Abstract
Background:
Most children with intermediate risk rhabdomyosarcoma (RMS) have gross disease (Group III) at the initiation of chemotherapy. Delayed primary excision (DPE) after induction chemotherapy allows for a reduction in adjuvant radiation dose, but with the risk of potential surgical morbidity. The aims of this study were to compare outcomes for Group III RMS with and without DPE and assess surgical morbidity.
Methods:
The study included 369 Clinical Group III patients at sites amenable to DPE from intermediate-risk COG studies D9803 (encouraged DPE) and ARST0531 (discouraged DPE).
Results:
Primary tumor site was bladder/prostate (136, 37%), extremity (97, 26%), trunk (24, 7%), retroperitoneum (91, 25%), or intrathoracic/perineum/perianal (21, 6%). DPE was performed in 112 (53.9%) in D9803 and 26 (16.2%) in ARST0531 (p<0.001) with loss of vital organ or function in 30/138 (22%). DPE allowed for reduction in RT dose in 81% (110/135) of patients (51% to 36 Gy, 30% to 42 Gy). DPE patients had improved unadjusted overall survival (p=0.013). In adjusted regression analysis, risk of death (HR 0.71, 95% CI 0.43–1.16) was similar for DPE and no-DPE and was improved for the subset of patients with tumors of the trunk and retroperitoneum (HR 0.44; 95% CI 0.20–0.97).
Conclusion:
Patients with Group III RMS have equivalent or improved outcomes with DPE and decreased RT dose for definitive local control. The choice of local control modality should weigh the potential morbidity of surgery versus that of higher dose irradiation.
Keywords: Pediatric, rhabdomyosarcoma, delayed primary excision
Lay Summary:
Most children with rhabdomyosarcoma, a tumor of muscle tissue, have residual tumor masses when they start chemotherapy. In addition to chemotherapy, these patients may be treated with radiation therapy, additional surgery or both. This study examined outcomes of patients who had delayed removal of their tumors after initial chemotherapy compared to those who just received radiation. Those who had surgery still required radiation, but in 81% of cases the surgery allowed for the dose of radiation to be reduced, which may alleviate some of the complications of radiation. In about one-fifth of patients this delayed surgery involved removing an important organ such as the bladder, or doing an amputation. After accounting for other factors which affect survival, the patients who had this delayed surgical excision had equivalent outcomes compared to those who had definitive radiation treatment. A subset of the patients – those with tumors in the retroperitoneum and trunk – actually had better survival with delayed surgery.
Precis:
In this study of 369 children with Group III rhabdomyosarcoma, 37% underwent delayed primary excision allowing for reduction in radiation dose in 81% of cases. This approach resulted in overall equivalent outcomes compared to patients who had definitive radiotherapy, and improved outcomes in the subset of patients with trunk and retroperitoneal tumors.
Introduction
Rhabdomyosarcoma (RMS) accounts for 3% of all pediatric cancers.1 The survival for children with RMS has improved over the last five decades even as the chemotherapy backbone has remained relatively unchanged. The multimodal treatment of pediatric RMS includes chemotherapy and surgery and/or radiation therapy (RT) for local control. However, approximately half of survivors of childhood sarcoma has at least one major adverse health status outcome as a result of therapy.2–4 Considerable recent effort has therefore been placed on optimizing risk stratification and modulating treatment intensity to reduce treatment morbidity.
The initial surgical approach for RMS includes primary excision only if it is anticipated to achieve complete resection without significant functional or cosmetic sequelae. This approach has been unchanged across multiple treatment protocols spanning several decades. With this approach, the majority of patients with RMS have gross disease at the start of chemotherapy (Clinical Group III) and are classified as intermediate risk. The local control paradigm for these patients has continued to evolve. In the IRS-IV protocol, Group III patients had excellent rates of local control with definitive RT (50.4 vs 59.4 Gy). D9803 examined the possibility of selectively decreasing the RT dose to 36 Gy for those with no evidence of disease, 41.4 Gy with microscopic residual disease, and 50.4 Gy with gross residual disease after delayed primary excision (DPE) at amenable sites. These RT doses were extrapolated from historical experience based on patients with upfront gross excision, where doses of 36–41.4 Gy were utilized, and where doses >40 Gy were superior for patients with microscopic residual disease. A total of 84% of those who had DPE were eligible for RT dose reduction and 79% of pathology specimens contained viable tumor. The local 5-year failure rate in D9803 after DPE and modulated radiation dose (0% for bladder dome, 7% for extremity and 20% for trunk) was similar to results from IRS-IV that utilized definitive RT for local control 5. Subsequently on Children’s Oncology Group (COG) ARST0531, DPE was discouraged because the study investigated early initiation of RT at week 4. Additionally, on that study, the cumulative dose of cyclophosphamide was significantly reduced. Local control rates on ARST0531 were inferior to those achieved in the prior D9803 study for patients with embryonal RMS, although it remains unknown whether that was due to the decreased intensity of chemotherapy, alteration in radiation schedule, and/or decreased use of DPE 6.
The primary aim of this study was to determine the effect of DPE on local failure and OS for the subset of patients with Group III RMS in anatomic locations amenable to DPE with an analysis controlled for the factors shown to be associated with the use of DPE, including study protocol (D9803 or ARST0531). The secondary aim was to determine rates of surgical morbidity, including loss of function or vital organ with DPE.
Methods
Patient Populations and Therapeutic Protocols
Patients from COG studies D9803 (1999–2005) and ARST0531 (2006–2012) with primary bladder/prostate, extremity, retroperitoneal, trunk, intrathoracic and perineal tumors were included. Clinical group was assigned prior to the initiation of chemotherapy. The analysis was restricted to 369 Clinical Group III patients (biopsy only or gross residual disease after resection without distant metastases). Median follow-up for the patients who remained alive was 5.9 years (range: 0.13–13.5 years) from study enrollment. Operative and pathology reports from the treating institution were reviewed to determine DPE procedures, associated loss of vital organ or function and pathologic findings of residual viable tumor in the DPE specimen. Central review of pathology specimens was not performed.
Details of the chemotherapy treatment have been previously published.7, 8 Briefly, for D9803, patients were randomized to standard VAC (vincristine, dactinomycin, and cyclophosphamide) chemotherapy or VAC alternating with vincristine, topotecan, and cyclophosphamide (VAC/VTC); for ARST0531 vincristine and irinotecan (VI) alternating with VAC. All treatment arms were combined for our analysis because there were no significant differences in outcomes by treatment arm within each study.
Local control regimens were different between D9803 and ARST0531. For D9803 clinical group III patients at these primary sites, local RT began after week 12.9 A dose of 50.4 Gy was used for definitive treatment of clinical group III tumors. By protocol design, patients with clinical group III tumors of the bladder dome, extremity, or trunk (including thorax, abdomen, and pelvis) were candidates for DPE at week 12 if the primary tumor appeared resectable. Those who had complete resection with negative margins (R0) then received 36 Gy, whereas those with microscopic residual (R1) or biopsy confirmation of complete response received 41.4 Gy. Patients with gross residual disease after delayed surgery received the full 50.4 Gy postoperatively. In contrast, on ARST0531 for patients > 24 months of age, definitive RT was the planned local control modality. Delayed primary resection before or after RT was allowed but not encouraged. For patients ≤ 24 months, individualized local control approaches for surgical resection and RT, were permitted. However, adherence to the RT algorithm whenever possible for patients < 24 months was encouraged. RT at a dose of 50.4 Gy started at week 4.
Some patients also had a surgical procedure during the last treatment period (week 30–42) or after relapse. These end-of-therapy procedures were excluded from the DPE analysis.
Definition of Treatment Response
In both D9803 and ARST0531, response was categorized by the best response achieved and was determined by radiographic measurement of tumor. Response was assessed at week 12 using two dimensional measurements in D9803 and at week 15 using three dimensional measurements in ARST0531. Complete response (CR: complete resolution of disease), partial response (PR: 50% decrease in the sum of the products of the maximum perpendicular diameters of measurable lesions on D9803; at least 64% reduction in 3-dimensional tumor volume compared to baseline on ARST0531), or no response (NR: less than PR and no more than 25% increase in the sum of the products of the maximum perpendicular diameters of measurable lesions on D9803 or 40% increase in tumor volume on ARST0531) was determined by local institutions without central imaging review. Local failure was defined as progression or relapse at the primary tumor site as a first event, including those with concurrent local plus regional or distant failure. OS was defined as the time from diagnosis to death from any cause. OS for participants who had not experienced the event of interest were censored at the patient’s last contact date.
Statistical Analysis Methods
The distributions of categorical participant characteristics were compared between the groups using the Fisher’s exact test. OS probabilities were estimated using the method of Kaplan and Meier with confidence intervals estimated by the Peto-Peto method and were compared between groups using the log-rank test.10 A Cox proportional hazards regression model was used to adjust comparisons based on covariates which varied significantly between the DPE and no-DPE groups, including study, age group (<1, 1–9, 10+) and gender as covariates. Because of the heterogeneous outcomes of tumors at different anatomic sites, subset analyses were performed, limited to patients with (1) extremity, (2) bladder-prostate, and (3) remaining anatomic site tumors. Cumulative incidence of local failure was estimated using a subdistribution proportional hazards analysis**, with regional/distant failures, second malignancy, and death as competing risks. The site of first recurrence was defined as local if the tumor recurred at the site of primary disease +/− regional or distant; as regional if regional lymph nodes were involved +/− distal recurrence; and as distant if any metastatic disease was present at recurrence in the absence of local or regional disease. Follow-up was current as of December 31, 2018.
Results
The study population included 369 Group III patients from D9803 (n=208) and ARST0531 (n=161). The primary site was bladder/prostate in 136 (37%), extremity in 97 (26%), trunk in 24 (7%), retroperitoneum in 91 (25%), intrathoracic in 4 (1%), and perineum/perianal in 17 (5%). DPE was performed in 112 (53.9%) patients in D9803 and 26 (16.2%) patients in ARST0531 (p<0.0001). Clinical factors between patients who did and did not undergo DPE are compared in Table 1. Participation on D9803 (p<0.0001), younger age (p=0.03) and female gender (p=0.0079) were associated with increased likelihood of DPE.
Table 1:
Demographics and clinical characteristics by DPE status
| DPE (%) | No-DPE (%) | p-value* | |
|---|---|---|---|
| Study | <0.0001 | ||
| D9803 | 112 (53.9) | 96 (46.2) | |
| ARST0531 | 26 (16.2) | 135 (83.9) | |
| Age | 0.03 | ||
| <1 | 12 (44.4) | 15 (55.6) | |
| 1–9 | 105 (40.5) | 154 (59.5) | |
| 10+ | 21 (25.3) | 62 (74.7) | |
| Sex | 0.0079 | ||
| Male | 72 (31.9) | 154 (68.1) | |
| Female | 66 (46.2) | 77 (53.9) | |
| Primary tumor site | 0.48 | ||
| Bladder/prostate | 49 (36.0) | 87 (64.0) | |
| Extremity | 43 (44.3) | 54 (55.7) | |
| Intrathoracic | 2 (50.0) | 2 (50.0) | |
| Perineum/anus | 4 (23.5) | 13 (76.5) | |
| Retroperitoneum | 33 (36.3) | 58 (63.7) | |
| Trunk | 7 (29.2) | 17 (70.8) | |
| Tumor size | 0.23 | ||
| ≤5 cm | 44 (42.7) | 59 (57.3) | |
| >5 cm | 93 (35.4) | 170 (64.6) | |
| Unknown | 1 (33.3) | 2 (66.7) | |
| T status | 0.19 | ||
| T1 | 79 (40.5) | 116 (59.5) | |
| T2 | 58 (33.5) | 115 (66.5) | |
| Unknown | 1 (100.0) | 0 | |
| N status | 0.23 | ||
| No, N0 | 115 (39.5) | 176 (60.5) | |
| Yes, N1 | 23 (31.1) | 51 (68.9) | |
| Unknown | 0 | 4 (100.0) | |
| Histology | 0.26 | ||
| ARMS | 40 (33.9) | 78 (66.1) | |
| ERMS | 68 (39.5) | 104 (60.5) | |
| BRMS | 18 (40.9) | 26 (59.1) | |
| NOS | 5 (31.3) | 11 (68.8) | |
| Spindle cell | 3 (21.4) | 11 (78.6) | |
| Mixed RMS | 4 (80.0) | 1 (20.0) | |
| Fusion Status | 0.11 | ||
| FOXO1−1 | 102 (39.5) | 156 (60.5) | |
| FOXO1+ | 23 (29.5) | 55 (70.5) | |
| Unknown | 13 (39.4) | 20 (60.61) | |
| Response at 12/15 weeks | 0.25 | ||
| CR | 9 (31.0) | 20 (69.0) | |
| PR | 75 (48.1) | 81 (51.9) | |
| NR | 25 (54.4) | 21 (45.7) | |
| PD | 2 (50.0) | 2 (50.0) | |
| Unknown | 27 (20.2) | 107 (79.9) | |
| End-of-therapy mass excision | 1.0 | ||
| Yes | 12 (36.4) | 21 (63.6) | |
| No | 126 (37.5) | 210 (62.5) |
Assuming FOX01− for ERMS, BRMS and Spindle cell.
Surgical outcomes for patients who underwent DPE on D9803 and ARST0531 are compared in Table 2. There was no difference in completeness of resection, findings of viable tumor at surgery, or procedure type between studies. Approximately 80% of DPE patients achieved R0 (50–52%) or R1 (30%) resection, which allowed for a reduction in RT dose (50% to 36Gy and 30% to 42Gy). Incomplete (R2) DPE occurred in 29% of bladder/prostate attempts, but only 5% of extremity tumor attempts. Approximately 80% of DPE specimens contained viable tumor. Of all DPE procedures performed only 15% were biopsy and the remaining 85% were excisional resections.
Table 2:
Surgical Outcomes from DPE in D9803 and ARST0531
| D9803 (%) | ARST0531 (%) | p-value | |
|---|---|---|---|
| Completeness of resection | 1.0 | ||
| R0 | 57 (50.9) | 12 (52.2) | |
| R1 | 34 (30.4) | 7 (30.4) | |
| R2 | 21 (18.8) | 4 (17.4) | |
| Pathologic findings | 0.40 | ||
| Viable RMS | 84 (77.8) | 21 (87.5) | |
| No viable RMS | 24 (22.2) | 3 (12.5) | |
| Procedure type | 0.36 | ||
| Biopsy | 19 (17.0) | 2 (8.0) | |
| Excision | 93 (83.0) | 23 (92.0) | |
| Loss of vital organ or function | 0.006 | ||
| Yes | 19 (16.8) | 11 (44.0) | |
| No | 94 (83.2) | 14 (56.0) |
Although DPE was much less common in ARST0531, there was a significantly higher risk of loss of vital organ or function on that study (p=0.006). Across the two studies, this loss of organ and/or function occurred in 31% of bladder/prostate DPE patients (13 cystectomy or cystoprostatectomy, 1 hysterectomy, 1 anterior exenteration); 20% of extremity DPE patients (6 amputation procedures, 3 wide excisions with functional deficits), 50% of perineal DPE patients (2 with colorectal resection and permanent stomas), and 12% of retroperitoneal DPE patients (4 with resection of bladder, uterus, and/or ureter). No cases of loss of vital organ or function were clearly attributable to unexpected surgical morbidity as opposed to planned resectional procedures.
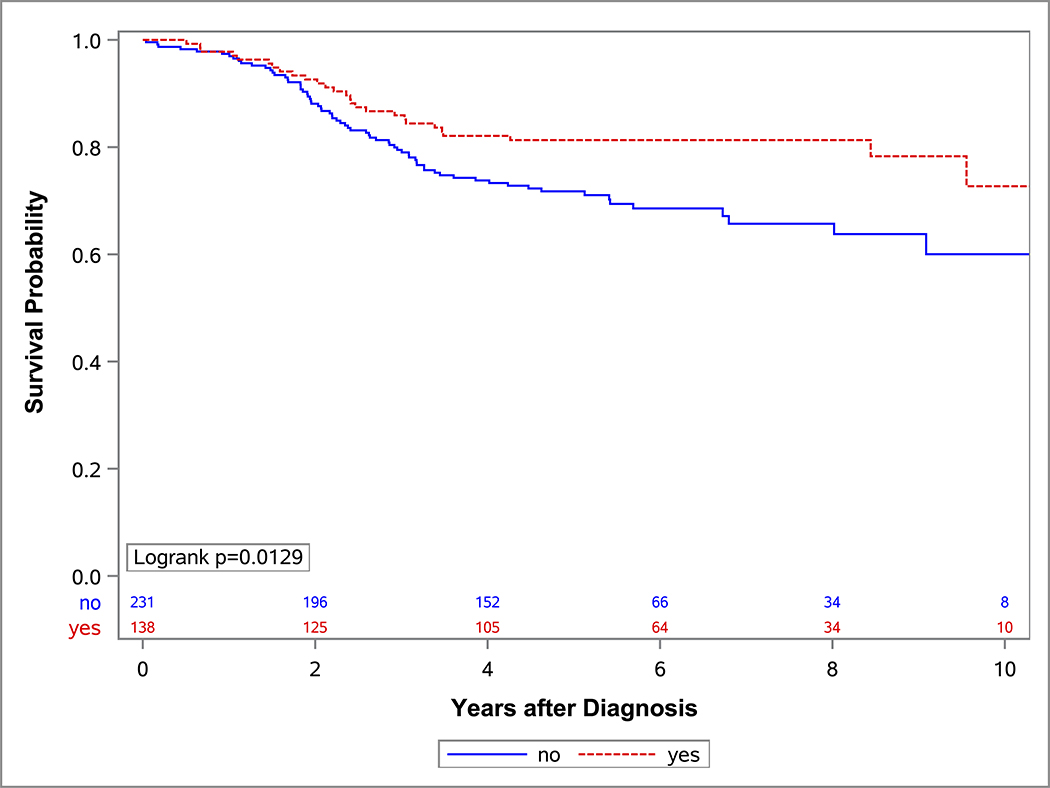
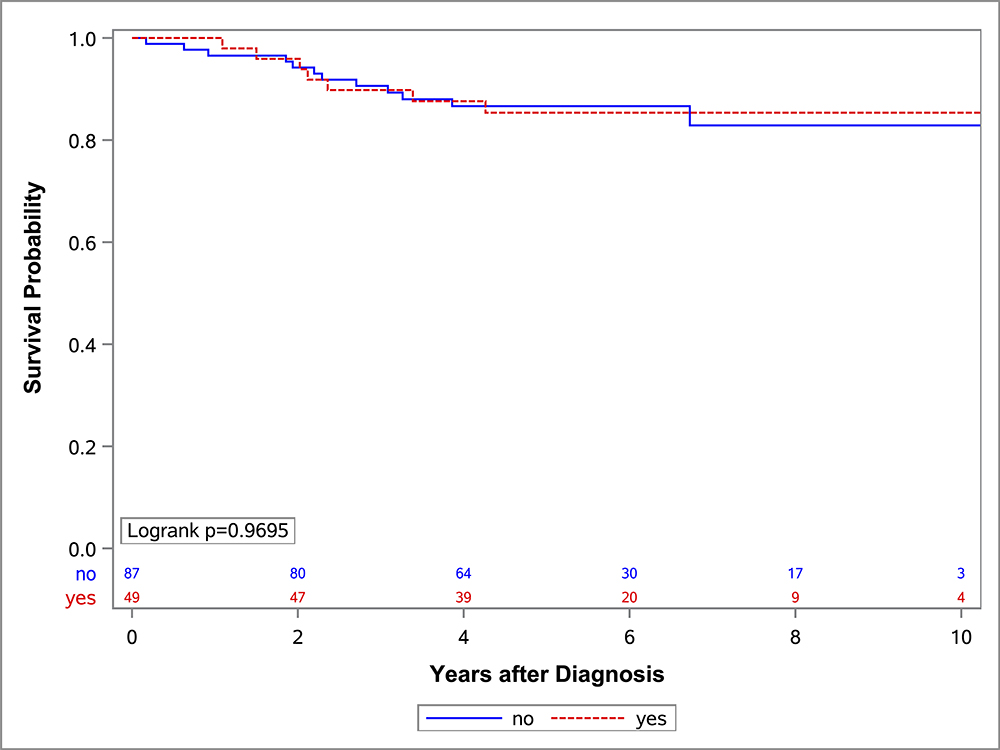
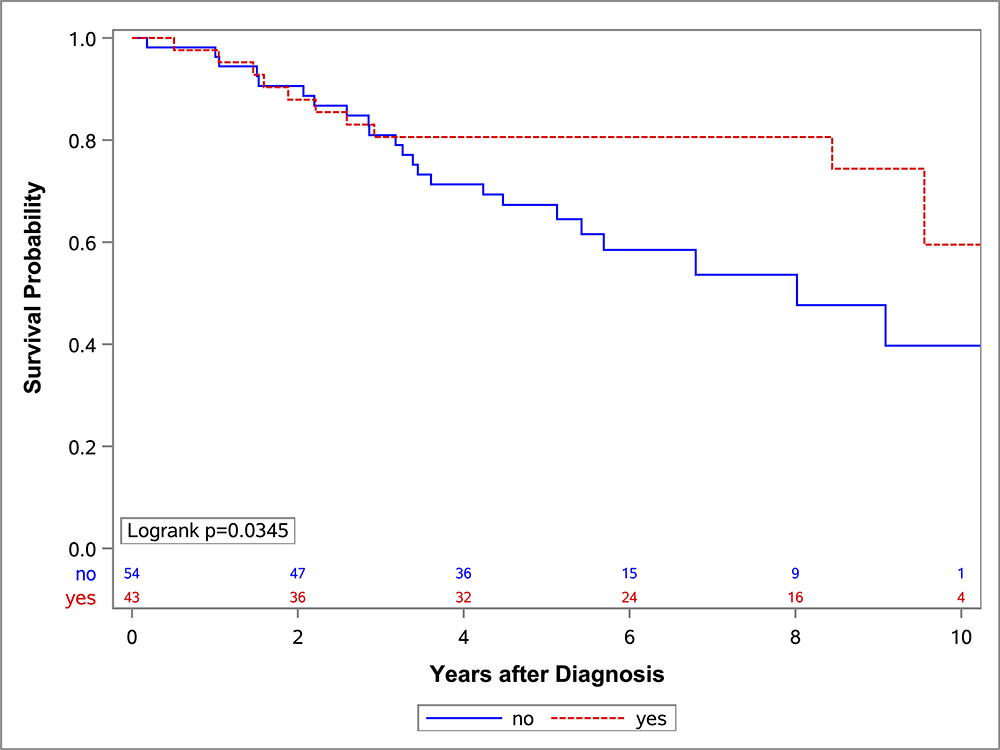
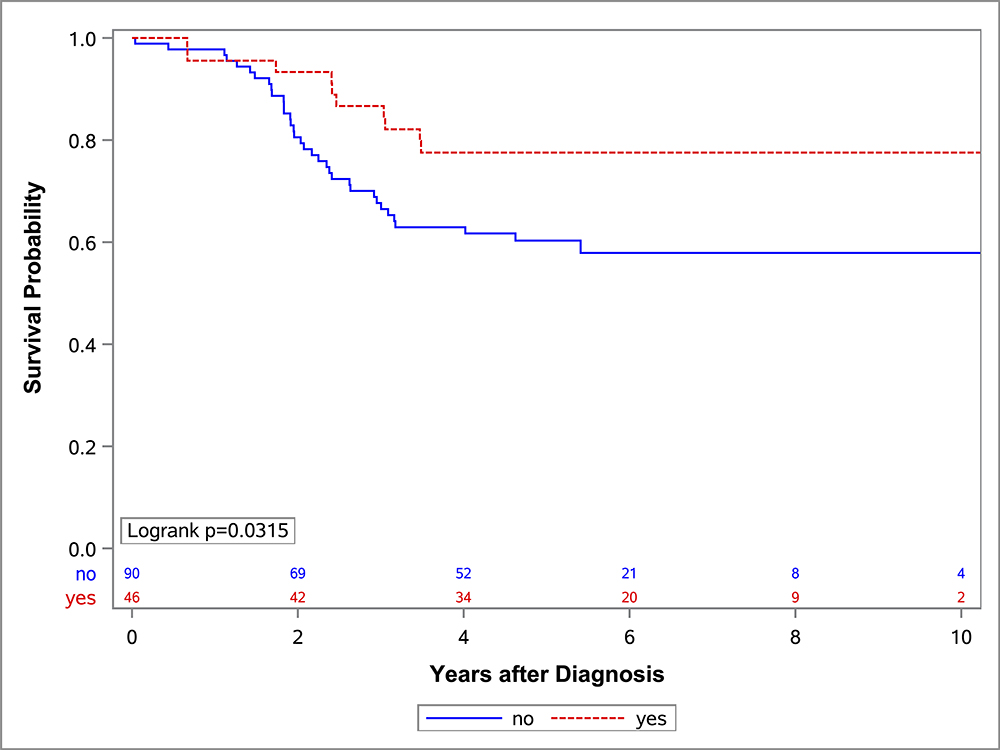
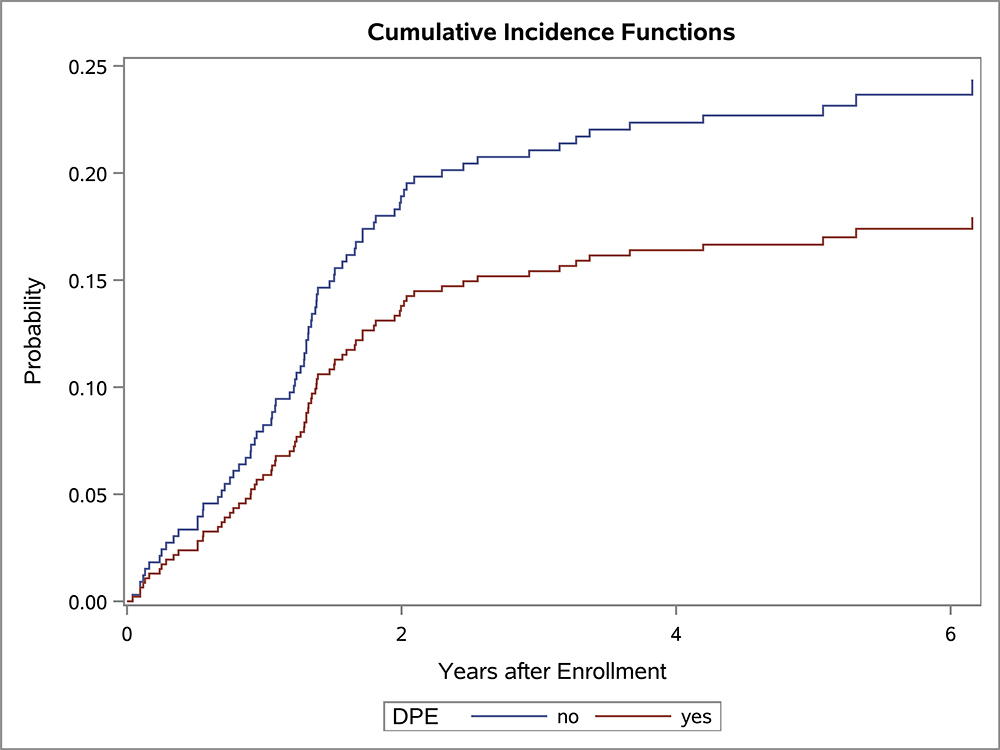
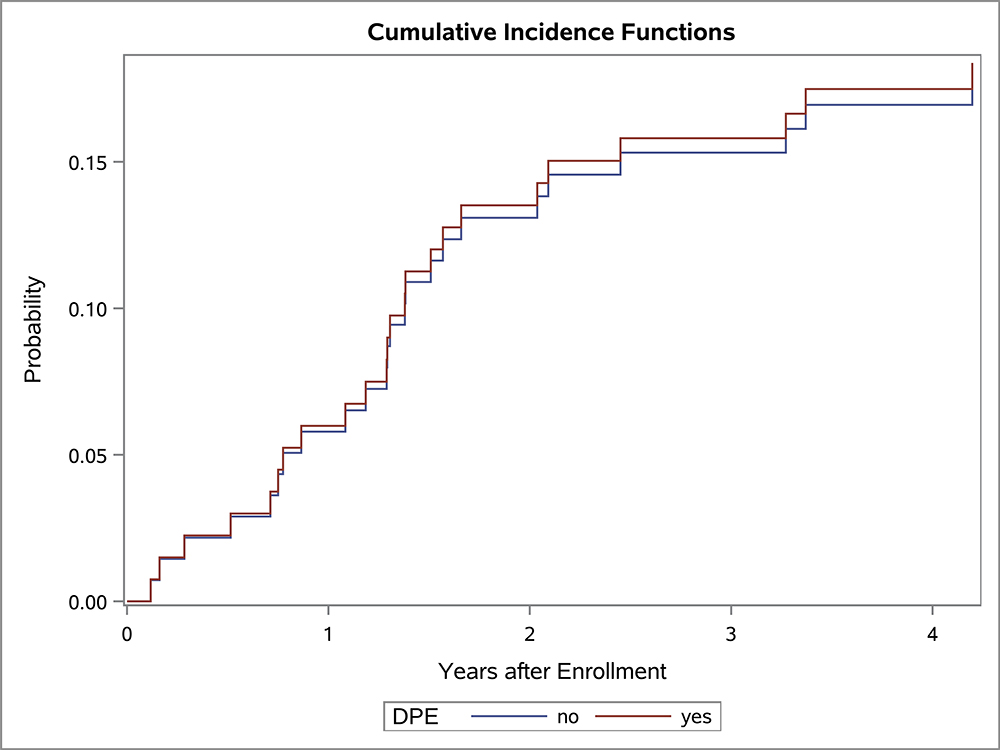
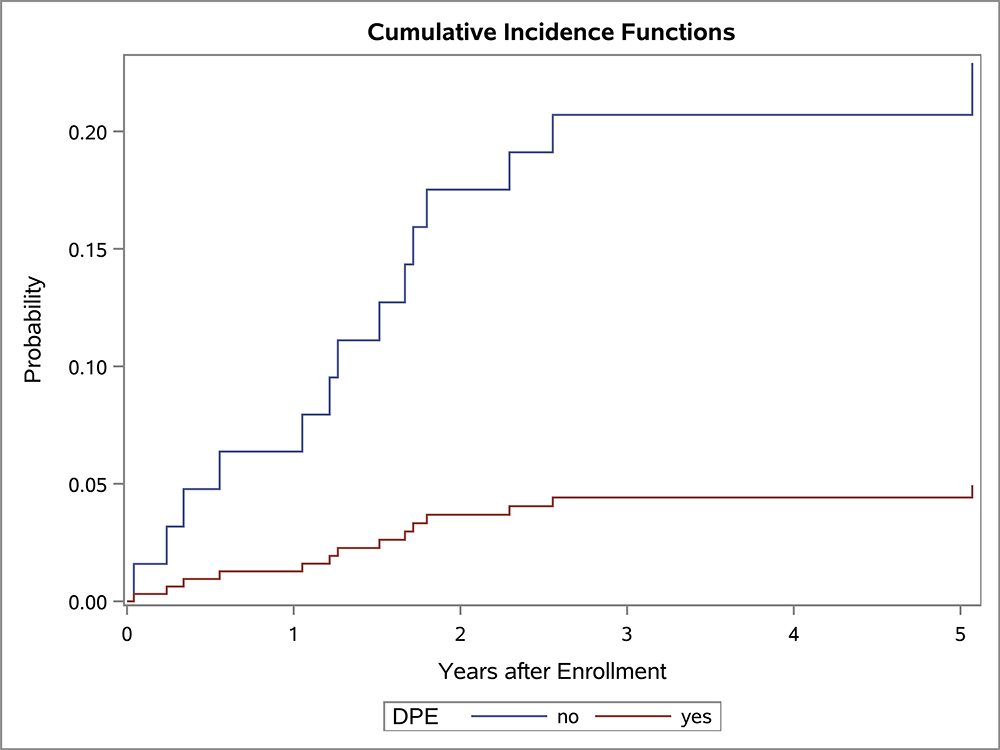
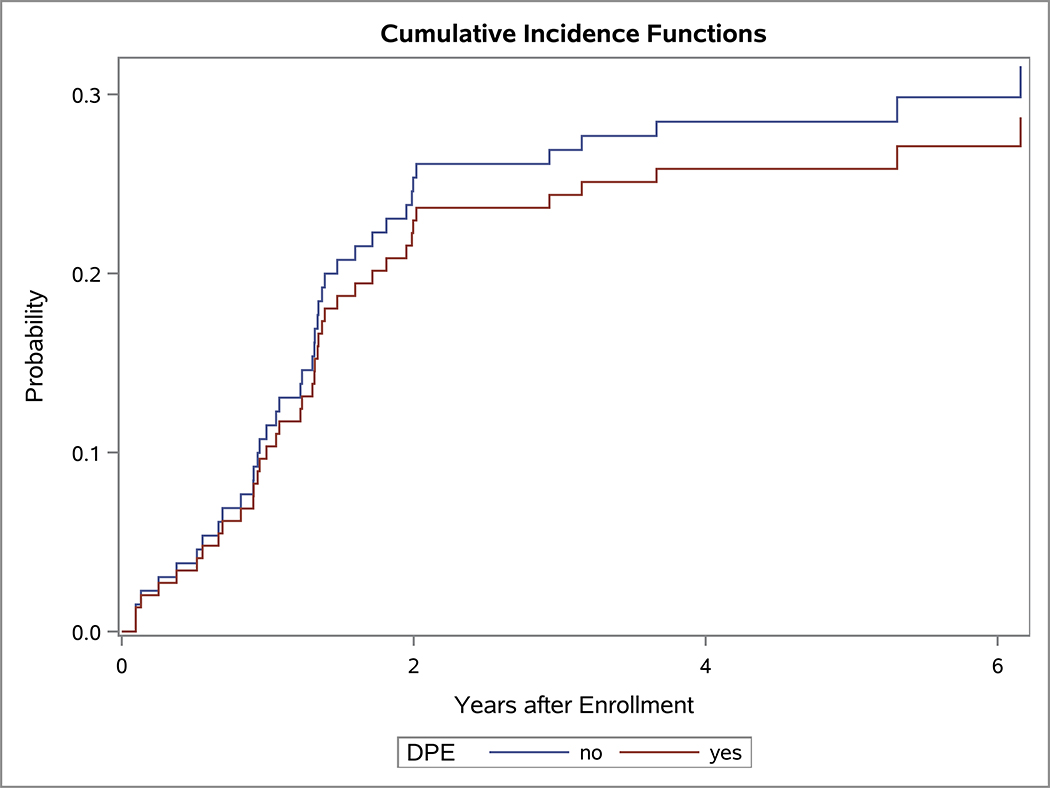
In the univariate OS analysis, patients who underwent DPE had improved survival compared to those who did not undergo DPE (Figure 1; p=0.013). This held true for the subset of patients with extremity tumors (p=0.035), and non-BP/non-extremity tumors (p=0.032). However, survival was similar with and without DPE for patients with bladder/prostate tumors (p=0.97). Survival also was similar with and without DPE for patients who were FOX01+ (p=0.15). When accounting for factors that differed significantly between the DPE and no-DPE groups, including the study on which the patient was enrolled, there was no difference in the risk of death for the entire cohort, bladder/prostate subset, nor extremity subset for patients with and without DPE (Table 3). However, for patients with non-BP/non-extremity tumors (a group which was largely composed of trunk and retroperitoneal tumors), DPE was still associated with a significantly reduced risk of mortality (HR 0.42; p=0.042).
Figure 1:
OS curves by the status of DPE for (A) all patients, (B) bladder-prostate, (C) extremity, (D) non-BP, non-extremity
Table 3:
Cox regression model for OS, adjusted by the effects of study, age, and gender
| Patients and regression model | Hazard ratio (DPE:no-DPE) | 95% CI | p-value |
|---|---|---|---|
| All participants, N=369 | 0.707 | (0.432, 1.156) | 0.17 |
| BP sites only, N=136 | 1.816 | (0.603, 5.466) | 0.29 |
| Extremity sites only, N=97 | 0.628 | (0.275, 1.434) | 0.27 |
| non-BP, non-extremity sites only, N=136 | 0.438 | (0.198, 0.970) | 0.042 |
The univariate competing risk analysis for local failure demonstrated that patients with extremity tumors had better outcomes (p=0.03) with DPE, however at other sites DPE did not impact local failure rates (Figure 2). Local failure rate also was similar with and without DPE for patients who were FOX01+ (p=0.78). After adjusting for potential confounding factors, the adjusted risk of local failure was comparable between the DPE and no-DPE groups for the entire cohort and all subsets by primary type (Table 4). Undergoing DPE did not have an overall impact on the site of first failure (Table 5). First failure was local (with or without additional regional or metastatic disease) in 77% of patients who underwent DPE and 60% of patients who did not undergo DPE (p=0.12).
Figure 2:
Cumulative incidence curves of local recurrence by the status of DPE for (A) all patients (p=0.15), (B) bladder-prostate (p=0.93), (C) extremity (p=0.03), (D) non-BP, non-extremity (p=0.73)
Table 4:
Competing risk analysis for local failure, adjusted by the effects of study, age, and gender
| Patients and regression model | Hazard ratio (DPE:no-DPE) | 95% CI | p-value |
|---|---|---|---|
| All participants | 0.725 | (0.401, 1.313) | 0.29 |
| BP sites only | 2.016 | (0.588, 6.914) | 0.27 |
| Extremity sites only | 0.109 | (0.006, 1.848) | 0.13 |
| non-BP, non-extremity sites only | 0.842 | (0.357, 1.984) | 0.69 |
Table 5:
Site of first failure
| Site | DPE (%) | no-DPE (%) | p-value |
|---|---|---|---|
| All participants | 0.12 | ||
| Local +/− regional or distant | 24 (77.4) | 53 (59.6) | |
| Regional or distant | 7 (22.6) | 36 (40.5) | |
| BP sites only | 1.0 | ||
| Local +/− regional or distant | 9 (90.0) | 15 (83.3) | |
| Regional or distant | 1 (10.0) | 3 (16.7) | |
| Extremity sites only | 0.68 | ||
| Local +/− regional or distant | 2 (25.0) | 12 (41.4) | |
| Regional or distant | 6 (75.0) | 17 (58.6) | |
| non-BP, non-extremity sites only | 0.01 | ||
| Local +/− regional or distant | 13 (100.0) | 26 (61.9) | |
| Regional or distant | 0 | 16 (38.1) |
Discussion
In this pooled analysis of patients with Group III RMS from the two most recent intermediate risk COG studies, children who underwent DPE combined with reduced-dose RT had at least equivalent outcomes compared to those who had definitive RT for local control. Although DPE is associated with moderate morbidity from resection of vital organs, including cystectomy and extremity amputations, the reduction in RT dose has potential long-term benefits. Decreased radiation dose and volume have been advocated as ways to minimize RT associated toxicity and complications.
In a previous study of 161 group III patients from D9803 with bladder dome, extremity and trunk tumors, 73 (45%) underwent DPE and had a reduction in radiation dose based on the completeness of resection 5. These patients were compared to a historic control of patients from IRS-IV who had a similar chemotherapy backbone treated with definitive RT, with no recommendation for DPE or contingency for reduction in the dose of RT based on the completeness of DPE. The local 5-year failure rate after DPE and reduced radiation dose (0% for bladder dome, 7% for extremity and 20% for trunk) was similar to IRS-IV. In a European study of 51 children with chest wall or intrathoracic RMS, patients who underwent DPE were more likely to achieve an R0 resection, and achieving a delayed R0 resection was associated with improved survival 11. These patients received between 36–50.4 Gy of radiation dependent upon completeness of DPE. Delayed surgery was also frequently utilized for patients with bladder and prostate tumors in the European CWS-2002P trial. Twenty of 26 patients treated with chemotherapy and surgery had a DPE, and these patients had a 75% 5-year EFS and 65% bladder preservation rate 12. In this study, RT was completely omitted for patients who had an R0 DPE, and 44.8 Gy given to those with an R1 resection. A prior Italian study of 39 patients with embryonal RMS also demonstrated good outcomes with DPE, although local relapses were more common when RT was completely omitted 13. All of these studies support the potential benefit of DPE, in conjunction with standard chemotherapy +/− RT, in patients with RMS.
The current study builds upon previous COG findings by expanding the cohort of patients from D9803 to include all bladder and prostate, intrathoracic, retroperitoneal, trunk and extremity primary sites. It also includes patients from ARST0531 with group III tumors in these sites for the analysis. DPE was not encouraged in ARST0531 but was performed at the discretion of the local treating institution. By pooling data from these two trials, this study evaluates the impact of DPE by comparing outcomes between patients with Group III tumors at these select primary sites. After controlling for factors which varied significantly between the groups who did or did not undergo DPE, we found the risk of local failure was comparable with and without DPE at all sites. Overall survival with and without DPE was comparable for patients with bladder, prostate or extremity tumors, and improved with DPE for tumors at other sites.
Choice of local control modality in children with intermediate risk RMS must balance the risks of morbidity from surgery and radiation while also ensuring excellent outcomes. RT is associated with significant late effects, particularly in younger children.14–17. Late effects from RT are dependent on the treatment dose, tumor site, field size, radiation technique, and age of the child at the time of RT. Long-term morbidity from RT for RMS can include growth impairment from irradiation of bones, fibrosis from irradiation of soft tissue, and the development of second cancers, especially secondary bone sarcomas.4 Many of the other late effects seen after RT for RMS are site specific, such as restrictive pulmonary disease after RT for intrathoracic tumors;18 edema, atrophy, and impairment in mobility after extremity RT;19 and proctitis and urinary dysfunction after pelvic RT.17 As the risk for many of these late effects (such as the risk of second cancers) increases linearly with dose,20 strategies to reduce RT dose (for example, from 50.4 Gy to 36 Gy with DPE) have the potential to decrease long-term toxicity from RT. However, surgery can also be associated with significant long term morbidity, particularly if vital organs are sacrificed.21 In a previous COG trial of children with low risk RMS, reduction of RT dose based upon the completeness of upfront surgical resection did not compromise local control or survival.22 However, for infants with localized RMS, treatment outside of protocol guidelines, often with delay or omission of RT, was associated with higher rates of local failure.23
Children with tumors of the bladder and/or prostate illustrate the balance between the morbidity of surgery and radiation therapy. Most studies of RMS outcomes rely on subjective, often retrospectively-defined, patient history rather than objective data to define outcomes. This lack of objective outcomes is compounded by differences in timing of events between treatment modalities; while the morbidity of surgery is immediately measurable, the full impact of radiation therapy is often delayed by years if not decades. As such, studies with sufficient long-term follow-up are essential for delineating the true morbidity of each therapy. For example, a study with a mean follow-up of >20 years found that patients undergoing partial resections and radiation therapy had a higher rate of both urinary incontinence and long-term need for surgical care as compared to radical resections alone.24 Small case series with 10- to 15-year follow-up after radical cystoprostatectomy have reported excellent continence and normal to moderately reduced erectile function after radical cystectomy.25 Additionally a single-center study with objective functional data has shown excellent rates of preserved continence and erectile function after partial cystectomy/prostatectomy followed by brachytherapy. However, on urodynamic assessment, irradiated teenagers whose bladders were left in situ were at high risk of detrusor sphincter dyssynergia with neurogenic bladder dysfunction. Additionally, female pelvic radiotherapy to treat pediatric RMS can contribute to late effects of infertility, proctitis and significant hypoplasia of the pelvic bones with complications in 23/24 patients, half of whom required surgical correction.17
Overall rates of local failure were higher on ARST0531 compared to D9803 for patients with Group III embryonal RMS.6 Differences between the two studies included (1) a reduced dose of cyclophosphamide, (2) earlier initiation of RT, and (3) discouragement of DPE on ARST0531 compared to D9803. The current analysis was controlled for study in order to minimize confounding findings based on the fact that more of the DPE patients came from D9803. However it is also possible that the lower rates of DPE on ARST0531 may be a factor explaining differences in local failure rates between the two studies.
This study has several important limitations, some of which have already been discussed. Importantly, ARST0531 and D9803 were sequential clinical trials conducted in different decades and patients within each trial were not randomized to undergo DPE or not. The cohort of patients who underwent DPE comes predominantly from the D9803 study. Furthermore, patients on D9803 had better rates of local control than those on ARST0531, which may bias results towards DPE although this should have been controlled in the regression analysis. It is also likely that patients who underwent DPE differed from those who did not, specifically that their tumors were larger or more aggressive than patients who did not undergo surgery. However, it is also possible that DPE was withheld for patients with more aggressive tumors deemed unresectable. This potential bias is thus likely to have biased our data toward the null hypothesis, as we demonstrated. While we did control for multiple factors through our multivariable model, no regression model can fully account for unknown or unrecognized confounding factors. Additionally, we do not consider this to represent the definitive list of anatomic sites appropriate for DPE. For instance, further investigation is required to compare the oncologic and functional outcomes for DPE in bladder tumors (particularly bladder dome tumors) compared to prostate tumors. Likewise, other rare sites such as the uterus and non-parameningeal head and neck locations may be appropriate for DPE in some cases. Further effort will also be required to understand the success of DPE at different anatomic locations. Higher rates of incomplete (R2) DPE for bladder-prostate tumors might suggest that these should be approached more cautiously with careful consideration of the feasibility of complete DPE as compared to extremity tumors where the risk of incomplete DPE is low.
In conclusion, children with Group III RMS at primary sites amenable to DPE have equivalent or better outcomes with DPE and subsequent reduction in RT dose when compared to definitive RT for local control. Given these results, multidisciplinary discussion on a case-by-case basis should weigh the risks and benefits of each treatment plan to determine the optimal therapeutic approach.
Acknowledgments
Funding was provided by: Children’s Oncology Group Grants U10CA180886, U10CA180899, U10CA098543, and U10CA098413; St. Baldrick’s Foundation; Seattle Children’s Foundation, from Kat’s Crew Guild through the Sarcoma Research Fund.
Footnotes
The authors have no conflicts of interest to disclose
REFERENCES
- 1.Hawkins DS, Spunt SL, Skapek SX, Committee COGSTS. Children’s Oncology Group’s 2013 blueprint for research: Soft tissue sarcomas. Pediatr Blood Cancer. 2013;60: 1001–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stevens MC. Treatment for childhood rhabdomyosarcoma: the cost of cure. Lancet Oncol. 2005;6: 77–84. [DOI] [PubMed] [Google Scholar]
- 3.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355: 1572–1582. [DOI] [PubMed] [Google Scholar]
- 4.Punyko JA, Mertens AC, Gurney JG, et al. Long-term medical effects of childhood and adolescent rhabdomyosarcoma: a report from the childhood cancer survivor study. Pediatr Blood Cancer. 2005;44: 643–653. [DOI] [PubMed] [Google Scholar]
- 5.Rodeberg DA, Wharam MD, Lyden ER, et al. Delayed primary excision with subsequent modification of radiotherapy dose for intermediate-risk rhabdomyosarcoma: a report from the Children’s Oncology Group Soft Tissue Sarcoma Committee. Int J Cancer. 2015;137: 204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casey DL, Chi YY, Donaldson SS, et al. Increased local failure for patients with intermediate-risk rhabdomyosarcoma on ARST0531: A report from the Children’s Oncology Group. Cancer. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arndt CA, Stoner JA, Hawkins DS, et al. Vincristine, actinomycin, and cyclophosphamide compared with vincristine, actinomycin, and cyclophosphamide alternating with vincristine, topotecan, and cyclophosphamide for intermediate-risk rhabdomyosarcoma: children’s oncology group study D9803. J Clin Oncol. 2009;27: 5182–5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hawkins DS, Chi YY, Anderson JR, et al. Addition of Vincristine and Irinotecan to Vincristine, Dactinomycin, and Cyclophosphamide Does Not Improve Outcome for Intermediate-Risk Rhabdomyosarcoma: A Report From the Children’s Oncology Group. J Clin Oncol. 2018: JCO2018779694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolden SL, Lyden ER, Arndt CA, et al. Local Control for Intermediate-Risk Rhabdomyosarcoma: Results From D9803 According to Histology, Group, Site, and Size: A Report From the Children’s Oncology Group. Int J Radiat Oncol Biol Phys. 2015;93: 1071–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peto RPJ. Asymptotically efficient rank invariant test procedures. J R Statist Soc A. 1972;135: 185–207. [Google Scholar]
- 11.Fuchs J, Urla C, Sparber-Sauer M, et al. Treatment and outcome of patients with localized intrathoracic and chest wall rhabdomyosarcoma: a report of the Cooperative Weichteilsarkom Studiengruppe (CWS). J Cancer Res Clin Oncol. 2018;144: 925–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seitz G, Fuchs J, Sparber-Sauer M, et al. Improvements in the Treatment of Patients Suffering from Bladder-Prostate Rhabdomyosarcoma: A Report from the CWS-2002P Trial. Ann Surg Oncol. 2016;23: 4067–4072. [DOI] [PubMed] [Google Scholar]
- 13.Cecchetto G, Carretto E, Bisogno G, et al. Complete second look operation and radiotherapy in locally advanced non-alveolar rhabdomyosarcoma in children: A report from the AIEOP soft tissue sarcoma committee. Pediatr Blood Cancer. 2008;51: 593–597. [DOI] [PubMed] [Google Scholar]
- 14.Heyn R, Haeberlen V, Newton WA, et al. Second malignant neoplasms in children treated for rhabdomyosarcoma. Intergroup Rhabdomyosarcoma Study Committee. J Clin Oncol. 1993;11: 262–270. [DOI] [PubMed] [Google Scholar]
- 15.Raney RB, Asmar L, Vassilopoulou-Sellin R, et al. Late complications of therapy in 213 children with localized, nonorbital soft-tissue sarcoma of the head and neck: A descriptive report from the Intergroup Rhabdomyosarcoma Studies (IRS)-II and - III. IRS Group of the Children’s Cancer Group and the Pediatric Oncology Group. Med Pediatr Oncol. 1999;33: 362–371. [DOI] [PubMed] [Google Scholar]
- 16.Sung L, Anderson JR, Donaldson SS, et al. Late events occurring five years or more after successful therapy for childhood rhabdomyosarcoma: a report from the Soft Tissue Sarcoma Committee of the Children’s Oncology Group. Eur J Cancer. 2004;40: 1878–1885. [DOI] [PubMed] [Google Scholar]
- 17.Spunt SL, Sweeney TA, Hudson MM, Billups CA, Krasin MJ, Hester AL. Late effects of pelvic rhabdomyosarcoma and its treatment in female survivors. J Clin Oncol. 2005;23: 7143–7151. [DOI] [PubMed] [Google Scholar]
- 18.Lucas JT Jr., Fernandez-Pineda I, Tinkle CL, et al. Late toxicity and outcomes following radiation therapy for chest wall sarcomas in pediatric patients. Pract Radiat Oncol. 2017;7: 411–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paulino AC. Late effects of radiotherapy for pediatric extremity sarcomas. Int J Radiat Oncol Biol Phys. 2004;60: 265–274. [DOI] [PubMed] [Google Scholar]
- 20.Berrington de Gonzalez A, Gilbert E, Curtis R, et al. Second solid cancers after radiation therapy: a systematic review of the epidemiologic studies of the radiation dose-response relationship. Int J Radiat Oncol Biol Phys. 2013;86: 224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alexander N, Lane S, Hitchcock R. What is the evidence for radical surgery in the management of localized embryonal bladder/prostate rhabdomyosarcoma? Pediatr Blood Cancer. 2012;58: 833–835. [DOI] [PubMed] [Google Scholar]
- 22.Raney RB, Walterhouse DO, Meza JL, et al. Results of the Intergroup Rhabdomyosarcoma Study Group D9602 protocol, using vincristine and dactinomycin with or without cyclophosphamide and radiation therapy, for newly diagnosed patients with low-risk embryonal rhabdomyosarcoma: a report from the Soft Tissue Sarcoma Committee of the Children’s Oncology Group. J Clin Oncol. 2011;29: 1312–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bradley JA, Kayton ML, Chi YY, et al. Treatment Approach and Outcomes in Infants With Localized Rhabdomyosarcoma: A Report From the Soft Tissue Sarcoma Committee of the Children’s Oncology Group. Int J Radiat Oncol Biol Phys. 2019;103: 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang HHZT, Shanahan M, Retik AB, Lee R. Long-term Urologic Outcomes in Genitourinary Rhabdomyosarcoma: A Single Center 48-Year Experience. Presented at the Societies for Pediatric Urology Annual Congress, Chicago, IL, May 2019. [Google Scholar]
- 25.Castagnetti M, Angelini L, Alaggio R, Scarzello G, Bisogno G, Rigamonti W. Oncologic outcome and urinary function after radical cystectomy for rhabdomyosarcoma in children: role of the orthotopic ileal neobladder based on 15-year experience at a single center. J Urol. 2014;191: 1850–1855. [DOI] [PubMed] [Google Scholar]