Abstract
Background
Korean ginseng (Panax ginseng Meyer) contains a variety of ginsenosides that can be metabolized to a biologically active substance, compound K. Previous research showed that compound K could be enriched in the red ginseng extract (RGE) after hydrolysis by pectinase. The current study investigated whether the enzymatically hydrolyzed red ginseng extract (HRGE) containing a notable level of compound K has cognitive improving and neuroprotective effects.
Methods
A scopolamine-induced hypomnesic mouse model was subjected to behavioral tasks, such as the Y-maze, passive avoidance, and the Morris water maze tests. After sacrificing the mice, the brains were collected, histologically examined (hematoxylin and eosin staining), and the expressions of antioxidant proteins analyzed by western blot.
Results
Behavioral assessment indicated that the oral administration of HRGE at a dosage of 300 mg/kg body weight reversed scopolamine-induced learning and memory deficits. Histological examination demonstrated that the hippocampal damage observed in scopolamine-treated mouse brains was reduced by HRGE administration. In addition, HRGE administration increased the expression of nuclear-factor-E2-related factor 2 and its downstream antioxidant enzymes NAD(P)H:quinone oxidoreductase and heme oxygenase-1 in hippocampal tissue homogenates. An in vitro assay using HT22 mouse hippocampal neuronal cells demonstrated that HRGE treatment attenuated glutamate-induced cytotoxicity by decreasing the intracellular levels of reactive oxygen species.
Conclusion
These findings suggest that HRGE administration can effectively alleviate hippocampus-mediated cognitive impairment, possibly through cytoprotective mechanisms, preventing oxidative-stress-induced neuronal cell death via the upregulation of phase 2 antioxidant molecules.
Keywords: Cognition, Korean Red Ginseng, Learning and memory, Neuroprotection, Pectinase-mediated hydrolysis
Abbreviations: ABTS, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid); BW, body weight; CCK-8, cell counting kit-8; DCF, dichlorofluorescein; DCFH, 2,7-dichlorodihydrofluorescein; DPPH, 2,2-diphenyl-1-picrylhydrazyl; H&E, hematoxylin and eosin; HO-1, heme oxygenase-1; HRGE, hydrolyzed red ginseng extract; KO, knockout; NQO1, NAD(P):quinone oxidoreductase 1; Nrf2, nuclear-factor-E2-related factor 2; PPD, protopanaxadiol; RGE, red ginseng extract; ROS, reactive oxygen species; WT, wild-type
1. Introduction
Korean ginseng (Panax ginseng Meyer) is a traditional medicine in East Asian countries. The root of P. ginseng is well known to contain diverse pharmacologically active compounds, such as ginsenosides, polyacetylenes, and phenolic compounds. Among the ginsenosides, Rb1, Rb2, Rc, Rd, Re, and Rg1 in white ginseng can be transformed into several dammarane ginsenosides Rg2, Rg3, Rg5, Rg6, Rg9, Rg10, Rh1, Rh4, Rk1, Rk3, Rz1, F4, and 20(E)-F4, along with the aglycones protopanaxadiol (PPD) and protopanaxatriol, during the heating process of red ginseng production [1,2]. In particular, orally administered PPD-type saponins, such as Rb1, Rb2, and Rc, can be further bioconverted into compound K (20-O-β-D-glucopyranosyl-20(S)-protopanaxadiol) by intestinal microflora [3].
Unlike other ginsenosides, compound K is absorbed through the gut lining and has numerous biological activities, such as antidiabetic [4], anticarcinogenic [5,6], antimelanogenic [7,8], neuroprotective [9,10], and cognitive-enhancing effects [11,12]. Multiple studies have focused on the transformation of ginsenosides into compound K by various methods, including enzymatic hydrolysis, to take advantage of the in vivo effects of compound K [13,14].
Previous studies have demonstrated that pectinase-mediated hydrolysis enriched compound K in Korean Red Ginseng extract (RGE) [15,16]. Hydrolyzed Korean Red Ginseng extract (HRGE) has been reported to exert diverse in vivo effects, such as restored antioxidant activity in aged rats [17,18], enhanced testicular function in rats [16,18], and lowered postprandial glucose levels in humans [19].
In this context, we investigated the benefits of HRGE in the improvement of learning and memory abilities using scopolamine-induced hypomnesic mice. Scopolamine is a muscarinic cholinergic receptor–antagonizing agent that is widely used to produce memory deficits in animal models [20,21]. Studies have shown that scopolamine can trigger the production of reactive oxygen species (ROS) by decreasing the activities of antioxidant enzymes and increasing lipid peroxidation, thereby aggravating oxidative stress in the cerebral cortex and hippocampus in mice [[22], [23], [24], [25], [26], [27]]. Oxidative stress is due to a disturbance in reduction–oxidation balance and is one of the critical causes of neurodegeneration and memory loss [[28], [29], [30], [31], [32]].
In the present study, we examined the neuroprotective and memory-improving effects of the compound-K-enriched HRGE in scopolamine-treated mice and compared these effects with nonhydrolyzed RGE.
2. Materials and methods
2.1. Preparation of RGE and HRGE
The samples RGE and HRGE were prepared in compliance with the methods approved by the Ministry of Food and Drug Safety in South Korea, as previously described [8,15]; these materials were generously provided by the Ginseng Biotech Research Team, Ilhwa Co., Ltd. (Guri, South Korea). Briefly, dried red ginseng was extracted in 50% (v/v) ethanol at 80–85°C for 8 h, filtered, and rotary-evaporated. The red ginseng ethanol extract (referred to as RGE in this paper) was hydrolyzed by treatment with pectinase (Connell Bros Company Australasia Pty Ltd., Croydon South, Australia) at 58°C for 24 h and then concentrated. The concentrate was denoted as HRGE. In vitro and in vivo examinations of RGE and HRGE, which presented solid contents of 60% (w/w) or higher, were conducted.
2.2. Analysis of ginsenoside contents
Ginsenosides in RGE and HRGE were quantified as previously described with minor modifications [33]. Briefly, the Acquity UPLC system (Waters Corporation, Milford, MA, USA) equipped with an Acquity UPLC BEH C18 column (100 mm × 2.1 mm, 1.7 mm; Waters Corporation) was used. The column temperature was 40°C, and the UV detector was set at 203 nm. The mobile phase consisted of a mixture of water and acetonitrile, applied at a gradient of 82:18–20:80 over 24.5 min, at a flow rate of 0.5 mL/min. The sample injection volume was 0.2 μL. Data were analyzed using Empower Pro software (Waters Corporation).
2.3. Animal behavioral tests
The protocol for this study was approved by the Animal Care and Use Committee of Kyungpook National University (approval number: KNU2015-0124). Five-week-old male C57BL/6J mice (nuclear-factor-E2-related factor 2 wild-type, Nrf2-WT) were purchased from Daehan Biolink Co., Ltd. (Eumseong, South Korea), and C57BL/6J/Nrf2-knockout (Nrf2-KO) mice were kindly provided by Professor Masayuki Yamamoto (Tohoku University, Sendai, Japan). Mice were housed under controlled laboratory conditions (temperature, 22 ± 2°C; relative humidity, 50 ± 5%; 12-h light/dark cycle with free access to chow and water).
After a week of acclimation, mice with a body weight of 18–22 g were divided into 10 groups (8 mice per group). A total of 56 Nrf2-WT mice were divided into seven groups; each group was administered one of the following options: (1) a vehicle only, and no scopolamine injected, (2) a vehicle and scopolamine, (3) tacrine at 10 mg/kg BW and scopolamine, (4) RGE at 300 mg/kg BW and scopolamine, (5) HRGE at 50 mg/kg BW and scopolamine, (6) HRGE at 100 mg/kg BW and scopolamine, or (7) HRGE at 300 mg/kg BW and scopolamine. A total of 24 Nrf2-KO mice were divided into three groups: (1) vehicle only and no scopolamine, (2) vehicle and scopolamine, and (3) HRGE at 300 mg/kg BW and scopolamine.
The test samples (RGE and HRGE) and tacrine (positive control) were dissolved in 10% (v/v) Tween 80 (Sigma-Aldrich, St. Louis, MO, USA) in sterilized saline (Sigma-Aldrich) and orally administered every day at a designated dosage using an oral gavage needle for 10 days. The samples or tacrine were given 1 h before subjecting the mice to the behavioral tasks. After 30 min of sample administration, scopolamine was peritoneally injected at a dosage of 1 mg/kg BW to induce learning and memory impairment.
Behavioral examinations were performed using three different tasks by following previous protocols [9,34,35]. The initial day of sample administration was set as Day 1. The Y-maze task was conducted on Day 2; spontaneous alteration of behavior was examined. The passive avoidance task was performed on Day 3–5. On the first day (Day 3), mice were allowed to experience the testing apparatus (Gemini Avoidance System, San Diego, CA, USA). On the second day (Day 4), they were trained to remain in the bright chamber by avoiding the dark chamber where an electrical foot shock was delivered. On the third day (Day 5), test trials were performed, and data were acquired. Lengthened latency times represented improved learning and memory ability. The Morris water maze task was conducted on Day 6–10. After adaptation to the swimming environment on Day 6 (the first day of swimming), the mice were dedicated to platform finding in the swimming pool for four consecutive days (Day 7–10). The arrival times for each mouse to the platform and the latency times in the platform quadrant were monitored.
2.4. Hematoxylin and eosin (H&E) staining
After the behavioral tests, the mice were sacrificed for brain tissue collection. Each tissue was fixed in formalin solution and further processed, as previously described with minor modifications [36]. Briefly, the tissues were dehydrated in ethanol and xylene successively, followed by paraffin embedding. Each paraffin block containing a whole-brain sample was coronally sectioned to 5-μm thickness using a microtome (RM-2125 RT, Leica, Nussloch, Germany). The sliced sections were stained with H&E, mounted, and then photographed using an optical microscope (Eclipse 80i, Nikon, Tokyo, Japan).
2.5. Western blot analysis
The hippocampi were dissected from the sacrificed mice and subjected to the NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher Scientific, Rockford, IL, USA). The cytoplasmic and nuclear fractions of the protein extracts were quantified, denatured, electrophoretically separated on sodium dodecyl sulfate–polyacrylamide gels, and transferred onto polyvinylidene fluoride membranes (Millipore, Bedford, MA, USA), as previously described [36].
The primary antibodies used in this study were against Nrf2, heme oxygenase-1 (HO-1) (both from Abcam, Cambridge, UK), lamin B, and β-actin (both from Santa Cruz Biotechnology, Santa Cruz, CA, USA). The secondary antibodies used were antirabbit or antimouse immunoglobulin G, conjugated to horseradish peroxidase (Santa Cruz Biotechnology).
2.6. Measurement of NAD(P):quinone oxidoreductase 1 (NQO1) activity
The dissected hippocampal tissues were homogenized using a microultrasonic cell disrupter (KT50, Kimble Kontes, Vineland, NJ, USA). After centrifugation at 12,000 × g, 4°C for 15 min, the protein content in each supernatant was quantified by the Lowry assay [37], and the enzymatic activity of NQO1 was assayed by spectrophotometrically monitoring the reduction of 2,6-dichlorophenolindophenol at 600 nm [38].
2.7. Cell viability assay
Mouse hippocampal HT22 cells were obtained and maintained as previously reported [39]. The cells were plated in a 96-well culture plate at a density of 4 × 103 cells per well. After 24 h of incubation, they were treated with the samples at various concentrations with or without 5 mM glutamate for 24 h, followed by determination of cell viability using the cell counting kit-8 (CCK-8, Dojindo Laboratories, Kumamoto, Japan) [40].
2.8. Dichlorofluorescein (DCF) assay
The intracellular ROS level was quantified as previously described [41,42] by measuring the level of highly fluorescent DCF, which is the oxidized form of 2,7-dichlorodihydrofluorescein (DCFH) intracellularly generated from DCFH diacetate (DCFH-DA; Sigma-Aldrich). Briefly, HT22 cells were plated in a 96-well black polystyrene plate (Nunc, Rochester, NY, USA) at 4 × 103 cells per well or in a 24-well transparent plate (Nunc) at 3 × 104 cells per well. After incubating for 24 h, they were treated with samples with or without 5 mM glutamate in 0.5% fetal bovine serum–containing culture medium for 6 h. The cells were then treated with 30 μM DCFH-DA at 37°C for 1 h. The fluorescence of intracellular DCF was detected at excitation and emission wavelengths of 485 and 535 nm, respectively, using a microplate reader (Infinite 200, Tecan, Grödig, Austria).
2.9. Determination of free-radical scavenging capacity and ferric-reducing antioxidant power (FRAP)
The antioxidant activities of RGE and HRGE were assessed using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) free-radical scavenging assays, as well as the FRAP method [41]. α-Tocopherol served as a positive control at concentrations of 50, 100, 500, and 1,000 μM.
2.10. Statistical analysis
Significance differences were determined by one-way analysis of variance, followed by Duncan's multiple-range post hoc test using SPSS software (SPSS Inc., Chicago, IL, USA). Significance was set at p < 0.05. Statistical differences were indicated by different alphabetical letters.
3. Results
3.1. Compositions of ginsenosides of RGE and HRGE
The HRGE was prepared by enzymatic hydrolysis of RGE with pectinase. Ten ginsenosides contained in RGE and HRGE were quantitatively analyzed (Table 1). The total content of ginsenosides was lower in HRGE than in RGE. In particular, the contents of the PPD-type ginsenosides were greatly decreased after pectinase treatment. Among the PPD-type ginsenosides, the major ones in RGE (Rb1, Rb2, and Rc) were found to exist at very low levels in HRGE. Simultaneously, their metabolites, such as F2, Rh2, and compound K, were remarkably increased in HRGE compared with those in RGE. These data indicated that there were more hydrolyzed PPD-type ginsenosides, including F2 and compound K, in HRGE than in RGE. In addition, RGE and HRGE were capable of concentration-dependently scavenging DPPH or ABTS radicals, as well as effectively reducing ferric ion (Suppl. Fig. S1).
Table 1.
Ginsenoside Compositions of RGE and HRGE
| Ginsenoside (mg/g dry matter1) | RGE | HRGE |
|---|---|---|
| Rb1 | 9.67 ± 0.13 | 0.40 ± 0.00 |
| Rg1 | 2.14 ± 0.01 | 2.02 ± 0.02 |
| Rb2 | 3.71 ± 0.03 | ND2 |
| Rc | 7.23 ± 0.12 | 0.86 ± 0.01 |
| Rd | 1.96 ± 0.00 | 0.65 ± 0.00 |
| F2 | 0.04 ± 0.00 | 5.76 ± 0.01 |
| Rg3s | 1.23 ± 0.02 | 0.93 ± 0.01 |
| Rg3r | 0.62 ± 0.01 | 0.53 ± 0.00 |
| Rh2 | ND | 0.57 ± 0.00 |
| Compound K | ND | 4.62 ± 0.03 |
| Total | 26.60 ± 0.15 | 16.34 ± 0.03 |
Total solid contents of RGE and HRGE were 61% and 66% (w/w), respectively.
ND, not detected.
3.2. Improvement of cognitive function by oral administration of RGE or HRGE in scopolamine-induced hypomnesic mice
C57BL/6J mice were orally administered HRGE (at 50, 100, or 300 mg/kg BW), RGE (at 300 mg/kg BW), or tacrine (at 10 mg/kg BW; positive control). The memory impairment was induced by intraperitoneal injection of scopolamine at 1 mg/kg BW 30 min before the mice were subjected to daily behavioral tests.
Scopolamine-induced memory loss was manifested by a decreased spontaneous exploration in the Y-maze test (Fig. 1A), a reduced latency staying in a bright chamber in the passive avoidance test (Fig. 2A), and a shortened time to find the platform and swim in the platform quadrant in the Morris water maze test (Fig. 3A, C). Moreover, HRGE or RGE administration at 300 mg/kg BW significantly restored exploration reduction (Fig. 1A), ameliorated associative learning impairment (Fig. 2A), and improved spatial learning and memory ability (Fig. 3A, C). The memory-improving effects of HRGE or RGE were found to be comparable to that of tacrine. Interestingly, the effects were observed only in Nrf2-WT mice, not in Nrf2-KO mice (Fig. 1, Fig. 2, Fig. 3B, D), suggesting that learning and memory enhancement by HRGE or RGE was mediated through Nrf2 signaling.
Fig. 1.
Oral administration of HRGE improved working memory deficits in scopolamine-treated C57BL/6J mice as assessed by the Y-maze test. (A, B) Nrf2-WT or Nrf2-KO mice were orally administered HRGE or RGE at a designated dosage daily and intraperitoneally injected with scopolamine at 1 mg/kg BW before the test. Spontaneous alterations were evaluated in Nrf2-WT (A) or Nrf2-KO mice (B). Values are expressed as mean ± SD (n = 8). Bars not sharing a common letter are significantly different from each other (p < 0.05).
Fig. 2.
Oral administration of HRGE improved associative learning and memory deficits in scopolamine-treated mice as assessed by the passive avoidance test. (A, B) The latency time for either Nrf2-WT (A) or Nrf2-KO (B) mice staying in the bright chamber, where no electric shock was given, was measured. Values are expressed as mean ± SD (n = 8). Bars not sharing a common letter are significantly different from each other (p < 0.05). There was no significant difference between the values for training trials.
Fig. 3.
Oral administration of HRGE improved spatial learning and memory deficits in scopolamine-treated mice as assessed by the Morris water maze test. The arrival time for each mouse to the platform (A, B) and latency time in the platform quadrant (C, D) were monitored. (A and C) Nrf2-WT mice. (B and D) Nrf2-KO mice. Values are expressed as mean ± SD (n = 8). Bars not sharing a common letter are significantly different from each other (p < 0.05).
3.3. Protective effect of HRGE administration from scopolamine-induced hippocampal damage
The animals were sacrificed at the termination of behavioral tests for the collection of the brain tissues, which were sectioned, stained, and observed for histological injury. While scopolamine treatment caused significant damage in the hippocampal CA1 region, as expected, HRGE administration at 300 mg/kg BW attenuated the scopolamine-induced abnormality (Fig. 4).
Fig. 4.
Oral administration of HRGE ameliorated scopolamine-induced hippocampal damage. The whole-brain tissues were obtained after the mice were sacrificed following completion of the behavioral tests, then sectioned to 5-μm thickness, and stained with H&E for microscopic observation. Representative images are shown at 100 × magnification; the CA1 pyramidal cell layer was further magnified.
3.4. Upregulation of antioxidant enzymes in the hippocampus by HRGE administration
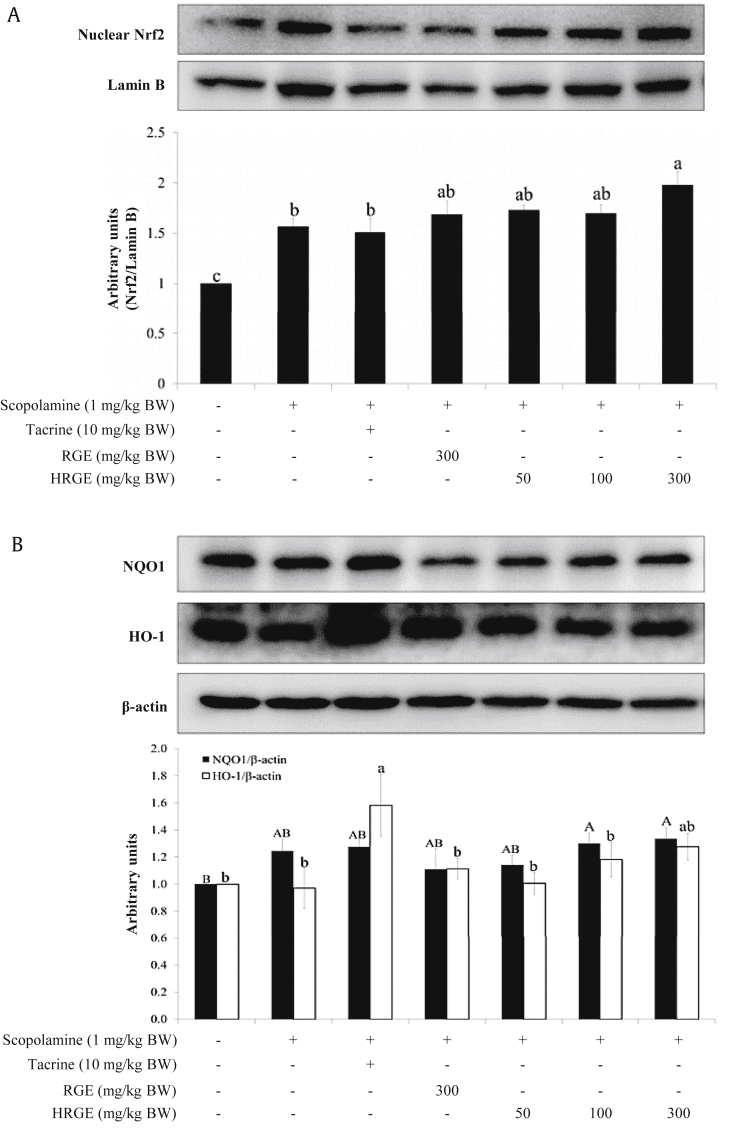
Western blot analysis demonstrated that the oral administration of HRGE or RGE increased the expressions of nuclear Nrf2 (Fig. 5A), as well as cytoplasmic NQO1 and HO-1 in the hippocampus (Fig. 5B). In particular, HRGE treatment at 300 mg/kg BW significantly increased the levels of nuclear Nrf2 and cytoplasmic NQO1 compared with the control (approximately 2- and 1.3-fold increase, respectively). Moreover, hippocampal NQO1 enzyme activity was enhanced by the administration of either RGE or HRGE (Fig. 5C).
Fig. 5.
Oral administration of HRGE upregulated Nrf2 and its downstream antioxidant enzymes in the hippocampal homogenates. The hippocampal tissues were collected from the decapitated Nrf2-WT mice. The protein expression levels of nuclear Nrf2 and cytoplasmic HO-1 (A, B) and the activity of NQO1 were examined (C). Values are expressed as mean ± SD (n = 6). Bars not sharing a common letter are significantly different from each other (p < 0.05).
3.5. Cytoprotective effect of HRGE by reducing intracellular oxidative stress
Mouse hippocampal HT22 cells were challenged with 5 mM glutamate, an inducer of oxidative stress and neuronal cell death, and treated with RGE or HRGE at concentrations of 0, 10, 50, 100, and 250 μg/mL (Fig. 6A). While glutamate treatment caused a prominent reduction of HT22 cell viability, pretreatment with RGE or HRGE attenuated the glutamate-induced cell death in a concentration-dependent manner. In addition, the intracellular ROS level was significantly increased by glutamate treatment (Fig. 6B–D). However, pretreatment with RGE or HRGE concentration-dependently lowered the ROS levels. These findings suggested that RGE and HRGE can protect hippocampal cells from the deleterious effect of ROS-induced oxidative stress.
Fig. 6.
Treatment with RGE or HRGE protected against glutamate-induced HT22 hippocampal neuronal cell death. HT22 cells were treated with 5 mM glutamate to generate cytotoxic conditions or intracellular ROS production. (A) Glutamate-induced cytotoxicity was reduced by RGE or HRGE. (B‒D) Intracellular ROS levels observed by fluorescence microscopy (B) and determined by the DCF assay (C and D) were increased in glutamate-treated cells, but decreased by concomitant treatment with RGE or HRGE. Values are expressed as mean ± SEM (n = 3). Bars not sharing a common letter are significantly different from each other (p < 0.05).
4. Discussion
Ginsenosides, a group of triterpene saponins contained in Korean Red Ginseng, are appreciated for their biologically beneficial effects [43]. Compound K, a bioactive metabolite of PPD-type ginsenosides [12], can be enriched in HRGE by pectinase-mediated hydrolysis of RGE, as reported elsewhere [15,16]. The present study examined the cognition-enhancing effect of orally administered HRGE in scopolamine-induced memory-deficit mice.
Multiple studies have reported that scopolamine, a classical muscarinic receptor antagonist [44], is capable of elevating oxidative stress in the murine hippocampus [[22], [23], [24], [25], [26]], which consequently affects neuronal cell survival and causes neurodegenerative disorders [[28], [29], [30], [31]]. The behavioral examinations in this study demonstrated that the intraperitoneal injection of scopolamine at 1 mg/kg BW induced significant learning and memory impairments and hippocampal damage in mice.
Behavioral tests indicated that oral administration of HRGE at 300 mg/kg BW improved working memory, as well as associative or spatial learning and memory performance in scopolamine-treated mice. Interestingly, these observations manifested only in Nrf2-WT mice, whereas the memory-improving effect of HRGE was not shown in Nrf2-KO mice. This finding indicates that HRGE would likely ameliorate scopolamine-induced cognitive impairment through Nrf2-mediated mechanisms. It is consistent with our unpublished data that neither RGE nor HRGE at the concentrations of up to 500 μg/mL affected acetylcholinesterase activity in vitro, while tacrine significantly inhibited the activity by about 20% at 10 μM and by about 40% at 50 μM (data not shown). Thus, the memory-improving effect of HRGE is presumed to be achieved by stimulating the Nrf2-mediated antioxidant defense system, rather than by affecting cholinergic neurotransmission in the scopolamine-treated mouse brain.
Nrf2 is a redox-sensitive transcription factor that triggers cellular antioxidant response and so protects cells from intrinsic or extrinsic oxidative stress [45,46]. Nrf2 exists in a dimeric form with its cytosolic inhibitor, Kelch-like ECH-associated protein 1 (Keap1), and undergoes ubiquitin-dependent proteasomal degradation in an unstressed condition. Upon exposure to oxidative stress or electrophiles, Nrf2 is liberated from Keap1 and translocated to the nucleus where it activates the transcription of a number of genes that encode antioxidant enzymes, including NQO1, HO-1, glutathione peroxidase, glutathione reductase, and γ-glutamylcysteine synthetase [47,48].
Consistent with previous studies [17,18], RGE and HRGE were found to have strong antioxidative activities by effectively scavenging free radicals and reducing ferric ions in a concentration-dependent manner. In addition, RGE or HRGE treatment decreased the intracellular ROS level and upregulated Nrf2-mediated antioxidant enzymes, thereby increasing cell viability in glutamate-treated hippocampal neuronal cells. These findings further support the notion that the neuronal-cell-protective effects of HRGE and RGE against ROS-producing glutamate insults are, in part, attributable to their antioxidative capabilities.
The brain, with its high oxygen consumption and lipid-rich content, is highly susceptible to oxidative stress, and ROS in the brain is strongly correlated with learning and memory impairment [49,50]. Considering that scopolamine treatment can elevate oxidative stress in the mammalian brain [22,25], it is reasonably speculated that the amelioration of cognitive impairment in the scopolamine-treated mice is a consequence of indirect antioxidant effects of RGE and HRGE or their metabolites. These effects decrease the intracellular ROS level in neuronal cells by promoting Nrf2 activation and subsequent induction of antioxidant enzymes, including NQO1 and HO-1, in the hippocampal region.
The total ginsenoside content was lower in HRGE than in RGE. Nevertheless, HRGE treatment was similar to or more potent than RGE treatment in increasing the expressions of NQO1 and HO-1 and attenuating histological injury in the hippocampus. Considering that compound K is the superior bioactive substance among the ginsenosides [12] and can protect against oxidative-stress-induced neuronal cell death [9], it is conceivable that compound K, which was enriched in HRGE, played a pivotal role in neuroprotection and cognitive improvement via upregulating the intracellular antioxidant defense system, such as antioxidant enzymes. For instance, HO-1 is involved in the conversion of heme into carbon monoxide (CO) and biliverdin. Subsequently, biliverdin is enzymatically transformed into bilirubin, one of the most potent endogenous antioxidants in the body. Furthermore, there are increasing lines of evidence to support that CO is a major player in anti-inflammation, mitochondrial biogenesis, and redox control [51].
In conclusion, orally administered HRGE can exert neuroprotective effects and alleviate scopolamine-induced learning and memory deficits in vivo, most likely through upregulation of Nrf2 and its downstream antioxidant enzymes. The functional components of HRGE and their mechanisms of action in the brain, including the transport of the ginsenoside metabolites across the blood–brain barrier, await further study.
Conflicts of interest
The authors declare that there are no potential conflicts of interest.
Acknowledgments
This work was supported by the “Food Functionality Evaluation program” under the Ministry of Agriculture, Food and Rural Affairs (2015). We thank Katrina Krogh, M.D., from essayreview.co.kr for editing a draft of this manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jgr.2019.12.005.
Contributor Information
Jisun Oh, Email: j.oh@knu.ac.kr.
Jong-Sang Kim, Email: vision@knu.ac.kr.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
Antioxidant activities of RGE or HRGE. (A) DPPH• scavenging capacity, (B) ABTS+• scavenging activity, and (C) ferric-reducing antioxidant power. Data are presented as mean ± SEM (n = 3). Bars not sharing a common letter are significantly different from each other (p < 0.05).
References
- 1.Lee S.M., Bae B.S., Park H.W., Ahn N.G., Cho B.G., Cho Y.L., Kwak Y.S. Characterization of Korean red ginseng (Panax ginseng Meyer): history, preparation method, and chemical composition. J Ginseng Res. 2015;39:384–391. doi: 10.1016/j.jgr.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee S.M., Kim S.C., Oh J., Kim J.H., Na M. 20(R)-Ginsenoside Rf: a new ginsenoside from red ginseng extract. Phytochem Lett. 2013;6:620–624. [Google Scholar]
- 3.Tawab M.A., Bahr U., Karas M., Wurglics M., Schubert-Zsilavecz M. Degradation of ginsenosides in humans after oral administration. Drug Metab Disposition. 2003;31:1065–1071. doi: 10.1124/dmd.31.8.1065. [DOI] [PubMed] [Google Scholar]
- 4.Kim K., Park M., Lee Y.M., Rhyu M.R., Kim H.Y. Ginsenoside metabolite compound K stimulates glucagon-like peptide-1 secretion in NCI-H716 cells via bile acid receptor activation. Arch Pharm Res. 2014;37:1193–1200. doi: 10.1007/s12272-014-0362-0. [DOI] [PubMed] [Google Scholar]
- 5.Shin D.H., Leem D.G., Shin J.S., Kim J.I., Kim K.T., Choi S.Y., Lee M.H., Choi J.H., Lee K.T. Compound K induced apoptosis via endoplasmic reticulum Ca2+ release through ryanodine receptor in human lung cancer cells. J Ginseng Res. 2018;42:165–174. doi: 10.1016/j.jgr.2017.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang Z., Wang J.R., Niu T., Gao S., Yin T.J., You M., Jiang Z.H., Hu M. Inhibition of P-glycoprotein leads to improved oral bioavailability of compound K, an anticancer metabolite of red ginseng extract produced by gut microflora. Drug Metab Disposition. 2012;40:1538–1544. doi: 10.1124/dmd.111.044008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang S., Kim J.E., Song N.R., Jung S.K., Lee M.H., Park J.S., Yeom M.H., Bode A.M., Dong Z.G., Lee K.W. The ginsenoside 20-O-beta-D-Glucopyranosyl-20(S)-Protopanaxadiol induces autophagy and apoptosis in human melanoma via AMPK/JNK phosphorylation. PLoS One. 2014;9 doi: 10.1371/journal.pone.0104305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han J.S., Sung J.H., Lee S.K. Antimelanogenesis activity of hydrolyzed ginseng extract (GINST) via inhibition of JNK mitogen-activated protein kinase in B16F10 cells. J Food Sci. 2016;81:H2085–H2092. doi: 10.1111/1750-3841.13380. [DOI] [PubMed] [Google Scholar]
- 9.Seo J.Y., Ju S.H., Oh J., Lee S.K., Kim J.S. Neuroprotective and cognition-enhancing effects of compound K isolated from red ginseng. J Agric Food Chem. 2016;64:2855–2864. doi: 10.1021/acs.jafc.5b05789. [DOI] [PubMed] [Google Scholar]
- 10.Park J.S., Shin J.A., Jung J.S., Hyun J.W., Le T.K.V., Kim D.H., Park E.M., Kim H.S. Anti-inflammatory mechanism of compound K in activated microglia and its neuroprotective effect on experimental stroke in mice. J Pharmacol Exp Ther. 2012;341:59–67. doi: 10.1124/jpet.111.189035. [DOI] [PubMed] [Google Scholar]
- 11.Hou J.G., Xue J.J., Lee M.R., Sun M.Q., Zhao X.H., Zheng Y.N., Sung C.K. Compound K is able to ameliorate the impaired cognitive function and hippocampal neurogenesis following chemotherapy treatment. Biochem Biophys Res Commun. 2013;436:104–109. doi: 10.1016/j.bbrc.2013.05.087. [DOI] [PubMed] [Google Scholar]
- 12.Oh J., Kim J.S. Compound K derived from ginseng: neuroprotection and cognitive improvement. Food Funct. 2016;7:4506–4515. doi: 10.1039/c6fo01077f. [DOI] [PubMed] [Google Scholar]
- 13.Park C.S., Yoo M.H., Noh K.H., Oh D.K. Biotransformation of ginsenosides by hydrolyzing the sugar moieties of ginsenosides using microbial glycosidases. Appl Microbiol Biotechnol. 2010;87:9–19. doi: 10.1007/s00253-010-2567-6. [DOI] [PubMed] [Google Scholar]
- 14.Zheng M.M., Xu F.X., Li Y.J., Xi X.Z., Cui X.W., Han C.C., Zhang X.L. Study on transformation of ginsenosides in different methods. Biomed Res Int. 2017;2017:8601027. doi: 10.1155/2017/8601027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin H., Seo J.H., Uhm Y.K., Jung C.Y., Lee S.K., Yim S.V. Pharmacokinetic comparison of ginsenoside metabolite IH-901 from fermented and non-fermented ginseng in healthy Korean volunteers. J Ethnopharmacol. 2012;139:664–667. doi: 10.1016/j.jep.2011.11.052. [DOI] [PubMed] [Google Scholar]
- 16.Kopalli S.R., Cha K.M., Lee S.H., Ryu J.H., Hwang S.Y., Jeong M.S., Sung J.H., Kim S.K. Pectinase-treated Panax ginseng protects against chronic intermittent heat stress-induced testicular damage by modulating hormonal and spermatogenesis-related molecular expression in rats. J Ginseng Res. 2017;41:578–588. doi: 10.1016/j.jgr.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramesh T., Kim S.W., Sung J.H., Hwang S.Y., Sohn S.H., Yoo S.K., Kim S.K. Effect of fermented Panax ginseng extract (GINST) on oxidative stress and antioxidant activities in major organs of aged rats. Exp Gerontol. 2012;47:77–84. doi: 10.1016/j.exger.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Won Y.J., Kim B.K., Shin Y.K., Jung S.H., Yoo S.K., Hwang S.Y., Sung J.H., Kim S.K. Pectinase-treated Panax ginseng extract (GINST) rescues testicular dysfunction in aged rats via redox-modulating proteins. Exp Gerontol. 2014;53:57–66. doi: 10.1016/j.exger.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Park S.H., Oh M.R., Choi E.K., Kim M.G., Ha K.C., Lee S.K., Kim Y.G., Park B.H., Kim D.S., Chae S.W. An 8-wk, randomized, double-blind, placebo-controlled clinical trial for the antidiabetic effects of hydrolyzed ginseng extract. J Ginseng Res. 2014;38:239–243. doi: 10.1016/j.jgr.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ebert U., Kirch W. Review - scopolamine model of dementia: electroencephalogram findings and cognitive performance. Eur J Clin Invest. 1998;28:944–949. doi: 10.1046/j.1365-2362.1998.00393.x. [DOI] [PubMed] [Google Scholar]
- 21.Klinkenberg I., Blokland A. The validity of scopolamine as a pharmacological model for cognitive impairment: a review of animal behavioral studies. Neurosci Biobehav Rev. 2010;34:1307–1350. doi: 10.1016/j.neubiorev.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 22.El-Sherbiny D.A., Khalifa A.E., Attia A.S., Eldenshary Eel D. Hypericum perforatum extract demonstrates antioxidant properties against elevated rat brain oxidative status induced by amnestic dose of scopolamine. Pharmacol Biochem Behav. 2003;76:525–533. doi: 10.1016/j.pbb.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 23.El-Khadragy M.F., Al-Olayan E.M., Abdel Moneim A.E. Neuroprotective effects of Citrus reticulata in scopolamine-induced dementia oxidative stress in rats. CNS Neurol Disord Drug Targets. 2014;13:684–690. doi: 10.2174/1871527313666140618105404. [DOI] [PubMed] [Google Scholar]
- 24.Budzynska B., Boguszewska-Czubara A., Kruk-Slomka M., Skalicka-Wozniak K., Michalak A., Musik I., Biala G. Effects of imperatorin on scopolamine-induced cognitive impairment and oxidative stress in mice. Psychopharmacology (Berl) 2015;232:931–942. doi: 10.1007/s00213-014-3728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan Y., Hu J., Li J., Yang Z., Xin X., Wang J., Ding J., Geng M. Effect of acidic oligosaccharide sugar chain on scopolamine-induced memory impairment in rats and its related mechanisms. Neurosci Lett. 2005;374:222–226. doi: 10.1016/j.neulet.2004.10.063. [DOI] [PubMed] [Google Scholar]
- 26.Skalicka-Wozniak K., Budzynska B., Biala G., Boguszewska-Czubara A. Scopolamine-induced memory impairment is alleviated by xanthotoxin: role of acetylcholinesterase and oxidative stress processes. ACS Chem Neurosci. 2018;9:1184–1194. doi: 10.1021/acschemneuro.8b00011. [DOI] [PubMed] [Google Scholar]
- 27.Venkatesan R., Subedi L., Yeo E.J., Kim S.Y. Lactucopicrin ameliorates oxidative stress mediated by scopolamine-induced neurotoxicity through activation of the NRF2 pathway. Neurochem Int. 2016;99:133–146. doi: 10.1016/j.neuint.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 28.Clausen A., Doctrow S., Baudry M. Prevention of cognitive deficits and brain oxidative stress with superoxide dismutase/catalase mimetics in aged mice. Neurobiol Aging. 2010;31:425–433. doi: 10.1016/j.neurobiolaging.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu R.L., Liu I.Y., Bi X.N., Thompson R.F., Doctrow S.R., Malfroy B., Baudry M. Reversal of age-related learning deficits and brain oxidative stress in mice with superoxide dismutase/catalase mimetics. Proc Natl Acad Sci U S A. 2003;100:8526–8531. doi: 10.1073/pnas.1332809100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Z., Zhou T., Ziegler A.C., Dimitrion P., Zuo L. Oxidative stress in neurodegenerative diseases: from molecular mechanisms to clinical applications. Oxid Med Cell Longev. 2017;2017:2525967. doi: 10.1155/2017/2525967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun K.Y., Bai Y.T., Zhao R., Guo Z.J., Su X., Li P.Q., Yang P.Y. Neuroprotective effects of matrine on scopolamine-induced amnesia via inhibition of AChE/BuChE and oxidative stress. Metab Brain Dis. 2019;34:173–181. doi: 10.1007/s11011-018-0335-y. [DOI] [PubMed] [Google Scholar]
- 32.Xiong W., MacColl Garfinkel A.E., Li Y., Benowitz L.I., Cepko C.L. NRF2 promotes neuronal survival in neurodegeneration and acute nerve damage. J Clin Invest. 2015;125:1433–1445. doi: 10.1172/JCI79735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park H.W., In G., Han S.T., Lee M.W., Kim S.Y., Kim K.T., Cho B.G., Han G.H., Chang I.M. Simultaneous determination of 30 ginsenosides in Panax ginseng preparations using ultra performance liquid chromatography. J Ginseng Res. 2013;37:457–467. doi: 10.5142/jgr.2013.37.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seo J.Y., Kim B.R., Oh J., Kim J.S. Soybean-Derived phytoalexins improve cognitive function through activation of Nrf2/HO-1 signaling pathway. Int J Mol Sci. 2018;19:268. doi: 10.3390/ijms19010268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seo J.Y., Lim S.S., Kim J., Lee K.W., Kim J.S. Alantolactone and isoalantolactone prevent amyloid beta25-35 -induced toxicity in mouse cortical neurons and scopolamine-induced cognitive impairment in mice. Phytother Res. 2017;31:801–811. doi: 10.1002/ptr.5804. [DOI] [PubMed] [Google Scholar]
- 36.Seo H., Oh J., Hahn D., Kwon C.S., Lee J.S., Kim J.S. Protective effect of glyceollins in a mouse model of dextran sulfate sodium-induced colitis. J Med Food. 2017;20:1055–1062. doi: 10.1089/jmf.2017.3960. [DOI] [PubMed] [Google Scholar]
- 37.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 38.Benson A.M., Hunkeler M.J., Talalay P. Increase of NAD(P)H:quinone reductase by dietary antioxidants: possible role in protection against carcinogenesis and toxicity. Proc Natl Acad Sci U S A. 1980;77:5216–5220. doi: 10.1073/pnas.77.9.5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oh J., Lee N.K. Schizandrin reduces cytoplasmic TDP-43 accumulation in hippocampal neuronal cells. Biotechnol Bioproc E. 2017;22:9–13. [Google Scholar]
- 40.Oh J., Jeon S.B., Lee Y., Lee H., Kim J., Kwon B.R., Yu K.Y., Cha J.D., Hwang S.M., Choi K.M. Fermented red ginseng extract inhibits cancer cell proliferation and viability. J Med Food. 2015;18:421–428. doi: 10.1089/jmf.2014.3248. [DOI] [PubMed] [Google Scholar]
- 41.Woo Y., Lee H., Jeong Y.S., Shin G.Y., Oh J.G., Kim J.S., Oh J. Antioxidant potential of selected Korean edible plant extracts. Biomed Res Int. 2017;2017:7695605. doi: 10.1155/2017/7695605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang H., Joseph J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radical Biol Med. 1999;27:612–616. doi: 10.1016/s0891-5849(99)00107-0. [DOI] [PubMed] [Google Scholar]
- 43.Shin B.K., Kwon S.W., Park J.H. Chemical diversity of ginseng saponins from Panax ginseng. J Ginseng Res. 2015;39:287–298. doi: 10.1016/j.jgr.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Falsafi S.K., Deli A., Hoger H., Pollak A., Lubec G. Scopolamine administration modulates muscarinic, nicotinic and NMDA receptor systems. PLoS One. 2012;7 doi: 10.1371/journal.pone.0032082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kensler T.W., Wakabayash N., Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 46.Itoh K., Chiba T., Takahashi S., Ishii T., Igarashi K., Katoh Y., Oyake T., Hayashi N., Satoh K., Hatayama I. An Nrf2 small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 47.Kaspar J.W., Niture S.K., Jaiswal A.K. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radical Biol Med. 2009;47:1304–1309. doi: 10.1016/j.freeradbiomed.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Itoh K., Wakabayashi N., Katoh Y., Ishii T., Igarashi K., Engel J.D., Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu R., Liu I.Y., Bi X., Thompson R.F., Doctrow S.R., Malfroy B., Baudry M. Reversal of age-related learning deficits and brain oxidative stress in mice with superoxide dismutase/catalase mimetics. Proc Natl Acad Sci U S A. 2003;100:8526–8531. doi: 10.1073/pnas.1332809100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Padurariu M., Ciobica A., Hritcu L., Stoica B., Bild W., Stefanescu C. Changes of some oxidative stress markers in the serum of patients with mild cognitive impairment and Alzheimer's disease. Neurosci Lett. 2010;469:6–10. doi: 10.1016/j.neulet.2009.11.033. [DOI] [PubMed] [Google Scholar]
- 51.Motterlini R., Otterbein L.E. The therapeutic potential of carbon monoxide. Nat Rev Drug Discov. 2010;9:728–743. doi: 10.1038/nrd3228. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Antioxidant activities of RGE or HRGE. (A) DPPH• scavenging capacity, (B) ABTS+• scavenging activity, and (C) ferric-reducing antioxidant power. Data are presented as mean ± SEM (n = 3). Bars not sharing a common letter are significantly different from each other (p < 0.05).