Abstract
Cytokine-related toxicity associated with the use of highly active chimeric antigen receptor T cells (CAR-T cells) is a significant clinical problem. By fusing the natural killer group 2D (NKG2D) ectodomain to 4-1BB and the DAP12 cytoplasmic domain containing only one immunoreceptor tyrosine-based activation motif, we have developed a 2nd-generation (2nd-Gen) NKG2D CAR for stable expression in human T cells. When compared to T cells modified with NKG2D CAR containing the commonly used CD3ζ activation domain, T cells expressing the NKG2D-DAP12 CAR stimulated lower level release of interferon gamma (IFN-γ), tumor necrosis factor alpha (TNF-α), and interleukin (IL)-2 during tumor cell lysis and their proliferative activity was lower upon repeated antigen stimulation, although no difference between the two CARs was observed in mediating in vitro tumor cell lysis. In tumor-bearing NSG mice, both types of CAR-T cells displayed similar anti-tumor activity, being able to completely eradicate established solid tumor xenografts. However, treatment with the NKG2D-CD3ζ CAR-T cells led to the death of most mice from xenogeneic graft versus host disease starting 30 days post-CAR-T cell injection, which was associated with a higher level of cytokine release, whereas all the mice treated with the NKG2D-DAP12 CAR-T cells survived well. Thus, the incorporation of the DAP12 activation domain in a CAR design may possibly provide a potential clinical advantage in mitigating the risk of cytokine release syndrome (CRS).
Keywords: NKG2D, CAR-T cells, CRS, DAP12, x-GVHD, CAR
Graphical Abstract
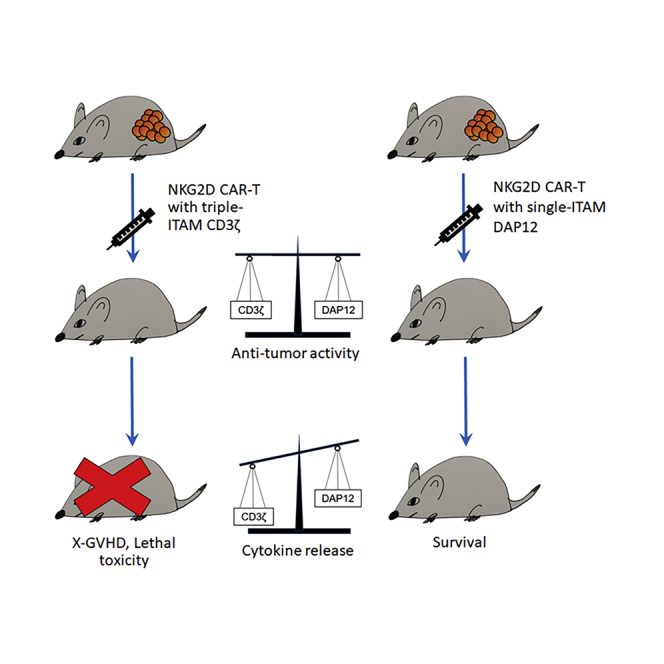
At similar levels of in vitro and in vitro anti-tumor activity, NKG2D-DAP12 CAR-T cells released less proinflammatory cytokines compared to NKG2D-CD3ζ counterparts. Consequently, mice treated with NKG2D-CD3ζ CAR-T cells suffered from death arising from xenogeneic graft versus host disease associated with pronounced cytokine release, whereas mice treated with NKG2D-DAP12 CAR-T cells survived.
Introduction
Adoptive transfer of chimeric antigen receptor (CAR)-modified T lymphocytes provides a highly promising strategy for cancer therapy and has produced impressive results in treating patients with B cell leukemia and lymphoma.1, 2, 3 A typical CAR configuration consists of an extracellular tumor antigen-targeting domain, a transmembrane domain, and one or more intracellular signaling domains for T cell activation. While the single-chain fragment variable (scFv) from a monoclonal antibody is often used as the CAR extracellular domain, a special group of CARs have been developed that uses a natural killer (NK) cell receptor, the NK group 2D (NKG2D) receptor, for tumor antigen recognition.4, 5, 6, 7 These NKG2D-based CARs comprise either the full-length NKG2D receptor (aa 1–216) or the NKG2D ectodomain (aa 83–216) and incorporate the cytoplasmic tail of the T cell receptor (TCR) CD3ζ chain containing three immunoreceptor tyrosine-based activation motifs (ITAMs) for intracellular signaling. The transfer of T cells modified with NKG2D CARs containing the CD3ζ activation domain is effective in cancer treatment in several preclinical animal models4, 5, 6,8, 9, 10, 11, 12, 13, 14 and has shown some promising results in early-stage clinical trials.15, 16, 17, 18
NKG2D, as an activating receptor, is expressed by several types of human cytotoxic lymphocytes, including NK cells, CD8+ αβ T cells, γδ T cells, and NK T (NKT) cells.19 The NKG2D receptor is composed of two homodimeric, transmembrane glycoproteins with a short cytoplasmic tail that is incapable of initiating intracellular signaling. Thus, NKG2D does not activate on its own, and signaling is mediated by specialized signaling adaptor molecules. In mouse immune cells, both the Tyr-X-X-Meth (YXXM) motif-containing DAP10 and ITAM-containing DAP12 (also known as TYROBP and KARAP) can be used as adaptors, while in human immune cells, NKG2D exclusively uses DAP10 as an adaptor.20 One NKG2D homodimer interacts noncovalently with two DAP10 homodimers, and this assembled hexameric structure then activates intracellular signaling. For DAP10, intracellular signaling is through phosphorylation of its cytoplasmic YXXM motif, while for DAP12, the only signaling domain that mediates all of the known effector functions of the protein is its single ITAM motif.21,22
To improve CAR function, much work has been done to test different costimulatory motifs, whereas only very limited work was performed on optimizing an activating domain with ITAMs. Based on the observation that DAP12 is expressed in NK cells and associates with several NK cell-activating receptors, Temme’s23,24 lab has developed two CARs with a scFv targeting the prostate stem cell Ag (PSCA) or epidermal growth factor receptor variant III (EGFRvIII) fused to DAP12, which was then used to modify NK cells. They demonstrated the phosphorylation of the DAP12-associated ZAP-70 kinase and interferon gamma (IFN-γ) release of CAR-engineered NK cells after contacting with target tumor cells. A recent study with the mRNA electroporation approach has further confirmed that 1st-generation (1st-Gen) NKG2D CAR generated by fusing the NKG2D ectodomain with DAP12 can improve tumor responses of human NK cells.25
However, no studies were reported so far in literature on effects of a DAP12-containing CAR in T cells, where in contrast to NK cells, DAP12 expression is rarely observed.22 In the present study, we constructed a 2nd-generation (2nd-Gen) CAR with the NKG2D ectodomain linked to 4-1BB and the cytoplasmic domain of human DAP12 and tested its function after stable expression in human T cells. We report here that this CAR configuration was effective in tumor killing yet stimulated lower cytokine release.
Results
Comparison between 1st-Gen and 2nd-Gen NKG2D DAP12-CARs: In Vitro
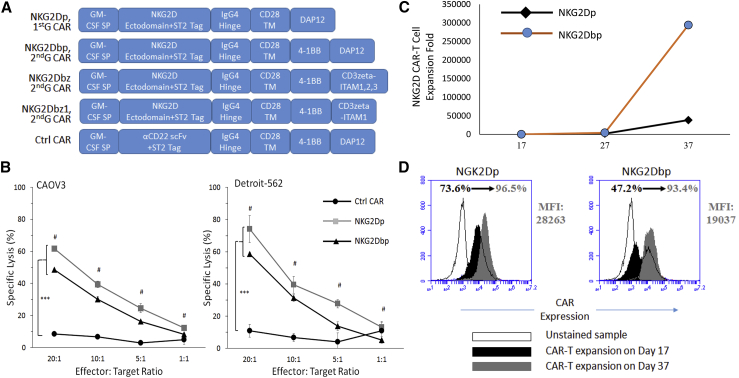
CAR expression cassettes used in the present study are shown in Figure 1A. In our initial effort to produce CAR T cells (CAR-T cells) (Figure S1A), human peripheral blood mononuclear cells (PBMCs) were activated with TransAct for 2 days before electroporation. Electroporated cells were then cultured with interleukin (IL)-2 for 5 days before CAR-T cell enrichment. We used the gamma-irradiated wild-type (WT) human K562 myelogenous leukemia cell line that naturally expresses NKG2D ligands (Figure S1B) for CAR-T cell enrichment at the low effector:target (E:T) ratio of 1:2 in the presence of IL-2. After repeated stimulation with the antigens presented by WT K562 cells, 50%–70% of T cells became NKG2D CAR positive by day 17 (Figure S1C). These CAR-T cells were tested for their ability to lyse target cancer cells expressing natural NKG2D ligands (Figure S2) using a 2-h Delfia Europium release in vitro cytotoxicity assay (Figure 1B). In a dose-dependent manner, NKG2D CAR-T cells efficiently lysed both CAOV3 and Detroit-562 target cells. At all E:T ratios, NKG2Dbp CAR-T cells exhibited lower killing efficiency compared to that of NKG2Dp CAR-T cells, likely due to their lower level of CAR expression relative to that on NKG2Dp CAR-T cells (Figure S1C).
Figure 1.
Characterization of NKG2D CAR-T Cells Bearing the DAP12-Activating Domain
(A) Schematic representations of CAR constructs used in the present study. NKG2Dp, 1st-Gen CAR construct containing the DAP12-activating domain only; NKG2Dbp, 2nd-Gen CAR construct containing a single 4-1BB costimulatory domain and DAP12; NKG2Dbz, 2nd-Gen CAR construct containing 4-1BB and the WT CD3ζ-activating domain with 3 ITAM motifs; NKG2Dbz1, 2nd-Gen CAR construct containing 4-1BB and a CD3ζ mutant with a single ITAM motif; and Ctrl CAR, an anti-CD22 scFv that was used to replace NKG2D ectodomain in NKG2Dbp. A Strep-Tag II (ST2) tag was included to facilitate the detection of CAR expression by flow cytometry analysis. (B) NKG2D CAR-T cells activated by DAP12 lyse cancer cells expressing NKG2D ligands. NKG2D ligand-positive CAOV3 (left) and Detroit-562 (right) cancer cell lines were used as target cells in a 2-h Delfia cytotoxicity assay. CAR-T cells were seeded with target cells at E:T ratios of 20:1, 10:1, 5:1, and 1:1. Data represent mean ± SD of triplicates, representative of three independent experiments. Statistical analysis was performed using one-way ANOVA, followed by Tukey’s multiple comparisons. ∗∗∗p < 0.001. Hash (#) signs indicates E:T ratios where NKG2Dp CAR is statistically different from NKG2Dbp CAR. (C) Antigen-dependent expansion of NKG2D CAR-T cells with γ-irradiated WT K562 feeder cells. Data indicate cell expansion folds obtained from cell counting by trypan blue exclusion assay on days 17, 27, and 37 post-DNA electroporation from one donor, representative of three independent experiments. (D) Intensity of CAR expression and proportion of CAR-expressing T cells increased consecutively upon repeated cultures with K562 feeder cells. Within each CAR group, CAR expression intensity (MFI values) and proportion of CAR-expressing cells (%) were analyzed in the middle (on day 17) and at the end (on day 37) of the expansion phase. Histogram diagrams from the middle and end of the expansion phase were superimposed to illustrate the enrichment of CAR-expressing T cells.
As demonstrated previously, T cells expressing 1st-Gen CD3ζ CARs are prone to undergo anergy, and a CAR design possessing both CD3ζ and costimulatory properties (2nd-Gen CARs) is required to obtain absolute expansion of human peripheral blood T cells upon repeated exposure to antigens.26 We examined the hypothesis that the incorporation of the 4-1BB costimulatory domain into DAP12 CARs would also positively modulate the antigen-dependent expansion of NKG2D DAP12 CAR-T cells. T cells stably expressing DAP12-bearing NKG2D CARs were continuously stimulated with K562 cells, as outlined in Figure S1A, and collected on days 17, 27, and 37 post-PBMC activation for counting and analysis. Both NKG2Dp and NKG2Dbp CAR-T cells underwent proliferation in response to antigenic stimulation from WT K562 cells. While expansion folds from both groups were comparable in the initial phases of co-culture with K562, a more pronounced expansion of CAR-T cells driven by 4-1BB signaling was observed: approximately 20 (±10) thousand-fold expansion for NKG2Dp CAR-T cells versus 70 (±50) thousand-fold expansion for NKG2Dbp CAR-T cells by day 37 (n = 3 per group). A representative cell expansion figure is shown in Figure 1C.
Pertinent to the persistent expansion of CAR-T cells, we observed that the incorporation of 4-1BB into NKG2D DAP12 CAR downregulated the expression of T cell exhaustion markers PD1 and LAG3 (Figures S3A and S3B). The 4-1BB costimulatory domain also improved the formation of memory T subsets (Figure S3C). We observed a clear divergence in memory T development by the end of the expansion phase: 39.6% effector memory T for NKG2Dp versus 87.5% for NKG2Dbp on day 37. Concomitantly, CAR expression intensity and the proportion of CAR-expressing T cells increased as antigen-dependent proliferation took place (Figure 1D). The frequencies of CAR-positive T cells were between 50% and 70% on day 17, and by day 37, around 90% of T cells in both NKG2D groups were CAR positive. While the proportions were comparable, the expression intensity of NKG2Dbp (mean fluorescence intensity [MFI] = 22.637 ± 5.091; n = 3) was lower compared to that of NKG2Dp (32,957 ± 6,638; n = 3).
Comparison between 1st-Gen and 2nd-Gen NKG2D DAP12-CARs: in a Human Colorectal Xenograft Model in NSG Mice
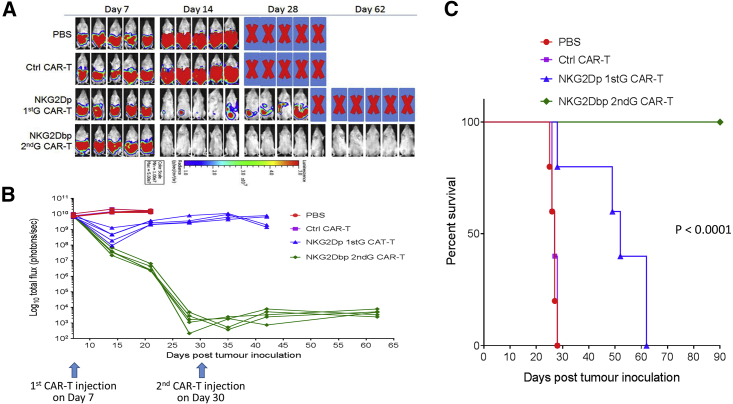
We went on to investigate whether there would be a difference in mediating in vivo tumor eradication between T cells stably expressing NKG2Dp and NKG2Dbp CARs. In view of the divergence in memory T cell development observed by day 37 (Figure S3C), we used day-27 NKG2D CAR-T cells in the experiment, as they had relatively comparable compositions of memory T subsets. We established a human colorectal cancer xenograft model in NSG mice by intraperitoneal (i.p.) injection of HCT116-Luc cells, which express NKG2D ligands (Figure S2). Tumor progression was monitored by whole-body bioluminescence imaging. As shown in Figures 2A and 2B, the bioluminescence intensities in the PBS group and the control CAR-T groups increased rapidly, and the mice in the two groups were all euthanized prior to day 28, when the moribund condition was developed. Mice that received 1st-Gen NKG2Dp CAR-T cells or 2nd-Gen NKG2Dbp CAR-T cells showed reduction in tumor burden on day 14 after receiving a single-dose T cell injection, especially after the injection of NKG2Dbp CAR-T cells, in which tumor xenografts were totally eliminated (Figures 2A and 2B). However, from day 14 onward, tumor burden for mice that received NKG2Dp CAR-T cells increased progressively, and although a second CAR T cell injection was given at day 30, the tumor burden did not decrease significantly (Figure 2B). All mice that received the NKG2Dp CAR-T cell injection were euthanized by day 62. In contrast, tumor burden in the group of mice that received NKG2Dbp CAR-T cells remained undetectable up to day 62 (Figure 2B). Mice continued to survive up to day 90 at the termination of the animal study (Figure 2C). These results demonstrated that the incorporation of a costimulatory domain is crucial in mediating the in vivo persistence of NKG2D DAP12 CAR-T cell function and, more importantly, in achieving in vivo tumor eradication. Of note, the superiority of 2nd-Gen over 1st-Gen DAP12 CAR T cells is consistent with what observed in both preclinical and clinical studies with CD3ζ CAR-T cells.
Figure 2.
Effects of NKG2D CARs with DAP12 in a Mouse Model of HCT116 Human CRC Cells
(A) Four groups of mice received i.p. injection of 2 × 106 HCT116 cells (day 0) followed by i.p. injection of PBS, control CAR-T cells, DAP12-containing NKG2D 1st- or 2nd-Gen CAR-T cells on day 7 and day 30 (1 × 107 cells per mouse). Growth of HCT116 was monitored by bioluminescent imaging on the indicated days. Bioluminescent images of 5 mice per group are shown. (B) Bioluminescence flux values from each mouse in the respective groups were plotted to monitor growth of tumors. (C) Kaplan-Meier analysis of survival of the in vivo experiment. Statistical analysis between groups was performed using the log-rank test.
Comparison between 2nd-Gen DAP12 CAR and 2nd-Gen CD3ζ CAR: In Vitro
After showing that 2nd-Gen NKG2D DAP12 CAR (NKG2Dbp) displayed impressive in vivo tumor killing effects, we moved on to compare this NKG2D CAR construct against an NKG2D CAR construct with CD3ζ, termed NKG2Dbz (Figure 1A). In particular, we constructed another NKG2Dbz CAR in which the zeta chain was mutated to contain the first ITAM only through deletion of the remaining CD3ζ chain and named it NKG2Dbz1 (Figure 1A). We continued to use the piggyBac transposon methodology for CAR-T cell generation. A modified 28-day protocol was adopted with the use of K562 cells engineered to express CD64, CD86, and CD137L, named K562C6,27 for CAR-T cell expansion and enrichment (Figure S4A). We examined and confirmed that electroporated T cells did not express NKG2D ligands before co-culturing with K562C6 and that CAR-T cell proliferation and enrichment could be significantly enhanced by co-culturing with K562C6 cells (Figure S5). This modified protocol shortened the entire CAR-T cell production process yet still provided a similar CAR-T cell production efficiency: all types of CAR-T cells were enriched to around 40% on day 7 to 90% on day 28 (Figure S4B).
Reports have shown that calibration of CAR activation potential directs alternative T cell fate and therapeutic potency.28 We investigated whether there is a difference in the generation of T cell memory subsets between NKG2Dbz, NKG2Dbz1, and NKG2Dbp CAR-T cells. Staining of CD45RO and CCR7 showed that, relative to NKG2Dbz with three ITAM motifs, the two CARs with a single ITAM, NKG2Dbz1 and NKG2Dbp, increased the fraction of effector memory CAR T cells and reduced the proportion of effector cells in response to in vitro stimulation with K562C6 cells (Figure S6).
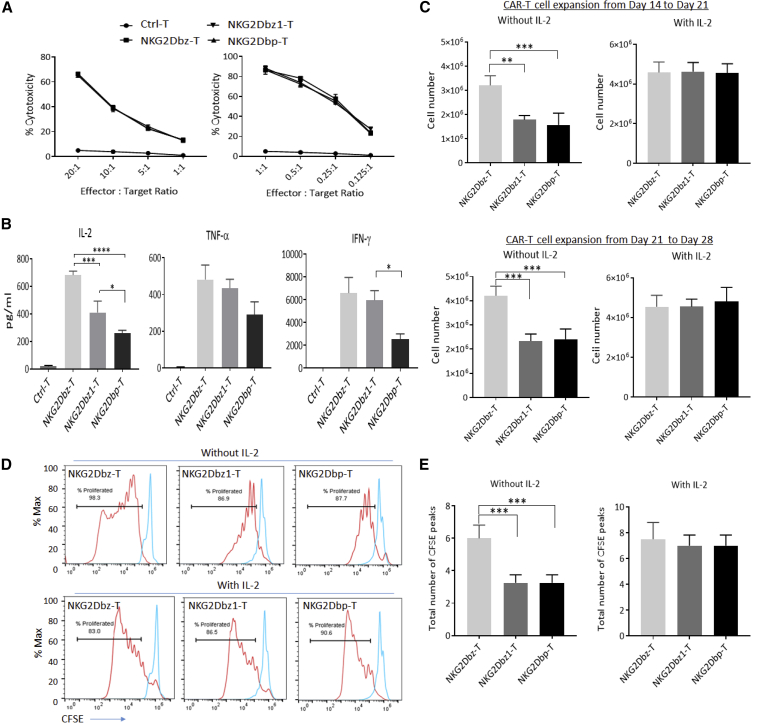
We then compared NKG2Dbz, NKG2Dbz1, and NKG2Dbp CAR-T cells for their in vitro tumor cell lysis activities against a panel of solid tumor cancer cells lines in 4-h and 16-h cytotoxicity assays. We found that the three CAR-T types showed indistinguishable tumor cell lysis activities, while all of them were highly effective in killing the investigated tumor cell lines (Figures 3A and S7). However, the cytokine production levels after co-culturing of CAR-T cells with target tumor cells were different, as assessed by ELISA assays. NKG2Dbz CAR-T cells, in general, promoted higher amounts of IL-2, IFN-γ, and tumor necrosis factor alpha (TNF-α) production as compared to NKG2Dbz1 and NKG2Dbp CAR-T cells, although the statistical differences were significant (p < 0.05) only between NKG2Dbz-T and NKG2Dbz1-T for IL-2 and between NKG2Dbz-T and NKG2Dbp-T for all three examined pro-inflammatory cytokines (Figures 3B and S8). Between NKG2Dbz1-T cells and NKG2Dbp-T cells, the latter stimulated the production of IL-2 and IFN-γ at lower levels (p < 0.05). In another set of experiments to compare NKG2Dbz and NKG2Dbp CAR-T cells using a sensitive multiplex cytokine quantification technology and an enzyme-linked immune absorbent spot (ELISpot) assay, NKG2Dbp CAR-T cells promoted significantly lower amounts of IFN-γ, TNF-α, and IL-2 production as compared to NKG2Dbz CAR-T cells (p < 0.05) (Figures S8 and S9).
Figure 3.
In Vitro Characterization of Three Different 2nd-Gen NKG2D CAR T Cells
(A) Cytolytic activity. Human HCT116 colorectal cancer cells were labeled with DELFIA BATDA Reagent (DELFIA EuTDA Cytotoxicity Reagents, PerkinElmer) followed by co-culture with CAR-T cells at indicated E:T ratios. Cytotoxicity assay was carried out over 4 h (left) and 16 h (right), and percent cytotoxicity was then calculated by measuring Europium release signal from the target tumor cells using a plate reader. The results shown are from one representative experiment out of two independent experiments with 2 different donors. (B) Cytokine release. HCT116 tumor cells were co-cultured with CAR-T cells at a 1:1 ratio for 16 h. Supernatants were collected to determine cytokine production by using the CBA (Cytometric Bead Array) Human Th1/Th2 Cytokine Kit (BD Biosciences). Data in bar graph represent mean ± SD for 3 cytokines in supernatants tested with CAR-T cells from 3 different PBMC donors. (C) CAR-T cell expansion. Antigen-dependent expansion of NKG2D CAR-T cells with γ-irradiated K562C6 feeder cells in the absence or presence of exogenous IL-2 (300 IU/mL) was examined. Data indicate total cell numbers obtained at the end of 1-week expansion from day 14 to day 21 (top) and day 21 to day 28 (bottom) from cell counting by trypan blue exclusion assay. Data represent mean ± SD of three independent experiments with CAR-T cells from 3 different PBMC donors. (D) CFSE T cell proliferation assay. At day 21, CAR-T cells were labeled with CFSE and co-cultured with K562C6 at a 1:1 ratio with or without IL-2 for 7 days, and proliferation was assessed at day 28 by flow cytometry. Flow cytometry plots for CFSE peak dilution are indicated, with the CFSE staining of stimulated CAR-T cells indicated as histograms on the left and the CFSE staining of non-stimulated CAR-T cells indicated as histograms on the right. The results shown are for one representative experiment out of three independent experiments with 3 different donors. (E) Total number of CFSE peaks. Data in bar chart graphs represent mean ± SD of the total number of CFSE peaks (left) in CAR-T samples from 3 different PBMC donors. Statistical significance was evaluated by one-way ANOVA followed by multiple correction. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001.
To determine whether changes in cytokine release exert any effects on CAR-T cell proliferation, in the next series of in vitro experiments, NKG2Dbz, NKG2Dbz1, and NKG2Dbp CAR-T cells were co-incubated with K562C6 cells either in the absence or presence of exogenous IL-2. Expanded cells were enumerated on day 21 and day 28, at the end of two consecutive 7-day co-culture phases. We observed that, in the absence of exogenous IL-2, cell numbers were consistently lower in the CAR-T samples modified with NKG2Dbz1 and NKG2Dbp as compared with those modified with NKG2Dbz (Figure 3C), indicating that NKG2Dbz1 and NKG2Dbp CAR-T cells proliferated less than NKG2Dbz CAR-T without the support of exogenous IL-2. The difference in cell proliferation was confirmed when a 7-day CFSE dilution assay without exogenous IL-2 was performed (Figures 3D, 3E, and S10), in which NKG2Dbz1 and NKG2Dbp CAR-T cell samples displayed a lower number of CFSE peaks, corresponding to low division numbers, than NKG2Dbz CAR-T cell samples. Nevertheless, in the presence of exogenous IL-2 (300 IU/mL), there were no obvious differences in the antigen-stimulated CAR-T cell proliferation between the three types of CAR-T cells (Figures 3C–3E, and S10).
Thus, our in vitro CAR-T cell characterization assays indicated that, while being similarly effective in mediating in vitro tumor cell killing, two types of CAR-T cells modified with CARs containing one single ITAM—NKG2Dbz1 and NKG2Dbp—were less active in antigen-stimulated cytokine production and CAR-T cell proliferation than NKG2Dbz CAR-T cells containing three ITAM signaling motifs. Since the activation of NKG2Dbp CAR stimulated lower levels of cytokine secretion than the activation of NKG2Dbz1 CAR, in the following experiments to compare in vivo effects between one single ITAM-containing and three ITAM-containing NKG2D CARs, we focused on the comparison between NKG2Dbz and NKG2Dbp CAR-T cells.
Comparison between 2nd-Gen DAP12 CAR and 2nd-Gen CD3ζ CAR: in a Human Colorectal Xenograft Model in NSG Mice
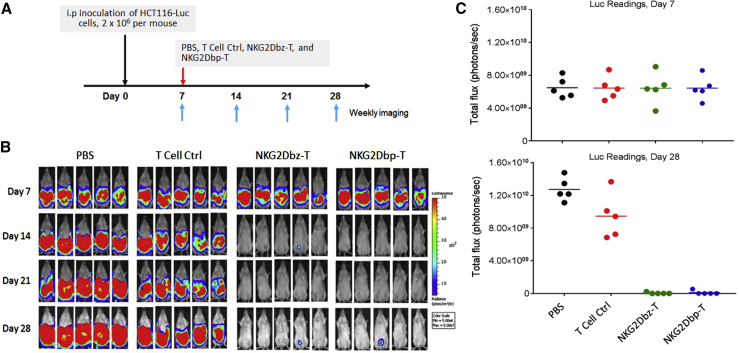
We then performed in vivo experiments to investigate whether there would be a difference between NKG2Dbp CAR-T cells and NKG2Dbz CAR-T cells in controlling tumor growth. The same mouse model with i.p.-injected HCT116-Luc cells as described earlier was used, and on day 7, animals were randomly divided into 4 groups for treatment, including PBS, control T cells, NKG2Dbp CAR-T cells, and NKG2Dbz CAR-T cells (Figure 4A). As shown in Figure 4B, the bioluminescence intensities in the PBS group and in the group of mice that received control T cells either increased or remained high during the imaging period from day 7 to day 28. NKG2Dbp and NKG2Dbz CAR-T cells eradicated peritoneal tumors efficiently: bioluminescence signals became undetectable on day 14 after a single-dose T cell injection. The signals on day 7 and day 28 are quantitatively indicated in bar graphs in Figure 4C. By day 28, while tumor burdens in most of the CAR-T-cell-treated mice remained undetectable, bioluminescence signals were detected at the injection site in two mice, one in the NKG2Dbp CAR-T cell group and another in the NKG2Dbz CAR-T cell group, suggesting tumor relapse (Figure 4B). The 2nd injection of CAR-T cells at a lower dose (2 × 106 cells per mice) was given to the two groups of mice, and the relapsed tumors were successfully eradicated.
Figure 4.
Effects of NKG2Dbp or NKG2Dbz CAR-T Cells in a Human CRC HCT116-Luc Xenograft Model
(A) Experimental outline for the animal study. Four groups of mice (5 mice per group) received an i.p. injection of 2 × 106 HCT116 cells (day 0) followed by an i.p. injection of PBS, control CAR-T cells, NKG2Dbz CAR-T cells, or NKG2Dbp CAR-T cells on day 7 (1 × 107 cells per mouse). (B) Growth of HCT116 xenografts was monitored by bioluminescent imaging on the indicated days. Bioluminescent images are shown. (C) Bioluminescence flux values on day 7 and day 28 from each mouse in the respective groups were plotted.
While being successful in tumor eradication, we noticed, however, that NKG2Dbz CAR-T cell injection resulted in significant body weight loss (>15%) in several mice after the 2nd injection of CAR-T cells. These mice started to display other symptoms of xenogeneic graft versus host disease (x-GVHD) such as ruffled fur, hunched posture and significantly reduced mobility from day 35 onward and had to be humanely euthanized according to the Institutional Animal Care and Use Committee (IACUC) protocol (Figure 5A). Post-mortem examination did not detect any tumors, confirming that death of the animals was not caused by tumor growth. Body weight loss and other x-GVHD symptoms were not observed in the group of mice that had received NKG2Dbp CAR-T cells. We terminated the animal study at day 150 post-tumor inoculation. All mice that received NKG2Dbp CAR-T cells remained healthy without signs of illness until the scheduled end of the experiment, whereas only 1 out of 5 mice from the NKG2Dbz CAR-T cell group survived by then (Figure 5B).
Figure 5.
Effects of Human CAR-T Cells in NSG Mice: Animal Death Caused by x-GVHD
(A) Growth of HCT116-Luc tumors was monitored by bioluminescent imaging on day 7 up to day 70. Mice from the NKG2Dbz CAR-T cell group were euthanized starting from day 35 onward due to evidence of xenogeneic GVHD (x-GVHD), despite negligible tumor burden based on bioluminescent imaging. Criteria of xenogeneic GVHD included >15% weight loss, hunched posture, ruffled fur, and significantly reduced mobility. (B) Survival of mice was monitored up to day 150 post-tumor inoculation and analyzed by the Kaplan-Meier method. Data shown were pooled from two separate animal experiments; ns = 3, 10, and 7 in the NKG2Dbz (1 injection), NKG2Dbz (2 injections), and NKG2Dbp (2 injections) groups, respectively. (C) Serum cytokine concentrations. Five days after PBS or T cell infusion, blood samples were collected through mouse tail vein bleeding. Serum cytokine concentrations were determined with the CBA (Cytometric Bead Array) Human Th1/Th2 Cytokine Kit (BD Biosciences). Data in bar graph represent mean ± SD of the blood samples collected from 3 NKG2Dbz-T-treated mice, 2 NKG2Dbp-T-treated mice, and 3 PBS-treated mice. ∗p < 0.05; ∗∗p < 0.01. (D) Different pathological effects of the two types of NKG2D CAR-T cells on the liver. Representative H&E staining images of formalin-fixed, paraffin-embedded mouse liver sections from tumor-bearing mice treated with NKG2Dbz-T or NKG2Dbp-T. Top: a liver tissue section from an NKG2Dbp-T-treated mouse showed minor focal necrosis (N). Bottom: a liver tissue section from an NKG2Dbz-T-treated mouse with multiple patchy areas of hepatic coagulative necrosis (N) associated with bile duct hyperplasia. On the right side is a higher magnification view of a necrotic area.
To confirm the aforementioned observation and also to examine whether x-GVHD was related to repeated injections of CAR-T cells, we performed another experiment with 4 groups of mice treated with PBS, 1 injection of NKG2Dbz-T, 2 injections of NKG2Dbz-T, and 2 injections of NKG2Dbp-T. We collected blood samples through tail vein bleeding on day 5 after PBS or CAR-T cell infusion and measured serum concentrations of IL-2, TNF-α, and IFN-γ. Similar to what was observed in vitro, the treatment with NKG2Dbp CAR-T cells triggered significantly lower levels of secretions of the 3 cytokines as compared to the treatment with NKG2Dbz CAR-T cells (p < 0.05) (Figure 5C). Importantly, we observed again that x-GVHD symptoms and animal death were associated with NKG2Dbz-T treatment, regardless of 1 or 2 injections, but not with NKG2Dbp-T treatment (Figures 5B and S11). At euthanization, livers were collected, and morphological detail was investigated using hematoxylin and eosin (H&E) staining. Multiple irregular patchy areas of hepatic coagulative necrosis were observed in liver sections from NKG2Dbz-T-treated mice but not in those from NKG2Dbp-T-treated mice (Figure 5D). The pathological necrosis was characterized by hepatocellular loss and absence of nuclei, being accompanied with the infiltration of mononuclear inflammatory cells and bile duct hyperplasia. These findings from the in vivo experiments support the interpretation that the robust expansion of highly active NKG2Dbz CAR-T cells during in vivo tumor cell lysis led to x-GVHD.
Evaluation of Acute Toxic Effects of 2nd-Gen DAP12 CAR-T Cells
Obviously, human CAR-T cell dose is critical in inducing x-GVHD in NSG mice. To further evaluate possible side effects of NKG2Dbp CAR-T cells, normal NSG mice (5 per group) were treated with i.p. injection of an increased dose of 6 × 107 CAR-T cells, and control mice were given PBS. Acute toxic effects were monitored over 7 days. Based on the body surface area (BSA) normalization method, the human equivalent dose of the 6 × 107 CAR-T cell dose used in this mouse study would be 24 × 107 cells/kg human body weight, or 1.48 × 1010 cells/62 kg (average body mass/weight globally).29 This total dose per person per injection is approximately 5-fold higher than the highest dose (3 × 109) in the THINK (Therapeutic Immunotherapy With NKR-2) trial (ClinicalTrials.gov: NCT03018405, an ongoing clinical trial to test NKG2D CAR-T cells.
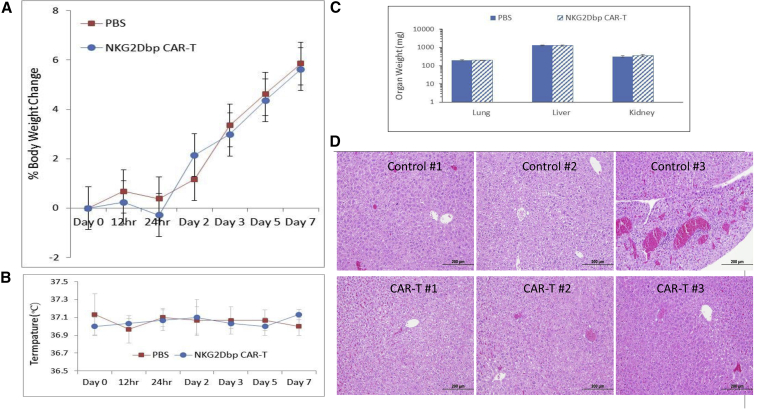
We did not observe any significant changes in body weight and body temperature following NKG2Dbp CAR-T injection (Figures 6A and 6B). No signs of toxicity (abnormal behaviors) and animal death were observed during the 7-day period. Animals were then sacrificed, and lungs, livers, and kidneys were harvested and weighed. There were no significant changes in organ weight between the CAR-T and PBS groups (Figure 6C). The lungs, livers, and kidneys from three mice per group were used for histological analysis. Representative photos of the liver sections from each mouse are shown in Figure 6D, and those from the lung and kidney are shown in Figure S12. The histological changes are summarized in Table S1. Overall, there was no histological evidence of NKG2Dbp CAR-induced acute and active toxicities (e.g., necrosis and inflammation) or pathological changes in all the examined organ sections. Thus, the findings from the acute toxicological study demonstrated that mice were able to tolerate a higher dose of 6 × 107 NKG2Dbp CAR-T cells administered via i.p. route.
Figure 6.
Preclinical Acute Toxicology Study of NKG2Dbp CAR-T Cells
PBS and the CAR-T cells (6 × 107 cells per mouse) were i.p. injected into NSG mice, 5 mice per group. (A and B) Temperatures (A), body weight change (B), and survival were monitored over the course of 7 days. (C) Animals were then sacrificed, and organs were harvested and weighted. (D) Pathological examination of liver tissues. Organs from 3 control mice and 3 CAR-T-treated mice were collected for H&E staining and histological examination.
Effects of 2nd-Gen DAP12 CAR-T Cells in a Human Ovarian Carcinoma Xenograft Model
To extend our studies to another tumor model, we established a xenograft mouse model of human ovarian carcinoma by i.p. injection of SKOV3-Luc human ovarian cancer cells, which also express NKG2D ligands (Figure S2), into NSG mice and tested in vivo tumor-killing effects of T cells expressing 2nd-Gen NKG2D DAP12 CAR. Seven days after tumor inoculation, three groups of mice started to receive treatments of PBS, control T cells without CAR, or NKG2Dbp CAR-T cells on day 7 and day 14 (Figure 7A). Growth of SKOV3 tumors was monitored by bioluminescent imaging. Tumors progressed rapidly in the PBS and control T cell groups (Figure 7B), resulting in animal death/euthanasia due to being moribund within 41 days (median survival: 38 days for the PBS group and 35 days for the control T cell group). Autopsies confirmed extensive i.p. cancer growth in all animals (data not shown). With the treatment of NKG2Dbp CAR-T cells, tumor xenografts were totally eliminated as detected by whole-body bioluminescence imaging from day 14 onward (Figure 7B). The tumor control effect was sustained, and the treated mice survived for at least 80 days (Figure 7C), the longest time point monitored in the experiment, demonstrating the potent effects of NKG2Dbp CAR-T cells in eradicating established peritoneal ovarian tumors.
Figure 7.
Effects of NKG2Dbp CAR-T Cells in a Mouse Model of SKOV3 Human Ovarian Cancer Cells
(A) The experimental outline for the investigation of anti-tumor effects of NKG2Dbp CAR-T cells in an i.p. injection SKOV3 xenograft model. Three groups of mice (5 mice per group) received an i.p. injection of 1 × 107 SKOV3 cells (day 0) followed by an i.p. injection of PBS, control CAR-T cells, or NKG2Dbp CAR-T cells on day 7 and day 14 (1 × 107 cells per injection per mouse). Growth of SKOV3 was monitored by bioluminescent imaging on the indicated days. (B) Tumor burden images by bioluminescent imaging from day 7 to day 42. (C) Kaplan-Meier analysis of survival of the in vivo experiment. Statistical analysis of survival between groups was performed using the log-rank test.
Discussion
DAP12 is a short 12-kDa transmembrane protein of 113 aa expressed on the cell surface, with a single ITAM harbored within the 48-aa cytoplasmic domain.21 The DAP12 has less than 25% homology with the ITAM motifs identified in human CD3ζ chain and FcεRI-γ chain. Ligation of a DAP12-associated receptor leads to activation of SRC-family kinases, phosphorylation of paired tyrosine residues in the ITAM of DAP12, and the subsequent recruitment of the cytoplasmic ZAP70 tyrosine kinases, converting the ligation event into downstream signaling and cytokine production.22
While DAP12 is not found to assemble with NKG2D in human immune cells, it does form noncovalent complexes with other receptors; for example, the killer Ig-like receptors (KIRs), of which expression is observed in both human T cells and NK cells, and the triggering receptor expressed on myeloid cell members (TREM).20 Wang and colleagues30 developed a KIR-based CAR (KIR-CAR) to target the tumor-associated antigen mesothelin through T cells by fusing a mesothelin-specific scFv to the transmembrane and short cytoplasmic domain of KIR2DS2. They then generated a bicistronic, lentiviral vector encoding SS1-KIR2DS2 and DAP12 to co-express both molecules in T cells. Primary human T cells modified with this bicistronic lentivirus exhibited superior antitumor activity compared with conventional 1st- and 2nd-Gen CD3ζ CARs in a xenograft model of mesothelioma. A similar CAR-T cell design was reported recently, in which a CD19- or mesothelin-specific scFv fused with TREM-1 was co-expressed with DAP12 in T cells.31 Although these two studies did not examine whether a single-chain CAR design with a scFv fused directly with DAP12 would be functioning in T cells, their results, together with the findings from the studies testing NK cells expressing DAP12-containing CARs, inspired us to investigate whether NKG2D ligand-targeting CARs with a single ITAM-motif-containing DAP12 activation domain could be used to improve T cell responses against cancer.23, 24, 25
The NKG2D receptor recognizes eight stress-induced ligands belonging to two families: two major histocompatibility complex (MHC) class I chain-related proteins, MICA and MICB, and six human cytomegalovirus (HCMV) UL16-binding proteins (ULBP1–6). These NKG2D ligands are not usually present on the cell surface of most healthy tissues but can be upregulated upon DNA damage, infection, and transformation of cells, thus being commonly detected on hematopoietic tumors and carcinomas.7,32 For example, MICA or MICB was found to be expressed by 100% of colorectal tumors, 97% of breast cancers, 95% of renal cell carcinomas, 81% of ovarian cancer, 77% of primary cutaneous melanomas, and 50% of primary uveal melanomas.33 Because of the tumor-associated overexpression, the NKG2D ligands have been a favorable therapeutic target for anticancer strategies.32 The widespread expression of NKG2D ligands in human cancer provides T cells armed with NKG2D CARs with great therapeutic potential to treat a broad range of tumor indications.4, 5, 6, 7
The natural NKG2D receptor has been well documented to act as a costimulatory molecule in T cells through the DAP10 signaling.19 All human CD8+ T cells constitutively express DAP10 but rarely express DAP12.22 Thus, T cells, although they display potent effector functions, cannot be directly activated by interaction between NKG2D and their ligands. Teng et al.34 have genetically engineered T cells by retroviral transduction to express DAP12. These DAP12-expressing T cells mediate effector function in an NKG2D-dependent manner: secreting IFN-γ following co-culture with NKG2D ligand-expressing, MHC class I-deficient tumor cells and mediating the specific lysis of the tumor cells. Another work has further shown that CD8+ T cells from DAP12 transgenic mice can proliferate in response to ligation with plate-bound anti-NKG2D monoclonal antibody (mAb).35 These results demonstrate the principle that DAP12 introduced through genetic engineering can function with NKG2D to activate T cells. Our study has extended the two previously reported DAP12 genetic engineering studies by generating NKG2D-DAP12 chimeric receptors with a single-molecule design.
Currently, CAR designs often use the cytoplasmic tail of TCR CD3ζ as the activation domain. By comparing CARs encoding a single ITAM derived from the CD3ζ chain with those containing the triple- and double-ITAM motifs, Sadelain and colleagues28 demonstrate that a single functional ITAM is sufficient for potent in vivo antitumor efficacy and superior to that afforded by the triple-ITAM-containing WT CD3ζ chain. Moreover, the single ITAM configuration favors the persistence of highly functional CARs, balancing the replicative capacity of long-lived memory cells and the acquisition of effective antitumor function, thereby yielding CAR designs with enhanced therapeutic profiles.
Even more attractive, a very recent study demonstrates that, decreasing the number of ITAMs of the CD3ζ chain in a CD19-specific CAR construct from three to two ITAMs, the CAR-T cells become substantially more selective for target cells expressing CD19 at a high density.36 With a CD19-blocking antibody that can artificially shift antigen densities required for CAR–T cell responses, the author has demonstrated that those target cells that expressed physiological amounts of CD19, but not those cells that overexpressed CD19, can be protected from CAR-T cell killing, suggesting that manipulating ITAM multiplicity may inform new strategies to improve CAR–T cell specificity.
This study focused primarily on DAP12 with a single ITAM. Upon interaction with target cells, our NKG2Dbp CAR-T cells with one ITAM motif per CAR molecule stimulate a relatively lower level release of cytokines as compared to that for T cells expressing NKG2Dbz that contain three ITAM motifs per CAR molecule, but the two CAR-T products generate similar in vivo anti-tumor activities. These observations suggest that cytokine release upon CAR activation could possibly be controlled by simply decreasing the ITAM density of a CAR construct. The reduction in cytokine release was also observed in the recent study reported by Feucht and colleagues28 for two of three single ITAM configurations tested in their CD3ζ CAR-T cells. We hypothesize that the reduction in cytokine release by NKG2Dbp is primarily attributable to the use of the single ITAM configuration, which can affect downstream functional output through the mitogen-activated protein kinase pathway.34 This effect provided by NKG2Dbp CAR-T would possibly provide a potential clinical advantage in reducing excess serum levels of cytokines and is one of the most important findings of this study.
The most common toxicities observed after CAR T cell therapy are cytokine release syndrome (CRS) and cerebral edema/neurotoxicity that involves release of excess amounts of cytokines and could be potentially life threatening.37 Several factors have been identified as associated with a higher risk of CRS and/or neurotoxicity among patients receiving CD19-targeted CAR-T cell therapies, including high tumor burden, high CAR-T cell dose, and lymphodepletion using cyclophosphamide and fludarabine. Using engineered T cell platforms associated with lower cytokine release has been suggested to improve safety and facilitate the extension of this therapeutic modality.1 Indeed, CAR T cells containing the CD8a hinge and transmembrane domain have been developed, which release lower levels of IFN-γ, TNF-α, and IL-2 as compared to those having the CD28 hinge and transmembrane domain during in vitro tumor cell lysis yet retain sufficient functional capability to eradicate established tumors from mice.38 The clinical advantage of the CAR-T platform is confirmed in a phase 1 dose-escalation trial, in which an anti-CD19 CAR T cell product with CD8 hinge and transmembrane region was observed to cause a 5% incidence rate of grade 3 or 4 neurologic toxicity, which is strikingly different from 55% of grade 3 or 4 neurologic toxicity observed in a previous clinical trial using anti-CD19 CAR T cells that incorporated CD28 hinge and transmembrane domain.39 The anti-lymphoma activities of the two CAR-T cell products were comparable.
While our NKG2D CAR constructs utilize the immunoglobulin G4 (IgG4) hinge and CD28 transmembrane domain, we adopted another strategy to control cytokine release, i.e., incorporating one ITAM motif per CAR molecule to reduce ITAM density. Whether the pre-clinical findings from our NKG2Dbp CAR-T cells, especially in reducing cytokine release and yet retaining tumor eradication capacity, can be translated into clinical benefit is worthy of investigation in future patient trials.
Materials and Methods
Cells and Cell Culture Conditions
Human PBMCs were isolated from fresh buffy coats by density gradient centrifugation using Ficoll-Paque (GE Healthcare, Milwaukee, WI, USA). PBMCs were cultured in T cell media (AIM-V + 5% AB serum) with recombinant IL-2 (PeproTech, Rocky Hill, NJ, USA) and activated with TransAct (Miltenyi Biotec, Bergisch Gladbach, Germany) or soluble OKT3 (eBioscience, San Diego, CA, USA). Human tumor cell lines were from ATCC (Manassas, VA, USA) and cultured as recommended.
Construction of Chimeric NKG2D CAR Vectors and Generation of CAR-T Cells
To construct NKG2D CAR vectors, the extracellular domain of human NKG2D (NKG2D ED; Uniprot: P26718-1; aa 83–216) was amplified by PCR from a PBMC cDNA library. The 1st-, and 2nd-Gen NKG2D CAR vectors were generated by fusing NKG2D-ED to the IgG4 hinge region, CD28 transmembrane region, and CD3ζ or DAP12 signaling moiety, with or without the intracellular costimulatory domain of 4-1BB. To construct a control CAR, an anti-CD22 scFv was used to replace NKG2D-ED in 2nd-Gen NKG2D CARs. Three Strep-Tag II (ST2; NWSHPQFEK) tags were included in all constructs to facilitate the detection of CAR expression by flow cytometry analysis. These constructs were placed into a piggyBac transposon plasmid. To generate CAR-T cells, a CAR-construct-containing plasmid was co-electroporated with a piggyBac-transposase-encoding plasmid into human peripheral blood T cells using the 4D-Nucleofector device (Lonza Biotech, Basel, Switzerland). Transfected cells were enriched as detailed in the Supplemental Materials and Methods.
Flow-Cytometric Analysis and Cytotoxicity Assay
Flow-cytometric analysis was performed with the Accuri C6 cytometer (BD Biosciences, Franklin Lakes, NJ, USA). The cytolytic activity of CAR-modified T cells was examined with a non-radioactive method (DELFIA EuTDA Cytotoxicity Reagents Kit, PerkinElmer, Waltham, MA, USA).
Animal Experiments
NSG mice were inoculated via i.p. injection of HCT116-luc or SKOV3-Luc cells to generate tumor models. To investigate in vivo anti-tumor effects of CAR-T cells, human T cells expressing an NKG2D CAR (1 × 107) were i.p. injected into tumor-bearing mice. Mice treated with PBS, control T cells, or T cells with the control CAR were used as controls. For the acute toxicology study, healthy NSG mice were given one dose of PBS or 6 × 107 CAR-T cells expressing NKG2Dbp CAR.
Statistical Analysis
Data are presented as mean ± standard deviation (SD). All statistics were performed with GraphPad Prism 7 (San Diego, CA, USA). p values < 0.05 were considered significant.
For the details of Materials and Methods, see Supplemental Information.
Author Contributions
Conceived and designed the experiments: S.W., J.Z., and J.W. Performed the experiments: Y.Y.N., J.C.K.T., and Z.L. Analyzed the data: Y.Y.N., J.C.K.T., and Z.L. Wrote the manuscript: S.W., Y.Y.N., J.C.K.T., and J.Z.
Conflicts of Interest
S.W., Y.Y.N., Z.L., and J.C.K.T. have filed patent applications related to CAR technology and could potentially receive licensing royalties in the future. The remaining authors declare no competing interests.
Acknowledgments
This work was supported by Singapore Ministry of Health’s National Medical Research Council (NMRC/CIRG/1406/2014 and NMRC/OFLCG/003/2018).
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.ymthe.2020.08.016.
Supplemental Information
References
- 1.Quintás-Cardama A. CD19 directed CARs in acute lymphoblastic leukemia: state of the art and beyond. Leuk. Lymphoma. 2019;60:1346–1348. doi: 10.1080/10428194.2018.1533132. [DOI] [PubMed] [Google Scholar]
- 2.Levin A., Shah N.N. Chimeric antigen receptor modified T cell therapy in B cell non-Hodgkin lymphomas. Am. J. Hematol. 2019;94(S1):S18–S23. doi: 10.1002/ajh.25403. [DOI] [PubMed] [Google Scholar]
- 3.Brown C.E., Mackall C.L. CAR T cell therapy: inroads to response and resistance. Nat. Rev. Immunol. 2019;19:73–74. doi: 10.1038/s41577-018-0119-y. [DOI] [PubMed] [Google Scholar]
- 4.Sentman C.L., Meehan K.R. NKG2D CARs as cell therapy for cancer. Cancer J. 2014;20:156–159. doi: 10.1097/PPO.0000000000000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilham D.E., Maher J. ‘Atypical’ CAR T cells: NKG2D and Erb-B as examples of natural receptor/ligands to target recalcitrant solid tumors. Immunotherapy. 2017;9:723–733. doi: 10.2217/imt-2017-0045. [DOI] [PubMed] [Google Scholar]
- 6.Demoulin B., Cook W.J., Murad J., Graber D.J., Sentman M.L., Lonez C., Gilham D.E., Sentman C.L., Agaugue S. Exploiting natural killer group 2D receptors for CAR T-cell therapy. Future Oncol. 2017;13:1593–1605. doi: 10.2217/fon-2017-0102. [DOI] [PubMed] [Google Scholar]
- 7.Frazao A., Rethacker L., Messaoudene M., Avril M.F., Toubert A., Dulphy N., Caignard A. NKG2D/NKG2-Ligand Pathway Offers New Opportunities in Cancer Treatment. Front. Immunol. 2019;10:661. doi: 10.3389/fimmu.2019.00661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang T., Lemoi B.A., Sentman C.L. Chimeric NK-receptor-bearing T cells mediate antitumor immunotherapy. Blood. 2005;106:1544–1551. doi: 10.1182/blood-2004-11-4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiss T., Weller M., Guckenberger M., Sentman C.L., Roth P. NKG2D-Based CAR T Cells and Radiotherapy Exert Synergistic Efficacy in Glioblastoma. Cancer Res. 2018;78:1031–1043. doi: 10.1158/0008-5472.CAN-17-1788. [DOI] [PubMed] [Google Scholar]
- 10.Han Y., Xie W., Song D.G., Powell D.J., Jr. Control of triple-negative breast cancer using ex vivo self-enriched, costimulated NKG2D CAR T cells. J. Hematol. Oncol. 2018;11:92. doi: 10.1186/s13045-018-0635-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tao K., He M., Tao F., Xu G., Ye M., Zheng Y., Li Y. Development of NKG2D-based chimeric antigen receptor-T cells for gastric cancer treatment. Cancer Chemother. Pharmacol. 2018;82:815–827. doi: 10.1007/s00280-018-3670-0. [DOI] [PubMed] [Google Scholar]
- 12.Deng X., Gao F., Li N., Li Q., Zhou Y., Yang T., Cai Z., Du P., Chen F., Cai J. Antitumor activity of NKG2D CAR-T cells against human colorectal cancer cells in vitro and in vivo. Am. J. Cancer Res. 2019;9:945–958. [PMC free article] [PubMed] [Google Scholar]
- 13.Yang D., Sun B., Dai H., Li W., Shi L., Zhang P., Li S., Zhao X. T cells expressing NKG2D chimeric antigen receptors efficiently eliminate glioblastoma and cancer stem cells. J. Immunother. Cancer. 2019;7:171. doi: 10.1186/s40425-019-0642-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun B., Yang D., Dai H., Liu X., Jia R., Cui X., Li W., Cai C., Xu J., Zhao X. Eradication of Hepatocellular Carcinoma by NKG2D-Based CAR-T Cells. Cancer Immunol. Res. 2019;7:1813–1823. doi: 10.1158/2326-6066.CIR-19-0026. [DOI] [PubMed] [Google Scholar]
- 15.Lonez C., Verma B., Hendlisz A., Aftimos P., Awada A., Van Den Neste E., Catala G., Machiels J.H., Piette F., Brayer J.B. Study protocol for THINK: a multinational open-label phase I study to assess the safety and clinical activity of multiple administrations of NKR-2 in patients with different metastatic tumour types. BMJ Open. 2017;7:e017075. doi: 10.1136/bmjopen-2017-017075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lonez C., Hendlisz A., Shaza L., Aftimos P., Vouche M., Donckier V., Machiels J.H., Van Den Eynde M., Canon J.L., Carrasco J. Celyad’s novel CAR T-cell therapy for solid malignancies. Curr. Res. Transl. Med. 2018;66:53–56. doi: 10.1016/j.retram.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Sallman D.A., Brayer J., Sagatys E.M., Lonez C., Breman E., Agaugué S., Verma B., Gilham D.E., Lehmann F.F., Davila M.L. NKG2D-based chimeric antigen receptor therapy induced remission in a relapsed/refractory acute myeloid leukemia patient. Haematologica. 2018;103:e424–e426. doi: 10.3324/haematol.2017.186742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baumeister S.H., Murad J., Werner L., Daley H., Trebeden-Negre H., Gicobi J.K., Schmucker A., Reder J., Sentman C.L., Gilham D.E. Phase I Trial of Autologous CAR T Cells Targeting NKG2D Ligands in Patients with AML/MDS and Multiple Myeloma. Cancer Immunol. Res. 2019;7:100–112. doi: 10.1158/2326-6066.CIR-18-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wensveen F.M., Jelenčić V., Polić B. NKG2D: A Master Regulator of Immune Cell Responsiveness. Front. Immunol. 2018;9:441. doi: 10.3389/fimmu.2018.00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosen D.B., Araki M., Hamerman J.A., Chen T., Yamamura T., Lanier L.L. A Structural basis for the association of DAP12 with mouse, but not human, NKG2D. J. Immunol. 2004;173:2470–2478. doi: 10.4049/jimmunol.173.4.2470. [DOI] [PubMed] [Google Scholar]
- 21.Olcese L., Cambiaggi A., Semenzato G., Bottino C., Moretta A., Vivier E. Human killer cell activatory receptors for MHC class I molecules are included in a multimeric complex expressed by natural killer cells. J. Immunol. 1997;158:5083–5086. [PubMed] [Google Scholar]
- 22.Lanier L.L. DAP10- and DAP12-associated receptors in innate immunity. Immunol. Rev. 2009;227:150–160. doi: 10.1111/j.1600-065X.2008.00720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Töpfer K., Cartellieri M., Michen S., Wiedemuth R., Müller N., Lindemann D., Bachmann M., Füssel M., Schackert G., Temme A. DAP12-based activating chimeric antigen receptor for NK cell tumor immunotherapy. J. Immunol. 2015;194:3201–3212. doi: 10.4049/jimmunol.1400330. [DOI] [PubMed] [Google Scholar]
- 24.Müller N., Michen S., Tietze S., Töpfer K., Schulte A., Lamszus K., Schmitz M., Schackert G., Pastan I., Temme A. Engineering NK Cells Modified With an EGFRvIII-specific Chimeric Antigen Receptor to Overexpress CXCR4 Improves Immunotherapy of CXCL12/SDF-1α-secreting Glioblastoma. J. Immunother. 2015;38:197–210. doi: 10.1097/CJI.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao L., Cen D., Gan H., Sun Y., Huang N., Xiong H., Jin Q., Su L., Liu X., Wang K. Adoptive Transfer of NKG2D CAR mRNA-Engineered Natural Killer Cells in Colorectal Cancer Patients. Mol. Ther. 2019;27:1114–1125. doi: 10.1016/j.ymthe.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sadelain M., Brentjens R., Rivière I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3:388–398. doi: 10.1158/2159-8290.CD-12-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Du S.H., Li Z., Chen C., Tan W.K., Chi Z., Kwang T.W., Xu X.H., Wang S. Co-Expansion of Cytokine-Induced Killer Cells and Vγ9Vδ2 T Cells for CAR T-Cell Therapy. PLoS ONE. 2016;11:e0161820. doi: 10.1371/journal.pone.0161820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feucht J., Sun J., Eyquem J., Ho Y.J., Zhao Z., Leibold J., Dobrin A., Cabriolu A., Hamieh M., Sadelain M. Calibration of CAR activation potential directs alternative T cell fates and therapeutic potency. Nat. Med. 2019;25:82–88. doi: 10.1038/s41591-018-0290-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reagan-Shaw S., Nihal M., Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 30.Wang E., Wang L.C., Tsai C.Y., Bhoj V., Gershenson Z., Moon E., Newick K., Sun J., Lo A., Baradet T. Generation of Potent T-cell Immunotherapy for Cancer Using DAP12-Based, Multichain, Chimeric Immunoreceptors. Cancer Immunol. Res. 2015;3:815–826. doi: 10.1158/2326-6066.CIR-15-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen B., Zhou M., Zhang H., Wang C., Hu X., Wang B., Wang E. TREM1/Dap12-based CAR-T cells show potent antitumor activity. Immunotherapy. 2019;11:1043–1055. doi: 10.2217/imt-2019-0017. [DOI] [PubMed] [Google Scholar]
- 32.Dhar P., Wu J.D. NKG2D and its ligands in cancer. Curr. Opin. Immunol. 2018;51:55–61. doi: 10.1016/j.coi.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le Bert N., Gasser S. Advances in NKG2D ligand recognition and responses by NK cells. Immunol. Cell Biol. 2014;92:230–236. doi: 10.1038/icb.2013.111. [DOI] [PubMed] [Google Scholar]
- 34.Teng M.W., Kershaw M.H., Hayakawa Y., Cerutti L., Jane S.M., Darcy P.K., Smyth M.J. T cells gene-engineered with DAP12 mediate effector function in an NKG2D-dependent and major histocompatibility complex-independent manner. J. Biol. Chem. 2005;280:38235–38241. doi: 10.1074/jbc.M505331200. [DOI] [PubMed] [Google Scholar]
- 35.Diefenbach A., Tomasello E., Lucas M., Jamieson A.M., Hsia J.K., Vivier E., Raulet D.H. Selective associations with signaling proteins determine stimulatory versus costimulatory activity of NKG2D. Nat. Immunol. 2002;3:1142–1149. doi: 10.1038/ni858. [DOI] [PubMed] [Google Scholar]
- 36.James J.R. Tuning ITAM multiplicity on T cell receptors can control potency and selectivity to ligand density. Sci. Signal. 2018;11:eaan1088. doi: 10.1126/scisignal.aan1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brudno J.N., Kochenderfer J.N. Recent advances in CAR T-cell toxicity: Mechanisms, manifestations and management. Blood Rev. 2019;34:45–55. doi: 10.1016/j.blre.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alabanza L., Pegues M., Geldres C., Shi V., Wiltzius J.J.W., Sievers S.A., Yang S., Kochenderfer J.N. Function of Novel Anti-CD19 Chimeric Antigen Receptors with Human Variable Regions Is Affected by Hinge and Transmembrane Domains. Mol. Ther. 2017;25:2452–2465. doi: 10.1016/j.ymthe.2017.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brudno J.N., Lam N., Vanasse D., Shen Y.W., Rose J.J., Rossi J., Xue A., Bot A., Scholler N., Mikkilineni L. Safety and feasibility of anti-CD19 CAR T cells with fully human binding domains in patients with B-cell lymphoma. Nat. Med. 2020;26:270–280. doi: 10.1038/s41591-019-0737-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.