Abstract
Objective
Linagliptin, a dipeptidyl peptidase-4 inhibitor, recently demonstrated cardiovascular (CV) safety versus placebo in Asians with advanced type 2 diabetes mellitus (T2DM) in the CARMELINA® trial. We assessed its CV safety compared with the sulfonylurea glimepiride in Asians with relatively early T2DM in the CAROLINA® trial.
Methods
Based on prespecified and post hoc subgroup analyses of the multinational CAROLINA® trial in which adults with relatively early T2DM and elevated CV risk were randomized to linagliptin or glimepiride added to usual care, we analyzed data for participants from Asian countries. This included the primary outcome defined as time to first CV death, non-fatal myocardial infarction, or non-fatal stroke [three-point major adverse cardiovascular events (3P-MACE)].
Results
Of the 6033 participants, 933 (15.5%) were from Asia. During a median follow-up of 6.2 years, 3P-MACE occurred in 9.5% and 11.1% of the linagliptin and glimepiride groups, respectively (hazard ratio [HR] 0.85; 95% confidence interval [CI] 0.57–1.26]), consistent with the overall population (HR 0.98; 95% CI 0.84–1.13; P = 0.17 for treatment by region interaction). Similarly, there were no significant differences between groups for other outcomes, including CV death (HR 0.73; 95% CI 0.38–1.38), non-CV mortality (HR 0.76; 95% CI 0.37–1.57) and hospitalization for heart failure (HR 0.89; 95% CI 0.36–2.19). Hypoglycemia adverse events occurred in 13.1% of linagliptin patients versus 42.1% of glimepiride patients (HR 0.25; 95% CI 0.19–0.33; P < 0.0001) despite similar glycemic control. Body weight was slightly lower with linagliptin relative to glimepiride: weighted average mean difference over 256 weeks of − 1.82 kg (95% CI − 2.28 to − 1.35).
Conclusions
In Asian patients, linagliptin demonstrated similar CV safety to glimepiride with a markedly lower rate of hypoglycemia and modestly lower weight.
Electronic supplementary material
The online version of this article (10.1007/s13340-020-00447-5) contains supplementary material, which is available to authorized users.
Keywords: Diabetes mellitus, type 2; Cardiovascular
Introduction
The prevalence of type 2 diabetes mellitus (T2DM) has increased rapidly in Asia in recent years [1–5]. In 2019, the International Diabetes Federation estimated that over half (251 million) of the 463 million people globally with diabetes were in Southeast Asia and the Western Pacific [6], including 116 million, 77 million and 7.4 million in China, India, and Japan, respectively [6]. Other estimates suggest an even higher prevalence of diabetes in Japan of 10 million people [7]. As T2DM is associated with elevated cardiovascular (CV) risk [8, 9], it is a leading cause of death from coronary heart disease and ischemic stroke, as illustrated in a recent pooled analysis of more than one million Asians with diabetes [10]. Thus, it is important that glucose-lowering medications considered for use in Asians with T2DM have demonstrated CV safety, or potentially even provide benefits.
As in Western countries, current clinical guidelines in China and India recommend metformin for first-line glucose-lowering pharmacotherapy, while dipeptidyl peptidase-4 (DPP-4) inhibitors and sulfonylureas (SUs) are among the options recommended for additional treatment [11, 12]. In Japan, the clinical practice guideline recommends individualized choice for each patient based on their disease status and the pharmacological and safety profiles of the available medications, which include DPP-4 inhibitors and SUs as options [13]. Both DPP-4 inhibitors and SUs are commonly prescribed in Asia [14]. A recent subgroup analysis of people with T2DM, high CV risk, and evidence of chronic kidney disease (CKD) in the CARMELINA® trial [15] demonstrated that the DPP-4 inhibitor linagliptin did not increase the risk of CV events in the sub-population living in Asia, compared with placebo [16]. However, little data to inform clinical decision making on the CV safety of many other commonly used medications, e.g. SUs, are available for people from Asia, with a particular scarcity of information on comparative effectiveness. This is especially important in view of the lingering controversy about the CV safety of SUs arising from the highly disputed University Group Diabetes Program (UGDP) clinical trial more than 50 years ago [17], which has been exacerbated by multiple, often contradictory, observational studies on this issue that had many limitations [18].
A recent multinational trial (CAROLINA®) evaluated the CV safety of linagliptin compared with an SU, both added to standard of care, in people with relatively early T2DM and elevated CV risk [19]. In this study, linagliptin was non-inferior to glimepiride for the composite outcome of CV death, non-fatal myocardial infarction, or non-fatal stroke (3-point major adverse CV events [3P-MACE]) [19]. Here, we report the results of prespecified and post hoc subgroup analyses of the effects of linagliptin versus glimepiride on CV outcomes, glycemic control, other parameters, and adverse events in the Asian participants.
Materials and methods
Study design
Previous publications have described in detail the rationale for the CAROLINA® trial [20], its design [21], and its conduct and overall findings [19]. In short, CAROLINA® was a randomized, double-blind, CV event-driven, multinational clinical trial comparing linagliptin with the second-generation SU glimepiride in people with relatively early T2DM and elevated CV risk. CAROLINA® was conducted in 43 countries between November 2010 and August 2018 according to the principles of the Declaration of Helsinki and the Harmonized Tripartite Guideline for Good Clinical Practice from the International Conference on Harmonisation, following approval by local authorities (ClinicalTrials.gov Identifier, NCT01243424).
To be eligible for inclusion, individuals with T2DM had to be aged 40–85 years with inadequate glycemic control and elevated CV risk. Inadequate glycemic control was defined as a glycated hemoglobin (HbA1c) level of 6.5–8.5% if treatment naïve or treated with metformin and/or an alpha-glucosidase inhibitor, or HbA1c 6.5–7.5% if receiving SU or glinide (meglitinide) monotherapy or dual therapy with either of those drugs combined with metformin or an alpha-glucosidase inhibitor (patients receiving dual therapy had to have been diagnosed with T2DM no more than 5 years previously). CV risk inclusion criteria were defined as ≥ 1 of the following factors: age ≥ 70 years; previous vascular disease; ≥ 2 CV risk factors (T2DM duration > 10 years, systolic blood pressure > 140 mmHg or ≥ 1 antihypertensive drug, current smoker, low-density lipoprotein cholesterol ≥ 135 mg/dl); and/or microvascular complications (estimated glomerular filtration rate 30–59 ml/min/1.73 m2, urinary albumin-to-creatinine ratio ≥ 30 µg/mg, or proliferative retinopathy). Patients with current or previous use of DPP-4 inhibitors, glitazones (thiazolidinediones), glucagon-like peptide-1 receptor agonists or insulin were excluded, as were those with New York Heart Association class III or IV heart failure.
Participants were randomized to receive double-dummy, once-daily, oral treatment with linagliptin 5 mg or glimepiride 1–4 mg. Individuals using SUs or glinides discontinued those medications at the randomization visit. Glimepiride was initiated at 1 mg and uptitrated every 4 weeks during the first 16 weeks to a maximal dose of 4 mg, while participants previously taking glimepiride started on their pre-trial dose. Investigators were encouraged to use glucose-lowering rescue medication if glycemic control was inadequate and to manage all other CV risk factors according to applicable guidelines and current standards of care. The primary end point was time to first occurrence of 3P-MACE. The key secondary end points were time to first occurrence of 4-point MACE (4P-MACE: CV death, non-fatal myocardial infarction, non-fatal stroke, or hospitalization for unstable angina), and two composite end points of treatment sustainability: (1) the proportion of participants with HbA1c ≤ 7.0% at the final visit without glycemic rescue medication, without any episodes of moderate or severe hypoglycemia and without > 2% weight gain between the end of titration and final visit; and (2) the proportion of participants with HbA1c ≤ 7.0% without rescue medication at the final visit and without > 2% weight gain between the end of titration and final visit. Additional prespecified secondary end points included the individual components of 3P-MACE and 4P-MACE, other CV events (including hospitalization for heart failure), non-CV mortality, and all-cause mortality. Clinical events committees blinded to treatment assignment centrally adjudicated all CV outcomes, deaths, and cases of pancreatitis or pancreatic cancer. The Medical Dictionary for Regulatory Activities Version 21.0 was used to classify adverse events reported by investigators.
CAROLINA® was designed to continue until at least 631 participants had an adjudication-confirmed 3P-MACE event. Assuming a hazard ratio (HR) of 1.0, this provided 90.9% power to demonstrate non-inferiority of linagliptin versus glimepiride with the prespecified non-inferiority margin of 1.3 at a one-sided alpha level of 2.5%. If non-inferiority was demonstrated, superiority was to be tested but the statistical power was considerably lower (80%, assuming an HR of 0.80).
Subgroup analysis of participants from Asia
We evaluated the clinical and metabolic outcomes and adverse events reported for participants from trial sites in Hong Kong, India, Japan, South Korea, Malaysia, the Philippines and Taiwan. Due to regional differences in type 2 diabetes risk factors and pathophysiology within Asia [1], we also conducted an analysis of clinical and metabolic outcomes for participants of Asian race in East Asia alone (Hong Kong, Japan, South Korea and Taiwan), who comprised the majority of Asian patients in the trial. All randomized participants treated with ≥ 1 dose of study drug were included in the analyses (the treated set). Clinical outcomes and hypoglycemia in the treatment groups were compared using Cox proportional hazards models with terms for treatment, geographic region, and treatment group by geographic region interaction. Changes from baseline in HbA1c and body weight were evaluated using mixed models for repeated measures that included terms for treatment, week repeated within patients, week by treatment interaction, continuous baseline value, and baseline value by week interaction applied to participants from Asia. Adverse events were summarized descriptively. For participants from Asia, analyses of 3P-MACE, 4P-MACE, hospitalization for heart failure, the key secondary end points, and adverse events (except cancer) were prespecified, while other analyses were post hoc; for East Asia, analyses of 3P-MACE and 4P-MACE were prespecified while others were post hoc. All subgroup analyses were exploratory; statistical significance was concluded based on an alpha level of 5%. Although the P values for treatment by region interaction provided for CV/mortality outcomes are based on analyses of all geographical regions, point estimates and confidence intervals (CI) for treatment comparison are presented only for the participants from Asia.
Results
Baseline characteristics and trial metrics
A total of 6033 patients were treated in the CAROLINA® trial, 933 (15.5%) of whom were from Asia. At baseline, the treatment groups in Asia (linagliptin: n = 465; glimepiride: n = 468) had similar demographic and clinical characteristics (Table 1). Overall, the Asian participants had characteristics typical of individuals with relatively early T2DM at elevated CV risk, as designed. On average, participants were 61.4 years old with mean BMI of 26.4 kg/m2, mean HbA1c of 7.1% and median duration of T2DM of 5.1 years. Nearly all participants (91.3%) were receiving glucose-lowering pharmacotherapy at study entry, most commonly regimens containing metformin (77.4%) and/or SUs (37.9%). Nearly half of the participants (45.7%) had established atherosclerotic CV disease (CVD), with the large majority (90.7%) taking antihypertensives (mean systolic and diastolic blood pressure was 133 and 79 mmHg, respectively). The majority of patients (70.5%) were taking statins at baseline (Table 1).
Table 1.
Baseline demographic and clinical characteristics of Asian patients
| Characteristic | Linagliptin (n = 465) | Glimepiride (n = 468) |
|---|---|---|
| Age, years | 61.4 ± 9.4 | 61.3 ± 9.4 |
| Sex, n (%) | ||
| Male | 311 (66.9) | 304 (65.0) |
| Female | 154 (33.1) | 164 (35.0) |
| Race, n (%) | ||
| Asian | 464 (99.8) | 468 (100.0) |
| White | 1 (0.2) | 0 |
| Smoking status, n (%) | ||
| Never smoker | 239 (51.4) | 269 (57.5) |
| Ex-smoker | 127 (27.3) | 127 (27.1) |
| Current smoker | 99 (21.3) | 72 (15.4) |
| CV risk entry criteria | ||
| Previous vascular disease | 199 (42.8) | 202 (43.2) |
| Microvascular complications | 58 (12.5) | 76 (16.2) |
| Age ≥ 70 years | 113 (24.3) | 112 (23.9) |
| ≥ 2 CV risk factors | 366 (78.7) | 349 (74.6) |
| None of above or missing data | 0 | 1 (0.2) |
| Heart failure, n (%) | 7 (1.5) | 20 (4.3) |
| Atherosclerotic CV disease, n (%) | 213 (45.8) | 213 (45.5) |
| Coronary artery disease | 155 (33.3) | 159 (34.0) |
| Cerebrovascular disease | 61 (13.1) | 69 (14.7) |
| Peripheral artery disease | 19 (4.1) | 18 (3.8) |
| Hypertension, n (%) | 413 (88.8) | 417 (89.1) |
| Microvascular disease, n (%) | 137 (29.5) | 154 (32.9) |
| Diabetic neuropathy | 51 (11.0) | 55 (11.8) |
| Diabetic nephropathy | 82 (17.6) | 86 (18.4) |
| Diabetic retinopathy | 39 (8.4) | 48 (10.3) |
| eGFR (MDRD), ml/min/1.73 m2 | 77.8 ± 20.9 | 77.1 ± 20.4 |
| eGFR (MDRD), n (%) | ||
| ≥ 90 ml/min/1.73 m2 | 127 (27.3) | 125 (26.7) |
| ≥ 60– < 90 ml/min/1.73 m2 | 242 (52.0) | 245 (52.4) |
| ≥ 30 − < 60 ml/min/1.73 m2 | 94 (20.2) | 95 (20.3) |
| ≥ 15 − < 30 ml/min/1.73 m2 | 1 (0.2) | 3 (0.6) |
| < 15 ml/min/1.73 m2 | 1 (0.2) | 0 |
| UACR, mg/g, median (25th–75th percentile) | 13.3 (7.1–33.6) | 13.3 (6.2–38.5) |
| UACR, n (%) | ||
| < 30 mg/g | 339 (72.9) | 328 (70.1) |
| 30–300 mg/g | 106 (22.8) | 111 (23.7) |
| > 300 mg/g | 20 (4.3) | 29 (6.2) |
| BMI, kg/m2a | 26.5 ± 3.7a | 26.3 ± 3.8 |
| HbA1c, % | 7.1 ± 0.5 | 7.1 ± 0.5 |
| Fasting plasma glucose, mg/dl | 129 ± 26 | 130 ± 24 |
| Diabetes duration, years, median (25th–75th percentile) | 5.2 (2.4–9.8) | 5.0 (2.3–9.1) |
| Diabetes duration ≤ 5 years, n (%) | 226 (48.6) | 233 (49.8) |
| Systolic blood pressure, mmHg | 133 ± 15 | 132 ± 16 |
| Diastolic blood pressure, mmHg | 79 ± 9 | 79 ± 10.0 |
| Pulse rate, beats per minute | 72 ± 11 | 72 ± 11 |
| Total cholesterol, mg/dlb | 164 ± 36 | 166 ± 41 |
| LDL cholesterol, mg/dlc | 85 ± 30 | 87 ± 37 |
| HDL cholesterol, mg/dlb | 50 ± 13 | 50 ± 13 |
| Triglycerides, mg/dlb | 150 ± 101 | 155 ± 149 |
| Glucose-lowering therapy, n (%) | ||
| Metformin | 360 (77.4) | 362 (77.4) |
| SU | 183 (39.4) | 171 (36.5) |
| Alpha-glucosidase inhibitor | 39 (8.4) | 34 (7.3) |
| Glinide (meglitinide) | 9 (1.9) | 8 (1.7) |
| Number of background glucose-lowering therapies, n (%) | ||
| 0 | 35 (7.5) | 46 (9.8) |
| 1 | 273 (58.7) | 269 (57.5) |
| 2 | 153 (32.9) | 152 (32.5) |
| 3 | 4 (0.9) | 1 (0.2) |
| Antihypertensives, n (%) | 424 (91.2) | 422 (90.2) |
| Angiotensin receptor blockers | 218 (46.9) | 216 (46.2) |
| Calcium antagonists | 194 (41.7) | 202 (43.2) |
| β-blockers | 180 (38.7) | 174 (37.2) |
| ACE inhibitors | 108 (23.2) | 118 (25.2) |
| Diuretics | 97 (20.9) | 104 (22.2) |
| Acetylsalicylic acid (aspirin), n (%) | 230 (49.5) | 217 (46.4) |
| Statins, n (%) | 325 (69.9) | 333 (71.2) |
Data are mean ± SD for patients treated with ≥ 1 dose of study medication unless otherwise specified
ACE angiotensin-converting enzyme, BMI body mass index, CV cardiovascular, eGFR estimated glomerular filtration rate, HbA1c glycated hemoglobin, HDL high-density lipoprotein, LDL low-density lipoprotein, MDRD Modification of Diet in Renal Disease, SD standard deviation, SE standard error, SU sulfonylurea, UACR urinary albumin-to-creatinine ratio
aData missing for 1 patient on linagliptin
bData missing for 17 patients (linagliptin: n = 7; glimepiride: n = 10)
cData missing for 39 patients (linagliptin: n = 19; glimepiride: n = 20)
The median (25th–75th percentile) duration of follow-up of Asian patients in the trial was 6.2 years (5.9–6.5) in both the linagliptin and glimepiride groups; median on-study medication exposure was 6.1 (5.5–6.4) and 6.1 (5.3–6.4) years, respectively. Overall, 921 (98.7%) of patients completed the study, with 236 (25.3% [linagliptin 23.2%; glimepiride 27.4%]) prematurely discontinuing study drug, most commonly because of adverse events, followed by patient refusal to continue taking study drug for reasons other than adverse events, and other reasons.
CV and mortality outcomes
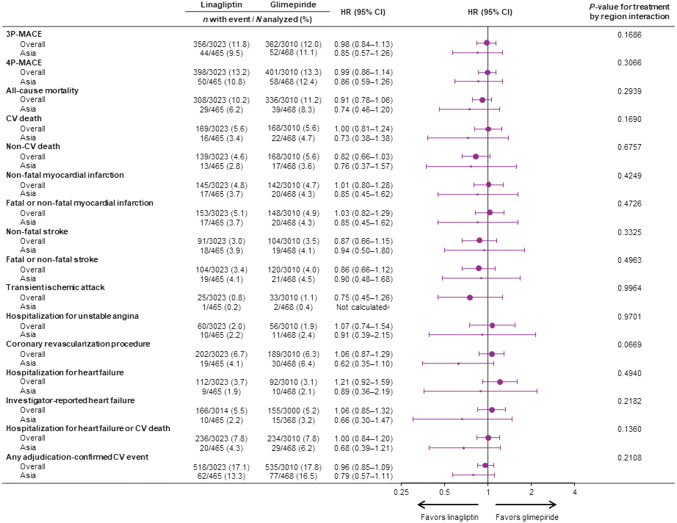
The primary endpoint of 3P-MACE occurred in 44 Asian patients (9.5%) randomized to linagliptin and 52 (11.1%) randomized to glimepiride (HR 0.85; 95% CI 0.57–1.26), consistent with the relative effects in the overall study population (HR 0.98; 95% CI 0.84–1.13; P value for treatment by region interaction: 0.1686) (Fig. 1). Similarly, 50 (10.8%) linagliptin-treated Asian patients experienced a 4P-MACE event compared with 58 (12.4%) glimepiride-treated patients (HR 0.86; 95% CI 0.59–1.26), which was also consistent with the overall study population (HR 0.99; 95% CI 0.86–1.14; P value for treatment by region interaction: 0.3066) (Fig. 1).
Fig. 1.
CV outcomes and mortality in overall trial population and Asian patients. aHR and 95% CI not calculated as < 14 patients with event. CI confidence interval, CV cardiovascular, HR hazard ratio, 3P-/4P-MACE three-point/four-point major adverse CV events
Linagliptin also did not increase patient risk for any of the individual outcomes included in 3P-MACE or 4P-MACE in Asian participants, consistent with the overall results, as indicated by all P values for treatment by region interaction being not significant (> 0.05) (Fig. 1).
Overall, 29 (6.2%) and 39 (8.3%) of Asian patients treated with linagliptin or glimepiride, respectively, died during the trial (HR 0.74; 95% CI 0.46–1.20), consistent with the observed effect of linagliptin treatment on all-cause mortality in the overall trial population (HR 0.91; 95% CI 0.78–1.06; P value for treatment by region interaction: 0.2939). Of these fatalities, CV death was the most frequent cause: 16 patients treated with linagliptin (3.4%) and 22 (4.7%) in the glimepiride group, with an HR of 0.73 (95% CI 0.38–1.38) compared with 1.00 (95% CI 0.81–1.24) in the overall population (P value for treatment by region interaction: 0.1690). Thirteen Asian patients in the linagliptin group (2.8%) died from non-CV causes compared with 17 in the glimepiride group (3.6%). The HR for non-CV death was 0.76 (95% CI 0.37–1.57) compared with 0.82 (95% CI 0.66–1.03) in the overall population (P value for treatment by region interaction: 0.6757).
In Asian patients, there was also no significant difference between linagliptin and glimepiride in the risk of hospitalization for heart failure (HR 0.89; 95% CI 0.36–2.19), the composite of hospitalization for heart failure or CV death (HR 0.68; 95% CI 0.39–1.21), or investigator-reported heart failure (HR 0.66; 95% CI 0.30–1.47). These findings were consistent with the overall population (all P values > 0.05 for treatment by region interactions).
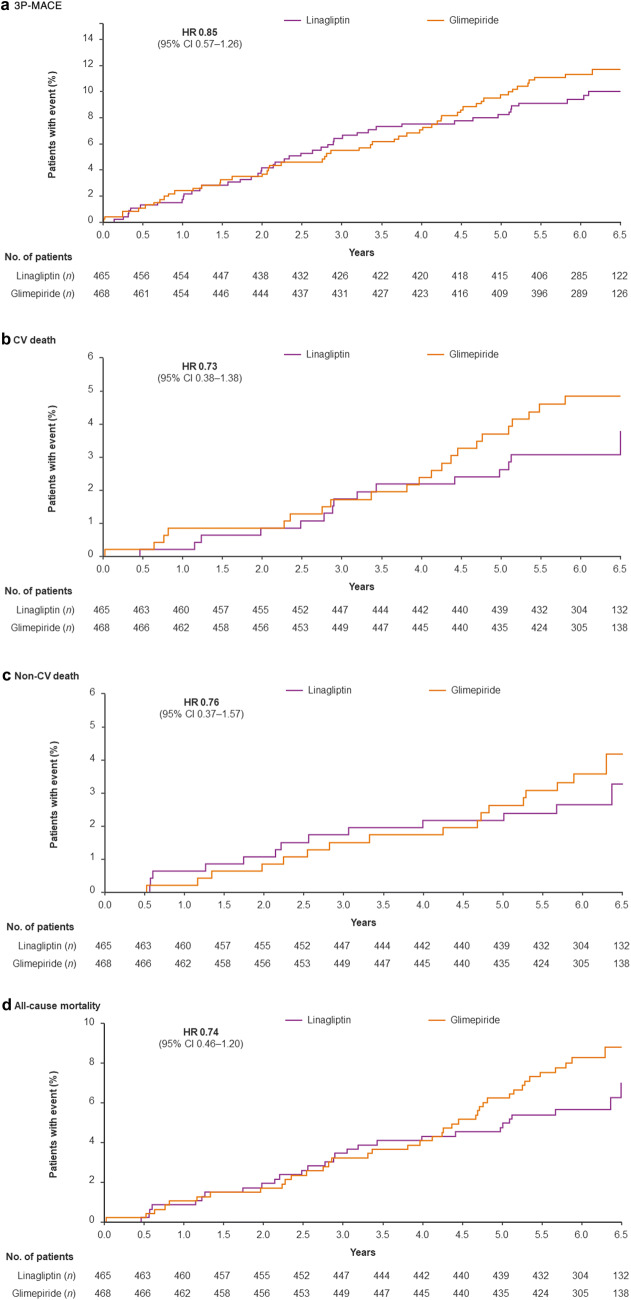
Figure 2 shows the time to first event for 3P-MACE, CV death, non-CV death, and all-cause mortality in the Asian cohort.
Fig. 2.
Time to first CV, CV death, event or death in Asian patients based on Kaplan–Meier estimates. CI confidence interval, CV cardiovascular, HR hazard ratio, 3P-MACE major adverse cardiovascular events (CV death, non-fatal myocardial infarction, non-fatal stroke)
CV and mortality outcomes in the subcohort of patients from East Asia (linagliptin: n = 284; glimepiride: n = 290) were very similar to those in the overall Asian cohort and overall trial population (Supplementary Fig. S1). The primary outcome, 3P-MACE, occurred in 8.5% and 9.3% of patients treated with linagliptin or glimepiride, respectively (HR 0.91; 95% CI 0.53–1.58; P value for treatment by region interaction: 0.2474), compared with 9.5% and 11.1% in Asia and 11.8% and 12.0% in the overall population. Similarly, 4.6% and 7.9% of linagliptin- and glimepiride-treated patients, respectively, from East Asia died during the trial (HR 0.57; 95% CI 0.29–1.13; P value for treatment by region interaction: 0.2977), compared with 6.2% and 8.3% of the linagliptin and glimepiride groups in Asia and 10.2% and 11.2% overall.
Metabolic outcomes and hypoglycemia events
The mean ± SD daily dose of glimepiride during the trial was 2.6 ± 1.2 mg in Asian patients, consistent with the mean dose in the overall trial population (2.9 ± 1.1 mg [19]). As shown in Supplementary Fig. S2, 37.7% of patients were using the highest dose of 4 mg by week 16 and 47.1% at week 256.
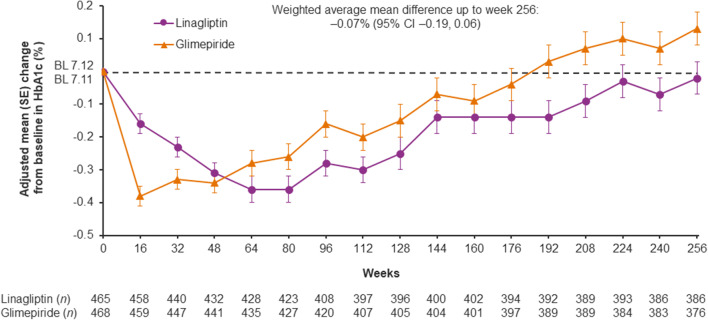
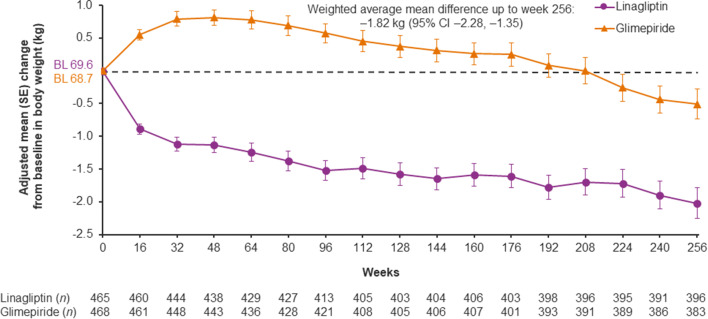
HbA1c initially decreased more with glimepiride than linagliptin, but there was no significant difference between the treatment groups over time: weighted average mean difference over 256 weeks of − 0.07% (95% CI − 0.19, 0.06) (Fig. 3). The introduction of new glucose-lowering medications during the trial occurred in similar proportions of the linagliptin and glimepiride groups (Supplementary Table S1). Mean body weight decreased initially in the linagliptin group and increased in the glimepiride group; this between-group difference was maintained over the study with a weighted average mean difference over 256 weeks of − 1.82 kg (95% CI − 2.28, − 1.35) (Fig. 4). Similar changes in body weight were seen in patients from East Asia (Supplementary Fig. S3). All these results were consistent with those in the overall trial population [19].
Fig. 3.
HbA1c over time in Asian patients. Baseline values are descriptive; post-baseline values are from a mixed model for repeated measures. BL baseline, CI confidence interval, HbA1c glycated hemoglobin, SE standard error
Fig. 4.
Body weight over time in Asian patients. Baseline values are descriptive; post-baseline values are from a mixed model for repeated measures. BL baseline, CI confidence interval, SE standard error
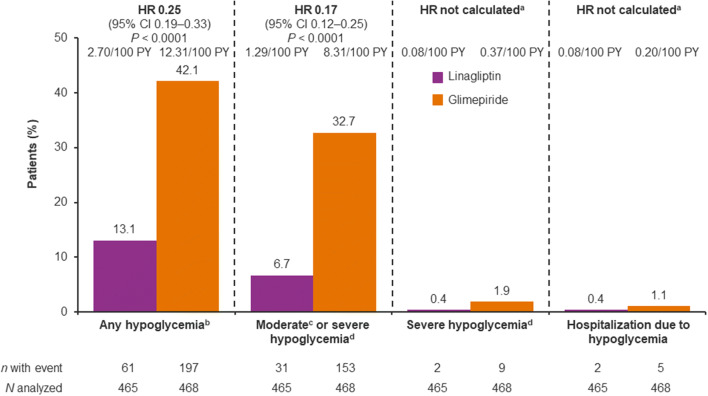
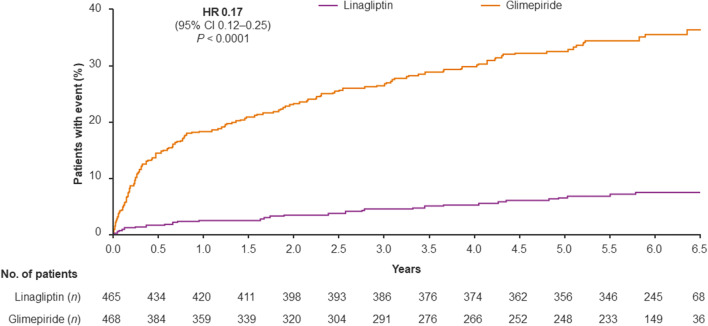
The incidence of hypoglycemia was substantially lower in the linagliptin group than in the glimepiride group, including severe episodes and those leading to hospitalization (Figs. 5, 6). During the study, at least one episode of hypoglycemia was reported for 13.1% of patients in the linagliptin group and 42.1% of the glimepiride group (HR 0.25; 95% CI 0.19–0.33, P < 0.0001), while 6.7% and 32.7%, respectively, reported at least one episode of moderate or severe hypoglycemia (HR 0.17; 95% CI 0.12–0.25, P < 0.0001). A similarly reduced incidence of hypoglycemia with linagliptin compared with glimepiride was also evident in East Asian patients (Supplementary Fig. S4). These findings are consistent with the overall study population, in which the risk of hypoglycemia was significantly lower with linagliptin than glimepiride in all prespecified categories of severity [16].
Fig. 5.
Hypoglycemia in Asian patients. aHR and 95% CI not calculated as < 14 patients with event; bAny investigator-defined hypoglycemia adverse event; chypoglycemia adverse event reported with typical symptoms of hypoglycemia and plasma glucose ≤ 70 mg/dl; dhypoglycemia event requiring the assistance of another person to actively administer carbohydrate, glucagon or other resuscitative actions. CI confidence interval, HR hazard ratio, PY patient-years
Fig. 6.
Time to first occurrence of moderatea or severeb hypoglycemia in Asian patients based on Kaplan–Meier estimates using data obtained until 7 days after treatment end. aHypoglycemia adverse event reported with typical symptoms of hypoglycemia and plasma glucose ≤ 70 mg/dl; bhypoglycemia event requiring the assistance of another person to actively administer carbohydrate, glucagon or other resuscitative actions. CI confidence interval, HR hazard ratio
Treatment sustainability
Among patients from Asian countries, more in the linagliptin group (99/465: 21.3%) than in the glimepiride group (52/468: 11.1%) achieved HbA1c ≤ 7.0% at the end of the study without—after week 16—using glycemic rescue medication, or having any episodes of moderate or severe hypoglycemia, or having > 2.0% weight gain (odds ratio 2.16; 95% CI 1.50–3.11). This was consistent with the results seen in the overall study population for this prespecified secondary end point: 16.0% and 10.2% of the linagliptin and glimepiride groups, respectively (odds ratio 1.68; 95% CI 1.43–1.96 [16]; P value for treatment by region interaction: 0.1210). Similarly, more linagliptin (106/465: 22.8%) than glimepiride patients (71/468: 15.2%) in Asia achieved the other treatment sustainability prespecified secondary end point of HbA1c ≤ 7.0% at study end without—after week 16—glycemic rescue medication or > 2.0% weight gain (odds ratio 1.65; 95% CI 1.18–2.30). Again, this was consistent with the overall study population (17.4% and 14.1% of linagliptin and glimepiride patients, respectively [odds ratio 1.29; 95% CI 1.11–1.48] [16]; P value for treatment by region interaction: 0.1491).
Adverse events
The adverse event profile of linagliptin compared with glimepiride in Asian patients was consistent with the profile of the overall study population [19]. Overall, adverse events in Asian patients were reported for comparable proportions of the linagliptin and glimepiride groups (Table 2). Serious adverse events were reported for 45.8% and 51.3% of the linagliptin and glimepiride groups, respectively, while adverse events leading to discontinuation of study drug were reported for 11.6% of the linagliptin group and 14.1% of the glimepiride group.
Table 2.
Adverse events in Asian patients
| Adverse event | Linagliptin (n = 465) | Glimepiride (n = 468) |
|---|---|---|
| Any adverse event | 443 (95.3) | 453 (96.8) |
| Serious adverse event | 213 (45.8) | 240 (51.3) |
| Adverse event leading to discontinuation | 54 (11.6) | 66 (14.1) |
| Any hospitalization | 184 (39.6) | 228 (48.7) |
| Hypersensitivity reactionsa | 87 (18.7) | 80 (17.1) |
| Pemphigoidb | 1 (0.2) | 0 |
| Skin lesionsc | 1 (0.2) | 0 |
| Acute pancreatitis (adjudication-confirmed) | 3 (0.6) | 2 (0.4) |
| Chronic pancreatitis (adjudication-confirmed) | 1 (0.2) | 0 |
| Cancerd | 22 (4.7) | 33 (7.1) |
| Colorectal cancere | 2 (0.4) | 5 (1.1) |
| Pancreatic cancer (adjudication-confirmed) | 3 (0.6) | 2 (0.4) |
| Gastric cancerf | 5 (1.1) | 1 (0.2) |
| Thyroid cancerg | 1 (0.2) | 0 |
Data are n (%) of patients treated with ≥ 1 dose of study medication until 7 days after the last intake of study medication except for hospitalization, pancreatitis and cancers, which include all events until study end
MedDRA Version 21.0 was used to classify adverse events
MedDRA Medical Dictionary for Regulatory Activities, SMQ Standardised MedDRA Query
aBased on the narrow SMQ “hypersensitivity”
bMedDRA preferred term
cBased on narrow SMQ “Severe cutaneous adverse reactions”
dBased on narrow SMQs “Malignant Tumors” and “Tumors of unspecified malignancy”
eMedDRA high-level term “Colorectal neoplasms malignant”
fMedDRA high-level term “Gastric neoplasms malignant”
gMedDRA high-level term “Thyroid neoplasms malignant”
One case of adjudication-confirmed chronic pancreatitis occurred in a linagliptin-treated patient, while adjudication-confirmed acute pancreatitis occurred in three patients receiving linagliptin and two receiving glimepiride. Cancer was reported for 4.7% of the linagliptin group and 7.1% of the glimepiride group. Adjudication-confirmed pancreatic cancer occurred in three patients receiving linagliptin and two receiving glimepiride (Table 2).
Discussion
These prespecified and post hoc subgroup analyses of the CAROLINA® trial found that the rate of CV events did not differ between linagliptin and glimepiride in the Asian sub-population or in the East Asian cohort, both comprising patients with relatively early T2DM at elevated CV risk who were appropriately receiving guideline-recommended treatments for CVD prevention. Notably, linagliptin was associated with a substantially lower incidence of hypoglycemia and modestly lower body weight compared with glimepiride. This subgroup analysis of the multinational CAROLINA® trial is consistent with the overall findings from this study [19].
CAROLINA® is to date the longest randomized, controlled, double-blind CV outcomes trial (CVOT) of a glucose-lowering drug, and the only one to have an active comparator. Over 15 placebo-controlled, multinational CVOTs of glucose-lowering drugs have taken place since 2008 when the U.S. Food and Drug Administration mandated that novel glucose-lowering drugs must demonstrate no excess risk for CVD [22]. This requirement arose from concerns about the CV safety of early-generation SUs [20] and the peroxisome proliferator-activated receptor agonists rosiglitazone [23] and muraglitazar [24]. However, none of these trials was conducted exclusively in Asians. In one of the few subgroup analyses of these CVOTs that reported detailed results for Asian participants, empagliflozin, a sodium-glucose co-transporter-2 inhibitor, was shown to reduce the risk of CV death, 3P-MACE and hospitalization for heart failure in Asian patients in the landmark EMPA-REG OUTCOME® trial [25]. A similar subgroup analysis of the multinational, placebo-controlled CARMELINA® trial [19] found that linagliptin did not increase the risk of CV events in Asian patients with T2DM and high CV and CKD risk [16]. Interestingly, in CARMELINA®, risk for albuminuria progression was reduced, and in particular, in participants from Asia, a nominally lower rate of hospitalization for heart failure was observed [16].
Until the comparative CAROLINA® trial, however, the CV safety of SUs had remained unresolved and highly debated. In the context of the established CV safety profile of linagliptin versus placebo demonstrated in CARMELINA® [15], CAROLINA® has largely resolved and put to rest the 50-year controversy about the CV safety of SUs that arose from the UGDP trial in the U.S. in the 1960s. Results from UGDP suggested that treatment with the first-generation SU tolbutamide was associated with an increased risk of CV death [17]—however, the findings were controversial due to the small number of events and purported methodological and operational issues with this study [26, 27]. Since then, there have been conflicting data on the CV safety of SUs from numerous observational studies, but no definitive trial had been conducted until CAROLINA®, which demonstrated no increased CV risk with glimepiride [19]. As a quality, active-controlled CVOT, CAROLINA® therefore provides important evidence to this long-debated issue, largely vindicating second-generation SUs (particularly glimepiride) [28]. Our subgroup analysis of CAROLINA® provides reassurance that in Asians with T2DM, glimepiride has similar CV safety to linagliptin, which itself was shown to have CV safety in Asians in the CARMELINA® trial [16]. These findings on the CV safety of linagliptin and glimepiride in Asians are important as both DPP-4 inhibitors and SUs are commonly prescribed in Asia. A recent study found that DPP‐4 inhibitors were the most frequently prescribed class of oral antidiabetes drug in Japan, followed by biguanides (including metformin) and SUs [14].
Other findings from CAROLINA® also have important implications for clinical care, as this study confirms and extends previous observations about the risk of hypoglycemia with SUs [29, 30]. The incidence of hypoglycemia (including severe episodes) was four- to sixfold greater with glimepiride than linagliptin [19]; this was even higher than had been predicted for CAROLINA® based on observational data [31]. We found the same increased risk for hypoglycemia with glimepiride compared with linagliptin in Asian patients. Glimepiride was not used at its highest licensed dose (6–8 mg) during the trial, as the 4-mg dose provides near-maximal antihyperglycemic efficacy [32]. Furthermore, throughout the study, among Asians, fewer than half the glimepiride-treated patients were receiving 4 mg. Despite this and the similar glycemic control between treatment groups, linagliptin had substantially lower risk for hypoglycemia. Renal function and age influence the risk of hypoglycemia [33], but these characteristics were balanced between treatment arms. As previously reported in CAROLINA® [19], linagliptin had a lower risk of hypoglycemia than glimepiride at all doses of the latter despite comparable overall HbA1c changes during the trial.
Many studies have found hypoglycemia to be associated with increased CV and heart failure risk, although it is unclear whether this is a causative relationship or if hypoglycemia is simply a marker of a vulnerable patient [34, 35]. The lack of increase in CV risk with glimepiride in CAROLINA® despite much higher incidence of hypoglycemia lends support to the latter theory. However, it is also conceivable that this is a reflection of CAROLINA® participants being relatively early in their T2DM disease course and hence being able to counterbalance and tolerate deleterious effects of hypoglycemia better than more fragile, higher-risk individuals. Irrespective of the link with CVD, prevention of hypoglycemia is an important goal in T2DM and a meaningful outcome to patients, as hypoglycemia is associated with increased risk of falls and fractures, hospitalizations and medical costs, as well as fear of hypoglycemia, impaired psychological outcomes, and reduced quality of life [36–39]. Fear of hypoglycemia also contributes to therapeutic inertia by physicians [40].
Overall, the findings from CAROLINA® reaffirm recent clinical recommendations in Western T2DM guidelines that patients with indicators of high CV risk or established CVD should receive second-line treatment (after metformin), or even first-line therapy, with a glucose-lowering drug that has proven CV benefit [41–44], which neither of the study drugs provide. However, when additional glucose-lowering therapy is required, or for third-line therapy, drugs with proven CV safety should be used [41, 42]. For second-line therapy of patients without indicators of high CV risk or established CVD, both DPP-4 inhibitors and SUs are valid options, and the low risk for hypoglycemia and weight gain of the former may be preferred to the latter unless cost issues override clinical considerations [41]. The findings from CAROLINA® have been integrated into the most recent update to these guidelines, which acknowledges that glimepiride has demonstrated similar CV safety to DPP-4 inhibitors and that linagliptin has benefit over glimepiride for hypoglycemia and body weight [42].
Our study is subject to limitations of subgroup analyses; most importantly, the reduction in statistical power from lower patient numbers compared with the overall trial population and the lack of adjustments for multiple subgroup comparisons. These limitations are balanced by strengths such as the use of randomized data for a still-substantial cohort of Asian patients (~ 1000 individuals) from a quality CVOT in which clinical events were rigorously adjudicated and fully consistent with the entire study cohort.
Conclusion
Linagliptin had similar CV safety to glimepiride in Asian patients with relatively early T2DM and elevated CV risk, but was associated with less hypoglycemia and modestly lower body weight than this SU.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The CAROLINA® trial was funded by the Boehringer Ingelheim & Eli Lilly and Company Diabetes Alliance. Medical writing assistance, supported financially by Boehringer Ingelheim, was provided by Giles Brooke, PhD, CMPP, of Elevate Scientific Solutions during the preparation of this manuscript.
Compliance with ethical standards
Conflict of interest
TK reports consulting/lecture fees from Abbott, Asahi Mutual Life Insurance, Astellas Pharma Inc., AstraZeneca KK, Bayer, Boehringer Ingelheim, Cosmic, Daiichi Sankyo Company, Limited, Eli Lilly and Company, Fujifilm, FUJIREBIO, Johnson & Johnson Co., Ltd., Kissei Pharmaceutical Co., Ltd., Kowa Co., Ltd., Kyowa Hakko Kirin Co., Ltd., Medical Review, Medscape Education, Medtronic Sofamor Danek, Mitsubishi Tanabe Pharma Corporation, MSD, Musashino Foods, Nipro, Novartis International AG, Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Ltd., Sanofi S.A., SANWA KAGAKU KENKYUSHO CO., LTD., Sumitomo Dainippon, Taisho Pharmaceutical Co., Ltd., Takeda Pharmaceutical Company Limited., and Terumo; grants from Astellas Pharma Inc., Daiichi Sankyo Company, Limited, Eli Lilly and Company, Kissei Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, MSD, Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Ltd., Sanofi S.A., Sumitomo Dainippon, Taisho Pharmaceutical Co., Ltd., and Takeda Pharmaceutical Company Limited.; contracted research from AstraZeneca KK and Takeda Pharmaceutical Company Limited.; joint research from Daiichi Sankyo Company, Limited.; endowed chair from Asahi Mutual Life Insurance, Boehringer Ingelheim, Kowa Co., Ltd., Mitsubishi Tanabe Pharma Corporation, MSD, Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Ltd, and Takeda Pharmaceutical Company Limited. JR has served on scientific advisory boards and received honoraria or consulting fees from Eli Lilly, Sanofi, Novo Nordisk, Janssen, AstraZeneca, Boehringer Ingelheim, and Intarcia; he has also received grants/research support from Merck, Pfizer, Sanofi, Novo Nordisk, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Genentech, Janssen, Lexicon, Boehringer Ingelheim, and Intarcia. DY has received consulting or speaker fees from MSD KK, Novo Nordisk Pharma Ltd. and Taisho Toyama Pharmaceutical Co. Ltd., and clinically commissioned/joint research grants from Taisho Toyama Pharmaceutical Co. Ltd., Ono Pharmaceutical Co. Ltd., Novo Nordisk Pharma Ltd., Arklay Co. Ltd. and Terumo Co. Ltd. KK has received lecture fees from Boehringer Ingelheim, Eli Lilly and Sanofi. Boehringer Ingelheim, Mitsubishi Tanabe Pharma and Ono Pharmaceutical contributed to establishing the Division of Anticipatory Molecular Food Science and Technology, Medical Research Institute, Kanazawa Medical University, and is under contract for consultancy with Boehringer Ingelheim. GW, YP, YM have no conflicts to disclose. MM, AK, TO, and OEJ are employees of Boehringer Ingelheim. NM is funded by the German Research Foundation SFB TRR 219 (projects M-03 and M-05); reports giving lectures for and receiving honoraria from Amgen, Boehringer Ingelheim, Sanofi-Aventis, Merck Sharp & Dohme, Bristol-Myers Squibb, AstraZeneca, Lilly, Novo Nordisk; receiving unrestricted research grants from Boehringer Ingelheim; serving as an advisor for Amgen, Bayer, Boehringer Ingelheim, Sanofi-Aventis, Merck Sharp & Dohme, Bristol-Myers Squibb, AstraZeneca, Novo Nordisk; serving in trial leadership for Boehringer Ingelheim and Novo Nordisk; and declining all personal compensation from pharmaceutical and device companies.
Ethical standards
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and/or with the Helsinki Declaration of 1964 and later versions.
Human rights statement
This research involves human participants. This report was limited to the Asian population from the CAROLINA® trial (ClinicalTrials.gov Identifier, NCT01243424). The study protocol was approved by the institutional review board or independent ethics committee from each site (approval numbers: not applicable) and all patients provided written informed consent before entering the trial. Full details of the approval process are provided in previous publications [19, 21].
Informed consent
Informed consent or substitute for it was obtained from all patients for being included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chan JC, Malik V, Jia W, Kadowaki T, Yajnik CS, Yoon KH, Hu FB. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301(20):2129–2140. doi: 10.1001/jama.2009.726. [DOI] [PubMed] [Google Scholar]
- 2.Ma RC, Chan JC. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci. 2013;1281:64–91. doi: 10.1111/nyas.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nanditha A, Ma RC, Ramachandran A, Snehalatha C, Chan JC, Chia KS, Shaw JE, Zimmet PZ. Diabetes in Asia and the Pacific: implications for the global epidemic. Diabetes Care. 2016;39(3):472–485. doi: 10.2337/dc15-1536. [DOI] [PubMed] [Google Scholar]
- 4.Ramachandran A, Snehalatha C, Ma RC. Diabetes in South-East Asia: an update. Diabetes Res Clin Pract. 2014;103(2):231–237. doi: 10.1016/j.diabres.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Lu J, Bi Y, Ning G. Curbing the obesity epidemic in China. Lancet Diabetes Endocrinol. 2016;4(6):470–471. doi: 10.1016/S2213-8587(16)30007-9. [DOI] [PubMed] [Google Scholar]
- 6.International Diabetes Federation. IDF Diabetes Atlas. 9th ed. Brussels, Belgium: International Diabetes Federation; 2019. https://www.diabetesatlas.org/en/resources/. Accessed 30 Jan 2020.
- 7.Ministry of Health, Labour, and Welfare, Japan. National Health and Nutrition Survey. 2016. https://www.mhlw.go.jp/english/. Accessed 30 Jan 2020
- 8.Hata J, Kiyohara Y. Epidemiology of stroke and coronary artery disease in Asia. Circ J. 2013;77(8):1923–1932. doi: 10.1253/circj.cj-13-0786. [DOI] [PubMed] [Google Scholar]
- 9.Ohira T, Iso H. Cardiovascular disease epidemiology in Asia: an overview. Circ J. 2013;77(7):1646–1652. doi: 10.1253/circj.cj-13-0702. [DOI] [PubMed] [Google Scholar]
- 10.Yang JJ, Yu D, Wen W, Saito E, Rahman S, Shu XO, Chen Y, Gupta PC, Gu D, Tsugane S, Xiang YB, Gao YT, Yuan JM, Tamakoshi A, Irie F, Sadakane A, Tomata Y, Kanemura S, Tsuji I, Matsuo K, Nagata C, Chen CJ, Koh WP, Shin MH, Park SK, Wu PE, Qiao YL, Pednekar MS, He J, Sawada N, Li HL, Gao J, Cai H, Wang R, Sairenchi T, Grant E, Sugawara Y, Zhang S, Ito H, Wada K, Shen CY, Pan WH, Ahn YO, You SL, Fan JH, Yoo KY, Ashan H, Chia KS, Boffetta P, Inoue M, Kang D, Potter JD, Zheng W. Association of diabetes with all-cause and cause-specific mortality in Asia: a pooled analysis of more than 1 million participants. JAMA Netw Open. 2019;2(4):e192696. doi: 10.1001/jamanetworkopen.2019.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weng J, Ji L, Jia W, Lu J, Zhou Z, Zou D, Zhu D, Chen L, Chen L, Guo L, Guo X, Ji Q, Li Q, Li X, Liu J, Ran X, Shan Z, Shi L, Song G, Yang L, Yang Y, Yang W, Chinese Diabetes Society Standards of care for type 2 diabetes in China. Diabetes Metab Res Rev. 2016;32(5):442–458. doi: 10.1002/dmrr.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bajaj S. RSSDI clinical practice recommendations for the management of type 2 diabetes mellitus 2017. Int J Diabetes Dev Ctries. 2018;38(Suppl 1):1–115. doi: 10.1007/s13410-018-0604-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haneda M, Noda M, Origasa H, Noto H, Yabe D, Fujita Y, Goto A, Kondo T, Araki E. Japanese clinical practice guideline for diabetes 2016. J Diabetes Investig. 2018;9(3):657–697. doi: 10.1111/jdi.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kadowaki T, Sarai N, Hirakawa T, Taki K, Iwasaki K, Urushihara H. Persistence of oral antidiabetic treatment for type 2 diabetes characterized by drug class, patient characteristics and severity of renal impairment: a Japanese database analysis. Diabetes Obes Metab. 2018;20(12):2830–2839. doi: 10.1111/dom.13463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenstock J, Perkovic V, Johansen OE, Cooper ME, Kahn SE, Marx N, Alexander JH, Pencina M, Toto RD, Wanner C, Zinman B, Woerle HJ, Baanstra D, Pfarr E, Schnaidt S, Meinicke T, George JT, von Eynatten M, McGuire DK, CARMELINA Investigators Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019;321(1):69–79. doi: 10.1001/jama.2018.18269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inagaki N, Yang W, Watada H, Ji L, Schnaidt S, Pfarr E, Okamura T, Johansen OE, George JT, von Eynatten M, Rosenstock J, Perkovic V, Wanner C, Cooper ME, Alexander JH, Komuro I, Nangaku M. Linagliptin and cardiorenal outcomes in Asians with type 2 diabetes mellitus and established cardiovascular and/or kidney disease: subgroup analysis of the randomized CARMELINA® trial. Diabetology Int. 2019;11(2):129–141. doi: 10.1007/s13340-019-00412-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meinert CL, Knatterud GL, Prout TE, Klimt CR. A study of the effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. II. Mortality results. Diabetes. 1970;19(Suppl):789–830. [PubMed] [Google Scholar]
- 18.Azoulay L, Suissa S. Sulfonylureas and the risks of cardiovascular events and death: a methodological meta-regression analysis of the observational studies. Diabetes Care. 2017;40(5):706–714. doi: 10.2337/dc16-1943. [DOI] [PubMed] [Google Scholar]
- 19.Rosenstock J, Kahn SE, Johansen OE, Zinman B, Espeland MA, Woerle HJ, Pfarr E, Keller A, Mattheus M, Baanstra D, Meinicke T, George JT, von Eynatten M, McGuire DK, Marx N, Investigators C Effect of linagliptin vs glimepiride on major adverse cardiovascular outcomes in patients with type 2 diabetes: The CAROLINA randomized clinical trial. JAMA. 2019;322(12):1155–1166. doi: 10.1001/jama.2019.13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenstock J, Marx N, Kahn SE, Zinman B, Kastelein JJ, Lachin JM, Bluhmki E, Patel S, Johansen OE, Woerle HJ. Cardiovascular outcome trials in type 2 diabetes and the sulphonylurea controversy: rationale for the active-comparator CAROLINA trial. Diab Vasc Dis Res. 2013;10(4):289–301. doi: 10.1177/1479164112475102. [DOI] [PubMed] [Google Scholar]
- 21.Marx N, Rosenstock J, Kahn SE, Zinman B, Kastelein JJ, Lachin JM, Espeland MA, Bluhmki E, Mattheus M, Ryckaert B, Patel S, Johansen OE, Woerle HJ. Design and baseline characteristics of the CARdiovascular Outcome Trial of LINAgliptin Versus Glimepiride in Type 2 Diabetes (CAROLINA®) Diab Vasc Dis Res. 2015;12(3):164–174. doi: 10.1177/1479164115570301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGuire DK, Marx N, Johansen OE, Inzucchi SE, Rosenstock J, George JT. FDA guidance on antihyperglycaemic therapies for type 2 diabetes: one decade later. Diabetes Obes Metab. 2019;21(5):1073–1078. doi: 10.1111/dom.13645. [DOI] [PubMed] [Google Scholar]
- 23.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356(24):2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 24.Nissen SE, Wolski K, Topol EJ. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA. 2005;294(20):2581–2586. doi: 10.1001/jama.294.20.joc50147. [DOI] [PubMed] [Google Scholar]
- 25.Kaku K, Lee J, Mattheus M, Kaspers S, George J, Woerle HJ, EMPA-REG OUTCOME Investigators Empagliflozin and cardiovascular outcomes in Asian patients with type 2 diabetes and established cardiovascular disease—results from EMPA-REG OUTCOME®. Circ J. 2017;81(2):227–234. doi: 10.1253/circj.CJ-16-1148. [DOI] [PubMed] [Google Scholar]
- 26.Kilo C, Miller JP, Williamson JR. The Achilles heel of the University Group Diabetes Program. JAMA. 1980;243(5):450–457. [PubMed] [Google Scholar]
- 27.O’Sullivan JB, D’Agostino RB. Decisive factors in the tolbutamide controversy. JAMA. 1975;232(8):825–829. [PubMed] [Google Scholar]
- 28.Riddle M. A verdict for glimepiride: effective and not guilty of cardiovascular harm. Diabetes Care. 2019;42(12):2161–2163. doi: 10.2337/dci19-0034. [DOI] [PubMed] [Google Scholar]
- 29.Wright AD, Cull CA, Macleod KM, Holman RR, Group U Hypoglycemia in type 2 diabetic patients randomized to and maintained on monotherapy with diet, sulfonylurea, metformin, or insulin for 6 years from diagnosis: UKPDS73. J Diabetes Complicat. 2006;20(6):395–401. doi: 10.1016/j.jdiacomp.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 30.ADVANCE Collaborative Group. Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 31.Patorno E, Schneeweiss S, Gopalakrishnan C, Martin D, Franklin JM. Using real-world data to predict findings of an ongoing phase IV cardiovascular outcome trial: cardiovascular safety of linagliptin versus glimepiride. Diabetes Care. 2019;42(12):2204–2210. doi: 10.2337/dc19-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldberg RB, Holvey SM, Schneider J. A dose-response study of glimepiride in patients with NIDDM who have previously received sulfonylurea agents. The Glimepiride Protocol #201 Study Group. Diabetes Care. 1996;19(8):849–856. doi: 10.2337/diacare.19.8.849. [DOI] [PubMed] [Google Scholar]
- 33.Perkovic V, Toto R, Cooper ME, Mann JFE, Rosenstock J, McGuire DK, Kahn SE, Marx N, Alexander JH, Zinman B, Pfarr E, Schnaidt S, Meinicke T, von Eynatten M, George JT, Johansen OE, Wanner C, CARMELINA investigators Effects of linagliptin on cardiovascular and kidney outcomes in people with normal and reduced kidney function: secondary analysis of the CARMELINA randomized trial. Diabetes Care. 2020 doi: 10.2337/dc20-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.International Hypoglycaemia Study Group Hypoglycaemia, cardiovascular disease, and mortality in diabetes: epidemiology, pathogenesis, and management. Lancet Diabetes Endocrinol. 2019;7(5):385–396. doi: 10.1016/S2213-8587(18)30315-2. [DOI] [PubMed] [Google Scholar]
- 35.Fitchett D, Inzucchi SE, Wanner C, Mattheus M, George JT, Vedin O, Zinman B, Johansen OE. Relationship between hypoglycaemia, cardiovascular outcomes, and empagliflozin treatment in the EMPA-REG OUTCOME® trial. Eur Heart J. 2020;41(2):209–217. doi: 10.1093/eurheartj/ehz621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson-Rabbett B, Seaquist ER. Hypoglycemia in diabetes: the dark side of diabetes treatment. A patient-centered review. J Diabetes. 2019;11(9):711–718. doi: 10.1111/1753-0407.12933. [DOI] [PubMed] [Google Scholar]
- 37.Seaquist ER, Anderson J, Childs B, Cryer P, Dagogo-Jack S, Fish L, Heller SR, Rodriguez H, Rosenzweig J, Vigersky R. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36(5):1384–1395. doi: 10.2337/dc12-2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hendrieckx C, Ivory N, Singh H, Frier BM, Speight J. Impact of severe hypoglycaemia on psychological outcomes in adults with type 2 diabetes: a systematic review. Diabet Med. 2019;36(9):1082–1091. doi: 10.1111/dme.14067. [DOI] [PubMed] [Google Scholar]
- 39.Frier BM. Hypoglycaemia in diabetes mellitus: epidemiology and clinical implications. Nat Rev Endocrinol. 2014;10(12):711–722. doi: 10.1038/nrendo.2014.170. [DOI] [PubMed] [Google Scholar]
- 40.Ahren B. Avoiding hypoglycemia: a key to success for glucose-lowering therapy in type 2 diabetes. Vasc Health Risk Manag. 2013;9:155–163. doi: 10.2147/VHRM.S33934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davies MJ, D'Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, Rossing P, Tsapas A, Wexler DJ, Buse JB. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2018;41(12):2669–2701. doi: 10.2337/dci18-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buse JB, Wexler DJ, Tsapas A, Rossing P, Mingrone G, Mathieu C, D'Alessio DA, Davies MJ. 2019 update to: Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2020;43(2):487–493. doi: 10.2337/dci19-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Das SR, Everett BM, Birtcher KK, Brown JM, Cefalu WT, Januzzi JL, Jr, Kalyani RR, Kosiborod M, Magwire ML, Morris PB, Sperling LS. 2018 ACC expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes and atherosclerotic cardiovascular disease: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2018;72(24):3200–3223. doi: 10.1016/j.jacc.2018.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, Federici M, Filippatos G, Grobbee DE, Hansen TB, Huikuri HV, Johansson I, Juni P, Lettino M, Marx N, Mellbin LG, Ostgren CJ, Rocca B, Roffi M, Sattar N, Seferovic PM, Sousa-Uva M, Valensi P, Wheeler DC, Group ESCSD 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41(2):255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.