Abstract
Liver fibrosis is associated with lifestyle-related diseases, including diabetes. The identification of diabetic patients with severe liver fibrosis is important, but a simple and reliable diagnostic procedure remains to be determined. We conducted an observational study to evaluate the performance of a FIB-4 index-based screening strategy for the diagnosis of advanced liver fibrosis in patients with diabetes or prediabetes. Two hundred and forty-two patients underwent abdominal imaging in our Study. According to the abdominal imaging findings, fatty liver, liver cirrhosis, and hepatocellular carcinoma were defined, and their association with FIB-4 index evaluated. The prevalences of liver cirrhosis and hepatocellular carcinoma in patients with a high (≥ 2.67; liver cirrhosis: 42.9%, hepatocellular carcinoma: 14.3%) FIB-4 index were significantly higher than in those with an intermediate (1.3 ≤ FIB-4 < 2.67; liver cirrhosis: 1.6%, hepatocellular carcinoma: 0.8%) or low FIB-4 index (< 1.3; liver cirrhosis: 1.2%, hepatocellular carcinoma: 0%). The diagnostic accuracy, specificity, and sensitivity of the FIB-4 index for the diagnosis of liver cirrhosis or hepatocellular carcinoma were 84.3%, 85.5%, and 89.3%, respectively, with an optimized cut-off value of 2.96 (sensitivity = 0.86, specificity = 0.98). Using an optimized cut-off value, FIB-4 index might be useful to identify liver cirrhosis or hepatocellular carcinoma in diabetes patients with high diagnostic accuracy.
Electronic supplementary material
The online version of this article (10.1007/s13340-020-00453-7) contains supplementary material, which is available to authorized users.
Keywords: Complications, Liver fibrosis, Liver cancer, Imaging
Introduction
Liver fibrosis is a critical finding in chronic liver disease and is associated with lifestyle-related diseases, including diabetes, chronic kidney disease, and cardiovascular disease [1–5]. The presence of concurrent chronic liver disease and another lifestyle-related disease promotes liver fibrosis, which is associated with the clinical outcome of chronic liver disease.
Non-alcoholic fatty liver disease (NAFLD) is strongly associated with obesity, metabolic syndrome, and other lifestyle-related diseases. The severity of lifestyle-related diseases is associated with the progression of NAFLD, including the development of liver cirrhosis (LC) and hepatocellular carcinoma (HCC) [6–8]. In particular, according to the results of previous epidemiologic studies, abnormal glucose metabolism and diabetes are common lifestyle-related comorbidities of NAFLD, and are associated with the progression of liver fibrosis and the incidence of HCC [9, 10]. Recent studies have shown that liver fibrosis is an independent and the most significant risk factor for mortality in NAFLD patients. Moreover, the severity of liver fibrosis is associated with both liver-related and non-liver-related mortality, including that due to cardiovascular disease (CVD) [11, 12].
Therefore, a simple and accurate screening procedure for liver fibrosis is required in clinical diabetes practice to identify patients with high-risk NAFLD, as a major diabetic complication, and especially for diabetologists receiving referrals from other departments. In addition, it remains to be determined whether liver fibrosis is indicative of a high risk of LC or HCC in diabetic patients attending a hospital. The FIB-4 index is a well-established, non-invasive index of the severity of liver fibrosis that is calculated using age, platelet count (PLT), and aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities [13]. We conducted an observational study to analyze the performance of a FIB-4 index-based screening strategy for the diagnosis of advanced liver fibrosis and HCC in diabetic patients.
Methods
Patients
We analyzed the medical records of 1091 outpatients who visited Saga University Hospital between April and June 2015, and had a diagnosis of diabetes (type 1 diabetes, type 2 diabetes, steroid-induced diabetes, or pancreatogenic diabetes) or prediabetes, with a hemoglobin A1c (HbA1c) value of ≥ 6% (42 mmol/mol) (Supplemental Figure S1). Of these patients, 364 who had been diagnosed with hepatic disease, including hepatitis virus infection, autoimmune hepatitis, primary biliary cholangitis, habitual alcohol intake > 30 g/day for men and > 20 g/day for women, or congestive hepatopathy, were excluded. Patients with abnormal endocrine function, including severe hypopituitarism or abnormal thyroid function, were also excluded, as were those with hematologic diseases, collagen diseases, multiple organ failure, shock, or malignancy other than HCC, because they might affect the measurements made. Finally, 671 diabetic patients without indicators of liver dysfunction were enrolled. The medical records of these participants were reviewed, including the abdominal imaging findings, for the 6-month period from their first hospital visit during the observational period, until December 31, 2015. Entire study period was from April 2015 to December 2019. According to the imaging findings, fatty liver (FL), LC, and HCC were defined and their associations with FIB-4 index were evaluated. The study protocol was approved by the Clinical Research Ethics Review Committee of Saga University Hospital (approval number; 28-4, Date; May 9. 2016) and the study was conducted in accordance with the principles of the 1975 Declaration of Helsinki, as revised in 2013. Information about the current study was provided on the web-site and right to reject to be involved in the study was announced for patients.
Physical examination and serum biochemical measurements
Body mass and height were measured, and body mass index (BMI) was calculated as body mass (kg) divided by the square of height (m2). Venous blood samples were obtained after overnight fasting and used to measure AST, ALT, total cholesterol (T-C), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), PLT, creatinine (Cr), estimated glomerular filtration rate (eGFR), and HbA1c, using conventional laboratory techniques. eGFR was calculated using the following equation: 194 × Cr (mg/dL)−1.094 × age (year)−0.287 for men, and the values obtained for women were multiplied by 0.739 [14]. FIB-4 Index was calculated using: Age × AST/PLT × (ALT)1/2] (10,11). Low (1.3) and high (2.67) cut-off values were defined for the analysis of FIB-4 data [15].
Evaluation of abdominal imaging findings
Abdominal imaging findings were extracted from medical records, including those from ultrasonography (US), computed tomography (CT), and magnetic resonance imaging (MRI) performed for any purpose. FL was diagnosed during US examination according to the criteria of the American Gastroenterology Association, on the basis of a marked increase in hepatic echogenicity, poor penetration of the posterior segment of the right lobe of the liver, and poor-to-no visualization of the hepatic vessels and diaphragm [16]. The severity of hepatic steatosis was not graded, because of the potential for errors due to differences between US equipment and operators. FL was diagnosed on CT examination if the liver–spleen attenuation ratio was < 1.0. On MRI examination, FL was diagnosed if the signal intensity of the image obtained with opposed-phase imaging was lower than that obtained with in-phase imaging, using chemical shift gradient echo [17–20]. LC was diagnosed when two or more of liver morphological abnormality, splenomegaly, and esophageal varix were identified using US, CT, or MRI imaging, or endoscopic examination.
For the diagnosis of LC, imaging data were evaluated with regard to the following five parameters and LC was diagnosed when two or more of the presence or absence of an irregular or nodular liver surface, a blunt liver edge, liver parenchymal abnormalities (coarseness, heterogeneity, or regenerative nodules), liver morphologic changes (including atrophy of the right lobe and the medial segment, hypertrophy of the lateral segment and the caudate lobe, or widened pericholecystic space and enlargement of the periportal space), or manifestations of portal hypertension (including splenomegaly, dilatation of the splenic vein, ascites, or collateral vessels [for example, gastroesophageal, paraesophageal, paraumbilical, splenorenal, retroperitoneal, or perisplenic mesenteric vessels]) were identified [21]. HCC was diagnosed using contrast-enhanced dynamic CT or MRI [22, 23]. All CT and MRI images were reviewed by two specialized radiologists (YE and JN) and all US examinations were reviewed by three ultrasonographers (HT, SI, and SO).
Statistics
The Mann–Whitney U and Kruskal–Wallis tests were used to compare quantitative data between the groups, and the Chi-square test or Fisher exact probability was used to compare categorical data between the groups. The diagnostic performance of the FIB-4 index was determined using a receiver operating characteristic (ROC) curve and optimal cut-off values were chosen to maximize the sum of the sensitivity and specificity in the Youden index [24]. Differences were considered significant when p < 0.05. All analyses were performed using IBM SPSS Statistics version 21 (IBM, Inc., Armonk, NY, USA).
Results
Characteristics of the participants
Of the 671 participants enrolled, there were 324 (48.3%) with FIB-4 indexes in the intermediate range and 76 (11.3%) with indexes in the high range (Supplemental Table S1). Age, body mass, and BMI significantly increased alongside the FIB-4 index. AST activity also increased with FIB-4 index; whereas, there was no significant trend with regard to ALT activity. Renal function significantly decreased with increases in FIB-4 index, and HbA1c significantly decreased with increases in FIB-4 index. There were no significant relationships between FIB-4 and circulating lipids, except for triglyceride, which significantly decreased with increases in FIB-4 index.
Characteristics of the participants who underwent imaging
A total of 242 patients (36%) underwent abdominal imaging (Supplemental Table S2). Compared with the participants who did not undergo imaging, there were significant differences in age (p = 0.03), AST activity (p = 0.005), and PLT (p = 0.003). FIB-4 index also differed between the participants who did or did not undergo imaging: it was significantly higher in the patients who did undergo imaging (p < 0.001). However, there were no significant differences in HbA1c or circulating lipids between these sets of participants.
Prevalences of FL, LC, and HCC in participants who underwent abdominal imaging, according to their FIB-4 index
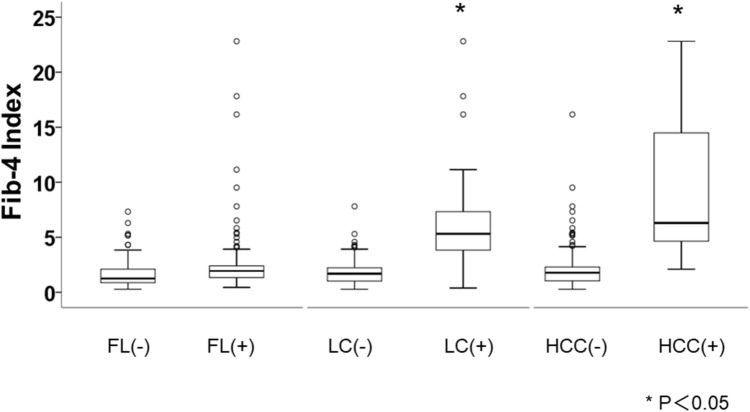
We compared the characteristics of the participants who underwent imaging according to their FIB-4 index range (Table 1). Similar to the findings for the participants as a whole, the parameters included in the FIB-4 index, such as age, AST activity, and platelet count, significantly differed among participants with FIB-4 indexes in the three ranges of values. According to the imaging findings, FL, LC, and HCC were identified in 28.6%, 42.9%, and 14.3% of the participants with FIB-4 indexes in the high range; 19.7%, 1.6%, and 0.8% of the participants with indexes in the intermediate range; and 47.4%, 1.2%, and 0% of the participants with indexes in the low range, respectively. The prevalences of LC or HCC were significantly higher in participants with FIB-4 indexes in the high range, whereas the prevalence of FL was significantly higher in participants with indexes in the low range. We also compared the FIB-4 index between participants who did or did not have FL, LC, or HCC (Fig. 1), and found that it was significantly higher in participants with LC or HCC than in those without.
Table 1.
Characteristics of the patients with imaging examination according to FIB-4 index range
| FIB-4 < 1.3 (n = 78) | 1.3 ≤ FIB-4 < 2.67 (n = 122) | 2.67 ≤ FIB-4 (n = 42) | p value | |
|---|---|---|---|---|
| Male/female | 37/41 | 77/45 | 26/16 | 0.077 |
| Age (year) | 54 [45–60] | 69 [65–74] | 72 [65–75] | < 0.001 |
| Body weight (kg) | 70.5 [60.5–79.8] | 62.0 [53.7–73.7] | 63.7 [56.3–74.1] | 0.002 |
| BMI | 25.9 [23.0–30.8] | 24.1 [21.9–27.4] | 25.6 [23.2–27.9] | 0.003 |
| AST (U/l) | 18 [15–23] | 22 [19–29] | 34 [23.7–46] | < 0.001 |
| ALT (U/l) | 19 [15–34] | 21 [15–33.25] | 26.5 [16–38.75] | 0.223 |
| PLT (104/μl) | 25.5 [21.5–28.6] | 18.1 [16.07–20.4] | 11.8 [8.6–14.0] | < 0.001 |
| FIB-4 Index | 0.91 [0.63–1.04] | 1.88 [1.56–2.20] | 4.10 [2.94–5.52] | < 0.001 |
| HbA1c (%) (mmol/mol) |
6.8 [6.2–7.6] 51 [44–60] |
6.7 [6.27–7.22] 50 [45–55] |
6.6 [6.2–7.12] 49 [44–54] |
0.304 |
| Cr (mg/dl) | 0.75 [0.60–0.97] | 0.84 [0.72–0.97] | 0.89 [0.76–1.06] | < 0.001 |
| eGFR (ml/min/1.73) | 77.6 [61.9–90.9] | 64.15 [54.6–71.5] | 56.3 [47.1–71.0] | < 0.001 |
| T-C (mg/dl) | 185 [160–210] | 182 [159–208.5] | 175 [152–198.5] | 0.610 |
| LDL-C (mg/dl) | 104 [93–126] | 99.9 [79–119] | 91 [80–123] | 0.114 |
| HDL-C (mg/dl) | 49 [42–63] | 56 [46–64] | 54 [45–68] | 0.263 |
| TG (mg/dl) | 136 [90–204] | 114 [82–168] | 122 [70.5–158.5] | 0.136 |
| FL (%) | 37 (47.4) | 24 (19.7) | 12 (28.6) | < 0.001* |
| LC (%) | 1 (1.2) | 2 (1.6) | 18 (42.9) | < 0.001* |
| HCC (%) | 0 (0) | 1 (0.8) | 6 (14.3) | < 0.001* |
Continuous variables were shown as median (quartile)
AST aspartate aminotransferase, ALT alanine aminotransferase, T-C total cholesterol, TG triglycerides, HDL-C HDL cholesterol, LDL-C LDL cholesterol, PLT platelet count, HbA1c hemoglobin A1c, Cr creatinine, eGFR estimated glomerular filtration rate, FL fatty liver, LC liver cirrhosis, HCC hepatocellular carcinoma
p values were obtained by Chi-Square test, *Fisher exact probability test or Kruskal–Wallis test
Fig. 1.
Distribution of FIB-4 Index according to the presence or absence of FL, LC, and HCC. The bold line in each box represents the median and the error bars the first and third quartiles. FL fatty liver, LC liver cirrhosis, HCC hepatocellular carcinoma. *p < 0.05, according to the Mann–Whitney U test
Diagnostic accuracy of FIB-4 index for the identification of LC or HCC in diabetes patients
To evaluate the diagnostic accuracy of FIB-4 index for the identification of LC or HCC, we performed ROC analysis (Fig. 2). The area under the ROC curve was 0.919. According to the Youden index, the cut-off value was determined to be 2.96 (sensitivity = 0.769, specificity = 0.938) and its diagnostic accuracy, positive predictive value, and negative predictive value were 84.3%, 94.1%, and 76.0%, respectively.
Fig. 2.
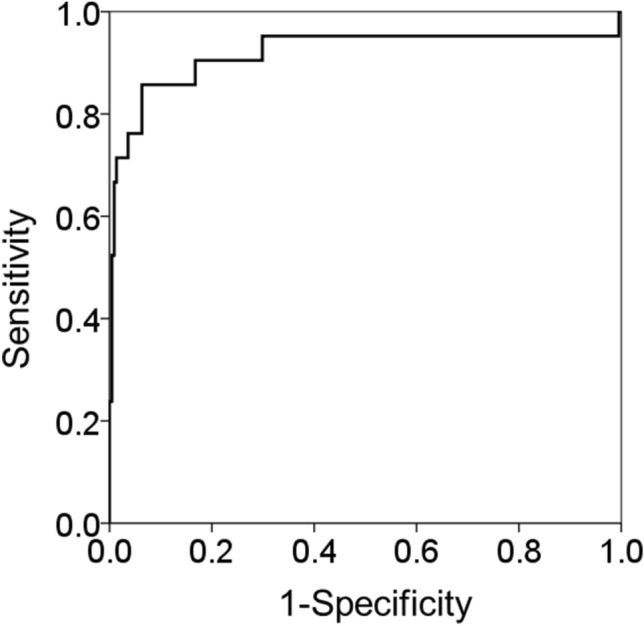
Receiver-operating characteristic curves for the diagnosis of LC and HCC in diabetes patients using FIB-4 Index. LC, liver cirrhosis; HCC, hepatocellular carcinoma
Estimate of the prevalence of LC or HCC in participants who did not undergo imaging
Using the ROC analysis and calculated cut-off value, we estimated the prevalence of LC or HCC in the participants who did not undergo imaging (Supplemental Figure S2). Surprisingly, 7% (28/429) of these participants had a FIB-4 index > 2.96, and were, therefore, considered to have LC or HCC.
Discussion
We have shown that FIB-4 index is capable of identifying LC or HCC in diabetic patients visiting a hospital who do not have viral hepatitis, alcoholic liver disease, or autoimmune liver disease. Using a cut-off value for the FIB-4 index of 2.96, LC or HCC can be diagnosed in diabetic patients with high sensitivity and specificity. According to a previous study, HCC and LC are major causes of death in diabetes patients [25]. Moreover, several meta-analyses have shown that diabetes increases the risk of mortality from liver cancer [26–28]. Diabetes is also associated with liver fibrosis and LC. Previous longitudinal studies have shown that insulin resistance is a predictor of the progression of liver fibrosis in NAFLD [29, 30]. On the other hand, a reduction in HbA1c is associated with the progression of liver fibrosis, independent of age, sex, and BMI, in diabetes patients with NAFLD [31].
The significant associations between diabetes, and LC and HCC, imply that an efficient screening strategy for the identification of liver fibrosis in diabetes patients, especially in those who may have NAFLD, is required. However, a practical and detailed screening algorithm for the identification of severe liver fibrosis or high-risk NAFLD patients with diabetes has not been provided by previously published guidelines for the management of diabetes. In the latest guidelines, published by the American Diabetes Association (ADA) in 2019, US was recommended for the first time for the identification of NAFLD in patients with type 2 diabetes or prediabetes [31, 32]. In 2016, the Clinical Practice Guidance published by the EASL, EASD, and EASO recommended abdominal US or the assessment of biomarkers of steatosis, including Fatty Liver Index, SteatoTest, or NAFLD Fat score, in the first instance for patients with metabolic risk factors, to diagnose liver steatosis [33]. If steatosis is present, the algorithm within these guidelines recommends further stratification of the diagnosis using non-invasive markers of fibrosis, including the NAFLD fibrosis score, Enhanced Liver Fibrosis, or FIB-4 index. This guidance indicates the importance of liver fibrosis, which is the strongest predictor of the risk of mortality, including from CVD and HCC [11, 12, 34]. The main recommendation in these guidelines is that liver steatosis should be identified first, and then the severity of liver fibrosis should be determined. However, it is important to be able to identify patients with advanced liver fibrosis or HCC in the wider population, such as among patients in a hospital; therefore, a simple, accurate, and cost-effective strategy is required that can be pursued as part of a routine health check. Because the number of patients with type 2 diabetes or prediabetes is very large and the cost of imaging is high [33], it is not feasible to perform imaging examinations in clinical practice on all patients. Consistent with this, we found that 63.9% of the diabetes patients did not undergo imaging, but 7% of these patients might have LC or HCC (Supplemental Figure S1).
There are a number of non-invasive procedures that can be used to diagnose liver fibrosis, but the advantage of the FIB-4 index is its simplicity: it is calculated using the patient’s age, AST and ALT activities, and platelet counts, which are frequently measured in routine clinical practice [13, 16]. The FIB-4 index has been well validated for the prediction of hepatic fibrosis and for determining the prognosis of NAFLD in numerous previous studies. According to previous reports, the appropriate FIB-4 cut-off values for the diagnosis of advanced fibrosis (stage ≥ 3 in the Brunt classification) are 1.30 as the lower cut-off value (negative predictive value 90–95%) and 2.67 (positive predictive value 80%) or 3.25 (positive predictive value 75%) as the higher cut-off value [13, 35–37]. In a longitudinal epidemiologic study of 4,083 patients with NAFLD, conducted in the USA, the hazard ratio for CVD-related mortality was 1.75 in NAFLD patients with FIB-4 indexes in the intermediate range (1.30–2.67) and 2.68 in those with FIB-4 indexes > 2.67 [38]. Furthermore, a retrospective cohort study of 320 patients with biopsy-confirmed NAFLD showed that the FIB-4 index is associated with overall mortality and liver-related events, including ascites, hemorrhage from gastroesophageal varices, and hepatic encephalopathy [39]. However, the cut-off values of 1.30 and 2.67 that were used in these studies were originally derived and validated in samples of NAFLD patients as a whole, rather than in the subgroup with [13]. In the longitudinal study reported by Bertot et al., noninvasive markers including FIB-4 index were tested whether they could predict the mortality and hepatic outcomes [40]. In the study, predictive accuracy of FIB-4 index was significantly diminished in the patients with diabetes comparing with non-diabetes patients. Because the calculation of FIB-4 index is not affected by glucose metabolism-related parameters like HbA1c, insulin and insulin resistance, it was possible that pathogenesis of diabetes including hyperglycemia, insulin resistance and diabetic complications might affect the mortality and hepatic outcome of the patients with diabetes in the longitudinal study of Bertot et al. On the other hand, in our current cross-sectional study including only diabetic patients, existence of LC and HCC could be clearly identified. Accuracy of FIB-4 index for the cross-sectional prediction of LC and HCC in non-diabetic patients should be tested further.
The NAFLD fibrosis and BARD scores are also well-established predictors of advanced liver fibrosis in NAFLD [13, 36, 37, 41] and are associated with NAFLD-related mortality [39, 42]. These scores are calculated using serum and clinical parameters, such as the FIB-4 index. However, because ‘diabetes’ or ‘impaired fasting glycemia’ is used in the calculation of both scores, their diagnostic accuracy may not be high in populations with NAFLD that also have diabetes or prediabetes. Moreover, diabetes patients frequently have multiple complications that affect prognosis, such as CVD and diabetic nephropathy. In this context, it is important to identify urgent cases that have comorbidities of severe fibrosis or HCC, because these findings might change the clinician’s priorities in the management of the diabetes and its complications. In the present study, the cut-off values for the FIB-4 index for the identification of LC and HCC were determined only in patients with diabetes or prediabetes. We believe these cut-off values should assist healthcare professionals to identify patients with diabetes that require urgent attention. However, because diabetes is associated with higher risks of colon cancer, pancreatic cancer, and uterine cancer, as well as HCC [26–28], imaging should form a part of the basic clinical assessment of a patient, and its frequency and cost-effectiveness, and the modality to be used, should be optimized in future studies.
There were a number of limitations to our study. Diagnoses of FL, LC, and HCC were made on the basis of imaging and the FIB-4 cut-off values determined were not validated using liver histology. Moreover, accurate diagnosis of LC is difficult in the conventional imaging examination; LC without morphological change of the liver could be diagnosed as non-cirrhosis. Further study using liver stiffness measurement such as FibroScan® and MR elastography, and other non-invasive tests such as type 3 procollagen and type 4 collagen 7 s should be performed. Whereas we obtained the cut-off value, 2.96, to identify LC and HCC in the diabetic patients, it should be noted that even the patients with FIB-4 index < 2.96 could have LC and HCC. Because FIB-4 index associates with age, optimal cut-off value should be obtained by age. However, our study conducted in a single center with relatively small sample size and limited number of the cases with LC and HCC. Therefore, age-specific cut-off value should be analyzed in larger cohort. We defined diabetes and prediabetes patients using HbA1c. However, it has been reported that HbA1c can be normal in patients with LC and hypersplenism due to the short lifespan of red blood cells [43], and such patients were not identified in the present cohort. Nevertheless, because this type of diabetes, referred to as ‘hepatogenous diabetes’, is associated with several distinct clinical features, including normal fasting glucose or hypoglycemia, and has a different pathogenesis from type 2 diabetes [44], it can generally be distinguished from type 2 diabetes. The usefulness of the FIB-4 index for patients with hepatogenous diabetes should be studied in the future.
In conclusion, the performance of the FIB-4 index for the diagnosis of LC and HCC in diabetic patients attending a hospital is excellent. Liver fibrosis should be evaluated in diabetes and prediabetes patients, even if they do not have viral hepatitis or alcoholic liver disease, because it is useful for the identification of patients with LC or HCC.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Mark Cleasby, PhD, from Edanz Group (https://www.edanzediting.com/ac) for editing a draft of this manuscript.
Author contributions
The involvement of each author was as follows: N.K. (1-5,7); H.T. (1,3,6,7); S.I. (1,2,7); K.I. (2,7); Mo.K. (2,7); Mi.K. (2,7); K.T. (2,7); H.M. (3,7); H.I. (3,7); S.O. (3,7); Y.M. (3,7); Y.E. (3,7); Y.E. (3,4,8); J.N. (3,4,8); H.I. (3,4,8); K.A. (1,3,6,7). Key: (1) conception and design; (2) acquisition of data; (3) analysis and interpretation of data; (4) drafting of the manuscript; (5) statistical analysis; (6) study supervision; (7) critical revision of the manuscript; (8) assistance for image interpretation. All authors read the final version of this article, approve its’ content and submission for publication. And all authors have agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Compliance with ethical standards
Conflict of interest
None of the authors have conflicts to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lemoine M, et al. Metabolic syndrome and obesity are the cornerstones of liver fibrosis in HIV-monoinfected patients. AIDS (London, England) 2017;31:1955–1964. doi: 10.1097/qad.0000000000001587. [DOI] [PubMed] [Google Scholar]
- 2.Khalili M, et al. Relationship between metabolic syndrome, alanine aminotransferase levels, and liver disease severity in a multiethnic North American Cohort with chronic hepatitis B. Diabetes Care. 2018;41:1251–1259. doi: 10.2337/dc18-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim SU, et al. Liver fibrosis assessed with transient elastography is an independent risk factor for ischemic stroke. Atherosclerosis. 2017;260:156–162. doi: 10.1016/j.atherosclerosis.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Yeung MW, et al. Advanced liver fibrosis but not steatosis is independently associated with albuminuria in Chinese patients with type 2 diabetes. J Hepatol. 2017 doi: 10.1016/j.jhep.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 5.Wijarnpreecha K, Thongprayoon C, Scribani M, Ungprasert P, Cheungpasitporn W. Noninvasive fibrosis markers and chronic kidney disease among adults with nonalcoholic fatty liver in USA. Eur J Gastroenterol Hepatol. 2018;30:404–410. doi: 10.1097/meg.0000000000001045. [DOI] [PubMed] [Google Scholar]
- 6.Duan XY, Zhang L, Fan JG, Qiao L. NAFLD leads to liver cancer: do we have sufficient evidence? Cancer Lett. 2014;345:230–234. doi: 10.1016/j.canlet.2013.07.033. [DOI] [PubMed] [Google Scholar]
- 7.White DL, Kanwal F, El-Serag HB. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin Gastroenterol Hepatol. 2012;10:1342–1359.e1342. doi: 10.1016/j.cgh.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yatsuji S, et al. Clinical features and outcomes of cirrhosis due to non-alcoholic steatohepatitis compared with cirrhosis caused by chronic hepatitis C. J Gastroenterol Hepatol. 2009;24:248–254. doi: 10.1111/j.1440-1746.2008.05640.x. [DOI] [PubMed] [Google Scholar]
- 9.Dyson J, et al. Hepatocellular cancer: the impact of obesity, type 2 diabetes and a multidisciplinary team. J Hepatol. 2014;60:110–117. doi: 10.1016/j.jhep.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Kawamura Y, et al. Large-scale long-term follow-up study of Japanese patients with non-alcoholic Fatty liver disease for the onset of hepatocellular carcinoma. Am J Gastroenterol. 2012;107:253–261. doi: 10.1038/ajg.2011.327. [DOI] [PubMed] [Google Scholar]
- 11.Angulo P, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–854. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 12.Dulai PS, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology (Baltimore, MD) 2017;65:1557–1565. doi: 10.1002/hep.29085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah AG, et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1104–1112. doi: 10.1016/j.cgh.2009.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horio M, Imai E, Yasuda Y, Watanabe T, Matsuo S. Performance of GFR equations in Japanese subjects. Clin Exp Nephrol. 2013;17:352–358. doi: 10.1007/s10157-012-0704-5. [DOI] [PubMed] [Google Scholar]
- 15.Yoneda M, et al. Noninvasive scoring systems in patients with nonalcoholic fatty liver disease with normal alanine aminotransferase levels. J Gastroenterol. 2013;48:1051–1060. doi: 10.1007/s00535-012-0704-y. [DOI] [PubMed] [Google Scholar]
- 16.Sanyal AJ. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology. 2002;123:1705–1725. doi: 10.1053/gast.2002.36572. [DOI] [PubMed] [Google Scholar]
- 17.Yajima Y, et al. Ultrasonographical diagnosis of fatty liver: significance of the liver-kidney contrast. Tohoku J Exp Med. 1983;139:43–50. doi: 10.1620/tjem.139.43. [DOI] [PubMed] [Google Scholar]
- 18.Yoneda M, et al. Nonalcoholic fatty liver disease: US-based acoustic radiation force impulse elastography. Radiology. 2010;256:640–647. doi: 10.1148/radiol.10091662. [DOI] [PubMed] [Google Scholar]
- 19.Saadeh S, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745–750. doi: 10.1053/gast.2002.35354. [DOI] [PubMed] [Google Scholar]
- 20.Hussain HK, et al. Hepatic fat fraction: MR imaging for quantitative measurement and display–early experience. Radiology. 2005;237:1048–1055. doi: 10.1148/radiol.2373041639. [DOI] [PubMed] [Google Scholar]
- 21.Kudo M, et al. Diagnostic accuracy of imaging for liver cirrhosis compared to histologically proven liver cirrhosis A multicenter collaborative study. Intervirology. 2008;51(Suppl 1):17–26. doi: 10.1159/000122595. [DOI] [PubMed] [Google Scholar]
- 22.Di Martino M, et al. Intraindividual comparison of gadoxetate disodium-enhanced MR imaging and 64-section multidetector CT in the Detection of hepatocellular carcinoma in patients with cirrhosis. Radiology. 2010;256:806–816. doi: 10.1148/radiol.10091334. [DOI] [PubMed] [Google Scholar]
- 23.Monzawa S, et al. Dynamic CT for detecting small hepatocellular carcinoma: usefulness of delayed phase imaging. AJR Am J Roentgenol. 2007;188:147–153. doi: 10.2214/ajr.05.0512. [DOI] [PubMed] [Google Scholar]
- 24.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura J, et al. Causes of death in Japanese patients with diabetes based on the results of a survey of 45,708 cases during 2001–2010: Report of the Committee on Causes of Death in Diabetes Mellitus. J Diabetes Invest. 2017;8:397–410. doi: 10.1111/jdi.12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y, et al. Association between type 2 diabetes and risk of cancer mortality: a pooled analysis of over 771,000 individuals in the Asia Cohort Consortium. Diabetologia. 2017;60:1022–1032. doi: 10.1007/s00125-017-4229-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noto H, Tsujimoto T, Noda M. Significantly increased risk of cancer in diabetes mellitus patients: a meta-analysis of epidemiological evidence in Asians and non-Asians. J Diabetes Investig. 2012;3:24–33. doi: 10.1111/j.2040-1124.2011.00183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sasazuki S, et al. Diabetes mellitus and cancer risk: pooled analysis of eight cohort studies in Japan. Cancer Sci. 2013;104:1499–1507. doi: 10.1111/cas.12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ekstedt M, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 30.Fassio E, Alvarez E, Dominguez N, Landeira G, Longo C. Natural history of nonalcoholic steatohepatitis: a longitudinal study of repeat liver biopsies. Hepatology. 2004;40:820–826. doi: 10.1002/hep.20410. [DOI] [PubMed] [Google Scholar]
- 31.Hamaguchi E, et al. Histological course of nonalcoholic fatty liver disease in Japanese patients: tight glycemic control, rather than weight reduction, ameliorates liver fibrosis. Diabetes Care. 2010;33:284–286. doi: 10.2337/dc09-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.American Diabetes, A. 4. Comprehensive Medical Evaluation and Assessment of Comorbidities: Standards of Medical Care in Diabetes-2019. Diabetes Care42, S34–S45. 10.2337/dc19-S004 (2019). [DOI] [PubMed]
- 33.EASL-EASD-EASO Clinical practice guidelines for the management of non-alcoholic fatty liver disease. Obesity facts9, 65–90. 10.1159/000443344 (2016). [DOI] [PMC free article] [PubMed]
- 34.Lonardo A, Nascimbeni F, Mantovani A, Targher G. Hypertension, diabetes, atherosclerosis and NASH: cause or consequence? J Hepatol. 2018;68:335–352. doi: 10.1016/j.jhep.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 35.Sterling RK, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology (Baltimore, MD) 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 36.McPherson S, et al. Age as a confounding factor for the accurate non-invasive diagnosis of advanced NAFLD fibrosis. Am J Gastroenterol. 2017;112:740–751. doi: 10.1038/ajg.2016.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. 2010;59:1265–1269. doi: 10.1136/gut.2010.216077. [DOI] [PubMed] [Google Scholar]
- 38.Kim D, Kim WR, Kim HJ, Therneau TM. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology (Baltimore, MD) 2013;57:1357–1365. doi: 10.1002/hep.26156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Angulo P, et al. Simple noninvasive systems predict long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2013;145:782–789.e784. doi: 10.1053/j.gastro.2013.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bertot LC, et al. Diabetes impacts prediction of cirrhosis and prognosis by non-invasive fibrosis models in non-alcoholic fatty liver disease. Liver Int. 2018;38:1793–1802. doi: 10.1111/liv.13739. [DOI] [PubMed] [Google Scholar]
- 41.Harrison SA, Oliver D, Arnold HL, Gogia S, Neuschwander-Tetri BA. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut. 2008;57:1441–1447. doi: 10.1136/gut.2007.146019. [DOI] [PubMed] [Google Scholar]
- 42.Unalp-Arida A, Ruhl CE. Liver fibrosis scores predict liver disease mortality in the United States population. Hepatology (Baltimore, MD) 2017;66:84–95. doi: 10.1002/hep.29113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Orsi E, Grancini V, Menini S, Aghemo A, Pugliese G. Hepatogenous diabetes: is it time to separate it from type 2 diabetes? Liver Int. 2017;37:950–962. doi: 10.1111/liv.13337. [DOI] [PubMed] [Google Scholar]
- 44.El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460–468. doi: 10.1053/j.gastro.2003.10.065. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.