Abstract
There are sex differences in the development of cocaine addiction. For example, the time that it takes for women from initial use to addiction is significantly shorter than for men. Thus, understanding why females are more vulnerable to cocaine addiction will provide insights into sex differences in the mechanisms underlying cocaine addiction. This study aimed to determine how cocaine demand intensity and elasticity might differ between sexes. In addition, the impact of estrous cycle and cocaine intake on demand was investigated. Male and female rats were trained to self-administer 0.125 mg of cocaine intravenously under a chained schedule in daily 2-h sessions for two weeks, and then, the cocaine demand function was determined with a modified within-session threshold procedure. Following the test, the rats began to self-administer a higher dose of cocaine (0.25 mg) to increase the cocaine intake. The demand function was then similarly determined in the same rats after two weeks of cocaine SA of the higher dose. No sex differences were found in either demand intensity or elasticity. Neither did the level of cocaine intake have an impact on the demand. The demand elasticity, but not intensity, was significantly lower during proestrus/estrus compared with diestrus. These data suggest that the faster transition to cocaine addiction in females cannot be explained by sex differences in the demand for cocaine and such a demand may change during different phases of estrus cycle.
Keywords: cocaine, self-administration, elasticity, sex differences, estrous cycle
Introduction
Recreational cocaine use can lead to addiction in vulnerable individuals (Gawin 1991; Wagner and Anthony 2002). Identifying the factors that promote the transition from recreational use to addiction is important to our understanding of cocaine addiction and to the development of much-needed treatment strategies. Sex appears to have a significant impact on the vulnerability to cocaine addiction. For example, women show a faster escalation of cocaine use, a shorter time from initial use to seeking treatment, and higher total consumption by the time they seek treatment (Becker and Hu 2008; Greenfield et al. 2010; Griffin et al. 1989; Haas and Peters 2000; Lozano et al. 2008). The faster transition to addiction, or so-called “telescoping” phenomenon, suggests that cocaine has a greater impact on the processes related to the development of cocaine addiction in women.
One hypothesis is that cocaine may have a greater motivational impact on women than men. The greater motivational effects are expected to result in greater amount of cocaine consumption and consequently, promote a faster transition to cocaine addiction. There is some evidence from animal studies supporting this hypothesis. For example, the level of motivation is often measured with the progressive-ratio (PR) schedule of reinforcement in the preclinical studies. With this schedule, the response requirement for earning a reward is increased after each reinforcement until animals reach the breaking point (BP), defined as the last response requirement at which animals stop responding or fail to earn the reward within a predefined time window (Hodos 1961; Roberts et al. 1989b). The BP is used as an index of the level of motivation for the reward. Under the PR schedule, female rats selectively bred for high intake of saccharin showed a higher level of motivation for cocaine than male rats with the same trait (Carroll et al. 2002). Higher motivation for cocaine was also observed in female Sprague-Dawley rats with daily 24 h access under a discrete trial schedule (Lynch and Taylor 2004) and in female Wistar rats with daily 4 h access under the fixed-ratio (FR) 1 schedule (Roberts et al. 1989a). One concern with the BP method is that the BP is not only dependent on the level of motivation, but also the dose of cocaine (Bedford et al. 1978; Roberts et al. 1989b). Because males and females differ in body weight, blood volume, body fat percentage, and metabolism (Becker et al. 2005; Festa et al. 2004) it is very difficult to find equivalent doses of cocaine between sexes. The body weight-adjusted dose cannot guarantee dose equivalency. Given such a concern, it is important to develop a methodology that can measure the level of motivation without the confounding effect of the dose.
Behavioral economics examines the relationship between price and consumption of a commodity, and the demand function is used to describe this relationship (Hursh and Silberberg 2008). A fundamental law described by the function is that consumption goes down as the price goes up. The rate of decrease in consumption in response to the price increase, referred to as elasticity, is used to measure the essential value of a commodity (Hursh and Silberberg 2008). The consumption at the minimum price reflects the demand intensity or hedonic “set point” (Bentzley et al. 2013). Because a commodity with a higher essential value is expected to have greater motivational effects or values, the elasticity can be used as an indicator of the motivational value of the commodity (Kawa et al. 2016; Newman and Ferrario 2020). More importantly, the demand elasticity does not depend on the dose of drugs or amount of rewards (Bickel et al. 1990; Kearns and Silberberg 2016; Winger 1993). Based on these previous studies, a within-session threshold procedure was recently developed to measure the demand elasticity and intensity of cocaine (Oleson et al. 2011). With this procedure, consumption of cocaine is defined as the self-administered amount of cocaine, and unit price is the response requirement for earning one unit of cocaine (responses/mg). Using this procedure, several studies demonstrate no sex-differences in the demand elasticity for cocaine (Kawa and Robinson 2018; Lacy et al. 2019; 2020) suggesting that cocaine has similar motivational effect in both sexes. Note that the unit price for cocaine can be increased by different approaches. One approach is to increase the response requirement for a fixed dose of cocaine (Winger et al. 2006). Alternatively, the price can be changed by altering the dose without changing the response requirement (Oleson et al. 2011). There is evidence that the two approaches are functionally equivalent (Bickel et al. 1990). In the recent studies, the price for cocaine was increased by sequentially reducing the dose of cocaine on a quarter-log scale. One concern with such a small decrease in the dose is that animals may not be able to discriminate the difference of the two consecutive doses and therefore, may not subjectively experience the increase in the price. Lack of such discrimination could reduce the power of these studies to detect sex differences in the elasticity. One goal of the current study aimed to address this issue.
Another factor that may contribute to the female vulnerability to cocaine addiction is natural fluctuations of sex hormones during menstrual cycles. There is evidence that the effects of cocaine is affected by the cycle. For example, cocaine produces stronger subjective effects during the follicular phase compared with luteal phase (Evans et al. 2002; Sofuoglu et al. 1999) and the levels of sex hormones including estrogen and progesterone are correlated with the stress- or cue-induced craving in cocaine-dependent women (Sinha et al. 2007). Given these observations, another goal of the current study was to determine the impact of estrous cycle on the cocaine demand.
Materials and Methods
Subjects and Drugs
Outbred Wistar rats (Male: 320–380 g, n = 20; Female: 230–300 g, n = 15, Charles River) were housed individually in plastic home cages in a temperature- and humidity-controlled colony room on a 12-h reverse light-dark cycle (lights off at 08:00). Male and female rats were fed 20 and 15 g per day of rat food chow during self-administration training, respectively. Water was freely available, except during the period in which they stayed in the operant chambers. Experiments were conducted during the dark phase (between 09:00 and 18:00). Cocaine hydrochloride (the National Institute on Drug Abuse, Bethesda, MD) was dissolved in physiological saline to prepare solutions with the concentrations of 2.5, and 5 mg/ml (salt), respectively. All procedures followed the Guidelines for the Care and Use of Laboratory Animals (National Research Council 2011) and were approved by University of Tennessee Health Science Center Animal Care and Use Committee.
Sucrose Self-Administration
The rats were first trained to press a lever for a sucrose pellet (45 mg, Research Diet, New Brunswick, NJ) in a standard operant conditioning chamber equipped with two metal levers (Med Associates Inc., St. Albans, VT). Once they were able to obtain 100 pellets within two hours, training with the chained schedule of reinforcement began. The chained schedule requires sequential responses on the two levers, respectively, to earn sucrose pellets (Olmstead et al. 2000). Only one lever is available at one time and pressing the first lever leads to its retraction and access to the second lever after a delay. Pressing the second lever results in delivery of one sucrose pellet and its retraction followed by re-extension of the first lever after a timeout period. Thus, the fixed-ratio 1 (FR1) schedule is effective for both levers (chained FR1FR1). During sucrose self-administration (SA), a delay of 2 s between extensions of the two levers and timeout of 5 s after each reinforcement were used in the daily 30-minute sessions. The training ended once rats can earn 60 (female) or 100 (male) pellets with 30 min based on the observations that some females did not earn 100 pellets within the session time.
Surgery
The catheterization was performed under anesthesia with isoflurane. The catheter was made of ~12-cm polyurethane tubing (MRE-037; Braintree Scientific, Inc. MA, USA) connected to a rat vascular access button (VAB95BS; Instech, PA, USA). The button was placed subcutaneously in the mid-scapular region and the tubing was tunneled under the skin and inserted ~3.5 cm into the right external jugular vein. After surgery, buprenorphine (0.03 mg/kg, subcutaneously) was given for the post-surgical analgesia. All rats were allowed to recover for one week during which 0.1 ml of the antibiotic gentamicin (10 mg/ml, Biowhitaker, Walkersville, MD) was injected through the catheter daily, and the catheter was flushed twice a day with heparinized physiological saline (30 U/ml). Catheter patency was evaluated by injecting 0.1 ml Brevital (1%) through the catheter and loss of muscle tone within five seconds indicated the patency of the catheter.
Cocaine SA
The rats were sequentially trained to self-administer 0.125 and 0.25 mg of intravenous cocaine in daily 2-hour sessions under the same chained schedule as described for sucrose SA except that a 20-s timeout and a 10-s compound stimulus consisting of the two flashing cue lights (0.2 sec on and 0.2 sec off) above the levers and tone (3000 Hz) were used after the reinforced responses. These doses were chosen based on our previous studies (Sun and Rebec 2003; Xue et al. 2011) and are on the descending limb of the inverted-U dose-response curve of cocaine (Piazza et al. 2000). The session ended either when two hours had passed or 80 infusions had been self-administered, whichever occurred first. The training was conducted 5–6 days per week until they reached the criterion: the number of cocaine infusions varied by < 20% for three consecutive sessions and a minimum of 10 sessions of training. After the demand function was determined, the rats continued to self-administer cocaine at a dose of 0.25 mg for another 10 sessions followed by the determination of the demand function again.
Impact of Cocaine Intake on the Demand for Cocaine
The unit price of cocaine is typically defined as the number of responses required to earn one mg of cocaine and can be manipulated by changing either the response requirement for a fixed dose of cocaine, or changing the dose while keeping the response requirement constant (Oleson et al. 2011). The recently developed threshold procedure uses a within-session design to measure the demand function by changing the dose in each of 11 bins (10 min/bin) under the fixed-ratio 1 (FR1) schedule (Oleson and Roberts 2009; Zittel-Lazarini et al. 2007). The dose is decreased in each bin on a quarter-log scale. The demand curve is constructed as the cocaine consumption (mg/kg body weight) during the 10-min bins vs the unit price of cocaine (response/mg). Several modifications of the procedure was made in the current study. First, the demand function was determined under the chained schedule. The 20-s timeout after each infusion of cocaine remained effective to be consistent with the training procedure. The timeout was used to minimize the toxicity of cocaine in the early bins in case the rats self-administer cocaine too fast. The timeout may, however, limit the number of infusions delivered per bin, in particular, in the later bins during which the doses of cocaine are low. Note that the maximum number of cocaine infusions that can be delivered with the 20-s timeout during the 10-min bin is ~27. Among all the rats tested, the maximum number of infusions obtained was 26, which occurred in four rats. Thus, it is unlikely that the maximum number of infusions was limited by the 20-s timeout in the current study. Second, the modified procedure consisted of nine consecutive 10-min bins. The number of bins was reduced to shorten the duration of the session so that the effects of the drugs targeting different neurotransmitter systems in the brain could be studied. Our past microinjection studies demonstrate that the effects of various drugs microinjected into several brain regions rarely last longer than 90 min (Sun et al. 2005; Xue et al. 2012). Third, the dose was sequentially decreased by half. The drug discrimination studies provide evidence that rats can discriminate a 2-fold difference in the dose of cocaine within a defined dose range (Callahan et al. 1995; Mantsch and Goeders 1999). At the training dose of 0.125 mg, the nine doses for determining the demand function included: 125, 62.5, 31.25, 15.63, 7.81, 3.91, 1.95, 0.98, and 0.49 μg/infusion. At the training dose of 0.25 mg, the nine doses were: 250, 125, 62.5, 31.25, 15.63, 7.81, 3.91, 1.95, and 0.98 μg/infusion. The doses were changed by varying the infusion duration of the pump as described by others (Bentzley et al. 2013). The demand function was only determined once after the training with 0.125 and 0.25 mg, respectively, to avoid the concern that learning after repeated tests may alter the demand function. For example, it is suggested that animals may learn the strategy to self-administer cocaine as much as possible during the low-price bins (Lacy et al. 2019; 2020).
The extent of exposure to cocaine plays an important role in the development of cocaine addiction (Deroche-Gamonet et al. 2004; Vanderschuren and Everitt 2004). Thus, the impact of the different levels of cocaine intake on the demand function was studied by using two different training doses using a within-subject design. The previous studies demonstrate that cocaine intake increases with the self-administered doses within a certain dose range (Lile et al. 2002; Smith et al. 2006). Thus, the rats were first trained to self-administer cocaine at the dose of 0.125 mg for ~10 daily session and the demand function was then determined. Subsequently, the same rats began to self-administer 0.25 mg for another ~10 daily sessions followed by the determination of the demand function again.
Impact of Estrous Cycle on Cocaine Demand
The effects of estrous cycle were determined for the female rats by comparing proestrus/estrus and diestrus using a mixed-subject design with estrous phase and dose as the between- and within-subject factors, respectively. To this end, a subset of rats (n = 7) from the experiment above were monitored for the estrous phase. The vaginal smears were collected 30 min before the test sessions and assessed by vaginal cytology to determine the phase (Marcondes et al. 2002). Proestrus and estrus were combined because most females did not show sequential transition of the phases in the dark phase as reported before (Becker et al. 2005; Perry et al. 2015). The rats went through two normal estrous cycles (4–5 days per cycle) before testing.
Relationship between Cocaine Demand and Brain Concentration
There is evidence that animals self-administer cocaine to maintain a preferred level of cocaine concentration in the brain (Zimmer et al. 2012). Because cocaine consumption depends on the price, it is of interest to know how the brain concentration may be related to demand elasticity. To this end, we estimated the brain concentration of cocaine during the session based on the following equation
with C as the concentration (mg/L), t (min) as the time after cocaine infusions, d as the dose (mg/kg), k (0.233/min) as the rate constant for transfer of cocaine from the blood to the brain, v (0.15 L/kg) as the apparent volume of distribution of the brain, α (0.642/min) and β (0.097/min) as the rate constants related to removal of cocaine via redistribution and elimination (Bentzley et al. 2013; Pan et al. 1991). The time course of the concentration after each intravenous infusion of cocaine was calculated with the equation and then summed over 30 s intervals based on the time of each infusion during the session. The area under the curve (AUC) and the time course of the concentration were calculated for each rat and the relationship between the AUC and demand elasticity was then examined.
Statistics
The rate of cocaine SA was calculated as the number of infusions divided by the session duration. For the demand function, the consumption in each bin was calculated as the milligram of cocaine per kilogram of body weight and the price as number of responses required to obtain one milligram of cocaine. The data were fitted to the exponentiated demand function (Koffarnus et al. 2015):
with Q as the consumption per bin, Q0 as the preferred consumption when the price is zero, an indicator of the demand intensity, k as the range of the intake in logarithmic units, α as the parameter indicating the rate change of consumption, an indicator of the demand elasticity, and C as the price. The parameter of α is thought to scale with the essential or motivational value of a commodity (Hursh and Silberberg 2008). Recent studies demonstrate that the exponentiated demand model avoids discarding zero consumption data points or arbitrary replacement of zero consumption with small values, a strategy used in fitting the original logarithmic function (Hursh and Silberberg 2008). As a result, the exponentiated model significantly improves the model fits (Koffarnus et al. 2015). In addition, because animals typically load up on cocaine in the beginning of the session, it was suggested that the data from the first bin should be discarded when fitting the demand function (Bentzley et al. 2013; Zimmer et al. 2012) and consequently, the model’s fits were significantly improved. In the current study, the cocaine intake in the first bin was more than two standard deviations above the mean of intake from bin 2 to bin 4 for each dose in both sexes. Thus, the first bin data point was excluded in the analysis. In addition, we noticed that some rats had unusually low consumption of cocaine in the second bin following the first “loading-up” bin. We suspected that the high consumption of cocaine in the first bin may have dampened the cocaine consumption in the second bin in these rats. Thus, we decided to exclude the data from the first two bins when fitting our data. The value of k reflects the range of consumption in the logarithmic unit and was set to 2.5 based on the range of consumption calculated from the third and last bin (Koffarnus et al. 2015). The nonlinear least square method was used to fit the function to obtain the parameters of α, and Q0 using the curve fitting toolbox in Matlab (The MathWorks 2019).
The D’Agostino & Pearson omnibus was used to determine whether the distribution of the data deviates from the normal distribution. Spearman correlation coefficients were calculated for the brain concentrations and elasticity because the distribution of elasticity failed to past the normality test. A two-way mixed ANOVA with sex and dose as between- and within-subject factor was used to analyze the significance of the effects of sex and dose on the demand intensity and elasticity. Similarly, the effects of estrous cycle and dose were analyzed with a two-way mixed AVOVA with estrous cycle and dose as between- and within-subject factors, respectively. The analyses were conducted with GraphPad Prism version 8.30 (GraphPad Software, La Jolla California) and MATLAB and Statistics Toolbox Release 2020a, (The MathWorks, Inc., Natick, Massachusetts). The significance level was set at 0.05.
Results
Impact of Cocaine Training Dose on Cocaine Demand
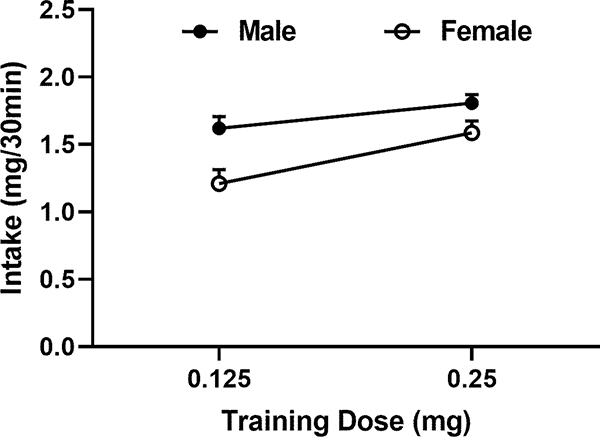
One of the male rats did not reach the training criteria at the dose of 0.125 mg and was excluded from the data analysis. The male and female rats reached the training criteria at the dose of 0.125 mg with an average of 8 ± 0.8 and 9 ± 1.1 (Mean ± SEM) sessions, respectively. At the dose of 0.25 mg, it took 3–4 days to reach the stabilized rates of cocaine SA. The average cocaine intake during the last three sessions before the determination of the demand functions is shown in Figure (Fig.) 1. There were significant main effects of dose (F1, 32 = 14.08, p = 0.0007) and sex (F1, 32 = 11.30, p = 0.002) on the intake. In either sex, the intake was higher for 0.25 vs 0.125 mg.
Fig. 1.
Impact of the training dose of cocaine on the intake. The intake of each rat was averaged across the first three sessions that met the training criteria. Data are represented as mean ± SEM across the rats. A two-way mixed ANOVA revealed a significant effect of dose on the intake (F1, 32 = 14.08, p = 0.0007). The intake was increased in both sexes but only reached the significance in female rats (t = 3.36, p < 0.05).
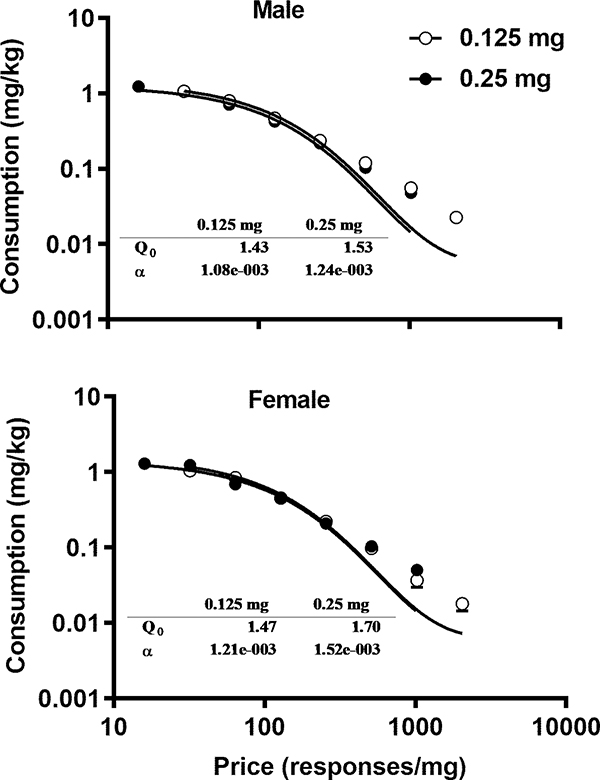
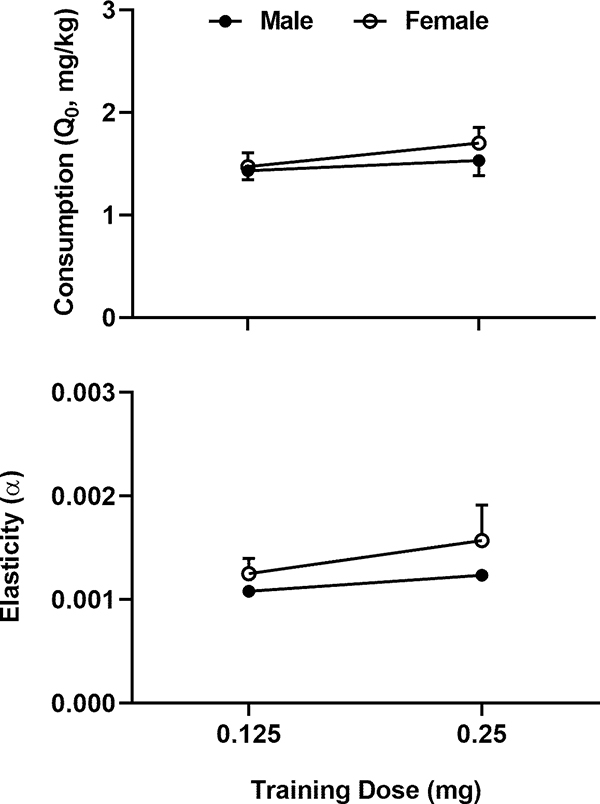
Consumption decreased as the unit price increased in both sexes as shown in Fig. 2. For the purpose of illustration, the data were averaged for each dose across the rats of each sex. The two-way mixed ANOVA analysis revealed no significant main effects of dose (F1, 32 = 2.270, p = 0.14) or sex (F1, 32 = 0.47, p = 0.49) on the demand intensity as shown in the upper panel of Fig. 3. The values of elasticity did not follow a normal distribution and were transformed by taking the inverse of the squares. The two-way mixed ANOVA analysis on the transformed data revealed no significant effects of dose (F1, 31 = 3.25, p = 0.08) or sex (F1, 31 = 0.03, p = 0.86) on the elasticity (the lower panel of Fig. 3). Thus, both demand elasticity and intensity were independent of the training dose of cocaine.
Fig. 2.
Impact of the price on cocaine consumption. The data were averaged across the rats under each condition and are represented as mean ± SEM. Upper Panel: Impact of the price on cocaine consumption after a history of cocaine SA of 0.125 and 0.25 mg of cocaine, respectively, in male rats. Lower Panel: Impact of the price on cocaine consumption after a history of cocaine SA of 0.125 and 0.25 mg of cocaine, respectively, in female rats. The insets show the averages of demand intensity and elasticity.
Fig. 3.
Effects of cocaine intake at different training doses and sex on the demand intensity and elasticity. The data from each individual rat were fitted to the exponentiated demand function and are represented as mean ± SEM. Upper Panel: Effects on the demand intensity. Lower Panel: Effects on the demand intensity. A two-way mixed ANOVA analysis did not reveal significant effects on either the demand intensity or elasticity.
Impact of Estrous Cycle on Cocaine Demand
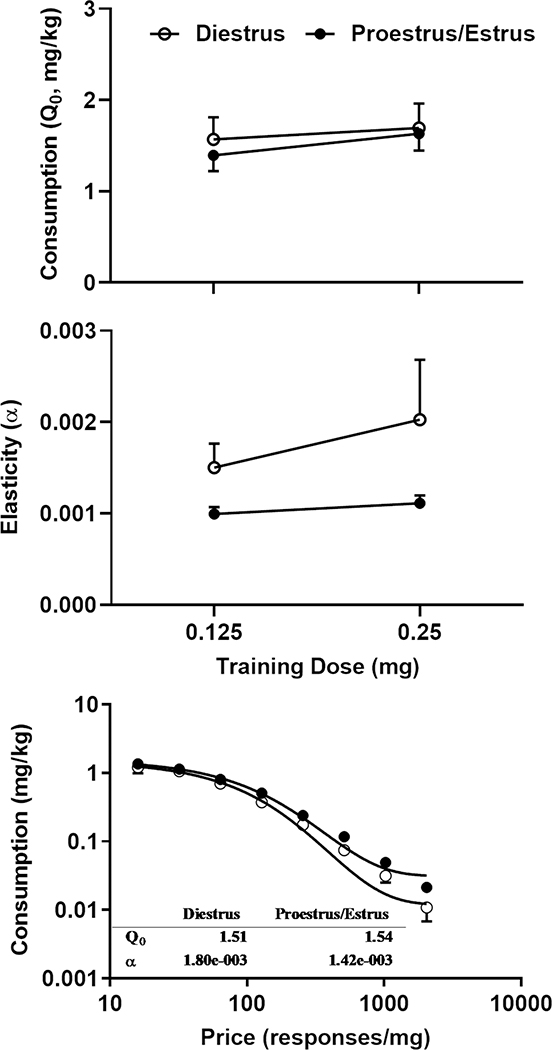
The estrous cycle was monitored for in a subpopulation of female rats. The phase of one rat was not determined. Consequently, two different sets of rats (n = 7 for each) were tested during proestrus/estrus and diestrus, respectively. The two-way mixed ANOVA analysis revealed that neither phase (F1, 12 = 0.22, p = 0.65) nor dose (F1, 12 = 0.99, p = 0.34) had significant effects on the demand intensity (the upper panel of Fig. 4). However, phase had significant effects on the elasticity (F1, 12 = 5.290, p = 0.04). Although there was an increasing trend during diestrus in response to the increase in the training dose, the two-way mixed ANOVA revealed no significant effects of dose on the elasticity (F1, 12 = 0.54, p = 0.47, the middle panel of Fig. 4). The demand curves during diestrus and proestrus/estrus were shown in the lower panel of Fig. 4. For the purpose of clarity, the data points based in the training doses of 0.125 and 0.25 mg were combined for each phase. This is based on the fact that one curve adequately fitted the data sets from two-dose series (Estrus: F 2, 94 = 1.15, p = 0.32; Diestrus: F 2, 94 =0.12, p = 0.88) indicating that the demand functions for the two data sets are not significantly different. Note that the error bars for the demand elasticity are relatively large (the middle panel of Fig. 4) compared with other figures. Because the phase was determined in a subset of rats, the smaller sample size likely contributed to the large errors. The individual data on the elasticity were presented in Table 1.
Fig. 4.
Effects of the estrous cycle on the demand intensity and elasticity. Two groups were tested during diestrus and proestrus/estrus, respectively. Top Panel: Effects on the demand intensity. Middle Panel: Effects on the demand intensity. Bottom Panel: the demand curves during diestrus and proestrus/estrus. A two-way mixed ANOVA analysis revealed a significant effect of the cycle on the elasticity (F1, 12 = 5.290, p = 0.04) but not intensity. The different levels of cocaine intake at two training doses had no significant effects.
Table 1.
Individual data on the demand elasticity during different estrous phases
| 0.125 mg a | 0.25 mg a | ||
|---|---|---|---|
| Diestrus | Estrus | Diestrus | Estrus |
| 0.00277 | 0.00139 | 0.00320 | 0.00092 |
| 0.00196 | 0.00083 | 0.00099 | 0.00145 |
| 0.00095 | 0.00091 | 0.00551 | 0.00098 |
| 0.00142 | 0.00095 | 0.00128 | 0.00089 |
| 0.00084 | 0.00093 | 0.00129 | 0.00134 |
| 0.00097 | 0.00084 | 0.00102 | 0.00098 |
| 0.00111 | 0.00122 | ||
the training dose before the demand function was determined.
Relationship between Cocaine Demand and Brain Concentration
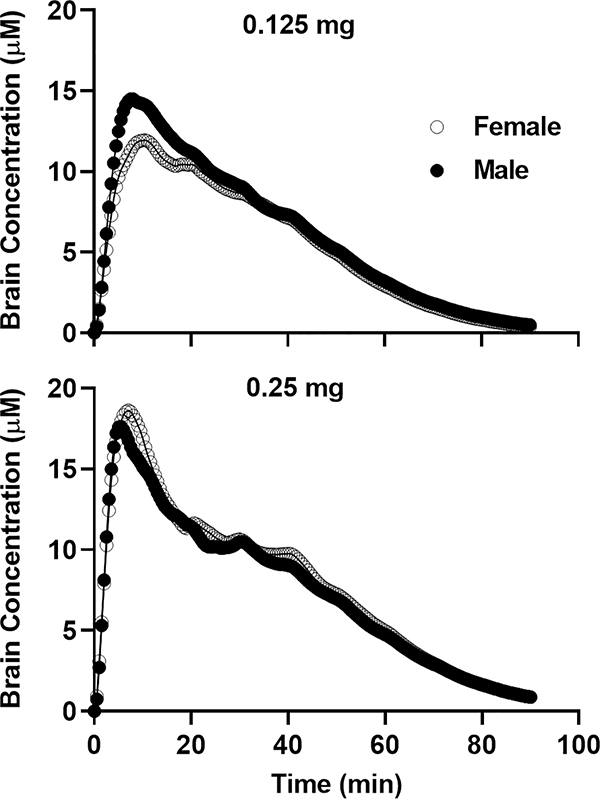
The time course of the calculated brain concentration of cocaine is shown in Fig. 5. For the purpose of illustration, the data were based on the demand testing session following the SA training with 0.125 or 0.25 mg of cocaine and averaged for all the rats of either sex. The AUC was calculated for each rat based on the time course of the concentration. As shown in the upper panel, male rats reached a higher peak concentration during the first 10-min bin (loading period) than female rats during the economic test session with the dose beginning at 0.125 mg. Such a difference disappeared at the dose of 0.25 mg. The Spearman correlation between the demand elasticity and AUC was calculated. As shown in Table 1, a significant correlation was found in three out of four conditions (two sexes by two doses).
Fig. 5.
Time course of the concentrations of cocaine in the brain during the demand test sessions. The concentrations of cocaine in the brain were calculated with 30-s intervals across the 90-min session. The average of the time courses across rats is shown here. Upper Panel: The time course during the session beginning with 0.125 mg of cocaine. Lower Panel: The time course during the session beginning with 0.25 mg of cocaine.
Discussion
Sex differences exist in the vulnerability to cocaine addiction, and such differences could result from the differential motivational effects of cocaine between sexes. Based on the theory of behavioral economics, the motivational value of a commodity should scale with its demand elasticity (negatively correlated). Using a modified threshold procedure, the current study measured the demand intensity and elasticity for cocaine in both sexes. Estrous cycle had a significant impact on elasticity that was significantly decreased during proestrus/estrus compared with diestrus. The demand was not affected by sex, or the level of cocaine intake.
Significant efforts have been directed at understanding the mechanism underlying sex differences in the vulnerability to cocaine addiction. One hypothesis is that the cocaine produces greater rewarding effects in females and consequently, increases the probability of future use and faster transition to cocaine addiction. Indeed, the greater reinforcing effects in female animals at low doses have been reported (Campbell et al. 2002; Lynch and Carroll 1999). Such a difference, however, disappears or even reverses at high doses (Caine et al. 2004; Haney et al. 1995). Recreational users likely sample doses to achieve the desired effects. Thus, sex differences in the rewarding effects of cocaine are unlikely to play a significant role in the faster transition to cocaine addiction in women. Alternatively, cocaine may have higher motivational value in females. The pathological motivation for cocaine is thought to play a key role in cocaine addiction (Flagel et al. 2009; Kalivas et al. 2005; Volkow and Fowler 2000). Thus, the faster transition to addiction may result from the greater motivational effects of cocaine in females. The evidence supporting this idea mainly comes from the studies using the PR schedule. Roberts’ group may be the first to report that female rats reach significantly higher BPs than male rats (Roberts et al. 1989a). Although these results have been replicated by others (Carroll et al. 2002), no sex differences in the BPs have also been reported (Cummings et al. 2011; Lynch and Taylor 2004; 2005). As discussed in introduction, BPs are a dose-dependent variable (Bedford et al. 1978; Depoortere et al. 1993; Roberts et al. 1989b) and it is difficult to determine the equivalent doses between two sexes. Thus, different doses used in these studies may contribute to the different results. On the other hand, the demand elasticity reflects intrinsic value of a commodity and thus, can be used as an indicator of the motivational value independent of the drug dose. Recent studies used the within-session threshold procedure to examine the demand function and demonstrated no sex differences in the demand elasticity or intensity of cocaine (Kawa and Robinson 2018; Lacy et al. 2019; 2020). Given the fact that the doses were decreased on a quarter-log scale in these studies, the difference between two consecutive doses may be too small to be discriminated by animals. The poor discrimination could contribute to the failure in detecting sex difference in the cocaine demand. To address this issue, the current study increased the difference between two consecutive doses that is expected to increase the dose discriminability. Indeed, the drug discrimination studies provide evidence that a 2-fold difference in the dose within a defined dose range is well discriminated by rats (Callahan et al. 1995; Mantsch and Goeders 1999). Consistent with the previous studies, our data demonstrate no sex differences in the elasticity. Given the same conclusion obtained under the different conditions, differential motivational impact of cocaine is unlikely to fully explain sex differences in the vulnerability to cocaine addiction.
The menstrual cycle alters the subjective effects of cocaine. For example, women experience more intense rewarding effects during the follicular vs luteal phase (Evans et al. 2002; Sofuoglu et al. 1999). Preclinical studies also demonstrate that female rats have higher BPs for cocaine during the estrous vs metestrus/diestrus (Hecht et al. 1999; Roberts et al. 1989a). However, the motivation measured as the demand elasticity is not different between metestrus/diestrus and estrus (Lacy et al. 2020). In addition, the latter study showed a significant increase in the demand intensity during estrus vs metestrus/diestrus. These results are different from the current study. Our data showed that the elasticity during proestrus/estrus was significantly higher compared with that during diestrus and no difference was found for the demand intensity. It is unclear how to reconcile these results. There are several procedural differences between the two studies. First, the demand function was determined under the chain schedule in the current study vs the regular FR1 schedule. The chained schedule in the current study is similar to the FR2 schedule. This difference, however, may not explain the different results because the consumption under low FR schedules do not differ significantly (Christensen et al. 2008; Koffarnus and Woods 2013; Wade-Galuska et al. 2007). Second, the estrus phases are not exactly the same. In the current study, proestrus and estrus were combined to compare with diestrus. The previous study combined metestrus and diestrus together to compare with estrus. Such a difference could contribute to the different results. Third and more importantly, the price for cocaine was increased at a steeper rate to increase the discriminability between the two consecutive doses. The steeper increase may have allowed us to detect the impact of the estrous cycle on the elasticity. The impact of the steepness of the price increase on the demand function has not been carefully examined. Given the limited data on this issue, further studies are warranted.
In the current study, the impact of cocaine intake on the demand function was determined by allowing the rats to self-administer two different doses of cocaine. The different levels of intake did not alter the elasticity or intensity of the demand for cocaine in either sex. The previous studies demonstrate that the extent of cocaine SA is important to the development of addiction-like behaviors in rats. For example, a subpopulation of rats developed addiction-like behaviors including increased motivation, persistent drug seeking behavior in the signaled absence of cocaine or in face of the negative consequences after a prolonged (> 70 days), but not limited (< 30 days), period of cocaine SA (Deroche-Gamonet et al. 2004). In addition, an extended (6 hr) but not limited (1 hr) daily access to cocaine for approximately two weeks also facilitated the development of the compulsive cocaine-seeking behavior (Pelloux et al. 2012; Pelloux et al. 2015). These data suggest that the level of cocaine intake may play an important role in the development of cocaine addiction. In contrast, the current study failed to observe the impact of cocaine intake on the demand elasticity or intensity. This could be related to the limited period training at each dose or relatively short session (2 hr). Indeed, with the long-access model (daily 6-h session), the demand intensity and elasticity are altered (Bentzley et al. 2014; James et al. 2018). Thus, lack of the impact of cocaine intake on the demand for cocaine should be interpreted within the current experimental conditions.
The regularly spaced cocaine SA observed during the FR training session maintains a relatively stable level of cocaine concentration in the brain (Zimmer et al. 2012). Under the within-session threshold procedure, the consumption gradually goes down as the price goes up. It is unknown how the demand function is related to the brain concentration of cocaine. The demand intensity indicates the preferred consumption when the price is zero. The elasticity is directly related to the motivational value of cocaine and thus, to the effort made to defend the preferred consumption. The inelastic rats are expected to be less sensitive to the price increase and thus, earn more infusions of cocaine compared with the elastic rats. It is expected that the brain concentration of cocaine should scale with the elasticity during the threshold session. To test this hypothesis, we calculated the AUC under the time course of the concentration from the two threshold sessions. The AUC was used to indicate the overall load of cocaine in the brain during the session. Among the four conditions (two sexes and two threshold sessions), we found that the AUC was correlated with elasticity for all conditions except for the threshold session conducted after the male rats were trained to self-administer 0.125 mg of cocaine. These data provide preliminary evidence that the AUC can be used as a correlate for the demand elasticity. Interestingly, sex hormones have an impact on the metabolism of cocaine. For example, estrogen decreased the brain concentration of cocaine after intraperitoneal administration (Niyomchai et al. 2006). Given the fact that the estrogen level is significantly higher during proestrus/estrus compared with diestrus (Smith et al. 1975; Staley and Scharfman 2005), the brain concentration of cocaine is likely lower during estrus. Thus, it is possible that rats have to increase the behavioral effort to defend the preferred level of cocaine in the brain. Thus, the differential impact of estrous phase on the metabolism could contribute to the phase effects on the demand elasticity of cocaine.
A couple of issues need to be considered when interpreting the results of the current study. Sex hormones including estrogen and progesterone fluctuate during estrous cycle (Smith et al. 1975; Staley and Scharfman 2005) and fluctuation of these hormones is largely responsible for the differential behavioral effects of cocaine during different phases of the estrous cycle (Becker and Koob 2016; Lynch and Taylor 2005; Perry et al. 2013). The estrous cycle was monitored based on cytology of vaginal smears in the current study. Ideally, the phase should be ascertained by monitoring the levels of sex hormones in the blood. To minimize stress, we elected to avoid collecting the blood sample before the cocaine SA session. It is also impractical to know the hormone levels before the session given the time needed to analyze the sample. The vaginal smear method has been widely used to determine the estrous phase. More importantly, the method has produced consistent results across a number of studies indicating the reliability of the method (Johnson et al. 2019; Lacy et al. 2019; 2020; Lynch et al. 2000; Roberts et al. 1987). Thus, it is unlikely that the conclusions from current study are significantly affected by lack of the data on the hormone levels in the blood. Another potential concern is that cocaine doses were not controlled for body weights in the current study. Given that the males were significantly heavier, the relative doses based on body weight were significantly higher in females. The reason that we did not base the cocaine dose on body weight was based on the fact that the demand function is not dependent on the dose (Bickel et al. 1990; Kearns and Silberberg 2016; Winger 1993). This is the main advantage of the behavioral economic methodology over the PR schedule. Thus, the negative impact of the dose-related issue on the conclusions of the current study is likely minimal. Another concern is that the demand function was fit to the data without first two bin points. It has been argued that the demand function is influenced by the economy type of a commodity, i.e., whether the commodity is available outside the economic session (Hursh 1980). A previous study demonstrated that the demand elasticity of cocaine was altered by the cheap alternative source of cocaine during the economic session (Kearns and Silberberg 2019). Thus, it can be argued that availability of cocaine during the first two bins may act as a cheap source and subsequently affect the demand elasticity. Note, however, that this study provided the cheap source of cocaine throughout the economic session based on variable time schedule. It had a significant impact on the consumption of cocaine at high unit prices. This should be not surprising because switching from the high price to low price source is expected. However, such a cheap source had little impact on the consumption at the low unit price (Kearns and Silberberg 2019). Given the fact that there were no cheap sources of cocaine when the unit prices were high in the current study and the minimal impact of the semi-economy on the consumption at the low prices, it is unlikely that our approach to the data analysis significantly affected the demand elasticity of cocaine.
In summary, the results from the current study showed that the estrous cycle had a significant impact on the demand elasticity for cocaine although no sex differences were found in the demand intensity or elasticity. Recent studies indicate that the reasons for men and women to use drugs are different. Pursuing the euphoric effect or thrill is the main driver for men whereas dealing with stress-related issues is the main driver for women (Becker et al. 2012; Kuntsche and Muller 2012). Pre-existing conditions such chronic stress and depression in women are thought to play a critical role in the faster transition to addiction (Becker et al. 2012). To better understand sex differences in the vulnerability to cocaine addiction, incorporating pre-existing conditions into animal models is needed to determine whether sex differences exist in the impact of these conditions on the development of cocaine addiction.
Table 2.
Correlation between the elasticity and brain concentration of cocaine
| 0.125 mg a | 0.25 mg | |||||
|---|---|---|---|---|---|---|
| p | r | n | p | r | n | |
| Male | 0.18 | −0.32 | 19 | 0.04 | −0.47 | 19 |
| Female | 0.0003 | −0.83 | 15 | 0.009 | −0.66 | 15 |
the training dose before the demand function was determined.
Data are shown as probability (p), Spearman correlation coefficient (r), and sample size (n)
Acknowledgements
The project was supported by Grant Number DA034776 (WLS) from the National Institute on Drug Abuse and its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIDA or NIH. All procedures followed the National Institute of Health Guidelines for the Care and Use of Laboratory Animals.
Footnotes
Conflicts of Interest
There are no conflicts of interest.
References
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E (2005) Strategies and methods for research on sex differences in brain and behavior. Endocrinology 146: 1650–73. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M (2008) Sex Differences in Drug Abuse. Front Neuroendocrin 29: 36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Koob GF (2016) Sex Differences in Animal Models: Focus on Addiction. Pharmacological reviews 68: 242–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Perry AN, Westenbroek C (2012) Sex differences in the neural mechanisms mediating addiction: a new synthesis and hypothesis. Biol Sex Differ 3: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford JA, Bailey LP, Wilson MC (1978) Cocaine reinforced progressive ratio performance in the rhesus monkey. Pharmacology, biochemistry, and behavior 9: 631–8. [DOI] [PubMed] [Google Scholar]
- Bentzley BS, Fender KM, Aston-Jones G (2013) The behavioral economics of drug self-administration: a review and new analytical approach for within-session procedures. Psychopharmacology 226: 113–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley BS, Jhou TC, Aston-Jones G (2014) Economic demand predicts addiction-like behavior and therapeutic efficacy of oxytocin in the rat. Proc Natl Acad Sci U S A 111: 11822–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, DeGrandpre RJ, Higgins ST, Hughes JR (1990) Behavioral economics of drug self-administration. I. Functional equivalence of response requirement and drug dose. Life Sci 47: 1501–10. [DOI] [PubMed] [Google Scholar]
- Caine SB, Bowen CA, Yu G, Zuzga D, Negus SS, Mello NK (2004) Effect of gonadectomy and gonadal hormone replacement on cocaine self-administration in female and male rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 29: 929–42. [DOI] [PubMed] [Google Scholar]
- Callahan PM, Bryan SK, Cunningham KA (1995) Discriminative stimulus effects of cocaine: antagonism by dopamine D1 receptor blockade in the amygdala. Pharmacology, Biochemistry & Behavior 51: 759–66. [DOI] [PubMed] [Google Scholar]
- Campbell UC, Morgan AD, Carroll ME (2002) Sex differences in the effects of baclofen on the acquisition of intravenous cocaine self-administration in rats. Drug and alcohol dependence 66: 61–9. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Lynch WJ, Campbell UC, Dess NK (2002) Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: phenotype and sex differences. Psychopharmacology 161: 304–13. [DOI] [PubMed] [Google Scholar]
- Christensen CJ, Silberberg A, Hursh SR, Huntsberry ME, Riley AL (2008) Essential value of cocaine and food in rats: tests of the exponential model of demand. Psychopharmacology 198: 221–9. [DOI] [PubMed] [Google Scholar]
- Cummings JA, Gowl BA, Westenbroek C, Clinton SM, Akil H, Becker JB (2011) Effects of a selectively bred novelty-seeking phenotype on the motivation to take cocaine in male and female rats. Biol Sex Differ 2: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depoortere RY, Li DH, Lane JD, Emmett-Oglesby MW (1993) Parameters of self-administration of cocaine in rats under a progressive-ratio schedule. Pharmacology, biochemistry, and behavior 45: 539–48. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV (2004) Evidence for addiction-like behavior in the rat. Science 305: 1014–7. [DOI] [PubMed] [Google Scholar]
- Evans SM, Haney M, Foltin RW (2002) The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology 159: 397–406. [DOI] [PubMed] [Google Scholar]
- Festa ED, Russo SJ, Gazi FM, Niyomchai T, Kemen LM, Lin SN, Foltz R, Jenab S, Quinones-Jenab V (2004) Sex differences in cocaine-induced behavioral responses, pharmacokinetics, and monoamine levels. Neuropharmacology 46: 672–87. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Akil H, Robinson TE (2009) Individual differences in the attribution of incentive salience to reward-related cues: Implications for addiction. Neuropharmacology 56 Suppl 1: 139–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawin FH (1991) Cocaine addiction: psychology and neurophysiology. Science 251: 1580–6. [DOI] [PubMed] [Google Scholar]
- Greenfield SF, Back SE, Lawson K, Brady KT (2010) Substance abuse in women. Psychiatr Clin North Am 33: 339–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin ML, Weiss RD, Mirin SM, Lange U (1989) A comparison of male and female cocaine abusers. Arch Gen Psychiatry 46: 122–6. [DOI] [PubMed] [Google Scholar]
- Haas AL, Peters RH (2000) Development of substance abuse problems among drug-involved offenders - Evidence for the telescoping effect. Journal of Substance Abuse 12: 241–253. [DOI] [PubMed] [Google Scholar]
- Haney M, Maccari S, Le Moal M, Simon H, Piazza PV (1995) Social stress increases the acquisition of cocaine self-administration in male and female rats. Brain research 698: 46–52. [DOI] [PubMed] [Google Scholar]
- Hecht GS, Spear NE, Spear LP (1999) Changes in progressive ratio responding for intravenous cocaine throughout the reproductive process in female rats. Dev Psychobiol 35: 136–45. [PubMed] [Google Scholar]
- Hodos W (1961) Progressive ratio as a measure of reward strength. Science 134: 943–4. [DOI] [PubMed] [Google Scholar]
- Hursh SR (1980) Economic concepts for the analysis of behavior. J Exp Anal Behav 34: 219–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, Silberberg A (2008) Economic demand and essential value. Psychol Rev 115: 186–98. [DOI] [PubMed] [Google Scholar]
- James MH, Bowrey HE, Stopper CM, Aston-Jones G (2018) Demand elasticity predicts addiction endophenotypes and the therapeutic efficacy of an orexin/hypocretin-1 receptor antagonist in rats. Eur J Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AR, Thibeault KC, Lopez AJ, Peck EG, Sands LP, Sanders CM, Kutlu MG, Calipari ES (2019) Cues play a critical role in estrous cycle-dependent enhancement of cocaine reinforcement. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J (2005) Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron 45: 647–50. [DOI] [PubMed] [Google Scholar]
- Kawa AB, Bentzley BS, Robinson TE (2016) Less is more: prolonged intermittent access cocaine self-administration produces incentive-sensitization and addiction-like behavior. Psychopharmacology 233: 3587–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawa AB, Robinson TE (2018) Sex differences in incentive-sensitization produced by intermittent access cocaine self-administration. Psychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns DN, Silberberg A (2016) Dose and elasticity of demand for self-administered cocaine in rats. Behavioural pharmacology 27: 289–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns DN, Silberberg A (2019) Opening the cocaine economy by providing within-session access to a cheaper source of cocaine makes demand for it more elastic. Behavioural pharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffarnus MN, Franck CT, Stein JS, Bickel WK (2015) A modified exponential behavioral economic demand model to better describe consumption data. Exp Clin Psychopharmacol 23: 504–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffarnus MN, Woods JH (2013) Individual differences in discount rate are associated with demand for self-administered cocaine, but not sucrose. Addiction biology 18: 8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntsche E, Muller S (2012) Why Do Young People Start Drinking? Motives for First-Time Alcohol Consumption and Links to Risky Drinking in Early Adolescence. Eur Addict Res 18: 34–39. [DOI] [PubMed] [Google Scholar]
- Lacy RT, Austin BP, Strickland JC (2019) The influence of sex and estrous cyclicity on cocaine and remifentanil demand in rats. Addiction biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy RT, Austin BP, Strickland JC (2020) The influence of sex and estrous cyclicity on cocaine and remifentanil demand in rats. Addiction biology 25: e12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lile JA, Morgan D, Birmingham AM, Wang Z, Woolverton WL, Davies HM, Nader MA (2002) The reinforcing efficacy of the dopamine reuptake inhibitor 2beta-propanoyl-3beta-(4-tolyl)-tropane (PTT) as measured by a progressive-ratio schedule and a choice procedure in rhesus monkeys. The Journal of pharmacology and experimental therapeutics 303: 640–8. [DOI] [PubMed] [Google Scholar]
- Lozano OM, Domingo-Salvany A, Martinez-Alonso M, Brugal MT, Alonso J, de la Fuente L, Investigators I (2008) Health-related quality of life in young cocaine users and associated factors. Qual Life Res 17: 977–985. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Arizzi MN, Carroll ME (2000) Effects of sex and the estrous cycle on regulation of intravenously self-administered cocaine in rats. Psychopharmacology 152: 132–9. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME (1999) Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology 144: 77–82. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Taylor JR (2004) Sex differences in the behavioral effects of 24-h/day access to cocaine under a discrete trial procedure. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 29: 943–51. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Taylor JR (2005) Persistent changes in motivation to self-administer cocaine following modulation of cyclic AMP-dependent protein kinase A (PKA) activity in the nucleus accumbens. Eur J Neurosci 22: 1214–20. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Goeders NE (1999) Ketoconazole blocks the stress-induced reinstatement of cocaine-seeking behavior in rats: relationship to the discriminative stimulus effects of cocaine. Psychopharmacology 142: 399–407. [DOI] [PubMed] [Google Scholar]
- Marcondes FK, Bianchi FJ, Tanno AP (2002) Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol 62: 609–14. [DOI] [PubMed] [Google Scholar]
- National Research Council (2011) Guide for the Care and Use of Laboratory Animals, Eighth Edition edn. The National Academies, Washington, DC [Google Scholar]
- Newman M, Ferrario CR (2020) An improved demand curve for analysis of food or drug consumption in behavioral experiments. Psychopharmacology 237: 943–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyomchai T, Akhavan A, Festa ED, Lin SN, Lamm L, Foltz R, Quinones-Jenab V (2006) Estrogen and progesterone affect cocaine pharmacokinetics in female rats. Brain Res Bull 68: 310–4. [DOI] [PubMed] [Google Scholar]
- Oleson EB, Richardson JM, Roberts DC (2011) A novel IV cocaine self-administration procedure in rats: differential effects of dopamine, serotonin, and GABA drug pre-treatments on cocaine consumption and maximal price paid. Psychopharmacology 214: 567–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleson EB, Roberts DC (2009) Behavioral economic assessment of price and cocaine consumption following self-administration histories that produce escalation of either final ratios or intake. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 34: 796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmstead MC, Parkinson JA, Miles FJ, Everitt BJ, Dickinson A (2000) Cocaine-seeking by rats: regulation, reinforcement and activation. Psychopharmacology 152: 123–31. [DOI] [PubMed] [Google Scholar]
- Pan HT, Menacherry S, Justice JB Jr. (1991) Differences in the pharmacokinetics of cocaine in naive and cocaine-experienced rats. J Neurochem 56: 1299–306. [DOI] [PubMed] [Google Scholar]
- Pelloux Y, Dilleen R, Economidou D, Theobald D, Everitt BJ (2012) Reduced Forebrain Serotonin Transmission is Causally Involved in the Development of Compulsive Cocaine Seeking in Rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 37: 2505–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelloux Y, Murray JE, Everitt BJ (2015) Differential vulnerability to the punishment of cocaine related behaviours: effects of locus of punishment, cocaine taking history and alternative reinforcer availability. Psychopharmacology 232: 125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry AN, Westenbroek C, Becker JB (2013) Impact of pubertal and adult estradiol treatments on cocaine self-administration. Hormones and behavior 64: 573–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry AN, Westenbroek C, Jagannathan L, Becker JB (2015) The Roles of Dopamine and alpha1-Adrenergic Receptors in Cocaine Preferences in Female and Male Rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 40: 2696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Deroche-Gamonent V, Rouge-Pont F, Le Moal M (2000) Vertical shifts in self-administration dose-response functions predict a drug-vulnerable phenotype predisposed to addiction. Journal of Neuroscience 20: 4226–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DC, Bennett SA, Vickers GJ (1989a) The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology 98: 408–11. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Dalton JC, Vickers GJ (1987) Increased self-administration of cocaine following haloperidol: effect of ovariectomy, estrogen replacement, and estrous cycle. Pharmacology, biochemistry, and behavior 26: 37–43. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Loh EA, Vickers G (1989b) Self-administration of cocaine on a progressive ratio schedule in rats: dose-response relationship and effect of haloperidol pretreatment. Psychopharmacology 97: 535–8. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fox H, Hong KI, Sofuoglu M, Morgan PT, Bergquist KT (2007) Sex steroid hormones, stress response, and drug craving in cocaine-dependent women: implications for relapse susceptibility. Exp Clin Psychopharmacol 15: 445–52. [DOI] [PubMed] [Google Scholar]
- Smith JE, Co C, Coller MD, Hemby SE, Martin TJ (2006) Self-administered heroin and cocaine combinations in the rat: additive reinforcing effects-supra-additive effects on nucleus accumbens extracellular dopamine. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 31: 139–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MS, Freeman ME, Neill JD (1975) The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology 96: 219–26. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK (1999) Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmacol 7: 274–83. [DOI] [PubMed] [Google Scholar]
- Staley K, Scharfman H (2005) A woman’s prerogative. Nat Neurosci 8: 697–699. [DOI] [PubMed] [Google Scholar]
- Sun W, Akins CK, Mattingly AE, Rebec GV (2005) Ionotropic glutamate receptors in the ventral tegmental area regulate cocaine-seeking behavior in rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 30: 2073–81. [DOI] [PubMed] [Google Scholar]
- Sun W, Rebec GV (2003) Lidocaine inactivation of ventral subiculum attenuates cocaine-seeking behavior in rats. J Neurosci 23: 10258–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The MathWorks I (2019) MATLAB and Statistics Toolbox Release 2019a. The MathWorks, Inc,, Natick, Massachusetts, United States. [Google Scholar]
- Vanderschuren LJ, Everitt BJ (2004) Drug seeking becomes compulsive after prolonged cocaine self-administration. Science 305: 1017–9. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS (2000) Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex 10: 318–25. [DOI] [PubMed] [Google Scholar]
- Wade-Galuska T, Winger G, Woods JH (2007) A behavioral economic analysis of cocaine and remifentanil self-administration in rhesus monkeys. Psychopharmacology 194: 563–72. [DOI] [PubMed] [Google Scholar]
- Wagner FA, Anthony JC (2002) From first drug use to drug dependence: Developmental periods of risk for dependence upon marijuana, cocaine, and alcohol. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 26: 479–488. [DOI] [PubMed] [Google Scholar]
- Winger G (1993) Fixed-ratio and time-out changes on behavior maintained by cocaine or methohexital in rhesus monkeys: II. Behavioral economic analysis. Exp Clin Psychopharm 1: 154–161. [Google Scholar]
- Winger G, Galuska CM, Hursh SR, Woods JH (2006) Relative reinforcing effects of cocaine, remifentanil, and their combination in rhesus monkeys. The Journal of pharmacology and experimental therapeutics 318: 223–9. [DOI] [PubMed] [Google Scholar]
- Xue Y, Steketee JD, Rebec GV, Sun W (2011) Activation of D(2)-like receptors in rat ventral tegmental area inhibits cocaine-reinstated drug-seeking behavior. Eur J Neurosci 33: 1291–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Steketee JD, Sun W (2012) Inactivation of the central nucleus of the amygdala reduces the effect of punishment on cocaine self-administration in rats. Eur J Neurosci 35: 775–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer BA, Oleson EB, Roberts DC (2012) The motivation to self-administer is increased after a history of spiking brain levels of cocaine. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 37: 1901–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zittel-Lazarini A, Cador M, Ahmed SH (2007) A critical transition in cocaine self-administration: behavioral and neurobiological implications. Psychopharmacology 192: 337–46. [DOI] [PubMed] [Google Scholar]