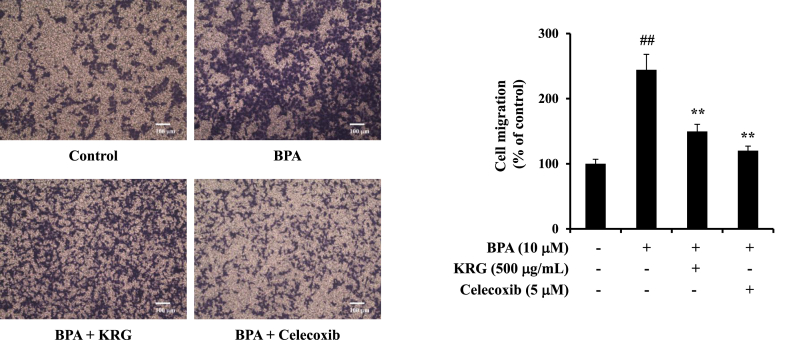
COX-2 is also strongly implicated in tumor progression by promoting important cellular functions, including cell migration [39]. To determine whether KRG inhibited the BPA-induced migration of A549 cells via COX-2, cell migration was investigated under the presence of the COX-2 inhibitor celecoxib. Celecoxib and KRG significantly suppressed the BPA-induced cell migration by approximately 39% and by 51% compared with BPA treatment (Fig. 3). Because celecoxib blocked cell migration induced by BPA, it suggests that COX-2 plays an important role in the regulation of cell migration by BPA. These results demonstrate that KRG suppresses BPA-induced cell migration.
Fig. 3.
Effects of KRG on BPA-induced cellular migration. A549 cells were coincubated with BPA or KRG or celecoxib in the upper chamber of transwell for 24 h. The migrated cells were counted using light microscopy. The bar graph shows the cells that have been migrated relatively. Scale bar represents 100 μm. ##p < 0.01, CON vs. BPA; **p < 0.01, BPA vs. BPA + KRG or BPA + celecoxib.
KRG, Korean Red Ginseng; BPA, bisphenol A.
3.4. KRG inhibits BPA-induced ROS production
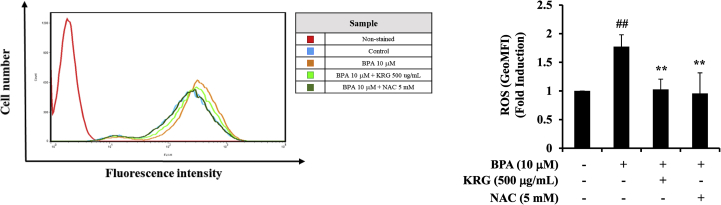
To understand the upstream modulation of NF-κB, we confirmed whether KRG inhibited BPA-induced ROS production in A549 cells by flow cytometry. ROS is involved in NF-κB activation and leads to increased cytokine expression [40]. Moreover, ROS activates signaling pathways, such as in cancer development and progression [41]. As shown Fig. 4, BPA markedly elevated the ROS levels in A549 cells compared with the control, whereas the ROS scavenger NAC almost completely blocked ROS production. Treatment with KRG (500 μg/mL) effectively suppressed the levels of BPA-induced ROS. These results indicate that KRG inhibited BPA-induced ROS production.
Fig. 4.
Effects of KRG on BPA-induced ROS production. A549 cells were pretreated with KRG for 1 h and treated with BPA. After 24h incubation, A549 cells were stained with cell-permeable dye 2′,7′-dichlorofluorescin diacetate (DCF-DA) (1 μM). ROS production was measured by flow cytometry. ##p < 0.01, CON vs. BPA; **p < 0.01, BPA vs. BPA + KRG or BPA + NAC.
KRG, Korean Red Ginseng; BPA, bisphenol A; ROS, reactive oxygen species; NAC, N-acetyl-L-cysteine.
3.5. KRG suppresses BPA-induced COX-2 and MMP-9
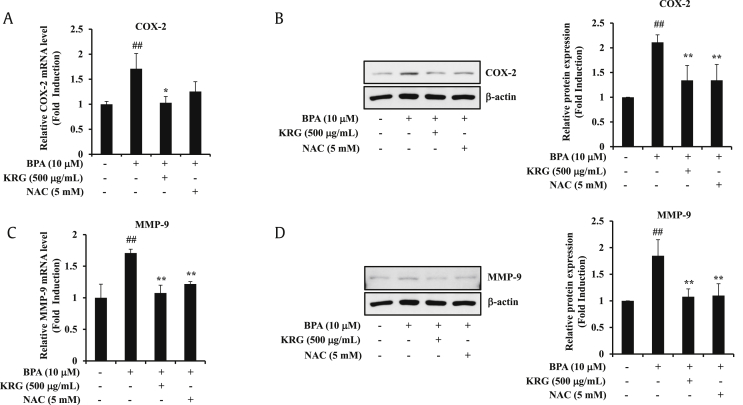
MMP-9 plays an important role in the migration and invasion of various cancer cells [42]. In addition, BPA increases MMP-9 expression in A549 cells [43]. NF-κB is centrally involved in the induction of MMP-9 expression by BPA [44,45]. To confirm whether the suppression of BPA-induced MMP-9 and COX-2 by KRG is associated with its ROS-scavenging activity, we confirmed the expression of MMP-9 and COX-2 on treatment of KRG and NAC. The BPA-induced COX-2 mRNA and protein levels were blocked by KRG and NAC (Fig. 5A and B). KRG and NAC also inhibited the BPA-induced MMP-9 mRNA and protein expression (Fig. 5C and D). These results demonstrate that KRG blocks BPA-induced COX-2 and MMP-9 via ROS signaling.
Fig. 5.
Effects of KRG on involvement of ROS in COX-2 and MMP-9 expression. (A) A549 cells were preincubated with KRG and NAC for 1 h and treated with BPA for 24 h. The levels of COX-2 mRNA were determined by qRT-PCR. (B) A549 cells were treated as described in (A). COX-2 and β-actin were evaluated by western blot analysis. (C) A549 cells were treated and total RNA was extracted as described in (A). The expression of MMP-9 mRNA was determined by qRT-PCR. (D) A549 cells were treated as described in (A). MMP-9 and β-actin were evaluated by western blot analysis. ##p < 0.01, CON vs. BPA; *p < 0.05 and **p < 0.01, BPA vs. BPA + KRG or BPA + NAC.
KRG, Korean Red Ginseng; BPA, bisphenol A; ROS, reactive oxygen species; COX-2, cyclooxygenase-2; qRT-PCR, quantitative reverse transcription polymerase chain reaction; MMP-9, matrix metalloproteinase-9; NAC, N-acetyl-L-cysteine.
4. Discussion
This study demonstrated that KRG inhibits the BPA-induced COX-2 expression and cell migration via the inhibition of ROS and downregulation of NF-κB activation and MMP-9 expression in A549 human lung cancer cells. These results point to a new possible therapeutic application for KRG to protect against inflammatory disorders induced by EDCs. We and others have shown that BPA induces COX-2 in cancer cell lines [15,46]. BPA induced COX-2 expression via the activation of mitogen-activated protein kinase and promoted the cellular migration of A549 and MDAMB-231 cells [15,47]. BPA induces COX-2 expression in human mesenchymal stem cells derived from uterine myoma tissue, human endometrial carcinoma cells [46,48]. In two epidemiological studies conducted with repeated urine and serum samples from 700 Korean elderly people, the associations of BPA with six inflammation markers (white blood cells, C-reactive protein, interleukin-10, alanine aminotransferase, aspartate transaminase and γ-glutamyl transpeptidase levels) were evaluated, and significant positive correlations between the levels of BPA and six inflammation markers were found [15]. A Taiwanese cohort study suggested that nonylphenol (NP) and BPA increase oxidative stress and decrease antioxidant activity during pregnancy and inflammation [49]. The generation of ROS increased with a decrease in mitochondrial membrane potential in BPA-exposed lymphoblastoid cell lines of children with autism [50]. Accumulating in vitro and in vivo studies strongly support the prooxidant role of BPA [51]. Induction of ROS and inflammation by BPA leads to activation of the mitogen-activated protein kinase, PI3K/AKT, and NF-κB pathways, inducing mitochondrial dysfunction and promoting changes in several cell signaling pathways, partly via nuclear or membrane ER signaling mechanisms [[52], [53], [54]]
Various studies have searched for protective and preventive natural products that are effective against EDC-induced pathological conditions [55,56]. Oleuropein- and hydroxytyrosol-rich extracts from olive leaves attenuated the liver injury and lipid accumulation induced by BPA in male rats via hypolipidemic and hepatoprotective effects by enhancing the antioxidative defense system and regulating inflammation [57]. Selenium and nanoselenium protected against the reproductive toxicity induced by BPA via improved antioxidant activity [58]. Data from 88,962 adults collected as part of the National Health Interview Survey to assess trends in the use of complementary health approaches found that ginseng was the ninth most commonly used nonvitamin, nonmineral dietary supplement in a question asking about usage in the previous 30 days after fish oil, glucosamine, probiotics, melatonin, coenzyme q-10, Echinacea, garlic supplements, and cranberry [59]. The many studies of the antioxidant activity of KRG induced by various oxidative stresses motivated our study, and our results show that KRG is effective against the increases in intracellular ROS and COX-2 induced by BPA. Only a few antioxidant studies have been conducted in humans, so clinical data on the beneficial effects of KRG on exposure to EDCs will be useful for extending the application of KRG.
MMP-9 plays a critical role in the progression of cancer, and the overexpression of MMP-9 is mostly related to the migration and invasion of various cancer cells [60]. In addition, MMP-9 is involved in the pathophysiology of cancer progression and inflammation-related diseases [61,62]. Recent research studies have supported that MMP-9 inhibitors have significant protective effects for tumor promotion by partially blocking the expression of proinflammatory enzymes, such as COX-2 [63]. Exposure of BPA induces cell migration and MMP-9 expression in several cancer cells [43,64]. Thus, targeting MMP-9 inhibition is another strategy for cancer prevention and treatment [65]. To identify the molecular mechanisms that determine the antimetastatic and antiinflammatory effect of KRG, a key question in this study asked whether KRG downregulates BPA-induced COX-2 and MMP-9 expression in A549 cells.
In summary, these above data demonstrated that KRG inhibits BPA-induced MMP-9 and COX-2 by inhibiting ROS in A549 cells. Therefore, our results suggest that KRG could be used as a potential therapeutic agent for inflammatory disorders by BPA in lung cancer cells.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This research was supported 2017 grant from the Korean Society of Ginseng to Y.J.L.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jgr.2020.01.002.
Contributor Information
Joonwoo Park, Email: jwpark4785@sejong.ac.kr.
YoungJoo Lee, Email: yjlee@sejong.ac.kr.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Kang J.H., Kondo F., Katayama Y. Human exposure to bisphenol A. Toxicology. 2006;226(2–3):79–89. doi: 10.1016/j.tox.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 2.Staples C.A., Dorn P.B., Klecka G.M., O'Block S.T., Harris L.R. A review of the environmental fate, effects, and exposures of bisphenol A. Chemosphere. 1998;36(10):2149–2173. doi: 10.1016/s0045-6535(97)10133-3. [DOI] [PubMed] [Google Scholar]
- 3.Gurmeet K., Rosnah I., Normadiah M.K., Das S., Mustafa A.M. Detrimental effects of bisphenol A on development and functions of the male reproductive system in experimental rats. EXCLI J. 2014;13:151–160. [PMC free article] [PubMed] [Google Scholar]
- 4.Hass U., Christiansen S., Boberg J., Rasmussen M.G., Mandrup K., Axelstad M. Low-dose effect of developmental bisphenol A exposure on sperm count and behaviour in rats. Andrology. 2016;4(4):594–607. doi: 10.1111/andr.12176. [DOI] [PubMed] [Google Scholar]
- 5.Washington W., Hubert L., Jones D., Gray W.G. Bisphenol a binds to the low-affinity estrogen binding site. Vitr Mol Toxicol. 2001;14(1):43–51. doi: 10.1089/109793301316882531. [DOI] [PubMed] [Google Scholar]
- 6.Sweeney M.F., Hasan N., Soto A.M., Sonnenschein C. Environmental endocrine disruptors: effects on the human male reproductive system. Rev Endocr Metab Disord. 2015;16(4):341–357. doi: 10.1007/s11154-016-9337-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer C., Mamillapalli R., Goetz L.G., Jorgenson E., Ilagan Y., Taylor H.S. Bisphenol A (BPA) exposure in utero leads to immunoregulatory cytokine dysregulation in the mouse mammary gland: a potential mechanism programming breast cancer risk. Horm Cancer. 2016;7(4):241–251. doi: 10.1007/s12672-016-0254-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trukhacheva E., Lin Z., Reierstad S., Cheng Y.H., Milad M., Bulun S.E. Estrogen receptor (ER) beta regulates ERalpha expression in stromal cells derived from ovarian endometriosis. J Clin Endocrinol Metab. 2009;94(2):615–622. doi: 10.1210/jc.2008-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bulun S.E. Endometriosis. N Engl J Med. 2009;360(3):268–279. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- 10.Arnal J.F., Laurell H., Fontaine C., Billon A., Calippe B., Lenfant F., Gourdy P. Estrogen receptor actions on vascular biology and inflammation: implications in vascular pathophysiology. Climacteric. 2009;12(Suppl 1):12–17. doi: 10.1080/13697130902820006. [DOI] [PubMed] [Google Scholar]
- 11.Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol. 2015;294(2):63–69. doi: 10.1016/j.cellimm.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monteiro R., Teixeira D., Calhau C. Estrogen signaling in metabolic inflammation. Mediators Inflamm. 2014;2014:615917. doi: 10.1155/2014/615917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panchanathan R., Liu H., Leung Y.K., Ho S.M., Choubey D. Bisphenol A (BPA) stimulates the interferon signaling and activates the inflammasome activity in myeloid cells. Mol Cell Endocrinol. 2015;415:45–55. doi: 10.1016/j.mce.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao H., Yang B.J., Li N., Feng L.M., Shi X.Y., Zhao W.H., Liu S.J. Bisphenol A and hormone-associated cancers: current progress and perspectives. Medicine (Baltimore) 2015;94(1):e211. doi: 10.1097/MD.0000000000000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song H., Park J., Bui P.T.C., Choi K., Gye M.C., Hong Y.C., Kim J.H., Lee Y.J. Bisphenol A induces COX-2 through the mitogen-activated protein kinase pathway and is associated with levels of inflammation-related markers in elderly populations. Environ Res. 2017;158:490–498. doi: 10.1016/j.envres.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Abedelhaffez A.S., El-Aziz E.A.A., Aziz M.A.A., Ahmed A.M. Lung injury induced by Bisphenol A: a food contaminant, is ameliorated by selenium supplementation. Pathophysiology. 2017;24(2):81–89. doi: 10.1016/j.pathophys.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 17.So S.H., Lee J.W., Kim Y.S., Hyun S.H., Han C.K. Red ginseng monograph. J Ginseng Res. 2018;42(4):549–561. doi: 10.1016/j.jgr.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim J.H. Pharmacological and medical applications of Panax ginseng and ginsenosides: a review for use in cardiovascular diseases. J Ginseng Res. 2018;42(3):264–269. doi: 10.1016/j.jgr.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christensen L.P. Ginsenosides chemistry, biosynthesis, analysis, and potential health effects. Adv Food Nutr Res. 2009;55:1–99. doi: 10.1016/S1043-4526(08)00401-4. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y., Choi H.K., Brinckmann J.A., Jiang X., Huang L. Chemical analysis of Panax quinquefolius (North American ginseng): a review. J Chromatogr A. 2015;1426:1–15. doi: 10.1016/j.chroma.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 21.Lee S.M., Bae B.S., Park H.W., Ahn N.G., Cho B.G., Cho Y.L., Kwak Y.S. Characterization of Korean red ginseng (Panax ginseng meyer): history, preparation method, and chemical composition. J Ginseng Res. 2015;39(4):384–391. doi: 10.1016/j.jgr.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakhjavani M., Hardingham J.E., Palethorpe H.M., Tomita Y., Smith E., Price T.J. Townsend A.R. Ginsenoside Rg3: potential molecular targets and therapeutic indication in metastatic breast cancer. Medicines (Basel) 2019;6(1) doi: 10.3390/medicines6010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee Y.M., Yoon H., Park H.M., Song B.C., Yeum K.J. Implications of red Panax ginseng in oxidative stress associated chronic diseases. J Ginseng Res. 2017;41(2):113–119. doi: 10.1016/j.jgr.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim K.H., Lee D., Lee H.L., Kim C.E., Jung K., Kang K.S. Beneficial effects of Panax ginseng for the treatment and prevention of neurodegenerative diseases: past findings and future directions. J Ginseng Res. 2018;42(3):239–247. doi: 10.1016/j.jgr.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahuja A., Kim J.H., Kim J.H., Yi Y.S., Cho J.Y. Functional role of ginseng-derived compounds in cancer. J Ginseng Res. 2018;42(3):248–254. doi: 10.1016/j.jgr.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saba E., Lee Y.Y., Kim M., Kim S.H., Hong S.B., Rhee M.H. A comparative study on immune-stimulatory and antioxidant activities of various types of ginseng extracts in murine and rodent models. J Ginseng Res. 2018;42(4):577–584. doi: 10.1016/j.jgr.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song H., Lee Y.J. Inhibition of hypoxia-induced cyclooxygenase-2 by Korean Red Ginseng is dependent on peroxisome proliferator-activated receptor gamma. J Ginseng Res. 2017;41(3):240–246. doi: 10.1016/j.jgr.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.In G., Ahn N.G., Bae B.S., Lee M.W., Park H.W., Jang K.H., Cho B.G., Han C.K., Park C.K., Kwak Y.S. In situ analysis of chemical components induced by steaming between fresh ginseng, steamed ginseng, and red ginseng. J Ginseng Res. 2017;41(3):361–369. doi: 10.1016/j.jgr.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim E., Kim D., Yoo S., Hong Y.H., Han S.Y., Jeong S., Jeong D., Kim J.H., Cho J.Y., Park J. The skin protective effects of compound K, a metabolite of ginsenoside Rb1 from Panax ginseng. J Ginseng Res. 2018;42(2):218–224. doi: 10.1016/j.jgr.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park C., Lee J., Kong B., Park J., Song H., Choi K., Guon T., Lee Y. The effects of bisphenol A, benzyl butyl phthalate, and di(2-ethylhexyl) phthalate on estrogen receptor alpha in estrogen receptor-positive cells under hypoxia. Environ Pollut. 2019;248:774–781. doi: 10.1016/j.envpol.2019.02.069. [DOI] [PubMed] [Google Scholar]
- 31.Kim H.J., Choi J.H., Hwang J.H., Kim K.S., Noh J.R., Choi D.H., Moon S.J., Kim H.Y., Kim S.W., Choi S. 3,5-Di-C-beta-D-glucopyranosyl phloroacetophenone, a major component of Melicope ptelefolia, suppresses fibroblast activation and alleviates arthritis in a mouse model: potential therapeutics for rheumatoid arthritis. Int J Mol Med. 2018;42(5):2763–2775. doi: 10.3892/ijmm.2018.3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X., Su G.Y., Zhao C., Qu F.Z., Wang P., Zhao Y.Q. Anticancer activity and potential mechanisms of 1C, a ginseng saponin derivative, on prostate cancer cells. J Ginseng Res. 2018;42(2):133–143. doi: 10.1016/j.jgr.2016.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim E.J., Kwon K.A., Lee Y.E., Kim J.H., Kim S.H., Kim J.H. Korean Red Ginseng extract reduces hypoxia-induced epithelial-mesenchymal transition by repressing NF-kappaB and ERK1/2 pathways in colon cancer. J Ginseng Res. 2018;42(3):288–297. doi: 10.1016/j.jgr.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sgambato A., Cittadini A. Inflammation and cancer: a multifaceted link. Eur Rev Med Pharmacol Sci. 2010;14(4):263–268. [PubMed] [Google Scholar]
- 35.Gandhi J., Khera L., Gaur N., Paul C., Kaul R. Role of modulator of inflammation cyclooxygenase-2 in gammaherpesvirus mediated tumorigenesis. Front Microbiol. 2017;8:538. doi: 10.3389/fmicb.2017.00538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lim J.W., Kim H., Kim K.H. Nuclear factor-kappaB regulates cyclooxygenase-2 expression and cell proliferation in human gastric cancer cells. Lab Invest. 2001;81(3):349–360. doi: 10.1038/labinvest.3780243. [DOI] [PubMed] [Google Scholar]
- 37.Wan F., Lenardo M.J. The nuclear signaling of NF-kappaB: current knowledge, new insights, and future perspectives. Cell Res. 2010;20(1):24–33. doi: 10.1038/cr.2009.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu T., Zhang L., Joo D., Sun S.C. NF-kappaB signaling in inflammation. Signal Transduct Target Ther. 2017;2 doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng H.H., Kuo C.C., Yan J.L., Chen H.L., Lin W.C., Wang K.H., Tsai K.K., Guvén H., Flaberg E., Szekely L. Control of cyclooxygenase-2 expression and tumorigenesis by endogenous 5-methoxytryptophan. Proc Natl Acad Sci U S A. 2012;109(33):13231–13236. doi: 10.1073/pnas.1209919109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morgan M.J., Liu Z.G. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011;21(1):103–115. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumari S., Badana A.K., MM G., S G., Malla R. Reactive oxygen species: a key constituent in cancer survival. Biomark Insights. 2018;13 doi: 10.1177/1177271918755391. 1177271918755391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gialeli C., Theocharis A.D., Karamanos N.K. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011;278(1):16–27. doi: 10.1111/j.1742-4658.2010.07919.x. [DOI] [PubMed] [Google Scholar]
- 43.Zhang K.S., Chen H.Q., Chen Y.S., Qiu K.F., Zheng X.B., Li G.C., Yang H.D., Wen C.J. Bisphenol A stimulates human lung cancer cell migration via upregulation of matrix metalloproteinases by GPER/EGFR/ERK1/2 signal pathway. Biomed Pharmacother. 2014;68(8):1037–1043. doi: 10.1016/j.biopha.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 44.Wang Z.Y., Lu J., Zhang Y.Z., Zhang M., Liu T., Qu X.L. Effect of Bisphenol A on invasion ability of human trophoblastic cell line BeWo. Int J Clin Exp Pathol. 2015;8(11):14355–14364. [PMC free article] [PubMed] [Google Scholar]
- 45.Lan X., Fu L.J., Zhang J., Liu X.Q., Zhang H.J., Zhang X., Ma M.F., Chen X.M., He J.L., Li L.B. Bisphenol A exposure promotes HTR-8/SVneo cell migration and impairs mouse placentation involving upregulation of integrin-beta1 and MMP-9 and stimulation of MAPK and PI3K signaling pathways. Oncotarget. 2017;8(31):51507–51521. doi: 10.18632/oncotarget.17882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang K.H., Kao A.P., Chang C.C., Lin T.C., Kuo T.C. Bisphenol A-induced epithelial to mesenchymal transition is mediated by cyclooxygenase-2 up-regulation in human endometrial carcinoma cells. Reprod Toxicol. 2015;58:229–233. doi: 10.1016/j.reprotox.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 47.Wang L., Hao J., Hu J., Pu J., Lu Z., Zhao L., Wang Q., Yu Q., Wang Y., Li G. Protective effects of ginsenosides against Bisphenol A-induced cytotoxicity in 15P-1 Sertoli cells via extracellular signal-regulated kinase 1/2 signalling and antioxidant mechanisms. Basic Clin Pharmacol Toxicol. 2012;111(1):42–49. doi: 10.1111/j.1742-7843.2012.00857.x. [DOI] [PubMed] [Google Scholar]
- 48.Wang K.H., Kao A.P., Chang C.C., Lin T.C., Kuo T.C. Bisphenol A at environmentally relevant doses induces cyclooxygenase-2 expression and promotes invasion of human mesenchymal stem cells derived from uterine myoma tissue. Taiwan J Obstet Gynecol. 2013;52(2):246–252. doi: 10.1016/j.tjog.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 49.Huang Y.F., Wang P.W., Huang L.W., Lai C.H., Yang W., Wu K.Y., Lu C.A., Chen H.C., Chen M.L. Prenatal nonylphenol and bisphenol A exposures and inflammation are determinants of oxidative/nitrative stress: a Taiwanese cohort study. Environ Sci Technol. 2017;51(11):6422–6429. doi: 10.1021/acs.est.7b00801. [DOI] [PubMed] [Google Scholar]
- 50.Kaur K., Chauhan V., Gu F., Chauhan A. Bisphenol A induces oxidative stress and mitochondrial dysfunction in lymphoblasts from children with autism and unaffected siblings. Free Radic Biol Med. 2014;76:25–33. doi: 10.1016/j.freeradbiomed.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 51.Gassman N.R. Induction of oxidative stress by bisphenol A and its pleiotropic effects. Environ Mol Mutagen. 2017;58(2):60–71. doi: 10.1002/em.22072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vinas R., Watson C.S. Mixtures of xenoestrogens disrupt estradiol-induced non-genomic signaling and downstream functions in pituitary cells. Environ Health. 2013;12:26. doi: 10.1186/1476-069X-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu J., Jiang L., Liu Y., Qian W., Liu J., Zhou J., Gao R., Xiao H., Wang J. MAPK and NF-kappaB pathways are involved in bisphenol A-induced TNF-alpha and IL-6 production in BV2 microglial cells. Inflammation. 2015;38(2):637–648. doi: 10.1007/s10753-014-9971-5. [DOI] [PubMed] [Google Scholar]
- 54.Ge L.C., Wang H.S. A commentary on "Involvement of activating ERK1/2 trough G protein coupled receptor 30 and estrogen receptor alpha/beta in low doses of bisphenol A promoting growth of Sertoli TM4 cells". Toxicol Lett. 2016;240(1):236–237. doi: 10.1016/j.toxlet.2015.09.021. [DOI] [PubMed] [Google Scholar]
- 55.Yang M., Lee H.S., Hwang M.W., Jin M. Effects of Korean red ginseng (Panax Ginseng Meyer) on bisphenol A exposure and gynecologic complaints: single blind, randomized clinical trial of efficacy and safety. BMC Complement Altern Med. 2014;14:265. doi: 10.1186/1472-6882-14-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saadeldin I.M., Hussein M.A., Suleiman A.H., Abohassan M.G., Ahmed M.M., Moustafa A.A., Moumen A.F., Abdel-Aziz Swelum A. Ameliorative effect of ginseng extract on phthalate and bisphenol A reprotoxicity during pregnancy in rats. Environ Sci Pollut Res Int. 2018;25(21):21205–21215. doi: 10.1007/s11356-018-2299-1. [DOI] [PubMed] [Google Scholar]
- 57.Mahmoudi A., Hadrich F., Feki I., Ghorbel H., Bouallagui Z., Marrekchi R., Fourati H., Sayadi S. Oleuropein and hydroxytyrosol rich extracts from olive leaves attenuate liver injury and lipid metabolism disturbance in bisphenol A-treated rats. Food Funct. 2018;9(6):3220–3234. doi: 10.1039/c8fo00248g. [DOI] [PubMed] [Google Scholar]
- 58.Khalaf A.A., Ahmed W., Moselhy W.A., Abdel-Halim B.R., Ibrahim M.A. Protective effects of selenium and nano-selenium on bisphenol-induced reproductive toxicity in male rats. Hum Exp Toxicol. 2019;38(4):398–408. doi: 10.1177/0960327118816134. [DOI] [PubMed] [Google Scholar]
- 59.Clarke T.C., Black L.I., Stussman B.J., Barnes P.M., Nahin R.L. Trends in the use of complementary health approaches among adults: United States, 2002-2012. Natl Health Stat Report. 2015;(79):1–16. [PMC free article] [PubMed] [Google Scholar]
- 60.Huang H. Matrix metalloproteinase-9 (MMP-9) as a cancer biomarker and MMP-9 biosensors: recent advances. Sensors (Basel) 2018;18(10) doi: 10.3390/s18103249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim J.H., Lee K.W., Lee M.W., Lee H.J., Kim S.H., Surh Y.J. Hirsutenone inhibits phorbol ester-induced upregulation of COX-2 and MMP-9 in cultured human mammary epithelial cells: NF-kappaB as a potential molecular target. FEBS Lett. 2006;580(2):385–392. doi: 10.1016/j.febslet.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 62.Kim S., Kim S.H., Hur S.M., Lee S.K., Kim W.W., Kim J.S., Kim J.H., Choe J.H., Nam S.J., Lee J.E. Silibinin prevents TPA-induced MMP-9 expression by down-regulation of COX-2 in human breast cancer cells. J Ethnopharmacol. 2009;126(2):252–257. doi: 10.1016/j.jep.2009.08.032. [DOI] [PubMed] [Google Scholar]
- 63.Winer A., Adams S., Mignatti P. Matrix metalloproteinase inhibitors in cancer therapy: turning past failures into future successes. Mol Cancer Ther. 2018;17(6):1147–1155. doi: 10.1158/1535-7163.MCT-17-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang X.L., Liu N., Weng S.F., Wang H.S. Bisphenol A increases the migration and invasion of triple-negative breast cancer cells via oestrogen-related receptor gamma. Basic Clin Pharmacol Toxicol. 2016;119(4):389–395. doi: 10.1111/bcpt.12591. [DOI] [PubMed] [Google Scholar]
- 65.Coussens L.M., Fingleton B., Matrisian L.M. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295(5564):2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.