Abstract
Panax species have gained numerous attentions because of their various biological effects on cardiovascular, kidney, reproductive diseases known for a long time. Recently, advanced analytical methods including thin layer chromatography, high-performance thin layer chromatography, gas chromatography, high-performance liquid chromatography, ultra-high performance liquid chromatography with tandem ultraviolet, diode array detector, evaporative light scattering detector, and mass detector, two-dimensional high-performance liquid chromatography, high speed counter-current chromatography, high speed centrifugal partition chromatography, micellar electrokinetic chromatography, high-performance anion-exchange chromatography, ambient ionization mass spectrometry, molecularly imprinted polymer, enzyme immunoassay, 1H-NMR, and infrared spectroscopy have been used to identify and evaluate chemical constituents in Panax species. Moreover, Soxhlet extraction, heat reflux extraction, ultrasonic extraction, solid phase extraction, microwave-assisted extraction, pressurized liquid extraction, enzyme-assisted extraction, acceleration solvent extraction, matrix solid phase dispersion extraction, and pulsed electric field are discussed. In this review, a total of 219 articles published from 1980 to 2018 are investigated. Panax species including P. notoginseng, P. quinquefolius, sand P. ginseng in the raw and processed forms from different parts, geographical origins, and growing times are studied. Furthermore, the potential biomarkers are screened through the previous articles. It is expected that the review can provide a fundamental for further studies.
Keywords: Analytical methods, Application, Content, Panax species, Sample preparations
1. Introduction
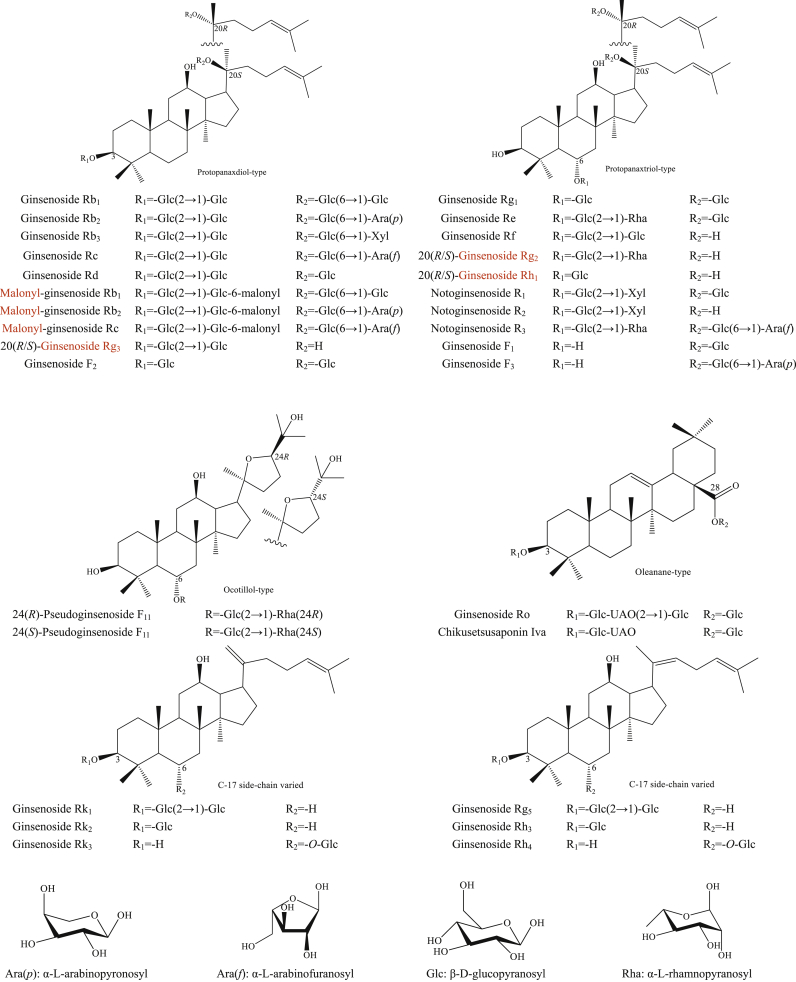
Genus Panax belonging to Family Araliaceae contains eleven species (three varieties) namely P. trifolius, P. notoginseng, P. quinquefolius, P. ginseng, P. pseudoginseng, P. zingiberensis, P. stipuleanatus, P. japonicus, P. japonicus var. angustifolius, P. japonicus var. major, and P. japonicus var. bipinnatifidus, which are mainly distributed in the Eastern Asia and Northern America [1]. Among them, most of the investigations have been conducted on P. notoginseng, P. quinquefolius, and P. ginseng for their pharmacological activity. Their use to treat cardiovascular, kidney, and reproductive diseases has a long history [2]. Various bioactive constituents including ginsenosides, polysaccharides, alkaloids, glucosides, and phenolic acids have been identified in P. ginseng in a previous study [3]. The main ginsenosides isolated from Panax species are shown in Fig. 1. They contain protopanaxadiol, protopanaxatriol, ocotillol, oleanolic acid, and C-17 side chain type [4,5]. Protopanaxadiol has a glucose moiety attached to C-20 and C-3, and protopanaxatriol has glycosylation sites at C-20, C-3, and C-6. The cleavage of glucose bond at C-20 is hydrolyzed before bond at C-3 and C-6 in processed condition [6]. The amount of isomer pairs is detected, and 20(S)-ginsenosides are always eluted more easily than 20(R)-ginsenosides [6]. Moreover, Δ20(21) ginsenosides are eluted before their Δ20(22) derivatives. Ocotillol-type and oleanane-type have a side chain at C-20. Yao et al have identified 945 ginsenosides from P. notoginseng leaves and 662 potentially novel ginsenosides [7]. Various species, parts, processings, regions, and growing times have a great influence on the chemical compounds of herbal medicines.
Fig. 1.
The main ginsenosides of Panax species (protopanaxadiol, protopanaxatriol, ocotillol, oleanane, and C-17 side chain type).
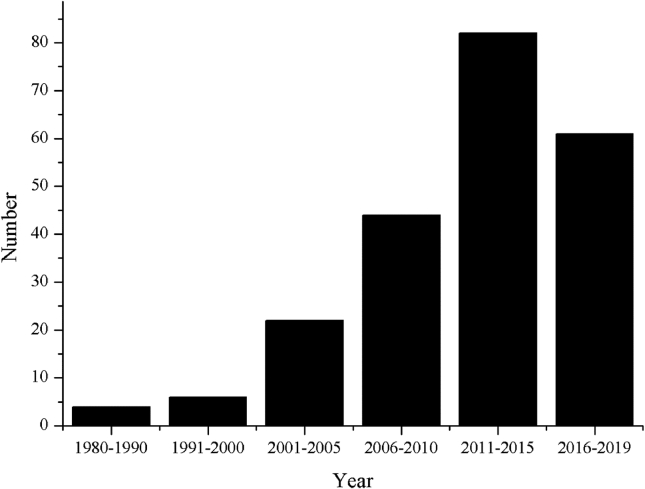
In the previous review, chemical and pharmacological diversity of ginsenosides of genus Panax L. was summarized [4,8,9]. Wang et al (2015) reviewed analytical techniques that were used in the evaluation of P. quinquefolius, while some advanced methods such as 2D-HPLC, micellar electrokinetic chromatography, and high-performance anion-exchange chromatography (HPAEC) were not investigated. In addition, P. ginseng and P. notoginseng with phenolic acids, dencichines, trilinoleins, flavonoids, and vitamins were not described [10]. Qi et al (2011) reviewed preparation, analytical advance, and applications of ginseng from January 2000 to September 2010 [11]. However, there are only few investigations in which analytical methods were applied to evaluate Panax species. Some advanced techniques such as ambient ionization mass spectrometry are hardly described in previous studies. In this review, we analyzed the published phytochemical analysis of Panax based on the keywords “Panax, ginseng” from Pubmed and Google Scholar. A total of 219 articles from 1980 to 2019 in the analytical methods of Panax species were investigated. As shown in Fig. 2, it is found that few researches are conducted during 1980 and 2000. The number of papers gradually grows with the time. It increased rapidly after 2011. Different sample preparations have significant influence on analysis of the bioactive compounds. The different analytical methods have different performances on the analysis of constituents of Panax species. Analytical methods including thin layer chromatography (TLC), high-performance thin layer chromatography (HPTLC), gas chromatography (GC), high-performance liquid chromatography (HPLC), ultra-high performance liquid chromatography (UHPLC) with tandem ultraviolet (UV) detector, diode array detector (DAD), evaporative light scattering detector (ELSD), and mass detector, two-dimensional high-performance liquid chromatography (2D-HPLC), ambient ionization mass spectrometry, high speed counter-current chromatography (HSCCC), and high speed centrifugal partition chromatography (HPCPC) are investigated. Furthermore, the methods have been applied to raw and processed ginseng of different species, from different parts, regions, growing ages, and biochemical analysis. The application in various fields is to screen the potential biomarkers for evaluating and quality control of Panax species. It is expected that the current review would have a solid fundamental for the future investigation.
Fig. 2.
The number of papers published during 1980 and 2019.
2. Sample preparations
During isolation and purification of bioactive components from natural products, extraction is the first and essential step [12]. A method with short extraction time, less extraction solvent, simple operation, low cost, and high extraction efficiency could be accepted. Sometimes many of factors are not satisfied because of the chemical profile of medicinal plants. In this review, the factors of sample preparations for Panax species are discussed (Table 1). As a traditional method, heat reflux extraction is used to extract ginsenosides, while it has the disadvantages of chemical transformation, wasting extraction solvent, and complicate operation [13]. Owing to convenient, simple, and high-efficient extraction, various extraction solvents (different concentrations of ethanol and methanol) and times have been used to extract ginsenosides, polyacetylenes, phenolic acids, flavonoids, and so on [[14], [15], [16]]. The operation time of microwave-assisted extraction is 60 times more efficient than that of Soxhlet extraction and 20 times more efficient than that of ultrasonic extraction [17]. Moreover, malonyl-ginsenosides Rb1, Rc, Rb2, and Rd can transform into corresponding neutral ginsenosides Rb1, Rc, Rb2, and Rd under high pressure microwave-assisted extraction at 400 kPa in 70% ethanol–water and at 600 kPa in methanol [18]. Compared with Soxhlet extraction, heat reflux extraction, ultrasonic extraction, and microwave-assisted extraction, pressurized liquid extraction has the highest extraction efficiency in the shortest time for P. quinquefolius, P. notoginseng, and red ginseng [12,19,20]. The amount of total ginsenosides (Rb1, Rb2, Rc, Rd, Re, and Rg1) increased with ultra-high-pressure extraction, whereas pressuring level and time have no influence on the content of ginsenosides [21]. The extraction time of pulsed electric field is less than 1 s, which is much less than that of the heat extraction method (6 h) [22]. In addition, matrix solid phase dispersion extraction has the advantages of short extraction time and less solvent usage, when compared with reflux extraction [23].
Table 1.
Various factors of sample preparation of Panax genus
| Technology | Extraction Time | Extraction Solvent | Extraction Efficiency | Operation | Cost | Reference |
|---|---|---|---|---|---|---|
| Soxhlet extraction | Long | More | High | Moderate | Low | [13] |
| Heat reflux extraction | Long | More | High | Moderate | Low | [125] |
| Ultrasonic extraction | Moderate | Moderate | High | Simple | Moderate | [126] |
| Solid phase extraction | Long | Moderate | Moderate | Simple | Moderate | [127] |
| Microwave-assisted extraction | Short | Less | High | Simple | High | [17] |
| Pressurized liquid extraction | Short | Less | High | Simple | High | [128] |
| Enzyme-assisted extraction | Long | Less | Low | Complex | Low | [113] |
| Accelerated solvent extraction | Short | Less | High | Simple | High | [129] |
| Matrix solid phase dispersion extraction | Short | Less | High | Simple | Moderate | [23] |
| Pulsed electric field | Short | More | High | Simple | Moderate | [22] |
3. Analytical methods
In the previous study, chromatographic methods including TLC/HPTLC, GC, HPLC, UHPLC (UV detector, DAD, ELSD, and MS detector), 2D-HPLC, HSCCC/HPCPC, and spectroscopic analysis, e.g., near infrared (NIR) spectroscopy and NMR, have been used to evaluate Panax species. Moreover, some advanced techniques such as ambient ionization mass spectrometry are applied to Panax. It is obvious that different techniques show different advantages and shortcomings. Detailed comparisons are provided in Table 2.
Table 2.
The advantages and shortcomings of technique analysis for Panax species
| Technique | Advantages | Shortcomings | Reference | |
|---|---|---|---|---|
| TLC/HPTLC | Rapid analysis Convenient operation High sensitivity and specificity Low cost |
Bad efficiency in separation Bad stability Need volatile organic solvents Low accuracy in quantification |
[[24], [25], [26]] | |
| GC | Rapid analysis Less solvent consuming High sensitivity Less time analysis |
Limited to volatile compounds Operation with the derivation High cost |
[76,130] | |
| HPLC/UHPLC | UV/DAD | Convenient operation High specificity High repeatability Low cost Combining with multiple detector |
Long analysis time Large solvent consuming Analytes with ultraviolet absorption Low sensitivity |
[[131], [132], [133]] |
| ELSD | High specificity Low cost |
Long analysis time Large solvent consuming Low sensitivity |
[52,77,104] | |
| MS | Convenient operation High sensitivity Less solvent consuming High resolution |
High cost Bad stability |
[93,134,135] | |
| 2D-LC | Wide coverage Good orthogonality High efficiency in separation |
Complicated operation Long analysis time Large solvent consuming |
[55,56] | |
| Ambient ionization mass spectrometry | Rapid analysis Convenient operation Less solvent consuming |
Bad stability High cost Low sensitivity Some compound with the derivation |
[59] | |
| HSCCC/HPCCC | High efficiency in separation | More solvent consuming Low sensitivity |
[62,136] | |
| 1H NMR | Fast analysis Less solvent consuming Easy operation |
High cost Low accuracy in quantification |
[65,66] | |
| Near infrared | Fast analysis No solvent consuming No sample preparation Low cost |
Low accuracy in quantification Low specificity |
[137,138] | |
3.1. TLC/HPTLC
As a rapid qualitative and quantitative analysis technology, TLC is recorded by Chinese Pharmacopoeia. Some scholars have applied TLC to evaluate Panax species (Table 3). In P. ginseng, ginsenosides Rb1, Rb2, Rc, Rd, Re, and Rg1 are determined simultaneously by HPTLC at an absorption of 275 nm. The method consists of a quaternary-solvents system (1,2-dichloroethane–100% ethanol–methanol–water, 56.8:19.2:19.2:4.8) to have an efficient saponins recovery and selective separation [24]. Different species with free mono- and oligo-saccharides are identified by HPTLC [25]. Moreover, to determine ginsenosides in P. trifolius, 2D-TLC with eluent A (chloroform–methanol–ethyl acetate–butanol–water, 4:4:8:1:2), eluent B (chloroform–butanol–methanol–water, 4:8:3:4), and eluent C (chloroform–methanol–water, 13:7:2) were used [26]. The TLC technology has some advantages of rapid, convenient, and sensitive characteristics to target compounds, whereas it always needs standards and there is a lack of uniqueness for bioactive compounds. In recent years, HPTLC-MS with rapid and accurate profile will hope for evaluating Panax species [27]. Two-dimensional HPTLC showed an efficient performance and good isolation profiles for Panax species in another study [28].
Table 3.
Chemical analysis of Panax species by TLC/HPTLC
3.2. Gas chromatography
Gas chromatography is employed to determine volatile organics, ginsenosides, and phenolic acids from Panax species (Table 4). Different derivatizations for chemical components were selected. For volatile organics, the GC–MS method can determine bioactive compounds of headspace without sample preparation for discriminating Panax species [29]. When determining ginsenosides in P. ginseng, it is applied to high-molecular-weight saponins after derivatization with trimethylsilylation [30]. Sample is subjected to trimethylsilane derivatization for evaluating phenolic acids in white and red ginsengs [31]. After derivatization with ethyl chloroformate, dencichine or other amino acids of P. notoginseng are determined [32]. GC–MS for volatile components can take some advantage with simple, fast, and effective characters, whereas for some non-volatile components, a complex operation is required. 2D-GC with high peak capacity, orthometric characteristic can be used to evaluate volatile components of samples, which is necessary to be discussed for the further study.
Table 4.
Chemical analysis of Panax species by GC–MS
| Method | Species | Part | Analytes | Reference |
|---|---|---|---|---|
| GC–MS | P. ginseng | Root | Ginsenosides Rg1, Re, Rd, Rc, Rb2, Rb1, F1 | [30] |
| GC–MS | Panax genus | Root | Panaxynol and panaxydol | [139] |
| GC–MS | P. ginseng | Root | Phenolic acids | [31] |
| GC–MS | P. notoginseng | Root | Dencichine | [32] |
| GC–MS | P. ginseng | Root | Volatile organic compounds | [76] |
| GC–MS | P. ginseng | Root | Volatile organic compounds | [130] |
| GC–MS | P. ginseng, P. notoginseng, P. quinquefolius | Root | Volatile organic compositions | [29] |
| GC–MS | P. ginseng, P. quinquefolius, P. notoginseng | Root | Volatile organic compounds | [140] |
3.3. HPLC/UHPLC
HPLC/UHPLC is the most frequently used method for Panax species in the qualitative and quantitative analysis. In this review, it is found that stationary phases including C18 column (250 × 4.6 mm, 5 μm) with different brands are used for ginsenosides, OV-170 (500 × 0.25 mm), LiChrosorb for polyacetylenes, polymer C18 column (250 × 4 mm, 10 μm) for trilinoleins, Waters Atlantis HILIC (hydrophilic interaction liquid chromatography) silica (50 × 2.1 mm, 3 μm) [33] for dencichine, and Zorbax SB-Aq column (150 × 4.6 mm, 5 μm) for nucleobases and nucleosides. Moreover, the small particle size ACQUITY UHPLC BEH C18 (2.1 × 100 mm, 1.7 μm) is used in UHPLC. Two-phase solvent systems contain water or buffer solution in water (formic acid, acetic acid, phosphoric acid, ammonium formate, or ammonium acetate) and acetonitrile or methanol. Formic acid in water improves resolution and eliminates peak tailing [[34], [35], [36]]. The solvent range of 1% to 100% is changed to obtain the appropriate gradient elution grogram. Ginsenosides could be eluted by the solvent range of 30–50% as observed in the literature. UHPLC with less analytical time has the better performance than HPLC.
3.3.1. UV/DAD and ELSD detector
UV detector is the traditional detector for the qualitative and quantitative analysis of chemical compounds in the Panax species (Table 5, Table 6). The detector with its low cost and simple operation has become the most commonly used analytical method in the laboratory. Therefore, it has been widely employed to determine the ginsenosides (malonyl ginsenoside, protopanaxadiol, protopanaxatriol, ocotillol, and oleanane), trilinoleins, polyacetylenes [37], phenolics [38], phytosterols [39], flavonoids, and vitamins [40]. The detection wavelengths for different types of biochemical compounds are various. It is reported that ginsenosides Rb1, Rb2, Rc, Rd, Re, Rf, Rg1, Rg2, F2, gypenoside XVII, and notoginsenoside R1 could be detected in the wavelength of 203 and 198 nm [[41], [42], [43], [44]]. The detection wavelength is set at 205 nm for trilinoleins [45], 254 nm for polyacetylenes [37], 260 nm for nucleobases and nucleosides [46], and 280 nm for phenolic compounds [38]. However, oleanane ginsenosides (ginsenoside Ro) are poor chromophores with weak UV absorption and are disturbed by solvents (the cut-off wavelength of methanol is 205 nm) that have low sensitivity with UV detection. DAD has the better recognition than conventional UV detection (Table 7). It is widely used to determine polar and non-polar [47], neutral and malonyl ginsenosides [48] in P. ginseng, P. quinquefolius, and P. notoginseng. As a mass detection, ELSD is mainly used for analysis of biological compounds that lack appropriate chromophores (Table 8). It can be used to identify and quantify neutral and acidic ginsenosides Rg1, Rg2, Ro, Rb1, Rb2, Rc, and Rd in P. ginseng, while the sensitivity of ELSD is five times lower than that with UV detection [49].
Table 5.
Ginsenosides analysis of Panax species by HPLC-UV
| Method | Species | Part | Analytes | Reference |
|---|---|---|---|---|
| HPLC-UV | P. ginseng | Root | Ginsenosides Rb1, Rb2, Rc, Rd, Rg1, Re, Rf | [141] |
| HPLC-UV | P. ginseng | Different parts and ages | Ginsenosides Rg1, Re, Rb1, Rc, Rb2, Rb3, Rd | [102] |
| HPLC-UV | P. ginseng | Root | Ginsenosides Rg1, Re, Rb1, Rc, Rb2, Rd | [22] |
| HPLC-UV | P. ginseng | Leaf | Ginsenosides F1, F2, F3, Re, Rg1, Rd, Rc, Rb2 | [23] |
| HPLC-UV | P. ginseng | Root | Ginsenosides Rg2, Rg3, Rg5, Rg6, Rh1, Rh4, Rk1, Rk3, F1, R4 | [73] |
| HPLC-UV | P. ginseng | Root | Ginsenosides Rg1, Re, Rb1, Rd | [142] |
| HPLC-UV | P. ginseng | Root | Ginsenosides Rb1, Rb2, Rc, Rd, Rf, Rg1, Rg2, Rg3, Rg5, Rg6, Rh1, Rh4, Rk1, Rk3, F1, F4 | [131] |
| HPLC-UV | P. ginseng | Root | Ginsenosides Rg1, Re, Ro | [143] |
| HPLC-UV | P. ginseng | Root | Malonyl ginsenosides | [144] |
| HPLC-UV | P. ginseng | Root | Ginsenosides and phenolic | [145] |
| HPLC-UV | P. quinquefolius | Root | Ginsenosides Rg1, Re, Rb1, Rc, Rb2, Rd | [132] |
| HPLC-UV | P. quinquefolius | Leaf, stem, root | Ginsenosides Rg1, Re, Rf, Rb1, Rc, Rb2, Rd | [125] |
| HPLC-UV | P. quinquefolius | Root | Ginsenosides Rb1, Rc, Rd, Re, Rg1 and F2, gypenoside XVII | [43] |
| HPLC-UV | P. quinquefolius | Root | Ginsenosides Rb1, Rc, Rd | [17] |
| HPLC-UV | P. quinquefolius | Root | Ginsenosides Rb1, Rb2, Rc, Rd, Re, Rf, Rg1 | [146] |
| HPLC-UV | P. quinquefolius | Root | Ginsenosides Rg1, Re, Rb1, Rc, Rd | [147] |
| HPLC-UV | P. quinquefolius | Root | Ginsenosides Rg1, Re, Rb1, Rb2, Rc, Rd | [12] |
| HPLC-UV | P. quinquefolius | Root | Ginsenosides Rb1, Rb2, Rc, Rd, Rg1, Rg2 | [113] |
| HPLC-UV | P. quinquefolius | Different parts and ages | Ginsenosides Rg1, Re, Rb1, Rc, Rb2, Rb3, Rd | [42] |
| HPLC-UV | P. quinquefolius | Root | Rare ginsenosides 20(S/R)-Rh1, Rg6, F4, Rk3, 20(S/R)-Rg3, Rk1, Rg5 | [148] |
| HPLC-UV | P. notoginseng | Root | Notoginsenoside R1, ginsenosides Rg1, Rb1, Rd | [133] |
| HPLC-UV | P. notoginseng | Root | Notoginsenoside R1, ginsenosides Rg1, Rb1, Rd, | [127] |
| HPLC-UV | P. notoginseng | Root | Notoginsenoside R1, ginsenosides Rg1, Rb1, Rd, | [119] |
| HPLC-UV | P. notoginseng | Rat tissue | Ginsenosides Rg1, Rb1, Rd | [149] |
| HPLC-UV | P. notoginseng | Flower bud | Notoginsenoside R1, ginsenosides Rg1, Re, Rb1, Rb2, Rb3, Rd, F2 | [150] |
| HPLC-UV | P. notoginseng | Different parts | Notoginsenoside R1, ginsenosides Rb1, Rb2, Rd, Re, Rg1, Rb3, Rg2, Rg3, Rh1 | [110] |
| HPLC-UV | P. notoginseng | Root | Notoginsenoside R1, ginsenosides Re, Rg1, Rb1, Rd | [151] |
| HPLC-UV | P. notoginseng | Root | Ginsenosides Rg1, Re, Rb1, 20(S/R)-Rh1, Rk3, Rh4, 20(S/R)-Rg3, notoginsenoside R1 | [152] |
| HPLC-UV | P. notoginseng | Root | Ginsenosides Rg1, Re, Rb1, Rd, notoginsenoside R1 | [153] |
| HPLC-UV | P. notoginseng | Root | Notoginsenoside R1, ginsenosides Rg1, Re, Rb1, Rd | [154] |
| HPLC-UV | P. notoginseng | Root, leaf, stem | Ginsenosides Rg1, Re, Rb1, Rd, notoginsenoside R1 | [155] |
| HPLC-UV | P. notoginseng | Root, rhizome | Notoginsenoside R1, R2, R3, ginsenosides Rg1, Rg2, Rg3, Rb1, Rd, Rh1, Re, quercetin | [13,156] |
| HPLC-UV | P. ginseng, P. quinquefolius, and ginseng drug preparations | Different parts | Ginsenosides Rb1, Rb2, Rc, Rd, Re, Rf, Rg1, Rg2 | [41] |
| HPLC-UV | P. sokpayensis, P. bipinnatifidus | Rhizomes | Ginsenosides Rg1, Rg2, Rf, Re, Rd, Rc, Rb1, Rb2 | [95] |
Table 6.
Chemical analysis of Panax species by HPLC-UV
| Method | Species | Part | Analytes | Reference |
|---|---|---|---|---|
| HPLC-UV | P. ginseng | Root | Ginsenosides and total phenolic | [157] |
| UHPLC-UV | P. ginseng | Fruit, leaf, root | Phenolic compounds | [38] |
| HPLC-UV | P. ginseng | Root | Phytosterols | [39] |
| HPLC-UV | P. ginseng | Main root, root hair, and leaf | Phenolic, flavonoid, vitamin | [14] |
| HPLC-UV | P. ginseng | Root, rhizome, and root hair | Trilinolein, 1,2-dilinoleoyl-3-oleoyl-glycerol | [45] |
| HPLC-UV | P. pseudoginseng | Root | Trilinolein | [45] |
| HPLC-UV | P. ginseng, P. quinquefolius, P. japonicus, P. notoginseng | Root | Polyacetylenes, ginsenosides | [37] |
| UHPLC-UV | P. notoginseng | Root | Fingerprinting analysis | [158] |
| HPLC-UV | P. notoginseng | Root | Fingerprinting analysis | [115] |
| HPLC-UV | P. ginseng, P. quinquefolius | Leaf | Metabolic profiling | [100] |
Table 7.
Chemical analysis of Panax species by HPLC-DAD
| Method | Species | Part | Analytes | Reference |
|---|---|---|---|---|
| HPLC-DAD | P. ginseng | Root | Polar and non-polar ginsenosides | [47] |
| UHPLC-DAD | P. ginseng | Root | Panaxfuraynes A and B | [101] |
| HPLC-DAD | P. ginseng | Root | Spectrum-efficacy relationship | [159] |
| HPLC-DAD | P. quinquefolius | Root | Ginsenosides Rb1, Rb2, Rc, Rd, Re, Rg1, Ro, gypenoside XVII, pseudoginsenoside-F11 | [160] |
| HPLC-DAD | P. quinquefolius | Root | Neutral and malonyl ginsenosides | [48] |
| HPLC-DAD | P. quinquefolius | Root | Ginsenosides Rg1, Re, Rb1, Rc, Rb2, Rd | [114] |
| HPLC-DAD | P. quinquefolius | Root | Ginsenosides Rb1, Rb2, Rc, Rd, Re, Rg1 | [126] |
| HPLC-DAD | P. quinquefolius | Fresh root | Ginsenosides and polyacetylenes | [161] |
| HPLC-DAD | P. notoginseng | Root | Notoginsenoside R1, ginsenosides Rg1, Re, Rf, Rb1, Rc, Rb2, Rb3, Rd | [19] |
| HPLC-DAD | P. notoginseng | Root | Notoginsenoside R1, ginsenosides Rg1, Re, Rb1, Rc, Rd | [44] |
| HPLC-DAD | P. notoginseng | Root | Ginsenosides Rb1, Rc, Rd, Re, Rg1, Rg5, Rk1, 20(R/S)-Rg3, 20(R/S)-Rh1, notoginsenosides R1 | [162] |
| HPLC-DAD | P. notoginseng | Root | Saponins | [79] |
| HPLC-DAD | P. notoginseng | Root | Ginsenosides Rb1, Rb2, Rc, Rd, Re, Rg1 | [21] |
| HPLC-DAD | P. notoginseng | Main root, rhizome, fibrous root | Notoginsenosides R1, R4, Fa, and K, ginsenosides Rg1, Rb1, Rd, Re, Rf, Rg2, Rh1 | [107] |
| HPLC-DAD | P. notoginseng | Root | Notoginsensides R1, ginsenosides Rg1, Re, Rb1, Rd | [163] |
| HPLC-DAD | P. notoginseng | Root | Notoginsenoside R1, ginsenosides Rg1, Rb1, Rd, Re | [164] |
| UHPLC-DAD | P. notoginseng | Root | Notoginsenoside R1, ginsenosides Rg1, Re, Rf, Rb1, Rg2, Rb3, Rd, Rg3 | [165] |
| UHPLC-DAD | P. notoginseng | Root | Notoginsenoside R1, ginsenosides Rg1, Re, Rb1, Rd | [166] |
| HPLC-DAD | P. notoginseng | Different parts | Fingerprint analysis | [109] |
| HPLC-DAD | P. notoginseng | Flower | Fingerprint analysis | [167] |
| HPLC-DAD | P. notoginseng, P. vietnamensis, P. stipuleanatus | Root | Fingerprint analysis | [97] |
| UPLC-PDA | P. ginseng | Root | Ginsenosides Rg1, Re, Rf, Rg2, Rb1, Rc, Rb2, Rd, Ro | [168] |
Table 8.
Chemical analysis of Panax species by HPLC-ELSD
| Method | Species | Part | Analytes | Reference |
|---|---|---|---|---|
| HPLC-ELSD | P. ginseng | Root | Ginsenosides Rg1, Re, Rb1, Rc, Rb2, Rd | [49] |
| HPLC-ELSD | Red ginseng | Root | Ginsenosides Rg1, Re, Rf, Rh1, Rg2, Rb1, Rc, Rb2, Rb3, Rd, Rg3, Rk1, Rg5, Rh2 | [77] |
| HPLC-ELSD | Black ginseng | Root | Less polar ginsenosides | [78] |
| HPLC-ELSD | P. ginseng | Root | Ginsenosides Rh1, Rg2, Rg3, Rg1, Rf, Re, Rd, Rb2, Rc, Rd | [169] |
| HPLC-ELSD | P. quinquefolius | Different parts | Ginsenosides Rg1, Re, F11, Rf, Rg2, Rh1, Rb1, Rc, Rb2, Rb3, Rd, Rh2 | [104] |
| HPLC-ELSD | P. quinquefolius | Different parts | 20(R)-dammarane-3β,6α,12β,20,25-pentol, 25(R)-ocotillol, 20(R)-protopanaxatriol, 20(S)-panaxatriol and 20(R)-dammarane-3β,12β,20,25-tetrol | [105] |
| HPLC-ELSD | P. ginseng, P. quinquefolius | Root | Ginsenoside Rf, 24(R)-pseudoginsenoside F11 | [90] |
| HPLC-ELSD | P. notoginseng | Root | Ginsenosides Rg1, Re, Rb1, Rc, Rb2, Rd | [170] |
| HPLC-ELSD | P. notoginseng | Different parts | Ginsenosides Rg1, Re, Rf, Rb1, Rc, Rb2, Rb3, Rd | [108] |
| HPLC-ELSD | P. notoginseng | Root | Ginsenosides Re, Rg1, Rb1, Rb2, Rc, Rd, notoginsenoside R1 | [52] |
| HPLC-ELSD | P. notoginseng, P. quinquefolius, P. ginseng | Root | Notoginsenoside R1, ginsenosides Rg1, Re, Rf, Rg2, Rc, Rb2, Rb3, Rd, Rg3 | [94,128] |
3.3.2. MS detector
Modern analytical techniques based on MS with chromatographic separation have the sensitivity and specificity characteristic when compared with traditional detection analysis of Panax species (Table 9) [10]. Ion sources including atmospheric pressure chemical ionization (APCI) and electrospray ionization (ESI) are used. The APCI can be applied to low molecule and polar compounds, such as 24(R)-pseudoginsenoside F11, ginsenoside Rf, and polyacetylenes [16,50,51]. The most of bioactive constituents of Panax species in the ESI mode has the better performance than that in the APCI mode, especially for the large and moderate polar compounds. Ginsenosides Rb1, Rb2, Rc, Rd, Re, Rf, Rg1, and notoginsenoside R1 have been analyzed with ESI mode in previous studies [52,53]. Dencichine, triterpenoid saponins, nucleobases, nucleosides, and polyacetylenes could be conducted by HPLC-MS as well (Table 10). In addition, MS hyphenations with Q-TOF, IT-TOF, Q-Trap, and Q-Orbitrap have been used to determine ginsenosides accurately and sensitively (Table 11). A total of 234 ginsenosides including 67 potential new ones were isolated tentatively by HPLC–QTOF-MS [54]. It is found that 646 ginsenosides were identified from stems and leaves of P. ginseng using linear ion-trap/Orbitrap mass spectrometry [55]. In the qualitative analysis, full scan, parent scan, daughter scan, and neutral loss scan have been employed. Selective ion monitoring and multiple reaction monitoring have been used to quantify bioactive compounds.
Table 9.
Ginsenosides analysis of Panax species using HPLC-MS
| Method | Species | Part | Analytes | Reference |
|---|---|---|---|---|
| HPLC-MS | P. ginseng | Root | Ginsenosides Rb1, Rb2, Rc, Rd, Re, Rf, Rg1, Rg2 | [118] |
| HPLC-ESI-MS | P. ginseng | Root | Ginsenosides Rg1, Re, Rb1, Rc, Rb2, Rd | [18] |
| HPLC-FD-MS | P. ginseng | Ginseng extract | Ginsenosides Rb1, Rb2, Rc, Rd, Re, Rf, Rg1 and Rg2 | [134] |
| HPLC-ESI-MS/MSn | P. ginseng | Root | Ginsenosides Rg1, 20(S)-Rg2, Rb1, Rc, Rh2, malonyl-ginsenoside Rb2 and Rc | [75] |
| HPLC-ESI-MS/MS | P. ginseng | Root | Low-polar ginsenosides | [80] |
| UHPLC-MS | P. ginseng | Root | Ginsenosides Rb1, Rb2, Rg1, Rg2, Rg3, Rc, Rd, Re, Rf | [171] |
| HPLC-MS/MS | P. ginseng | Root | Ginsenosides Rg1, Re, Rf, Rb1, Rc, Rb2, Rd, Rg2, Rh1, F1, F2, Rg3, PPT | [122] |
| HPLC-MS/MS | P. ginseng | Fresh root | Ginsenosides Rg1, Re, Rf, Rb1, Rb2, Rd, 20(S)-Rg2, Rc, 20(S)-Rh1, F1, F2, 20(S)-Rg3, 20(S)-protopanaxatriol, compound K, 20(S)-Rh2 | [172] |
| HPLC-Qtrap-MS | P. ginseng | Root | Ginsenosides | [173] |
| HPLC-MS | P. ginseng | Root | Notoginsenoside R1, ginsenoside Rb2, Re, Rb1, Rc, Rg1, Rb3, Rf, F1, Rd, Rh1, Rg2, F2, Rg3, Rh2, compound K | [174] |
| LC-MS/MS | P. ginseng | Root | 15 ginsenosides | [175] |
| UHPLC-HRMS | P. quinquefolius | Root | Ginsenosides Rb1, Rb2, Rb3, Rc, Rd, Re, Rf, Rg1, Rg2, Rg3, Rh1, Rh2, Ro, F1, F2, F3, pseudoginsenoside F11, notoginsenosides R1, R2 | [93] |
| HPLC-APCI-MS | P. quinquefolius | Root | 24(R)-pseudoginsenoside F11 | [50] |
| UPLC-MS/MS | P. quinquefolius | Different parts | 22 ginsenosides | [176] |
| HPLC-MS | P. ginseng, P. quinquefolius | Root | Ginsenosides Rb1, Rb2, Rc, Ro, Rd, Re, Rf, Rg1, pseudoginsenoside F11 | [88] |
| HPLC-MS | P. ginseng, P. quinquefolius | Root | Ginsenoside Rf, 24(R)-pseudoginsenoside F11 | [89] |
| UHPLC-ESI-MS | P. notoginseng | Different parts | Metabolite profiling | [112] |
| HPLC-MS | P. notoginseng | extraction | Ginsenosides Rg1, Rb1, notoginsenoside R1 | [177,178] |
| UHPLC-MS/MS | P. notoginseng | Extract | Notoginsenoside R1, ginsenosides Rg1, Rb1, Re, Rd | [120] |
| UPLC-MS/MS | P. notoginseng | Compounds | Notoginsenoside R1, ginsenosides Rg3, Rd, Rg2, Rb2, Rf, Rg1, Rb1, Re | [179] |
| HPLC-MS | P. notoginseng | Root | Notoginsenoside R1, ginsenosides Rg1, Rb1, Rd, F2, Re | [180] |
| LC-Q-Trap-MS | P. notoginseng | Extraction | Notoginseng total saponins | [181] |
| LC-MS/MS | Steamed notoginseng | Rat plasma | 23 triterpenoids | [182] |
| UHPLC-MS | P. japonicus | Leaf | Chikusetsusaponins V, Ib, IV, IVa, IV ethyl ester | [183] |
| HPLC-MS | P. ginseng, P. quinquefolius, P. notoginseng | Root | Ginsenosides Ro, Ra2, Ra3, Rb1, Rb2, Rb3, Rc, Rd, Re, Rg1, Rg2, 20(S)-Rg3, Rf, notoginsenosides R1, R2, R4 and 24(R)-pseudoginsenoside F11 | [91] |
| HPLC-APCI-MS | P. quinquefolius, P. ginseng, P. notoginseng | Root | Ginsenosides Rf, F11, notoginsenoside R1 | [51] |
Table 10.
Other chemical constituents of Panax species using HPLC-MS
| Method | Species | Part | Analytes | Reference |
|---|---|---|---|---|
| HPLC-MS | Panax | Root | Dencichine | [33] |
| HPLC-ESI-MS | P. notoginseng | Root | Triterpenoid saponins | [184] |
| HPLC-MS | P. notoginseng | Root | Nucleobases, nucleosides, and saponins | [46] |
| HPLC-APCI-MS | P. ginseng | Root | Polyacetylenes | [16] |
| NanoESI-MS | P. ginseng | Different parts | Lipidomics | [185] |
| UPLC-MS/MS | P. quinquefolius | Root | Zoxamide | [186] |
| LC-Q-TOF-MS | P. ginseng | Root | Malonyl ginsenoside, amino acids, polysaccharides | [187] |
Table 11.
Qualitative analysis of Panax species by HPLC-MS, HPLC-QTOF-MS, LC-IT-TOFMS
| Method | Species | Part | Analytes | Reference |
|---|---|---|---|---|
| HPLC-ESI-MS/MSn | P. ginseng | Root | Multicomponent quantification fingerprint | [188] |
| UHPLC-QTOF-MS | P. ginseng | Different parts | Qualitative analysis | [189] |
| LC-QTOF/MS | P. ginseng | Root | Fingerprint analysis | [190] |
| LC-QTOF-MS/MS | P. ginseng | Root | Ginsenosides Rc, Rb2, Rb3, malonyl-ginsenosides | [191] |
| UHPLC-QTOF MS | P. ginseng | Root | Metabolomics analysis | [117] |
| UHPLC-QTOF-MS | P. ginseng | Hairy root | Metabolomics analysis | [116] |
| LC-QTOF/MS | P. ginseng | Root | Metabolite profiling | [35] |
| UPLC-QTOF-MS | P. ginseng | Ginseng extract | 22 ginsenosides | [6] |
| UPLC-QTOF-MS | P. ginseng | Rhizome and root | 59 ginsenosides | [103] |
| UHPLC-QTOF-MS | P. ginseng | Root | Original neutral, malonyl, and chemically transformed ginsenosides | [192] |
| UPLC-DAD-QTOF-MS/MS | P. ginseng | Root | Qualitative and quantitative analysis | [193] |
| UPLC-QTOF-MS | P. ginseng | Root | Metabolomics analysis | [121] |
| UHPLC-Q-TOF MS | P. ginseng | Root | Metabolite profiling | [194] |
| UPLC-QTOF-MS | P. ginseng | Root | Metabolite profiling | [195] |
| UHPLC/QTOF-MS | P. ginseng | Leaf | Metabolite profiling | [196] |
| UPLC-QTOF-MS | P. ginseng | Root | Ginsenosides | [197] |
| UPLC-QTOF-MS | P. ginseng | Root | Metabolite profiling | [198] |
| UPLC-QTOF-MS | Red ginseng | Root | Metabolite profiling | [199] |
| UPLC-QTOF-MS | P. ginseng | Root | 44 ginsenosides | [200] |
| UPLC-QTOF-MS | P. ginseng | Different parts | 58 ginsenosides | [201] |
| UPLC-QTOF-MS | P. ginseng | Root | Cell-based neuroactivity screening | [202] |
| UPLC-QTOF-MS | P. ginseng | Flower | Transformation of ginsenosides | [203] |
| UPLC-QTOF-MS | P. ginseng (different processed) | Root | Metabolite profiling | [204] |
| UHPLC QTOF-MS | P. ginseng (different age) | Root | Metabolite profiling | [205] |
| UHPLC-QTOF-MS | White and red ginseng | Root | Fingerprint analysis | [206] |
| UPLC-QTOF-MS | P. ginseng (different age) | Root | Metabolomics analysis | [207] |
| LC-TOF-MS | P. quinquefolius | Root | Ginsenosides | [208] |
| UPLC-QTOF-MS | P. quinquefolius | Root | Metabolomics analysis | [209] |
| LC–MS | P. quinquefolius | Root | Fingerprint analysis | [210] |
| HPLC-ESI-MS | P. quinquefolius | Root | Metabolomics analysis | [211] |
| HPLC-MSn | P. quinquefolius | Root | 59 ginsenosides of protopanaxadiol, protopanaxatriol, oleanane and ocotillol types | [81] |
| UHPLC-QTOF-MS/MS | P. quinquefolius | Root | Metabolite profiling | [74] |
| UHPLC-QTOF-MS | P. ginseng, P. quinquefolius | Leaf | Metabolomics analysis | [36] |
| UHPLC-QTOF MS | P. ginseng, P. quinquefolius | Root | Metabolite profiling | [99] |
| HPLC-ESI-MS | P. notoginseng | Different parts | Metabolomics analysis | [111] |
| LC-MS | P. notoginseng | Root | Metabolite profiling | [15] |
| UHPLC-QTOF-MS | P. notoginseng | Root | Metabolite profiling | [53] |
| UHPLC-QTOF-MS | P. notoginseng | Root | Metabolite profiling | [72] |
| LC-QTOF-MS | P. notoginseng | Extract | Metabolomics analysis | [212] |
| LC-QTOF-MS | P. notoginseng | Leaf | Metabolite profiling | [34] |
| LC-IT-MS and UHPLC-QTOF-MS | P. notoginseng | Flower bud | Metabolite profiling | [70] |
| UPLC-ESI-QTOF-MS | P. notoginseng | Root | Fingerprint analysis | [213] |
| HPLC-QTOF-MS | P. notoginseng | Root | Metabolite profiling | [54] |
| LC-triple-TOF/MS | P. notoginseng | Extraction | Ginsenosides Rb1, Rb2, Rd, Re, Rf, Rg1 and notoginsenoside R1 | [214] |
| UPLC/Q-TOF MS | P. notoginseng | Leaf | Ginsenosides Rb1, Rc, Rb2, Rb3, notoginsenosides Fc, Fe, Fd | [215] |
| HPLC-QTOF-MS | P. ginseng, P. notoginseng, P. japonicus, P. quinquefolius | Root | Metabolite profiling | [98] |
| LC-MS-IT-QTOF | P. ginseng, P. quinquefolius, P. notoginseng | Root | Qualitative analysis | [87] |
| UHPLC-IMC-NLF | P. ginseng, P. quinquefolius, P. notoginseng | Root | Malonyl-ginsenosides | [216] |
| UPLC-LTQ-Qrbitrap-MS | P. ginseng, P. quinquefolius, P. notoginseng | Different parts | Malonyl-ginsenosides | [217] |
| UHPLC-QE-HRMS | P. ginseng, P. quinquefolius, P. notoginseng | Root | 101 compounds | [135] |
3.3.3. 2D-HPLC
The traditional methods for comprehensive chemical analysis of Panax species are of low-efficiency and incomplete. Recently, two-dimensional liquid chromatography has been used to analyze the complicated bioactive constituents (Table 12). Online and offline systems are constructed to obtain a high orthogonality and peak capacity. On offline 2D LC system, the first dimensional HILIC analysis for separation of polar compounds and the second dimensional ACQUITY UPLC BEH C18 are used to determine ginsenosides in P. notoginseng; the results indicated that orthogonality could be up to 81%, and the peak capacity is found to be 10200 [56]. It is similar that two-dimensional liquid chromatography, hybrid linear ion-trap/Orbitrap mass spectrometry could discover the new natural molecules, and some even trace amount in P. ginseng [55]. Online 2D LC systems have a simpler operation than offline ones. For instance, a quick, reproducible, and fast method for separation of saponins from P. notoginseng is established by using an online two-dimensional chromatography [57].
Table 12.
2D-LC applied to Panax species
| Method | Species | Part | Analytes | Reference |
|---|---|---|---|---|
| 2D LC/LTQ-Orbitrap-MS/NMR | P. ginseng | Stems and leaves | A total of 646 ginsenosides were characterized, and 427 have not been isolated from the genus of Panax L. | [55] |
| 2D LC-ESI | P. ginseng | Extraction | Triterpenoid saponins | [218] |
| 2DLC-MS | P. ginseng | Extraction | Ginsenosides Rd, Rc, Rb2, Rb1, Re | [219] |
| 2D chromatographic method | P. notoginseng | Root | Ginsenosides Rb1, Rg1, Rg2, Rh1, Rh4, Rd, 20(S)-Rg3, notoginsenosides R1, T5 | [57] |
| HILIC × RPLC | P. notoginseng | Root | Metabolomics analysis | [56] |
| 2D LC-QTOF-MS | P. notoginseng | Extraction | Total saponins | [220] |
3.4. Ambient ionization mass spectrometry
Recently, the developed ambient ionization mass spectrometry such as DART-MS and MALDI TOF-MSI are used to evaluate Panax (Table 13) [40,58]. For these methods, direct sampling and ionization are conducted in the open air with no or minimal sample preparation [59]. The most of ginsenosides need derivatization, whereas pseudoginsenoside F11, compound K, protopanaxatriol, and protopanaxadiol are detected without derivatization [59]. In addition, notoginsenoside R1, ginsenosides Rb1, Rg1, and Re from P. ginseng, and P. notoginseng are simultaneously determined by DART-MS [58,60].
Table 13.
Ambient ionization mass spectrometry applied to Panax species
3.5. HSCCC/HPCPC
As shown in Table 14, the similar techniques including HSCCC and HPCPC are liquid–liquid partition chromatography. The appropriate solvent systems composed of n-hexane, n-butanol, methylene chloride, methanol, isopropanol, ethyl acetate, and water are employed to isolate the bioactive compounds. In addition, ammonium acetate could reduce the separation time and eliminate emulsification [61]. Ginsenosides Rb1, Re, Rg1, Rb2, Rd, Rf, Rh1, and notoginsenoside R1 could be isolated by HSCCC, and the purity of ginsenosides are more than 95% [62].
Table 14.
HPCCC and HSCCC applied to Panax species
| Method | Species | Part | Analytes | Reference |
|---|---|---|---|---|
| HSCCC-ELSD | P. ginseng | Root | Ginsenosides Rg3, Rk1, Rg5, F4 | [62] |
| HSCCC-DAD | P. ginseng | Leaf | Ginsenosides Rk1, Rg5, Rs5, 20(R)-Rg3, Rs4 | [129] |
| HPCCC-ESLD | P. ginseng | Root | Ginsenosides Rf, Rd, Re, Rb1 | [61] |
| HSCCC-ELSD | P. ginseng | Root | Ginsenosides Rg1, Re, Rf, Rh1, Rb1, Rc, Rb2, Rd | [221] |
| HPCCC | P. ginseng | Root | Ginsenosides Re, Rb1, Rc, Rb2 | [222] |
| HSCCC-MCI gel column | P. ginseng | Root | Ginsenosides Re, Rg1 | [223] |
| CPC-ELSD | P. notoginseng | Root | Notoginsenoside R1, ginsenosides Rg1, Re, Rb1 | [224] |
| HSCCC | P. ginseng | Root | Ginsenosides Rg1, Re, Rf, Rg2, Rb1, Rb2, Rd, Rg3, Rk1, Rg5, Rg6, and F4 | [225] |
| HPCCC | P. notoginseng | Root | Notoginsenosides R6, R1, Spt1, ginsenosides Rb1, F4, Rh3, Rg3, Rs3, Rk1 | [136] |
| HSCCC | P. notoginseng | Root | Ginsenosides Rg1, Rd, R1, Re, Rb1 | [68] |
3.6. Others
Micellar electrokinetic chromatography could measure the ginsenosides Rg1, Re, Rf, Rb1, Rc, Rb2, Rb3, Rd, Rf, Rh1, Rg1, and notoginsenoside R1 in high separation efficiency without any organic solvent and with shorter run time when compared to chromatographic analysis (Table 15) [63]. It can extract dencichine from P. notoginseng with a purity of 98.5% [64]. Moreover, NMR technique in the qualitative analysis is used to discriminate geographical origins of P. ginseng and to obtain the potential markers [65]. It also quantifies malonyl-ginsenosides Re, Rb1, Rb2, Rc, and Rd [66]. HPAEC-PAD could analyze amadori compounds in processed ginseng within 15 min of single chromatographic run and eliminate the complex derivatization [67]. Enzyme immunoassay by anti-Rf antiserum quantifies ginsenosides Rg2 and Rf in P. ginseng [68]. Dencichine is measured by HPAEC for discrimination of P. notoginseng, P. ginseng, and P. quinquefolius [69]. In addition, MCI gel column chromatography combining with LC-MS could analyze metabolic profiling qualitatively [70]. To determine the various constituents of Panax species, multiple techniques have been used (Table 16). HPLC-UV coupled with GC-MS has been used to evaluate ginsenosides and volatile compounds [20]. Zhu et al using HPLC, CE, and NIR discriminated different parts of P. notoginseng [71].
Table 15.
Other analysis of Panax species
| Method | Species | Part | Analytes | Reference |
|---|---|---|---|---|
| Micellar electrokinetic chromatography | P. ginseng | Root | Ginsenosides Rg1, Re, Rf, Rb1, Rc, Rb2, Rd | [226] |
| Micellar electrokinetic chromatography | P. notoginseng | Root | Ginsenosides Rd, Rc, Rb3, Rb1, Rh1, Rg2, Rf, Rg1, Re, notoginosides R1 | [227] |
| High-performance anion-exchange chromatography | P. ginseng | Extraction and rat plasma | Arginyl-fructose, arginyl-fructosyl-glucose | [67] |
| High-performance anion-exchange chromatography | P. notoginseng, P. ginseng, P. quinquefolius | Root | Dencichine | [69] |
| Molecularly imprinted polymer | P. notoginseng | Root | Dencichine | [64] |
| Enzyme immunoassay | P. ginseng | Root | Ginsenosides Rf and Rg2 | [63] |
| 1H NMR | P. ginseng | Root | Qualitative analysis | [65] |
| 1H NMR | P. ginseng | Flower bud | Malonyl-ginsenosides Re, Rb1, Rb2, Rc, Rd | [66] |
| 1H NMR | P. quinquefolius | Root | Qualitative analysis | [228] |
| SFC-MS | P. ginseng, P. quinquefolius | Root | Nucleobases, nucleosides, ginsenosides | [229] |
| UHPSFC-QTOF-MS | P. ginseng, P. quinquefolius, P. notoginseng | Lipids | [230] | |
| FT-IR spectroscopy | P. notoginseng | Root | Protein | [231] |
| Near-infrared spectroscopy | P. ginseng | Root | Ginsenosides Rg1, Rb1, Re, Rf, Rc, Rb2, Rg2, Rb3, Rd | [137] |
| Near-infrared spectroscopy | P. notoginseng | Root | Fingerprint analysis | [232] |
| Infrared and ultraviolet spectroscopy | P. notoginseng | Root | Notoginsenoside R1, ginsenosides Rg1, Re, Rb1, Rd | [138] |
| FT-IR spectroscopy | P. ginseng | Different parts | Fingerprint analysis | [233] |
Table 16.
Multiple techniques applied to Panax species
| Method | Species | Part | Analytes | Reference |
|---|---|---|---|---|
| HPLC-UV, UHPLC-PDA, CE-UV, IR | P. notoginseng | Main root, rhizome | Fingerprint analysis | [71] |
| HPLC-UV, GC-MS | P. ginseng | Root | Ginsenosides Rg1, Re, Rf, Rh1, Rg2, Rb1, Rc, Rb2, Rg3, F2, compound K, Rk1, Rg5, Rh2 | [20] |
| HPLC-UV, HPLC-MS | P. notoginseng | Extract | Fingerprinting and quantitative analysis | [234] |
| HPLC-UV, HPLC-MS | P. ginseng | Rhizome | Ginsenosides Rb1, Rb2, Rc, Rd, Re, Rf, Rg1, Rg2 | [235] |
| HPLC-DAD, LC-ESI-MSn | P. notoginseng | Leaf | Chemical profiles and anticancer | [236] |
| GC–MS, LC–MS | P. ginseng, P. quinquefolius | Different parts | Primary and second metabolites | [106] |
| LC-ELSD, LC-Q-TOF-MS | P. vietnamensis | Radix and rhizome | Ginsenosides Rg1, Re, Rb1, Rc, Rb2, Rd, majonoside R1, R2 and vina-ginsenoside R2 | [96] |
4. Analytical methods applied to Panax species
As we all know, the different processing methods, species, parts, regions, and ages have different chemical information. To display the chemical markers of different conditions, we have reviewed the advanced techniques evaluating samples of Panax. In addition, the mechanisms of chemical compounds changing for Panax are illustrated.
4.1. Raw and processed ginseng
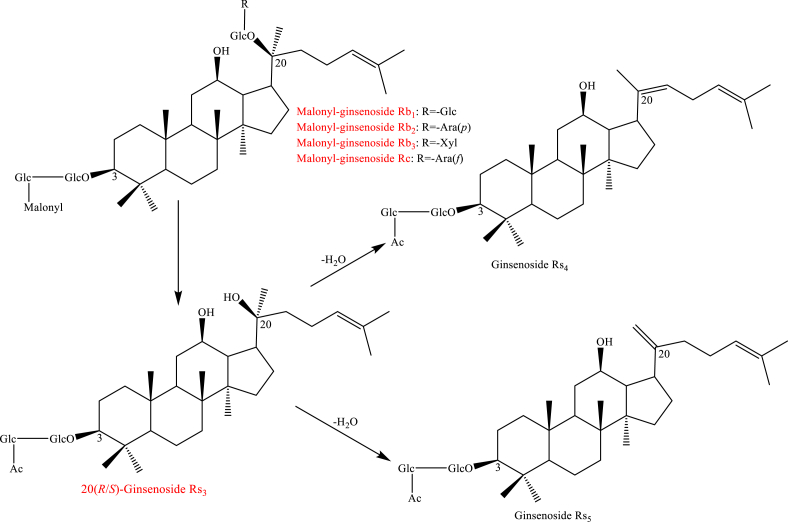
Processing Panax species leads to various bioactive characteristics, which have been used in the treatment of different diseases when compared to raw ginseng. In the Chinese medicine, “Sheng Da Shu Bu” and “Sheng Leng Shu Wen” with regard to raw P. notoginseng are used for hemostasis and cardiovascular diseases, whereas the steamed form is used to “nourish” blood [10]. Those theories suggested that raw and processing have the opposite effect on some illness. Different chemical profiles in the processing have been investigated in the previous study. As a formal method, from raw to processed material steaming with different temperatures and times has been used. P. ginseng is steamed at 98°C and 120°C at 2 h, 6 h, and 9 h, which shows the various bioactive constituents. Time-dependent profiling of raw and steamed Panax species is studied [[72], [73], [74]]. “Red ginseng” is formed at two- or three-time steaming and “black ginseng” is formed with cyclic nine-time steaming at 98°C for 3 h. Therefore, phytochemical components including saponins and volatile oils are reviewed in this investigation. It is found that chemical constituents with polar ginsenosides can be transformed to low polar ginsenosides by hydrolysis, isomerization, and dehydration [75]. The concentration of polar ginsenosides, notoginsenoside R1, ginsenosides Rg1, Re, Rb1, Rc, Rb1, Rc, Rb2, Rb3, and Rd, decreased by steaming, wheras that of low polar ginsenosides, Rh1, Rg2, Rg3, Rh2, Rs3, Rk1, Rs5, and Rs4, increased, and ginsenosides Rg3, Rg5, and Rk1 are the unique compounds from steamed ginseng [44,[76], [77], [78], [79], [80]].
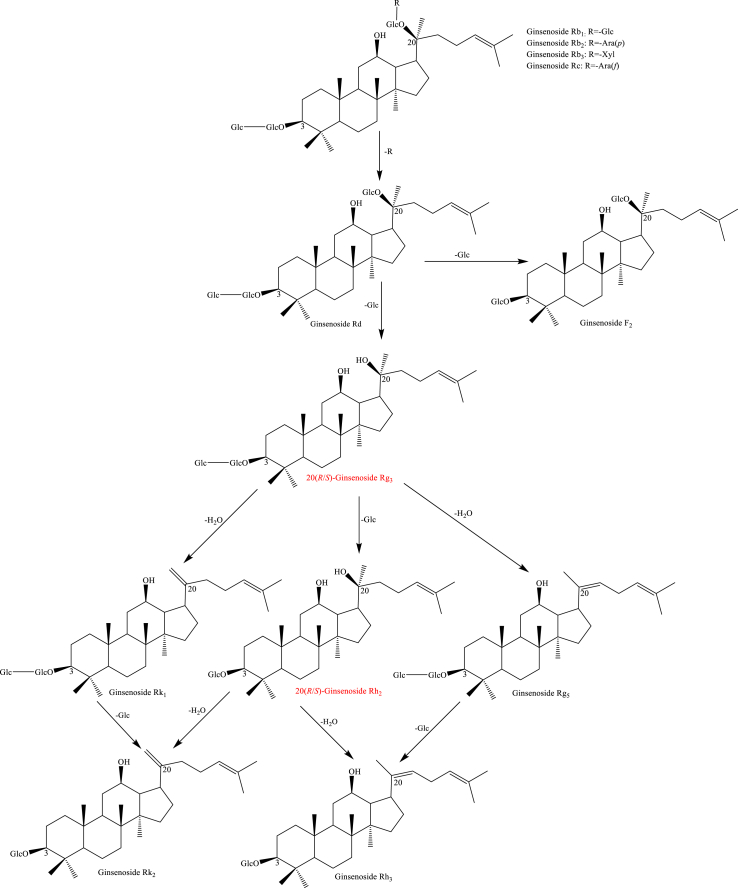
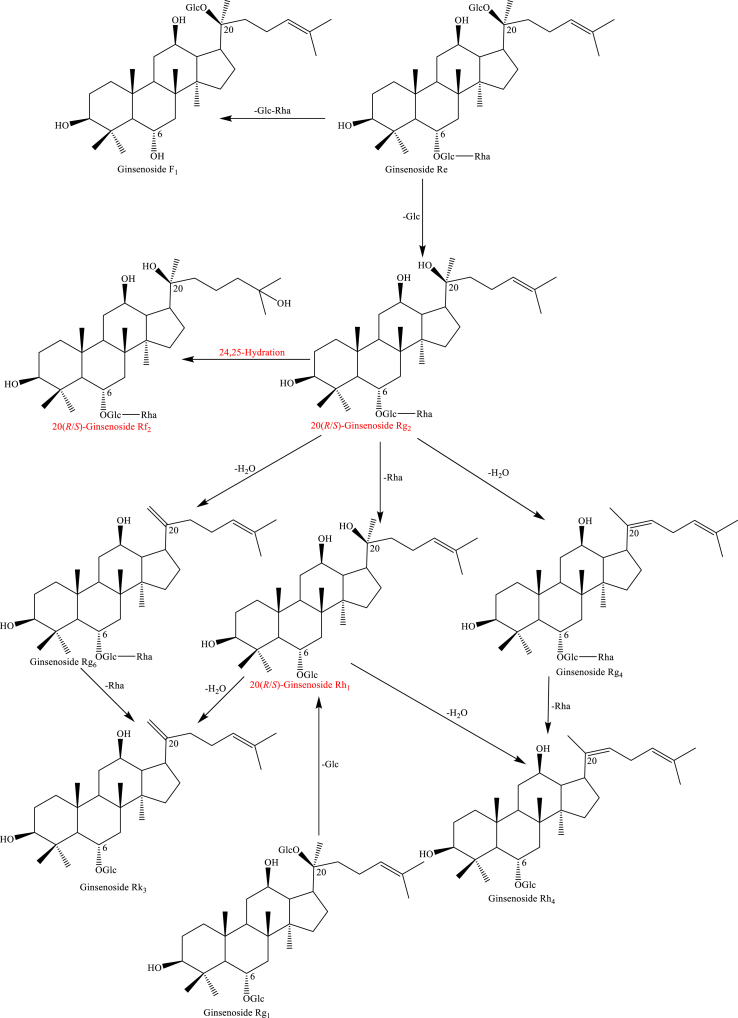
Usually, the types of saponins in the Panax species are mainly protopanaxadiol, protopanaxatriol, ocotillol, and oleanane. As shown in Scheme 1, protopanaxadiol including ginsenosides Rb1, Rb2, Rb3, and Rc converted to Rd by hydrolysis of a glycosylation moiety at C-20. Then, the loss of glycosylation moiety at C-20 and C-3 of Rd through hydrolysis generated ginsenosides 20(R/S)-Rg3 and F2. 20(R/S)-Rh2, Rk1, and Rg5 under the reaction conditions gradually increased [44,74,75,[77], [78], [79], [80], [81]]. Interestingly, ginsenosides Rk1 and Rg5 were deduced to 20(R/S)-Rg3 by Δ20(21) and Δ20(22) dehydration at C-20. Ginsenosides Rk1 and Rg5 are hydrolyzed to generate Rk2 and Rh3 by loss of a glycosylation moiety at C-20 [74,75,80,81]. Protopanaxatriol including ginsenosides Re and Rg1 produced 20(R/S)-Rg2, F1, Rg6, 20(R/S)-Rh1, and Rg4 through hydrolysis of a glycosylation moiety at C-20 and C-6 when the creaming with high-temperature and long-time shown in Scheme 2 [74,75,77,80,81]. Specifically, ginsenosides 20(R/S)-Rf2 was deduced by C-24 and C-25 hydration of Rg2 [81]. In addition, malonyl and acetyl ginsenosides could convert to the corresponding neutral ginsenosides through demalonylation and deacetylation reaction shown in Scheme 3 [74,75]. Such as acetyl-ginsenosides 20(R/S)-Rs3, Rs4, and Rs5 were deduced to be generated from malonyl-ginsenosides Rb1, Rb2, and Rc through hydrolysis, decarboxylation, and dehydration [74,75]. For oleanane type, the chemical transformations have not been studied up to now. The possible transformation pathways deduced are shown in Scheme 4 [81].
Scheme 1.
The potential transformation pathway of protopanaxadiol ginsenosides after processing.
Scheme 2.
The potential transformation pathway of protopanaxatriol ginsenosides after processing.
Scheme 3.
The potential transformation pathway of malonyl and acetyl ginsenosides after processing.
Scheme 4.
The potential transformation pathway of oleanane ginsenosides after processing.
4.2. Different species
Different species of Panax have different effects on diseases. P. ginseng is used for its anticancer effect [82]. While P. quinquefolius has a good performance on antidiabetic, anti-inflammatory, and neuroprotective effects [[83], [84], [85]], P. notoginseng always have effects on the cardiovascular system, hemostatic, and antioxidant activities [86]. P. japonicus, P. vietnamensis, P. stipuleanatus, P. sokpayensis, and P. bipinnatifidus are also used to protect and treat diseases all over the world. Usually, ginsenosides are the main bioactive components for the Panax species. Yao et al have reported that 623 ginsenosides in the ethanol extract of P. ginseng, P. quinquefolius, and P. notoginseng are discovered, and among those, 437 are potentially novel ginsenosides [87]. Polysaccharides, essential oils, phenolic acids, alkaloids, and others were also investigated in a previous study [3]. The similar morphological characteristics especially medicinal power and its extraction are hard to evaluate them in the markets. The fake and inferior goods may arise owing to price difference for Panax species largely. It is therefore necessary to select some quality markers for distinguishing Panax.
For saponins, ginsenoside Rf is only detected in P. ginseng, whereas 24(R)-pseudoginsenoside F11 is mainly detected in P. quinquefolius [[88], [89], [90]]. Ginsenoside Rs1 is used to differentiate P. ginseng and P. quinquefolius also [91]. Furthermore, the higher amount of Rg1 group (Rf, Rg1) is in P. ginseng and that of the Rb1 group is in the P. quinquefolius [92]. A higher protopanaxadiol/protopanaxatriol ratio for P. quinquefolius is about 3, while the value is between 1 and 3 for P. ginseng [93]. When P. notoginseng and P. quinquefolius are compared, the former has the highest ginsenoside content (9.176%), and the latter has the highest polyacetylene content (0.08%) [37]. Notoginsenoside R1 is detected in both P. notoginseng and P. ginseng [51], whereas ginsenoside Rg3 is observed in the red ginseng [94]. Ginsenoside Rc was not detected in P. sokpayensis, and ginsenosides Rf, Rc, and Rb2 are not detected in P. bipinnatifidus [95]. P. vietnamensis mainly has ocotillol type of ginsenosides [96]. To describe the more chemical information, metabolic components combined with multivariate statistical analysis, hierarchical clustering analysis, principal component analysis, and partial least squares discriminant analysis have been applied to evaluate different species and to select the appropriate chemomarkers [97]. The results indicated that ginsenoside Rf, 20(S)-pseudoginsenoside F11, malonyl-ginsenoside Rb1, and ginsenoside Rb2 could be used to differentiate P. ginseng, P. notoginseng, P. japonicus, and P. quinquefolius [98]. 24(R)-Pseudoginsenoside F11, ginsenoside Rf, Ra1, F2, and 20-glucoginsenoside Rf can differentiate processed P. ginseng and P. quinquefolius [99]. The metabolic constituents of leaves to avoid damaging the roots can separate Panax species [100]. Pseudoginsenoside F11, Rb3, malonyl-notoginsenoside Fd, malonyl-ginsenosides F2, Rb3, Re, F3, R2, and F1 are selected as the chemical markers for leaves of P. ginseng and P. quinquefolius [36]. For essential oil, hexanal, 2-pyrrolidnone, (E)-2-heptenal, (E)-2-octenal, heptanal, isospathulenol, (E, E)-2,4-decadienal, 3-octen-2-one, benzaldehyde, 2-pentylfuran, and (E)-2-nonenal can discriminate P. ginseng and P. notoginseng [29]. Mono- and oligo-saccharide are similar in the different regions and Panax species [25]. However, dencichine varied in Panax species, the highest (0.36 ± 0.02%) is in P. notoginseng, then P. ginseng (0.31 ± 0.06%) and P. quinquefolium (0.1 ± 0.01%), and the lowest (0.03 ± 0.07%) was in steamed P. ginseng. The contents of panaxfuraynes A and B are less than 3 and 2 ng/g in the roots of P. quinquefolius, P. japonicum, P. notoginseng, and P. ginseng, whereas they were not found in P. japonicum [101].
4.3. Different parts
Different parts include aerial parts (flower, leaf, and stem) and underground parts (rhizome, main root, lateral root, and root hair) in Panax species, which have been used for medicinal purposes. As a medicinal tea, flower and leaf are used to prevent disease for the human in the eastern world, especially in China. An official herbal medicine, leaf of P. ginseng is recorded in Chinese Pharmacopoeia. Different parts of Panax species have long been used. For instance, rhizomes of P. notoginseng and P. ginseng are called as “Jinkou” and “Lutou” in the traditional medicine, respectively. Different parts have various pharmacological activities [86]. The chemical profile for different parts of Panax species is significant.
In P. ginseng, the content of ginsenosides is higher in the leaf and root hair and lower in stem and other parts. The content of ginsenosides in the root and root hair increases with age from one to five years [102]. More kinds of ginsenosides are found in cork than those in cortex, phloem, xylem, and resin canals; the content of ginsenosides of phloem, xylem, and resin canals from branch root is high than that from main root [103]. The content of total phenols in fruit and leaf is higher than in roots, including major phenolic compounds chlorogenic acid, gentisic acid, p- and m-coumaric acid, and rutin [38]. Moreover, the order for triacylglycerol content is rhizome > main root > root hair. Ginsenosides in P. quinquefolius follow this order leaf > root hair > rhizome > stem [104]. Sapogenins are found more in stem and leaves than other parts of P. quinquefolius [105]. Both P. ginseng and P. quinquefolius mainly have ginsenosides Rg1, Re, and Rd for leaves, and ginsenosides Re, Rb1, and Rc for root hair [41]. The reason for ginsenosides accumulation in P. ginseng main root and P. quinquefolius lateral roots may be high rates of C assimilation to C accumulation [106]. In P. notoginseng, different parts can be identified based on saponin content difference [107]. The type of 20(S)-protopanaxatriol is mainly distributed in the underground parts, whereas 20(S)-protopanaxadiol is mainly distributed in the aerial parts [108,109]. Different parts could be identified by metabolomic combined with principal component analysis [71,110,111]. Notoginsenosides R4, Fa, Q, S, Fc, R1, H, A, B, ginsenosides Rb1, Rb2, Rb3, Rc, Rd, F2, Rh2, Rg1, Re, Rf, Rg2, malonyl-ginsenoside-Rb1, and 20-O-glucoginsenoside-Rf contribute to up- or down-regulation of different parts of P. notoginseng [112]. The main roots have 31% higher ginsenosides content than rhizome [96].
4.4. Different region and age
P. ginseng is mainly distributed in Korea, North Korea, and Northeastern China, P. quinquefolius in America and Canada, and P. notoginseng in Southwestern China. Geographical origin is a major influential factor for quality control [35]. Metabolomics combined with OPLS-DA could be used to discriminate P. ginseng of different regions [65]. The contents of 1,2-dilinoleoyl-3-oleoyl-glycerol of P. ginseng from Korea, Japan, and China are 0.41 ± 0.009 mg/g, 0.45 ± 0.01 mg/g, and 0.22 ± 0.008 mg/g, and those of trilinolein are 0.37 ± 0.009 mg/g, 0.39 ± 0.016 mg/g, and 0.27 ± 0.009 mg/g . Furthermore, P. quinquefolius roots cultivated in Jilin Province are similar to those cultivated in Canada in the compositions [113], whereas those grown in China and North America showed no major difference [93]. Ginsenosides Rb1, Rc, Rb2, Rg1, and Rd are influenced by location [114]. The highest polyacetylene content is distributed in Nagano, Japan [37]. Chemical constituents of rhizome and main roots of P. notoginseng from Wenshan, Honghe, and Kunming have no significant difference [115]. Different growing years may lead to different chemical profiles. For P. ginseng, seven ginsenosides show age-dependent variations [116]. Metabolites combining with multivariate statistical methods could classify different ages, especially for 4, 5, and 6 years [117]. The total contents of ginsenosides for main root and fibrous root in four years are highest [118]. The highest concentrations of stigmasterol and β-sitosterol are found in 6-year-old P. ginseng cultivated in Jinan, Korea [39]. For notoginseng, different growth years can be identified by the saponin content, the content of most and total saponins in the order is 3 > 2>1-year-old in the main root samples [107]. The best season for harvesting is September to October [13].
4.5. Biochemical analysis
Metabolism of Panax species in the different tissues could obtain a better understanding of biological effects. Ginsenosides Rg1, Rb1, and Rd of P. notoginseng in rat tissues (kidney, liver, heart, spleen, and lung) are determined. The highest concentrations of three saponins were at 90 min except for spleen after oral dose, whereas after intravenous administration, they could not detect in all tissues after 8 h [119]. After nasal administration, notoginsenoside R1, ginsenosides Rg1, Rb1, Rd, and Re from P. notoginseng have been determined in brain [120]. The metabolites in the urine after being administered orally ginseng decoction were used to distinguish normal control group, deficiency of vital energy model group, and ginseng treatment group and to find potential biomarkers [121]. Biotransformation of P. ginseng in the rat intestinal microflora indicated that protopanaxadiol-type ginsenosides were more easily metabolized than protopanaxatriol-type ginsenosides [122].
5. Conclusion
In this review, different sample preparations including Soxhlet extraction, heat reflux extraction, ultrasonic extraction, solid phase extraction, microwave-assisted extraction, pressurized liquid extraction, enzyme-assisted extraction, accelerated solvent extraction, matrix solid phase dispersion extraction, and pulsed electric field were compared. The TLC technique has been used to quantify and identify Panax species quickly, although it always needs standards and lacks uniqueness for bioactive compounds. GC–MS could be used to determine ginsenosides, phenolic acids, dencichine, pesticide residues, and volatile components, although for some non-volatile components complex operation is required. UHPLC with less analytical time has the better performance than HPLC, and DAD has the better recognition than conventional UV detection. HPLC tandem MS has the sensitivity and specificity characteristic when compared with traditional detection. In the liquid–liquid partition chromatography (HSCCC and HPCPC), ammonium acetate could reduce the separation time and eliminate emulsification. After processing ginseng, chemical constituents with polar ginsenosides can be transformed to low polar ginsenosides by hydrolysis, isomerization, and dehydration. Ginsenoside Rf is only detected in P. ginseng, whereas 24(R)-pseudoginsenoside F11 is mainly detected in P. quinquefolius. When P. notoginseng and P. quinquefolius are compared, the former has the highest ginsenoside content (9.176%) and the latter has the highest polyacetylene content (0.08%). The content of ginsenosides in the leaf and root hair is higher, and it is lower in stem and other parts of P. ginseng. In addition, the content of total phenols in fruit and leaf is higher than in roots. For P. notoginseng, the type of 20(S)-protopanaxatriol is mainly distributed in the underground parts, whereas 20(S)-protopanaxadiol is mainly distributed in the aerial parts. P. ginseng is mainly distributed in Korea, North Korea, and Northeastern China, P. quinquefolius in America and Canada, and P. notoginseng in Southwestern China. Protopanaxadiol-type ginsenosides were more easily metabolized than protopanaxatriol-type ginsenosides in the rat intestinal microflora.
From the current review, the present analysis of Panax species is not sufficient. The following aspects need to be investigated.
-
(1)
According to previous studies, the different sample preparations and analytical methods have been used to evaluate ginsenosides of Panax species. It is necessary that the harmonious and practical standard criteria method is established for determining ginsenosides of different species, parts, and ages quickly and accurately.
-
(2)
As we all know, ginseng has been widely used for prevention and treatment of diseases all over the world. Meanwhile, the criteria of Chinese Pharmacopoeia, United States Pharmacopeia, Japanese Pharmacopoeia, and South Korean Pharmacopoeia for P. ginseng have been developed. Different countries have different criteria. It is expected that the uniform criteria for ginseng should be established for development of the ginseng industry.
-
(3)
As an oleanane type, ginsenoside Ro was only detected in the P. ginseng and P. quinquefolius, which could be used to inhibit testosterone 5α-reductase and for testosterone-treated disease [123]. Both Ro and its transformation products in red ginseng are the bioactive constituents [124]. The chemical transformation pathway and the metabolism in vitro and in vivo are the key research in the further investigation. Furthermore, in Chinese Pharmacopoeia, quality markers for P. ginseng and red ginseng are ginsenosides Rg1, Re, and Rb1, although they have the various pharmacological effects. It is reported that red ginseng has the better performance biological activity than fresh ginseng [92]. What has not been investigated until now is the different bioactive components. The condition of ginseng from raw to processed, temperature, time, and pressure are necessary to be optimized for future studies.
-
(4)
Notoginsenoside R1 and ginsenoside Rg3 are discovered in P. notoginseng and red ginseng, although they are not unique. Several biomarkers have been selected to discriminate Panax species by metabolite coupled to chemometrics. The possible biomarkers need to be verified through large number of samples. In Chinese Pharmacopoeia, Rg1+Re + Rb1≥2% for P. quinquefolius, Rg1+Re ≥ 0.3% and Rb1 ≥ 0.2% for P. ginseng, Rg1+Re ≥ 2.25% for leaves of P. ginseng, and Rg1+Rb1+R1 ≥ 5% for P. notoginseng are quality control. Obviously, the biomarkers are unique for each one. The comprehensive evaluation of quality control for Panax species needs further investigation.
-
(5)
Rhizome and main root of Panax species with different chemical profiles are recorded in official documents. Most of the time, main root is used and rhizome is not, such as “Qulu” (cutting out rhizome) in traditional medicine. Up to now, the differences between rhizome and main root have not been investigated. A comprehensive, accurate, and convenient method is necessary to establish in the further study.
Conflicts of interest
All authors declare that they have no conflict of interest.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (81920108033, 81903804, 81703682, 81530096, and 81573581).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jgr.2019.12.009.
Contributor Information
Li Yang, Email: yl7@shutcm.edu.cn.
Zhengtao Wang, Email: ztwang@shutcm.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Lu Q. A review on studies of Panax plant taxonomy. J Jilin Agr Univ. 1992;14:107–111. [Google Scholar]
- 2.The State Pharmacopoeia Commission . 2015. Chinese Pharmacopoeia. Beijing. [Google Scholar]
- 3.Ru W., Wang D., Xu Y., He X., Sun Y.E., Qian L., Zhou X., Qin Y. Chemical constituents and bioactivities of Panax ginseng (C. A. Mey.) Drug Discov Ther. 2015;9:23–32. doi: 10.5582/ddt.2015.01004. [DOI] [PubMed] [Google Scholar]
- 4.Kim D.H. Chemical diversity of Panax ginseng, Panax quinquifolium, and Panax notoginseng. J Ginseng Res. 2012;36:1–15. doi: 10.5142/jgr.2012.36.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang W.Z., Hu Y., Wu W.Y., Ye M., Guo D.A. Saponins in the genus Panax L. (Araliaceae): a systematic review of their chemical diversity. Phytochemistry. 2014;106:7–24. doi: 10.1016/j.phytochem.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Yang H., Lee D.Y., Kang K.B., Kim J.Y., Kim S.O., Yoo Y.H., Sung S.H. Identification of ginsenoside markers from dry purified extract of Panax ginseng by a dereplication approach and UPLC-QTOF/MS analysis. J Pharm Biomed Anal. 2015;109:91–104. doi: 10.1016/j.jpba.2015.02.034. [DOI] [PubMed] [Google Scholar]
- 7.Yao C.L., Pan H.Q., Wang H., Yao S., Yang W.Z., Hou J.J., Jin Q.H., Wu W.Y., Guo D.A. Global profiling combined with predicted metabolites screening for discovery of natural compounds: characterization of ginsenosides in the leaves of Panax notoginseng as a case study. J Chromatogr A. 2018;1538:34–44. doi: 10.1016/j.chroma.2018.01.040. [DOI] [PubMed] [Google Scholar]
- 8.Shin B.K., Kwon S.W., Park J.H. Chemical diversity of ginseng saponins from Panax ginseng. J Ginseng Res. 2015;39:287–298. doi: 10.1016/j.jgr.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qi L.W., Wang C.Z., Yuan C.S. Ginsenosides from American ginseng: chemical and pharmacological diversity. Phytochemistry. 2011;72:689–699. doi: 10.1016/j.phytochem.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y., Choi H.K., Brinckmann J.A., Jiang X., Huang L. Chemical analysis of Panax quinquefolius (North American ginseng): a review. J Chromatogr A. 2015;1426:1–15. doi: 10.1016/j.chroma.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 11.Qi L.W., Wang C.Z., Yuan C.S. Isolation and analysis of ginseng: advances and challenges. Nat Prod Rep. 2011;28:467–495. doi: 10.1039/c0np00057d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang S., Chen R., Wu H., Wang C. Ginsenoside extraction from Panax quinquefolium L. (American ginseng) root by using ultrahigh pressure. J Pharm Biomed Anal. 2006;41:57–63. doi: 10.1016/j.jpba.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 13.Dong T.T., Cui X.M., Song Z.H., Zhao K.J., Ji Z.N., Lo C.K., Tsim K.W. Chemical assessment of roots of Panax notoginseng in China: regional and seasonal variations in its active constituents. J Agric Food Chem. 2003;51:4617–4623. doi: 10.1021/jf034229k. [DOI] [PubMed] [Google Scholar]
- 14.Kim J.S. Investigation of phenolic, flavonoid, and vitamin contents in different parts of Korean Ginseng (Panax ginseng C.A. Meyer) Prev Nutr Food Sci. 2016;21:263–270. doi: 10.3746/pnf.2016.21.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J.R., Yau L.F., Gao W.N., Liu Y., Yick P.W., Liu L., Jiang Z.H. Quantitative comparison and metabolite profiling of saponins in different parts of the root of Panax notoginseng. J Agric Food Chem. 2014;62:9024–9034. doi: 10.1021/jf502214x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yeo C.R., Yong J.J., Popovich D.G. Isolation and characterization of bioactive polyacetylenes Panax ginseng Meyer roots. J Pharm Biomed Anal. 2017;139:148–155. doi: 10.1016/j.jpba.2017.02.054. [DOI] [PubMed] [Google Scholar]
- 17.Yang Y., Chen L., Zhang X.X., Guo Z. Microwave assisted extraction of major active ingredients in Panax quinquefolium L. J Liq Chromatogr R T. 2009;27:3203–3211. [Google Scholar]
- 18.Wang Y., You J., Yu Y., Qu C., Zhang H., Ding L., Zhang H., Li X. Analysis of ginsenosides in Panax ginseng in high pressure microwave-assisted extraction. Food Chem. 2008;110:161–167. doi: 10.1016/j.foodchem.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 19.Wan J.B., Lai C.M., Li S.P., Lee M.Y., Kong L.Y., Wang Y.T. Simultaneous determination of nine saponins from Panax notoginseng using HPLC and pressurized liquid extraction. J Pharm Biomed Anal. 2006;41:274–279. doi: 10.1016/j.jpba.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 20.Lee H.S., Lee H.J., Yu H.J., Ju do W., Kim Y., Kim C.T., Kim C.J., Cho Y.J., Kim N., Choi S.Y. A comparison between high hydrostatic pressure extraction and heat extraction of ginsenosides from ginseng (Panax ginseng CA Meyer) J Sci Food Agric. 2011;91:1466–1473. doi: 10.1002/jsfa.4334. [DOI] [PubMed] [Google Scholar]
- 21.Shin J.S., Ahn S.C., Choi S.W., Lee D.U., Kim B.Y., Baik M.Y. Ultra high pressure extraction (UHPE) of ginsenosides from Korean Panax ginseng powder. Food Sci Biotechno. 2010;19:743–748. [Google Scholar]
- 22.Hou J., He S., Ling M., Li W., Dong R., Pan Y., Zheng Y. A method of extracting ginsenosides from Panax ginseng by pulsed electric field. J Sep Sci. 2010;33:2707–2713. doi: 10.1002/jssc.201000033. [DOI] [PubMed] [Google Scholar]
- 23.Shi X., Jin Y., Liu J., Zhou H., Wei W., Zhang H., Li X. Matrix solid phase dispersion extraction of ginsenosides in the leaves of Panax ginseng C. M. Mey. Food Chem. 2011;129:1253–1257. doi: 10.1016/j.foodchem.2011.04.067. [DOI] [PubMed] [Google Scholar]
- 24.Vanhaelen-Fastre R.J., Faes M.L., Vanhaelen M.H. High-performance thin-layer chromatographic determination of six major ginsenosides in Panax ginseng. J Chromatogr A. 2000;868:269–276. doi: 10.1016/s0021-9673(99)01253-4. [DOI] [PubMed] [Google Scholar]
- 25.Cheong K.L., Wu D.T., Hu D.J., Zhao J., Cao K.Y., Qiao C.F., Han B.X., Li S.P. Comparison and characterization of the glycome of Panax species by high-performance thin-layer chromatography. J Planar Chromatogr. 2014;27:449–453. [Google Scholar]
- 26.Lee T.M., Der Marderosian A. Two-dimensional TLC analysis of ginsenosides from root of dwarf ginseng (Panax trifolius L.) Araliaceae. J Pharm Sci. 1981;70:89–91. doi: 10.1002/jps.2600700119. [DOI] [PubMed] [Google Scholar]
- 27.Kasote D., Ahmad A., Chen W., Combrinck S., Viljoen A. HPTLC-MS as an efficient hyphenated technique for the rapid identification of antimicrobial compounds from propolis. Phytochem Lett. 2015;11:326–331. [Google Scholar]
- 28.Tuzimski T. Two-dimensional TLC with adsorbent gradients of the type silica-octadecyl silica and silica-cyanopropyl for separation of mixtures of pesticides. J Planar Chromatogr. 2005;18:349–357. [Google Scholar]
- 29.Cho I.H., Lee H.J., Kim Y.S. Differences in the volatile compositions of ginseng species (Panax sp.) J Agric Food Chem. 2012;60:7616–7622. doi: 10.1021/jf301835v. [DOI] [PubMed] [Google Scholar]
- 30.Bombardelli E.B.A., Gabetta B., Martinelli E.M. Gas-liquid chromatographic method for determination of ginsenosides in Panax ginseng. J Chromatogr A. 1980;196:121–132. [Google Scholar]
- 31.Jung M.Y., Jeon B.S., Bock J.Y. Free, esterified, and insoluble-bound phenolic acids in white and red Korean ginsengs (Panax ginseng C.A. Meyer) Food Chem. 2002;79:105–111. [Google Scholar]
- 32.Xie G.X., Qiu Y.P., Qiu M.F., Gao X.F., Liu Y.M., Jia W. Analysis of dencichine in Panax notoginseng by gas chromatography-mass spectrometry with ethyl chloroformate derivatization. J Pharm Biomed Anal. 2007;43:920–925. doi: 10.1016/j.jpba.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 33.Koh H.L., Lau A.J., Chan E.C. Hydrophilic interaction liquid chromatography with tandem mass spectrometry for the determination of underivatized dencichine (beta-N-oxalyl-L-alpha,beta-diaminopropionic acid) in Panax medicinal plant species. Rapid Commun Mass Spectrom. 2005;19:1237–1244. doi: 10.1002/rcm.1928. [DOI] [PubMed] [Google Scholar]
- 34.Mao Q., Yang J., Cui X.M., Li J.J., Qi Y.T., Zhang P.H., Wang Q. Target separation of a new anti-tumor saponin and metabolic profiling of leaves of Panax notoginseng by liquid chromatography with eletrospray ionization quadrupole time-of-flight mass spectrometry. J Pharm Biomed Anal. 2012;59:67–77. doi: 10.1016/j.jpba.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 35.Lee D.Y., Kim J.K., Shrestha S., Seo K.H., Lee Y.H., Noh H.J., Kim G.S., Kim Y.B., Kim S.Y., Baek N.I. Quality evaluation of Panax ginseng roots using a rapid resolution LC-QTOF/MS-based metabolomics approach. Molecules. 2013;18:14849–14861. doi: 10.3390/molecules181214849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mao Q., Bai M., Xu J.D., Kong M., Zhu L.Y., Zhu H., Wang Q., Li S.L. Discrimination of leaves of Panax ginseng and P. quinquefolius by ultra high performance liquid chromatography quadrupole/time-of-flight mass spectrometry based metabolomics approach. J Pharm Biomed Anal. 2014;97:129–140. doi: 10.1016/j.jpba.2014.04.032. [DOI] [PubMed] [Google Scholar]
- 37.Washida D., Kitanaka S. Determination of polyacetylenes and ginsenosides in Panax species using high performance liquid chromatography. Chem Pharm Bull. 2003;51:1314–1317. doi: 10.1248/cpb.51.1314. [DOI] [PubMed] [Google Scholar]
- 38.Chung I.M., Lim J.J., Ahn M.S., Jeong H.N., An T.J., Kim S.H. Comparative phenolic compound profiles and antioxidative activity of the fruit, leaves, and roots of Korean ginseng (Panax ginseng Meyer) according to cultivation years. J Ginseng Res. 2016;40:68–75. doi: 10.1016/j.jgr.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee D.G., Lee J., Kim K.T., Lee S.W., Kim Y.O., Cho I.H., Kim H.J., Park C.G., Lee S. High-performance liquid chromatography analysis of phytosterols in Panax ginseng root grown under different conditions. J Ginseng Res. 2018;42:16–20. doi: 10.1016/j.jgr.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bai H., Wang S., Liu J., Gao D., Jiang Y., Liu H., Cai Z. Localization of ginsenosides in Panax ginseng with different age by matrix-assisted laser-desorption/ionization time-of-flight mass spectrometry imaging. J Chromatogr B Analyt Technol Biomed Life Sci. 2016;1026:263–271. doi: 10.1016/j.jchromb.2015.09.024. [DOI] [PubMed] [Google Scholar]
- 41.Soldati F., Sticher O. HPLC separation and quantitative determination of ginsenosides from Panax ginseng, Panax quinquefolium and from ginseng drug preparations. 2nd communication. Planta Med. 1980;39:348–357. doi: 10.1055/s-2008-1074929. [DOI] [PubMed] [Google Scholar]
- 42.Zhang K., Wang X., Ding L., Li J., Qu C.L., Chen L.G., Jin H.Y., Mang H.Q. Determination of seven major ginsenosides in different parts of Panax quinquefolius L.(American Ginseng) with different ages. Chem Res Chinese U. 2008;24:707–711. [Google Scholar]
- 43.Gafner S., Bergeron C., McCollom M.M., Cooper L.M., Mcphail K.L., Gerwick W.H., Angerhofer C.K. Evaluation of the efficiency of three different solvent systems to extract triterpene saponins from roots of Panax quinquefolius using high-performance liquid chromatography. J Agr Food Chem. 2004;52:1546–1550. doi: 10.1021/jf0307503. [DOI] [PubMed] [Google Scholar]
- 44.Lau A.J., Woo S.O., Koh H.L. Analysis of saponins in raw and steamed Panax notoginseng using high-performance liquid chromatography with diode array detection. J Chromatogr A. 2003;1011:77–87. doi: 10.1016/s0021-9673(03)01135-x. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y.H., Hong C.Y., Chen C.F., Tsai T.H. Determination of triacylglycerols in Panax Pseudo-ginseng by HPLC polymeric column. J Liq Chromatogr R T. 2006;19:2497–2503. [Google Scholar]
- 46.Qian Z.M., Wan J.B., Zhang Q.W., Li S.P. Simultaneous determination of nucleobases, nucleosides and saponins in Panax notoginseng using multiple columns high performance liquid chromatography. J Pharm Biomed Anal. 2008;48:1361–1367. doi: 10.1016/j.jpba.2008.09.038. [DOI] [PubMed] [Google Scholar]
- 47.Lee S.I., Kwon H.J., Lee Y.M., Lee J.H., Hong S.P. Simultaneous analysis method for polar and non-polar ginsenosides in red ginseng by reversed-phase HPLC-PAD. J Pharm Biomed Anal. 2012;60:80–85. doi: 10.1016/j.jpba.2011.08.030. [DOI] [PubMed] [Google Scholar]
- 48.Du X.W., Wills R.B.H., Stuart D.L. Changes in neutral and malonyl ginsenosides in American ginseng (Panax quinquefolium) during drying, storage and ethanolic extraction. Food Chem. 2004;86:155–159. [Google Scholar]
- 49.Li W., Fitzloff J.F. HPLC analysis of ginsenosides in the roots of Asian ginseng (Panax ginseng) and North American ginseng (Panax quinquefolius) with in-line photodiode array and evaporative light scattering detection. J Liq Chromatogr R T. 2006;25:29–41. [Google Scholar]
- 50.Ma X., Xiao H., Liang X. Identification of ginsenosides in Panax quinquefolium by LC-MS. Chromatographia. 2006;64:31–36. [Google Scholar]
- 51.Leung K.S.Y., Chan K., Bensoussan A., Munroe M.J. Application of atmospheric pressure chemical ionisation mass spectrometry in the identification and differentiation of Panax Species. Phytochem Analysis. 2007;18:146–150. doi: 10.1002/pca.962. [DOI] [PubMed] [Google Scholar]
- 52.Li L., Tsao R., Dou J., Song F., Liu Z., Liu S. Detection of saponins in extract of Panax notoginseng by liquid chromatography-electrospray ionisation-mass spectrometry. Anal Chim Acta. 2005;536:21–28. [Google Scholar]
- 53.Chan E.C., Yap S.L., Lau A.J., Leow P.C., Toh D.F., Koh H.L. Ultra-performance liquid chromatography/time-of-flight mass spectrometry based metabolomics of raw and steamed Panax notoginseng. Rapid Commun Mass Spectrom. 2007;21:519–528. doi: 10.1002/rcm.2864. [DOI] [PubMed] [Google Scholar]
- 54.Lai C.J., Tan T., Zeng S.L., Qi L.W., Liu X.G., Dong X., Li P., Liu E.H. An integrated high resolution mass spectrometric data acquisition method for rapid screening of saponins in Panax notoginseng (Sanqi) J Pharm Biomed Anal. 2015;109:184–191. doi: 10.1016/j.jpba.2015.02.028. [DOI] [PubMed] [Google Scholar]
- 55.Qiu S., Yang W.Z., Shi X.J., Yao C.L., Yang M., Liu X., Jiang B.H., Wu W.Y., Guo D.A. A green protocol for efficient discovery of novel natural compounds: characterization of new ginsenosides from the stems and leaves of Panax ginseng as a case study. Anal Chim Acta. 2015;893:65–76. doi: 10.1016/j.aca.2015.08.048. [DOI] [PubMed] [Google Scholar]
- 56.Xing Q., Liang T., Shen G., Wang X., Jin Y., Liang X. Comprehensive HILIC x RPLC with mass spectrometry detection for the analysis of saponins in Panax notoginseng. Analyst. 2012;137:2239–2249. doi: 10.1039/c2an16078a. [DOI] [PubMed] [Google Scholar]
- 57.Lelu J.K., Liu Q., Alolga R.N., Fan Y., Xiao W.L., Qi L.W., Li P. A new two-dimensional chromatographic method for separation of saponins from steamed Panax notoginseng. J Pharm Biomed Anal. 2016;125:355–359. doi: 10.1016/j.jpba.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 58.Liu W., He Y., Li L., Liu S. Fast quantitative analysis of ginsenosides in Asian ginseng (Panax ginseng C. A. Mayer) by using solid-phase methylation coupled to direct analysis in real time. Rapid Commun Mass Spectrom. 2016;30(Suppl 1):111–115. doi: 10.1002/rcm.7627. [DOI] [PubMed] [Google Scholar]
- 59.Wang Y., Li C., Huang L., Liu L., Guo Y., Ma L., Liu S. Rapid identification of traditional Chinese herbal medicine by direct analysis in real time (DART) mass spectrometry. Anal Chim Acta. 2014;845:70–76. doi: 10.1016/j.aca.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 60.Zeng S., Wang L., Chen T., Qu H. On-line coupling of macroporous resin column chromatography with direct analysis in real time mass spectrometry utilizing a surface flowing mode sample holder. Anal Chim Acta. 2014;811:43–50. doi: 10.1016/j.aca.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 61.Qi X., Ignatova S., Luo G., Liang Q., Jun F.W., Wang Y., Sutherland I. Preparative isolation and purification of ginsenosides Rf, Re, Rd and Rb1 from the roots of Panax ginseng with a salt/containing solvent system and flow step-gradient by high performance counter-current chromatography coupled with an evaporative light scattering detector. J Chromatogr A. 2010;1217:1995–2001. doi: 10.1016/j.chroma.2010.01.057. [DOI] [PubMed] [Google Scholar]
- 62.Ha Y.W., Lim S.S., Ha I.J., Na Y.C., Seo J.J., Shin H., Son S.H., Kim Y.S. Preparative isolation of four ginsenosides from Korean red ginseng (steam-treated Panax ginseng C. A. Meyer), by high-speed counter-current chromatography coupled with evaporative light scattering detection. J Chromatogr A. 2007;1151:37–44. doi: 10.1016/j.chroma.2007.01.038. [DOI] [PubMed] [Google Scholar]
- 63.Glockl I., Veit M., Blaschke G. Determination of ginsenosides from Panax ginseng using micellar electrokinetic chromatography. Planta Medica. 2002;68:158–161. doi: 10.1055/s-2002-20241. [DOI] [PubMed] [Google Scholar]
- 64.Ji W., Xie H., Zhou J., Wang X., Ma X., Huang L. Water-compatible molecularly imprinted polymers for selective solid phase extraction of dencichine from the aqueous extract of Panax notoginseng. J Chromatogr B Analyt Technol Biomed Life Sci. 2016;1008:225–233. doi: 10.1016/j.jchromb.2015.11.053. [DOI] [PubMed] [Google Scholar]
- 65.Nguyen H.T., Lee D.K., Choi Y.G., Min J.E., Yoon S.J., Yu Y.H., Lim J., Lee J., Kwon S.W., Park J.H. A 1H NMR-based metabolomics approach to evaluate the geographical authenticity of herbal medicine and its application in building a model effectively assessing the mixing proportion of intentional admixtures: a case study of Panax ginseng: metabolomics for the authenticity of herbal medicine. J Pharm Biomed Anal. 2016;124:120–128. doi: 10.1016/j.jpba.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 66.Wang Y.S., Jin Y.P., Gao W., Xiao S.Y., Zhang Y.W., Zheng P.H., Wang J., Liu J.X., Sun C.H., Wang Y.P. Complete 1H-NMR and 13C-NMR spectral assignment of five malonyl ginsenosides from the fresh flower buds of Panax ginseng. J Ginseng Res. 2016;40:245–250. doi: 10.1016/j.jgr.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Joo K.M., Park C.W., Jeong H.J., Lee S.J., Chang I.S. Simultaneous determination of two Amadori compounds in Korean red ginseng (Panax ginseng) extracts and rat plasma by high-performance anion-exchange chromatography with pulsed amperometric detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;865:159–166. doi: 10.1016/j.jchromb.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 68.Yoon S.R., Nah J.J., Kim S.K., Kim S.C., Nam K.Y., Jung D.W., Nah S.Y. Determination of ginsenoside Rf and Rg2 from Panax ginseng using enzyme immunoassy. Chem Pharm Bull. 1997;46:1144–1147. doi: 10.1248/cpb.46.1144. [DOI] [PubMed] [Google Scholar]
- 69.Qiao C.F., Liu X.M., Cui X.M., Hu D.J., Chen Y.W., Zhao J., Li S.P. High-performance anion-exchange chromatography coupled with diode array detection for the determination of dencichine in Panax notoginseng and related species. J Sep Sci. 2013;36:2401–2406. doi: 10.1002/jssc.201300334. [DOI] [PubMed] [Google Scholar]
- 70.Yang W.Z., Bo T., Ji S., Qiao X., Guo D.A., Ye M. Rapid chemical profiling of saponins in the flower buds of Panax notoginseng by integrating MCI gel column chromatography and liquid chromatography/mass spectrometry analysis. Food Chem. 2013;139:762–769. doi: 10.1016/j.foodchem.2013.01.051. [DOI] [PubMed] [Google Scholar]
- 71.Zhu J., Fan X., Cheng Y., Agarwal R., Moore C.M., Chen S.T., Tong W. Chemometric analysis for identification of botanical raw materials for pharmaceutical use: a case study using Panax notoginseng. PLoS One. 2014;9 doi: 10.1371/journal.pone.0087462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Toh D.F., New L.S., Koh H.L., Chan E.C. Ultra-high performance liquid chromatography/time-of-flight mass spectrometry (UHPLC/TOFMS) for time-dependent profiling of raw and steamed Panax notoginseng. J Pharm Biomed Anal. 2010;52:43–50. doi: 10.1016/j.jpba.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 73.Lee S.A., Jo H.K., Im B.O., Kim S., Whang W.K., Ko S.K. Changes in the contents of prosapogenin in the red ginseng (Panax ginseng) depending on steaming batches. J Ginseng Res. 2012;36:102–106. doi: 10.5142/jgr.2012.36.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sun B.S., Xu M.Y., Li Z., Wang Y.B., Sung C.K. UPLC-Q-TOF-MS/MS analysis for steaming times-dependent profiling of steamed Panax quinquefolius and its ginsenosides transformations induced by repetitious steaming. J Ginseng Res. 2012;36:277–290. doi: 10.5142/jgr.2012.36.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sun B.S., Pan F.Y., Sung C.K. Repetitious steaming-induced chemical transformations and global quality of black ginseng derived from Panax ginseng by HPLC-ESI-MS/MSn based chemical profiling approach. Biotechnol Bioproc E. 2011;16:956–965. [Google Scholar]
- 76.Abd El-Aty A.M., Kim I.K., Kim M.R., Lee C., Shim J.H. Determination of volatile organic compounds generated from fresh, white and red Panax ginseng (C. A. Meyer) using a direct sample injection technique. Biomed Chromatogr. 2008;22:556–562. doi: 10.1002/bmc.969. [DOI] [PubMed] [Google Scholar]
- 77.Kim S.N., Ha Y.W., Shin H., Son S.H., Wu S.J., Kim Y.S. Simultaneous quantification of 14 ginsenosides in Panax ginseng C.A. Meyer (Korean red ginseng) by HPLC-ELSD and its application to quality control. J Pharm Biomed Anal. 2007;45:164–170. doi: 10.1016/j.jpba.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 78.Sun B.S., Gu L.J., Fang Z.M., Wang C.Y., Wang Z., Lee M.R., Li Z., Li J.J., Sung C.K. Simultaneous quantification of 19 ginsenosides in black ginseng developed from Panax ginseng by HPLC-ELSD. J Pharm Biomed Anal. 2009;50:15–22. doi: 10.1016/j.jpba.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 79.Sun S., Wang C.Z., Tong R., Li X.L., Fishbein A., Wang Q., He T.C., Du W., Yuan C.S. Effects of steaming the root of Panax notoginseng on chemical composition and anticancer activities. Food Chem. 2010;118:307–314. [Google Scholar]
- 80.Zhang Y.C., Pi Z.F., Liu C.M., Song F.R., Liu Z.Q., Liu S.Y. Analysis of low-polar ginsenosides in steamed Panax ginseng at high-temperature by HPLC-ESI-MS/MS. Chem Res Chinese U. 2012;28:31–36. [Google Scholar]
- 81.Huang X., Liu Y., Zhang Y., Li S.P., Yue H., Chen C.B., Liu S.Y. Multicomponent assessment and ginsenoside conversions of Panax quinquefolium L. roots before and after steaming by HPLC-MS(n) J Ginseng Res. 2019;43:27–37. doi: 10.1016/j.jgr.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Castro-Aceituno V., Ahn S., Simu S.Y., Singh P., Mathiyalagan R., Lee H.A., Yang D.C. Anticancer activity of silver nanoparticles from Panax ginseng fresh leaves in human cancer cells. Biomed Pharmacother. 2016;84:158–165. doi: 10.1016/j.biopha.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 83.Park K.S., Ko S.K., Chung S.H. Comparisons of antidiabetic effect between ginseng radix alba, ginseng radix rubra and Panax quinquefolium radix in MLD STZ-induced diabetic rats. J Ginseng Res. 2003;27:56–61. [Google Scholar]
- 84.Darshan S., Doreswamy R. Patented antiinflammatory plant drug development from traditional medicine. Phytother Res. 2004;18:343–357. doi: 10.1002/ptr.1475. [DOI] [PubMed] [Google Scholar]
- 85.Chen Z., Lu T., Yue X., Wei N., Jiang Y., Chen M., Ni G., Liu X., Xu G. Neuroprotective effect of ginsenoside Rb1 on glutamate-induced neurotoxicity: with emphasis on autophagy. Neurosci Lett. 2010;482:264–268. doi: 10.1016/j.neulet.2010.07.052. [DOI] [PubMed] [Google Scholar]
- 86.Ng T.B. Pharmacological activity of sanchi ginseng (Panax notoginseng) J Pharm Pharmacol. 2006;58:1007–1019. doi: 10.1211/jpp.58.8.0001. [DOI] [PubMed] [Google Scholar]
- 87.Yang W.Z., Ye M., Qiao X., Liu C.F., Miao W.J., Bo T., Tao H.Y., Guo D.A. A strategy for efficient discovery of new natural compounds by integrating orthogonal column chromatography and liquid chromatography/mass spectrometry analysis: its application in Panax ginseng, Panax quinquefolium and Panax notoginseng to characterize 437 potential new ginsenosides. Anal Chim Acta. 2012;739:56–66. doi: 10.1016/j.aca.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 88.Chan T.W.D., But P.P.H., Cheng S.W., Kwok I.M.Y., Lau F.W., Xu H.X. Differentiation and authentication of Panax ginseng, Panax quinquefolius, and ginseng products by using HPLC/MS. Anal Chem. 2000;72:1281–1287. doi: 10.1021/ac990819z. [DOI] [PubMed] [Google Scholar]
- 89.Li W., Gu C., Zhang H., Awang D.V.C., Fitzloff J.F., Fong H.H.S., Breemen R.B.V. Use of high-performance liquid chromatography-tandem mass spectrometry to distinguish Panax ginseng. Anal Chem. 2000;72:5417–5422. doi: 10.1021/ac000650l. [DOI] [PubMed] [Google Scholar]
- 90.Li W., Fitzloff J.F. HPLC with evaporative light scattering detection as a tool to distinguish Asian ginseng (Panax ginseng) and North American ginseng (Panax quinquefolius) J Liq Chromatogr R T. 2006;25:17–27. [Google Scholar]
- 91.Yang W., Qiao X., Li K., Fan J., Bo T., Guo D.A., Ye M. Identification and differentiation of Panax ginseng, Panax quinquefolium, and Panax notoginseng by monitoring multiple diagnostic chemical markers. Acta Pharm Sin B. 2016;6:568–575. doi: 10.1016/j.apsb.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang T., Guo R., Zhou G., Zhou X., Kou Z., Sui F., Li C., Tang L., Wang Z. Traditional uses, botany, phytochemistry, pharmacology and toxicology of Panax notoginseng (Burk.) F.H. Chen: a review. J Ethnopharmacol. 2016;188:234–258. doi: 10.1016/j.jep.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 93.Huang X., Liu Y., Zhang N., Sun X., Yue H., Chen C., Liu S. UPLC Orbitrap HRMS Analysis of Panax quinquefolium L. for authentication of Panax genus with chemometric methods. J Chromatogr Sci. 2018;56:25–35. doi: 10.1093/chromsci/bmx077. [DOI] [PubMed] [Google Scholar]
- 94.Wan J.B., Li S.P., Chen J.M., Wang Y.T. Chemical characteristics of three medicinal plants of the Panax genus determined by HPLC-ELSD. J Sep Sci. 2007;30:825–832. doi: 10.1002/jssc.200600359. [DOI] [PubMed] [Google Scholar]
- 95.Gurung B., Bhardwaj P.K., Rai A.K., Sahoo D. Major ginsenoside contents in rhizomes of Panax sokpayensis and Panax bipinnatifidus. Nat Prod Res. 2018;32:234–238. doi: 10.1080/14786419.2017.1343322. [DOI] [PubMed] [Google Scholar]
- 96.Yunusova N., Kim J.Y., Lee G.J., Hong J.Y., Shin B.K., Cai S.Q., Piao X.L., Park J.H., Kwon S.W. Comparison of ginsenosides in radix and rhizome of wild Panax species using LC-ELSD and LC-Q-TOF-MS. Int J Food Sci Tech. 2015;50:1607–1614. [Google Scholar]
- 97.Xia P., Bai Z., Liang T., Yang D., Liang Z., Yan X., Liu Y. High-performance liquid chromatography based chemical fingerprint analysis and chemometric approaches for the identification and distinction of three endangered Panax plants in Southeast Asia. J Sep Sci. 2016;39:3880–3888. doi: 10.1002/jssc.201600460. [DOI] [PubMed] [Google Scholar]
- 98.Xie G., Plumb R., Su M., Xu Z., Zhao A., Qiu M., Long X., Liu Z., Jia W. Ultra-performance LC/TOF MS analysis of medicinal Panax herbs for metabolomic research. J Sep Sci. 2008;31:1015–1026. doi: 10.1002/jssc.200700650. [DOI] [PubMed] [Google Scholar]
- 99.Park H.W., In G., Kim J.H., Cho B.G., Han G.H., Chang I.M. Metabolomic approach for discrimination of processed ginseng genus (Panax ginseng and Panax quinquefolius) using UPLC-QTOF MS. J Ginseng Res. 2014;38:59–65. doi: 10.1016/j.jgr.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yang S.O., Lee S.W., Kim Y.O., Sohn S.H., Kim Y.C., Hyun D.Y., Hong Y.P., Shin Y.S. HPLC-based metabolic profiling and quality control of leaves of different Panax species. J Ginseng Res. 2013;37:248–253. doi: 10.5142/jgr.2013.37.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee S.M., Lee H.B., Lee C.G. A convenience UPLC/PDA method for the quantitative analysis of panaxfuraynes A and B from Panax ginseng. Food Chem. 2010;123:955–958. [Google Scholar]
- 102.Shi W., Wang Y., Li J., Zhang H., Ding L. Investigation of ginsenosides in different parts and ages of Panax ginseng. Food Chem. 2007;102:664–668. [Google Scholar]
- 103.Liang Z., Chen Y., Xu L., Qin M., Yi T., Chen H., Zhao Z. Localization of ginsenosides in the rhizome and root of Panax ginseng by laser microdissection and liquid chromatography-quadrupole/time of flight-mass spectrometry. J Pharm Biomed Anal. 2015;105:121–133. doi: 10.1016/j.jpba.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 104.Qu C., Bai Y., Jin X., Wang Y., Zhang K., You J., Zhang H. Study on ginsenosides in different parts and ages of Panax quinquefolius L. Food Chem. 2009;115:340–346. [Google Scholar]
- 105.Zhang X., Ma X., Si B., Zhao Y. Simultaneous determination of five active hydrolysis ingredients from Panax quinquefolium L. by HPLC-ELSD. Biomed Chromatogr. 2011;25:646–651. doi: 10.1002/bmc.1495. [DOI] [PubMed] [Google Scholar]
- 106.Liu J., Liu Y., Wang Y., Abozeid A., Zu Y.G., Tang Z.H. The integration of GC-MS and LC-MS to assay the metabolomics profiling in Panax ginseng and Panax quinquefolius reveals a tissue- and species-specific connectivity of primary metabolites and ginsenosides accumulation. J Pharm Biomed Anal. 2017;135:176–185. doi: 10.1016/j.jpba.2016.12.026. [DOI] [PubMed] [Google Scholar]
- 107.Jia X.H., Wang C.Q., Liu J.H., Li X.W., Wang X., Shang M.Y., Cai S.Q., Zhu S., Komatsu K. Comparative studies of saponins in 1-3-year-old main roots, fibrous roots, and rhizomes of Panax notoginseng, and identification of different parts and growth-year samples. J Nat Med. 2013;67:339–349. doi: 10.1007/s11418-012-0691-6. [DOI] [PubMed] [Google Scholar]
- 108.Wan J.B., Yang F.Q., Li S.P., Wang Y.T., Cui X.M. Chemical characteristics for different parts of Panax notoginseng using pressurized liquid extraction and HPLC-ELSD. J Pharm Biomed Anal. 2006;41:1596–1601. doi: 10.1016/j.jpba.2006.01.058. [DOI] [PubMed] [Google Scholar]
- 109.Wang Z., Chen Y.Y., Pan H.J., Wei L., Wang Y.H., Zeng C.H. Saponin accumulation in flower buds of Panax notoginseng. Chinese Herbal Medicines. 2015;7:179–184. [Google Scholar]
- 110.Wang C.Z., Ni M., Sun S., Li X.L., He H., Mehendale S.R., Yuan C.S. Detection of adulteration of notoginseng root extract with other panax species by quantitative HPLC coupled with PCA. J Agric Food Chem. 2009;57:2363–2367. doi: 10.1021/jf803320d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wan J.B., Zhang Q.W., Hong S.J., Li P., Li S.P., Wang Y.T. Chemical investigation of saponins in different parts of Panax notoginseng by pressurized liquid extraction and liquid chromatography-electrospray ionization-tandem mass spectrometry. Molecules. 2012;17:5836–5853. doi: 10.3390/molecules17055836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dan M., Su M., Gao X., Zhao T., Zhao A., Xie G., Qiu Y., Zhou M., Liu Z., Jia W. Metabolite profiling of Panax notoginseng using UPLC-ESI-MS. Phytochemistry. 2008;69:2237–2244. doi: 10.1016/j.phytochem.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 113.Chen J., Xie M., Fu Z., Lee F.S.C., Wang X. Development of a quality evaluation system for Panax quinquefolium. L based on HPLC chromatographic fingerprinting of seven major ginsenosides. Microchem J. 2007;85:201–208. [Google Scholar]
- 114.Lim W., Mudge K.W., Vermeylen F. Effects of population, age, and cultivation methods on ginsenoside content of wild American ginseng (Panax quinquefolium) J Agric Food Chem. 2005;53:8498–8505. doi: 10.1021/jf051070y. [DOI] [PubMed] [Google Scholar]
- 115.Yang Z., Zhu J., Zhang H., Fan X. Investigating chemical features of Panax notoginseng based on integrating HPLC fingerprinting and determination of multiconstituents by single reference standard. J Ginseng Res. 2018;42:334–342. doi: 10.1016/j.jgr.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kim N., Kim K., Lee D., Shin Y.S., Bang K.H., Cha S.W., Lee J.W., Choi H.K., Hwang B.Y., Lee D. Nontargeted metabolomics approach for age differentiation and structure interpretation of age-dependent key constituents in hairy roots of Panax ginseng. J Nat Prod. 2012;75:1777–1784. doi: 10.1021/np300499p. [DOI] [PubMed] [Google Scholar]
- 117.Kim N., Kim K., Choi B.Y., Lee D., Shin Y.S., Bang K.H., Cha S.W., Lee J.W., Choi H.K., Jang D.S. Metabolomic approach for age discrimination of Panax ginseng using UPLC-Q-Tof MS. J Agric Food Chem. 2011;59:10435–10441. doi: 10.1021/jf201718r. [DOI] [PubMed] [Google Scholar]
- 118.Wang Y., Pan J.Y., Xiao X.Y., Lin R.C., Cheng Y.Y. Simultaneous determination of ginsenosides in Panax ginseng with different growth ages using high-performance liquid chromatography-mass spectrometry. Phytochem Analysis. 2006;17:424–430. doi: 10.1002/pca.944. [DOI] [PubMed] [Google Scholar]
- 119.Li L., Sheng Y., Zhang J., Guo D. Determination of four active saponins of Panax notoginseng in rat feces by high-performance liquid chromatography. J Chromatogr Sci. 2005;43:421–425. doi: 10.1093/chromsci/43.8.421. [DOI] [PubMed] [Google Scholar]
- 120.Guo Q., Li P., Wang Z., Cheng Y., Wu H., Yang B., Du S., Lu Y. Brain distribution pharmacokinetics and integrated pharmacokinetics of Panax Notoginsenoside R1, ginsenosides Rg1, Rb1, Re and Rd in rats after intranasal administration of Panax notoginseng saponins assessed by UPLC/MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;969:264–271. doi: 10.1016/j.jchromb.2014.08.034. [DOI] [PubMed] [Google Scholar]
- 121.Lin H., Pi Z., Men L., Chen W., Liu Z., Liu Z. Urinary metabonomic study of Panax ginseng in deficiency of vital energy rat using ultra performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. J Ethnopharmacol. 2016;184:10–17. doi: 10.1016/j.jep.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 122.Dong W.W., Zhao J., Zhong F.L., Zhu W.J., Jiang J., Wu S., Yang D.C., Li D., Quan L.H. Biotransformation of Panax ginseng extract by rat intestinal microflora: identification and quantification of metabolites using liquid chromatography-tandem mass spectrometry. J Ginseng Res. 2017;41:540–547. doi: 10.1016/j.jgr.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Matsuda H., Yamazaki M., Asanuma Y., Kubo M. Promotion of hair growth by ginseng radix on cultured mouse vibrissal hair follicles. Phytother Res. 2003;17:797–800. doi: 10.1002/ptr.1241. [DOI] [PubMed] [Google Scholar]
- 124.Murata K., Takeshita F., Samukawa K., Tani T., Matsuda H. Effects of ginseng rhizome and ginsenoside Ro on testosterone 5α-reductase and hair re-growth in testosterone-treated mice. Phytother Res. 2012;26:48–53. doi: 10.1002/ptr.3511. [DOI] [PubMed] [Google Scholar]
- 125.Assinewe V.A., Baum B.R., Gagnon D., Arnason J.T. Phytochemistry of wild populations of Panax quinquefolius L. (North American ginseng) J Agric Food Chem. 2003;51:4549–4553. doi: 10.1021/jf030042h. [DOI] [PubMed] [Google Scholar]
- 126.Corbit R.M., Ferreira J.F., Ebbs S.D., Murphy L.L. Simplified extraction of ginsenosides from American ginseng (Panax quinquefolius L.) for high-performance liquid chromatography-ultraviolet analysis. J Agric Food Chem. 2005;53:9867–9873. doi: 10.1021/jf051504p. [DOI] [PubMed] [Google Scholar]
- 127.Li L., Zhang J.L., Sheng Y.X., Ye G., Guo H.Z., Guo D.A. Liquid chromatographic method for determination of four active saponins from Panax notoginseng in rat urine using solid-phase extraction. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;808:177–183. doi: 10.1016/j.jchromb.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 128.Wan J.-B., Li P., Li S., Wang Y., Dong T.T.X., Tsim K.W.K. Simultaneous determination of 11 saponins in Panax notoginseng using HPLC-ELSD and pressurized liquid extraction. J Sep Sci. 2006;29:2190–2196. doi: 10.1002/jssc.200600103. [DOI] [PubMed] [Google Scholar]
- 129.Zhang Y., Zhang J., Liu C., Yu M., Li S. Extraction, isolation, and aromatase inhibitory evaluation of low-polar ginsenosides from Panax ginseng leaves. J Chromatogr A. 2017;1483:20–29. doi: 10.1016/j.chroma.2016.12.068. [DOI] [PubMed] [Google Scholar]
- 130.Lee K.S., Kim G.H., Kim H.H., Chang Y.I., Lee G.H. Volatile compounds of Panax ginseng C.A. Meyer cultured with different cultivation methods. J Food Sci. 2012;77:C805–C810. doi: 10.1111/j.1750-3841.2012.02765.x. [DOI] [PubMed] [Google Scholar]
- 131.Yoon S.H., Nam Y.M., Hong J.T., Kim S.J., Ko S.K. Modification of ginsenoside composition in red ginseng (Panax ginseng) by ultrasonication. J Ginseng Res. 2016;40:300–303. doi: 10.1016/j.jgr.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Smith R.G., Caswell D., Carriere A., Zielke B. Variation in the ginsenoside content of American ginseng, Panax quinquefolius L, roots. Can J Bot. 1996;74:1616–1620. [Google Scholar]
- 133.Li L., Sheng Y., Zhang J., Wang C., Guo D. HPLC determination of four active saponins from Panax notoginseng in rat serum and its application to pharmacokinetic studies. Biomed Chromatogr. 2004;18:849–856. doi: 10.1002/bmc.400. [DOI] [PubMed] [Google Scholar]
- 134.Schulten H.R.S.F. Identification of ginsenosides from Panax ginseng in fractions obtained by high-performance liquid chromatography by field desorption mass spectrometry, multiple internal reflection infrared spectroscopy and thin-layer chromatography. J Chromatogr A. 1981;212:37–49. [Google Scholar]
- 135.Du Z., Li J., Zhang X., Pei J., Huang L. An Integrated LC-MS-based strategy for the quality assessment and discrimination of three Panax species. Molecules. 2018;23 doi: 10.3390/molecules23112988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang Y., Liu C., Qi Y., Li S., Wang J. Application of accelerated solvent extraction coupled with counter-current chromatography to extraction and online isolation of saponins with a broad range of polarity from Panax notoginseng. Sep Purif Technol. 2013;106:82–89. [Google Scholar]
- 137.Haibo B., Lixing N., Dan W., Shaoxiong Y., Shan L., Zhiyong G., Xiaojia X., Gangli W., Xiangri L. Rapid determination of Panax ginseng by near-infrared spectroscopy. Anal Methods. 2013;5 [Google Scholar]
- 138.Jiang C., Qu H. A comparative study of using in-line near-infrared spectra, ultraviolet spectra and fused spectra to monitor Panax notoginseng adsorption process. J Pharm Biomed Anal. 2015;102:78–84. doi: 10.1016/j.jpba.2014.08.029. [DOI] [PubMed] [Google Scholar]
- 139.Liu J.H., Lee C.S., Leung K.M., Yan Z.K., Shen B.H., Zhao Z.Z., Jiang Z.H. Quantification of two polyacetylenes in radix ginseng and roots of related Panax species using a gas chromatography-mass spectrometric method. J Agr Food Chem. 2007;55:8830–8835. doi: 10.1021/jf070735o. [DOI] [PubMed] [Google Scholar]
- 140.Chen X.J., Qiu J.F., Wang Y.T., Wan J.B. Discrimination of three Panax species based on differences in volatile organic compounds using a static headspace GC-MS-based metabolomics approach. Am J Chin Med. 2016;44:663–676. doi: 10.1142/S0192415X16500361. [DOI] [PubMed] [Google Scholar]
- 141.Bonfill M., Casals I., Palazon J., Mallol A., Morales C. Improved high performance liquid chromatographic determination of ginsenosides in Panax ginseng-based pharmaceuticals using a diol column. Biomed Chromatogr. 2002;16:68–72. doi: 10.1002/bmc.120. [DOI] [PubMed] [Google Scholar]
- 142.Li C.Y., Lau D.T., Dong T.T., Zhang J., Choi R.C., Wu H.Q., Wang L.Y., Hong R.S., Li S.H., Song X. Dual-index evaluation of character changes in Panax ginseng C. A. Mey stored in different conditions. J Agr Food Chem. 2013;61:6568–6573. doi: 10.1021/jf400456w. [DOI] [PubMed] [Google Scholar]
- 143.Liu Z., Wang C.Z., Zhu X.Y., Wan J.Y., Zhang J., Li W., Ruan C.C., Yuan C.S. Dynamic changes in neutral and acidic ginsenosides with different cultivation ages and harvest seasons: identification of chemical characteristics for Panax ginseng quality control. Molecules. 2017;22 doi: 10.3390/molecules22050734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu Z., Li Y., Li X., Ruan C.C., Wang L.J., Sun G.Z. The effects of dynamic changes of malonyl ginsenosides on evaluation and quality control of Panax ginseng C. A. Meyer. J Pharm Biomed Anal. 2012;64–65:56–63. doi: 10.1016/j.jpba.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 145.Chung I.M., Kim J.W., Seguin P., Jun Y.M., Kim S.H. Ginsenosides and phenolics in fresh and processed Korean ginseng (Panax ginseng C.A. Meyer): effects of cultivation location, year, and storage period. Food Chem. 2012;130:73–83. [Google Scholar]
- 146.Wang A.B., Wang C.Z., Wu J.A., Osinski J., Yuan C.S. Determination of major ginsenosides in Panax quinquefolius (American ginseng) using, high-performance liquid chromatography. Phytochem Analysis. 2005;16:272–277. doi: 10.1002/pca.838. [DOI] [PubMed] [Google Scholar]
- 147.Schlag E.M., McIntosh M.S. Ginsenoside content and variation among and within American ginseng (Panax quinquefolius L.) populations. Phytochemistry. 2006;67:1510–1519. doi: 10.1016/j.phytochem.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 148.Yao H., Li X., Liu Y., Wu Q., Jin Y. An optimized microwave-assisted extraction method for increasing yields of rare ginsenosides from Panax quinquefolius L. J Ginseng Res. 2016;40:415–422. doi: 10.1016/j.jgr.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Li L., Sheng Y.X., Zhang J.L., Wang S.S., Guo D.A. High-performance liquid chromatographic assay for the active saponins from Panax notoginseng in rat tissues. Biomed Chromatogr. 2006;20:327–335. doi: 10.1002/bmc.567. [DOI] [PubMed] [Google Scholar]
- 150.Gao X., Dan M., Zhao A., Xie G., Jia W. Simultaneous determination of saponins in flower buds of Panax notoginseng using high performance liquid chromatography. Biomed Chromatogr. 2008;22:244–249. doi: 10.1002/bmc.915. [DOI] [PubMed] [Google Scholar]
- 151.Liu X., Qiu Z., Wang L., Chen Y. Quality evaluation of Panax notoginseng extract dried by different drying methods. Food Bioprod Proc. 2011;89:10–14. [Google Scholar]
- 152.Wang D., Liao P.Y., Zhu H.T., Chen K.K., Xu M., Zhang Y.J., Yang C.R. The processing of Panax notoginseng and the transformation of its saponin components. Food Chem. 2012;132:1808–1813. [Google Scholar]
- 153.Li S.P., Qiao C.F., Chen Y.W., Zhao J., Cui X.M., Zhang Q.W., Liu X.M., Hu D.J. A novel strategy with standardized reference extract qualification and single compound quantitative evaluation for quality control of Panax notoginseng used as a functional food. J Chromatogr A. 2013;1313:302–307. doi: 10.1016/j.chroma.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 154.Gong X., Chen H., Pan J., Qu H. Optimization of Panax notoginseng extraction process using a design space approach. Sep Purif Technol. 2015;141:197–206. [Google Scholar]
- 155.Xia P., Guo H., Ru M., Yang D., Liang Z., Yan X., Liu Y. Accumulation of saponins in Panax notoginseng during its growing seasons. Ind Crop Prod. 2017;104:287–292. [Google Scholar]
- 156.Dong T.T., Zhao K.J., Huang W.Z., Leung K.W., Tsim K.W. Orthogonal array design in optimizing the extraction efficiency of active constituents from roots of Panax notoginseng. Phytother Res. 2005;19:684–688. doi: 10.1002/ptr.1728. [DOI] [PubMed] [Google Scholar]
- 157.Bae H.J., Chung S.I., Lee S.C., Kang M.Y. Influence of aging process on the bioactive components and antioxidant activity of ginseng (Panax ginseng L.) J Food Sci. 2014;79:H2127–H2131. doi: 10.1111/1750-3841.12640. [DOI] [PubMed] [Google Scholar]
- 158.Du X., Zhao Y., Yang D., Liu Y., Fan K., Liang Z., Han R. A correlation model of UPLC fingerprints and anticoagulant activity for quality assessment of Panax notoginseng by hierarchical clustering analysis and multiple linear regression analysis. Anal Methods. 2015;7:2985–2992. [Google Scholar]
- 159.Shan S.M., Luo J.G., Huang F., Kong L.Y. Chemical characteristics combined with bioactivity for comprehensive evaluation of Panax ginseng C.A. Meyer in different ages and seasons based on HPLC-DAD and chemometric methods. J Pharm Biomed Anal. 2014;89:76–82. doi: 10.1016/j.jpba.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 160.Wang Y.H., Hong C.Y., Chen C.F., Tsai T.H. Reversed-phase high-performance liquid chromatography determination of ginsenosides of Panax quinquefolium. J Liq Chromatogr Re T. 1996;19:2497–2503. [Google Scholar]
- 161.Christensen L.P., Jensen M., Kidmose U. Simultaneous determination of ginsenosides and polyacetylenes in American ginseng root (Panax quinquefolium L.) by high-performance liquid chromatography. J Agr Food Chem. 2006;54:8995–9003. doi: 10.1021/jf062068p. [DOI] [PubMed] [Google Scholar]
- 162.Lau A.J., Seo B.H., Woo S.O., Koh H.L. High-performance liquid chromatographic method with quantitative comparisons of whole chromatograms of raw and steamed Panax notoginseng. J Chromatogr A. 2004;1057:141–149. doi: 10.1016/j.chroma.2004.09.069. [DOI] [PubMed] [Google Scholar]
- 163.Chen T., Gong X., Chen H., Qu H. Process development for the decoloration of Panax notoginseng extracts: a design space approach. J Sep Sci. 2015;38:346–355. doi: 10.1002/jssc.201400808. [DOI] [PubMed] [Google Scholar]
- 164.Zhang H.Z., Liu D.H., Zhang D.K., Wang Y.H., Li G., Yan G.L., Cao L.J., Xiao X.H., Huang L.Q., Wang J.B. Quality assessment of Panax notoginseng from different regions through the analysis of marker chemicals, biological potency and ecological factors. PLoS One. 2016;11 doi: 10.1371/journal.pone.0164384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Guan J., Lai C.M., Li S.P. A rapid method for the simultaneous determination of 11 saponins in Panax notoginseng using ultra performance liquid chromatography. J Pharm Biomed Anal. 2007;44:996–1000. doi: 10.1016/j.jpba.2007.03.032. [DOI] [PubMed] [Google Scholar]
- 166.Chen T., Gong X., Chen H., Zhang Y., Qu H. Chromatographic elution process design space development for the purification of saponins in Panax notoginseng extract using a probability-based approach. J Sep Sci. 2016;39:306–315. doi: 10.1002/jssc.201500976. [DOI] [PubMed] [Google Scholar]
- 167.Xie R.F., Yang B.R., Cheng P.P., Wu S., Li Z.C., Tang J.Y., Li S., Tang N., Lee S.M.Y., Wang Y.H. Study on the HPLC chromatograms and pro-angiogenesis activities of the flowers of Panax notoginseng. J Liq Chromatogr R T. 2015;38:1286–1295. [Google Scholar]
- 168.Kim D., Kim M., Rana G.S., Han J. Seasonal variation and possible biosynthetic pathway of ginsenosides in Korean Ginseng Panax ginseng Meyer. Molecules. 2018;23 doi: 10.3390/molecules23071824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lee D.Y., Cho J.G., Lee M.K., Lee J.W., Lee Y.H., Yang D.C., Baek N.I. Discrimination of Panax ginseng roots cultivated in different areas in Korea using HPLC-ELSD and principal component analysis. J Ginseng Res. 2011;35:31–38. [Google Scholar]
- 170.Li W.K., Fitzloff J.F. A validated method for quantitative determination of saponins in notoginseng (Panax notoginseng) using high-performance liquid chromatography with evaporative light-scattering detection. J Pharm Pharmacol. 2001;53:1637–1643. doi: 10.1211/0022357011778241. [DOI] [PubMed] [Google Scholar]
- 171.Koh E., Jang O.H., Hwang K.H., An Y.N., Moon B. Effects of steaming and air-drying on ginsenoside composition of Korean ginseng (Panax ginseng C.A. Meyer) J Food Process Pres. 2015;39:207–213. [Google Scholar]
- 172.Dong W.W., Han X.Z., Zhao J., Zhong F.L., Ma R., Wu S., Li D., Quan L.H., Jiang J. Metabolite profiling of ginsenosides in rat plasma, urine and feces by LC-MS/MS and its application to a pharmacokinetic study after oral administration of Panax ginseng extract. Biomed Chromatogr. 2018;32 doi: 10.1002/bmc.4105. [DOI] [PubMed] [Google Scholar]
- 173.Song Y., Zhang N., Shi S., Li J., Zhao Y., Zhang Q., Jiang Y., Tu P. Homolog-focused profiling of ginsenosides based on the integration of step-wise formate anion-to-deprotonated ion transition screening and scheduled multiple reaction monitoring. J Chromatogr A. 2015;1406:136–144. doi: 10.1016/j.chroma.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 174.Stavrianidi A., Stekolshchikova E., Porotova A., Rodin I., Shpigun O. Combination of HPLC-MS and QAMS as a new analytical approach for determination of saponins in ginseng containing products. J Pharm Biomed Anal. 2017;132:87–92. doi: 10.1016/j.jpba.2016.09.041. [DOI] [PubMed] [Google Scholar]
- 175.Ye J., Gao Y., Tian S., Su J., Zhang W. A novel and effective mode-switching triple quadrupole mass spectrometric approach for simultaneous quantification of fifteen ginsenosides in Panax ginseng. Phytomedicine. 2018;44:164–172. doi: 10.1016/j.phymed.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 176.Xia Y.G., Song Y., Liang J., Guo X.D., Yang B.Y., Kuang H.X. Quality analysis of American ginseng cultivated in Heilongjiang using UPLC-ESI(-)-MRM-MS with chemometric methods. Molecules. 2018;23 doi: 10.3390/molecules23092396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chen W., Dang Y., Zhu C. Simultaneous determination of three major bioactive saponins of Panax notoginseng using liquid chromatography-tandem mass spectrometry and a pharmacokinetic study. Chin Med-Uk. 2010;5:12. doi: 10.1186/1749-8546-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Feng H., Chen W., Zhu C. Pharmacokinetics study of bio-adhesive tablet of Panax notoginseng saponins. Int Arch Med. 2011;4:18. doi: 10.1186/1755-7682-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhou L., Xing R., Xie L., Rao T., Wang Q., Ye W., Fu H., Xiao J., Shao Y., Kang D. Development and validation of an UFLC-MS/MS assay for the absolute quantitation of nine notoginsenosides in rat plasma: application to the pharmacokinetic study of Panax Notoginseng Extract. J Chromatogr B Analyt Technol Biomed Life Sci. 2015;995–996:46–53. doi: 10.1016/j.jchromb.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 180.Lai C.J., Tan T., Zeng S.L., Dong X., Liu E.H., Li P. Relative quantification of multi-components in Panax notoginseng (Sanqi) by high-performance liquid chromatography with mass spectrometry using mobile phase compensation. J Pharm Biomed Anal. 2015;102:150–156. doi: 10.1016/j.jpba.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 181.Chen J., Guo X., Song Y., Zhao M., Tu P., Jiang Y. MRM-based strategy for the homolog-focused detection of minor ginsenosides from notoginseng total saponins by ultra-performance liquid chromatography coupled with hybrid triple quadrupole-linear ion trap mass spectrometry. RSC Advances. 2016;6:96376–96388. [Google Scholar]
- 182.Zhu D., Zhou Q., Li H., Li S., Dong Z., Li D., Zhang W. Pharmacokinetic characteristics of steamed notoginseng by an efficient LC-MS/MS method for simultaneously quantifying twenty-three triterpenoids. J Agr Food Chem. 2018;66:8187–8198. doi: 10.1021/acs.jafc.8b03169. [DOI] [PubMed] [Google Scholar]
- 183.Li S., Liu C., Liu C., Zhang Y. Extraction and in vitro screening of potential acetylcholinesterase inhibitors from the leaves of Panax japonicus. J Chromatogr B Analyt Technol Biomed Life Sci. 2017;1061–1062:139–145. doi: 10.1016/j.jchromb.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 184.Liu Y., Li J., He J., Abliz Z., Qu J., Yu S., Ma S., Liu J., Du D. Identification of new trace triterpenoid saponins from the roots of Panax notoginseng by high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom. 2009;23:667–679. doi: 10.1002/rcm.3917. [DOI] [PubMed] [Google Scholar]
- 185.Kim S.H., Shin Y.S., Choi H.K. Nano ESI-MS-based lipidomics to discriminate between cultivars, cultivation ages, and parts of Panax ginseng. Anal Bioanal Chem. 2016;408:2109–2121. doi: 10.1007/s00216-016-9314-5. [DOI] [PubMed] [Google Scholar]
- 186.Podhorniak L.V. A rapid miniaturized residue analytical method for the determination of zoxamide and its two acid metabolites in ginseng roots using UPLC-MS/MS. J Agr Food Chem. 2014;62:3702–3709. doi: 10.1021/jf405403v. [DOI] [PubMed] [Google Scholar]
- 187.Wan J.Y., Fan Y., Yu Q.T., Ge Y.Z., Yan C.P., Alolga R.N., Li P., Ma Z.H., Qi L.W. Integrated evaluation of malonyl ginsenosides, amino acids and polysaccharides in fresh and processed ginseng. J Pharm Biomed Anal. 2015;107:89–97. doi: 10.1016/j.jpba.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 188.Xie Y.Y., Luo D., Cheng Y.J., Ma J.F., Wang Y.M., Liang Q.L., Luo G.A. Steaming-induced chemical transformations and holistic quality assessment of red ginseng derived from Panax ginseng by means of HPLC-ESI-MS/MS(n)-based multicomponent quantification fingerprint. J Agr Food Chem. 2012;60:8213–8224. doi: 10.1021/jf301116x. [DOI] [PubMed] [Google Scholar]
- 189.Qiu S., Yang W.Z., Yao C.L., Qiu Z.D., Shi X.J., Zhang J.X., Hou J.J., Wang Q.R., Wu W.Y., Guo D.A. Nontargeted metabolomic analysis and "commercial-homophyletic" comparison-induced biomarkers verification for the systematic chemical differentiation of five different parts of Panax ginseng. J Chromatogr A. 2016;1453:78–87. doi: 10.1016/j.chroma.2016.05.051. [DOI] [PubMed] [Google Scholar]
- 190.Lee D.Y., Cho J.G., Bang M.H., Han M.W., Lee M.H., Yang D.C., Baek N.I. Discrimination of Korean ginseng (Panax ginseng) roots using rapid resolution LC-QTOF/MS combined by multivariate statistical analysis. Food Sci Biotechnol. 2011;20:1119–1124. [Google Scholar]
- 191.Sun J., Mi J., Qin Q., Yu Q., Wu W., Liu S. Identification of ginsenosides Rc, Rb2, Rb3 and related malonyl-ginsenosides in Panax ginseng extracts by using RRLC-Q-TOF-MS/MS. International Conference on Human Health and Biomedical Engineering. 2011:1140–1143. [Google Scholar]
- 192.Wu W., Sun L., Zhang Z., Guo Y., Liu S. Profiling and multivariate statistical analysis of Panax ginseng based on ultra-high-performance liquid chromatography coupled with quadrupole-time-of-flight mass spectrometry. J Pharm Biomed Anal. 2015;107:141–150. doi: 10.1016/j.jpba.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 193.Wang H.P., Zhang Y.B., Yang X.W., Zhao D.Q., Wang Y.P. Rapid characterization of ginsenosides in the roots and rhizomes of Panax ginseng by UPLC-DAD-QTOF-MS/MS and simultaneous determination of 19 ginsenosides by HPLC-ESI-MS. J Ginseng Res. 2016;40:382–394. doi: 10.1016/j.jgr.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wang H.P., Liu Y., Chen C., Xiao H.B. Screening specific biomarkers of herbs using a metabolomics approach: a case study of Panax ginseng. Sci Rep. 2017;7:4609. doi: 10.1038/s41598-017-04712-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zhang H.M., Li S.L., Zhang H., Wang Y., Zhao Z.L., Chen S.L., Xu H.X. Holistic quality evaluation of commercial white and red ginseng using a UPLC-QTOF-MS/MS-based metabolomics approach. J Pharm Biomed Anal. 2012;62:258–273. doi: 10.1016/j.jpba.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 196.Chang X., Zhang J., Li D., Zhou D., Zhang Y., Wang J., Hu B., Ju A., Ye Z. Nontargeted metabolomics approach for the differentiation of cultivation ages of mountain cultivated ginseng leaves using UHPLC/QTOF-MS. J Pharm Biomed Anal. 2017;141:108–122. doi: 10.1016/j.jpba.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 197.Zhu H., Shen H., Xu J., Xu J.D., Zhu L.Y., Wu J., Chen H.B., Li S.L. Comparative study on intestinal metabolism and absorption in vivo of ginsenosides in sulphur-fumigated and non-fumigated ginseng by ultra performance liquid chromatography quadruple time-of-flight mass spectrometry based chemical profiling approach. Drug Test Anal. 2015;7:320–330. doi: 10.1002/dta.1675. [DOI] [PubMed] [Google Scholar]
- 198.Song H.H., Moon J.Y., Ryu H.W., Noh B.S., Kim J.H., Lee H.K., Oh S.R. Discrimination of white ginseng origins using multivariate statistical analysis of data sets. J Ginseng Res. 2014;38:187–193. doi: 10.1016/j.jgr.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.In G., Seo H.K., Park H.W., Jang K.H. A metabolomic approach for the discrimination of red ginseng root parts and targeted validation. Molecules. 2017;22 doi: 10.3390/molecules22030471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Xu X.F., Xu S.Y., Zhang Y., Zhang H., Liu M.N., Liu H., Gao Y., Xue X., Xiong H., Lin R.C. Chemical comparison of two drying methods of mountain cultivated ginseng by UPLC-QTOF-MS/MS and multivariate statistical analysis. Molecules. 2017;22 doi: 10.3390/molecules22050717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lee J.W., Choi B.R., Kim Y.C., Choi D.J., Lee Y.S., Kim G.S., Baek N.I., Kim S.Y., Lee D.Y. Comprehensive profiling and quantification of ginsenosides in the root, stem, leaf, and berry of Panax ginseng by UPLC-QTOF/MS. Molecules. 2017;22 doi: 10.3390/molecules22122147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Yuan J., Chen Y., Liang J., Wang C.Z., Liu X., Yan Z., Tang Y., Li J., Yuan C.S. Component analysis and target cell-based neuroactivity screening of Panax ginseng by ultra-performance liquid chromatography coupled with quadrupole-time-of-flight mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2016;1038:1–11. doi: 10.1016/j.jchromb.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Li X., Yao F., Fan H., Li K., Sun L., Liu Y. Intraconversion of polar ginsenosides, their transformation into less-polar ginsenosides, and ginsenoside acetylation in ginseng flowers upon baking and steaming. Molecules. 2018;23 doi: 10.3390/molecules23040759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lee J.W., Ji S.H., Choi B.R., Choi D.J., Lee Y.G., Kim H.G., Kim G.S., Kim K., Lee Y.H., Baek N.I. UPLC-QTOF/MS-based metabolomics applied for the quality evaluation of four pocessed Panax ginseng products. Molecules. 2018;23 doi: 10.3390/molecules23082062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Huang B.M., Zha Q.L., Chen T.B., Xiao S.Y., Xie Y., Luo P., Wang Y.P., Liu L., Zhou H. Discovery of markers for discriminating the age of cultivated ginseng by using UHPLC-QTOF/MS coupled with OPLS-DA. Phytomedicine. 2018;45:8–17. doi: 10.1016/j.phymed.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 206.Zhao Q., Zhao N., Ye X., He M., Yang Y., Gao H., Zhang X. Rapid discrimination between red and white ginseng based on unique mass-spectrometric features. J Pharm Biomed Anal. 2019;164:202–210. doi: 10.1016/j.jpba.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 207.Zhu H., Lin H., Tan J., Wang C., Wang H., Wu F., Dong Q., Liu Y., Li P., Liu J. UPLC-QTOF/MS-based nontargeted metabolomic analysis of mountain- and garden-cultivated ginseng of different ages in Northeast China. Molecules. 2018;24 doi: 10.3390/molecules24010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Qi L.W., Wang H.Y., Zhang H., Wang C.Z., Li P., Yuan C.S. Diagnostic ion filtering to characterize ginseng saponins by rapid liquid chromatography with time-of-flight mass spectrometry. J Chromatogr A. 2012;1230:93–99. doi: 10.1016/j.chroma.2012.01.079. [DOI] [PubMed] [Google Scholar]
- 209.Lin H., Zhu H., Tan J., Wang H., Dong Q., Wu F., Liu Y., Li P., Liu J. Non-targeted metabolomic analysis of methanolic extracts of wild-simulated and field-grown American ginseng. Molecules. 2019;24 doi: 10.3390/molecules24061053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sun J., Chen P. Differentiation of Panax quinquefolius grown in the USA and China using LC/MS-based chromatographic fingerprinting and chemometric approaches. Anal Bioanal Chem. 2011;399:1877–1889. doi: 10.1007/s00216-010-4586-7. [DOI] [PubMed] [Google Scholar]
- 211.Sun X., Chen P., Cook S.L., Jackson G.P., Harnly J.M., Harrington P.B. Classification of cultivation locations of Panax quinquefolius L samples using high performance liquid chromatography-electrospray ionization mass spectrometry and chemometric analysis. Anal Chem. 2012;84:3628–3634. doi: 10.1021/ac2032832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Wen X.D., Yang J., Ma R.H., Gao W., Qi L.W., Li P., Bauer B.A., Du G.J., Zhang Z., Somogyi J. Analysis of Panax notoginseng metabolites in rat bile by liquid chromatography-quadrupole time-of-flight mass spectrometry with microdialysis sampling. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;895–896:162–168. doi: 10.1016/j.jchromb.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Liu P., Yu H.S., Zhang L.J., Song X.B., Kang L.P., Liu J.Y., Zhang J., Cao M., Yu K., Kang T.G. A rapid method for chemical fingerprint analysis of Panax notoginseng powders by ultra performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. Chin J Nat Med. 2015;13:471–480. doi: 10.1016/S1875-5364(15)30042-X. [DOI] [PubMed] [Google Scholar]
- 214.Xiao J., Chen H., Kang D., Shao Y., Shen B., Li X., Yin X., Zhu Z., Li H., Rao T. Qualitatively and quantitatively investigating the regulation of intestinal microbiota on the metabolism of Panax notoginseng saponins. J Ethnopharmacol. 2016;194:324–336. doi: 10.1016/j.jep.2016.09.027. [DOI] [PubMed] [Google Scholar]
- 215.Liu F., Ma N., He C., Hu Y., Li P., Chen M., Su H., Wan J.B. Qualitative and quantitative analysis of the saponins in Panax notoginseng leaves using ultra-performance liquid chromatography coupled with time-of-flight tandem mass spectrometry and high performance liquid chromatography coupled with UV detector. J Ginseng Res. 2018;42:149–157. doi: 10.1016/j.jgr.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Shi X.J., Yang W.Z., Qiu S., Yao C.L., Shen Y., Pan H.Q., Bi Q.R., Yang M., Wu W.Y., Guo D.A. An in-source multiple collision-neutral loss filtering based nontargeted metabolomics approach for the comprehensive analysis of malonyl-ginsenosides from Panax ginseng, P. quinquefolius, and P. notoginseng. Anal Chim Acta. 2017;952:59–70. doi: 10.1016/j.aca.2016.11.032. [DOI] [PubMed] [Google Scholar]
- 217.Shi X., Yang W., Huang Y., Hou J., Qiu S., Yao C., Feng Z., Wei W., Wu W., Guo D. Direct screening of malonylginsenosides from nine Ginseng extracts by an untargeted profiling strategy incorporating in-source collision-induced dissociation, mass tag, and neutral loss scan on a hybrid linear ion-trap/Orbitrap mass spectrometer coupled to ultra-high performance liquid chromatography. J Chromatogr A. 2018;1571:213–222. doi: 10.1016/j.chroma.2018.08.026. [DOI] [PubMed] [Google Scholar]
- 218.Wang S., Qiao L., Shi X., Hu C., Kong H., Xu G. On-line stop-flow two-dimensional liquid chromatography-mass spectrometry method for the separation and identification of triterpenoid saponins from ginseng extract. Anal Bioanal Chem. 2015;407:331–341. doi: 10.1007/s00216-014-8219-4. [DOI] [PubMed] [Google Scholar]
- 219.Wu Y., Liu J., Gu S., Lin L., Chen Y., Ma M., Chen B. Orthogonal strategy development using reversed macroporous resin coupled with hydrophilic interaction liquid chromatography for the separation of ginsenosides from ginseng root extract. J Sep Sci. 2017;40:4128–4134. doi: 10.1002/jssc.201700487. [DOI] [PubMed] [Google Scholar]
- 220.Yang W., Zhang J., Yao C., Qiu S., Chen M., Pan H., Shi X., Wu W., Guo D. Method development and application of offline two-dimensional liquid chromatography/quadrupole time-of-flight mass spectrometry-fast data directed analysis for comprehensive characterization of the saponins from Xueshuantong Injection. J Pharm Biomed Anal. 2016;128:322–332. doi: 10.1016/j.jpba.2016.05.035. [DOI] [PubMed] [Google Scholar]
- 221.Shehzad O., Ha I.J., Park Y., Ha Y.W., Kim Y.S. Development of a rapid and convenient method to separate eight ginsenosides from Panax ginseng by high-speed counter-current chromatography coupled with evaporative light scattering detection. J Sep Sci. 2011;34:1116–1122. doi: 10.1002/jssc.201000932. [DOI] [PubMed] [Google Scholar]
- 222.Cheng Y., Zhang M., Liang Q., Hu P., Wang Y., Jun F.W., Luo G. Two-step preparation of ginsenoside-Re, Rb1, Rc and Rb2 from the root of Panax ginseng by high-performance counter-current chromatography. Sep Purif Technol. 2011;77:347–354. [Google Scholar]
- 223.Chen F., Luo J., Kong L. Fast isolation of ginsenosides Re and Rg1 from the roots of Panax ginseng by HSCCC-ELSD combined with MCI gel CC guided by HPLC-MS. J Liq Chromatogr R T. 2012;35:912–923. [Google Scholar]
- 224.Wang J., Liu C.M., Li L., Bai H.L. Isolation of four high-purity dammarane saponins from extract of Panax notoginseng by centrifugal partition chromatography coupled with evaporative light scattering detection in one operation. Phytochem Anal. 2011;22:263–267. doi: 10.1002/pca.1274. [DOI] [PubMed] [Google Scholar]
- 225.Shehzad O., Kim H.P., Kim Y.S. State-of-the-art separation of ginsenosides from Korean white and red ginseng by countercurrent chromatography. Anal Bioanal Chem. 2013;405:4523–4530. doi: 10.1007/s00216-012-6609-z. [DOI] [PubMed] [Google Scholar]
- 226.Cao X.L., Tian Y., Zhang T.Y., Liu Q.H., Jia L.J., Ito Y. Separation of dammarane-saponins from notoginseng, root of Panax notoginseng (Burk.) F. H. Chen, by HSCCC coupled with evaporative light scattering detector. J Liq Chromatogr R T. 2007;26:1579–1591. [Google Scholar]
- 227.Wang S., Ye S., Cheng Y. Separation and on-line concentration of saponins from Panax notoginseng by micellar electrokinetic chromatography. J Chromatogr A. 2006;1109:279–284. doi: 10.1016/j.chroma.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 228.Zhao H., Xu J., Ghebrezadik H., Hylands P.J. Metabolomic quality control of commercial Asian ginseng, and cultivated and wild American ginseng using 1H NMR and multi-step PCA. J Pharm Biomed Anal. 2015;114:113–120. doi: 10.1016/j.jpba.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 229.Huang Y., Zhang T., Zhao Y., Zhou H., Tang G., Fillet M., Crommen J., Jiang Z. Simultaneous analysis of nucleobases, nucleosides and ginsenosides in ginseng extracts using supercritical fluid chromatography coupled with single quadrupole mass spectrometry. J Pharm Biomed Anal. 2017;144:213–219. doi: 10.1016/j.jpba.2017.03.059. [DOI] [PubMed] [Google Scholar]
- 230.Shi X., Yang W., Qiu S., Hou J., Wu W., Guo D. Systematic profiling and comparison of the lipidomes from Panax ginseng, P. quinquefolius, and P. notoginseng by ultrahigh performance supercritical fluid chromatography/high-resolution mass spectrometry and ion mobility-derived collision cross section measurement. J Chromatogr A. 2018;1548:64–75. doi: 10.1016/j.chroma.2018.03.025. [DOI] [PubMed] [Google Scholar]
- 231.Liu Y., Xie M.X., Kang J., Zheng D. Studies on the interaction of total saponins of Panax notoginseng and human serum albumin by Fourier transform infrared spectroscopy. Spectrochim Acta Part A. 2003;59:2747–2758. doi: 10.1016/s1386-1425(03)00055-6. [DOI] [PubMed] [Google Scholar]
- 232.Chen H., Lin Z., Tan C. Fast discrimination of the geographical origins of notoginseng by near-infrared spectroscopy and chemometrics. J Pharm Biomed Anal. 2018;161:239–245. doi: 10.1016/j.jpba.2018.08.052. [DOI] [PubMed] [Google Scholar]
- 233.Lee B.J., Kim H.Y., Lim S.R., Huang L., Choi H.K. Discrimination and prediction of cultivation age and parts of Panax ginseng by Fourier-transform infrared spectroscopy combined with multivariate statistical analysis. PLoS One. 2017;12 doi: 10.1371/journal.pone.0186664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Yao H., Shi P.Y., Shao Q., Fan X.H. Chemical fingerprinting and quantitative analysis of a Panax notoginseng preparation using HPLC-UV and HPLC-MS. Chin Med-Uk. 2011;6 doi: 10.1186/1749-8546-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.MacCrehan W.A., White C.M. Simplified ultrasonically- and microwave-assisted solvent extractions for the determination of ginsenosides in powdered Panax ginseng rhizomes using liquid chromatography with UV absorbance or electrospray mass spectrometric detection. Anal Bioanal Chem. 2013;405:4511–4522. doi: 10.1007/s00216-013-6871-8. [DOI] [PubMed] [Google Scholar]
- 236.Mao Q., Li Y., Li S.L., Yang J., Zhang P.H., Wang Q. Chemical profiles and anticancer effects of saponin fractions of different polarity from the leaves of Panax notoginseng. Chin J Nat Med. 2014;12:30–37. doi: 10.1016/S1875-5364(14)60006-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.