Abstract
Background:
Lymphedema is a side effect of breast cancer treatment, causing swelling and pain in the arm and hand. We tested two lymphedema prevention interventions and their impact on health-related quality of life (HRQL) in a group-randomized trial in 38 U.S. cooperative group sites.
Methods:
Patients were recruited before breast surgery. Sites were randomized to lymphedema-prevention education only (EO) or EO with exercise and physical therapy (LEAP). Lymphedema was defined as a ≥10% difference in arm volume at any time from baseline to 18-months post-surgery. HRQL was assessed using the Functional Assessment of Cancer Therapy–Breast plus 4 lymphedema items (FACT-B+4). Longitudinal mixed model regression analysis, adjusting for key demographic and clinical variables, examined participants’ HRQL by: 1) intervention group, and 2) lymphedema status.
Results:
547 patients (56% LEAP) were enrolled and completed HRQL assessments. Results showed no differences between the interventions in preventing lymphedema (p=0.37) or HRQL (i.e., FACT-B+4 total score, p=0.8777). At 18 months, the presence of lymphedema was borderline significantly associated with HRQL (p=0.0825). However, African-Americans reported greater lymphedema symptoms (p=0.0002) and better emotional functioning (p=0.0335) than other race/ethnicities. Lower HRQL during the intervention was associated with younger age (p=<0.0001), ECOG performance status >0 (p=0.0002), ≥1 positive lymph node(s) (p=0.0009), ≤high school education (p<0.0001), chemotherapy (p=0.0242), and having only axillary node dissection or sentinel node biopsy, as compared to both (p=0.0007).
Conclusions:
The tested interventions did not differ in preventing lymphedema or in HRQL outcomes. African-American women reported greater HRQL impacts due to lymphedema symptoms than other race/ethnicities.
Keywords: lymphedema, breast cancer, quality of life, symptoms, race
Precis:
Two tested lymphedema prevention interventions did not differ in preventing lymphedema or in quality of life outcomes. African-American women, however, reported greater lymphedema symptoms than other race/ethnicities.
INTRODUCTION
Lymphedema is often reported following breast cancer surgery and is characterized by swelling and/or pain in the arm or hand on the same side as the affected breast1–5. Symptoms can also include tightness, numbness, and decreased range of motion1. Lymphedema affects between 20–94% of women2 and results from a malfunction of the lymph system causing excess fluid to collect in the affected area2. Rates of lymphedema have changed over time, reflecting a shift in treatment techniques, yet estimates suggest close to one million women are still affected by lymphedema symptoms4. Common risk factors for the development of lymphedema include the type of lymph node surgery (axillary dissection vs. sentinel node biopsy), the type of treatment (mastectomy vs lumpectomy, radiation, chemotherapy), and the number of positive lymph nodes involved. Patient characteristics associated with lymphedema include a higher body mass index (BMI) and the presence of infection6–10.
Prior research has consistently reported poorer health-related quality of life (HRQL) among women with lymphedema11–18, affecting both their physical and mental health. Decreased strength and function of the arm can disrupt daily activities and fine motor skills15, resulting in poorer functional capabilities. For some, the swelling and continual reminder of breast cancer can increase feelings of anxiety and depression1. Across studies, poorer HRQL in women with lymphedema has been associated with younger patient age (<40 years), surgical and other treatment characteristics, and minority race16. However, swelling has not been found to be related to swelling of the dominant versus non-dominant arm14. In general, those with more severe swelling report worse physical functioning and poorer mental health than those with less severe swelling13. A linear dose relationship was also found in one study between decreasing HRQL scores and increasing number of lymphedema-related arm symptoms15. In addition, research has shown that any potential increases in HRQL after breast reconstructive surgery may be negated when lymphedema is present11. When controlling for factors affecting HRQL, such as BMI, clinical and demographic characteristics, and decreased range of motion, lymphedema has still been found to be significantly associated with lower HRQL scores18. Longer time since treatment, however, is associated with better HRQL among patients12.
There have been intervention studies focusing on breast cancer survivors with lymphedema aimed at reducing their swelling and improving function19–23, but few studies have been aimed at preventing lymphedema occurrence. In 2006, the Cancer and Leukemia Group B (CALGB) initiated a Phase III, group randomized clinical trial to prevent lymphedema among women after surgery for breast cancer (protocol # CALGB 70305; ClinicalTrials.gov identifier: NCT00376597) (Paskett et al., in press). This trial compared the effectiveness of two interventions: 1) lymphedema prevention education only (EO); or 2) education + exercise (including use of a compression sleeve) titled the Lymphedema Education and Prevention (LEAP) group. In this paper, we report on a planned secondary endpoint, participants’ HRQL by intervention group and lymphedema status, adjusting for key demographic and clinical variables known to be associated with lymphedema in this population. CALGB is now a part of the Alliance for Clinical Trials in Oncology.
METHODS
Participants
Eligible participants included women newly diagnosed with breast cancer (stage I – III), aged 18 years or older, with no prior history of lymphedema, carcinoma in situ, lobular carcinoma in situ, ductal carcinoma in situ or invasive breast cancer. Patients who received neoadjuvant chemotherapy were eligible if pre-surgery measurements and self-reported assessments were completed prior to their first chemotherapy treatment. Eligible patients also had to have medical clearance to participate in a mild exercise program and have an upper arm size that accommodated a standard-size elastic compression sleeve and gauntlet. Patients who underwent bilateral mastectomies or axillary node dissection (ALND) and/or radiation bilaterally were ineligible. Patients were recruited from 38 CALGB and National Clinical Trials Network sites across the United States (U.S.) between December 2006 and September 2013, with follow-up continuing until December 2015. Sites were randomly assigned to one of the two intervention groups: 1) education only (EO) or 2) education + exercise with physical therapy (LEAP). All patients at a participating institution were assigned to the same intervention group to minimize contamination bias. The trial was approved by the Institutional Review Board (IRB) of each participating site, and each participant signed an IRB-approved, protocol-specific written informed consent in accordance with federal and institutional guidelines.
Participant Measures
Participants were recruited at their first pre-operative visit, using a two-step eligibility process. In Step 1, eligible participants were consented and registered to the study prior to surgery, so that baseline measurements could be collected. Baseline measurements of height, weight, range of motion, and arm circumference were taken by a trained institutional nurse pre-surgery or pre-neoadjuvant systemic treatment, as applicable. Participants completed self-reports of demographics, lymphedema knowledge, body image, self-efficacy24, fear of cancer recurrence, self-reported pain and swelling, HRQL25, and adherence to lymphedema prevention practices. These assessments were repeated after surgery and at 6- (by mail), 12-, and 18-months post-surgery.
In Step 2, women were randomized to one of the two study intervention groups only if they had either axillary node dissection or sentinel node biopsy. All eligible participants registered to Step 2 met with a trained lymphedema prevention educator to review lymphedema etiology, signs, symptoms, treatments, and preventive self-care practices (i.e., education only [EO] intervention). Participants randomized to the LEAP intervention also received a physical therapy-focused intervention, in which they were assessed by a physical therapist and instructed in an individualized exercise regimen involving breathing, stretching, strengthening, and ROM exercises varying the amount of weight used, body position and number of repetitions performed based on the participant’s ability (i.e., lymphedema education plus exercise). Participants were instructed to perform these exercises daily, using an instructional video for home use. LEAP participants also were given 2-pound hand weights for use during daily exercises and an elastic compression sleeve and gauntlet (Juzo Class I 20–30mmHg) to wear during exercise, air travel, and/or vigorous activity. At both 12- and 18-months post-surgery, participants in both groups met again briefly with the study educator. Study educators also contacted participants by phone at 9- and 15-months post-surgery to reinforce prevention practices, answer questions, and remind participants of upcoming study appointments. Adherence to the exercise components were self-reported using study calendars throughout the 18-month trial period.
Outcome Measures
Lymphedema was defined as: 1) limb volume increase of ≥ 10% in the affected arm between the pre-operative and 12- or 18-month visits, after controlling for percentage change in BMI; or 2) a diagnosis of lymphedema by a participant’s physician at any time following the post-operative assessment (up to 18 months post-surgery)26–29.
HRQL outcomes were assessed using the Functional Assessment of Cancer Therapy–Breast plus 4 lymphedema items (FACT-B+4)25. This 42-item scale is comprised of 6 subscales: physical, emotional, functional, and social well-being, other concerns related to breast cancer, and lymphedema symptoms (4 items). Individual scores are calculated for each of the subscales, as well as a total score comprised of all items across the 6 subscales. The subscale and total scores are transformed to a scale from 0–100, with higher scores indicating better HRQL/functioning. The FACT has demonstrated sensitivity to change over time and meets all requirements for use in oncology clinical trials, including ease of administration, brevity, reliability, and validity25.
Statistical Methods
In order to investigate the impact of the interventions on the participants’ quality of life over the 18-month study period, mixed-model regression analysis was used to examine differences in FACT scores by intervention group assignment (EO versus LEAP), adjusting for demographic variables (race, age, education) and clinical/treatment variables (Eastern Cooperative Oncology Group [ECOG] performance status [PS] at baseline, immediate reconstructive surgery, definitive primary surgery, number of positive lymph nodes, chemotherapy [yes/no], type of node surgery, and time since surgery). In addition, to assess the general impact of lymphedema status (yes/no) on HRQL at 18-months post-recruitment, linear regression analysis was used adjusting for the same demographic and clinical/treatment variables as listed above, as well as for intervention group assignment and baseline HRQL scores.
Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center (SDC). All analyses were completed on the study database frozen on April 30, 2016. Data quality was ensured by review of data by the Alliance SDC and by the study chairperson following Alliance policies. The trial was monitored at least twice annually by the Data and Safety Monitoring Board.
RESULTS
A total of 554 participants were enrolled in the main trial (56% in LEAP; 44% in the EO intervention). Main trial results indicated that there were no significant differences between the two intervention groups in preventing the occurrence of lymphedema. Lymphedema-free rates were 58% in the EO and 55% in LEAP (p=0.73). Kaplan-Meier estimates of 18-month lymphedema-free probabilities were also similar (84% EO vs. 81% LEAP) (Paskett et al., in press). The HRQL data were examined, however, in order to investigate whether there were any HRQL impacts (either positive or negative) to participating in the interventions that might inform the study results.
Of the 554 total participants, 547 completed at least the baseline FACT-B+4 and were included in the HRQL analyses. Participants’ demographic and clinical characteristics are provided in Table 1. On average, the women were 57.6 years old, non-Hispanic White, with approximately 75% having completed at least some college/training after high school. The majority of the women were either employed (52%), retired (24%) or homemakers (11%). There were several significant differences between the two study arms in several demographic factors. The LEAP participants had a higher level of educational attainment than women in the EO group (p=0.0321). However, the EO intervention had a higher proportion of African-American (p=0.0368) and Hispanic/Latina participants (p<0.0001) than the LEAP group. The higher proportion of Hispanic/Latina and African-American women was due primarily to one recruitment site that treated, and thus enrolled, primarily underrepresented minorities, which caused an imbalance in the racial/ethnic composition between the two intervention arms.
Table 1.
Baseline Demographic and Clinical Characteristics of Study Participants**
| Educational Only (N=238) |
LEAP (N=309) |
Total (N=547) |
p value | |
|---|---|---|---|---|
| Age | 0.40671 | |||
| N | 238 | 309 | 547 | |
| Mean (SD) | 58.0 (11.5) | 57.4 (11.2) | 57.6 (11.3) | |
| Range | (24.0–83.0) | (27.0–88.0) | (24.0–88.0) | |
| Race | 0.03682 | |||
| African-American | 37 (15.7%) | 30 (9.9%) | 67 (12.4%) | |
| Other (Asian, American Indian, Alaska Native, More than 1 race) | 6 (2.5%) | 17 (5.6%) | 23 (4.3%) | |
| White | 193 (81.8%) | 256 (84.5%) | 449 (83.3%) | |
| Ethnicity | <0.00012 | |||
| Hispanic or Latino | 39 (17.0%) | 10 (3.3%) | 49 (9.2%) | |
| Non-Hispanic | 190 (83.0%) | 293 (96.7%) | 483 (90.8%) | |
| Performance Status (PS) | 0.06202 | |||
| 0 | 193 (97.0%) | 236 (91.8%) | 429 (94.1%) | |
| 1 | 6 (3.0%) | 20 (7.8%) | 26 (5.7%) | |
| 2 | 0 (0.0%) | 1 (0.4%) | 1 (0.2%) | |
| Educational Background | 0.03212 | |||
| < HS | 19 (8.8%) | 11 (3.6%) | 30 (5.8%) | |
| HS Grad | 38 (17.7%) | 65 (21.3%) | 103 (19.8%) | |
| Some college/Jr College | 75 (34.9%) | 90 (29.5%) | 165 (31.7%) | |
| BA/BS College Degree | 32 (14.9%) | 64 (21.0%) | 96 (18.5%) | |
| > BA/BS College Degree | 51 (23.7%) | 75 (24.6%) | 126 (24.2%) | |
| Marital Status | 0.88222 | |||
| Married | 130 (61.0%) | 188 (62.0%) | 318 (61.6%) | |
| Separated/divorced/widowed | 65 (30.5%) | 93 (30.7%) | 158 (30.6%) | |
| Single/never married | 18 (8.5%) | 22 (7.3%) | 40 (7.8%) | |
| Employment Status | 0.72792 | |||
| Disabled | 16 (7.5%) | 19 (6.3%) | 35 (6.8%) | |
| Employed | 117 (54.9%) | 154 (50.7%) | 271 (52.4%) | |
| Homemaker | 19 (8.9%) | 38 (12.5%) | 57 (11.0%) | |
| Retired | 50 (23.5%) | 74 (24.3%) | 124 (24.0%) | |
| Student | 2 (0.9%) | 2 (0.7%) | 4 (0.8%) | |
| Unemployed | 9 (4.2%) | 17 (5.6%) | 26 (5.0%) | |
| Definitive primary surgery | 0.94762 | |||
| Partial mastectomy/lumpectomy/excisional biopsy | 152 (65.5%) | 200 (65.8%) | 352 (65.7%) | |
| Mastectomy, NOS | 80 (34.5%) | 104 (34.2%) | 184 (34.3%) | |
| Type of axillary lymph node dissection | 0.10582 | |||
| Axillary node dissection only | 61 (25.6%) | 63 (20.4%) | 124 (22.7%) | |
| Both Axillary node dissection and sentinel node biopsy | 74 (31.1%) | 87 (28.2%) | 161 (29.4%) | |
| Both missing | 1 (0.4%) | 0 (0.0%) | 1 (0.2%) | |
| Neither axillary node dissection nor sentinel node biopsy | 3 (1.3%) | 1 (0.3%) | 4 (0.7%) | |
| Sentinel node biopsy but axillary node dissection missing | 1 (0.4%) | 0 (0.0%) | 1 (0.2%) | |
| Sentinel node biopsy only | 98 (41.2%) | 158 (51.1%) | 256 (46.8%) | |
| Number of positive lymph nodes | 0.08371 | |||
| N | 217 | 295 | 512 | |
| Mean (SD) | 2.4 (5.2) | 2.1 (5.5) | 2.2 (5.3) | |
| Range | (0.0–41.0) | (0.0–60.0) | (0.0–60.0) | |
| Immediate reconstructive surgery | 0.00052 | |||
| No | 213 (91.0%) | 247 (80.2%) | 460 (84.9%) | |
| Yes | 21 (9.0%) | 61 (19.8%) | 82 (15.1%) | |
| Receptor status, ER | 0.38952 | |||
| Negative | 55 (23.2%) | 60 (19.4%) | 115 (21.1%) | |
| Positive | 182 (76.8%) | 248 (80.3%) | 430 (78.8%) | |
| Not Done | 0 (0.0%) | 1 (0.3%) | 1 (0.2%) | |
| Receptor status, PgR | 0.04792 | |||
| Negative | 89 (37.6%) | 87 (28.2%) | 176 (32.2%) | |
| Positive | 148 (62.4%) | 221 (71.5%) | 369 (67.6%) | |
| Not Done | 0 (0.0%) | 1 (0.3%) | 1 (0.2%) | |
| HER-2/neu receptors | 0.66192 | |||
| Negative | 193 (82.1%) | 246 (80.4%) | 439 (81.1%) | |
| Positive | 39 (16.6%) | 53 (17.3%) | 92 (17.0%) | |
| Not Done | 3 (1.3%) | 7 (2.3%) | 10 (1.8%) | |
| Pathologic primary tumor size | 0.91511 | |||
| N | 228 | 302 | 530 | |
| Mean (SD) | 2.2 (1.8) | 2.4 (3.9) | 2.3 (3.2) | |
| Range | (0.0–12.5) | (0.0–60.0) | (0.0–60.0) | |
| Grade | 0.16912 | |||
| Low | 55 (23.4%) | 65 (22.2%) | 120 (22.7%) | |
| Intermediate | 90 (38.3%) | 135 (46.1%) | 225 (42.6%) | |
| High | 90 (38.3%) | 93 (31.7%) | 183 (34.7%) | |
| Chemotherapy | 0.41492 | |||
| Missing | 4 (1.7%) | 4 (1.3%) | 8 (1.5%) | |
| No | 138 (58.2%) | 197 (63.8%) | 335 (61.4%) | |
| Yes | 95 (40.1%) | 108 (35.0%) | 203 (37.2%) | |
| Radiation prior to lymphedema diagnosis or within 18 months for those who were lymphedema-free | 0.85702 | |||
| 0 No | 73 (30.7%) | 97 (31.4%) | 170 (31.1%) | |
| 1 Yes | 165 (69.3%) | 212 (68.6%) | 377 (68.9%) | |
| Body Mass Index (BMI) | 0.96501 | |||
| N | 238 | 309 | 547 | |
| Mean (SD) | 28.4 (6.0) | 28.0 (5.4) | 28.2 (5.7) | |
| Range | (18.0–57.9) | (16.4–53.3) | (16.4–57.9) |
Not all participants answered all questions
Kruskal Wallis (unadjusted)
Chi-Square (unadjusted)
In terms of clinical characteristics, there were few significant differences between the EO and the LEAP participants, with the exceptions that more women in the LEAP intervention had immediate reconstructive surgery (19.8% vs. 9%, p=0.0005) and were PgR receptor status positive (71.5% vs. 62.4%, p=0.048) than the EO participants. Performance status (PS) was borderline significantly different between the two arms, with women in the EO group reporting a better PS than women in the LEAP group (p=0.062). Lastly, there were no significant differences at baseline between the intervention groups on the unadjusted FACT-B+4 total or subscale scores (Table 2).
Table 2.
Comparisons of the Unadjusted Baseline FACT-B+4 Total and Subscale Scores by Intervention Group
| Education Only (EO) Intervention Group (N=238) |
LEAP Intervention Group (N=309) |
Total of All Participants (N=547) |
p value (between intervention groups) |
|||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Month 18 | Baseline | Month 18 | Baseline | Month 18 | Baseline | Month18 | |
| Physical Subscale* | 0.1213 | 0.7682 | ||||||
| N | 212 | 174 | 291 | 215 | 503 | 389 | ||
| Mean (SD) | 90.1 (14.9) | 87.5 (13.9) | 89.2 (13.1) | 86.4 (15.4) | 89.6 (13.8) | 86.9 (14.7) | ||
| Median | 92.9 | 92.9 | 92.9 | 92.9 | 92.9 | 92.9 | ||
| Q1, Q3 | 85.7, 100 | 82.1, 96.4 | 85.7, 100 | 78.6, 96.4 | 85.7, 100 | 82.1, 96.4 | ||
| Range | (0.0–100) | (7.1–100) | (0.0–100) | (3.6–100) | (0.0–100) | (3.6–100) | ||
| Social Subscale | 0.5884 | 0.1614 | ||||||
| N | 233 | 177 | 308 | 218 | 541 | 395 | ||
| Mean (SD) | 85.7 (15.4) | 84.4 (17.8) | 84.6 (16.6) | 82.5 (17.7) | 85.1 (16.0) | 83.4 (17.7) | ||
| Median | 91.7 | 92.9 | 89.3 | 87.5 | 89.3 | 89.3 | ||
| Q1, Q3 | 75.0, 100 | 75.0, 100 | 75.0, 100 | 75.0, 100 | 75.0, 100 | 75.0, 100 | ||
| Range | (17.9–100) | (14.3–100) | (25.0–100) | (25.0–100) | (17.9–100) | (14.3–100) | ||
| Emotional Subscale | 0.8511 | 0.0253 | ||||||
| N | 225 | 174 | 297 | 212 | 522 | 386 | ||
| Mean (SD) | 73.8 (17.7) | 85.7 (14.3) | 73.7 (17.1) | 83.5 (13.6) | 73.7 (17.3) | 84.5 (13.9) | ||
| Median | 75.0 | 87.5 | 75.0 | 83.3 | 75.0 | 87.5 | ||
| Q1, Q3 | 62.5, 87.5 | 79.2, 95.8 | 62.5, 87.5 | 79.2, 91.7 | 62.5, 87.5 | 79.2, 95.8 | ||
| Range | (20.0–100) | (25.0–100) | (20.8–100) | (25.0–100) | (20.0–100) | (25.0–100) | ||
| Functional Subscale | 0.6581 | 0.2696 | ||||||
| N | 238 | 177 | 307 | 220 | 545 | 397 | ||
| Mean (SD) | 75.8 (20.2) | 82.1 (16.4) | 75.4 (19.3) | 79.9 (18.1) | 75.6 (19.6) | 80.9 (17.3) | ||
| Median | 78.6 | 85.7 | 78.6 | 82.1 | 78.6 | 82.1 | ||
| Q1, Q3 | 64.3, 92.9 | 71.4, 96.4 | 64.3, 92.9 | 67.9, 92.9 | 64.3, 92.9 | 71.4, 96.4 | ||
| Range | (10.7–100) | (29.2–100) | (20.0–100) | (21.4–100) | (10.7–100) | (21.4–100) | ||
| Fact G Total Score | 0.6061 | 0.1239 | ||||||
| N | 209 | 172 | 287 | 210 | 496 | 382 | ||
| Mean (SD) | 82.1 (12.9) | 85.1 (12.1) | 81.7 (12.4) | 83.2 (12.7) | 81.9 (12.6) | 84.0 (12.5) | ||
| Median | 84.3 | 87.0 | 83.7 | 84.3 | 84.3 | 86.1 | ||
| Q1, Q3 | 75.8, 91.0 | 77.9, 95.4 | 74.5, 91.7 | 76.9, 93.5 | 75.0, 91.7 | 76.9, 94.4 | ||
| Range | (29.3–100) | (36.1–100) | (30.6–100) | (38.9–100) | (29.3–100) | (36.1–100) | ||
| Additional Concerns Subscale | 0.9286 | 0.4768 | ||||||
| N | 222 | 176 | 294 | 219 | 516 | 395 | ||
| Mean (SD) | 67.7 (13.7) | 70.1 (12.1) | 67.7 (13.2) | 69.1 (12.2) | 67.7 (13.4) | 69.5 (12.2) | ||
| Median | 67.5 | 71.5 | 67.5 | 72.5 | 67.5 | 72.5 | ||
| Q1, Q3 | 58.3, 77.5 | 62.5, 77.5 | 60.0, 77.5 | 60.0, 77.5 | 60.0, 77.5 | 62.5, 77.5 | ||
| Range | (20.0–100) | (22.5–100) | (27.5–100) | (35.0–100) | (20.0–100) | (22.5–100) | ||
| Plus 4 Subscale | 0.2310 | 0.1303 | ||||||
| N | 200 | 175 | 278 | 217 | 478 | 392 | ||
| Mean (SD) | 95.3 (12.0) | 91.7 (13.2) | 94.7 (12.0) | 89.5 (15.7) | 94.9 (12.0) | 90.5 (14.7) | ||
| Median | 100.0 | 100.0 | 100.0 | 93.8 | 100.0 | 100.0 | ||
| Q1, Q3 | 100, 100 | 87.5, 100 | 93.8, 100 | 87.5, 100 | 93.8, 100 | 87.5, 100 | ||
| Range | (25.0–100) | (31.3–100) | (18.8–100) | (18.8–100) | (18.8–100) | (18.8–100) | ||
| FACT-B Total Score | 0.3284 | 0.1730 | ||||||
| N | 200 | 172 | 284 | 209 | 484 | 381 | ||
| Mean (SD) | 79.3 (9.8) | 81.0 (10.6) | 78.0 (11.0) | 79.5 (10.9) | 78.5 (10.5) | 80.2 (10.8) | ||
| Median | 79.6 | 83.0 | 79.3 | 80.4 | 79.5 | 81.8 | ||
| Q1, Q3 | 74.6, 87.2 | 74.3, 89.2 | 72.0, 86.2 | 74.3, 88.5 | 73.0, 86.5 | 74.3, 88.5 | ||
| Range | (46.8–100) | (35.1–97.7) | (38.5–98.6) | (40.5–97.3) | (38.5–100) | (35.1–97.7) | ||
| FACT-B+4 Total Score | 0.2962 | 0.1626 | ||||||
| N | 191 | 172 | 271 | 209 | 462 | 381 | ||
| Mean (SD) | 81.2 (9.0) | 82.0 (10.1) | 79.8 (10.3) | 80.5 (10.6) | 80.3 (9.8) | 81.2 (10.4) | ||
| Median | 81.5 | 83.8 | 81.1 | 81.4 | 81.4 | 82.3 | ||
| Q1, Q3 | 76.8, 88.2 | 75.9, 89.9 | 74.4, 87.2 | 75.6, 89.0 | 75.6, 87.7 | 75.6, 89.6 | ||
| Range | (44.7–100) | (41.5–98.0) | (38.4–98.8) | (38.4–97.6) | (38.4–100) | (38.4–98.0) | ||
Higher scores indicated better HRQL on the FACT-B+4 total and all subscale scores
Mixed model regression analyses indicated no significant differences by intervention group assignment on HRQL, as measured by the FACT-B+4 total score (p=0.8777) (Table 3). Worse HRQL over the 18 month study period was associated with an ECOG PS >0 (p=0.0002), being 6 months or less from breast cancer surgery (p=0.0001), ≥1 positive lymph node(s) (p=0.0009), no education beyond high school (p<0.0001), having had any chemotherapy (p=0.0242), having only axillary node dissection or sentinel node biopsy, as compared to both (p=0.0007), and younger age (p=<0.0001). In general, these factors associated with poorer quality of life for the FACT-B+4 total score, were mirrored in the subscale results (Appendix Table 3a). There was one notable exception, however, in the results for the lymphedema 4-item subscale. HRQL impacts for lymphedema symptoms were significantly worse for African-American women than non-Hispanic white participants or women of other race/ethnicities over the intervention study period (p=0.0002). However, African-American women reported better emotional functioning than non-Hispanic white or other racial groups (p=0.0335) during the 18 month study period.
Table 3.
Mixed Model Regression Analysis of the Impact of the Lymphedema Intervention Group, Clinical and Demographic Characteristics on Participants’ Health-Related Quality of Life (i.e., FACT-B+4 total score) Over the 18 Month Study Period
| Variable | Level | Estimate | Standard Error | P-Value | Overall P-Value |
|---|---|---|---|---|---|
| Lymphedema Intervention Group | LEAP | −0.2089 | 1.3476 | 0.8777 | 0.8777 |
| Education Only (EO) | Reference | ||||
| Immediate reconstructive surgery | Yes | −0.6987 | 0.9281 | 0.4595 | 0.4595 |
| No | Reference | ||||
| Definitive primary surgery | Mastectomy, NOS | 1.3136 | 0.7948 | 0.1088 | 0.1088 |
| Partial mastectomy/lumpectomy/ excisional biopsy | Reference | ||||
| Race | African-American | −0.2886 | 0.9659 | 0.7668 | 0.7220 |
| Other | 1.0089 | 1.4270 | 0.4842 | ||
| White | Reference | ||||
| ECOG Performance Status | 1 & 2 | −7.5739 | 1.3944 | 0.0002 | 0.0002 |
| 0 | Reference | ||||
| Follow-up period | Pre-Surgery | Reference | 0.0001 | ||
| 6 Months | −2.6163 | 0.7760 | 0.0011 | ||
| 12 Months | 0.6066 | 0.7972 | 0.4484 | ||
| 18 Months | 0.7700 | 0.8008 | 0.3385 | ||
| Number of positive lymph nodes | 1–3 | −2.6191 | 0.8867 | 0.0048 | 0.0009 |
| 4+ | −4.3304 | 1.1191 | 0.0003 | ||
| 0 | Reference | ||||
| Education | At least some college | 3.1241 | 0.7765 | 0.0002 | 0.0001 |
| Post College work/degree | 3.8446 | 0.8872 | <.0001 | ||
| HS Grad or less | Reference | ||||
| Chemotherapy | Yes | −1.7769 | 0.7440 | 0.0242 | 0.0242 |
| No | Reference | ||||
| Axillary Node Dissection | Axillary node dissection only | −2.1234 | 0.9360 | 0.0280 | 0.0007 |
| Sentinel node biopsy only | −3.9897 | 0.9768 | 0.0002 | ||
| Both Axillary node dissection and sentinel node biopsy | Reference | ||||
| Age | 1 more year | 0.1829 | 0.02942 | <.0001 | <.0001 |
To examine changes in the adjusted FACT-B+4 subscale and total scores during the trial, we plotted the change scores from pre-surgery to 18 months for each subscale, as well as for the total score (Figure 1). In general, there was some decline in quality of life domains from pre-surgery to 6 months, with some gradual improvement at 12 and 18 months. This pattern was seen particularly in the functional and additional concerns subscales, and the FACT-B+4 total score. However, it was notable that both the physical and the social functioning scores did not reach their pre-surgery levels by 18 months, indicating some lingering decrements in function. Similarly, lymphedema symptoms became prevalent at 6 months, with only some modest improvement in symptoms at 12 and 18 months post-surgery. What was striking, however, was the positive increase in emotional functioning during the course of the study, beginning at month 6 and continuing through the end of the trial.
Figure 1.
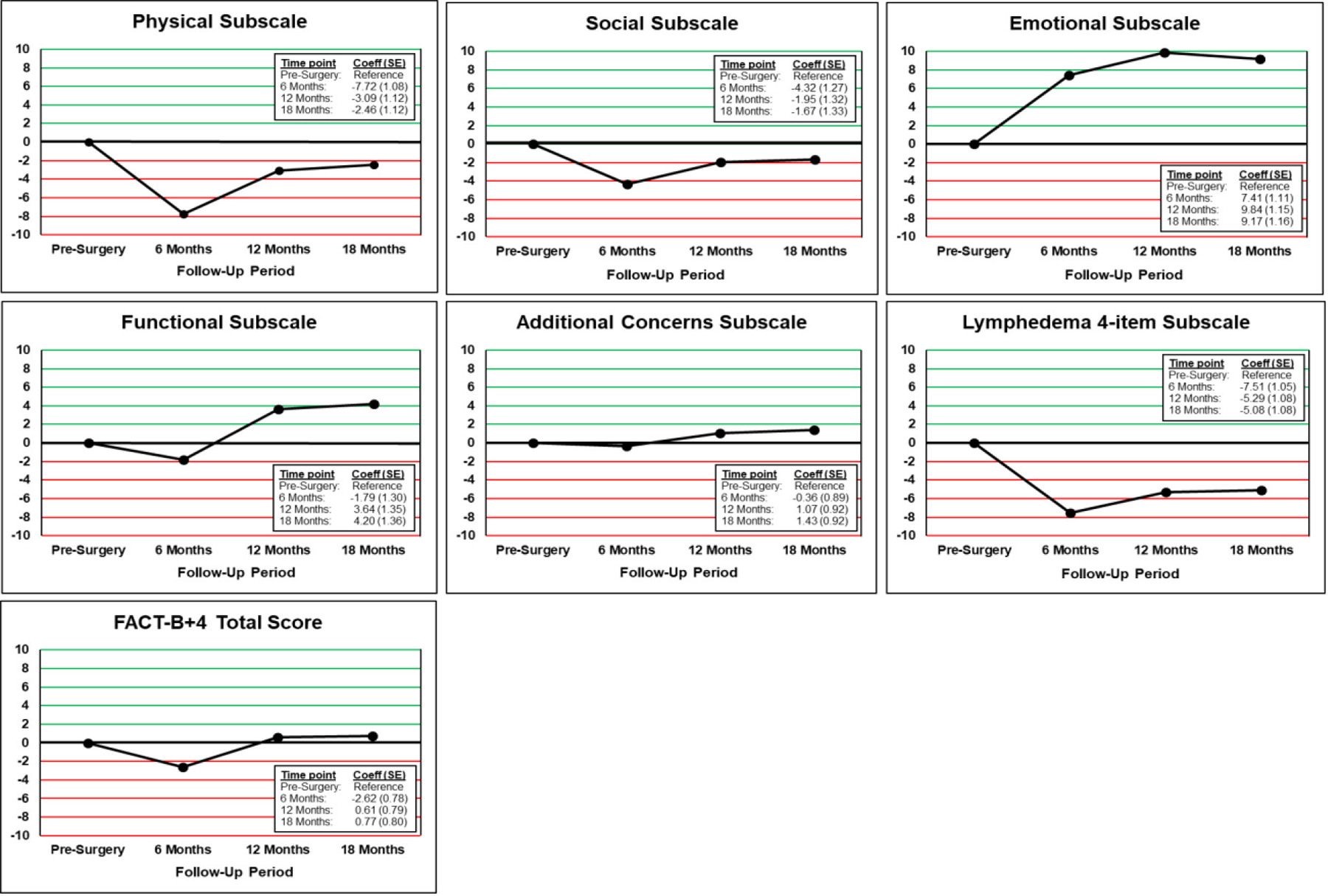
Plots of Changes in Adjusted FACT-B+4 Subscale and Total Scores from Baseline to 18 Months
Lastly, in our final analysis, we examined the impact of developing lymphedema (yes/no) by 18-months post-surgery on the FACT-B+4 total (Table 4) and subscales scores (Appendix Table 4a), adjusting for baseline scores. The presence of lymphedema was only found to be borderline significantly related to the FACT-B+4 total score (p=0.0825), and the functional well-being subscale (p=0.094) at 18 months post-surgery, trending toward worse HRQL among the participants with lymphedema.
Table 4.
Linear Regression Model of the Impact of the Presence of Lymphedema on Participants’ HRQL at 18 Months adjusted for Intervention Group, Demographic and Clinical Variables
| Variable | Level | Estimate | Standard Error | P-Value | Overall P-Value |
|---|---|---|---|---|---|
| Lymphedema Intervention Group | LEAP | −0.6161 | 1.1482 | 0.5921 | 0.5921 |
| Education Only (EO) | Reference | ||||
| Immediate reconstructive surgery | Yes | −1.8887 | 1.5627 | 0.2281 | 0.2281 |
| No | Reference | ||||
| Definitive primary surgery | Mastectomy, NOS | 1.1734 | 1.4323 | 0.4135 | 0.4135 |
| Partial mastectomy/lumpectomy/excisional biopsy | Reference | ||||
| Race | African-American | −2.0784 | 1.6039 | 0.1964 | 0.3549 |
| Other | 1.4292 | 2.8702 | 0.6190 | ||
| White | Reference | ||||
| ECOG Performance Status | 1 & 2 | −4.2272 | 2.3780 | 0.0768 | 0.0768 |
| 0 | Reference | ||||
| Number of positive lymph nodes | 1–3 | 0.8704 | 1.5469 | 0.5742 | 0.2989 |
| 4+ | −2.1654 | 2.1082 | 0.3055 | ||
| 0 | Reference | ||||
| Lymphedema | Yes | −2.3175 | 1.3288 | 0.0825 | 0.0825 |
| No | Reference | ||||
| Education | At least some college | 0.6585 | 1.3911 | 0.6364 | 0.4045 |
| Post College work/degree | 2.0368 | 1.5706 | 0.1961 | ||
| HS Grad or less | Reference | ||||
| Chemotherapy | Yes | −1.1679 | 1.3790 | 0.3980 | 0.3980 |
| No | Reference | ||||
| AND/SND Status | Axillary node dissection only | −0.4700 | 1.6435 | 0.7752 | 0.9123 |
| Sentinel node biopsy only | −0.6072 | 1.5351 | 0.6928 | ||
| Both Axillary node dissection and sentinel node biopsy | Reference | ||||
| Age | 1 more year | 0.04319 | 0.05546 | 0.4369 | 0.4369 |
| Baseline FACT-B+4 Total Score | 0.6572 | 0.05971 | <.0001 | <.0001 |
DISCUSSION
Lymphedema is an unwanted side effect of treatment for breast cancer. We examined the HRQL impacts of two lymphedema prevention interventions after breast cancer surgery. In the main trial, (Paskett et al., in press), lymphedema-free rates by 18 months were 58% vs 55% in the EO and LEAP groups, respectively. The LEAP intervention, which combined lymphedema education with daily exercise and the use of compression garments, was not found to be superior to the EO treatment arm. Low adherence to the LEAP intervention components may have been a factor in the lack of a significant difference between the two groups. Adherence to the LEAP exercises was approximately 50% overall, and 31% wore the elastic garments as prescribed. Primary reasons that the participants gave for not completing the daily prescribed exercises were lack of time (average of 45.9% across all exercises and time points), and low perceived benefit in completing the exercises (average of 19.2% across all exercises and time points). In addition, a study limitation was that exercise and sleeve use in the EO arm was not tracked in this study, so EO participants who engaged in these behaviors could not be accounted for in the analysis.
The results of the HRQL analyses were similar to the results of the main trial, and also indicated no significant differences in participants’ HRQL by intervention group. During the 18-month study period, lower FACT-B+4 total scores were related to common clinical, treatment and demographic variables. In addition, the presence of lymphedema symptoms by 18 months was only found to be borderline significantly related to the participants’ HRQL, suggesting that participants with lymphedema may not have been experiencing severe symptoms and/or had learned to manage these symptoms over the course of the study period using the EO intervention materials and information common to both study groups. It is also not known whether the EO participants used compression garments or completed any exercises on their own that may have assisted in reducing lymphedema symptoms, even if they did not prevent the occurrence of lymphedema.
As has been reported previously, worse HRQL in this study was associated with closer time to breast surgery and chemotherapy treatment12,17. We also observed that women with a higher number of positive lymph nodes and a higher ECOG PS were more likely to have lymphedema symptoms and worse HRQL, suggesting a relationship between lymphedema and higher stage disease. However, Beaulac et al (18) found that early-stage breast cancer patients with lymphedema reported lower FACT-B scores compared to women without lymphedema.
In this trial, higher education and older age were associated with better HRQL, comparable to other related research9,16, with the exception that younger age was not related to a worse global HRQL in research by Chachaj et al (14). Interestingly, we observed that women who had both axillary node dissection and sentinel node biopsy had higher HRQL than those who had only one procedure alone. This finding has not been reported previously in any HRQL study, and should be explored for possible reasons/mechanisms for this association. In addition, consistent with a systematic review by Pusic et al., our findings suggest that African-American women may have worse lymphedema symptoms compared to non-Hispanic White women16. Not only did these women in our study report greater lymphedema symptoms, they also reported better emotional functioning than non-Hispanic white or women in other racial groups. Examining lymphedema and HRQL in underrepresented minorities is under-studied and needs to be further explored to help improve the lives of these women following breast cancer surgery and treatment. Focusing on racial/ethnic minority women’s HRQL will be important in future lymphedema studies to design more effective prevention and symptom intervention studies.
In summary, a group-randomized trial to test the effectiveness of two interventions to prevent lymphedema in women newly diagnosed with breast cancer had no major HRQL impacts – either positively or negatively – by intervention group. HRQL in the study arms tended to mirror some previous findings of lymphedema among newly diagnosed breast cancer patients. However, two important findings are apparent. First, women who had both axillary node dissection and sentinel node biopsy had higher HRQL than those who had only one procedure alone. This finding has not been reported previously in any study. Secondly, we found that African-American women experienced more severe lymphedema symptoms, but reported better emotional functioning than women of other racial/ethnic groups. Both of these findings should be further explored to reduce the comorbidity associated with breast cancer surgery and treatment, and improve patient HRQL.
Supplementary Material
Acknowledgments
Support: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under the Award Number UG1CA189823 (Alliance for Clinical Trials in Oncology NCORP Grant), UG1CA189817, UG1CA189819, U10CA180790, U10CA180836, U10CA180850, and UL1TR001409, https://acknowledgments.alliancefound.org. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclaimers: None
ClinicalTrials.gov Identifier: NCT00376597
Conflict of Interest Statement: Dr. Loprinzi reports grants from NCI during the conduct of the study. Dr. Paskett reports grants from Merck Foundation and Pfizer during the conduct of the study.
Poster presentation at the International Society for Quality of Life Research (ISOQOL). October 26, 2018. Dublin, Ireland.
REFERENCES
- 1.DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysi. The Lancet Oncology. 2013;14(6):500–515. [DOI] [PubMed] [Google Scholar]
- 2.Armer JM, Stewart BR. Post-breast cancer lymphedema: incidence increases from 12 to 30 to 60 months. Lymphology. 2010;43(3):118–127. [PMC free article] [PubMed] [Google Scholar]
- 3.Shih YC, Xu Y, Cormier JN, et al. Incidence, treatment costs, and complications of lymphedema after breast cancer among women of working age: a 2-year follow-up study. J Clin Oncol. 2009;27(12):2007–2014. [DOI] [PubMed] [Google Scholar]
- 4.Shah C, Arthur D, Riutta J, Whitworth P, Vicini FA. Breast-cancer related lymphedema: a review of procedure-specific incidence rates, clinical assessment AIDS, treatment paradigms, and risk reduction. Breast J. 2012;18(4):357–361. [DOI] [PubMed] [Google Scholar]
- 5.Rockson SG. Update on the biology and treatment of lymphedema. Curr Treat Options Cardiovasc Med. 2012;14(2):184–192. [DOI] [PubMed] [Google Scholar]
- 6.Swenson KK, Nissen MJ, Leach JW, Post-White J. Case-control study to evaluate predictors of lymphedema after breast cancer surgery. Oncol Nurs Forum. 2009;36(2):185–193. [DOI] [PubMed] [Google Scholar]
- 7.Hidding JT, Beurskens CH, van der Wees PJ, van Laarhoven HW, Nijhuis-van der Sanden MW. Treatment related impairments in arm and shoulder in patients with breast cancer: a systematic review. PLoS One. 2014;9(5):e96748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wernicke AG, Goodman RL, Turner BC, et al. A 10-year follow-up of treatment outcomes in patients with early stage breast cancer and clinically negative axillary nodes treated with tangential breast irradiation following sentinel lymph node dissection or axillary clearance. Breast Cancer Res Treat. 2011;125(3):893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paskett ED, Naughton MJ, McCoy TP, Case LD, Abbott JM. The epidemiology of arm and hand swelling in premenopausal breast cancer survivors. Cancer Epidemiol Biomarkers Prev. 2007;16(4):775–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ribeiro Pereira ACP, Koifman RJ, Bergmann A. Incidence and risk factors of lymphedema after breast cancer treatment: 10 years of follow-up. Breast. 2017;36:67–73. [DOI] [PubMed] [Google Scholar]
- 11.Penha TR, Botter B, Heuts EM, Voogd AC, von Meyenfeldt MF, van der Hulst RR. Quality of Life in Patients with Breast Cancer-Related Lymphedema and Reconstructive Breast Surgery. J Reconstr Microsurg. 2016;32(6):484–490. [DOI] [PubMed] [Google Scholar]
- 12.Kibar S, Dalyan Aras M, Unsal Delialioglu S. The risk factors and prevalence of upper extremity impairments and an analysis of effects of lymphoedema and other impairments on the quality of life of breast cancer patients. Eur J Cancer Care (Engl). 2017;26(4). [DOI] [PubMed] [Google Scholar]
- 13.Oliveri JM, Day JM, Alfano CM, et al. Arm/hand swelling and perceived functioning among breast cancer survivors 12 years post-diagnosis: CALGB 79804. J Cancer Surviv. 2008;2(4):233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chachaj A, Malyszczak K, Pyszel K, et al. Physical and psychological impairments of women with upper limb lymphedema following breast cancer treatment. Psychooncology. 2010;19(3):299–305. [DOI] [PubMed] [Google Scholar]
- 15.Ahmed RL, Prizment A, Lazovich D, Schmitz KH, Folsom AR. Lymphedema and quality of life in breast cancer survivors: the Iowa Women’s Health Study. J Clin Oncol. 2008;26(35):5689–5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pusic AL, Cemal Y, Albornoz C, et al. Quality of life among breast cancer patients with lymphedema: a systematic review of patient-reported outcome instruments and outcomes. J Cancer Surviv. 2013;7(1):83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee SH, Min YS, Park HY, Jung TD. Health-related quality of life in breast cancer patients with lymphedema who survived more than one year after surgery. J Breast Cancer. 2012;15(4):449–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beaulac SM, McNair LA, Scott TE, LaMorte WW, Kavanah MT. Lymphedema and quality of life in survivors of early-stage breast cancer. Archives Of Surgery (Chicago, Ill: 1960). 2002;137(11):1253–1257. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Brown JC, Paskett ED, Zemel BS, Cheville AL, Schmitz KH. Changes in arm tissue composition with slowly progressive weight-lifting among women with breast cancer-related lymphedema. Breast Cancer Res Treat. 2017;164(1):79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winters-Stone KM, Laudermilk M, Woo K, Brown JC, Schmitz KH. Influence of weight training on skeletal health of breast cancer survivors with or at risk for breast cancer-related lymphedema. J Cancer Surviv. 2014;8(2):260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johansson K, Hayes S, Speck RM, Schmitz KH. Water-based exercise for patients with chronic arm lymphedema: a randomized controlled pilot trial. Am J Phys Med Rehabil. 2013;92(4):312–319. [DOI] [PubMed] [Google Scholar]
- 22.Sener HO, Malkoc M, Ergin G, Karadibak D, Yavuzsen T. Effects of Clinical Pilates Exercises on Patients Developing Lymphedema after Breast Cancer Treatment: A Randomized Clinical Trial. J Breast Health (2013). 2017;13(1):16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buchan J, Janda M, Box R, Schmitz K, Hayes S. A Randomized Trial on the Effect of Exercise Mode on Breast Cancer-Related Lymphedema. Med Sci Sports Exerc. 2016;48(10):1866–1874. [DOI] [PubMed] [Google Scholar]
- 24.Lorig K, Stewart A, Ritter P, Gonzalez v, Laurent D, Lynch J. Outcome Measures for Health Education and Other Health Care Interventions. Thousand Oaks, CA: Sage; 1996. [Google Scholar]
- 25.Coster S, Poole K, Fallowfield LJ. The validation of a quality of life scale to assess the impact of arm morbidity in breast cancer patients post-operatively. Breast Cancer Res Treat. 2001;68(3):273–282. [DOI] [PubMed] [Google Scholar]
- 26.Mahamaneerat WK, Shyu CR, Stewart BR, Armer JM. Breast cancer treatment, BMI, post-op swelling/lymphoedema. J Lymphoedema. 2008;3(2):38–44. [PMC free article] [PubMed] [Google Scholar]
- 27.Ferguson CM, Swaroop MN, Horick N, et al. Impact of Ipsilateral Blood Draws, Injections, Blood Pressure Measurements, and Air Travel on the Risk of Lymphedema for Patients Treated for Breast Cancer. J Clin Oncol. 2016;34(7):691–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casley-Smith JR. Measuring and representing peripheral oedema and its alterations. Lymphology. 1994;27(2):56–70. [PubMed] [Google Scholar]
- 29.Loprinzi CL, Kugler JW, Sloan JA, et al. Lack of effect of coumarin in women with lymphedema after treatment for breast cancer. N Engl J Med. 1999;340(5):346–350. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


