Abstract
Precision medicine aims to better individualize healthcare. It requires that biomaterials be designed for the physiological characteristics of a specific patient. To make this a reality, biomaterials research and development must address differences of biological sex. More specifically, biomaterials should be designed with properties optimized and appropriate for male and female patients. In analyzing research articles from seven prominent biomaterials journals, sex as a biological variable is missing from an overwhelming majority of in vitro biomaterial studies. From the survey, the reporting of the sex of primary cell cultures happened only 10.3% of the time. Contributing to this trend is that commercial vendors bias cell lines toward one sex or another by not disclosing information of cell line sex at the time of purchase; researchers do not communicate this pertinent information in published studies; and many journal policies have little to no requirements for reporting cell line characteristics. Omitting this valuable information leads to a gap in the understanding of sex-specific cell-biomaterial interactions and it creates a bias in research findings towards one sex or another. To curb this concerning trend and make precision biomaterials a reality will require the biomaterials field to “talk about sex” by reporting cell sex more broadly.
Keywords: sex differences, precision biomaterials, hormones, patient-specific, cell-material interactions
Graphical Abstract
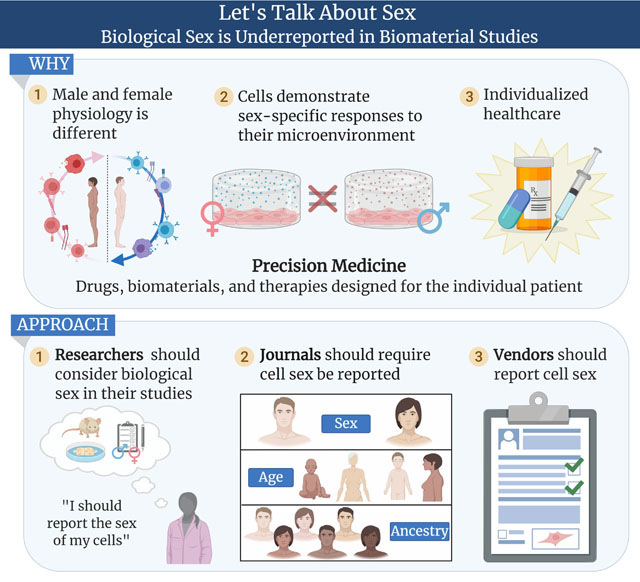
Since 1999, sex has been recognized as a significant biological variable in biomedical research; yet, 20+ years later it remains unreported in much of the biomaterials literature. Opportunities exist for investigators, cell vendors, and journals to reverse this trend. To make precision biomaterials a reality will require the biomaterials research community to actively address this challenge by reporting cell sex more broadly.
1. Introduction
Over the last two decades material properties have increasingly been recognized as stimuli capable of controlling cell fate. In no uncertain terms cells have been found to respond to a multitude of material properties including a material’s mechanics, architecture, and surface topography.[1–9] To take advantage of this additional mechanism for directing cell fate, materials engineers have pursued creating material systems resembling the extracellular matrix (ECM).[10–13] But we ask, who’s ECM? Because ECM differs in males and females. For both healthy and diseased tissue, it diverges in composition, structure, mechanics, and remodeling behavior.[14–23] Have we missed designing our biomaterials for males or females?
Biologically, humans are different from one another because of their genetics, environment, and/or lifestyle. Biological sex is one of the most impactful manifestations of these differences. The National Institutes of Health Office of Research on Women’s Health defines “sex” as the biological differences between females and males related to chromosomes, organs, and endogenous hormone profiles.[24] It is important to clarify that this is different from “gender”, which is a social construct assumed from historical and cultural customs across space and time. Nonetheless in medicine, sex and gender interact to both contribute to health and disease.[25]
In the clinic
The differences between females and males manifest as physiological differences, which have major implications for health, disease, medicine and of course biomaterials.[26] From the perspective of signaling, male and female hormone profiles are different.[27] Sex hormone levels in males gradually decrease after adulthood whereas sex hormone levels in females drastically drop later in life at menopause.[27] Many diseases can occur and progress due to changes in a patient’s endocrinology such as with osteoporosis, which afflicts females more than males. This is largely in part to the significant drop of endogenous estrogen seen in females after menopause.[28]. From an immunology perspective, male and female immune systems develop differently.[29] For instance, T cells activate and proliferate more in females than in males.[29] While at the same time this protective feature of the female immune system acts as a double-edged sword. 80% of autoimmune diseases occur in females.[29] In the context of cardiology, a troubling statistic is that cardiovascular disease is the number one killer of both males and females. Yet, males suffer from cardiovascular disease throughout their lifetimes, while females show a significant prevalence for the disease during and after menopause.[30–34] For an oncologist, treating cancer can be a matter of sex. Different cancers present at different frequencies in males and females and cancers use different metabolic pathways between the sexes.[35] These pathophysiological differences then translate into treatment outcome disparities. Vaccine efficacy differs between the sexes.[29] Females show more adverse reactions to vaccines and have shown equivalent antibody titers when immunized with half a vaccination dose compared to males receiving a full vaccination dose.[29] Organ transplants from male donors are more successful than those from female donors.[36] Drug and cell therapy potencies and responses are different for male and female recipients.[37–39] This case is illustrated by the fact that at one point, 8 separate drugs had to be removed from the marketplace because they led to significantly adverse effects in females.[40] This list of important biological reasons to consider sex as a variable continues to grow reinforcing that sex differences touch on every facet of medicine. The most recent illustration of this has been with COVID-19. Strong evidence shows, across all ages, males are more seriously afflicted by the disease than females.[41] Collectively, these outcomes start to beg the questions: How can biomaterials sequester or release chemical factors at relevant, sex-specific dosages? What needs to change (if anything) for a biomaterial to take advantage/suffer from these immunological differences?? How should biomaterials be designed for male and female tissue?
At the bench
While work is underway to understand the epidemiology of disease and disease treatment, sex-based differences manifest as observable differences at the bench, as well. In vitro, male and female cells will respond differently to biological, chemical, and mechanical stimuli used by biomaterial researchers. Common metrics used to evaluate biomaterial performance such as cell proliferation, cell morphometrics, cell migration, and cell response to stressors are different for male and female cells.[42–47] For instance, sex-based and age-based differences in male and female primary osteoblasts translate into functional differences in cell behavior at the bench that influence cell-biomaterial interactions.[48] Osteoblast response to estrogen has been shown to be both sex-dependent and surface property-dependent.[49,50] Similar differences have been observed for endothelial cells as a function of sex and tissue source.[42] Mice show sex-dependent in vivo toxicity to gold nanoparticles and sex-dependent blood-brain barrier permeability to polystyrene nanoparticles and macromolecular tracers.[51,52] These findings highlight one area (nanoparticles) in which the response to a material is sex-dependent and such differences likely exist more broadly. Important to tissue engineering and regenerative medicine, pluripotent stem cell differentiation efficiency varies with cell sex such that the differentiation strategy needs to be designed for both male and female cells.[53] In the context of mechanobiology, male and female cells respond differently to the same mechanical stimulation (e.g. fluid shear stress).[42,54] For instance, this has been observed for endothelial cells exposed to laminar fluid shear stress resulting in a 1000+ difference in gene regulation between male and female cells upon stimulation.[54] Clearly, biological sex impacts pre-clinical studies. Placed within the design framework of tissue engineering, the cells are different, the signals are different, and the matrices are different for males and females. So, we need to start asking ourselves: How should scaffolds be designed for the differences in the composition of male and female tissue? What aspects of male and female physiology need to be captured for in vitro models? What new design considerations need to be included in biomaterial research to account for sex differences?
In the weeds of the biology
In this section we highlight some of the main genetic mechanisms owing to sex differences. First and foremost, males and females are different chromosomally. Females have two X chromosomes while males have a single X and a single Y chromosome. Because it is thought that only one X chromosome is necessary for biological function, to equalize the genetic dosage contributed by the X chromosome between females and males, female cells undergo X inactivation. This means that one of the two X chromosomes in female cells is randomly silenced. Inactivation takes place during the early stages of development and continues for all subsequent cell divisions.[55] However; despite, inactivation some genes can escape (~15%); a process that is variable among individuals and their cells.[56] The implication of X inactivation is that female cells exhibit mosaicism in the use of their X chromosomes i.e. female tissue is composed of two populations of cells distinct in their active X chromosome. Clinically, this often manifests as a protection for females against many X-linked genetic diseases as compared to males.[57,58] This is because male cells have only one copy of the X chromosome such that if their X chromosome carries a mutation it is always expressed. Conversely, only male cells have a Y chromosome, which has primarily been thought to be for sex determination.[59] However, recent evidence suggests many of the genes coded by the Y chromosome are involved in cancer, cardiovascular disease, and Parkinson’s disease, among others.[59–63] In fact, since the 1960s it has been known that male cells; namely, in leukocytes, exhibit a loss of the Y chromosome with age and smoking.[64–68] Loss of the Y chromosome in somatic cells leads to male cell mosaicism and has been implicated with increased risk of pathogenicity and mortality.[59] For both male and female somatic cells, their sex chromosomes contribute a great deal to their expressed phenotypes.
In pursuit of precision medicine
With the ever growing body of research on sex differences, to consolidate the field’s knowledge, in November 1999, the Institute of Medicine (now the U.S. National Academy of Medicine) formed a committee for “Understanding the Biology of Sex and Gender Differences,” that produced the final report Exploring the Biological Contributions to Human Health. The report compiled our understanding of sex and gender in human health and enumerated recommendations for the biomedical research enterprise to adopt for studying sex as a biological variable.[69] Many of the ideas expressed in the previous paragraphs were highlighted in the report. Specifically, the tenth recommendation of the report stated,
“Determine and disclose the sex of origin of biological research materials. The origins and sex chromosome constitutions of cells or tissue cultures used for cell biological, molecular biological, or biochemical experiments should be stated when they are known. Attempts should be made to discern the sex of origin when it is unknown. Journal editors should encourage inclusion of such information in Materials and Methods sections as standard practice.
(The committee acknowledges that inclusion of people, animals, or cells and tissues of or from both sexes in all studies is not always feasible or appropriate. Rather, the committee is urging researchers to regard sex, that is, being male or female, as an important basic human variable that should be considered when designing, analyzing, and reporting findings from studies in all areas and at all levels of biomedical research. Determining and disclosing the sex of origin of biological research materials are important steps in that direction.)”
Not reporting this information can lead to non-reproducible experiments, data that is not translatable to the unincluded sex, advancements in technology that benefit one sex over the other, and/or pharmaceuticals that illicit sex-specific side effects.[25,38,70,71]
To start addressing these challenges facing biomedical research, in 2015 the NIH changed its policy requiring all NIH-funded pre-clinical studies to include sex as a biological variable.[72] This policy change paralleled the growing pursuit for precision medicine fueled by our knowledge of sex-based differences in the clinic and at the bench. Precision medicine looks to cast aside a “one-size fits all” approach to disease treatment and prevention in exchange for an individualized approach that considers a patient’s genetic, environmental, and lifestyle variability. Formally, in 2015 precision medicine was initiated by former President Barack Obama and is championed by the ongoing National Institutes of Health (NIH) All of Us program. In essence it aims to individualize healthcare and improve our understanding of sex-based differences in medicine, which has historically been underrepresented.[26,70,73,74] To accomplish the goals of precision medicine requires precision biomaterials–biomaterials designed with the biology of the individual patient in mind for eliciting desired and predictable outcomes.[75–84] This includes considering a patient’s sex, age, and ancestry in developing a precision biomaterial.
Now some 20 years after the committee convened, in light of the push for precision medicine, the NIH policy change, and the increasing appreciation for sex differences in the literature, we asked, “Are biomaterials scientists and engineers following recommendation #10 outlined in the Institute of Medicine report? Do they know the sex of the cells used for their in vitro studies? Is the sex of the cells being reported? And are they investigating sex-based differences in cell-biomaterial interactions?” In writing this essay and answering these questions we hope to compel the field to include sex as a biological variable and more importantly to always report the sex of their cells.
2. Biological Sex is Underreported in Biomaterial Studies
We took a snapshot of the current biomaterials literature by surveying the articles published during December 2019 in 7 prominent biomaterials journals: ACS Biomaterials Science & Engineering, Acta Biomaterialia, Biomaterials, Biomaterials Science, Journal of Biomedical Materials Part A, Macromolecular Bioscience, and Advanced Healthcare Materials. We reviewed the main text and supplementary files of all communications and full articles published (Supplementary Information), which totaled to 303 journal articles that described in vitro cell culture experiments (Table 1). To be clear, the studies surveyed were not necessarily focused on reporting sex-based differences (in fact we found none focused on this). All studies that reported the use of any cells were included in the survey regardless of the nature of the biomaterial study.
Table 1.
Breakdown of articles published by each journal surveyed
| Journal | Journal Abbreviation | Number of Articles |
|---|---|---|
| ACS Biomaterials Science & Engineering | ACS Biomater. Sci. Eng. | 43 |
| Acta Biomaterialia | Acta Biomater. | 38 |
| Advanced Healthcare Materials | Adv. Heathcare Mater. | 25 |
| Biomaterials | Biomaterials | 90 |
| Biomaterials Science | Biomater. Sci. | 78 |
| Journal of Biomedical Materials Research Part A | J. Biomed. Mater. Res., Part A | 18 |
| Macromolecular Bioscience | Macromol. Biosci. | 11 |
For every cellular assay reported to be used in the study, we recorded its: cell source (commercial, harvested/isolated, unknown), commercial vendor or supplier, the cell type, sex of the cells, and if the cells were transformed, immortalized, or established (TIE) cell lines or primary cell lines. After the fact, we investigated the TIE cell lines for their sex, tissue, and species. This information was collected from cell line product pages available from American Type Culture Collection (ATCC) and/or the Cellosaurus database.[85] For our survey, in the category of cell sex we recorded whether the cells used were male, female, or if the sex went unreported. For those articles that did not report the sex of the cells, we further noted that a large fraction (27%) of those did not provide enough information for the reader of the article to look up the sex information on either the vendor’s product page or a cell resource such as Cellosaurus. This later observation highlights the importance of researchers providing adequate information so that readers interested in sex-based differences have context even when sex-based differences were not the direct focus of the published work.
From the 303 articles we surveyed, there were 352 different cell cultures (Figure 1). 11 cell culture studies (3.7%) reported the sex of the cells used in the study, of which 4 were reported as male and 7 were reported as female. Meaning that 96.3% of the cell culture studies did not report the sex of the cells used in the study. Of those 11 cultures, sex was not considered as a variable; it was only reported. Only 1 of the 303 articles reported using both male and female cells. However, there was no reference to the sex of the cells in the author’s analyses of their data. After removing the transformed, immortalized, or established (TIE) cell lines from the total number of different cell cultures the statistic did not greatly improve–89.7% of primary cell cultures used in studies did not report the sex of the cells. In effect, this suggests that there remains a tremendous opportunity and need in the field to study the sex differences of cell-biomaterial interactions.
Figure 1.
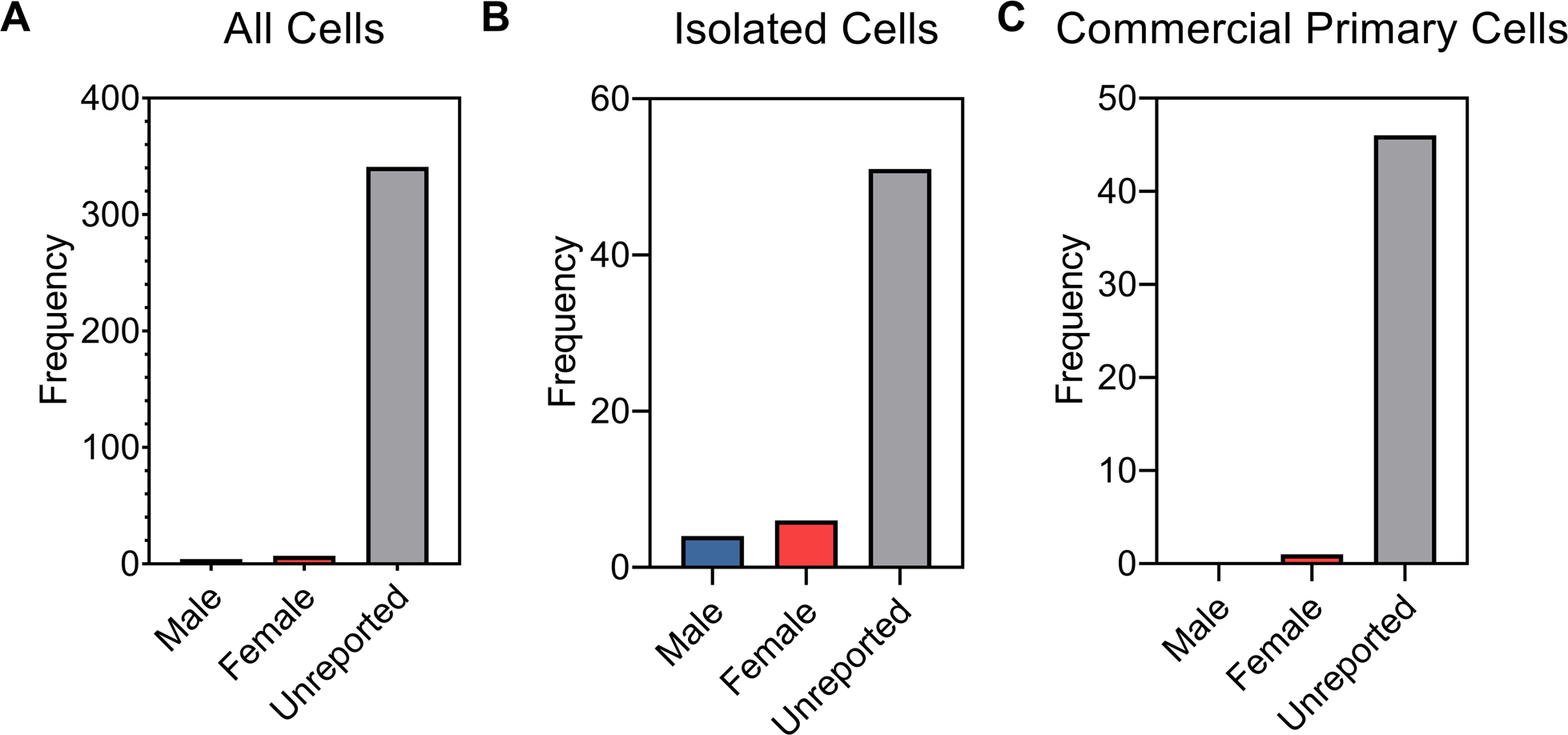
Breakdown of cell sex for all the cell cultures reported (A); for all primary cells isolated or harvested “in-house” (B); and for all primary cells purchased from commercial vendors (C). For our survey, we recorded whether the cells used were reported as male, female, or if the sex was unreported.
Given the growing evidence demonstrating that male and female cells respond differently in culture, this is a concerning outcome. Like our findings, a 2017 analysis of 40 non-reproductive organ-on-a-chip technologies reported that 62% did not specify sex and that of those that did specify sex they were predominantly male. In response to these findings, the authors of the review stated, “These data highlight that it is of upmost importance for the organ‐on‐a‐chip field moving forward to at least report the sex of utilized tissues and cells as well as to use sex‐balanced systems when studying drug effects and disease mechanisms.”[86] The author’s commentary echoes our own and those of the U.S. National Academy of Medicine’s some 18 years earlier. Though this trend of underreporting is not exclusive to the biomaterials community. To a slightly lesser degree it has also been seen in leading cardiovascular journals, whose articles did not report the sex of cells 72–80% of the time.[31,87] Overall, similar findings have been reported across the span of biomedical and biological research.[36,88–91] Though our survey is a snapshot of a single month, and only includes 7 journals, it is likely consistent across the field. Indeed, few investigators report the sex of the cells under investigation or any of the other demographic information relevant to developing precision biomaterials. This is not to say that all studies must include sex as a biological variable. It may not be appropriate for all studies; but rather, it should be reported in all cases to provide context to the data being presented. Through consistent reporting of cell sex, advancements can be made in developing biomaterial technologies that address sex-based health disparities in disease onset, progression, and treatment. With the goal to understand why sex is missing in biomaterials research, raise awareness for its importance, and share opportunities that exist to change this trend, we took a deeper look at the source of cells used in research, the challenge investigators face, and the guidelines provided by journals.
3. Let’s Start Talking– Opportunities to Reverse This Trend
3.1. Cell Vendors
We start our discussion with the origin of the cells used in biomaterial studies, either commercially available primary cells, commercially available cell lines, or primary isolated or harvested cells. For all cell lines, commercial vendors provide certificates of analysis. This document should include basic demographic information such as sex, age, and ancestry for single donor primary cells. Likewise, demographic information for “in-house” lab-specific harvested or isolated primary cells is either unrestricted for animal-derived cells or codified for human-derived cells following institutional review board procedures. Surprisingly, our results indicate that in both instances, whether the primary cell lines are purchased or isolated “in-house”, the reporting of cell sex was near zero (Figure 1). We identified only 10 cell culture studies from the pool of 352 that reported the sex of their isolated primary cell lines. In contrast, we identified only 1 cell culture study that reported the sex of their commercially sourced primary cell lines. Overall, reporting the sex of cells goes largely unreported for both purchased and isolated primary cell lines.
Cell vendors can play a role in reversing this trend by providing sex information on their cells at the time of purchase. Many vendor websites organize their products by cell type without readily providing lot-specific information for each cell line of a given cell type. For some vendors, in addition to lot information, the sex of the cells and other demographic information is not readily available, but rather available upon request. While making a request for this information is not inhibitory on its own, the need for taking the additional step to get it reinforces the notion that sex as a biological variable in cell culture is still largely missing. In our work to study sex-based differences in the mechanoresponsiveness of vascular cells, we sought to purchase both male and female vascular cells. We found that there was a significant bias towards one sex or the other for vascular cells from two vendors, depending on the vascular cell type and vendor, either Lifeline Cell Technologies or Cell Applications, respectively (Figure 2).
Figure 2.
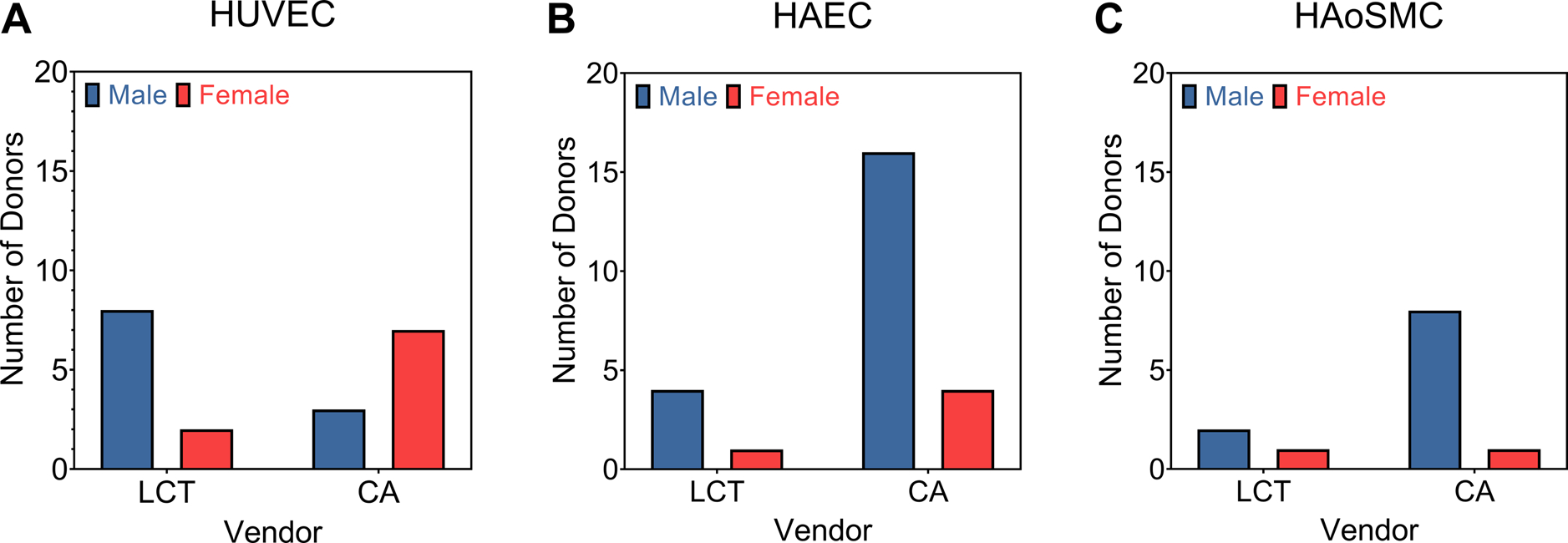
The comparison of the number of male and female primary cell lines (A) HUVEC = Human Umbilical Vein Endothelial Cell; (B) HAEC = Human Aortic Endothelial Cell; and (C) HAoSMC = Human Aortic Smooth Muscle Cells available from two commercial vendors for three vascular-derived cell types. LCT = Lifeline Cell Technologies. CA = Cell Applications. This is a snapshot from August 2019.
When investigators purchase cells from some commercial vendors and a specific sex is not requested, additional challenges exist. For instance, our data suggests that when purchasing from Cell Applications in August 2019 if a specific cell sex was not requested, there was a ~75% chance of receiving a vial of male human aortic endothelial cells compared to a vial of female human aortic endothelial cells. This means that there is an inadvertent bias in studies when cell sex is not considered as a biological variable, and that bias may be cell type specific and/or vendor specific. Moreover, because the information on cell sex then goes unreported almost exclusively in published work there is no way for the biomaterials community to know if the advancements we are making are biased towards one sex or the other.
For studies whose focus is on studying sex differences additional challenges exist in obtaining enough cell populations with which to complete a statistically robust study. A follow-up several months later with Cell Applications revealed continual bias toward one sex or the other depending on the cell type. More importantly, the number of separate cell populations was in the single digits with some cell types having only 1 population of a specific sex (Figure 3). Similar biases and insufficient descriptions of cell sex have been reported for the cell banks American Type Culture Collection (ATCC), European Collection of Cell Cultures (ECACC), and Japanese Collection of Research Bioresources (JCRB).[92] In the case that the sex of a cell line is not provided on its certificate of analysis, it can be recovered by subsequent confirmation with the vendor. However, internal verification of each cell line’s sex is recommended and can be determined by polymerase chain reaction (PCR) for sex-specific genes.[54,87] Overall, vendors can help to increase the reporting of cell sex by disclosing cell donor information such as sex, age, and ancestry at the time of sale.
Figure 3.
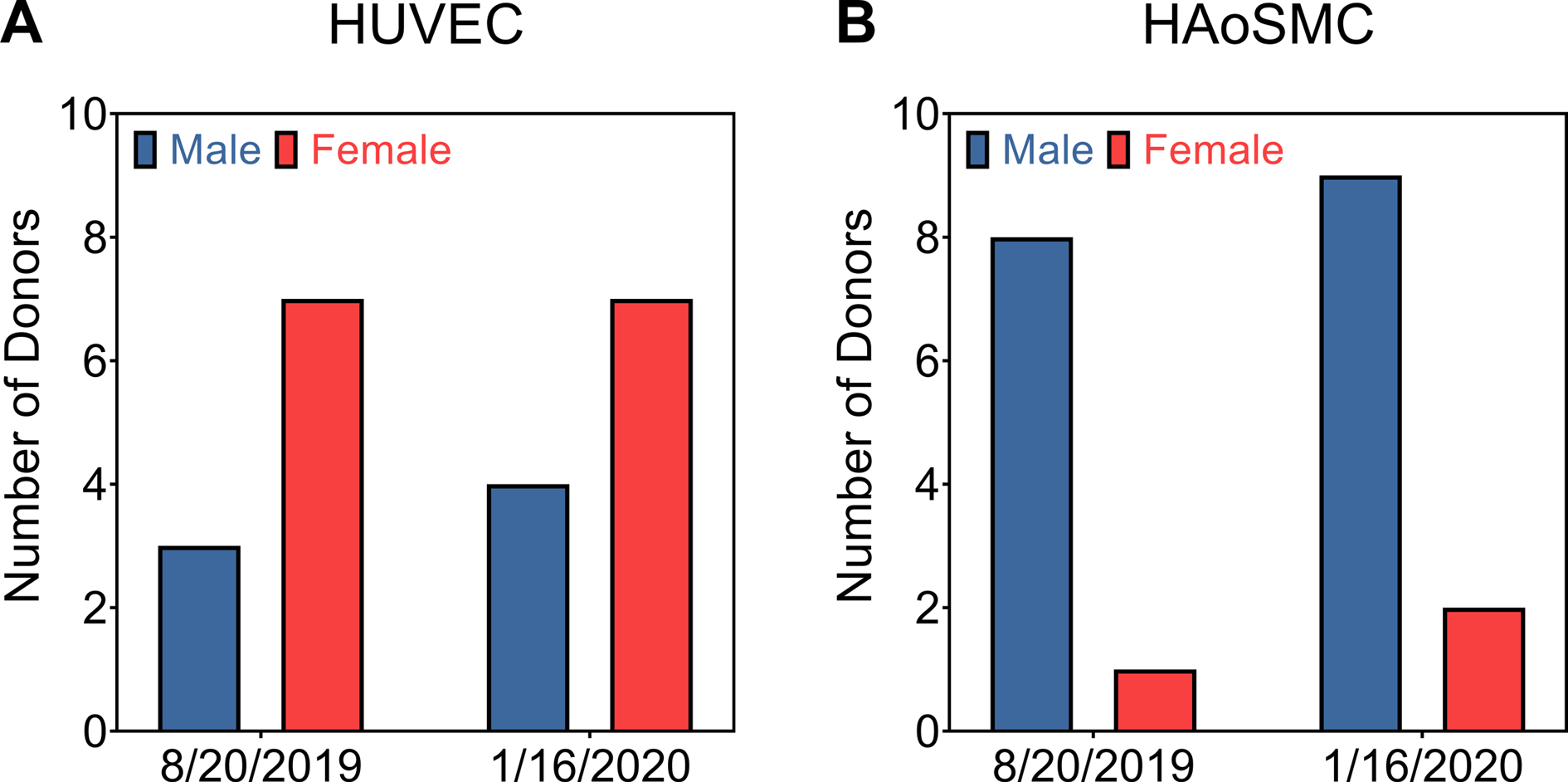
Comparison of the number of male and female primary cell lines (A) HUVEC = Human Umbilical Vein Endothelial Cell; and (B) HAoSMC = Human Aortic Smooth Muscle Cells available from Cell Applications at two separate times of year.
3.2. Investigators and Cell Lines
We briefly explored the lack of cell sex reporting by researchers in the field of biomaterials (Figure 1). Many of the cells used by for the studies included in our survey were transformed, immortalized, or established (TIE) cell lines. These cells almost always have their sex recorded in the Cellosaurus database. Because of this, we further investigated the heavy use of TIE cell lines. We found that there is a bias in biomaterials research towards one sex or another for a given tissue when using TIE cell lines. As an example of this bias, consider the existing collection of TIE cell lines catalogued. Performing a simple search within the Cellosaurus database (Version 34, March 2020) using the search phrases, “male Homo sapiens,” “female Homo sapiens,” “male Mus musculus,” and “female Mus musculus” provided 42,456 instances of male human cell lines, 38,029 instances of female human cell lines, 12,193 instances of male mouse cell lines, and 1,026 instances of female mouse cell lines, respectively. The results of this search provide evidence of a slight bias towards male human cell lines, but a very significant bias towards male mouse cell lines used by the research community. Among the 303 articles analyzed in our survey, TIE cell lines were the most prevalent class of cell used (222 instances of distinct cultures of the total 352 cultures). From our findings, it appeared that male and female TIE cell lines were used at the same frequency (Figure 4). However, this is an artifact of sex-specific tissues (female breasts, ovaries, cervix, male prostates, etc.) in that they can only have cell lines derived from either male or female specific tissues. When comparing across non-specific tissues, there was a significant bias towards male cells (Figure 4).
Figure 4.
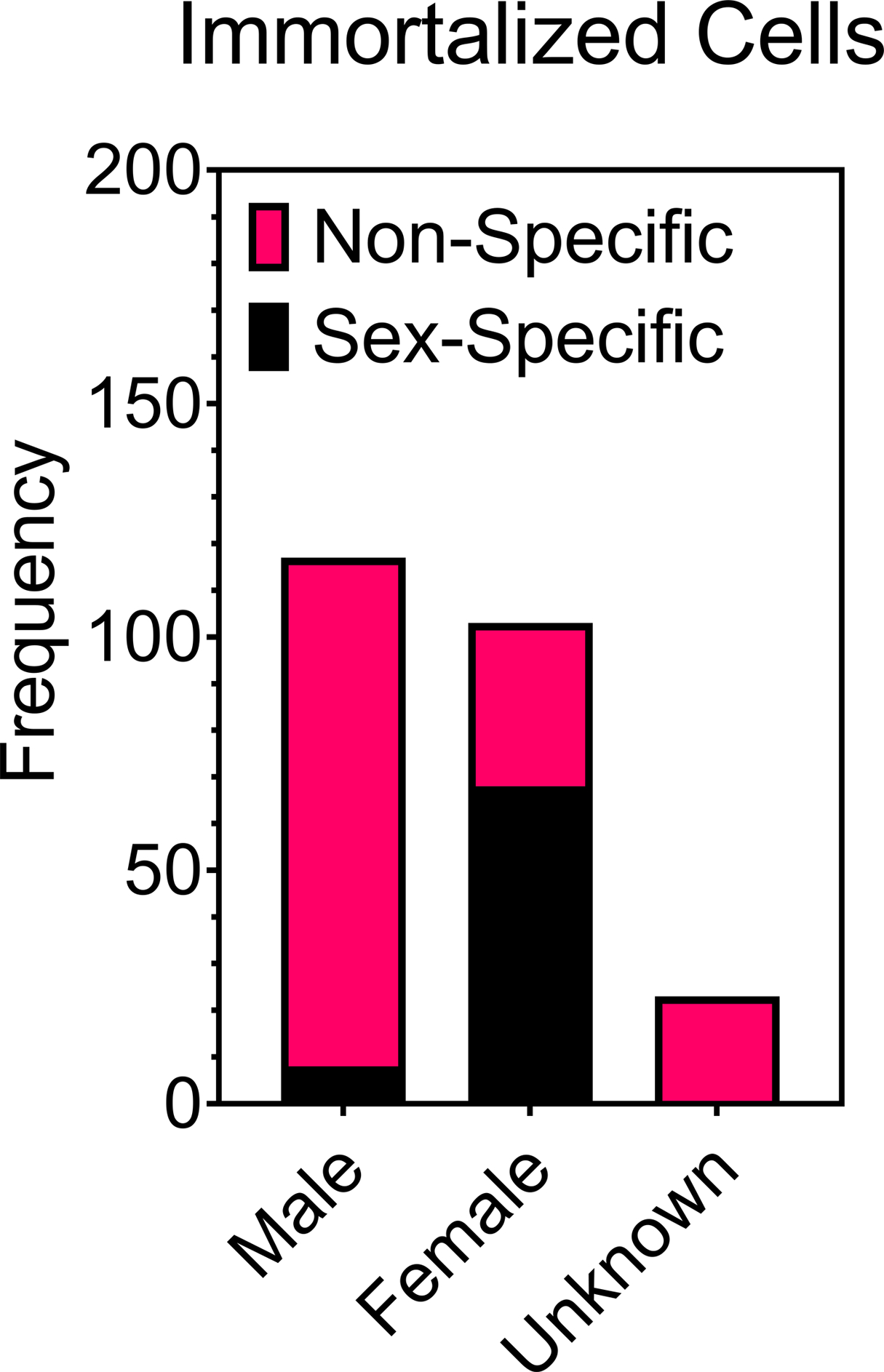
Comparison of the number of male and female immortalized/established/transformed cell lines derived from sex-specific tissue (female breasts, ovaries, gonads, cervix, male prostates etc.) and non-specific tissue (all other tissue).
Our analyses showed that the amount of bias for one sex or another was a function of the tissue being studied. There was reduced bias towards human blood, bone, brain, eye, and pancreatic derived TIE cell lines in the biomaterials literature. Male and female cells were used at approximately the same frequency (Figure 5). In the case of human colon, kidney, liver, lung, and skin derived human TIE cell lines there was a bias toward one sex over another. Worse yet, mouse TIE cell lines showed complete bias toward a single sex for each type of tissue. There was an overall bias toward male cell lines (Figure 5). The sex bias that we observed for these types of mouse cell lines is likely the product of there being nearly 12 times more male mouse cell lines than female mouse cell lines. Additionally, there are several TIE cell lines with their sex unreported in the Cellosaurus. For instance, this is the case for MC-3T3s, the commonly used cell line for bone studies. When these cells of partially unknown origin are used it is unclear to which sex the study results would be relevant, if at all. Moreover, in the process of making immortalized cell lines these cells may develop abnormal gene expression, “The large number of malignant immortal cell lines available from the American Type Culture Collection and similar sources are rarely identified by sex, but even if they were it would be extremely difficult to interpret differences among donor lines, as the expression of many genes is presumably abnormal by virtue of the malignant transformation process.”[69] This can be the case for male-derived cell lines, which after immortalization and heavy passage have been shown to lose expression of genes contained on the Y-chromosome.[93] Such chromosomal instabilities can make it impossible identify or confirm the sex of TIE cell lines.[94] At the same time, cell lines have been misidentified as “female” until after the fact PCR analyses were used to verify their sex.[95] These issues make it challenging to assess sex differences between male and female TIE cell lines without careful characterization of the sex of the cell line by the investigator. Overall, researchers should be mindful to know, confirm, and report the sex of their primary and TIE cell lines to reduce bias, increase reproducibility, and enable their research results to be contextualized for sex.
Figure 5.
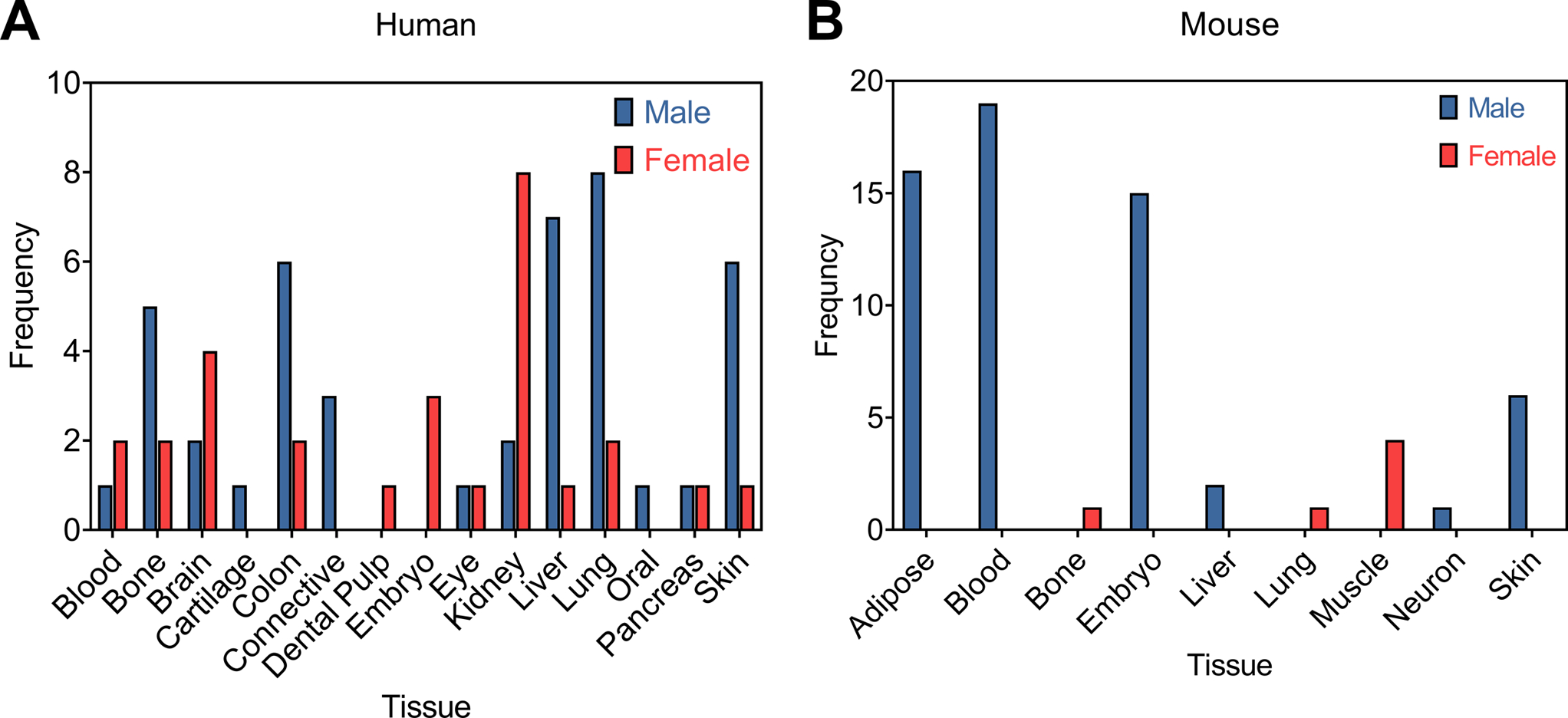
Comparison of the number of male and female transformed/immortalized/established cell lines reported in the 303 journal articles that described in vitro cell culture experiments from 7 prominent biomaterials journals (See Table 1). Cell lines have been separated by non-specific tissue for those cell lines derived from (A) humans and (B) mice.
3.3. Journal Editors
Opportunities exist for the journals that publish biomaterials research to reverse this trend in underreporting. At the time of this writing, the journals, ACS Biomaterials Science & Engineering, Acta Biomaterialia, Advanced Healthcare Materials, Biomaterials, Biomaterials Science, the Journal of Biomedical Materials Research Part A, and Macromolecular Bioscience did not have requirements for reporting the sex of cell lines used in studies. Only Biomaterials attempted to account for sex differences in animal studies. Biomaterials outlined this requirement within their editorial policy stating, “The sex of animals must be indicated, and where appropriate, the influence (or association) of sex on the results of the study.”[96] A comparison of several high impact biomaterial focused journals revealed that the reporting of cell sex is not a requirement (Figure 6) and provides an opportunity for journals to make this change.
Figure 6.
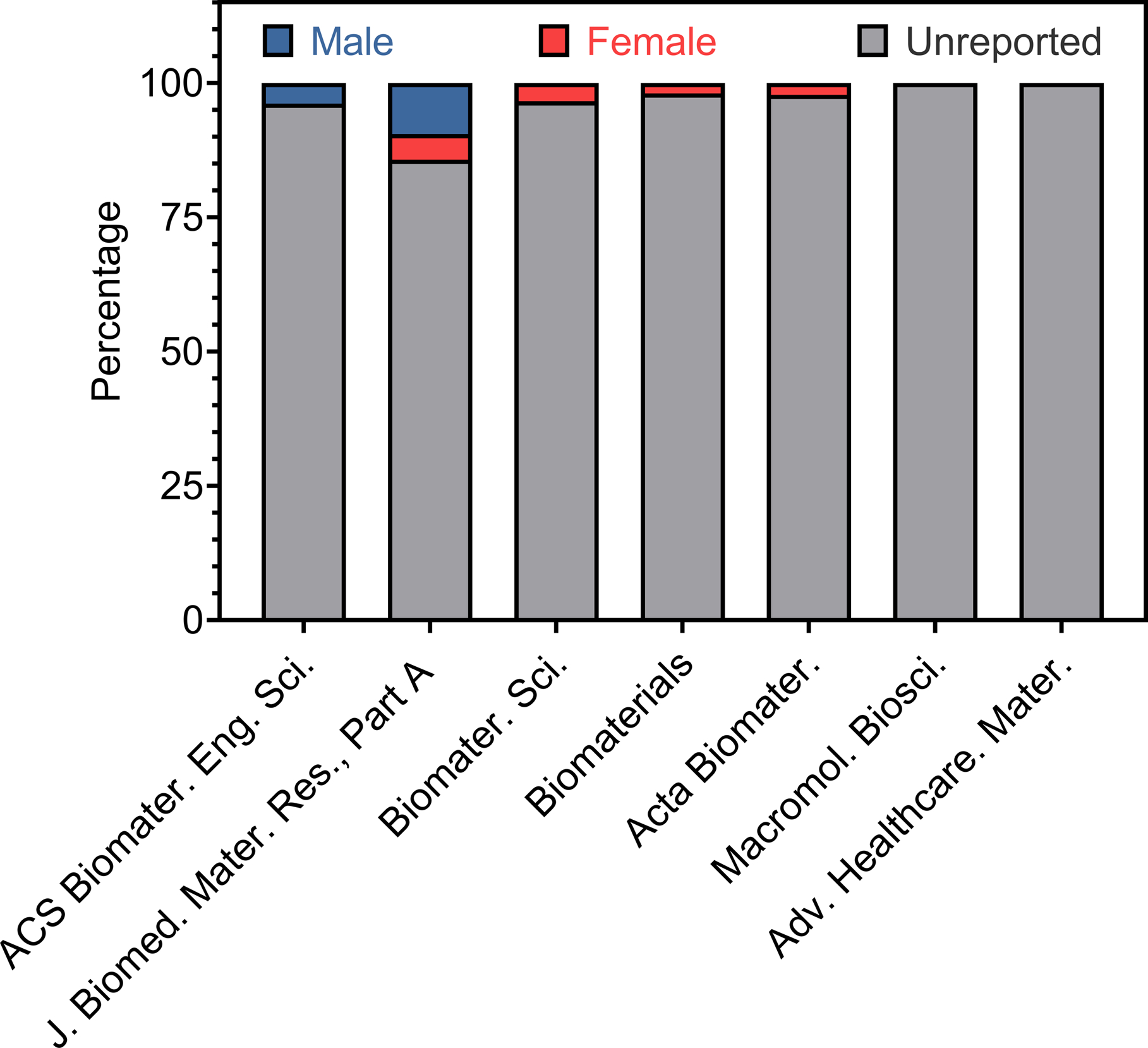
Breakdown of cell sex for all the cell cultures recorded for each journal surveyed for articles published in December 2019.
This absence in reporting is not exclusive to the journals surveyed. In fact, in other high impact publications such as Science journals, we saw similar editorial policies. Like Biomaterials, their policy only concerned animal studies and required of their authors’ to state the, “species, strain, sex, and age of laboratory animals … in the main text or Supplementary Materials.”[97] Nonetheless, this is slowly changing as researchers are increasingly raising awareness and attention to treating sex as a biological variable some 20 years following the U.S. National Academy of Medicine’s original report, which made the original case for it.[98,99,108–112,100–107] Nature journals have begun requiring for a Reporting Summary document to be provided with research article publications, which includes line items for cell line specifics. However, their instructions are general, and there is no standard to which this must be held. The detail of this reporting is at the discretion of the researcher, and documents exemplifying underreporting have been identified. Comparatively, journals published by the American Physiological Society have more explicit reporting guidelines for cell lines requesting that authors disclose the, “source of cells utilized (species, sex (for vertebrates), strain, race, age of donor, whether primary or established and associated Research Resource Identifiers (RRIDs)).”[113] To raise awareness and heighten the relevance of biomaterials research in the coming era of precision biomaterials will require journals to incorporate guidelines for reporting cell sex. Even in the case where the study is not primarily focused on reporting sex-based differences, the reporting of cell sex provides context to the reader for the data presented and should be included in an article’s methods section.
4. Conclusions and Future Outlook
Clearly, cell sex goes largely unreported in biomaterials research. Luckily, this trend can be easily flipped with specific actions taken by commercial cell vendors to diversify their cell populations and make cell demographic information readily available. In addition, journal editorial policies can and should require reporting the sex of cells used for in vitro studies, regardless of the primary focus of the article. This is not to say that every study published requires the inclusion of both male and female cells, simply that if cells are used, the sex (male/female/both or mixed/pooled populations) be included. Finally, the burden of this task primarily falls upon the biomaterial investigators conducting research. Within the biomaterials field there is consensus that sex-based differences exist; therefore, we should include sex as a biological variable and continue to advance the goals of precision medicine. At a minimum, in all accounts of in vitro studies, cell sex should be reported. We believe the universal reporting of this valuable piece of information by investigators can be a first step for them to consider and include sex as a biological variable in their research.
After recognizing sex as a biological variable, certain practices common to cell culture should be reconsidered when studying sex differences. Many of these center around sex hormones to which male and female cells respond differently.[27] One such practice is limiting the use of phenol red in cell culture media.[114] This chemical has an estrogenic effect that can differentially influence male and female cells.[115,116] Many cell culture media formulations are sold supplemented with phenol red and in fact even polystyrene can have an inadvertent weak estrogenic effect from leached phenolic compounds.[117] When dealing with sex, it becomes that much more important to recognize external influences on our systems. At the same time, serum is a black box added to cell culture media to introduce the necessary hormones for culturing cells.[118] Unsurprisingly, expressed differences have been observed in culture when using fetal bovine serum versus human-derived serum.[119] Recently, there has been a consensus to use human-derived serum or serum-free media in cell culture, especially in the development of cell-based therapies to limit xenogenic contamination.[120–122] More importantly, the use of these supplements improves cell performance. For instance, human-derived serum has been shown to enhance 3D culture.[123] This is significant as biomaterial design strives to emulate the 3D microenvironment and as such using human-derived serum or serum-free media could lead to more promising and relevant in vitro results for organoids and tissue models.[124–127] Recent work has shown that male and female sera following transcatheter aortic valve replacement elicits sex-specific cellular responses from vascular interstitial cells cultured on soft and stiff substrates. Especially fruitful was that results from this in vitro model corroborated with clinical outcomes illustrating the immediate translational benefit to including sex as a biological variable.[128] Pertinent to biomaterial development is that cells respond to sex hormones with extracellular matrix specificity and dose-dependency.[129] This means that common ECM proteins used to coat culture surfaces to promote cell adhesion (collagen, fibronectin, laminin, etc.) can lead to cells exhibiting differential hormone interactions. In fact, even common biomaterials can influence the hormone composition of our cell cultures. PDMS is the main offender, capable of releasing and absorbing small, hydrophobic molecules, including sex hormones to and from solution.[130,131] To realize active, dynamic precision biomaterials for male and female patients, it is imperative to understand these chemo-mechanical interactions. With the appropriate combination of sera/media, cells, and biomaterials clinically relevant in vitro tissue and disease models can be made that recapitulate sex differences.[86,99,128,132–134] However, these advances rely on knowledge of sex-specific cell-biomaterial interactions and cannot be made without them. Biological sex has been lauded as potentially being, “the most impactful of any of the individual differences between humans and it is certainly the most universally relevant.”[135] As we increase the complexity of our biomaterials, we cannot forget the basic characteristics and properties of the cells we use when designing them. So, as a community, let’s talk about sex and start reporting it.
Supplementary Material
Acknowledgements
Research reported in this publication was supported in part by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number F31HL147445 to Bryan D. James. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Table of Contents figure was created by co-author PG with BioRender.com
Biography

Bryan D. James is a National Heart, Lung, and Blood Institute Predoctoral Fellow and Ph.D. candidate at the University of Florida in the Department of Materials Science & Engineering. He obtained his B.A.Sc. in Materials Engineering from the University of Toronto in 2017. Currently, his work aims to understand the sex-specific cell-material interactions of vascular cells. Additionally, he has pioneered the use of nucleic acid-collagen complex (NACC) biomaterials for tissue engineering and regenerative medicine.

Josephine B. Allen, Ph.D. is an Associate Professor and head of the Allen Research Group in the Department of Materials Science and Engineering at the University of Florida. She received her Ph.D. in Biological Sciences from Northwestern University. Dr. Allen’s research interests lay at the interface of cell biology and biomaterial science within the fields of tissue engineering and regenerative medicine. The goal of the Allen Research Group is to design advanced bioactive biomaterials capable of eliciting a specific cellular response that promotes healing and tissue formation.
Footnotes
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
References
- [1].Engler AJ, Sen S, Sweeney HL, Discher DE, Cell 2006, 126, 677. [DOI] [PubMed] [Google Scholar]
- [2].Discher DE, Science (80-.) 2005, 310, 1139. [DOI] [PubMed] [Google Scholar]
- [3].Levental I, Georges PC, Janmey PA, Soft Matter 2007, 3, 299. [DOI] [PubMed] [Google Scholar]
- [4].Wen Q, Janmey PA, Exp. Cell Res 2013, 319, 2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chaudhuri O, Gu L, Klumpers D, Darnell M, Bencherif SA, Weaver JC, Huebsch N, Lee HP, Lippens E, Duda GN, et al. , Nat. Mater 2016, 15, 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Discher DE, Mooney DJ, Zandstra PW, Science (80-.) 2009, 324, 1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, Bencherif SA, Rivera-Feliciano J, Mooney DJ, Nat. Mater 2010, 9, 518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yang C, Tibbitt MW, Basta L, Anseth KS, Nat. Mater 2014, 13, 645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Khetan S, Guvendiren M, Legant WR, Cohen DM, Chen CS, Burdick JA, Nat. Mater 2013, 12, 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Galarraga JH, Burdick JA, Nat. Mater 2019, 18, 914. [DOI] [PubMed] [Google Scholar]
- [11].Burdick JA, Vunjak-Novakovic G, Tissue Eng. Part A 2009, 15, 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tibbitt MW, Anseth KS, Biotechnol. Bioeng 2009, 103, 655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rosales AM, Vega SL, DelRio FW, Burdick JA, Anseth KS, Angew. Chemie Int. Ed 2017, 56, 12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sarver DC, Kharaz YA, Sugg KB, Gumucio JP, Comerford E, Mendias CL, J. Orthop. Res 2017, 35, 2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mirzaali MJ, Schwiedrzik JJ, Thaiwichai S, Best JP, Michler J, Zysset PK, Wolfram U, Bone 2016, 93, 196. [DOI] [PubMed] [Google Scholar]
- [16].Osakabe T, Hayashi M, Hasegawa K, Okuaki T, Ritty TM, Mecham RP, Wachi H, Seyama Y, Ann. Clin. Biochem. An Int. J. Biochem. Lab. Med 2001, 38, 527. [DOI] [PubMed] [Google Scholar]
- [17].Dworatzek E, Baczko I, Kararigas G, PROTEOMICS - Clin. Appl 2016, 10, 84. [DOI] [PubMed] [Google Scholar]
- [18].Butler JE, Hammond TH, Gray SD, Laryngoscope 2001, 111, 907. [DOI] [PubMed] [Google Scholar]
- [19].Li Z, Wang Z, Yin Z, Zhang Y, Xue X, Han J, Zhu Y, Zhang J, Emmert MY, Wang H, Oncotarget 2017, 8, 53714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Karastergiou K, Fried SK, in Sex Gend. Factors Affect. Metab. Homeostasis, Diabetes Obesity. Adv. Exp. Med. Biol (Ed: Mauvais-Jarvis F), Springer, Cham, 2017, pp. 29–51. [Google Scholar]
- [21].DuPont JJ, Kenney RM, Patel AR, Jaffe IZ, Br. J. Pharmacol 2019, 176, 4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ruff CB, Hayes WC, J. Orthop. Res 1988, 6, 886. [DOI] [PubMed] [Google Scholar]
- [23].Pan Q, O’Connor MI, Coutts RD, Hyzy SL, Olivares-Navarrete R, Schwartz Z, Boyan BD, Biol. Sex Differ 2016, 7, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].National Institutes of Health Office of Research on Women’s Health, “Sex and Gender,” can be found under https://orwh.od.nih.gov/sex-gender, 2020.
- [25].Tannenbaum C, Ellis RP, Eyssel F, Zou J, Schiebinger L, Nature 2019, 575, 137. [DOI] [PubMed] [Google Scholar]
- [26].Mauvais-Jarvis F, Bairey Merz N, Barnes PJ, Brinton RD, Carrero J-J, DeMeo DL, De Vries GJ, Epperson CN, Govindan R, Klein SL, et al. , Lancet 2020, 396, 565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Decaroli MC, Rochira V, Virulence 2017, 8, 545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Alswat KA, J. Clin. Med. Res 2017, 9, 382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Klein SL, Flanagan KL, Nat. Rev. Immunol 2016, 16, 626. [DOI] [PubMed] [Google Scholar]
- [30].Mendelsohn ME, Science (80-.) 2005, 308, 1583. [DOI] [PubMed] [Google Scholar]
- [31].Maric-Bilkan C, Arnold AP, Taylor DA, Dwinell M, Howlett SE, Wenger N, Reckelhoff JF, Sandberg K, Churchill G, Levin E, et al. , Hypertension 2016, 67, 802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Jelani Q, Petrov M, Martinez SC, Holmvang L, Al-Shaibi K, Alasnag M, Curr. Atheroscler. Rep 2018, 20, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Srivaratharajah K, Abramson BL, Can. J. Cardiol 2018, 34, 356. [DOI] [PubMed] [Google Scholar]
- [34].Mosca L, Barrett-Connor E, Kass Wenger N, Circulation 2011, 124, 2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Rubin JB, Lagas JS, Broestl L, Sponagel J, Rockwell N, Rhee G, Rosen SF, Chen S, Klein RS, Imoukhuede P, et al. , Biol. Sex Differ 2020, 11, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wizeman T, Sex-Specific Reporting of Scientific Research, National Academies Press, Washington, D.C., 2012. [PubMed] [Google Scholar]
- [37].Klein SL, Morgan R, Biol. Sex Differ 2020, 11, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Carey JL, Nader N, Chai PR, Carreiro S, Griswold MK, Boyle KL, Clin. Ther 2017, 39, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Deasy BM, Lu A, Tebbets JC, Feduska JM, Schugar RC, Pollett JB, Sun B, Urish KL, Gharaibeh BM, Cao B, et al. , J. Cell Biol 2007, 177, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Heinrich J, Most Drugs Withdrawn in Recent Years Had Greater Health Risks for Women, Washington, D.C., 2001. [Google Scholar]
- [41].Salje H, Tran Kiem C, Lefrancq N, Courtejoie N, Bosetti P, Paireau J, Andronico A, Hozé N, Richet J, Dubost C-L, et al. , Science (80-.) 2020, eabc3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Huxley VH, Kemp SS, Schramm C, Sieveking S, Bingaman S, Yu Y, Zaniletti I, Stockard K, Wang J, J. Physiol 2018, 596, 3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Lorenz M, Blaschke B, Benn A, Hammer E, Witt E, Kirwan J, Fritsche-Guenther R, Gloaguen Y, Bartsch C, Vietzke A, et al. , Atherosclerosis 2019, 291, 99. [DOI] [PubMed] [Google Scholar]
- [44].Penaloza C, Estevez B, Orlanski S, Sikorska M, Walker R, Smith C, Smith B, Lockshin RA, Zakeri Z, FASEB J. 2009, 23, 1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Antoniucci D, Miller VM, Sieck GC, Fitzpatrick LA, Endothelium 2001, 8, 137. [DOI] [PubMed] [Google Scholar]
- [46].Cattaneo MG, Vanetti C, Decimo I, Di Chio M, Martano G, Garrone G, Bifari F, Vicentini LM, Sci. Rep 2017, 7, 9612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Zhang Y, Dong X, Shirazi J, Gleghorn JP, Lingappan K, Am. J. Physiol. Circ. Physiol 2018, 315, H1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Katzburg S, Lieberherr M, Ornoy A, Klein BY, Hendel D, Somjen D, Bone 1999, 25, 667. [DOI] [PubMed] [Google Scholar]
- [49].Olivares-Navarrete R, Hyzy SL, Chaudhri RA, Zhao G, Boyan BD, Schwartz Z, Biol. Sex Differ 2010, 1, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Berger MB, Cohen DJ, Olivares-Navarrete R, Williams JK, Cochran DL, Boyan BD, Schwartz Z, Biol. Sex Differ 2018, 9, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Bharadwaj VN, Copeland C, Mathew E, Newbern J, Anderson TR, Lifshitz J, Kodibagkar VD, Stabenfeldt SE, Tissue Eng. Part A 2020, 26, 688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zhang X-D, Chen, Song, Wang, Shen, Wu, Fan, Fan, Sun Y, Liu P, et al. , Int. J. Nanomedicine 2013, 2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Randolph LN, Bao X, Oddo M, Lian XL, Sci. Rep 2019, 9, 16696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Lorenz M, Koschate J, Kaufmann K, Kreye C, Mertens M, Kuebler WM, Baumann G, Gossing G, Marki A, Zakrzewicz A, et al. , Atherosclerosis 2015, 240, 61. [DOI] [PubMed] [Google Scholar]
- [55].Garieri M, Stamoulis G, Blanc X, Falconnet E, Ribaux P, Borel C, Santoni F, Antonarakis SE, Proc. Natl. Acad. Sci 2018, 115, 13015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Berletch JB, Yang F, Xu J, Carrel L, Disteche CM, Hum. Genet 2011, 130, 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Machiela MJ, Zhou W, Karlins E, Sampson JN, Freedman ND, Yang Q, Hicks B, Dagnall C, Hautman C, Jacobs KB, et al. , Nat. Commun 2016, 7, 11843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Migeon BR, J. Am. Soc. Nephrol 2008, 19, 2052. [DOI] [PubMed] [Google Scholar]
- [59].Guo X, Dai X, Zhou T, Wang H, Ni J, Xue J, Wang X, Hum. Genet 2020, 139, 421. [DOI] [PubMed] [Google Scholar]
- [60].Molina E, Chew GS, Myers SA, Clarence EM, Eales JM, Tomaszewski M, Charchar FJ, Sci. Rep 2017, 7, 16710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Murakami S, Chishima S, Uemoto H, Sakamoto E, Sato T, Kurabe N, Kawasaki Y, Shibata T, Akiyama H, Tashiro F, Oncogene 2014, 33, 2978. [DOI] [PubMed] [Google Scholar]
- [62].Lee J, Pinares-Garcia P, Loke H, Ham S, Vilain E, Harley VR, Proc. Natl. Acad. Sci 2019, 116, 16577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Maan AA, Eales J, Akbarov A, Rowland J, Xu X, Jobling MA, Charchar FJ, Tomaszewski M, Eur. J. Hum. Genet 2017, 25, 1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Dumanski JP, Rasi C, Lönn M, Davies H, Ingelsson M, Giedraitis V, Lannfelt L, Magnusson PKE, Lindgren CM, Morris AP, et al. , Science (80-.) 2015, 347, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Forsberg LA, Gisselsson D, Dumanski JP, Nat. Rev. Genet 2017, 18, 128. [DOI] [PubMed] [Google Scholar]
- [66].Thompson DJ, Genovese G, Halvardson J, Ulirsch JC, Wright DJ, Terao C, Davidsson OB, Day FR, Sulem P, Jiang Y, et al. , Nature 2019, 575, 652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Forsberg LA, Hum. Genet 2017, 136, 657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Jacobs PA, Brunton M, Court Brown WM, Doll R, Goldstein H, Nature 1963, 197, 1080. [DOI] [PubMed] [Google Scholar]
- [69].Wizemann TM, Pardue M-L, Eds., Exploring the Biological Contributions to Human Health, National Academies Press, Washington, D.C., 2001. [PubMed] [Google Scholar]
- [70].Clayton JA, Collins FS, Nature 2014, 509, 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Beery AK, Zucker I, Neurosci. Biobehav. Rev 2011, 35, 565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].National Institutes of Health, “Consideration of Sex as a Biological Variable in NIH-funded Research,” can be found under https://grants.nih.gov/grants/guide/notice-files/NOT-OD-15-102.html, 2015. [Google Scholar]
- [73].Engl N. J. Med 2019, 381, 668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Collins FS, Varmus H, Engl N. J. Med 2015, 372, 793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Aguado BA, Grim JC, Rosales AM, Watson-Capps JJ, Anseth KS, Sci. Transl. Med 2018, 10, eaam8645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Guzzi EA, Tibbitt MW, Adv. Mater 2020, 32, 1901994. [DOI] [PubMed] [Google Scholar]
- [77].Raman R, Langer R, Adv. Mater 2020, 32, 1901969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Facklam AL, Volpatti LR, Anderson DG, Adv. Mater 2020, 32, 1902005. [DOI] [PubMed] [Google Scholar]
- [79].Moore EM, Maestas DR Jr., Comeau HY, Elisseeff JH, Tissue Eng. Part B Rev 2020, ten. TEB.2019.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Zhou J, Kroll AV, Holay M, Fang RH, Zhang L, Adv. Mater 2020, 32, 1901255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Prendergast ME, Burdick JA, Adv. Mater 2020, 32, 1902516. [DOI] [PubMed] [Google Scholar]
- [82].Wang J, Wang Z, Yu J, Kahkoska AR, Buse JB, Gu Z, Adv. Mater 2020, 32, 1902004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Ferber S, Gonzalez RJ, Cryer AM, Andrian UH, Artzi N, Adv. Mater 2020, 32, 1903847. [DOI] [PubMed] [Google Scholar]
- [84].McCrary MW, Bousalis D, Mobini S, Song YH, Schmidt CE, Acta Biomater. 2020, 111, 1. [DOI] [PubMed] [Google Scholar]
- [85].Bairoch A, J. Biomol. Tech 2018, 29, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Nawroth J, Rogal J, Weiss M, Brucker SY, Loskill P, Adv. Healthc. Mater 2018, 7, 1700550. [DOI] [PubMed] [Google Scholar]
- [87].Taylor K, Vallejo-Giraldo C, Schaible NS, Zakeri R, Miller VM, Biol. Sex Differ 2011, 2, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, Gold MS, Holdcroft A, Lautenbacher S, Mayer EA, et al. , Pain 2007, 132, S26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Mogil JS, Chanda ML, Pain 2005, 117, 1. [DOI] [PubMed] [Google Scholar]
- [90].Geller SE, Koch A, Pellettieri B, Carnes M, J. Women’s Heal 2011, 20, 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Lee SK, BMB Rep. 2018, 51, 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Park M-N, Park JH, Paik HY, Lee SK, Am. J. Physiol. Physiol 2015, 308, C578. [DOI] [PubMed] [Google Scholar]
- [93].Shah K, McCormack CE, Bradbury NA, Am. J. Physiol. Physiol 2014, 306, C3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Klein SL, Schiebinger L, Stefanick ML, Cahill L, Danska J, de Vries GJ, Kibbe MR, McCarthy MM, Mogil JS, Woodruff TK, et al. , Proc. Natl. Acad. Sci 2015, 112, 5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Durkin AS, Cedrone E, Sykes G, Boles D, Reid YA, Vitr. Cell. Dev. Biol.- Anim 2000, 36, 344. [DOI] [PubMed] [Google Scholar]
- [96].Biomaterials, “Guide for Authors,” can be found under https://www.elsevier.com/journals/biomaterials/0142-9612/guide-for-authors, n.d.
- [97].Science, “Science Journals: editorial policies,” can be found under https://www.sciencemag.org/authors/science-journals-editorial-policies?_ga=2.229016220.1428687232.1589067733-2121765867.1589067733, n.d.
- [98].Porras AM, McCoy CM, Masters KS, Circ. Res 2017, 120, 604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Simon LR, Masters KS, ACS Biomater. Sci. Eng 2019, acsbiomaterials.9b01067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Graves JAM, Cell 2001, 107, 285. [Google Scholar]
- [101].Wizemann TM, Pardue M-L, Eds., Exploring the Biological Contributions to Human Health: Does Sex Matter?, National Academies Press, Washington, D.C., 2001. [PubMed] [Google Scholar]
- [102].Pollitzer E, Nature 2013, 500, 23. [DOI] [PubMed] [Google Scholar]
- [103].Woitowich NC, Woodruff TK, Proc. Natl. Acad. Sci 2019, 116, 7154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Karczmar AL, Woodruff TK, Clin. Chem 2019, 65, 36. [DOI] [PubMed] [Google Scholar]
- [105].Woitowich NC, Woodruff TK, J. Women’s Heal 2019, 28, 9. [DOI] [PubMed] [Google Scholar]
- [106].Woitowich NC, Woodruff TK, Nature 2019, 565, 25. [DOI] [PubMed] [Google Scholar]
- [107].Prakash VS, Mansukhani NA, Helenowski IB, Woodruff TK, Kibbe MR, J. Women’s Heal 2018, 27, 1342. [DOI] [PubMed] [Google Scholar]
- [108].Rabesandratana T, Science (80-.) 2014, DOI 10.1126/science.caredit.a1400067. [DOI] [Google Scholar]
- [109].Miller VM, Am. J. Physiol. Physiol 2012, 302, C1269. [DOI] [PubMed] [Google Scholar]
- [110].Fuller CM, Insel PA, Am. J. Physiol. Physiol 2014, 306, C1. [DOI] [PubMed] [Google Scholar]
- [111].Arnegard ME, Whitten LA, Hunter C, Clayton JA, J. Women’s Heal 2020, jwh. 2019.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Woitowich NC, Beery A, Woodruff T, Elife 2020, 9, DOI 10.7554/eLife.56344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].American Physiological Society, “Experimental Details to Report in Your Manuscript,” can be found under https://journals.physiology.org/author-info.experimental-details-to-report, n.d.
- [114].Miller VM, Kaplan JR, Schork NJ, Ouyang P, Berga SL, Wenger NK, Shaw LJ, Webb RC, Mallampalli M, Steiner M, et al. , Biol. Sex Differ 2011, 2, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Berthois Y, Katzenellenbogen JA, Katzenellenbogen BS, Proc. Natl. Acad. Sci 1986, 83, 2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Welshons WV, Wolf MF, Murphy CS, Jordan VC, Mol. Cell. Endocrinol 1988, 57, 169. [DOI] [PubMed] [Google Scholar]
- [117].de Souza Santos R, ALTEX 2018, 435. [DOI] [PubMed] [Google Scholar]
- [118].Hayashi I, Larner J, Sato G, In Vitro 1978, 14, 23. [DOI] [PubMed] [Google Scholar]
- [119].Mazlyzam AL, Aminuddin BS, Saim L, Ruszymah BHI, Arch. Med. Res 2008, 39, 743. [DOI] [PubMed] [Google Scholar]
- [120].Geraghty RJ, Capes-Davis A, Davis JM, Downward J, Freshney RI, Knezevic I, Lovell-Badge R, Masters JRW, Meredith J, Stacey GN, et al. , Br. J. Cancer 2014, 111, 1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Karnieli O, Friedner OM, Allickson JG, Zhang N, Jung S, Fiorentini D, Abraham E, Eaker SS, tan K. Yong, Chan A, et al. , Cytotherapy 2017, 19, 155. [DOI] [PubMed] [Google Scholar]
- [122].Cimino M, Gonçalves RM, Barrias CC, Martins MCL, Stem Cells Int. 2017, 2017, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Heger JI, Froehlich K, Pastuschek J, Schmidt A, Baer C, Mrowka R, Backsch C, Schleußner E, Markert UR, Schmidt A, Exp. Cell Res 2018, 365, 57. [DOI] [PubMed] [Google Scholar]
- [124].James BD, Allen JB, ACS Biomater. Sci. Eng 2018, 4, 3818. [DOI] [PubMed] [Google Scholar]
- [125].Moore EM, Ying G, West JL, Adv. Biosyst 2017, 1, 1600021. [Google Scholar]
- [126].Caliari SR, Burdick JA, Nat. Methods 2016, 13, 405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Kratochvil MJ, Seymour AJ, Li TL, Paşca SP, Kuo CJ, Heilshorn SC, Nat. Rev. Mater 2019, 4, 606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Aguado BA, Schuetze KB, Grim JC, Walker CJ, Cox AC, Ceccato TL, Tan A-C, Sucharov CC, Leinwand LA, Taylor MRG, et al. , Sci. Transl. Med 2019, 11, eaav3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Cid MC, Esparza J, Schnaper HW, Juan M, Yague J, Grant DS, Urbano-Márquez A, Hoffman GS, Kleinman HK, Angiogenesis 1999, 3, 271. [DOI] [PubMed] [Google Scholar]
- [130].Toepke MW, Beebe DJ, Lab Chip 2006, 6, 1484. [DOI] [PubMed] [Google Scholar]
- [131].Regehr KJ, Domenech M, Koepsel JT, Carver KC, Ellison-Zelski SJ, Murphy WL, Schuler LA, Alarid ET, Beebe DJ, Lab Chip 2009, 9, 2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Chou DB, Frismantas V, Milton Y, David R, Pop-Damkov P, Ferguson D, MacDonald A, Vargel Bölükbaşı Ö, Joyce CE, Moreira Teixeira LS, et al. , Nat. Biomed. Eng 2020, 4, 394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Zhang B, Korolj A, Lai BFL, Radisic M, Nat. Rev. Mater 2018, 3, 257. [Google Scholar]
- [134].U. S. F. & D. Administration, “Organs-On-Chips for Radiation Countermeasures,” 2019.
- [135].Green DJ, Hopkins ND, Jones H, Thijssen DHJ, Eijsvogels TMH, Yeap BB, Exp. Physiol 2016, 101, 230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


