Abstract
Volumetric muscle loss injury is a common health problem with long-term disabilities. One common treatment is using muscle flaps from donor site, which has limited potentials due to donor site availability and morbidity. Although several stem cell therapies have been evaluated so far, most suffer from limited availability, immune incompatibility, or differentiation potential. Therefore, induced pluripotent stem cells (iPSCs) have a great promise for this purpose due to their unique differentiation, self-renewal, and immunocompatibility. Current study was designed to determine therapeutic potential of human iPSCs (hiPSCs) in a mouse model of volumetric muscle loss. Muscles were subjected to excision to generate 30%–40% muscle loss. Next, hiPSCs were differentiated toward skeletal myogenic progenitors and used with fibrin hydrogel to reconstruct the lost muscle. Histologic evaluation of the treated muscles indicated abundant engraftment of donor-derived mature fibers expressing human markers. Donor-derived fibers were also positive for the presence of neuromuscular junction (NMJ), indicating their proper innervation. Evaluation of the engrafted region indicated the presence of donor-derived satellite cells expressing human markers and Pax7. Finally, in situ muscle function analysis demonstrated significant improvement of the muscle contractility in muscles treated with hiPSCs. These results therefore provide key evidence for the therapeutic potential of human iPSCs in volumetric muscle loss injuries.
Keywords: human iPSCs, volumetric muscle loss (VML), stem cells, engraftment, innervation, satellite cells, muscle stem cells, functional recovery
Graphical Abstract

Volumetric muscle loss (VML) injuries are common during trauma and combat or after tissue resection. Unfortunately, large VMLs are often incurable, leading to functional disabilities. In this article, Darabi and colleagues describe a novel method using human iPSCs for the treatment of VML injury in a mouse model.
Introduction
Muscle-mass-loss injuries are one of the major causes of hospitalization and disabilities in the United States.1 They are very common during severe trauma and accidents or in training and combat situations in armed forces and usually involve severe musculoskeletal injuries, such as multiple wounds in extremities.2, 3, 4 Although skeletal muscle has a tremendous regenerative potential to recover after minor to moderate tissue injuries,5, 6, 7 once the damage and muscle loss exceed this capacity, it leads to massive volumetric muscle loss (VML).8,9 Due to extensive loss of muscle mass, its stem cells, and bio-scaffold, the endogenous repair mechanism of the muscle cannot self-repair.8,10,11 Therefore, damaged muscle is often replaced by fibrotic tissue, leading to defective muscle function and different levels of disability.9 Indeed, musculoskeletal injuries are one of the leading causes of disability in traumatic patients and veterans and create a substantial financial cost for the health system.2,3
Although tissue-replacement therapy using muscle flaps from a donor site is a potential therapeutic option, it is often limited to the small injuries due to limitations of the donor muscle site availability and its possible morbidity.12 Therefore, there is substantial need for outsourcing muscle mass for replacement. The ideal muscle transplant must be immune compatible and, upon implantation, be able to integrate with the recipient muscle to support proper perfusion and innervation and eventually contribute to functional recovery.13 To this end, one of the ideal candidates for VML repair would be using stem cells along with biocompatible scaffolds to generate 3D muscle constructs and implants.14
Considering limited source availability, expansion/differentiation potential, or immunogenicity of different stem cell types or muscle progenitors (such as satellite cells or myoblasts), one of the ideal stem cell types for this application would be human induced pluripotent stem cells (hiPSCs).15 With the recent advancement of integration-free reprogramming of the iPSCs16,17 and availability of efficient myogenic differentiation protocols developed by several groups, including us,18, 19, 20, 21, 22 hiPSCs can be considered as a great candidate for generation of biocompatible muscle implants for VML injuries. Therefore, the primary goal of this study—as a proof of principle—was to evaluate the engraftment and functional improvement potential of hiPSC-derived myogenic progenitors in a VML mouse model. hiPSCs were differentiated into myogenic progenitors using a recently developed protocol, which allows efficient myogenic induction and purification in hiPSCs in a very short time.18 To provide a biocompatible scaffold, a fibrin gel along with hiPSC-derived myogenic progenitors was used to cast the defect in situ—as previously validated.23 Using cohort control groups, in situ muscle functional assay was also performed for a sensitive and precise evaluation of the functional recovery of the muscles at the end of study time course.
Results
Differentiation and Purification of the hiPSC-Derived Myogenic Progenitors
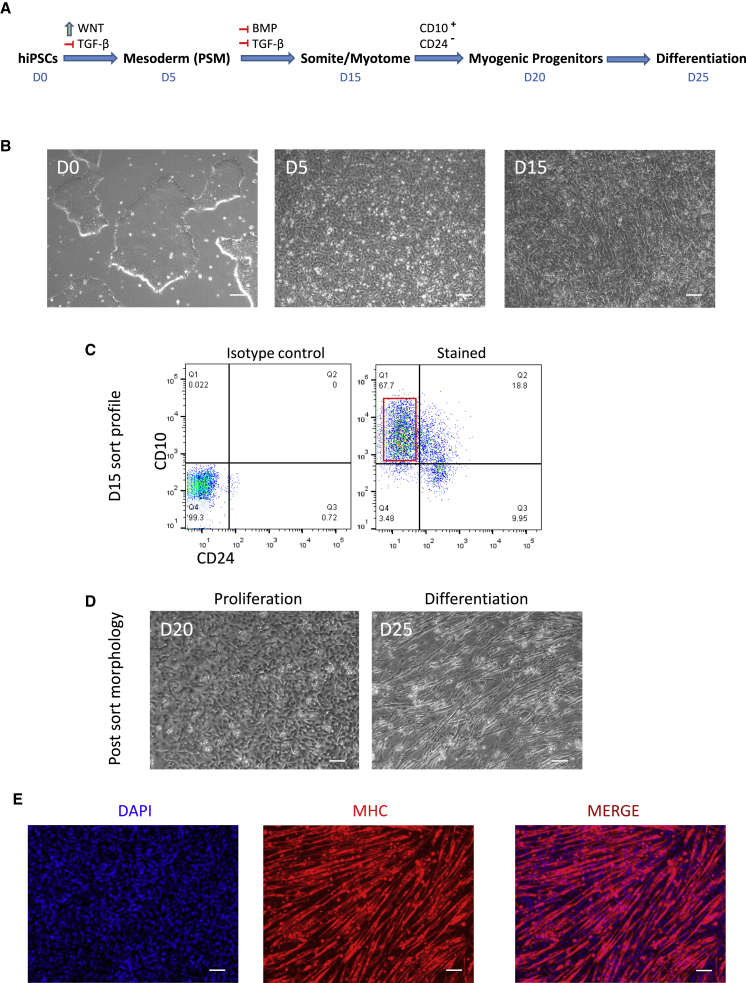
In order to induce myogenic progenitors from hiPSCs, we have used a recently developed protocol.18 As demonstrated in Figures 1A and 1B, hiPSCs were differentiated toward presomitic mesoderm (PSM) by induction of WNT signaling. Next, further maturation of somite/myogenic progenitors were induced by inhibition of BMP and transforming growth factor β (TGF-β) for another 10 days. Finally, at day 15, myogenic progenitors were enriched by sorting for CD10+CD24− fraction.18 As displayed in Figure 1C, 67.7% of the cells were positive for myogenic lineage at day 15 of differentiation, which is in agreement with the published method.18 After the sort (Figure 1D), myogenic progenitors were expanded for another week and finally differentiated to myotubes to confirm their myogenic potential. This is demonstrated in Figure 1E, which indicates uniform formation of myotubes expressing myosin heavy chain (MHC) after 5 days of differentiation (detailed characterization, gene expression, and quantification profile of the cells during different stages of differentiation has been previously published).18 After validation of the differentiation potential, myogenic cells were used for in vivo assays below.
Figure 1.
Derivation of Skeletal Myogenic Progenitors from Human iPSCs
Directed differentiation of hiPSCs toward myogenic lineage using small molecules and purification of the cells using surface markers. (A) Schematic figure shows stepwise differentiation of hiPSCs toward mesoderm and myotomal cells using small molecules and growth factors and their timeline. (B) Bright-field images demonstrate morphology transition of the cells during D0–D15. (C) Dot plots demonstrate expression profile of the differentiated cells at day 15 for CD10 and CD24. Red box marks sorting gate for CD10+CD24− myogenic progenitors. (D) Bright-field images demonstrate morphology of the sorted myogenic cells during proliferation and after differentiation. (E) Differentiated myotubes stained for myosin heavy chain and DAPI are shown. Scale bars: 100 μm.
Engraftment Potential of hiPSC-Derived Myogenic Progenitors into the VML Mouse Model
In order to evaluate the engraftment potential of the hiPSC-derived myogenic progenitors into VML model, tibialis anterior (TA) muscles of the adult immunodeficient NSG mice (n = 6 per group) were used to create a VML model as described before.23 As shown in Figure 2A, after the creation of a 3 × 3 × 6 mm wedge excision (VML), mice were divided into three experimental groups (untreated, gel alone, and gel + cells) and treated accordingly. Figure 2B demonstrates the extent of the muscle bio-scaffold loss (laminin staining) after creation of VML model and its restoration upon treatment. As shown (marked area, Figure 2B), the VML creates a large defect in muscle bio-scaffold. Next, the defects were either left untreated as control or treated with gel alone or gel + cells. For the gel + cells, 3 × 105 hiPSC-derived myogenic cells were resuspended in a 10 μL fibrinogen solution and used for in situ casting as described previously.23 Figure 2C demonstrates the site of VML injury before and after in situ casting with gel + cell compound. 5 weeks after the treatment, muscles were harvested for histological evaluation. As expected, untreated or gel-alone-treated muscles did not contain any human-derived cells and showed signs of tissue scarring and fibrosis (white arrows, Figure 2D, upper panels). However, muscles treated with combination of hiPSC-derived myogenic progenitors and gel demonstrated efficient engraftment of the donor cells into the defect zone and formed regenerated fibers expressing human-specific markers. As shown in Figures 2D and 2E, engrafted cells were able to generate newly formed donor-derived fibers expressing human dystrophin and human lamin A/C proteins. Quantification of the fibers within the defect zone (Figure 2F) indicated the majority of the myofibers in the damaged region express human dystrophin (73% ± 2.6%; mean ± SEM). Interestingly, remaining areas in the damaged zones contained mouse-derived fibers (i.e., negative for human-specific markers; 27% ± 3.2%; mean ± SEM), indicating mutual partnership between donor and host myogenic cells to regenerate the damaged region. Further quantification based on the zone of engraftment (Figure 2G) indicated better engraftment of the hiPSC-derived cells and hybrid fibers in the contact zone area between host and implant region, while with more abundance of the host-derived fibers in periphery region (away from the contact points between host muscle and implant, as marked in Figure 2G). This might be due to better migration or survival potential of the host myogenic progenitors away from contact points of the graft. All together, these data confirmed the engraftment ability of hiPSC-derived myogenic progenitors into the VML mouse model.
Figure 2.
Engraftment Potential of hiPSC-Derived Myogenic Progenitors into VML Injury Model
(A) Schematic figure shows generation of the VML injury model in the TA muscle and experimental groups (n = 6 mice per group). (B) Cross section images stained for laminin demonstrate the loss of muscle bio-scaffold following VML injury and its restoration after the treatment. (C) Images demonstrate the VML injury site before and after in situ gel casting using 3 × 105 cells resuspended in a 10 μL fibrinogen solution. (D) Treated muscles cross-sections stained for human-specific markers are shown (human dystrophin in red and human lamin A/C in green). Upper panel demonstrates a control section treated with gel alone. There is no evidence of human cell markers, and white arrows mark fibrotic areas. Lower panel demonstrates a representative section of the stem-cell-treated groups, indicating the presence of the myofibers positive for human dystrophin and lamin A/C. (E) High magnification of the engrafted region demonstrates myofibers positive with human-specific dystrophin and their associated human nuclei stained for human lamin A/C (peripherally located marked with yellow and centrally located marked with white arrows). (F) Quantification of the human-dystrophin-positive and negative fibers in damaged zone is shown. Data are presented as mean ± SEM. (G) Regional quantification and percent abundance of donor, hybrid, and mouse fibers within the transplanted region. Schematic image on the left marks contact zone and the periphery area of the transplanted region. Data are presented as mean ± SEM. ∗∗∗p < 0.001. Scale bars: 100 μm.
Innervation Potential of the hiPSC-Derived Fibers after Engraftment into the VML Mouse Model
As one of the important aspects of skeletal muscle regeneration is the proper innervation of the newly formed muscle fibers, we evaluated donor-derived fibers for the presence of innervation sites. This was done by specific staining for neuromuscular junctions (NMJs), which is the synapse between the axon terminal of the motor neuron and the highly excitable part of the fiber membrane, the motor endplate.24 Therefore, if engrafted myofibers are truly contributing to the contractile function of the muscle and its force generation, they should also be innervated by motor neurons through formation of new NMJs. In order to identify the presence of the NMJs, engrafted muscles were stained for the presence of a donor marker (human dystrophin), synaptophysin, and α-bungarotoxin. Synaptophysin is a presynaptic regulator of vesicle fusion and is expressed at the presynaptic nerve terminal of the motor neuron at its target muscle's NMJ.25 On the other hand, α-bungarotoxin has a highly specific affinity to the α-subunit of the nicotinic acetylcholine receptor (AChR) of the postsynaptic membrane of the NMJ.26 Thus, if the new fibers are properly innervated by motor neurons, both markers should be expressed and co-localized accordingly at the NMJ site. These data are presented in Figures 3A and 3B. As demonstrated, we were able to identify the presence of donor-derived fibers (positive for human dystrophin in red) containing properly formed NMJs (expressing both synaptophysin and AChR). Figure 3B demonstrates higher magnification of the selected area (yellow box in Figure 3A). As shown, donor-derived fibers expressing human dystrophin (marked with yellow arrows) contained properly formed NMJs with co-localized presynaptic and postsynaptic membranes positive for synaptophysin and AChR. Analysis of the longitudinal sections of the treated muscles also indicated similar topographical and well-localized distribution of NMJs compared to control muscles (Figure S1). Furthermore, evaluation of the myofiber nuclei position (Figure 3C) indicated that majority of the hiPSC donor-derived fibers contain peripherally located nuclei (82% ± 7%, mean ± SEM), which can be considered in favor of terminal maturation and possible innervation. Higher levels of mature donor fibers with peripherally located nuclei in VML model might also be related to the absence of ongoing degeneration/regeneration cycle in this model (as opposed to dystrophic mice models, such as mdx), which allows faster maturation of the donor fibers in VML. Altogether, these data confirmed innervation of hiPSC-derived myofibers through formation of NMJs.
Figure 3.
Evaluation of the hiPSC-Derived Myofibers for the Presence of NMJ
(A) Engrafted muscles were stained for human-specific dystrophin, synaptophysin, and AChR (α-bungarotoxin) to identify hiPSC-derived myofibers positive for NMJ. (B) High-magnification images from the engrafted site (yellow box) indicate co-localization of synaptophysin and AChR (marked by α-bungarotoxin), indicating the presence of NMJ in human-iPSC-derived fibers. Yellow arrows mark positive donor fibers for human dystrophin, synaptophysin, and α-bungarotoxin marking NMJs. White arrow marks a host-derived fiber positive for NMJ and negative for human dystrophin in the engrafted region. (C) Quantification of the donor-derived fibers for the location of the myofibers nuclei is shown. Percent of the regenerating (with centrally located nuclei) and mature fibers (with peripherally located nuclei) is shown. Data are presented as mean ± SEM. Scale bars: 100 μm.
Restoration of Muscle Stem Cells and Vasculature after Engraftment of hiPSC-Derived Myogenic Cells
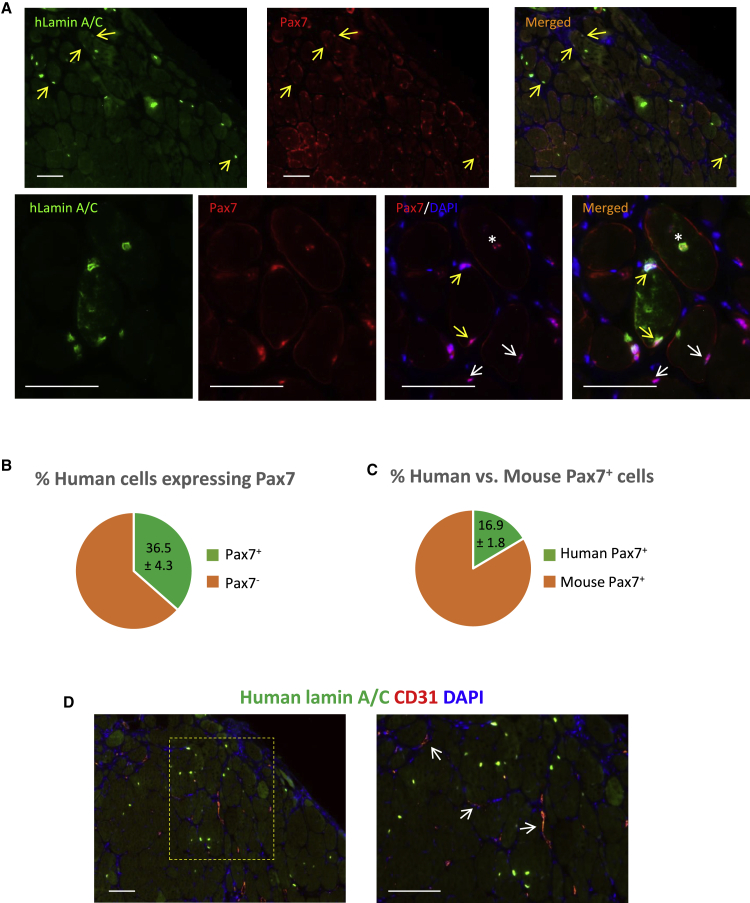
As one of the important aspects of skeletal muscle regeneration is the restoration of muscle stem cell compartment, we searched for any evidence supporting seeding of the satellite cells with donor hiPSC-donor-derived cells. Therefore, engrafted muscles were sectioned and stained for human-specific lamin A/C antibody, which marks human cell nuclei, along with Pax7, which stains muscle stem cells (satellite cells). These data are presented in Figure 4A. As demonstrated, we were able to identify a number of human-iPSC-derived Pax7-positive satellite cells in the engrafted region (Figure 4A, upper panel). As demonstrated in high-magnification images in the lower panel, human-derived satellite cells (marked with yellow arrows) can be identified as positive nuclei expressing both Pax7 and human lamin A/C while positioned in the right location of the satellite cells at the periphery of the myofibers. As expected, recipient (mouse) satellite cells expressing Pax7 and negative for human lamin A/C marker (marked with white arrows), as well as regenerating/activated donor-derived myogenic progenitors positive for both markers and located central to the regenerating fiber (marked with white asterisk), were also detectable in the representative section. Further quantitative analysis of the sections (Figure 4B) indicated a considerable percentage of human-derived cells express Pax7 (36.6% ± 4.3%; mean ± SEM) and are being positioned in the correct location of the satellite cell. In addition, 16.4% ± 1.8% (mean ± SEM) of the total Pax7-positive satellite cells (mouse + human) in the engrafted regions were donor derived (Figure 4C). These data indicated that hiPSC-derived myogenic progenitors are able to restore muscle stem cells upon transplantation into VML mouse model.
Figure 4.
Evaluation of the Presence of Donor-Derived Pax7+ Muscle Stem Cells and Restoration of Vasculature in Engrafted Muscles
In order to evaluate in vivo satellite cell formation potential of hiPSC-derived cell, sections were stained for a human marker (lamin A/C) and Pax7. (A) Low and high magnifications of the engrafted muscles demonstrate the presence of donor-derived satellite cells. Yellow arrows mark donor-derived satellite cells. White arrows in lower panel mark recipient satellite cells. White asterisk in lower panel marks a regenerating human cell nucleus located at the center of myofiber while still positive for Pax7. (B) Percent of hiPSC-derived cells positive for Pax7 is shown. (C) Percent of human- versus mouse-derived Pax7-positive cells in engrafted regions is shown. (D) Images stained for CD31 and human lamin A/C demonstrate formation of the host-derived vessels in the engrafted region. Data are mean ± SEM. Scale bars: 100 μm.
Finally, to identify vasculature regrowth and the source of the newly formed vessels in the engrafted regions, sections were stained with CD31 to mark endothelial cells and human lamin A/C to identify donor cells. These data are demonstrated in Figure 4D, which indicates restoration of the blood vessels in the regenerated region. As expected, CD31-positive vessels are host-derived (mouse) and human iPSC-derived myogenic cells only contributed to the formation of myofibers as expected. These data highlight the important role of the host-derived endothelial cells in revascularization of the engrafted region in VML model after cell/bio-scaffolds repair.
Functional Recovery of the VML Muscles Treated with hiPSC-Derived Myogenic Progenitors
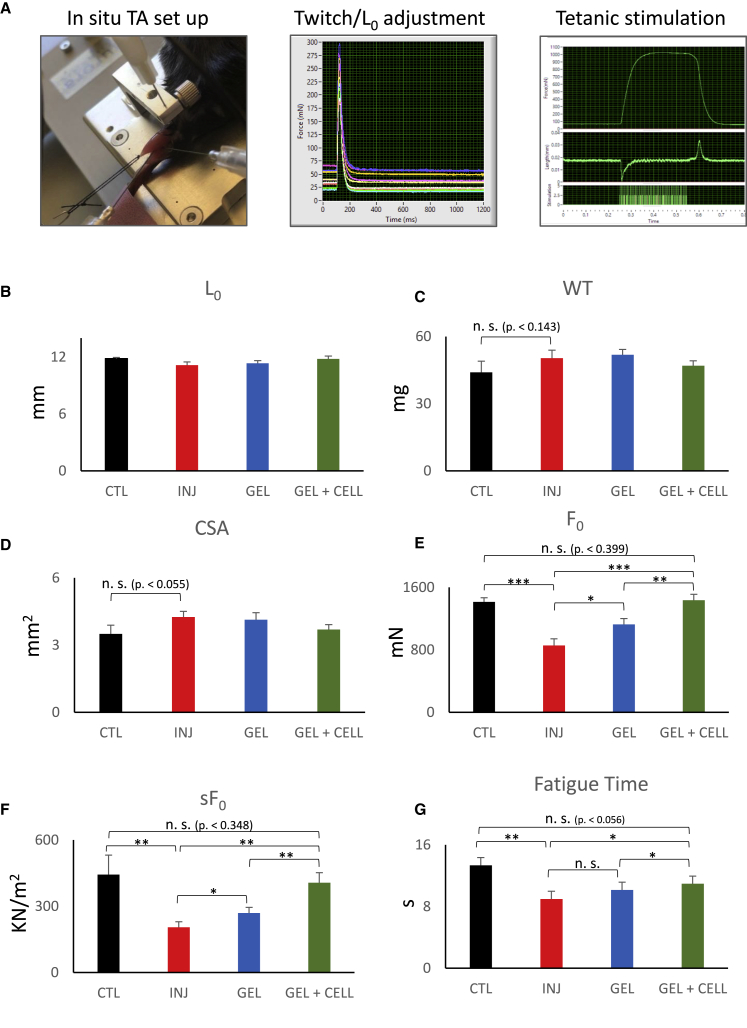
As one of the most common morbidities in VML injuries is muscle function deficit and disability, measurement of muscle function is an important parameter for evaluation of any treatment efficacy. Although in vitro organ bath muscle physiologic studies are a common approach for small murine muscles (such as extensor digitorum longus [EDL]), its sensitivity is reduced in case of larger muscles, such as TA, due to the reduced perfusion and subsequent muscle hypoxia in the organ bath. To circumvent this, in situ muscle function platform is advantageous, as it allows physiological perfusion of the muscle and therefore a more-sensitive readout of the TA contractility. As represented in Figure 5A, after general anesthesia, in this method the distal tendon of the TA muscle is dissected and connected to a force transducer (while still perfused physiologically) and will be stimulated using a pair of electromyography electrodes. Next, muscles are stimulated by a set of incremental twitches (Figure 5A, middle panel) to determine optimal length (L0), followed by tetanic and fatigue test protocols (Figure 5A, right panel). We therefore evaluated muscle function parameters in four experimental groups of control (uninjured), VML injured without treatment, gel-alone-treated, and gel + cell-treated cohort groups (n = 6 mice/group) using this set up.
Figure 5.
Evaluation of the Functional Recovery in VML Injury Muscles Treated with hiPSC-Derived Myogenic Progenitors
Muscle function assays were done using in situ muscle function analysis method (n = 6 mice/group). (A) In situ muscle setup demonstration and examples of twitch contraction optimization and the tetanic stimulation of the muscle. (B) Graph bars demonstrate the average of optimal muscle lengths among experimental groups. (C) Average weight of the TA muscles among experimental groups is shown. (D) Cross section area (CSA) of the studied muscles is shown. (E) Graph demonstrates average values of the F0 among studied groups and their statistical significance. As demonstrated, F0 was significantly reduced following VML injury in untreated group. Cell + gel-treated group demonstrated highest significant force recovery. (F) Specific force (sF0) value of the experimental groups indicates significant force recovery after treatment. (G) Fatigue resistance time among experimental groups is shown. Data are presented as mean ± SEM. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
As demonstrated in Figures 5B and 5C, we did not observe any significant differences in the length and weight of the studied muscles. Although average weight of the TA muscle inclined to be higher in injured and gel-alone-treated groups compared to control, it was not statistically significant. Similar observation was noted in cross section area (CSA) of the studied muscles (Figure 5D). However, maximal isometric tetanic force (F0) showed significant differences among studied groups. In case of the injury without treatment group, the average F0 was significantly reduced to almost 60% of its normal value, indicating inability of the endogenous muscle repair to recover from VML injury (Figure 5E). Interestingly, gel-alone-treated group demonstrated a slight improvement in F0 value compared to injury. The most-significant F0 recovery was found in the gel + cell-treated muscles, which was able to restore F0 to control value (Figure 5E). When normalized to muscle CSA, specific force (sF0) value also demonstrated similar trends, indicating the bio-scaffold and stem cell therapy can improve overall muscle function in the VML model (Figure 5F). In addition, as demonstrated in the last graph, fatigue resistance time was also recovered back to almost normal levels after therapy only in the gel + cell-treated animals (Figure 5G). Altogether, these data confirmed the ability of the hiPSC therapy in functional improvement of the VML in current murine model.
Discussion
VML injury is a very common type of muscle damage, which mainly happens after trauma, combat injuries, or during surgical resection of tumors.3,27 As the endogenous machinery of the muscle regeneration (i.e., muscle stem cells) generally fails to completely repair and reconstitute the lost muscle due to massive tissue destruction, VML often leads to different degrees of muscle dysfunction and disability.2,9 Current treatments for VML include wound cleaning, autologous muscle flap transfer, and rehabilitative therapies.12,28 However, they often have limited benefits, especially in case of massive VMLs, mainly due to donor-site morbidity and graft failure.29,30 Therefore, during recent years, several attempts have been implemented toward using different extracellular matrices (such as hydrogels and acellularized bio-scaffolds) and stem cells to determine optimal therapeutic options.14 In this regard, hiPSCs are a major game changer among other stem cell types due to their unique pluripotency/differentiation as well as their self-renewal abilities.31, 32, 33, 34, 35 In addition, because they can be derived from the same patient (autologous), they have the advantage of full immunocompatibility. Considering the source limitation of other stem cell candidates, such as satellite cells, iPSCs have another advantage of unlimited expansion and self-renewal potential and therefore are on the top of the candidate list for regenerative medicine.15 Among all these advantages, surprisingly, the field of VML therapy is lagging behind in using hiPSCs for this purpose. One of the main reasons for this delay can be attributed to the lack of efficient myogenic differentiation method for hiPSCs. Fortunately, with the recent advancement in the field, now there are several protocols for derivation of engraftable myogenic progenitors from hiPSCs.18, 19, 20, 21, 22 As our lab is interested in iPSC-based muscle regeneration, the main aim of the current study was to investigate the therapeutic efficacy of hiPSCs for VML injuries. Because the focus was on the stem cell side of the regeneration, for the bio-scaffold, we used a well-known, fibrin-based hydrogel, which was previously validated for VML reconstruction in similar murine model.23 One advantage of the current myogenic differentiation method is using directed differentiation approach for myogenic induction, lack of transgene integration or gene overexpression, and finally purification of the myogenic progenitors using surface markers.18 Current engraftment data also clearly confirm formation of abundant donor myofibers expressing human proteins within the regenerated VML site. Interestingly, new donor-derived fibers not only demonstrated mature fiber phenotypes in vivo (such as peripherally located nuclei and expression of human dystrophin), they were well integrated with host-derived myofibers repairing damaged area. This also points to the importance of the host myogenic cells in reconstruction of the defect, not only in formation of new/hybrid fibers with donor cells but also in formation of new vessels in the engrafted region, as demonstrated in the Results section. Moreover, as one of the necessities of the engrafted tissue is the capability to repopulate muscle stem cell compartment, this ability was well-confirmed in the current study. Indeed, the presence of Pax7/human marker double-positive muscle stem cells at the site of engraftment is a clear indication of formation of muscle stem cells from engrafted hiPSCs. Considering high regeneration and renewal rate of the skeletal muscle tissue, the presence of a robust muscle stem cell compartment supports longevity of the engraftment in VML model, although more studies, such as re-injury assays, still need to be done to prove this ability.
Of note, when compared to other mice models, such as NSG-mdx, hiPSC-derived cells formed a larger fraction of satellite cells in VML model (16.4% ± 1.8% in VML compared to 12% ± 1.9% in NSG-mdx using same cells as published before).18 The lower percent of hiPSC-derived satellite cells in NSG-mdx model might be related to continuous and ongoing muscle degeneration-regeneration cycles within the dystrophic mdx model, leading to constant activation and possible depletion of muscle stem cells. This can be further investigated by exploration of the symmetric/asymmetric division of hiPSC-derived satellite cells and their long-term quantification in these models.
Finally, as one of the most important aspects of the VML therapy is to restore muscle function,36 proper innervation of the engrafted tissue is essential and indeed highly desired.37,38 Another advantage of the current study is to provide clear evidence for innervation of the hiPSC-derived myofibers by demonstration of the presence of NMJ in myofibers positive for human markers. Presence of well-localized NMJs in donor-derived fibers containing both pre- (synaptophysin) and postsynaptic (AChR) structures, along with peripherally located nuclei in majority of the donor-derived fibers provides more evidence for terminal maturation and innervation of the regenerated fibers. This was further supported when considering the muscle function data. These data provided further evidence for functional recovery of the treated muscles following hiPSC therapy. Indeed, stem-cell-treated muscles showed significantly improved maximal isometric tetanic (F0) and specific forces (sF0) compared to untreated group. In addition, engraftment was also able to improve the fatigue resistance near to normal level, which is an important finding considering the importance of muscle endurance during continuous contractions.
Taken together, current study provides proof of principle for the therapeutic potential of the hiPSCs to treat VML injury and their innervation to improve contractile muscle function. However, to move forward, there are still several steps to be done. On the top of the list is to evaluate the hiPSCs regenerative potential in larger VMLs.39 Though the current study’s VML model in mouse TA muscle represents 30%–40% muscle mass loss, it is still a small model compared to actual patients. Major problems with large VMLs, such as lack of vascularity and hypoxic conditions within the defect, and problems with cell migration and perfusion of the large transplants are among the major hurdles to overcome.40,41 These might need different design in the bioengineered tissue to include different bio-scaffold material or cell types, such as vessel progenitors and other cellular compartments supporting different lineages, as well as the need for bioprinting of 3D large constructs to support vasculature and other necessary structures.14,42, 43, 44 Therefore, scale-up studies and using larger animal models along with hiPSCs are the next steps to advance current research. Nevertheless, our findings confirming the therapeutic potential of hiPSCs in VML injury is the first step forward to prove their potential for skeletal muscle regeneration in this model.
Materials and Methods
hiPSC Culture and Skeletal Myogenic Differentiation
hiPSCs were maintained and expanded on Matrigel-coated flasks (BD Biosciences) in mTeSR1 medium (STEMCELL Technologies). For differentiation toward skeletal myogenic lineage, we used a recently developed protocol by our lab.18 Briefly, hiPSCs were differentiated toward PSM using a WNT agonist (CHIR99021; Selleck Chemical) and TGF-β inhibitor (SB431542; Selleck Chemical) for 5 days. Then, the cells were further differentiated toward skeletal myogenic progenitors using a BMP inhibitor (LDN193189; Stemgent) for 10 more days. Finally, for purification of myogenic progenitors, the cells were sorted for CD10+CD24− fraction. Myogenic cells were then expanded for a few days before transplantation or terminal differentiation. Terminal myotube differentiation was induced using IMDM (Invitrogen) supplemented with 15% knockout serum replacement (Invitrogen), as previously described.18
VML Model and Stem Cell/Biomaterial Transplantation
For generation of VML models and transplantation studies, 3- to 4-month-old NSG mice (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ; The Jackson Laboratory) were used (n = 6 per experimental group of 4). Experimental groups included control (uninjured), injured without treatment, gel-alone, and gel + cell-treated cohorts. Mice were maintained in a barrier facility, and all experimental studies were carried out in accordance and approved by the Institutional Animal Care and Use Committee (IACUC) guidelines of The University of Texas Health Science Center at Houston. VML model was generated using a method developed before by us.23 Briefly, after general anesthesia using isoflurane, TA muscle was exposed through skin incision. VML model was then created by excising a 3 × 3 × 6 mm longitudinal defect using a fine iris scissor. Then, iPSC-derived myogenic progenitors (3 × 105) resuspended in a 10 μL fibrinogen solution (4 mg/mL fibrinogen; Sigma) were carefully used to fill in the defect. Solidification was induced by addition of 2 μL thrombin solution (5IU; Sigma), followed by wound closure using a nylon suture.
In Situ TA Muscle Function Study
For accurate measurement of TA muscle function parameters, an in situ muscle function assay was performed using 3-in-1 animal system (Aurora Scientific). Mice (n = 6 per experimental group) were anesthetized using avertin, and after skin incision, the TA muscle was exposed. After knee stabilization on the heated pad using a vertical knee pin and clamp, the TA distal tendon was carefully released and attached to the force transducer hook using a silk suture. Then, the needle electrodes were placed under the TA, and muscle was stimulated using a series of rectangular unipolar pulses of 0.2 ms at different muscle base tensions to determine the optimal length (L0). Then, the maximal tetanic force and fatigue time were determined at 150 Hz using same rectangular unipolar pulses of 0.2 ms for 300 ms or 2 minrespectively, as described before.19,45,46 At the end, muscle optimal length and weight were recorded and used to calculate the muscle CSA and the specific force (sF0).
Histology and Immunostaining
For histologic and immunostaining of the samples, fresh TA muscles were harvested and embedded in O.C.T. compound (n = 6 mice per group). Samples were then frozen using liquid-nitrogen-cooled isopenthane and sectioned for staining. Sections were used unfixed or after fixation with 4% paraformaldehyde (PFA) for H&E or immunofluorescent staining as described before.18,19 Primary antibodies include mouse monoclonal anti-Pax7 (Developmental Studies Hybridoma Bank, DSHB), mouse monoclonal anti-human dystrophin (Millipore), rabbit anti-human lamin A/C (Abcam), rabbit anti-laminin (Sigma), mouse-anti MHC (DSHB), rabbit monoclonal anti-synaptophysin (SP11; Thermo Fisher Scientific) and α-bungarotoxin (Thermo Fisher Scientific). Sections were then stained using appropriate secondary antibodies (Alexa Fluor; Thermo Fisher Scientific) and counter-stained with DAPI to mark nuclei.
Quantifications and Statistical Analysis
Engraftment quantification was done using serial sectioning of the muscle at 30 μm intervals. For each muscle/mouse, five representative (low-magnification cross section) images from the engraftment site per mice were used for quantitative evaluation (n = 6 mice per group). Number of mouse and human nuclei (positive for human lamin A/C) and human-dystrophin-positive and negative fibers were counted using low-magnification images (10×) and used for statistical analysis as described before.18 Data were analyzed using ANOVA and Student’s t test. p < 0.05 was considered significant.
Author Contributions
J.W. conducted the experiments, analyzed the data, and helped write the manuscript. N.M. helped with transplantation and functional studies and analyzed the data. S.B. performed quantifications. R.D. designed the study, supervised experiments, analyzed the data, and wrote the manuscript.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
Research reported in this publication was supported by the National Institute of Arthritis, and Musculoskeletal and Skin Diseases (NIAMS) of the NIH under award numbers 1R01AR068293 and 1R21AR071583. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.ymthe.2020.09.012.
Supplemental Information
References
- 1.Greising S.M., Dearth C.L., Corona B.T. Regenerative and rehabilitative medicine: a necessary synergy for functional recovery from volumetric muscle loss injury. Cells Tissues Organs (Print) 2016;202:237–249. doi: 10.1159/000444673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corona B.T., Rivera J.C., Owens J.G., Wenke J.C., Rathbone C.R. Volumetric muscle loss leads to permanent disability following extremity trauma. J. Rehabil. Res. Dev. 2015;52:785–792. doi: 10.1682/JRRD.2014.07.0165. [DOI] [PubMed] [Google Scholar]
- 3.Cross J.D., Ficke J.R., Hsu J.R., Masini B.D., Wenke J.C. Battlefield orthopaedic injuries cause the majority of long-term disabilities. J. Am. Acad. Orthop. Surg. 2011;19(Suppl 1):S1–S7. doi: 10.5435/00124635-201102001-00002. [DOI] [PubMed] [Google Scholar]
- 4.Grogan B.F., Hsu J.R., Skeletal Trauma Research Consortium Volumetric muscle loss. J. Am. Acad. Orthop. Surg. 2011;19(Suppl 1):S35–S37. doi: 10.5435/00124635-201102001-00007. [DOI] [PubMed] [Google Scholar]
- 5.Morgan J., Partridge T. Skeletal muscle in health and disease. Dis. Model. Mech. 2020;13 doi: 10.1242/dmm.042192. dmm042192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seale P., Rudnicki M.A. A new look at the origin, function, and “stem-cell” status of muscle satellite cells. Dev. Biol. 2000;218:115–124. doi: 10.1006/dbio.1999.9565. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y.X., Rudnicki M.A. Satellite cells, the engines of muscle repair. Nat. Rev. Mol. Cell Biol. 2011;13:127–133. doi: 10.1038/nrm3265. [DOI] [PubMed] [Google Scholar]
- 8.Corona B.T., Wenke J.C., Ward C.L. Pathophysiology of volumetric muscle loss injury. Cells Tissues Organs (Print) 2016;202:180–188. doi: 10.1159/000443925. [DOI] [PubMed] [Google Scholar]
- 9.Garg K., Ward C.L., Hurtgen B.J., Wilken J.M., Stinner D.J., Wenke J.C., Owens J.G., Corona B.T. Volumetric muscle loss: persistent functional deficits beyond frank loss of tissue. J. Orthop. Res. 2015;33:40–46. doi: 10.1002/jor.22730. [DOI] [PubMed] [Google Scholar]
- 10.Caldwell C.J., Mattey D.L., Weller R.O. Role of the basement membrane in the regeneration of skeletal muscle. Neuropathol. Appl. Neurobiol. 1990;16:225–238. doi: 10.1111/j.1365-2990.1990.tb01159.x. [DOI] [PubMed] [Google Scholar]
- 11.Lepper C., Partridge T.A., Fan C.M. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development. 2011;138:3639–3646. doi: 10.1242/dev.067595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin C.H., Lin Y.T., Yeh J.T., Chen C.T. Free functioning muscle transfer for lower extremity posttraumatic composite structure and functional defect. Plast. Reconstr. Surg. 2007;119:2118–2126. doi: 10.1097/01.prs.0000260595.85557.41. [DOI] [PubMed] [Google Scholar]
- 13.Quarta M. Volumetric muscle loss: including nerves into the equation. Muscle Nerve. 2018;57:705–706. doi: 10.1002/mus.26080. [DOI] [PubMed] [Google Scholar]
- 14.Passipieri J.A., Christ G.J. The potential of combination therapeutics for more complete repair of volumetric muscle loss injuries: the role of exogenous growth factors and/or progenitor cells in implantable skeletal muscle tissue engineering technologies. Cells Tissues Organs (Print) 2016;202:202–213. doi: 10.1159/000447323. [DOI] [PubMed] [Google Scholar]
- 15.Del Carmen Ortuño-Costela M., García-López M., Cerrada V., Gallardo M.E. iPSCs: a powerful tool for skeletal muscle tissue engineering. J. Cell. Mol. Med. 2019;23:3784–3794. doi: 10.1111/jcmm.14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haridhasapavalan K.K., Borgohain M.P., Dey C., Saha B., Narayan G., Kumar S., Thummer R.P. An insight into non-integrative gene delivery approaches to generate transgene-free induced pluripotent stem cells. Gene. 2019;686:146–159. doi: 10.1016/j.gene.2018.11.069. [DOI] [PubMed] [Google Scholar]
- 17.Heng B.C., Fussenegger M. Integration-free reprogramming of human somatic cells to induced pluripotent stem cells (iPSCs) without viral vectors, recombinant DNA, and genetic modification. Methods Mol. Biol. 2014;1151:75–94. doi: 10.1007/978-1-4939-0554-6_6. [DOI] [PubMed] [Google Scholar]
- 18.Wu J., Matthias N., Lo J., Ortiz-Vitali J.L., Shieh A.W., Wang S.H., Darabi R. A myogenic double-reporter human pluripotent stem cell line allows prospective isolation of skeletal muscle progenitors. Cell Rep. 2018;25:1966–1981.e4. doi: 10.1016/j.celrep.2018.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darabi R., Arpke R.W., Irion S., Dimos J.T., Grskovic M., Kyba M., Perlingeiro R.C. Human ES- and iPS-derived myogenic progenitors restore DYSTROPHIN and improve contractility upon transplantation in dystrophic mice. Cell Stem Cell. 2012;10:610–619. doi: 10.1016/j.stem.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hicks M.R., Hiserodt J., Paras K., Fujiwara W., Eskin A., Jan M., Xi H., Young C.S., Evseenko D., Nelson S.F. ERBB3 and NGFR mark a distinct skeletal muscle progenitor cell in human development and hPSCs. Nat. Cell Biol. 2018;20:46–57. doi: 10.1038/s41556-017-0010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chal J., Oginuma M., Al Tanoury Z., Gobert B., Sumara O., Hick A., Bousson F., Zidouni Y., Mursch C., Moncuquet P. Differentiation of pluripotent stem cells to muscle fiber to model Duchenne muscular dystrophy. Nat. Biotechnol. 2015;33:962–969. doi: 10.1038/nbt.3297. [DOI] [PubMed] [Google Scholar]
- 22.Shelton M., Kocharyan A., Liu J., Skerjanc I.S., Stanford W.L. Robust generation and expansion of skeletal muscle progenitors and myocytes from human pluripotent stem cells. Methods. 2016;101:73–84. doi: 10.1016/j.ymeth.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 23.Matthias N., Hunt S.D., Wu J., Lo J., Smith Callahan L.A., Li Y., Huard J., Darabi R. Volumetric muscle loss injury repair using in situ fibrin gel cast seeded with muscle-derived stem cells (MDSCs) Stem Cell Res. (Amst.) 2018;27:65–73. doi: 10.1016/j.scr.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Omar A., Marwaha K., Bollu P.C. StatPearls; 2020. Physiology, neuromuscular junction. In StatPearls. [PubMed] [Google Scholar]
- 25.Colasante C., Brouard M.O., Pécot-Dechavassine M. Synaptophysin (p38) immunolabelling at the mouse neuromuscular junction. Neuromuscul. Disord. 1993;3:395–400. doi: 10.1016/0960-8966(93)90084-w. [DOI] [PubMed] [Google Scholar]
- 26.Utkin Y.N. Three-finger toxins, a deadly weapon of elapid venom--milestones of discovery. Toxicon. 2013;62:50–55. doi: 10.1016/j.toxicon.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Rivera J.C., Corona B.T. Muscle-related disability following combat injury increases with time. U.S. Army Med. Dep. J. 2016:30–34. [PubMed] [Google Scholar]
- 28.Lin S.H., Chuang D.C., Hattori Y., Chen H.C. Traumatic major muscle loss in the upper extremity: reconstruction using functioning free muscle transplantation. J. Reconstr. Microsurg. 2004;20:227–235. doi: 10.1055/s-2004-823110. [DOI] [PubMed] [Google Scholar]
- 29.Lawson R., Levin L.S. Principles of free tissue transfer in orthopaedic practice. J. Am. Acad. Orthop. Surg. 2007;15:290–299. doi: 10.5435/00124635-200705000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Klinkenberg M., Fischer S., Kremer T., Hernekamp F., Lehnhardt M., Daigeler A. Comparison of anterolateral thigh, lateral arm, and parascapular free flaps with regard to donor-site morbidity and aesthetic and functional outcomes. Plast. Reconstr. Surg. 2013;131:293–302. doi: 10.1097/PRS.0b013e31827786bc. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 33.Inoue H., Nagata N., Kurokawa H., Yamanaka S. iPS cells: a game changer for future medicine. EMBO J. 2014;33:409–417. doi: 10.1002/embj.201387098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi Y., Inoue H., Wu J.C., Yamanaka S. Induced pluripotent stem cell technology: a decade of progress. Nat. Rev. Drug Discov. 2017;16:115–130. doi: 10.1038/nrd.2016.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takahashi K., Yamanaka S. Induced pluripotent stem cells in medicine and biology. Development. 2013;140:2457–2461. doi: 10.1242/dev.092551. [DOI] [PubMed] [Google Scholar]
- 36.Corona B.T., Flanagan K.E., Brininger C.M., Goldman S.M., Call J.A., Greising S.M. Impact of volumetric muscle loss injury on persistent motoneuron axotomy. Muscle Nerve. 2018;57:799–807. doi: 10.1002/mus.26016. [DOI] [PubMed] [Google Scholar]
- 37.Gilbert-Honick J., Iyer S.R., Somers S.M., Takasuka H., Lovering R.M., Wagner K.R., Mao H.Q., Grayson W.L. Engineering 3D skeletal muscle primed for neuromuscular regeneration following volumetric muscle loss. Biomaterials. 2020;255:120154. doi: 10.1016/j.biomaterials.2020.120154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilbert-Honick J., Grayson W. Vascularized and innervated skeletal muscle tissue engineering. Adv. Healthc. Mater. 2020;9:e1900626. doi: 10.1002/adhm.201900626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sarrafian T.L., Bodine S.C., Murphy B., Grayson J.K., Stover S.M. Extracellular matrix scaffolds for treatment of large volume muscle injuries: a review. Vet. Surg. 2018;47:524–535. doi: 10.1111/vsu.12787. [DOI] [PubMed] [Google Scholar]
- 40.Greising S.M., Rivera J.C., Goldman S.M., Watts A., Aguilar C.A., Corona B.T. Unwavering pathobiology of volumetric muscle loss injury. Sci. Rep. 2017;7:13179. doi: 10.1038/s41598-017-13306-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Corona B.T., Greising S.M. Challenges to acellular biological scaffold mediated skeletal muscle tissue regeneration. Biomaterials. 2016;104:238–246. doi: 10.1016/j.biomaterials.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 42.Willett N.J., Krishnan L., Li M.T., Guldberg R.E., Warren G.L. Guidelines for models of skeletal muscle injury and therapeutic assessment. Cells Tissues Organs (Print) 2016;202:214–226. doi: 10.1159/000445345. [DOI] [PubMed] [Google Scholar]
- 43.Fuoco C., Petrilli L.L., Cannata S., Gargioli C. Matrix scaffolding for stem cell guidance toward skeletal muscle tissue engineering. J. Orthop. Surg. Res. 2016;11:86. doi: 10.1186/s13018-016-0421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mertens J.P., Sugg K.B., Lee J.D., Larkin L.M. Engineering muscle constructs for the creation of functional engineered musculoskeletal tissue. Regen. Med. 2014;9:89–100. doi: 10.2217/rme.13.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Darabi R., Pan W., Bosnakovski D., Baik J., Kyba M., Perlingeiro R.C. Functional myogenic engraftment from mouse iPS cells. Stem Cell Rev. Rep. 2011;7:948–957. doi: 10.1007/s12015-011-9258-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Darabi R., Gehlbach K., Bachoo R.M., Kamath S., Osawa M., Kamm K.E., Kyba M., Perlingeiro R.C. Functional skeletal muscle regeneration from differentiating embryonic stem cells. Nat. Med. 2008;14:134–143. doi: 10.1038/nm1705. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.