Abstract
Obesity and insulin resistance are closely associated with a state of low-grade inflammation in the body, and adipose tissue macrophages (ATMs) play central roles in this inflammation. ATMs are known to exhibit marked functional heterogeneity. M1 ATMs produce inflammatory cytokines and induce insulin resistance. On the other hand, the majority of ATMs in lean individuals are M2 ATMs, which have anti-inflammatory potential. We found that M1 and M2 ATMs can be clearly distinguished using CD11c and CD206 as markers, and that both the number of the M1 and M2 ATMs and the M1/M2 ratio are correlated with the degree of insulin resistance. M1/M2 polarity in the adipose tissue is influenced not only by the level of secretion of various polarizing adipokines and chemokines, but also by factors in the local microenvironment, such as hypoxia. M1 ATMs acquire their polarity via activation of hypoxia-inducible factor-1α (HIF-1α) by local hypoxia, and absence of HIF-1α in the myeloid cells appears to enhance insulin sensitivity by promoting angiogenesis in adipose tissue. On the other hand, the resident M2 ATMs interact with adipose tissue progenitors to control adiposity. Thus, beyond their role as immunoregulatory cells, the M1/M2 ATMs also regulate the microenvironment in the adipose tissue and control insulin sensitivity. Recently, we have shown that interventions in the gut microbiota may be effective in controlling obesity-induced chronic inflammation. Control of M1/M2 ATM polarity is a potential therapeutic target for the treatment of insulin resistance associated with obesity.
Keywords: Insulin resistance, Macrophage, Inflammation, Obesity, Microbiota
Introduction
Development of obesity-associated insulin resistance is promoted by systemic inflammation caused by increased levels of inflammatory cytokines, such as TNFα, IL-6, MCP-1, and IL-1β, and the major site of inflammation is the adipose tissue. Increased number of ATMs strongly contributes to its mechanisms [1, 2]. ATMs show highly heterogeneous characteristics, and include at least two major populations called the classically activated, or M1, ATMs and alternatively activated, or M2, ATMs [3]. Originally, the concept of M1 and M2 macrophages was derived from in vitro studies. M1 macrophages are mainly induced by Th1 signaling, involving factors such as lipopolysaccharide (LPS) and IFNγ, and express high levels of inflammatory cytokines. M2 macrophages, on the other hand, are induced by Th2 signaling, involving factors such as IL-4 and IL-13, and are associated with anti-inflammatory reactions. In healthy adipose tissue, more than 90% of the ATMs are of the M2 phenotype, which exhibit anti-inflammatory potential. As obesity progresses, Ly6c+ inflammatory monocytes recruited into the adipose tissue differentiate into M1 ATMs and cluster around necrotic adipocytes, forming crown-like structures (CLSs) to phagocytose dead adipocytes [4, 5]. In 2007, Lumeng et al. proposed CD11c as a marker of M1 ATMs and reported that CD11c+ macrophages are a major population of cells recruited into obese adipose tissue [6]. While, through the use of this marker, we recognized the relationship between the recruitment of M1 ATMs and insulin resistance, the precise involvement of M2 ATMs remains poorly understood.
Characteristics of M1/M2 macrophages in the pathophysiology of insulin resistance
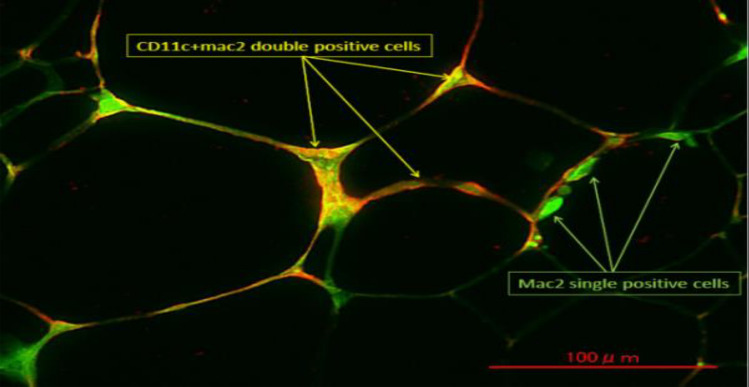
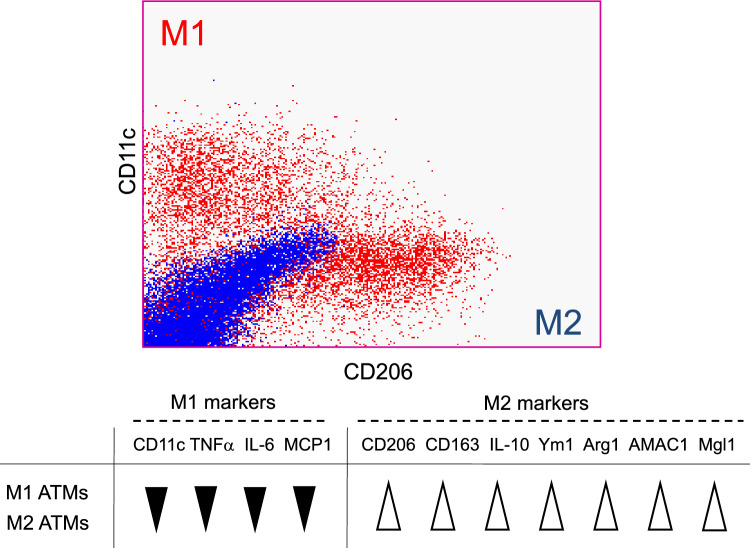
Immunohistochemistry of obese adipose tissue showed that Mac2+/CD11c+ M1 ATMs formed CLS, while Mac2+/CD11c− ATMs, which include M2 ATMs, locate in isolation (Fig. 1). To identify CD11c− M2 macrophages more precisely, we separated the ATMs by flow cytometry using CD11c and CD206 as markers of M1 and M2 macrophages, respectively; this method allowed CD11c+ CD206− M1 ATMs and CD11c− CD206+ M2 ATMs to be clearly distinguished (Fig. 2). In addition, the gene expression profiles of each of these cell fractions were significantly different. While M1 ATMs showed higher expression levels of inflammation-related genes, such as TNFα, IL-6 and MCP-1 than M2 ATMs, the M2 ATMs expressed higher expression levels of anti-inflammatory genes, such as IL-10 (Fig. 2). Under the lean condition, more than 90% of ATMs are of the M2 phenotype, with only around 1−5% being of the M1 phenotype. In obese adipose, both the numbers of M1 and M2 ATMs are markedly increased, although recruitment of M1 ATMs is more striking, resulting in an increase of the M1/M2 ratio. This M1/M2 phenotypic switch is observed specifically in the visceral adipose tissue, and the increase in M1 macrophages is not as significant in the subcutaneous adipose tissue [7, 8]. Treatment with the insulin-sensitizing PPARγ agonist, pioglitazone, or with the PPARγ partial agonist, telmisartan, decreased not only the number of both types of ATMs, but also the M1/M2 ratio, suggesting that both the absolute numbers of M1 and M2 ATMs and the M1/M2 ratio are associated with the insulin sensitivity [7, 9].
Fig. 1.
Immunohistochemistry of adipose tissue in obese mice. Green: Mac2 Red: CD11c
Fig. 2.
Representative flow cytometry result and gene expression patterns of M1/M2 ATMs. Blue dots show isotype control
Adipose tissue hypoxia induces M1 polarity via activation of HIF-1α in obesity
As obesity progresses, adipose tissue is exposed to hypoxia due to decreased capillary density [10], lower blood flow [11] and insufficient induction of VEGF expression in obesity [8, 12]. We found that M1 ATMs are more hypoxic and show higher expression levels of inflammation- and hypoxia-related genes than M2 ATMs, such as TNFα, IL-6 and IL-1β than M2 ATMs. Increased levels of these genes were observed in bone marrow-derived macrophages (BMDMs) cultured under the hypoxic condition, suggested that hypoxia in obese adipose tissue induces the inflammatory phenotypes of macrophages. Furthermore, this acquisition of M1 polarity induced by hypoxia was partially abrogated in HIF-1α knockout BMDMs, indicating the significance of HIF1 α in inducing the inflammatory M1 ATMs in obese adipose tissue [8].
Next, we examined the involvement of HIF-1α in macrophages in glucose metabolism. We found that myeloid cell-specific HIF-1α deletion protected against high-fat diet (HFD)-induced inflammation, CLS formation, and poor vasculature development in obese adipose tissue and systemic insulin resistance. Despite the reduced VEGFa expression in the epididymal adipose tissue (eWAT), the endothelial cells and preadipocytes in HIF-1α-knockout mice showed higher expression levels of angiogenic genes, such as VEGFa, Angpt1, Fgf1, and Fgf10, associated with enhanced angiogenesis in the eWAT [13]. Thus, in obesity, adipose tissue hypoxia promotes the acquisition of M1 polarity via HIF-1α activation, and the HIF-1α in M1 ATMs suppresses neoangiogenesis through interacting with preadipocytes and endothelial cells; thus, hypoxia triggers a vicious cycle and exacerbates the development of insulin resistance.
A novel role of CD206+ M2 ATMs: a niche for adipocyte progenitors
Several lines of evidence have reported that the resident ATMs in the adipose tissue are involved in maintaining insulin sensitivity through their anti-inflammatory functions, while interacting with other immune cells, such as Treg cells, eosinophils and invariant natural killer cells [14–18]. Although the majority of ATMs are of the M2 phenotype in the lean state, the roles of M2 ATMs in maintaining the adipose tissue microenvironment are not yet fully understood. To investigate the involvement of M2 ATMs in adipose tissue and glucose metabolism, we generated a mouse model with partial depletion of CD206+ M2 macrophages induced by diphtheria toxin (DT) administration. We found that the depletion of CD206+ M2 macrophages promoted the proliferation of adipocyte progenitors (APs), which subsequently differentiated into mature adipocytes. As a result, while the adipocyte sizes significantly reduced, the numbers of adipocytes increased in the eWAT, which were associated with improved glucose metabolism. In regard to the underlying mechanism, M2 ATMs show high expression levels of transforming growth factor (TGF)-β, which is reportedly involved in inhibiting proliferation/differentiation of various cells, including preadipocytes, and melanocyte- and hematopoietic stem cells [19–21]. Indeed, genes downstream of TGF-β and pSmad 2/3 signaling were downregulated after CD206+ M2 A™ depletion, followed by proliferation of APs. Thus, CD206+ M2 ATMs appear to suppress the proliferation of APs via the TGF-β signaling pathway [22]. Moreover, we found that CD206+ M2 A™ depletion promoted browning of cold-stimulated inguinal adipose tissue, by inducing an increase in the number of PDGFRα+ Sca1+ progenitors. Taken together, CD206+ M2 ATMs constitute a microenvironment for APs, in a TGF-β-dependent manner, to maintain systemic insulin sensitivity by tuning the quiescence/proliferation balance of APs to adapt to changes in the nutritional status.
Intervention of the gut microbiota affected diet-induced inflammation and glucose metabolism
We elucidated the role of M1/M2 ATMs, which have a great impact on the control of chronic inflammation and adipose tissue microenvironment. As the next step, we focused on the gut microbiota as a novel modifier of glucose metabolism. The gut microbiota is reported to be involved in the regulation of the host’s energy balance, glucose and lipid metabolism, and the immune response [23–26]. We have previously shown that different strains of mice and mice from different vendors exhibit different rates of obesity following HFD feeding. C57BL/6 J mice from Jax Labs (B6J) and 129 mice from Taconic (129 T) are obesity-prone, while 129 mice from Jax (129 J) are obesity-resistant. This difference between the 129 mice is, at least in part, due to differences in the gut microbiome between the mice from these two vendors [27]. To further explore the role of the microbiome in obesity, we treated these three strains of mice with either vancomycin (to kill gram-positive bacteria) or metronidazole (to kill anaerobic bacteria), and challenged them with an HFD. 16S rRNA sequencing showed that both antibiotics changed the microbiome composition in all the strains. In the B6J mice, which tend to have genetically inflammatory potential and show insulin resistance, antibiotics improved the glucose metabolism, decreased the serum TNFα levels, decreased the number of inflammatory macrophages in the liver and adipose tissue, and improved insulin signaling. This improvement of glucose metabolism could be reproduced by transferring the gut bacteria isolated from antibiotic-treated donors to HFD-fed B6J mice. Metabolomic analysis showed that HFD and antibiotics changed the serum metabolite profile, including prominent changes in the bile acids and levels of the anti-inflammatory bile acid receptor TGR5 expressed in the liver Kupffer cells. These changes could also be reproduced, in part, by treatment with a TGR5 agonist in the B6J mice. In contrast, antibiotic treatment of HFD-fed 129 J or 129 T mice, which are genetically resistant to diet-induced inflammation, did not improve the metabolic profile, despite causing equally dramatic changes in the gut microbiota and in the levels of different serum bile acids. These mice also failed to show improved glucose tolerance in response to treatment with the TGR5 agonist. Thus, antibiotic modification of the gut microbiota, acting through changes in the bile acids and inflammatory signaling, can improve insulin signaling and glucose metabolism. However, these effects are strain-dependent and partly dependent on bile acids, indicating the important interactions of the gut microbiome with the host genetics, bile acid metabolism and inflammatory potential [28].
To further explore the relationships between the metabolic phenotype, gut microbiota and other host factors, we performed LC–MS-based metabolomics of the cecal contents and plasma of B6J, 129 J and 129 T mice reared on chow, an HFD, and an HFD containing vancomycin or metronidazole. The cecal and plasma metabolite profiles showed multifold differences reflecting the combined and integrated effects of the antibiotics contained in the diet, the host genetic background, and the gut microbiome. Interestingly, among the approximately 400 identified metabolites profiled in the plasma using untargeted LC–MS, 18 plasma metabolites showed strong positive or negative correlations with the host insulin resistance across strains and diets. Thus, the diet, host genetics and gut microbiota interact to create distinct responses in the plasma metabolites, contributing to regulation of the metabolism and degree of insulin resistance [29].
Bofutsushosan improves chronic inflammation and insulin resistance through modification of the gut microbiota-pleiotropic effect of a Japanese herbal medicine
Recent studies have revealed that obesity and intake of a high-fat diet cause dysbiosis, characterized by decreased bacterial diversity and a decrease in the population of Akkermansia muciniphila (Akk); the mucous layer of the intestinal epithelium becomes thin and the expressions of tight-junction-related proteins decrease. In this “leaky gut,” the intestinal permeability increases and the lipopolysaccharides derived from Gram-negative bacteria flow into the circulation. This causes the activation of Toll-like receptor 4 (TLR-4) signaling, promoting systemic inflammation [30]. Indeed, obese subjects and rodents exhibit higher plasma endotoxin levels, which have been shown to be correlated with insulin resistance. Therefore, it would be beneficial to identify factors that can restore the barrier function, as possible targets for alleviating obesity-related inflammation and insulin resistance.
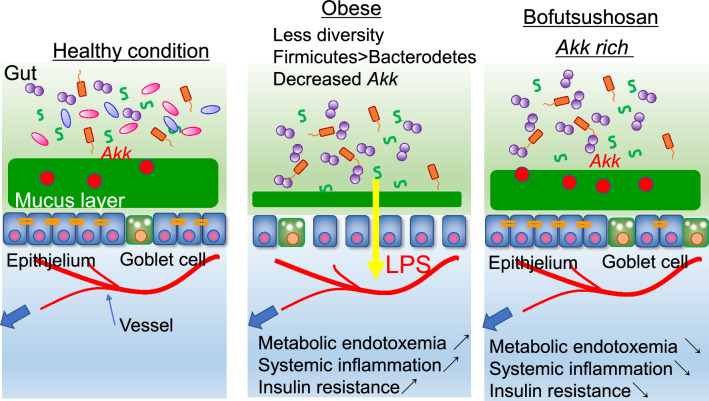
Recently, many products available in the market, besides antibiotics, have been reported to have an impact on the gut microbiota [31]. We found that Bofutsushosan (BFT), a Japanese herbal medicine, which has been clinically used for constipation in obese patients, ameliorates systemic inflammation and glucose metabolism by modifying the gut microbiota in mice fed an HFD. The changes in the bacterial composition mainly involved a bloom of Akk, which are bacteria that are known to improve the gut barrier function. BFT administration increased the expression levels of claudin-1, a tight-junction-related protein, in the colon, and alleviated endotoxemia. Antibiotic treatment abrogated the metabolic improvement that accrued from BFT treatment. Furthermore, the improved glucose metabolism could be reproduced by cecal bacterial transfer, suggesting that BFT modifies the gut microbiota with an increase of Akk, contributing to improving the gut barrier function and preventing metabolic endotoxemia, and consequently, to attenuation of diet-induced inflammation and insulin resistance (Fig. 3) [32]. Intervention of the gut microbiota can be a novel concept in improving insulin sensitivity and glucose metabolism through alleviating obesity-induced systemic inflammation.
Fig. 3.
Gut microbiota modified by Bofutsushosan strengthens gut barrier and alleviates endotoxemia
Conclusions and future perspectives
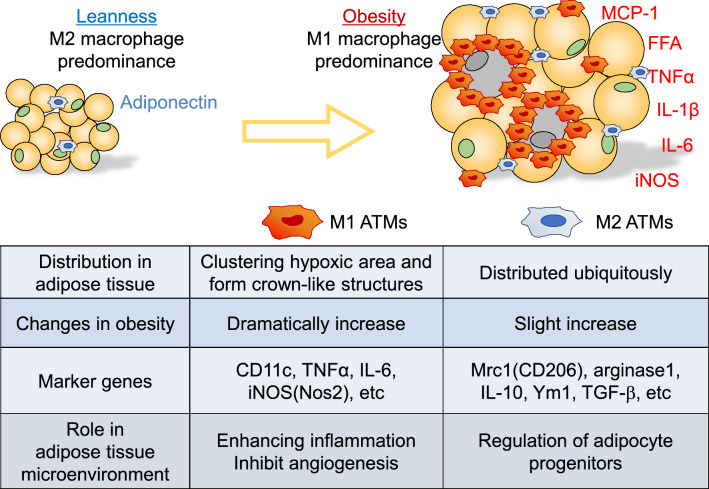
We focused on the role of chronic inflammation in the pathophysiology of obesity-induced insulin resistance. To analyze the effect of macrophage polarity in the adipose tissue on glucose metabolism in greater detail, we developed a method for accurately separating M2 macrophages using CD206 as a marker, and investigated the involvement of the M1/M2 macrophages in the development of insulin resistance and the adipose tissue microenvironment (Fig. 4). Today, many researchers use this method to evaluate chronic inflammation in the adipose tissue, to further our understanding of the pathophysiology of insulin resistance. Furthermore, we clarified the impact of the gut microbiota on the control of chronic inflammation caused by obesity. Demonstration of the pleiotropic actions of drugs that are already in clinical use, such as Bofutsushosan, including modification of the gut microbiota and intestinal barrier function, provides insights into new therapeutic targets to improve glucose metabolism. We strongly expect future studies to lead to the development of new therapeutic strategies for metabolic syndrome via control of obesity-related inflammation.
Fig. 4.
General characteristics of M1/M2 ATMs
Acknowledgements
A summary of this review was presented in the Lilly Award Lecture at the 63rd Annual Meeting of the Japan Diabetes Society, Shiga, Japan. I would like to express sincere gratitude to Professor Kazuyuki Tobe, Professor C. Ronald Kahn, and Professor Masashi Kobayashi, for their mentoring, and to my colleagues, collaborators and family for their support. The author was supported by JSPS KAKENHI (17K09821, 20K08882), the AMED PRIME (JP18gm6010023h0001), the Japan Diabetes Foundation, the Takeda Science Foundation and the Mochida Memorial Foundation for Medical and Pharmaceutical Research, the Yakult Bio-Science Foundation, the Daiichi Sankyo Foundation of Life Science.
Compliance with ethical standards
Conflict of interest
Shiho Fujisaka declares that she has no conflict of interest associated with this research.
Human or animal rights
This article does not include data collected from any studies involving human or animal subjects.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 2.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25(12):677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 4.Weisberg SP, Hunter D, Huber R, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116(1):115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cinti S, Mitchell G, Barbatelli G, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46(11):2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 6.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117(1):175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujisaka S, Usui I, Bukhari A, et al. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes. 2009;58(11):2574–2582. doi: 10.2337/db08-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujisaka S, Usui I, Ikutani M, et al. Adipose tissue hypoxia induces inflammatory M1 polarity of macrophages in an HIF-1alpha-dependent and HIF-1alpha-independent manner in obese mice. Diabetologia. 2013;56(6):1403–1412. doi: 10.1007/s00125-013-2885-1. [DOI] [PubMed] [Google Scholar]
- 9.Fujisaka S, Usui I, Kanatani Y, et al. Telmisartan improves insulin resistance and modulates adipose tissue macrophage polarization in high-fat-fed mice. Endocrinology. 2011;152(5):1789–1799. doi: 10.1210/en.2010-1312. [DOI] [PubMed] [Google Scholar]
- 10.Pasarica M, Sereda OR, Redman LM, et al. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes. 2009;58(3):718–725. doi: 10.2337/db08-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolinder J, Kerckhoffs DA, Moberg E, Hagstrom-Toft E, Arner P. Rates of skeletal muscle and adipose tissue glycerol release in nonobese and obese subjects. Diabetes. 2000;49(5):797–802. doi: 10.2337/diabetes.49.5.797. [DOI] [PubMed] [Google Scholar]
- 12.Elias I, Franckhauser S, Ferre T, et al. Adipose tissue overexpression of vascular endothelial growth factor protects against diet-induced obesity and insulin resistance. Diabetes. 2012;61(7):1801–1813. doi: 10.2337/db11-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takikawa A, Mahmood A, Nawaz A, et al. HIF-1alpha in myeloid cells promotes adipose tissue remodeling toward insulin resistance. Diabetes. 2016;65(12):3649–3659. doi: 10.2337/db16-0012. [DOI] [PubMed] [Google Scholar]
- 14.Odegaard JI, Chawla A. Alternative macrophage activation and metabolism. Annu Rev Pathol. 2011;6:275–297. doi: 10.1146/annurev-pathol-011110-130138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qiu Y, Nguyen KD, Odegaard JI, et al. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 2014;157(6):1292–1308. doi: 10.1016/j.cell.2014.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121(6):2094–2101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee MW, Odegaard JI, Mukundan L, et al. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell. 2015;160(1–2):74–87. doi: 10.1016/j.cell.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohsen-Kanson T, Hafner AL, Wdziekonski B, Villageois P, Chignon-Sicard B, Dani C. Expression of cell surface markers during self-renewal and differentiation of human adipose-derived stem cells. Biochem Biophys Res Commun. 2013;430(3):871–875. doi: 10.1016/j.bbrc.2012.12.079. [DOI] [PubMed] [Google Scholar]
- 19.Yamazaki S, Ema H, Karlsson G, et al. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell. 2011;147(5):1146–1158. doi: 10.1016/j.cell.2011.09.053. [DOI] [PubMed] [Google Scholar]
- 20.Nishimura EK, Suzuki M, Igras V, et al. Key roles for transforming growth factor beta in melanocyte stem cell maintenance. Cell Stem Cell. 2010;6(2):130–140. doi: 10.1016/j.stem.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ignotz RA, Massague J. Type beta transforming growth factor controls the adipogenic differentiation of 3T3 fibroblasts. Proc Natl Acad Sci USA. 1985;82(24):8530–8534. doi: 10.1073/pnas.82.24.8530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nawaz A, Aminuddin A, Kado T, et al. CD206(+) M2-like macrophages regulate systemic glucose metabolism by inhibiting proliferation of adipocyte progenitors. Nat Commun. 2017;8(1):286. doi: 10.1038/s41467-017-00231-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Velagapudi VR, Hezaveh R, Reigstad CS, et al. The gut microbiota modulates host energy and lipid metabolism in mice. J Lipid Res. 2010;51(5):1101–1112. doi: 10.1194/jlr.M002774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Chatelier E, Nielsen T, Qin J, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500(7464):541–546. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- 25.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 26.Ridaura VK, Faith JJ, Rey FE, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341(6150):1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ussar S, Griffin NW, Bezy O, et al. Interactions between gut microbiota, host genetics and diet modulate the predisposition to obesity and metabolic syndrome. Cell Metab. 2015;22(3):516–530. doi: 10.1016/j.cmet.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujisaka S, Ussar S, Clish C, et al. Antibiotic effects on gut microbiota and metabolism are host dependent. J Clin Invest. 2016;126(12):4430–4443. doi: 10.1172/JCI86674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujisaka S, Avila-Pacheco J, Soto M, et al. Diet, genetics, and the gut microbiome drive dynamic changes in plasma metabolites. Cell Rep. 2018;22(11):3072–3086. doi: 10.1016/j.celrep.2018.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 31.Maier L, Pruteanu M, Kuhn M, et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555(7698):623–628. doi: 10.1038/nature25979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fujisaka S, Usui I, Nawaz A, et al. Bofutsushosan improves gut barrier function with a bloom of Akkermansia muciniphila and improves glucose metabolism in mice with diet-induced obesity. Sci Rep. 2020;10(1):5544. doi: 10.1038/s41598-020-62506-w. [DOI] [PMC free article] [PubMed] [Google Scholar]