Abstract
Purpose:
Lymphedema affects many women treated for breast cancer. We examined the effectiveness of an education-only (EO) versus education + sleeve compression/exercise intervention (LEAP) on lymphedema incidence and range of motion (ROM) in a group-randomized trial (NCT00376597) across 38 cooperative group sites.
Patients and Methods:
Women newly-diagnosed at stage I-III, who underwent either sentinel or full axillary node dissection, were randomized stratified by their treating institution to EO or LEAP. Participants completed surveys, arm volume measurements, and self-reported ROM assessments pre-surgery and at 12- and 18-months post-surgery. Lymphedema was defined as ≥ 10% difference in limb volume at any time from pre-surgery to 18 months post-surgery or diagnosis by a health provider. Cochran-Mantel-Haenszel (CMH) tests compared lymphedema-free rates between groups, stratified by lymph node surgery type. Self-reported ROM differences were compared between groups.
Results:
554 participants (56% LEAP) were included in the analyses. At 18 months, lymphedema-free rates were 58% (EO) vs 55% (LEAP) (p=0.37). ROM for both arms was greater in LEAP versus EO at 12 months; by 18 months, most women reported full ROM, regardless of group. In LEAP, only one-third wore the sleeve ≥75% of the time; 50% performed lymphedema exercises at least weekly.
Conclusion:
Lymphedema incidence did not differ by intervention group at 18 months. Poor adherence in the LEAP group may have contributed. However, physical therapy may speed recovery of ROM. Further research is needed to effectively reduce the incidence and severity of lymphedema in breast cancer patients.
ClinicalTrials.gov Identifier:
Keywords: lymphedema, breast cancer, education, exercise, range of motion, prevention
Precis:
Women in the exercise plus education group (LEAP) had faster return of full range of motion, but there was no difference by intervention group in lymphedema incidence at 18 months. Poor adherence in the LEAP group may have contributed to similar lymphedema rates in the intervention groups; however, physical therapy may speed recovery of range of motion.
INTRODUCTION
Lymphedema, a possible complication of breast cancer treatment, is commonly distinguished by swelling and/or pain in the affected arm and/or hand. The prevalence of lymphedema varies from 21% to 94%, due to lack of standardized diagnostic criteria and surveillance 1-3. Most women are diagnosed with lymphedema within 3 years of treatment 3. Common risk factors include treatment factors (e.g., axillary lymph node dissection (ALND), mastectomy, radiation, chemotherapy, greater number of positive lymph nodes), and patient factors (e.g., higher body mass index (BMI), infection 4-8). Studies consistently report poorer quality of life (QOL) among patients with lymphedema 9,10, and full range of motion (ROM) in the affected arm is often impeded 11.
While intervention studies have been conducted to reduce swelling in women with lymphedema 12-16, lymphedema risk-reduction strategies have not been rigorously tested. The overall goal of this clinical trial was to compare the effectiveness of two interventions on lymphedema incidence in a randomized cooperative group setting. Secondary aims were to compare: (1) severity of lymphedema; (2) ROM of affected arm; (3) adherence to lymphedema prevention exercises; and (4) health-related QOL (HRQL) by intervention group (reported in a separate manuscript [ref – manuscript will be submitted to Cancer as a companion paper to this one]).
METHODS
Participants
The study was designed as a randomized phase III trial within Cancer and Leukemia Group B (CALGB, now Alliance – CALGB 70305). The eligibility process utilized a two-step procedure, meaning eligible participants were enrolled in Step 1 and then registered for Step 2 if they had either ALND or sentinel lymph node dissection (SLND). Women newly diagnosed with breast cancer (stage I – III), aged 18 years or older, with no history of lymphedema, lobular or ductal carcinoma in situ, or invasive breast cancer were eligible. Patients who received neoadjuvant chemotherapy were eligible if pre-surgery arm measurements were completed prior to first chemotherapy treatment. Eligible patients had medical clearance to participate in a mild exercise program and had an upper arm size that accommodated a standard-size elastic compression sleeve and gauntlet. Patients who underwent bilateral mastectomies, bilateral ALND, and/or bilateral radiation were ineligible, but those who had immediate or delayed reconstruction were eligible.
Patients were recruited from 38 CALGB and National Cancer Institute Cooperative Group Clinical Trials sites across the United States from December 2006-September 2013, with follow-up until December 2015. Eligible sites were providing no more than lymphedema prevention education to patients prior to being randomly assigned to intervention groups of education only (EO) or Lymphedema Education and Prevention (LEAP) for focused education + exercise (including sleeve use). A stratified permuted block approach (stratified by the annual number of stage I-III breast cancer patients treated at the institution) was used for randomization. Participating institutions were randomized to EO or LEAP so that all participants at an institution received identical treatment plans to minimize contamination bias. The trial was approved by institutional review boards (IRB) of participating sites, and participants signed IRB-approved, protocol-specific informed consents in accordance with federal and institutional guidelines.
Participant Assessments
Participants were recruited at their first pre-operative visit, consented and registered to Step 1. Baseline measurements of height, weight, self-reported ROM and arm circumference 17 were taken by trained nurses prior to treatment. Participants completed questionnaires regarding demographics, lymphedema knowledge, body image, self-efficacy 18, fear of cancer recurrence, self-reported pain and swelling, self-reported ROM, HRQL 19, and adherence to lymphedema risk-reduction practices. Assessments were repeated after surgery and at 6 (by mail), 12, and 18 months post-surgery.
Intervention Components
All eligible participants were registered to Step 2 ≤6 weeks after surgery. A trained lymphedema prevention educator reviewed lymphedema etiology, signs, symptoms, treatments, and preventative self-care practices (Table 1). Participants at LEAP institutions also received an intervention of assessment and instruction by a physical therapist in an individualized exercise regimen involving breathing, stretching, strengthening, and personalized ROM exercises; all patients were taught the same exercises. Participants successfully demonstrated study exercises and were instructed to perform exercises daily, provided an instructional video for home use, and asked to document exercise performance in study calendars. On average, these activities required approximately 15 minutes/day to complete. LEAP participants also received 2-pound (0.91kg) hand weights for daily use and an elastic compression sleeve and gauntlet (Juzo Class I 20-30mmHg) to wear during exercise (study-related or personal), air travel, and/or vigorous activity. Participants met with the study educator for a brief visit at 12- and 18-months post-surgery and by phone at 9- and 15-months post-surgery to reinforce adherence to study interventions and answer questions.
Table 1.
Overview of intervention group components
| Education-only Components | LEAP Components |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outcomes
The primary endpoint of this trial was lymphedema-free rates at 18 months. Lymphedema was defined as an increase of ≥10% in the volume of the affected arm between the pre-operative and 12- or 18-month visits, after controlling for percentage change in BMI, or as a diagnosis by a participant’s physician (using his/her own clinical judgment) any time following the post-operative assessment (up to 18-months post-surgery) 20,21. While there is no “gold standard” definition for lymphedema, ≥10% limb volume change (LVC) is a conservative and commonly used definition 22. Secondary outcomes included: (1) severity of lymphedema defined as change in arm circumference at the site of greatest difference; (2) self-reported ROM; and (3) adherence to sleeve/gauntlet use and exercises (LEAP group).
Statistical Methods
All analyses accounted for cluster (site) randomization as specified in the protocol. The primary study objective was to compare lymphedema-free rates between groups. The study was designed to detect an improvement in the 18-month lymphedema-free rate from 65% (EO group) to 77% (LEAP group). A total of 560 participants provided 81% power with a one-sided type I error of 0.05 using the method by Donner 23 to account for the group-randomized design, assuming an intra-cluster correlation (ICC) of 0.02, based on estimates of rates of lymphedema provided by Alliance institutions as well as from CALGB 79804.
The primary intent-to-treat analysis (ITT) included all eligible participants. Participants with missing lymphedema measurements at 12- and/or 18-months post-surgery, and whose lymphedema status could not be determined, were classified as not lymphedema-free. Lymphedema-free rates were adjusted for BMI at 18-months post-surgery, stratified by type of lymph node dissection, institution size (small: ≤ 275 new breast cancer patients annually; moderate: 276-450 new breast cancer patients annually; and large: ≥ 450 new breast cancer patients annually) and compared between the two groups using an adjusted Cochran-Mantel-Haenszel test to account for ICC 24. Sensitivity analyses using complete case and simple and multiple imputations for missing data were conducted and confirmed original results. Imputation-based models produced similar findings.
The difference in arm circumference from baseline to 12 and 18 months post-surgery at the site of the greatest difference was summarized and compared using the GEE model to account for cluster randomization. Self-reported ROM was assessed by participants choosing one of six illustrations best indicating how high they could reach above their head with each arm. Responses were dichotomized to reflect full ROM vs. not full ROM. ROM differences were compared between study groups at pre-surgery vs 12 and 18 months post-surgery using GEE models to account for cluster randomization. Each arm was assessed independently, adjusting for baseline BMI, surgery type, type of lymph node dissection, affected arm being dominant, and receipt of radiation and/or chemotherapy. Analyses of these secondary endpoints were limited to the complete cases only.
At 12- and 18-months, LEAP group participants were assessed for adherence to performing individualized arm exercises and wearing an elastic sleeve at least 75% of the time during heavy arm use, exercise, or travel. Statistical analyses concerning adherence were descriptive.
Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center (SDC). All analyses were completed on the study database frozen on April 30, 2016. Data quality was ensured by review of data by the Alliance SDC and by the study chairperson following Alliance policies. The trial was monitored at least twice annually by the Alliance Data and Safety Monitoring Committee. All analyses were conducted using the SAS software v.9.4.
RESULTS
A total of 876 participants were registered to Step 1. Of those registered, 568 (65%) underwent surgery and were registered to Step 2 (Figure 1). Nineteen sites (253 participants) were randomized to the EO group and 22 sites (315 participants) were randomized to the LEAP group. A total of 554 participants (98%) were included in the analyses (14 were excluded as they were registered to Step 2 in error). Participant characteristics are provided in Table 2. Differences in race and ethnicity between the groups were observed resulting from demographic differences of patient populations at participating institutions.
Figure 1:
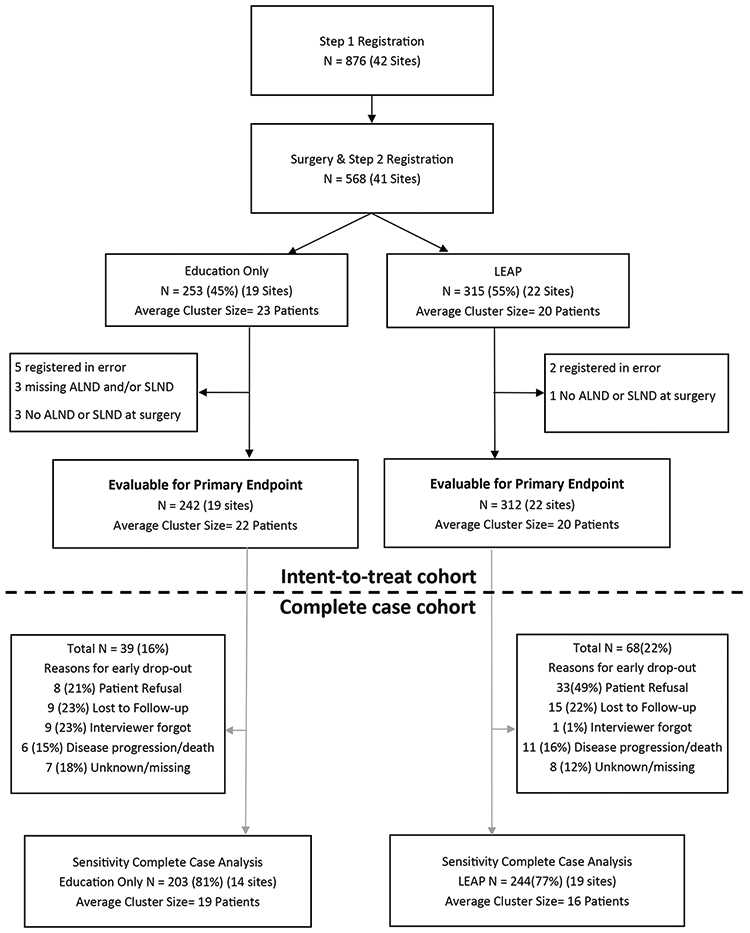
CONSORT diagram for CALGB 70305 (Alliance)
Table 2:
Baseline demographic and clinical characteristics by intervention group
| Education Only (N=242) |
LEAP (N=312) |
Total (N=554) |
|
|---|---|---|---|
| Age | |||
| N | 242 | 312 | 554 |
| Median | 59.0 | 58.0 | 58.0 |
| Range | (24.0-83.0) | (27.0-88.0) | (24.0-88.0) |
| Race | |||
| Missing | 3 | 6 | 9 |
| Black | 38 (15.9%) | 31 (10.1%) | 69 (12.7%) |
| Other (Asian, American Indian/Alaska Native, more than 1 race) | 7 (2.9%) | 17 (5.6%) | 24 (4.4%) |
| White | 194 (81.2%) | 258 (84.3%) | 452 (82.9%) |
| Ethnicity | |||
| Missing | 9 | 6 | 15 |
| Hispanic or Latino | 35 (15.0%) | 10 (3.3%) | 45 (8.3%) |
| Non-Hispanic | 198 (85.0%) | 296 (96.7%) | 494 (91.7%) |
| ECOG Performance Status | |||
| Missing | 42 | 52 | 94 |
| 0 | 194 (97.0%) | 239 (91.9%) | 433 (94.1%) |
| 1 | 6 (3.0%) | 20 (7.7%) | 26 (5.7%) |
| 2 | 0 (0.0%) | 1 (0.4%) | 1 (0.2%) |
| Education | |||
| Missing | 27 | 8 | 35 |
| < HS | 17 (7.9%) | 11 (3.6%) | 28 (5.4%) |
| > BA/BS College Degree | 52 (24.2%) | 75 (24.7%) | 127 (24.5%) |
| BA/BS College Degree | 34 (15.8%) | 64 (21.1%) | 98 (18.9%) |
| HS Grad | 36 (16.7%) | 65 (21.4%) | 101 (19.5%) |
| Some college/Jr College | 76 (35.3%) | 89 (29.3%) | 165 (31.8%) |
| Marital Status | |||
| Missing | 28 | 10 | 38 |
| Married | 131 (61.2%) | 187 (61.9%) | 318 (61.6%) |
| Separated/divorced/widowed | 65 (30.4%) | 93 (30.8%) | 158 (30.6%) |
| Single/never married | 18 (8.4%) | 22 (7.3%) | 40 (7.8%) |
| Employment Status | |||
| Missing | 28 | 9 | 37 |
| Disabled | 16 (7.5%) | 19 (6.3%) | 35 (6.8%) |
| Employed | 118 (55.1%) | 153 (50.5%) | 271 (52.4%) |
| Homemaker | 19 (8.9%) | 38 (12.5%) | 57 (11.0%) |
| Retired | 51 (23.8%) | 74 (24.4%) | 125 (24.2%) |
| Student | 2 (0.9%) | 2 (0.7%) | 4 (0.8%) |
| Unemployed | 8 (3.7%) | 17 (5.6%) | 25 (4.8%) |
| Definitive primary surgery | |||
| Missing | 4 | 5 | 9 |
| Partial mastectomy/lumpectomy/excisional biopsy | 155 (65.1%) | 199 (64.8%) | 354 (65.0%) |
| Mastectomy, NOS | 83 (34.9%) | 108 (35.2%) | 191 (35.0%) |
| Type of axillary lymph node dissection | |||
| Axillary lymph node dissection only | 64 (26.4%) | 67 (21.5%) | 131 (23.6%) |
| Both axillary and sentinel lymph node dissection | 78 (32.2%) | 87 (27.9%) | 165 (29.8%) |
| Sentinel lymph node dissection only | 100 (41.3%) | 158 (50.6%) | 258 (46.6%) |
| Number of positive lymph nodes | |||
| N | 225 | 298 | 523 |
| Mean (SD) | 2.3 (5.1) | 2.1 (5.5) | 2.2 (5.3) |
| Immediate reconstructive surgery | |||
| Missing | 3 | 1 | 4 |
| No | 218 (91.2%) | 249 (80.1%) | 467 (84.9%) |
| Yes | 21 (8.8%) | 62 (19.9%) | 83 (15.1%) |
| Tumor laterality | |||
| Missing | 1 | 1 | 2 |
| Left | 130 (53.9%) | 159 (51.1%) | 289 (52.4%) |
| Right | 111 (46.1%) | 151 (48.6%) | 262 (47.5%) |
| Bilateral | 0 (0.0%) | 1 (0.3%) | 1 (0.2%) |
| Receptor status,ER | |||
| Negative | 57 (23.6%) | 60 (19.2%) | 117 (21.1%) |
| Positive | 184 (76.0%) | 251 (80.4%) | 435 (78.5%) |
| Not Done | 1 (0.4%) | 1 (0.3%) | 2 (0.4%) |
| Receptor status,PR | |||
| Negative | 92 (38.0%) | 87 (27.9%) | 179 (32.3%) |
| Positive | 149 (61.6%) | 224 (71.8%) | 373 (67.3%) |
| Not Done | 1 (0.4%) | 1 (0.3%) | 2 (0.4%) |
| HER-2/neu receptors | |||
| Missing | 2 | 3 | 5 |
| Negative | 194 (80.8%) | 249 (80.6%) | 443 (80.7%) |
| Positive | 42 (17.5%) | 53 (17.2%) | 95 (17.3%) |
| Not Done | 4 (1.7%) | 7 (2.3%) | 11 (2.0%) |
| Pathologic primary tumor size | |||
| N | 233 | 305 | 538 |
| Median | 1.7 | 1.8 | 1.7 |
| Grade | |||
| Missing | 2 | 16 | 18 |
| Low | 54 (22.5%) | 65 (22.0%) | 119 (22.2%) |
| Intermediate | 93 (38.8%) | 138 (46.6%) | 231 (43.1%) |
| High | 93 (38.8%) | 93 (31.4%) | 186 (34.7%) |
| Receipt of chemotherapy | |||
| Missing | 4 (1.7%) | 4 (1.3%) | 8 (1.4%) |
| No | 143 (59.1%) | 199 (63.8%) | 342 (61.7%) |
| Yes | 95 (39.3%) | 109 (34.9%) | 204 (36.8%) |
| Radiation prior to lymphedema diagnosis or within 18 months for those who were lymphedema-free | |||
| No | 76 (31.4%) | 98 (31.4%) | 174 (31.4%) |
| Yes | 166 (68.6%) | 214 (68.6%) | 380 (68.6%) |
| Baseline BMI | |||
| N | 242 | 312 | 554 |
| Median | 27.0 | 27.5 | 27.3 |
Lymphedema-free rates in the EO and LEAP groups were 69% vs 70%, respectively, for participants with SLND alone; 47% vs 33% for participants with ALND alone; and 54% vs 47% for those with both SLND and ALND. Approximately 15% of patients in both groups at 12- and 18-months had missing data and were by default not lymphedema-free. In the EO group, 141 (58%) remained free of lymphedema 18 months after surgery, compared to 172 (55%) in the LEAP group (OR = 0.77; 95%CI: (0.74, 2.15); p=0.73). A clinician diagnosed 36% of lymphedema cases, while the remainder were assessed as having lymphedema per the study protocol. The estimate of adjusted ICC of lymphedema, after adjusting for type of lymph node dissection and BMI, was 0.0485 (95% CI: (−0.004, 0.101)), suggesting a small intra-cluster correlation25. A complete case analysis was also undertaken as a sensitivity analysis. Results comparing the efficacy of the two intervention groups were not significantly different (p=0.39).
Among patients who had arm measurements at baseline as well as at post-surgery, severity of lymphedema as defined by changes in arm circumference at the site of greatest difference as a continuous variable between the groups indicated no difference in arm volume at 12 months post-surgery (estimated difference (LEAP – EO) = −.04; 95% CI = (−.97, 0.88); p =0.93) and at 18 months post-surgery (estimated difference (LEAP – EO) = .34; 95% CI = (−.97, 1.64); p =0.61). Since 95% (364 of 383) patients with both arm measurement and self-reported swelling data at 18 months reported none or mild swelling, no statistical test was performed on the agreement between patient self-reported swelling and the difference of change in arm circumference at the site of the greatest difference.
At pre-surgery, women in the LEAP group were less likely to report full ROM for both arms compared to women in the EO group (75% vs. 58%; p=0.0004). At 12 months post-surgery, women in the LEAP group reported greater ROM for both arms (right arm: 90% vs. 83%, p=0.02; left arm: 91% vs. 84%, p=0.16). There was a 32% (95% CI: (24%, 40%)) increase in those who reported full ROM in the LEAP group vs 6% (95% CI: (−2%, 13%)) increase (EO group) from pre-surgery to 12 months post-surgery (p<0.0001), and similar increases were observed in those same groups from pre-surgery to 18 months post-surgery (p<0.0001) (Table 3). At 18 months post-surgery, an equal percentage (93%) of women in both groups reported full ROM. Women in the LEAP group reported higher odds of having full ROM in the affected arm than women in the EO group at 12 months compared to baseline (OR: 5.62 (95% CI: (3.54, 8.93)) vs. 1.39 (95% CI: (0.81, 2.38)) as well as at 18 months (OR: 7.08 (95% CI: (4.32, 11.58)) vs. 1.55 (95% CI: (0.88, 2.71))) compared to baseline (Table 4).
Table 3:
Increase in Percent Reporting Full ROM by Treatment Group (Affected Arm Only)
| Treatment Group |
Time Period | Difference in % Full ROM (post follow-up – baseline) |
P-value |
|---|---|---|---|
| EO (n = 177) | 12 months vs pre-surgery | 6% increase (81% - 75%) | <0.0001* |
| LEAP (n = 215) | 32% increase (86% - 54%) | ||
| EO (n = 164) | 18 months vs pre-surgery | 13% increase (90% - 77%) | <0.0001* |
| LEAP (n = 211) | 36% increase (91% - 55%) |
chi-square
Table 4:
Results of Logistic Regression Model of Full ROM by Treatment Group by Time (Affected Arm Only)
| Treatment Group | Time Period | OR (Full vs Not Full ROM) | 95% CI | P-value |
|---|---|---|---|---|
| EO | 12 months vs pre-surgery | 1.39 | 0.81 –2.38 | 0.23 |
| EO | 18 months vs pre-surgery | 1.55 | 0.88 –2.71 | 0.13 |
| LEAP | 12 months vs pre-surgery | 5.62 | 3.54 –8.93 | <0.0001 |
| LEAP | 18 months vs pre-surgery | 7.08 | 4.34 –11.58 | <0.0001 |
Adherence to intervention activities in the LEAP group was low (Table 5). About half of the LEAP participants performed breathing/lymph flow, stretching and strengthening exercises as instructed, and even fewer (31%) wore the elastic garments as prescribed. Participants were more likely to wear the garments during exercise at 6 months, but by 18 months, participants reported wearing the garments with similar frequency across all activities (i.e., heavy arm use, exercise, air travel). Further analysis in the LEAP group showed no association between sleeve usage at 6 months (≥75% vs. <75%) and lymphedema-free rates (OR = 0.74, 95% CI: 0.40 – 1.36, p=0.3316). Lack of time (average 45.9% across all exercises and time points) and low perceived benefit (average 19.2% across all exercises and time points) were the most commonly reported reasons for not performing exercises as prescribed (data not shown).
Table 5.
Frequency of elastic sleeve use and exercises by visit for LEAP group
| 12 Months n (%) |
18 Months n (%) |
|
|---|---|---|
| Elastic sleeve use during heavy arm use, exercise or travel | ||
|
66 (31.6%) | 67 (31.3%) |
|
94 (45.0%) | 92 (43.0%) |
|
49 (23.4%) | 55 (25.7%) |
| Breathing & lymph flow exercises | ||
|
114 (54.0%) | 103 (47.9%) |
|
65 (30.8%) | 69 (32.1%) |
|
32 (15.2%) | 43 (20.0%) |
| Stretching exercises | ||
|
128 (60.7%) | 118 (55.4%) |
|
62 (29.4%) | 65 (30.5%) |
|
21 (10.0%) | 30 (14.1%) |
| Strengthening exercises | ||
|
117 (56.3%) | 100 (47.6%) |
|
55 (26.4%) | 60 (28.6%) |
|
36 (17.3%) | 50 (23.8%) |
DISCUSSION
Lymphedema is a potential adverse effect of breast cancer treatment. Prior studies have evaluated the impact of exercise, including weight training and stretching exercises, on either lymphedema development in women at-risk 26,27 or safety in women with diagnosed lymphedema 12,14,16,28. Focusing on women at risk for lymphedema, the current results are similar to those of Anderson et al. of no intervention effect with exercise 27. Another study, however, found that a lymphedema diagnosis was significantly higher (p=0.01) in the control (25%) than the intervention group (7%), and developed faster in the control group (HR, 0.26, p=0.01) 26. The major differences in that study were the exclusion of women who only had SLND and inclusion of several procedures performed by a physiotherapist (e.g., manual lymphatic drainage, massage of the surgical scar, etc.) in addition to instruction for prescribed exercises. Exercise has benefits, such as restoring strength, function, and ROM, improving overall well-being 29,30, and maintaining or developing muscle tone that helps mobilize fluid from the limb with or without compression garments 31,32, and its promise as a means of lymphedema risk-reduction is emerging. Recent studies of breast cancer patients that incorporate aerobic and/or resistance exercise, as well as regular compression garment use, suggest that these activities may reduce the incidence and severity of lymphedema, improve ROM and improve QOL33-35.
Lacomba et al. suggests that the addition of trained therapists conducting assisted activities that help establish drainage routes in the arm, might be necessary to reduce lymphedema in at-risk women 26. This technique should be further studied for effectiveness with a randomized controlled design as well as feasibility of implementation in varied settings, as not all therapists are trained in this technique and many patients do not have insurance to cover all lymphedema services. There is some evidence of the benefit of early rehabilitation following breast cancer surgery36.
The current study finding that women in the LEAP group experienced faster improvement in ROM in the affected arm at 12 months from baseline, compared to women in the EO group, is consistent with previous findings 37,38. This suggests that even minimal exercise may help women who undergo lymph node dissection to regain ROM quickly after surgery.
The lack of intervention effect in the current study might be due, in part, to low adherence with intervention components. About half of the LEAP group performed the breathing, stretching and strengthening exercises as instructed, but less than one-third used the compression garments as instructed. This result is similar to other studies examining self-management practices, including compression garment use 39-41. Thus, compression garment adherence may be one of the most challenging risk-reduction practices to adopt 42. Understanding which components of interventions have low adherence is important for developing and implementing interventions acceptable to patients.
This study has several strengths. With 554 women evaluable at Step 2, this represents the largest study for lymphedema risk-reduction to-date. Additionally, women were recruited from 38 National Clinical Trials Network (NCTN) sites across the U.S., assuring a representative sample of breast cancer patients that contributes to generalizability of results. Moreover, this trial used a standard lymphedema definition, with objective measurements taken by trained staff regularly assessed for quality.
Study weaknesses included the fact that information about exercise and/or sleeve use among EO participants was not collected; additional prevention activities aimed at reducing lymphedema risk may have occurred among EO women. In addition, the ITT analysis included all women; however, those lost to follow-up (19.3%) were assigned as treatment failures. Reassuringly, all sensitivity analyses confirmed the ITT analysis results. Finally, while we specified a definition of lymphedema to evaluate the main outcome, one-third of the women who developed lymphedema in this study were diagnosed using individual clinician-specified definitions; however, this was explored in sensitivity analyses and did not change the overall findings.
In summary, a group-randomized trial to test the effectiveness of two interventions to reduce lymphedema risk in women with primary breast cancer who underwent any type of LND failed to find a difference in lymphedema incidence, with about 20% of all participants developing lymphedema. This study established several important findings, including the positive impact of the intervention on a quicker return to full ROM of the affected arm, the ability to train staff at multiple NCTN institutions in the assessment and intervention protocol, and the need to assess adherence to intervention components. Future research could test adherence-enhancing strategies and assisted activities among women at risk for developing breast cancer-related lymphedema.
Acknowledgments
Support: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under the Award Number UG1CA189823 (Alliance for Clinical Trials in Oncology NCORP Grant), UG1CA189817, UG1CA189819, U10CA180790, U10CA180836, U10CA180850, UL1TR001409, Susan G Komen for the Cure (POP0600316), Lance Armstrong Foundation (66426) and a private donor. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST STATEMENT:
Dr. Loprinzi reports grants from NCI during the conduct of the study. Dr. Paskett reports grants from Merck Foundation and Pfizer during the conduct of the study.
Presented at the American Society of Clinical Oncology Cancer Survivorship Symposium: Advancing Care and Research; San Diego, CA; January 27, 2017
References
- 1.DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysi. Lancet Oncol. 2013;14(6):500–515. [DOI] [PubMed] [Google Scholar]
- 2.Armer JM, Stewart BR. Post-breast cancer lymphedema: incidence increases from 12 to 30 to 60 months. Lymphology. 2010;43(3):118–127. [PMC free article] [PubMed] [Google Scholar]
- 3.Shah C, Arthur D, Riutta J, Whitworth P, Vicini FA. Breast-cancer related lymphedema: a review of procedure-specific incidence rates, clinical assessment AIDS, treatment paradigms, and risk reduction. Breast J. 2012;18(4):357–361. [DOI] [PubMed] [Google Scholar]
- 4.Swenson KK, Nissen MJ, Leach JW, Post-White J. Case-control study to evaluate predictors of lymphedema after breast cancer surgery. Oncol Nurs Forum. 2009;36(2):185–193. [DOI] [PubMed] [Google Scholar]
- 5.Hidding JT, Beurskens CH, van der Wees PJ, van Laarhoven HW, Nijhuis-van der Sanden MW. Treatment related impairments in arm and shoulder in patients with breast cancer: a systematic review. PLoS One. 2014;9(5):e96748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wernicke AG, Goodman RL, Turner BC, et al. A 10-year follow-up of treatment outcomes in patients with early stage breast cancer and clinically negative axillary nodes treated with tangential breast irradiation following sentinel lymph node dissection or axillary clearance. Breast Cancer Res Treat. 2011;125(3):893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paskett ED, Naughton MJ, McCoy TP, Case LD, Abbott JM. The epidemiology of arm and hand swelling in premenopausal breast cancer survivors. Cancer Epidemiol Biomarkers Prev. 2007;16(4):775–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ribeiro Pereira ACP, Koifman RJ, Bergmann A. Incidence and risk factors of lymphedema after breast cancer treatment: 10 years of follow-up. Breast. 2017;36:67–73. [DOI] [PubMed] [Google Scholar]
- 9.Penha TR, Botter B, Heuts EM, Voogd AC, von Meyenfeldt MF, van der Hulst RR. Quality of life in patients with breast cancer-related lymphedema and reconstructive breast surgery. J Reconstr Microsurg. 2016;32(6):484–490. [DOI] [PubMed] [Google Scholar]
- 10.Kibar S, Dalyan Aras M, Unsal Delialioglu S. The risk factors and prevalence of upper extremity impairments and an analysis of effects of lymphoedema and other impairments on the quality of life of breast cancer patients. Eur J Cancer Care (Engl). 2017;26(4). [DOI] [PubMed] [Google Scholar]
- 11.Belmonte R, Messaggi-Sartor M, Ferrer M, Pont A, Escalada F. Prospective study of shoulder strength, shoulder range of motion, and lymphedema in breast cancer patients from pre-surgery to 5 years after ALND or SLNB. Support Care Cancer. 2018;26(9):3277–3287. [DOI] [PubMed] [Google Scholar]
- 12.Zhang X, Brown JC, Paskett ED, Zemel BS, Cheville AL, Schmitz KH. Changes in arm tissue composition with slowly progressive weight-lifting among women with breast cancer-related lymphedema. Breast Cancer Res Treat. 2017;164(1):79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winters-Stone KM, Laudermilk M, Woo K, Brown JC, Schmitz KH. Influence of weight training on skeletal health of breast cancer survivors with or at risk for breast cancer-related lymphedema. J Cancer Surviv. 2014;8(2):260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johansson K, Hayes S, Speck RM, Schmitz KH. Water-based exercise for patients with chronic arm lymphedema: a randomized controlled pilot trial. Am J Phys Med Rehabil. 2013;92(4):312–319. [DOI] [PubMed] [Google Scholar]
- 15.Sener HO, Malkoc M, Ergin G, Karadibak D, Yavuzsen T. Effects of clinical pilates exercises on patients developing lymphedema after breast cancer treatment: a randomized clinical trial. J Breast Health (2013). 2017;13(1):16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buchan J, Janda M, Box R, Schmitz K, Hayes S. A Randomized Trial on the Effect of Exercise Mode on Breast Cancer-Related Lymphedema. Med Sci Sports Exerc. 2016;48(10):1866–1874. [DOI] [PubMed] [Google Scholar]
- 17.Armer JM, Stewart BR. A comparison of four diagnostic criteria for lymphedema in a post-breast cancer population. Lymphat Res Biol. 2005;3(4):208–217. [DOI] [PubMed] [Google Scholar]
- 18.Lorig K, Stewart A, Ritter P, Gonzalez v, Laurent D, Lynch J. Outcome Measures for Health Education and Other Health Care Interventions. Thousand Oaks, CA: Sage; 1996. [Google Scholar]
- 19.Coster S, Poole K, Fallowfield LJ. The validation of a quality of life scale to assess the impact of arm morbidity in breast cancer patients post-operatively. Breast Cancer Res Treat. 2001;68(3):273–282. [DOI] [PubMed] [Google Scholar]
- 20.Mahamaneerat WK, Shyu CR, Stewart BR, Armer JM. Breast cancer treatment, BMI, post-op swelling/lymphoedema. J Lymphoedema. 2008;3(2):38–44. [PMC free article] [PubMed] [Google Scholar]
- 21.Ferguson CM, Swaroop MN, Horick N, et al. Impact of Ipsilateral Blood Draws, Injections, Blood Pressure Measurements, and Air Travel on the Risk of Lymphedema for Patients Treated for Breast Cancer. J Clin Oncol. 2016;34(7):691–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armer JM, Ballman KV, McCall L, et al. Lymphedema symptoms and limb measurement changes in breast cancer survivors treated with neoadjuvant chemotherapy and axillary dissection: results of American College of Surgeons Oncology Group (ACOSOG) Z1071 (Alliance) substudy. Support Care Cancer. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donner A, Klar N. Design and Analysis of Cluster Randomization Trials in Health Research. New York: Oxford Press; 2000. [Google Scholar]
- 24.Donner A, Donald A. The statistical analysis of multiple binary measurements. J Clin Epidemiol. 1988;41:899–905. [DOI] [PubMed] [Google Scholar]
- 25.Ridout MS, Demetrio CG, Firth D. Estimating intraclass correlation for binary data. Biometrics. 1999;55(1):137–148. [DOI] [PubMed] [Google Scholar]
- 26.Torres Lacomba M, Yuste Sanchez MJ, Zapico Goni A, et al. Effectiveness of early physiotherapy to prevent lymphoedema after surgery for breast cancer: randomised, single blinded, clinical trial. BMJ (Clinical research ed). 2010;340:b5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson RT, Kimmick GG, McCoy TP, et al. A randomized trial of exercise on well-being and function following breast cancer surgery: the RESTORE trial. J Cancer Surviv. 2012;6(2):172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmitz KH, Ahmed RL, Troxel A, et al. Weight lifting in women with breast-cancer-related lymphedema. The New England journal of medicine. 2009;361(7):664–673. [DOI] [PubMed] [Google Scholar]
- 29.Ahmed RL, Thomas W, Yee D, Schmitz KH. Randomized controlled trial of weight training and lymphedema in breast cancer survivors. J Clin Oncol. 2006;24(18):2765–2772. [DOI] [PubMed] [Google Scholar]
- 30.Courneya KS, McKenzie DC, Mackey JR, et al. Effects of exercise dose and type during breast cancer chemotherapy: multicenter randomized trial. J Natl Cancer Inst. 2013;105(23):1821–1832. [DOI] [PubMed] [Google Scholar]
- 31.Winters-Stone KM, Dobek J, Nail L, et al. Strength training stops bone loss and builds muscle in postmenopausal breast cancer survivors: a randomized, controlled trial. Breast Cancer Res Treat. 2011;127(2):447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winters-Stone KM, Dobek J, Bennett JA, Nail LM, Leo MC, Schwartz A. The effect of resistance training on muscle strength and physical function in older, postmenopausal breast cancer survivors: a randomized controlled trial. J Cancer Surviv. 2012;6(2):189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sweeney FC, Demark-Wahnefried W, Courneya KS, et al. Aerobic and resistance exercise improves shoulder function in women who are overweight or obese and have breast cancer: randomized, controlled trial. Phys Ther. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Omar MTA, Gwada RFM, Omar GSM, El-Sabagh RM, Mersal AAE. Low-intensity resistance training and compression garment in the management of breast cancer-related lymphedema: single-blinded randomized controlled trial. J Cancer Educ. 2019. [DOI] [PubMed] [Google Scholar]
- 35.Ochalek K, Partsch H, Gradalski T, Szygula Z. Do compression sleeves reduce the incidence of arm lymphedema and improve quality of life? Two-year results from a prospective randomized trial in breast cancer survivors. Lymphat Res Biol. 2019;17(1):70–77. [DOI] [PubMed] [Google Scholar]
- 36.Scaffidi M, Vulpiani MC, Vetrano M, et al. Early rehabilitation reduces the onset of complications in the upper limb following breast cancer surgery. Eur J Phys Rehabil Med. 2012;48(4):601–611. [PubMed] [Google Scholar]
- 37.Mazor M, Lee JQ, Peled A, et al. The effect of yoga on arm volume, strength, and range of motion in women at risk for breast cancer-related lymphedema. J Altern Complement Med. 2018;24(2):154–160. [DOI] [PubMed] [Google Scholar]
- 38.Park JH. The effects of complex exercise on shoulder range of motion and pain for women with breast cancer-related lymphedema: a single-blind, randomized controlled trial. Breast Cancer. 2017;24(4):608–614. [DOI] [PubMed] [Google Scholar]
- 39.Sherman KA, Miller SM, Roussi P, Taylor A. Factors predicting adherence to risk management behaviors of women at increased risk for developing lymphedema. Support Care Cancer. 2015;23(1):61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alcorso J, Sherman KA, Koelmeyer L, Mackie H, Boyages J. Psychosocial factors associated with adherence for self-management behaviors in women with breast cancer-related lymphedema. Support Care Cancer. 2016;24(1):139–146. [DOI] [PubMed] [Google Scholar]
- 41.Brown JC, Cheville AL, Tchou JC, Harris SR, Schmitz KH. Prescription and adherence to lymphedema self-care modalities among women with breast cancer-related lymphedema. Support Care Cancer. 2014;22(1):135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tidhar D, Katz-Leurer M. Aqua lymphatic therapy in women who suffer from breast cancer treatment-related lymphedema: a randomized controlled study. Support Care Cancer. 2010;18(3):383–392. [DOI] [PubMed] [Google Scholar]


