Abstract
Antiretroviral therapy has markedly reduced morbidity and mortality for persons living with HIV. Individual tailoring of antiretroviral regimens has the potential to further improve the long-term management of HIV through the mitigation of treatment failure and drug-induced toxicities. While the mechanisms underlying anti-HIV drug adverse outcomes are multifactorial, the application of drug-specific pharmacogenomic knowledge is required in order to move toward the personalization of HIV therapy. Thus, detailed understanding of the metabolism and transport of antiretrovirals and the influence of genetics on these pathways is of importance. To this end, this review provides an up-to-date overview of the metabolism of anti-HIV therapeutics, and the impact of genetic variation in drug metabolism and transport on the treatment of HIV. The future perspectives and current challenges in pursuing personalized HIV treatment are also discussed.
Keywords: pharmacogenomics, drug metabolism, HIV medication, drug-drug interactions, personalized medicine
1. INTRODUCTION
Human immunodeficiency virus (HIV) continues to be one of the greatest public health concerns worldwide. Although a cure for HIV infection has not been found, the advancement of antiretroviral therapy (ART), and pre- and post-exposure prophylaxis is moving humanity ever closer to the total eradication of HIV by decreasing the risk of viral transmission. There are two major subtypes of HIV, namely HIV-1 and HIV-2, with HIV-1 being most virulent and prevalent (1, 2). A 2019 report by The Joint United Nations Programme on HIV/AIDS (UNAIDS) shows a significant global decrease in HIV-related mortality in the last decade from an approximate 1.5 million in 2008 to 0.8 million in 2018 (3). Meanwhile, though gradually, the number of new HIV infections per year continues to decline (4). This decrease in new HIV infections is largely due to the development of more effective ART, which reduces the risk of the transmission of the virus between serodiscordant sexual partners by lowering the viral load of the infected partner to an undetectable level (5). Although ART has improved HIV outcomes, novel anti-HIV drugs are still needed to improve quality of life and further prevent viral transmission. This pursuit is not without challenges. Successful HIV treatment currently requires lifelong adherence to therapeutics, some of which are associated with adverse events such as dyslipidemia, hyperglycemia, hyperlactatemia, hepatotoxicity, and osteoporosis (6). Personalization of HIV treatment may help to abrogate these adverse effects (7).
Drug Metabolism and Pharmacogenomics.
Drug metabolism refers to the biotransformation of a drug by metabolizing enzymes in a living organism. The enzymes involved in drug metabolism are expressed throughout the body but are predominantly present in the liver. During biotransformation, drugs can undergo modifications in which a functional group is introduced or revealed. This process is denoted as phase I metabolism. Phase II metabolism involves conjugation of a drug with a charged species such as glutathione, sulfate, or glucuronic acid (Figure 1). Drug oxidation and glucuronic acid conjugation, catalyzed by the cytochromes P450 (P450; CYP) superfamily of enzymes and UDP-glucuronosyltransferases (UGTs), respectively, are central to the metabolism of a wide range of drugs, including many anti-HIV drugs (8, 9). The metabolites that are produced from these reactions are generally polar, and therefore, more readily excreted from the body than the drug itself (9, 10). As a result, drug metabolism plays an important role in governing therapeutic responses, including the duration and magnitude of a drug’s pharmacological action. Additionally, drug metabolites can be more or less pharmacologically or toxicologically active than the parent drug (11–13). Beyond metabolizing enzymes, the therapeutic effects of a drug can also be regulated by drug transporters, which are cell membrane proteins that transport drugs into and out of the cell (Figure 1 and Figure 2). Genetic variation in drug metabolism and transport can contribute to interindividual differences in treatment outcomes (7, 14). Thus, personalizing ART requires understanding and prediction of the impact of genetics on drug metabolism and/or transport through the application of pharmacogenomics.
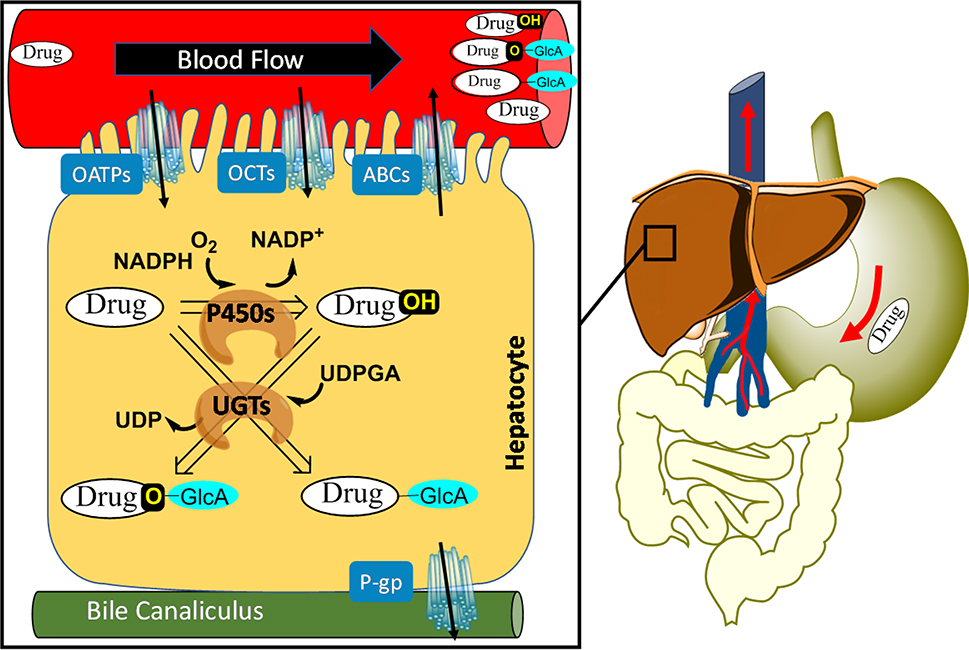
Figure 1.
The first-pass metabolism of HIV drugs upon oral administration. After being swallowed, a drug is absorbed via the gut wall and intestine. It then enters the hepatic portal system. An abundance of drug metabolizing enzymes are present in the intestine and liver. Prior to metabolism, the drug is actively transported (OATPs = organic-anion-transporting peptides, OCTs = organic cation transporters, ABCs = ATP-binding cassette transporters, P-gp = P-glycoprotein, a member of ABCs) or passively diffuses into the cell, or both. In the liver, drug metabolism occurs inside hepatocytes where the drug undergoes modifications (e.g., oxidation by P450s) or conjugations (e.g., glucuronidation by UGTs). The resulting metabolites are excreted into the bile canaliculus or re-enter the blood, after which they can be excreted by the kidneys.
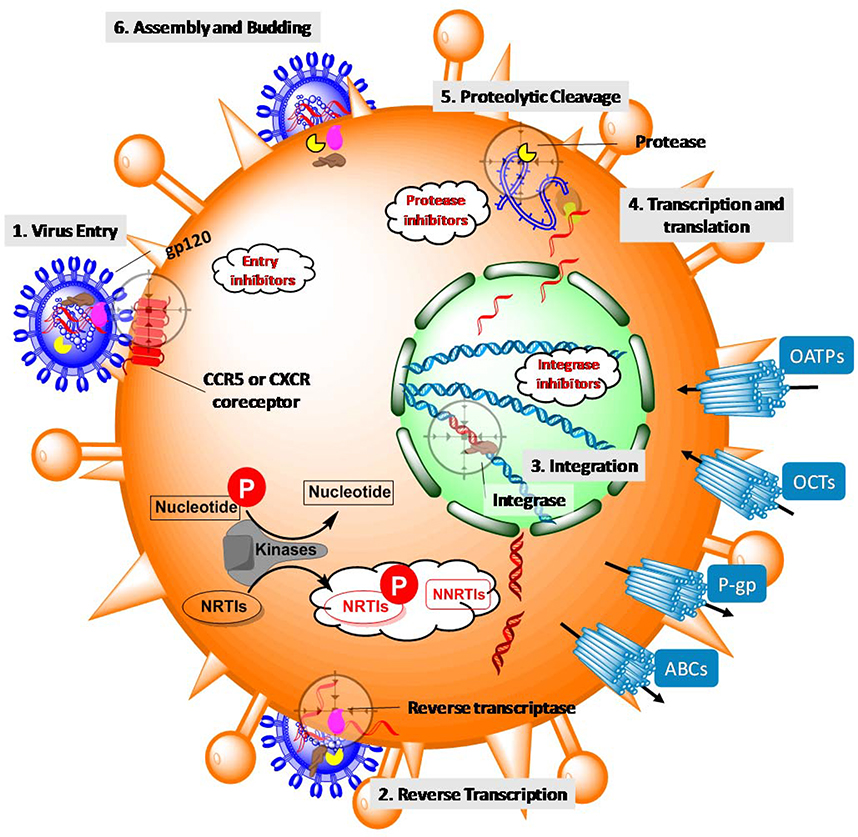
Figure 2.
Lifecycle of HIV-1 and action of antiretrovirals in CD4+ cells. The lifecycle is initiated by the binding of viral envelope protein gp120 to the receptors on a CD4+ cell (process targeted by entry inhibitors) (step 1). Once inside the cell, HIV releases and uses reverse transcriptase to convert viral RNA into DNA (process targeted by NRTIs and NNRTIs; NRTIs must be phosphorylated by host kinases inside the cell in order to become pharmacologically active) (step 2). Subsequently, viral integrase catalyzes the incorporation of viral DNA into the host genome (process targeted by integrase inhibitors) (step 3), which allows HIV to hijack host cellular machinery to produce long chains of viral proteins (step 4). Hydrolysis of these long chains of viral proteins by viral protease furnishes each individual component (process targeted by protease inhibitors) (step5) that is assembled into newly formed HIV progeny ready to bud off (step 6).
Pharmacogenomics, which is the study of how genetics influences a person’s response to drugs, seeks to guide the rational selection and dosing of therapeutics. To this end, much of the analyses within pharmacogenomics are aimed at understanding the impact of single nucleotide polymorphisms (SNPs) on drug metabolism and disposition. SNPs of particular interest are those that impact the expression and/or activity of metabolizing enzymes and drug transporters, and result in a change in drug efficacy and safety profiles (15). In order to standardize the annotation of these SNPs, pharmacogenomics employs a star nomenclature system (e.g., CYP2B6*6) to denote genetic variants that are prevalent and may have clinical relevance. In this nomenclature system, *1 is typically assigned to the so-called wild-type allele. The impact of several individual SNPs on disposition of HIV drugs and/or clinical outcomes discussed in this review are summarized in Table 1. The goal of this review is to provide a comprehensive guide to current knowledge of anti-HIV drug metabolism and transport pharmacogenomics, with an eye toward leveraging these insights to inform the personalization of HIV treatment and prevention.
Table 1.
The influence of genetic variation on antiretroviral drug exposure and clinical outcomes.
| Gene or protein | Drug affected | Alleles evaluated | Reported consequences (compared to wildtype) |
|---|---|---|---|
| CYP3A5 | Maraviroc | *2, *3, *6, and *7 | 41% higher plasma concentrations and 66% lower apparent clearance in homozygous dysfunctional groups (24). |
| CYP2C19 | Etravirine | *2 | 8–38% decrease in intrinsic clearance (95). |
| Nelfinavir | Rate of metabolism to hydroxy-t-butylamide metabolite decreases by 50%; no significant impact on efficacy or toxicity (134). | ||
| CYP2B6 | Efavirenz | Loss-of-function alleles | Neuropsychiatric adverse events associated with decreased intrinsic clearance (88, 89). |
| Nevirapine | G516T | Decreased intrinsic clearance; no clear association with adverse events (74). | |
| UGT1A1 | Dolutegravir | *6, *28, and other reduced-function alleles | Neuropsychiatric adverse events associated with decreased intrinsic clearance (125). |
| Atazanavir | *6, *28 | Hyperbilirubinemia associated with decreased intrinsic clearance (140, 141). | |
| Indinavir | |||
| Raltegravir | *28 | Decreased intrinsic clearance; no clear association with adverse events (111). | |
| HLA-B | Abacavir | *5701 | Strongly correlated with hypersensitivity (46). |
| OCT1 | Lamivudine | P283L, P341L | Significantly decreased intrinsic clearance (56). |
| OCT2 | Lamivudine | T199I, T201M, A270S | |
| ABCB1 | Nevirapine | C3435T | Decreased risk of hepatotoxicity (73). |
2. ENTRY INHIBITORS
Classification of HIV drugs.
Based on the action of drugs on specific phases of the HIV lifecycle (Figure 2), antiretroviral medications can be generally classified into four categories: entry inhibitors, reverse transcriptase inhibitors, integrase inhibitors, and protease inhibitors. A typical ART regimen consists of three drug components from at least two different classes (16). Due to the high mutation rate of HIV, no individual antiretroviral drug has been shown to have long-term therapeutic effects when administered alone. The combination of antiretrovirals overcomes drug resistance by suppressing the pools of potential resistance mutations. However, the nature of multidrug regimens and the necessity of long-term adherence to antiretrovirals present challenges in chronic management and often requires pharmacovigilance to avoid potential drug-drug interactions and adverse reactions, especially in the presence of comorbidities.
HIV infection begins with viral particles anchoring on the surface of a host cell (e.g., helper T-cell) by interacting with the surface CD4 glycoprotein. Once it binds to CD4, the viral envelope glycoprotein gp120 and a subunit gp41 engage one or both coreceptors CCR5 and CXCR4 and undergoes conformation changes that lead to the fusion of the two membranes (17). Although an attractive target for anti-HIV intervention, the viral gp120 has remained elusive in the context of drug discovery due to its high degree of variability and limited access to the binding sites (18). For example, fostemsavir is currently being developed as an entry inhibitor that blocks the conformational change of gp120; however, due to the high variation in amino acid sequence and flexibility of gp120 of HIV-1, some HIV strains are naturally resistant to fostemsavir (19). Therefore, most entry inhibitors act by targeting the coreceptors CCR5 and/or CXCR4.
Approved by the FDA in 2003, enfuvirtide was the first-in-class entry inhibitor used in combination therapy for the treatment of HIV. As a synthetic polypeptide, enfuvirtide mimics a conserved amino acid sequence of the envelope protein gp41 that serves as a key domain for the binding of the coreceptors and therefore disrupts the fusion process. Enfuvirtide has not been found to be a substrate of P450s or UGTs (20). Additionally, since enfuvirtide is active extracellularly, cell membrane transport is unlikely to be important for its actions (21).
The second entry inhibitor, maraviroc, was approved by the FDA in 2007 and is a CCR5 antagonist that blocks the viral gp120 from interacting with the coreceptor. Maraviroc is principally metabolized by CYP3A5, and to a lesser extent by CYP3A4 (22). CYP3A4 and CYP3A5 share almost identical substrate specificity due to significant sequence homology (32); therefore, their distinct activities towards maraviroc is of interest. N-dealkylation, mono- and di-oxygenation, as well as oxygenation followed by glucuronidation, were reported for maraviroc metabolism (22, 23). Meanwhile, an in vitro study using human liver microsomes indicated that the homozygous loss-of-function allele CYP3A5*3/*3 had 79% less enzymatic activity compared to the wild-type homozygous CYP3A5*1/*1 (22), while a clinical study observed 41% lower maraviroc plasma concentrations and 66% higher clearance in the homozygous wild-type group compared to the homozygous dysfunctional groups (CYP3A5*2, *3, *6, and *7) (24). These results may partially explain clinically observed interindividual variation in maraviroc drug responses, suggesting that maraviroc may be underdosed in patients possessing homozygous CYP3A5*1 alleles.
3. REVERSE TRANSCRIPTASE INHIBITORS
Upon entering the cell, the HIV RNA genome is converted into DNA by reverse transcriptase (Figure 2). As an essential enzyme in viral replication, reverse transcriptase has been a primary target for anti-HIV therapeutics. Reverse transcriptase inhibitors are divided into subcategories of nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) and non-nucleoside reverse transcriptase inhibitors (NNRTIs), each having distinctly different mechanisms of action. In addition to being used for the treatment of HIV, the combination of NRTIs emtricitabine and tenofovir disoproxil fumarate is prescribed for pre-exposure and post-exposure prophylaxis (25, 26). Therefore, understanding the metabolism and pharmacogenomics of these NRTIs is important to ensure their success in prevention of HIV transmission.
3.1. Nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs)
NRTIs are analogues of naturally occurring nucleosides or nucleotides that lack a necessary hydroxyl for continuing polymerization. As a result, incorporation of NRTIs into the nascent proviral DNA by reverse transcriptase prevents its elongation and thus terminates the DNA synthesis process. NRTIs are substrates of both viral reverse transcriptase and host cell DNA synthase; the former accounts for NRTI antiretroviral activity while the latter contributes to drug toxicity.
Inactive in their formulated forms, NRTIs must be phosphorylated (by kinases in host cells) to their pharmacologically-active metabolites: nucleoside triphosphate analogues that inhibit HIV reverse transcription. This inhibition is achieved by competition with the natural reverse transcriptase substrates and incorporation into DNA, resulting in chain termination. The kinases that perform phosphorylation of NRTIs are less well understood from a drug metabolism perspective than are classic metabolizing enzymes such as P450s and UGTs. This is in part attributable to the predominant biological role of these kinases in the phosphorylation of endogenous substrates, as opposed to the clearance of xenobiotics. Of note, the phosphorylation of NRTIs occurs within the target cell, and the presence of NRTIs in the plasma is not a robust marker of clinical efficacy or toxicity (27). However, the intracellular concentration of the phosphorylated metabolites has been demonstrated to serve as a better indicator of virologic effectiveness (28). NRTIs require sequential phosphorylation events to form a pharmacologically active nucleoside triphosphate analogue, and multiple kinases have been noted to catalyze these phosphorylation steps (29) (summarized in Table 2). Thus, activation of NRTIs is likely sensitive to a range of factors including cell-type specific expression of the relevant kinases and disease states that impact on kinase expression (27). Further, although currently unknown, genetic variation in NRTI-phosphorylating kinases may alter drug response (30). This uncertainty represents an important knowledge gap since differential ability to activate NRTIs could explain interindividual variability in treatment outcomes that cannot be attributed to adherence alone.
Table 2.
Summary of proposed stepwise activation of NRTIs and the major enzymes for corresponding biotransformation. Abbreviations: Phs = phosphorylation, Dea = deamination, Amn = amination, TK1 = thymidine kinase 1, TMPK = thymidylate kinases, NDPK = nucleoside-diphosphate kinases, AK2 = Adenylate kinase 2, PKM = pyruvate kinase muscle, PKLR = pyruvate kinase liver and blood cells, CKM = creatine kinase muscle, DCK = deoxycytidine kinase, CMPK = cytidine monophosphate kinase 1, PGK1 = phosphoglycerate kinase 1, AK = adenosine kinases, ADAL1 = adenosine deaminase-like protein 1, GUK1 = guanylate kinase 1, C5NT = cytosolic 5’-nucleotidases, ADSS = adenylosuccinate synthetase, ASL = adenylosuccinate lyase, U/CMPK = uridine/cytidine monophosphate kinase, PGK = 3-phosphoglycerate kinase, NDPK = nucleoside diphosphate kinases, TK = thymidine kinases.
| NRTI⟍Activation | Step 1 | Step 2 | Step 3 | Step 4 | Ref | ||||
|---|---|---|---|---|---|---|---|---|---|
| Reaction | Enzyme | Reaction | Enzyme | Reaction | Enzyme | Reaction | Enzyme | ||
| Zidovudine | Phs | TK1 | Phs | TMPK | Phs | NDPK | (27, 32) | ||
| Tenofovir | Phs | AK2 | Phs | PKM, PKLR, CKM | (29) | ||||
| Emtricitabine | Phs | DCK | Phs | TK1 | Phs | CMPK1, PGK1 | (42) | ||
| Abacavir | Phs | AK | Dea | ADAL1 | Phs | GUK1 | Phs | * | (45, 46) |
| Didanosine | Phs | C5NT | Amn | ADSS, ASL | Phs | AK | Phs | AK | (50) |
| Lamivudine | Phs | DCK | Phs | U/CMPK | Phs | PGK, NDPK | (54, 55) | ||
| Stavudine | Phs | TK | Phs | TMPK | Phs | NDPK | (59) | ||
various enzymes are involved and the principal enzymes have not been well characterized.
Zidovudine was the first antiretroviral medication for HIV approved by the FDA in 1987. Zidovudine undergoes three phosphorylation steps to form the active triphosphate, with the second phosphorylation being rate-limiting (31). As a thymidine analogue, the first phosphorylation to monophosphate is primarily catalyzed by thymidine kinase 1 (32); the monophosphate is then phosphorylated to diphosphate by thymidylate kinases, followed by the final phosphorylation by nucleoside-diphosphate kinases to produce the active triphosphate metabolite (27). Zidovudine is also subject to glucuronidation by UGT2B7 (33), accounting for approximately 75% elimination of the drug via renal excretion (34). Induction of UGTs by rifampin significantly increases the oral clearance of zidovudine (35). In addition, the reduction of the 3’-azido to 3’-amino group results in the formation of 3′‐amino‐3′‐deoxythymidine, which is highly toxic for human bone marrow cells (36). Interestingly, P450 enzymes were suggested to be involved in 3′‐amino‐3′‐deoxythymidine formation, though the contribution of specific isozymes remains unknown (37).
Approved in 2001 by the FDA for treatment of HIV as well as in 2008 for hepatitis B, tenofovir (administered as tenofovir alafenamide or tenofovir disoproxil fumarate) is widely prescribed to treat HIV infections. In addition, tenofovir is prescribed for HIV pre- and post-exposure prophylaxis. In its original form, which contains a phosphonate group, tenofovir is highly hydrophilic and difficult to diffuse through cell membranes. To improve its bioavailability, tenofovir is prescribed as prodrugs, tenofovir disoproxil fumarate or tenofovir alafenamide, that are hydrolyzed by esterases to release tenofovir. As an NRTI, tenofovir must be phosphorylated to its pharmacologically active tenofovir-diphosphate metabolite. Unlike other NRTIs, tenofovir contains a pseudo phosphate group (phosphonate) and therefore only two phosphorylation steps are required to form the pharmacologically active triphosphate (tenofovir-diphosphate). Several kinases have been found to phosphorylate tenofovir in a cell/tissue specific manner (29). In peripheral blood mononuclear cells and vaginal tissue, the first phosphorylation is carried out by adenylate kinase 2, while the second phosphorylation is catalyzed by either pyruvate kinase muscle or pyruvate kinase liver/red blood cell (29). In colonic tissue, the first phosphorylation is also catalyzed by adenylate kinase 2 but the second phosphorylation is catalyzed primarily by muscle creatine kinase (29). Of note, while these pyruvate and creatine kinases are named for the cell and tissues in which they were first identified and found to be active, they are expressed in a range of cells/tissues. Genetic variation has been found in these kinases (29, 38, 39), but the influence of these polymorphisms on phosphorylation of NRTIs remains largely unexplored. Genetic variants of these kinases has been studied in a cohort of 505 individuals from the United States, Thailand, and South Africa (39). In that study, 19 of the 505 subjects (~4%) carried at least one kinase variant that is predicted (using in silico tools) to be deleterious, which could result in low or no tenofovir activation; further in vitro assays using recombinant enzymes confirmed decreased activity towards tenofovir phosphorylation by certain naturally occurring adenylate kinase 2 variants such as K28R, T194I, V19G, A55V, and K62E compared to the wild type (39), indicating that genetics may impact tenofovir activation. Apart from phosphorylation, tenofovir undergoes minimal hepatic metabolism and is primarily cleared, unchanged, via renal excretion (40). In addition, tenofovir and tenofovir disoproxil fumarate have been shown to inhibit CYP2C9 and CYP2E1 (41).
Emtricitabine is used in combination for HIV treatment and prevention. Emtricitabine undergoes three phosphorylation steps to form the active metabolite, emtricitabine-triphosphate. In peripheral blood mononuclear cells, the first and second phosphorylations are catalyzed by deoxycytidine kinase and thymidine kinase 1 respectively, whereas the final phosphorylation is carried out by both cytidine monophosphate kinase 1 and phosphoglycerate kinase 1 (42). A quantitative reverse transcriptase-PCR assay indicated that the abundance of cytidine monophosphate kinase 1 mRNA was 8-fold greater in colon tissue than in vaginal tissue, whereas phosphoglycerate kinase 1 mRNA abundance is 4-fold greater in vaginal than colonic tissue, which could result in differential formation of the active emtricitabine-triphosphate in these tissues (43). A genetic study of 498 HIV-uninfected participants reported that 44 of the 498 individuals (9%) carried at least one variant of a kinase involved in phosphorylation of emtricitabine (42). Emtricitabine has not been found to undergo metabolism by P450s or UGTs (23). However, it is both a substrate and inhibitor of ATP-binding cassette transporter C1 (44).
Abacavir was approved by the FDA in 1998 for the treatment of HIV. Unlike most NRTIs, abacavir activation involves four metabolic reactions. Adenosine kinases carry out the formation of abacavir monophosphate, which undergoes deamination by adenosine deaminase-like protein 1 (45) to form carbovir monophosphate; the second phosphorylation is catalyzed by guanylate kinase 1, followed by the conversion to triphosphate by a number of kinases including creatine kinases, pyruvate kinases, nucleoside diphosphate kinases, phosphoglycerate kinases, and phosphoenolpyruvate carboxykinase (46). Interestingly, abacavir diphosphate or triphosphate are not detectable within cells (47), which suggests that deamination of abacavir monophosphate to carbovir monophosphate is necessary for the subsequent phosphorylation. Besides intracellular phosphorylation, abacavir is also subject to hepatic metabolism, including oxidation catalyzed by alcohol dehydrogenases and glucuronidation by UGTs (48); however, the contribution of individual enzymes within these families to abacavir metabolism has not been fully characterized. The pharmacogenetic study of abacavir is almost exclusively focused on human leukocyte antigen B (HLA-B) gene. The variant allele HLA-B *5701 is strongly correlated with abacavir hypersensitivity that is clinically observed in 5–8% of patients during the first 6 weeks of abacavir treatment (46). A double-blind, randomized study with 1956 patients showed that prospective screening of the HLA-B*5701 allele could prevent abacavir hypersensitivity (49).
Didanosine, in 1991, became the second FDA-approved HIV medication. Phosphorylation of didanosine to didanosine-monophosphate is carried out by cytosolic 5’-nucleotidases. Didanosine-monophosphate is then converted to dideoxy adenosine monophosphate by the synergistic action of adenylosuccinate synthetase and adenylosuccinate lyase (50). The second and third phosphorylation of dideoxy adenosine monophosphate to dideoxy adenosine diphosphate and dideoxy adenosine triphosphate may be carried out by adenylate kinases (51, 52). Didanosine is not subject to hepatic metabolism and no inhibitory effect on P450s has been demonstrated (53).
Lamivudine is an NRTI that was approved by the FDA in 1995. Deoxycytidine kinase carries out the first phosphorylation of lamivudine to lamivudine-monophosphate (54). Uridine/cytidine monophosphate kinase catalyzes the phosphorylation of lamivudine-monophosphate to lamivudine-diphosphate (54), whereas both 3-phosphoglycerate kinase and nucleoside diphosphate kinases catalyze the third phosphorylation step to generate the active lamivudine-triphosphate (55). Lamivudine is mostly excreted unchanged and undergoes minimal hepatic metabolism (27). On the other hand, drug transporters involved in renal excretion have an important impact on the clearance of lamivudine. A transport kinetics study using oocytes expressing organic cation transporter (OCT) 1 and OCT2 suggested that, compared to the wild-type, the intrinsic clearance of lamivudine decreased significantly in the presence of OCT1 variants P283L and P341L, and OCT2 variants T199I, T201M, and A270S (56). These variants are all commonly found in Asian populations (57). Further in vivo study is required to evaluate the impact of drug transporter pharmacogenetics on lamivudine disposition.
Stavudine has relatively high toxicity compared to other NRTIs and this has limited the use of this drug (58). Stavudine is metabolized intracellularly to mono-, di-, and triphosphate metabolites sequentially by thymidine kinases, thymidylate kinases, and nucleoside diphosphate kinases (59). Interestingly, as a 2’,3’-didehydro-2’,3’-dideoxy analogue of thymidine, stavudine has only 0.17% affinity of that of thymidine towards thymidine kinases (60), and the intracellular accumulation of stavudine triphosphate may be associated with adverse reactions such as lipodystrophy (61). Similar to tenofovir, FTC, and 3TC, stavudine is not susceptible to hepatic metabolism and limited study has been carried out on its interactions with these metabolizing enzymes.
3.2. Non-nucleoside/nucleotide reverse transcriptase inhibitors (NNRTIs)
Unlike NRTIs, NNRTIs are chemically distinct from nucleotides/nucleosides, do not get incorporated into DNA and therefore do not require intracellular activation via phosphorylation. The NNRTI binding pocket is located on the p66 subunit of HIV-1 reverse transcriptase that is approximately 10 Å from the active site for DNA polymerization (62, 63). Generally, NNRTIs bind to the allosteric site of HIV-1 reverse transcriptase and induce a conformational change, inhibiting catalysis. As such, NNRTIs act as non-competitive inhibitors in contrast to the competitive inhibition by NRTIs (62). Although HIV-2 reverse transcriptase shares significant homology with HIV-1 reverse transcriptase, most NNRTIs are inactive towards HIV-2, primarily due to HIV-2 lacking Tyr181 and Tyr 188 residues that are required for binding (64).
The first-in-class NNRTI nevirapine was approved by the FDA in 1996 as a first-line medication for HIV infections. In some settings, nevirapine is prescribed as a single-dose to prevent mother-to-child transmission of HIV (65). Despite its efficacy, nevirapine can have side effects, including severe skin rash and hepatoxicity, and as such, nevirapine carries an FDA black box warning (66). Pharmacovigilance of the plasma nevirapine concentration is warranted for patients with compromised livers (67). Several studies suggest that biotransformation of nevirapine to 12-hyroxy-nevirapine is likely to underlie nevirapine-induced toxicity (68–70). In addition to 12-hydroxy-nevirapine, other monooxygenated metabolites including 2-, 3-, and 8-hydroxy-nevirapine have been identified (71). Nevirapine is primarily metabolized by CYP3A4, CYP2D6, CYP2B6, and to a lesser extent CYP3A5, giving rise to the above monooxygenated metabolites. A phenotyping study using cDNA-expressed P450s demonstrated that the formation of 2- and 3-hydroxy-nevirapine was exclusively catalyzed by the CYP3A subfamily (CYP3A4 and CYP3A5) and CYP2B6, respectively, whereas formation of 8- and 12-hydroxy-nevirapine was likely mediated by multiple P450s (72). All four monooxygenated metabolites can be further glucuronidated in subsequent phase II metabolism, which accounts for 80% of the elimination of NVP (71). Besides hepatic metabolizing enzymes, several clinical studies have shown that ATP-binding cassette transporters (ABCs) are involved in the hepatoxicity of nevirapine-containing regimens (73–75). Specifically, homozygosity for the loss of function ABCB1 3435C>T SNP is associated with a decreased risk of hepatoxicity (73). In addition, the CYP2B6 516G>T variant affects nevirapine clearance: those with the homozygous CYP2B6 516TT genotype exhibit an estimated nevirapine clearance of 1.86 L/h compared to that of 2.62 L/h and 2.95 L/h for heterozygous CYP2B6 516GT and homozygous CYP2B6 516GG, respectively (74).
Since being approved by the FDA in 1998, efavirenz has been one of the most widely used antiretroviral drugs. Efavirenz is primarily metabolized by CYP2B6 to 8-hydroxy-efavirenz and 8,14-dihydroxy-efavirenz. CYP2A6 catalyzes the formation of 7-hydroxy-efavirenz. The 8-hydroxy-efavirenz metabolite is the predominant P450-dependent metabolite of efavirenz (76, 77). As with nevirapine, chronic use of efavirenz has been associated with hepatoxicity, resulting in ~10% of patients discontinuing treatment due to intolerable side effects (78). It has been suggested that efavirenz induces hepatic cell death but a study using primary human hepatocytes demonstrated that 8-hydroxy-efavirenz stimulates cell death via activation of c-Jun N-terminal kinase and Bcl-2 interacting mediator of cell death (79). Neurotoxicity resulting in cognitive and mood disorders has also been reported for efavirenz-containing regimens (80–83). A study using primary neurons found that efavirenz and its metabolites 7- and 8-hyroxy-efavirenz induce dendritic spine injury in a concentration-dependent manner (84). Notably, 8-hyroxy-efavirenz produced at least an order of magnitude more damage to the neurons than the parent efavirenz or the other monooxygenated metabolite 7-hydroxyefavirenz. Compartmentalization of efavirenz metabolites was revealed by studying bodily fluid of patients on an efavirenz-based regimen (77). In this study, 8-hydroxy-efavirenz was detected in blood plasma, seminal plasma, and cerebrospinal fluid, whereas 7-hydroxy-efavirenz and 8,14-dihdryoxyefavirenz were only found in blood and seminal plasma, and none of the metabolites were found to exhibit pharmacologic activity towards HIV-1. Besides P450-mediated metabolism, efavirenz is glucuronidated by UGT2B7 to form efavirenz-N-glucuronide (85). Formation of secondary metabolites, such as efavirenz-7-O-glucuronide, efavirenz-8-O-glucuronide (these metabolites represent oxidation of efavirenz to the 7- or 8-hydroxy-efavirenz metabolite, followed by glucuronidation), is carried out by several UGT isoforms (86). Interestingly, in vivo, a decrease in the levels of oxygenated metabolites and commensurate increase in the levels of efavirenz-N-glucuronide was observed for CYP2B6 loss-of-function alleles (47, 87). In the context of efavirenz-mediated toxicity, Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines for CYP2B6 and efavirenz suggest an increased risk of adverse effects for carriers of decreased function variant alleles (88). A clinical study has suggested an a priori 35% dosage reduction in patients carrying homozygous CYP2B6*6 variant alleles that are prevalent among people of African origin (89).
Unfortunately, chronic use of first-generation NNRTIs such as nevirapine and efavirenz has led to the emergence of drug-resistant viral strains, which often results in treatment failure (90, 91). The second-generation NNRTI etravirine was approved by the FDA in 2008 and has proven resilient towards resistant viral strains (92). A structural study demonstrated that the potency of etravirine against drug-resistance mutations was due to torsional flexibility of the diarylpyrimidine structure of etravirine that can bind to reverse transcriptase in multiple conformations (92). Therefore, etravirine has been suggested for treatment-experienced patients who have developed drug resistance to nevirapine and efavirenz (93). Based on an in vitro study using c-DNA expressed P450s and inhibition assays, etravirine was found to be primarily metabolized by CYP2C19 and CYP3A4/5, and to a lesser extent by CYP2B6, and CYP2C9 (94). In addition, assays performed using human liver microsomes that were genotyped as homozygous for the loss-of-function CYP2C19*2 allele indicated a 75–100% decrease in the formation of oxygenated metabolites of etravirine (94). Similarly, a clinical study showed that carriers of the CYP2C19*2 variant allele (both homozygous and heterozygous) had 8–38% less clearance compared to the wild-type (95). Although etravirine has been shown to induce CYP3A4/5 via pregnane X receptor-mediated modulation in vitro (94, 96), no clinically significant etravirine-mediated drug-drug interactions have been observed and therefore dosage adjustment is generally not required (97).
Rilpivirine is another second-generation NNRTI and a diarylpyrimidine derivative that provides the advantage of flexible binding to the HIV-1 viral reverse transcriptase. Rilpivirine was approved by the FDA in 2011 for the treatment of HIV infection. An in vitro study using a panel of cDNA expressed P450s and UGTs indicated that rilpivirine was primarily metabolized by CYP3A4 and CYP3A5, leading to the formation of mono- and dioxygenated metabolites, whereas glucuronidation was mostly carried out by UGT1A1 and UGT1A4 to form rilpivirine-O-glucuronide (oxidation followed by glucuronidation) and rilpivirine-N-glucuronide (direct glucuronidation), respectively (98). A population pharmacokinetics study in 249 adult HIV-positive patients suggested minimal impact of genetic variance in CYP3A4, CYP3A5, CYP2C19, UGT1A1, or UGT1A4 on rilpivirine clearance, likely due to the multiple metabolic pathways involved (99). Although no significant drug-drug interactions have been reported, rilpivirine has been shown to significantly inhibit P-glycoprotein, organic-anion-transporting peptide 1B1, 1B3, and CYP3A4, CYP2C19, and CYP2B6, while inducing the mRNA expression of CYP3A4 and UGT1A3 (100). Therefore, further investigations on the potential interactions of rilpivirine with substrates of these transporters and metabolizing enzymes is warranted.
Doravirine was approved by the FDA in 2018 for treatment-naïve patients and has shown noninferiority as well as an improved safety profile compared to the standard of care regimens (101, 102). An in vivo study involving healthy human volunteers suggested that doravirine is primarily metabolized by CYP3A4 and CYP3A5 (103). Doravirine has not been found to exhibit inhibition towards major P450s, UGTs, or P-glycoprotein (101), while several clinical investigations indicate a low propensity of doravirine for causing drug-drug interactions (104, 105). Therefore, doravirine has the potential to become a preferred drug in its class and is currently being investigated in treatment-experienced patients (101).
4. INTEGRASE INHIBITORS
Integrase is one of three viral enzymes (the other two being protease and reverse transcriptase) essential to HIV replication, and is an excellent therapeutic target since there is no equivalent enzyme within host cells; therefore, the inhibition of viral integrase does not interfere with normal cell functions (106). After reverse transcription, the viral DNA is integrated into the host DNA by integrase, enabling the transcription of viral DNA to produce viral proteins (Figure 2). As such, blocking the function of integrase can halt the retroviral replication process and terminate the viral lifecycle. Integrase inhibitors generally act by chelating Mg2+, a critical cofactor for viral DNA binding in the integrase active site, thereby preventing integrase from interacting with the viral DNA (107). Four integrase inhibitors – raltegravir, elvitegravir, dolutegravir, and bictegravir – have been approved by the FDA to treat HIV infections. Of note, due to the high barrier to resistance and tolerability of drugs in this class, particularly dolutegravir, the 2018 World Health Organization recommendations suggest dolutegravir in combination with a two NRTI backbone as the preferred first-line HIV treatment regimen (108).
The first-in-class integrase inhibitor raltegravir received FDA approval in 2007 and despite its inconvenient twice-daily dosing schedule, has proven effective against drug-resistant HIV-1 infection when the standard of care regimen has failed (109). Based on a study conducted with healthy volunteers, raltegravir is primarily metabolized by UGT1A1; this represents a major route of elimination (110). Although clinically significant drug-drug interactions have not been reported for raltegravir, caution should be exercised when it is co-administered with strong UGT1A1 inhibitors or inducers. The UGT1A1*28 genetic variant can have significant impact on raltegravir metabolism: patients carrying homozygous UGT1A1*28 variant alleles were found to have greater raltegravir plasma concentrations when compared to wild-type (111). However, no correlation between this pharmacokinetic effect and treatment outcomes has been established.
In 2008, elvitegravir became the second integrase inhibitor approved by the FDA as a part of a fixed dose combination (elvitegravir/cobicistat/emtricitabine/tenofovir). Elvitegravir is rapidly metabolized by CYP3A subfamily enzymes, resulting in an average half-life of 3.5 hours after a single dose (112). As such, elvitegravir is prescribed with pharmacokinetic boosters such as cobicistat and ritonavir that inhibit human CYP3A subfamily resulting in plasma concentrations and a longer half-life of elvitegravir than can be achieved with lower doses (113). For example, when 100 mg elvitegravir was co-administered twice daily with 100 mg ritonavir, a 20-fold increase in AUC and three-fold increase of half-life to 9.5 hours were observed, compared to twice-daily administration of 100 mg elvitegravir alone (112). Due to the success of such pharmacokinetic boosting, pharmacokinetic boosters are now broadly utilized in HIV treatment; this strategy has been applied to nearly all protease inhibitors (114–116). Despite the benefits, an altered metabolic profile resulting from concomitant use of pharmacokinetic boosters also represents a challenge for dose adjustment of other co-administered drugs in order to avoid drug-drug interactions. Serious and sometimes fatal drug-drug interactions have been reported with administration of pharmacokinetic boosters (117–119). Therefore, evaluation of potential drug-drug interactions is warranted for the treatment of HIV-associated comorbidities under boosted antiretroviral regimens. However, most NRTIs are not subject to P450-mediated metabolism and therefore are generally not affected when co-administered with pharmacokinetic boosters (120).
Raltegravir and elvitegravir have overlapping and modest genetic barriers for resistance (121), which has spurred interest in the development of second-generation integrase inhibitors, one of which – dolutegravir– was approved by the FDA in 2013. Unlike twice-daily raltegravir, dolutegravir is dosed once-daily and, unlike elvitegravir, does not require pharmacokinetic boosting (122). Dolutegravir is extensively metabolized by UGT1A1 to the inactive dolutegravir-O-glucuronide metabolite and to a lesser extent by CYP3A4-mediated oxidation (123). Decreased oral clearance was observed among carriers of UGT1A1 reduced function alleles (124). Neuropsychiatric adverse events were more often observed for patients carrying UGT1A1*6, UGT1A1*28 reduced-function alleles than those with normal alleles (125). In addition, a clinical study reported potential drug-drug interactions with abacavir, which is likely due to competition of dolutegravir and abacavir for UGT1A1 (126). Thus, further investigation of potential interactions of dolutegravir with other UGT1A1 substrates is warranted.
Bictegravir, the newest addition to the integrase inhibitors family, was approved by the FDA in 2018. Bictegravir is currently only available as a part of a bictegravir/emtricitabine/tenofovir alafenamide combination tablet (127). Similar to dolutegravir, bictegravir is metabolized by CYP3As and UGT1A1 (128). More information on drug-drug interactions, influence of pharmacogenomics, and metabolite profiles of bictegravir is expected in future studies.
5. PROTEASE INHIBITORS
After the integration of viral DNA into the host genome, transcription of viral DNA produces polyproteins that are inactive until cleaved by viral protease into individual functional parts (Figure 2). Inhibition of the viral protease prevents the maturation of viral particles and blocks the infectivity of nascent virions (129). Generally, protease inhibitors resemble the tetrahedral intermediate of the substrate by competitively binding to the protease active site to disable its enzymatic function (130). However, due to the high mutation rate of HIV, the protease active site can change rapidly to block accessibility to protease inhibitors, rendering them ineffective.
To date, nine protease inhibitors have been approved by the FDA for HIV treatment, including saquinavir, indinavir, ritonavir, nelfinavir, amprenavir, lopinavir, atazanavir, tipranavir, and darunavir. Except for tipranavir, all protease inhibitors are peptidomimetics and share a common feature: a chiral secondary hydroxyl group that makes critical contact with the protease catalytic Asp25/25’ residues (131). Most protease inhibitors are primarily metabolized by the CYP3A subfamily during phase I metabolism, except for nelfinavir, which is metabolized primarily by CYP2C19 (132). Biotransformation of nelfinavir by CYP2C19 leads to the formation of an active hydroxy-t-butylamide metabolite with antiretroviral activities similar to the parent nelfinavir (133). The rate of metabolism of nelfinavir to hydroxy-t-butylamide metabolite is decreased by 50% in patients carrying the CYP2C19*2 loss-of-function allele as compared to wild-type but no significant change in efficacy or toxicities due to this genetic variation were found (134). The oral bioavailability of protease inhibitors is generally low (< 68%) with a median half-life of ~~6 hours (135), thus a frequent dosing schedule is required. To overcome their short half-lives, concomitant use of pharmacokinetic boosters that selectively inhibit CYP3A4 activity have made once-daily dosing possible for protease inhibitors (136). While the protease inhibitor ritonavir is often used as a booster due to its potent CYP3A4 inhibition, another commonly used booster, cobicistat, does not have antiretroviral activity (137). Despite the similarities between ritonavir and cobicistat, switching of the boosters should be systematically reviewed to anticipate proper dosage adjustment (138). Concurrent administration of protease inhibitors with CYP3A4 inducers are often problematic in that the resulting decrease of protease inhibitor plasma concentrations often leads to reduced efficacy and development of drug resistance (129). In addition to being substrates of CYP3A4, all protease inhibitors also inhibit CYP3A and other P450 enzymes with varying degrees of potency (23, 129). This often results in altered pharmacokinetic profiles of other co-administered drugs such as ethinyl estradiol and statins (129, 139). Therefore caution should be exercised when co-administering protease inhibitors with drugs that are known substrates of the corresponding P450 enzymes.
In addition to phase I metabolism, several protease inhibitors undergo glucuronidation during phase II metabolism. Of note, atazanavir and indinavir reportedly induce hyperbilirubinemia among patients with Gilbert’s syndrome carrying UGT1A1*28 and/or UGT1A1*6 alleles, which ultimately led to discontinuation of this treatment in this subpopulation (140, 141).
Most protease inhibitors are substrates of drug transporters, such as multidrug resistance proteins (e.g., P-glycoprotein) (142) and organic-anion-transporting polypeptides (143), thus affecting their intracellular accumulation at the site of viral replication. Significant inter-individual variation in protease inhibitor plasma concentrations can be attributed in part to genetic polymorphisms in genes that encode these drug transporters (144). For example, overexpression of P-glycoprotein has been associated with accelerated acquisition of drug resistance (145). Targeted inhibition of P-glycoprotein has been shown to increase the penetration of HIV protease inhibitors into sanctuary sites, e.g., brain and testes (146). Overall, pharmacogenomic factors that modulate metabolism and active transport can have significant implications on the disposition and distribution of protease inhibitors and thereby influence their pharmacokinetic and safety profiles.
6. CONCLUSION AND FUTURE CONSIDERATIONS
Understanding the metabolism of HIV drugs can provide important insights into the mechanisms that govern interindividual variability in treatment outcomes. The application of pharmacogenomic insights has the potential to inform the personalization of HIV treatment, and the rational selection and dosing of drugs. Further, as next-generation antiretroviral therapies are developed to address important issues such as end organ disease, drug-drug interactions and adherence, the abundance of existing knowledge of anti-HIV drug metabolism and transport can be leveraged to facilitate the development of new drugs. The impact of genetics on susceptibility to drug-drug interactions at the level of drug metabolism and transport is largely unexplored. Research in this area is required in order to mitigate and better predict adverse outcomes due to drug-drug interactions involving HIV therapies. Primary challenges and barriers to broad scale implementation of pharmacogenomics for use in individualizing HIV treatment include difficulties in performing testing as part of routine clinical practice, a lack of clinical data required to solidify gene-drug associations, and the expense of genetic tests. If these factors can be overcome, an exciting new era of personalized HIV therapy could be on the horizon.
7. LIST OF ABBREVIATIONS
- ART
Antiretroviral therapy
- P450s
cytochrome P450 enzymes
- UGTs
glucuronosyltransferases
- SNP
single nucleotide polymorphism
- NRTIs
nucleoside/nucleotide reverse transcriptase inhibitors
- NNRTIs
non-nucleoside reverse transcriptase inhibitors
- HLA-B
human leukocyte antigen B
- OCTs
organic cation transporters
- ABCs
ATP-binding cassette transporters
8. REFERENCES
- 1.Prince PD, Matser A, van Tienen C, Whittle HC, Schim van der Loeff MF. 2014. Mortality rates in people dually infected with HIV-1/2 and those infected with either HIV-1 or HIV-2: a systematic review and meta-analysis. AIDS. 28(4):549–58 [DOI] [PubMed] [Google Scholar]
- 2.Cock KMD, Adjorlolo G, Ekpini E, Sibailly T, Kouadio J, et al. 1993. Epidemiology and Transmission of HIV-2: Why There Is No HIV-2 Pandemic. JAMA. 270(17):2083–86 [DOI] [PubMed] [Google Scholar]
- 3.HIV/AIDS JUNP on, others. 2019. UNAIDS Data 2019. 2019
- 4.Organization WH, others. 2017. Policy brief: Consolidated guidelines on HIV prevention, diagnosis, treatment and care for key populations. World Health Organization; [PubMed] [Google Scholar]
- 5.Eisinger RW, Dieffenbach CW, Fauci AS. 2019. HIV Viral Load and Transmissibility of HIV Infection: Undetectable Equals Untransmittable. JAMA. 321(5):451–52 [DOI] [PubMed] [Google Scholar]
- 6.Montessori V, Press N, Harris M, Akagi L, Montaner JSG. 2004. Adverse effects of antiretroviral therapy for HIV infection. CMAJ. 170(2):229–38 [PMC free article] [PubMed] [Google Scholar]
- 7.Pirmohamed M, Back DJ. 2001. The pharmacogenomics of HIV therapy. Pharmacogenomics J. 1(4):243–53 [DOI] [PubMed] [Google Scholar]
- 8.Lewis DF. 2003. Human cytochromes P450 associated with the phase 1 metabolism of drugs and other xenobiotics: a compilation of substrates and inhibitors of the CYP1, CYP2 and CYP3 families. Current medicinal chemistry. 10(19):1955–1972 [DOI] [PubMed] [Google Scholar]
- 9.Dutton G 2019. Glucuronidation of drugs and other compounds. CRC press [Google Scholar]
- 10.Lin C, Shi J, Moore A, Khetani SR. 2016. Prediction of Drug Clearance and Drug-Drug Interactions in Microscale Cultures of Human Hepatocytes. Drug Metab Dispos. 44(1):127–36 [DOI] [PubMed] [Google Scholar]
- 11.Zimmermann M, Zimmermann-Kogadeeva M, Wegmann R, Goodman AL. 2019. Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature. 570(7762):462–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirchmair J, Göller AH, Lang D, Kunze J, Testa B, et al. 2015. Predicting drug metabolism: experiment and/or computation? Nature Reviews Drug Discovery. 14(6):387–404 [DOI] [PubMed] [Google Scholar]
- 13.Niyonsaba E, Easton MW, Feng E, Yu Z, Zhang Z, et al. 2019. Differentiation of Deprotonated Acyl-, N-, and O-Glucuronide Drug Metabolites by Using Tandem Mass Spectrometry Based on Gas-Phase Ion–Molecule Reactions Followed by Collision-Activated Dissociation. Anal. Chem. 91(17):11388–96 [DOI] [PubMed] [Google Scholar]
- 14.Lee VHL, Sporty JL, Fandy TE. 2001. Pharmacogenomics of drug transporters: the next drug delivery challenge. Advanced Drug Delivery Reviews. 50:S33–40 [DOI] [PubMed] [Google Scholar]
- 15.Garte S, Gaspari L, Alexandrie A-K, Ambrosone C, Autrup H, et al. 2001. Metabolic Gene Polymorphism Frequencies in Control Populations. Cancer Epidemiol Biomarkers Prev. 10(12):1239–48 [PubMed] [Google Scholar]
- 16.Adults P on AG for, Adolescents. 2018. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. Department of Health and Human Services; Washington, DC [Google Scholar]
- 17.Esté JA, Telenti A. 2007. HIV entry inhibitors. The Lancet. 370(9581):81–88 [DOI] [PubMed] [Google Scholar]
- 18.Kadow J, Wang HG, Lin PF. 2006. Small-molecule HIV-1 gp120 inhibitors to prevent HIV-1 entry: an emerging opportunity for drug development. Curr Opin Investig Drugs. 7(8):721–26 [PubMed] [Google Scholar]
- 19.Alessandri-Gradt E, Charpentier C, Leoz M, Mourez T, Descamps D, Plantier J-C. 2018. Impact of natural polymorphisms of HIV-1 non-group M on genotypic susceptibility to the attachment inhibitor fostemsavir. J Antimicrob Chemother. 73(10):2716–20 [DOI] [PubMed] [Google Scholar]
- 20.Patel IH, Zhang X, Nieforth K, Salgo M, Buss N. 2005. Pharmacokinetics, Pharmacodynamics and Drug Interaction Potential of Enfuvirtide. Clin Pharmacokinet. 44(2):175–86 [DOI] [PubMed] [Google Scholar]
- 21.Joly V, Jidar K, Tatay M, Yeni P. 2010. Enfuvirtide: from basic investigations to current clinical use. Expert Opinion on Pharmacotherapy. 11(16):2701–13 [DOI] [PubMed] [Google Scholar]
- 22.Lu Y, Hendrix CW, Bumpus NN. 2012. Cytochrome P450 3A5 Plays a Prominent Role in the Oxidative Metabolism of the Anti-Human Immunodeficiency Virus Drug Maraviroc. Drug Metab Dispos. 40(12):2221–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiang TKL, Wilby KJ, Ensom MHH. 2016. In Vitro Reaction Phenotyping and Drug Interaction Data In Pharmacokinetic and Pharmacodynamic Drug Interactions Associated with Antiretroviral Drugs, ed Kiang TKL, Wilby KJ, Ensom MHH, pp. 27–41. Singapore: Springer [Google Scholar]
- 24.Lu Y, Fuchs EJ, Hendrix CW, Bumpus NN. 2014. CYP3A5 Genotype Impacts Maraviroc Concentrations in Healthy Volunteers. Drug Metab Dispos. 42(11):1796–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siedner MJ, Tumarkin E, Bogoch II. 2018. HIV post-exposure prophylaxis (PEP). BMJ. 363: [DOI] [PubMed] [Google Scholar]
- 26.Coelho LE, Torres TS, Veloso VG, Landovitz RJ, Grinsztejn B. 2019. Pre-exposure prophylaxis 2.0: new drugs and technologies in the pipeline. The Lancet HIV. 6(11):e788–99 [DOI] [PubMed] [Google Scholar]
- 27.Piliero PJ. 2004. Pharmacokinetic Properties of Nucleoside/Nucleotide Reverse Transcriptase Inhibitors. JAIDS Journal of Acquired Immune Deficiency Syndromes. 37:S2. [DOI] [PubMed] [Google Scholar]
- 28.Solas C, Li Y-F, Xie M-Y, Sommadossi J-P, Zhou X-J. 1998. Intracellular Nucleotides of (−)-2′,3′-Deoxy-3′-Thiacytidine in Peripheral Blood Mononuclear Cells of a Patient Infected with Human Immunodeficiency Virus. Antimicrobial Agents and Chemotherapy. 42(11):2989–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lade JM, To EE, Hendrix CW, Bumpus NN. 2015. Discovery of Genetic Variants of the Kinases That Activate Tenofovir in a Compartment-specific Manner. EBioMedicine. 2(9):1145–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamlin AN, Tillotson J, Bumpus NN. 2019. Genetic variation of kinases and activation of nucleotide analog reverse transcriptase inhibitor tenofovir. Pharmacogenomics. 20(2):105–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen J, Flexner C, Liberman RG, Skipper PL, Louissaint N, et al. 2012. Biphasic Elimination of Tenofovir Diphosphate and Nonlinear Pharmacokinetics of Zidovudine Triphosphate in a Microdosing Study. J Acquir Immune Defic Syndr. 61(5):593–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furman PA, Fyfe JA, Clair MHS, Weinhold K, Rideout JL, et al. 1986. Phosphorylation of 3’-azido-3’-deoxythymidine and selective interaction of the 5’-triphosphate with human immunodeficiency virus reverse transcriptase. PNAS. 83(21):8333–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barbier O, Turgeon D, Girard C, Green MD, Tephly TR, et al. 2000. 3′-azido-3′-deoxythimidine (AZT) is glucuronidated by human UDP-glucuronosyltransferase 2B7 (UGT2B7). Drug Metab Dispos. 28(5):497–502 [PubMed] [Google Scholar]
- 34.Acosta EP, Page LM, Fletcher CV. 1996. Clinical Pharmacokinetics of Zidovudine. Clin-Pharmacokinet. 30(4):251–62 [DOI] [PubMed] [Google Scholar]
- 35.Gallicano KD, Sahai J, Shukla VK, Seguin I, Pakuts A, et al. 1999. Induction of zidovudine glucuronidation and amination pathways by rifampicin in HIV-infected patients. British Journal of Clinical Pharmacology. 48(2):168–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cretton EM, Xie MY, Bevan RJ, Goudgaon NM, Schinazi RF, Sommadossi JP. 1991. Catabolism of 3’-azido-3’-deoxythymidine in hepatocytes and liver microsomes, with evidence of formation of 3’-amino-3’-deoxythymidine, a highly toxic catabolite for human bone marrow cells. Mol Pharmacol. 39(2):258–66 [PubMed] [Google Scholar]
- 37.Cretton EM, Sommadossi JP. 1993. Reduction of 3’-azido-2’,3’-dideoxynucleosides to their 3’-amino metabolite is mediated by cytochrome P-450 and NADPH-cytochrome P-450 reductase in rat liver microsomes. Drug Metab Dispos. 21(5):946–50 [PubMed] [Google Scholar]
- 38.Bowman JE, Frischer H, Ajmar F, Carson PE, Gower MK. 1967. Population, Family and Biochemical Investigation of Human Adenylate Kinase Polymorphism. Nature. 214(5093):1156–58 [DOI] [PubMed] [Google Scholar]
- 39.Figueroa DB, Tillotson J, Li M, Piwowar-Manning E, Hendrix CW, et al. 2018. Discovery of genetic variants of the kinases that activate tenofovir among individuals in the United States, Thailand, and South Africa: HPTN067. PLOS ONE. 13(4):e0195764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim YK, Choi MJ, Oh TY, Yu K-S, Lee S. 2017. A comparative pharmacokinetic and tolerability analysis of the novel orotic acid salt form of tenofovir disoproxil and the fumaric acid salt form in healthy subjects. Drug Des Devel Ther. 11:3171–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nekvindová J, Mašek V, Veinlichová A, Anzenbacherová E, Anzenbacher P, et al. 2006. Inhibition of human liver microsomal cytochrome P450 activities by adefovir and tenofovir. Xenobiotica. 36(12):1165–77 [DOI] [PubMed] [Google Scholar]
- 42.Figueroa DB, Madeen EP, Tillotson J, Richardson P, Cottle L, et al. 2018. Genetic Variation of the Kinases That Phosphorylate Tenofovir and Emtricitabine in Peripheral Blood Mononuclear Cells. AIDS Research and Human Retroviruses. 34(5):421–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.To E, Bumpus NN. 2013. Mucosal Expression of the Cytochromes P450 (CYP) and Nucleotide Kinases Involved in the Biotransformation of Drugs Used in Human Immunodeficiency Virus (HIV) Pre-exposure Prophylaxis (PrEP). The FASEB Journal. 27(1_supplement):664.7–664.7 [Google Scholar]
- 44.Bousquet L, Pruvost A, Didier N, Farinotti R, Mabondzo A. 2008. Emtricitabine: inhibitor and substrate of multidrug resistance associated protein. European journal of pharmaceutical sciences. 35(4):247–256 [DOI] [PubMed] [Google Scholar]
- 45.Schinkmanová M, Votruba I, Holý A. 2006. N6-Methyl-AMP aminohydrolase activates N6-substituted purine acyclic nucleoside phosphonates. Biochemical Pharmacology. 71(9):1370–76 [DOI] [PubMed] [Google Scholar]
- 46.Barbarino JM, Kroetz DL, Altman RB, Klein TE. 2014. PharmGKB summary: abacavir pathway. Pharmacogenet Genomics. 24(5):276–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Faletto MB, Miller WH, Garvey EP, Clair MHS, Daluge SM, Good SS. 1997. Unique intracellular activation of the potent anti-human immunodeficiency virus agent 1592U89. Antimicrobial Agents and Chemotherapy. 41(5):1099–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grilo NM, Antunes AMM, Caixas U, Marinho AT, Charneira C, et al. 2013. Monitoring abacavir bioactivation in humans: Screening for an aldehyde metabolite. Toxicology Letters. 219(1):59–64 [DOI] [PubMed] [Google Scholar]
- 49.Mallal S, Phillips E, Carosi G, Molina J-M, Workman C, et al. 2008. HLA-B*5701 Screening for Hypersensitivity to Abacavir. New England Journal of Medicine. 358(6):568–79 [DOI] [PubMed] [Google Scholar]
- 50.Pruvost A, Negredo E, Benech H, Theodoro F, Puig J, et al. 2005. Measurement of Intracellular Didanosine and Tenofovir Phosphorylated Metabolites and Possible Interaction of the Two Drugs in Human Immunodeficiency Virus-Infected Patients. Antimicrobial Agents and Chemotherapy. 49(5):1907–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahluwalia G, Cooney DA, Hartman NR, Mitsuya H, Yarchoan R, et al. 1993. Anomalous accumulation and decay of 2’,3’-dideoxyadenosine-5’-triphosphate in human T-cell cultures exposed to the anti-HIV drug 2’,3’-dideoxyinosine. Drug Metab Dispos. 21(2):369–76 [PubMed] [Google Scholar]
- 52.Holec AD, Mandal S, Prathipati PK, Destache CJ. 2017. Nucleotide reverse transcriptase inhibitors: a thorough review, present status and future perspective as HIV therapeutics. Current HIV research. 15(6):411–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Makinson A, Pujol J-L, Le Moing V, Peyriere H, Reynes J. 2010. Interactions Between Cytotoxic Chemotherapy and Antiretroviral Treatment in Human Immunodeficiency Virus-Infected Patients with Lung Cancer. Journal of Thoracic Oncology. 5(4):562–71 [DOI] [PubMed] [Google Scholar]
- 54.Liou J-Y, Dutschman GE, Lam W, Jiang Z, Cheng Y-C. 2002. Characterization of Human UMP/CMP Kinase and Its Phosphorylation of d- and l-Form Deoxycytidine Analogue Monophosphates. Cancer Res. 62(6):1624–31 [PubMed] [Google Scholar]
- 55.Zhou Z, Rodman JH, Flynn PM, Robbins BL, Wilcox CK, D’Argenio DZ. 2006. Model for Intracellular Lamivudine Metabolism in Peripheral Blood Mononuclear Cells Ex Vivo and in Human Immunodeficiency Virus Type 1-Infected Adolescents. Antimicrobial Agents and Chemotherapy. 50(8):2686–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Choi M-K, Song I-S. 2012. Genetic variants of organic cation transporter 1 (OCT1) and OCT2 significantly reduce lamivudine uptake. Biopharmaceutics & Drug Disposition. 33(3):170–78 [DOI] [PubMed] [Google Scholar]
- 57.Kang H-J, Song I-S, Shin HJ, Kim W-Y, Lee C-H, et al. 2007. Identification and Functional Characterization of Genetic Variants of Human Organic Cation Transporters in a Korean Population. Drug Metab Dispos. 35(4):667–75 [DOI] [PubMed] [Google Scholar]
- 58.Magula N, Dedicoat M. 2015. Low dose versus high dose stavudine for treating people with HIV infection. Cochrane Database of Systematic Reviews [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moketla MB, Wadley AL, Kamerman P, Rosa D de A. 2018. Pharmacogenetic variation influences sensory neuropathy occurrence in Southern Africans treated with stavudine-containing antiretroviral therapy. PLOS ONE. 13(10):e0204111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ho HT, Hitchcock MJ. 1989. Cellular pharmacology of 2’,3’-dideoxy-2’,3’-didehydrothymidine, a nucleoside analog active against human immunodeficiency virus. Antimicrobial Agents and Chemotherapy. 33(6):844–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Domingo P, Cabeza MC, Pruvost A, Torres F, Salazar J, et al. 2011. Association of Thymidylate Synthase Gene Polymorphisms with Stavudine Triphosphate Intracellular Levels and Lipodystrophy. Antimicrobial Agents and Chemotherapy. 55(4):1428–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sluis-Cremer N, Tachedjian G. 2008. Mechanisms of inhibition of HIV replication by non-nucleoside reverse transcriptase inhibitors. Virus Research. 134(1):147–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kohlstaedt LA, Wang J, Friedman JM, Rice PA, Steitz TA. 1992. Crystal structure at 3.5 A resolution of HIV-1 reverse transcriptase complexed with an inhibitor. Science. 256(5065):1783–90 [DOI] [PubMed] [Google Scholar]
- 64.Condra JH, Emini EA, Gotlib L, Graham DJ, Schlabach AJ, et al. 1992. Identification of the human immunodeficiency virus reverse transcriptase residues that contribute to the activity of diverse nonnucleoside inhibitors. Antimicrobial Agents and Chemotherapy. 36(7):1441–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lallemant M, Jourdain G, Le Coeur S, Mary JY, Ngo-Giang-Huong N, et al. 2004. Single-Dose Perinatal Nevirapine plus Standard Zidovudine to Prevent Mother-to-Child Transmission of HIV-1 in Thailand. New England Journal of Medicine. 351(3):217–28 [DOI] [PubMed] [Google Scholar]
- 66.Jao J, Sturdevant M, Martin JDR, Schiano T, Fiel MI, Huprikar S. 2010. Nevirapine-Induced Stevens Johnson–Syndrome and Fulminant Hepatic Failure Requiring Liver Transplantation. American Journal of Transplantation. 10(7):1713–16 [DOI] [PubMed] [Google Scholar]
- 67.González de Requena D, Núñez M, Jiménez-Nácher I, Soriano V. 2002. Liver toxicity caused by nevirapine. AIDS. 16(2):290. [DOI] [PubMed] [Google Scholar]
- 68.Chen J, Mannargudi BM, Xu L, Uetrecht J. 2008. Demonstration of the Metabolic Pathway Responsible for Nevirapine-Induced Skin Rash. Chem. Res. Toxicol. 21(9):1862–70 [DOI] [PubMed] [Google Scholar]
- 69.Antunes AMM, Godinho ALA, Martins IL, Oliveira MC, Gomes RA, et al. 2010. Protein Adducts As Prospective Biomarkers of Nevirapine Toxicity. Chem. Res. Toxicol. 23(11):1714–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Antunes AMM, Godinho ALA, Martins IL, Justino GC, Beland FA, Marques MM. 2010. Amino Acid Adduct Formation by the Nevirapine Metabolite, 12-Hydroxynevirapine—A Possible Factor in Nevirapine Toxicity. Chem. Res. Toxicol. 23(5):888–99 [DOI] [PubMed] [Google Scholar]
- 71.Riska P, Lamson M, MacGregor T, Sabo J, Hattox S, et al. 1999. Disposition and Biotransformation of the Antiretroviral Drug Nevirapine in Humans. Drug Metab Dispos. 27(8):895–901 [PubMed] [Google Scholar]
- 72.Erickson DA, Mather G, Trager WF, Levy RH, Keirns JJ. 1999. Characterization of the In Vitro Biotransformation of the HIV-1 Reverse Transcriptase Inhibitor Nevirapine by Human Hepatic Cytochromes P-450. Drug Metab Dispos. 27(12):1488–95 [PubMed] [Google Scholar]
- 73.Ciccacci C, Borgiani P, Ceffa S, Sirianni E, Marazzi MC, et al. 2009. Nevirapine-induced hepatotoxicity and pharmacogenetics: a retrospective study in a population from Mozambique. Pharmacogenomics. 11(1):23–31 [DOI] [PubMed] [Google Scholar]
- 74.Chou M, Bertrand J, Segeral O, Verstuyft C, Borand L, et al. 2010. Population Pharmacokinetic-Pharmacogenetic Study of Nevirapine in HIV-Infected Cambodian Patients. Antimicrobial Agents and Chemotherapy. 54(10):4432–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Haas DW, Bartlett JA, Andersen JW, Sanne I, Wilkinson GR, et al. 2006. Pharmacogenetics of Nevirapine-Associated Hepatotoxicity: An Adult AIDS Clinical Trials Group Collaboration. Clin Infect Dis. 43(6):783–86 [DOI] [PubMed] [Google Scholar]
- 76.Ward BA, Gorski JC, Jones DR, Hall SD, Flockhart DA, Desta Z. 2003. The Cytochrome P450 2B6 (CYP2B6) Is the Main Catalyst of Efavirenz Primary and Secondary Metabolism: Implication for HIV/AIDS Therapy and Utility of Efavirenz as a Substrate Marker of CYP2B6 Catalytic Activity. J Pharmacol Exp Ther. 306(1):287–300 [DOI] [PubMed] [Google Scholar]
- 77.Avery LB, VanAusdall JL, Hendrix CW, Bumpus NN. 2013. Compartmentalization and Antiviral Effect of Efavirenz Metabolites in Blood Plasma, Seminal Plasma, and Cerebrospinal Fluid. Drug Metab Dispos. 41(2):422–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kappelhoff BS, van Leth F, Robinson PA, MacGregor TR, Baraldi E, et al. 2005. Are adverse events of nevirapine and efavirenz related to plasma concentrations. Antivir Ther. 10(4):489–498 [PubMed] [Google Scholar]
- 79.Bumpus NN. 2011. Efavirenz and 8-hydroxyefavirenz induce cell death via a JNK- and BimEL-dependent mechanism in primary human hepatocytes. Toxicology and Applied Pharmacology. 257(2):227–34 [DOI] [PubMed] [Google Scholar]
- 80.Marzolini C, Telenti A, Decosterd LA, Greub G, Biollaz J, Buclin T. 2001. Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS. 15(1):71. [DOI] [PubMed] [Google Scholar]
- 81.Pérez-Molina JA. 2002. Safety and Tolerance of Efavirenz in Different Antiretroviral Regimens: Results from a National Multicenter Prospective Study in 1,033 HIV-Infected Patients. HIV Clinical Trials. 3(4):279–86 [DOI] [PubMed] [Google Scholar]
- 82.Lochet P, Peyrière H, Lotthé A, Mauboussin JM, Delmas B, Reynes J. 2003. Long-term assessment of neuropsychiatric adverse reactions associated with efavirenz. HIV Medicine. 4(1):62–66 [DOI] [PubMed] [Google Scholar]
- 83.Rihs TA, Begley K, Smith DE, Sarangapany J, Callaghan A, et al. 2006. Efavirenz and chronic neuropsychiatric symptoms: a cross-sectional case control study. HIV Medicine. 7(8):544–48 [DOI] [PubMed] [Google Scholar]
- 84.Tovar-y-Romo LB, Bumpus NN, Pomerantz D, Avery LB, Sacktor N, et al. 2012. Dendritic Spine Injury Induced by the 8-Hydroxy Metabolite of Efavirenz. J Pharmacol Exp Ther. 343(3):696–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bélanger A-S, Caron P, Harvey M, Zimmerman PA, Mehlotra RK, Guillemette C. 2009. Glucuronidation of the Antiretroviral Drug Efavirenz by UGT2B7 and an in Vitro Investigation of Drug-Drug Interaction with Zidovudine. Drug Metab Dispos. 37(9):1793–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bae SK, Jeong Y-J, Lee C, Liu K-H. 2011. Identification of human UGT isoforms responsible for glucuronidation of efavirenz and its three hydroxy metabolites. Xenobiotica. 41(6):437–44 [DOI] [PubMed] [Google Scholar]
- 87.Iulio J di, Fayet A, Arab-Alameddine M, Rotger M, Lubomirov R, et al. 2009. In vivo analysis of efavirenz metabolism in individuals with impaired CYP2A6 function. Pharmacogenetics and Genomics. 19(4):300. [DOI] [PubMed] [Google Scholar]
- 88.Desta Z, Gammal RS, Gong L, Whirl‐Carrillo M, Gaur AH, et al. 2019. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2B6 and Efavirenz-Containing Antiretroviral Therapy. Clinical Pharmacology & Therapeutics. 106(4):726–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nyakutira C, Röshammar D, Chigutsa E, Chonzi P, Ashton M, et al. 2008. High prevalence of the CYP2B6 516G→T(*6) variant and effect on the population pharmacokinetics of efavirenz in HIV/AIDS outpatients in Zimbabwe. Eur J Clin Pharmacol. 64(4):357–65 [DOI] [PubMed] [Google Scholar]
- 90.Richman DD, Havlir D, Corbeil J, Looney D, Ignacio C, et al. 1994. Nevirapine resistance mutations of human immunodeficiency virus type 1 selected during therapy. Journal of Virology. 68(3):1660–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bacheler L, Jeffrey S, Hanna G, D’Aquila R, Wallace L, et al. 2001. Genotypic Correlates of Phenotypic Resistance to Efavirenz in Virus Isolates from Patients Failing Nonnucleoside Reverse Transcriptase Inhibitor Therapy. Journal of Virology. 75(11):4999–5008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Das K, Clark Arthur D, Lewi PJ, Heeres J, de Jonge MR, et al. 2004. Roles of Conformational and Positional Adaptability in Structure-Based Design of TMC125-R165335 (Etravirine) and Related Non-nucleoside Reverse Transcriptase Inhibitors That Are Highly Potent and Effective against Wild-Type and Drug-Resistant HIV-1 Variants. J. Med. Chem. 47(10):2550–60 [DOI] [PubMed] [Google Scholar]
- 93.Madruga JV, Cahn P, Grinsztejn B, Haubrich R, Lalezari J, et al. 2007. Efficacy and safety of TMC125 (etravirine) in treatment-experienced HIV-1-infected patients in DUET-1: 24-week results from a randomised, double-blind, placebo-controlled trial. The Lancet. 370(9581):29–38 [DOI] [PubMed] [Google Scholar]
- 94.Yanakakis LJ, Bumpus NN. 2012. Biotransformation of the Antiretroviral Drug Etravirine: Metabolite Identification, Reaction Phenotyping, and Characterization of Autoinduction of Cytochrome P450-Dependent Metabolism. Drug Metab Dispos. 40(4):803–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lubomirov R, Arab-Alameddine M, Rotger M, Fayet-Mello A, Martinez R, et al. 2013. Pharmacogenetics-based population pharmacokinetic analysis of etravirine in HIV-1 infected individuals. Pharmacogenetics and Genomics. 23(1):9–18 [DOI] [PubMed] [Google Scholar]
- 96.Schöller-Gyüre M, Kakuda TN, Raoof A, De Smedt G, Hoetelmans RMW. 2009. Clinical Pharmacokinetics and Pharmacodynamics of Etravirine. Clin Pharmacokinet. 48(9):561–74 [DOI] [PubMed] [Google Scholar]
- 97.Kakuda TN, Schöller-Gyüre M, Hoetelmans RMW. 2011. Pharmacokinetic Interactions between Etravirine and Non-Antiretroviral Drugs. Clin Pharmacokinet. 50(1):25–39 [DOI] [PubMed] [Google Scholar]
- 98.Lade JM, Avery LB, Bumpus NN. 2013. Human Biotransformation of the Nonnucleoside Reverse Transcriptase Inhibitor Rilpivirine and a Cross-Species Metabolism Comparison. Antimicrobial Agents and Chemotherapy. 57(10):5067–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Aouri M, Barcelo C, Guidi M, Rotger M, Cavassini M, et al. 2017. Population Pharmacokinetics and Pharmacogenetics Analysis of Rilpivirine in HIV-1-Infected Individuals. Antimicrobial Agents and Chemotherapy. 61(1): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Weiss J, Haefeli WE. 2013. Potential of the novel antiretroviral drug rilpivirine to modulate the expression and function of drug transporters and drug-metabolising enzymes in vitro. International Journal of Antimicrobial Agents. 41(5):484–87 [DOI] [PubMed] [Google Scholar]
- 101.Colombier M-A, Molina J-M. 2018. Doravirine: a review. Current Opinion in HIV and AIDS. 13(4):308. [DOI] [PubMed] [Google Scholar]
- 102.Anderson MS, Gilmartin J, Cilissen C, De Lepeleire I, Van Bortel L, et al. 2015. Safety, tolerability and pharmacokinetics of doravirine, a novel HIV non-nucleoside reverse transcriptase inhibitor, after single and multiple doses in healthy subjects. Antivir Ther. 20(4):397–405 [DOI] [PubMed] [Google Scholar]
- 103.Sanchez RI, Fillgrove KL, Yee KL, Liang Y, Lu B, et al. 2019. Characterisation of the absorption, distribution, metabolism, excretion and mass balance of doravirine, a non-nucleoside reverse transcriptase inhibitor in humans. Xenobiotica. 49(4):422–32 [DOI] [PubMed] [Google Scholar]
- 104.Anderson MS, Khalilieh S, Yee KL, Liu R, Fan L, et al. 2017. A Two-Way Steady-State Pharmacokinetic Interaction Study of Doravirine (MK-1439) and Dolutegravir. Clin Pharmacokinet. 56(6):661–69 [DOI] [PubMed] [Google Scholar]
- 105.Khalilieh S, Yee KL, Sanchez RI, Triantafyllou I, Fan L, et al. 2017. Results of a Doravirine-Atorvastatin Drug-Drug Interaction Study. Antimicrobial Agents and Chemotherapy. 61(2): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pommier Y, Johnson AA, Marchand C. 2005. Integrase inhibitors to treat HIV/Aids. Nature Reviews Drug Discovery. 4(3):236–48 [DOI] [PubMed] [Google Scholar]
- 107.Chen X, Tsiang M, Yu F, Hung M, Jones GS, et al. 2008. Modeling, Analysis, and Validation of a Novel HIV Integrase Structure Provide Insights into the Binding Modes of Potent Integrase Inhibitors. Journal of Molecular Biology. 380(3):504–19 [DOI] [PubMed] [Google Scholar]
- 108.Organization WH, others. 2018. Updated recommendations on first-line and second-line antiretroviral regimens and post-exposure prophylaxis and recommendations on early infant diagnosis of HIV: interim guidelines: supplement to the 2016 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. World Health Organization [Google Scholar]
- 109.Steigbigel RT, Cooper DA, Kumar PN, Eron JE, Schechter M, et al. 2008. Raltegravir with Optimized Background Therapy for Resistant HIV-1 Infection. New England Journal of Medicine. 359(4):339–54 [DOI] [PubMed] [Google Scholar]
- 110.Kassahun K, McIntosh I, Cui D, Hreniuk D, Merschman S, et al. 2007. Metabolism and Disposition in Humans of Raltegravir (MK-0518), an Anti-AIDS Drug Targeting the Human Immunodeficiency Virus 1 Integrase Enzyme. Drug Metab Dispos. 35(9):1657–63 [DOI] [PubMed] [Google Scholar]
- 111.Belkhir L, Seguin-Devaux C, Elens L, Pauly C, Gengler N, et al. 2018. Impact of UGT 1 A1 polymorphisms on Raltegravir and its glucuronide plasma concentrations in a cohort of HIV-1 infected patients. Scientific Reports. 8(1):1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ramanathan S, Mathias AA, German P, Kearney BP. 2011. Clinical Pharmacokinetic and Pharmacodynamic Profile of the HIV Integrase Inhibitor Elvitegravir. Clin Pharmacokinet. 50(4):229–44 [DOI] [PubMed] [Google Scholar]
- 113.Mathias AA, German P, Murray BP, Wei L, Jain A, et al. 2010. Pharmacokinetics and Pharmacodynamics of GS-9350: A Novel Pharmacokinetic Enhancer Without Anti-HIV Activity. Clinical Pharmacology & Therapeutics. 87(3):322–29 [DOI] [PubMed] [Google Scholar]
- 114.Moyle G 2001. Use of HIV protease inhibitors as pharmacoenhancers. The AIDS reader. 11(2):87–98 [PubMed] [Google Scholar]
- 115.Larson KB, Wang K, Delille C, Otofokun I, Acosta EP. 2014. Pharmacokinetic Enhancers in HIV Therapeutics. Clin Pharmacokinet. 53(10):865–72 [DOI] [PubMed] [Google Scholar]
- 116.Lepist E-I, Phan TK, Roy A, Tong L, MacLennan K, et al. 2012. Cobicistat Boosts the Intestinal Absorption of Transport Substrates, Including HIV Protease Inhibitors and GS-7340, In Vitro. Antimicrobial Agents and Chemotherapy. 56(10):5409–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mykietiuk A, Bonvehì P, Temporiti E, Uruena A, Herrera F, Vila A. 2001. Clinical Ergotism Induced by Ritonavir. Scandinavian Journal of Infectious Diseases. 33(10):788–89 [DOI] [PubMed] [Google Scholar]
- 118.Zhou S-F, Xue CC, Yu X-Q, Li C, Wang G. 2007. Clinically important drug interactions potentially involving mechanism-based inhibition of cytochrome P450 3A4 and the role of therapeutic drug monitoring. Therapeutic drug monitoring. 29(6):687–710 [DOI] [PubMed] [Google Scholar]
- 119.Josephson F 2010. Drug–drug interactions in the treatment of HIV infection: focus on pharmacokinetic enhancement through CYP3A inhibition. Journal of Internal Medicine. 268(6):530–39 [DOI] [PubMed] [Google Scholar]
- 120.Ramanathan S, Shen G, Hinkle J, Enejosa J, Kearney BP. 2007. Pharmacokinetics of Coadministered Ritonavir-Boosted Elvitegravir and Zidovudine, Didanosine, Stavudine, or Abacavir. JAIDS Journal of Acquired Immune Deficiency Syndromes. 46(2):160. [DOI] [PubMed] [Google Scholar]
- 121.Karmon SL, Markowitz M. 2013. Next-Generation Integrase Inhibitors. Drugs. 73(3):213–28 [DOI] [PubMed] [Google Scholar]
- 122.Katlama C, Murphy R. 2012. Dolutegravir for the treatment of HIV. Expert Opinion on Investigational Drugs. 21(4):523–30 [DOI] [PubMed] [Google Scholar]
- 123.Castellino S, Moss L, Wagner D, Borland J, Song I, et al. 2013. Metabolism, Excretion, and Mass Balance of the HIV-1 Integrase Inhibitor Dolutegravir in Humans. Antimicrobial Agents and Chemotherapy. 57(8):3536–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen S, St Jean P, Borland J, Song I, Yeo AJ, et al. 2013. Evaluation of the effect of UGT1A1 polymorphisms on dolutegravir pharmacokinetics. Pharmacogenomics. 15(1):9–16 [DOI] [PubMed] [Google Scholar]
- 125.Yagura H, Watanabe D, Kushida H, Tomishima K, Togami H, et al. 2017. Impact of UGT1A1 gene polymorphisms on plasma dolutegravir trough concentrations and neuropsychiatric adverse events in Japanese individuals infected with HIV-1. BMC Infectious Diseases. 17(1):622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.de Boer MGJ, van den Berk GEL, van Holten N, Oryszcyn JE, Dorama W, et al. 2016. Intolerance of dolutegravir-containing combination antiretroviral therapy regimens in real-life clinical practice. AIDS. 30(18):2831. [DOI] [PubMed] [Google Scholar]
- 127.Devanathan AS, Anderson DJC, Cottrell ML, Burgunder EM, Saunders AC, Kashuba ADM. 2019. Contemporary Drug–Drug Interactions in HIV Treatment. Clinical Pharmacology & Therapeutics. 105(6):1362–77 [DOI] [PubMed] [Google Scholar]
- 128.Deeks ED. 2018. Bictegravir/Emtricitabine/Tenofovir Alafenamide: A Review in HIV-1 Infection. Drugs. 78(17):1817–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Flexner C 1998. HIV-protease inhibitors. New England Journal of Medicine. 338(18):1281–1293 [DOI] [PubMed] [Google Scholar]
- 130.Kick EK, Ellman JA. 1995. Expedient Method for the Solid-Phase Synthesis of Aspartic Acid Protease Inhibitors Directed toward the Generation of Libraries. J. Med. Chem. 38(9):1427–30 [DOI] [PubMed] [Google Scholar]
- 131.Ali A, Bandaranayake RM, Cai Y, King NM, Kolli M, et al. 2010. Molecular Basis for Drug Resistance in HIV-1 Protease. Viruses. 2(11):2509–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hirani VN, Raucy JL, Lasker JM. 2004. Conversion of the Hiv Protease Inhibitor Nelfinavir to a Bioactive Metabolite by Human Liver Cyp2c19. Drug Metab Dispos. 32(12):1462–67 [DOI] [PubMed] [Google Scholar]
- 133.Zhang KE, Wu E, Patick AK, Kerr B, Zorbas M, et al. 2001. Circulating Metabolites of the Human Immunodeficiency Virus Protease Inhibitor Nelfinavir in Humans: Structural Identification, Levels in Plasma, and Antiviral Activities. Antimicrobial Agents and Chemotherapy. 45(4):1086–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hirt D, Mentré F, Tran A, Rey E, Auleley S, et al. 2008. Effect of CYP2C19 polymorphism on nelfinavir to M8 biotransformation in HIV patients. British Journal of Clinical Pharmacology. 65(4):548–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hughes PJ, Cretton-Scott E, Teague A, Wensel TM. 2011. Protease Inhibitors for Patients With HIV-1 Infection. P T. 36(6):332–45 [PMC free article] [PubMed] [Google Scholar]
- 136.Moyle GJ, Back D. 2001. Principles and practice of HIV-protease inhibitor pharmacoenhancement. HIV Medicine. 2(2):105–13 [DOI] [PubMed] [Google Scholar]
- 137.Tseng A, Hughes CA, Wu J, Seet J, Phillips EJ. 2017. Cobicistat Versus Ritonavir: Similar Pharmacokinetic Enhancers But Some Important Differences. Ann Pharmacother. 51(11):1008–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Marzolini C, Gibbons S, Khoo S, Back D. 2016. Cobicistat versus ritonavir boosting and differences in the drug–drug interaction profiles with co-medications. J Antimicrob Chemother. 71(7):1755–58 [DOI] [PubMed] [Google Scholar]
- 139.Fichtenbaum CJ, Gerber JG, Rosenkranz SL, Segal Y, Aberg JA, et al. 2002. Pharmacokinetic interactions between protease inhibitors and statins in HIV seronegative volunteers: ACTG Study A5047. AIDS. 16(4):569. [DOI] [PubMed] [Google Scholar]
- 140.Zucker SD, Qin X, Rouster SD, Yu F, Green RM, et al. 2001. Mechanism of indinavir-induced hyperbilirubinemia. PNAS. 98(22):12671–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gammal RS, Court MH, Haidar CE, Iwuchukwu OF, Gaur AH, et al. 2016. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for UGT1A1 and Atazanavir Prescribing. Clinical Pharmacology & Therapeutics. 99(4):363–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lee CGL, Gottesman MM, Cardarelli CO, Ramachandra M, Jeang K-T, et al. 1998. HIV-1 Protease Inhibitors Are Substrates for the MDR1 Multidrug Transporter. Biochemistry. 37(11):3594–3601 [DOI] [PubMed] [Google Scholar]
- 143.Hartkoorn RC, San Kwan W, Shallcross V, Chaikan A, Liptrott N, et al. 2010. HIV protease inhibitors are substrates for OATP1A2, OATP1B1 and OATP1B3 and lopinavir plasma concentrations are influenced by SLCO1B1 polymorphisms. Pharmacogenet Genomics. 20(2):112–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rodríguez-Nóvoa S, Barreiro P, Jiménez-Nácher I, Soriano V. 2006. Overview of the pharmacogenetics of HIV therapy. Pharmacogenomics J. 6(4):234–45 [DOI] [PubMed] [Google Scholar]
- 145.Jones K, Bray PG, Khoo SH, Davey RA, Meaden ER, et al. 2001. P-glycoprotein and transporter MRP1 reduce HIV protease inhibitor uptake in CD4 cells: potential for accelerated viral drug resistance? AIDS. 15(11):1353. [DOI] [PubMed] [Google Scholar]
- 146.Choo EF, Leake B, Wandel C, Imamura H, Wood AJJ, et al. 2000. Pharmacological Inhibition of P-glycoprotein Transport Enhances the Distribution of HIV-1 Protease Inhibitors into Brain and Testes. Drug Metab Dispos. 28(6):655–60 [PubMed] [Google Scholar]