Abstract
The serotonin 5-HT1A receptor has attracted wide attention as a target for treatment of psychiatric disorders. Although this receptor is important in the pharmacological mechanisms of action of new-generation antipsychotics, its characterization remains incomplete. Studies based on in vitro molecular imaging on brain tissue by autoradiography, and more recently in vivo PET imaging, have not yielded clear results, in particular due to the limitations of current 5-HT1A radiotracers, which lack specificity and/or bind to all 5-HT1A receptors, regardless of their functional status. The new concept of PET neuroimaging of functionally active G-protein-coupled receptors makes it possible to revisit PET brain exploration by enabling new research paradigms. For the 5-HT1A receptor it is now possible to use [18F]-F13640, a 5-HT1A receptor radioligand with high efficacy agonist properties, to specifically visualize and quantify functionally active receptors, and to relate this information to subjects’ pathophysiological or pharmacological state. We therefore propose imaging protocols to follow changes in the pattern of functional 5-HT1A receptors in relation to mood deficits or cognitive processes. This could allow improved discrimination of different schizophrenia phenotypes and greater understanding of the basis of therapeutic responses to antipsychotic drugs. Finally, as well as targeting functionally active receptors to gain insights into the role of 5-HT1A receptors, the concept can also be extended to the study of other receptors involved in the pathophysiology or therapy of psychiatric disorders.
Subject terms: Schizophrenia, Biomarkers
Introduction
Serotonin (5-hydroxytryptamine, 5-HT) receptors constitute a large family of seven receptor classes (5-HT1 to 5-HT7) with 14 different subtypes. Five subgroups are distinguished within the 5-HT1 receptor group, i.e., 5-HT1A, 5-HT1B, 5-HT1D, 5-HT1E and 5-HT1F receptors. The 5-HT1A subtype was the first of the serotonin receptors to be sequenced and is nowadays one of the most thoroughly studied. Its distribution within the brain is classically divided into two functional groups: presynaptic autoreceptors which are present at high densities in the midbrain raphe nuclei, and postsynaptic heteroreceptors in other brain regions, notably the hippocampus, the cingulate cortex, the lateral septum and other brain regions that are important for control of mood and cognition. Presynaptic 5-HT1A autoreceptors on serotonergic neurons mediate an important negative feedback influence on neuronal firing, thus eliciting inhibition of 5-HT release. Postsynaptic 5-HT1A receptors are located on different cell types, including GABAergic interneurons and excitatory pyramidal and granule cells. Due to its modulating influence on the whole serotonergic system and its large distribution throughout the brain, the 5-HT1A receptor is considered as one of the most important 5-HT receptor subtypes, and is of primary interest when investigating serotonergic neurotransmission in brain1,2.
The 5-HT1A receptor is strongly associated with alterations in mood and emotion, as demonstrated by numerous preclinical and clinical studies1. Many of these are focused on depression and anxiety, but compelling evidence also points to an important role of 5-HT1A receptor dysfunction in schizophrenia3. This chronic psychiatric disease affects around 20 million people worldwide4, creating a substantial global burden of care, with significant morbidity and functional impact4,5. Notably, life expectancy in patients with schizophrenia has been estimated to be 10–20 years shorter than in the general population6. Although schizophrenia is associated with an imbalance in dopamine transmission, with an hyperactivity in the mesolimbic system and an hypoactivity in the prefrontal cortex, other neurotransmitters, including glutamate, GABA and serotonin, are also involved in its physiopathological process7,8. The main symptoms of schizophrenia comprise delusions, hallucinations, disorganization and negative symptoms, i.e., blunted affect or aboulia. Diagnosis is based on the presence of at least two of these main symptoms for 6 months or more, leading to significant functional impairment. Cognitive impairment is a core feature of schizophrenia and may be present at any stage of the disease9. Both negative and cognitive symptoms are associated with poor functional outcomes10 and are currently not well-managed by available pharmacological treatments. A better understanding of the physiopathology of schizophrenia is thus required to identify and validate pharmacological targets and, ideally, lead to novel therapeutic strategies for patients.
The involvement of serotonin 5-HT1A receptors in the pharmacology of antipsychotics
Early classical (i.e., typical) antipsychotics (e.g., phenothiazine, butyrophenone, and benzamide derivatives) possess dopamine D2 receptor blocking activity and show efficacy for control of positive symptoms. These agents, however, are not very effective for treatment of negative or cognitive symptoms and frequently induce extra-pyramidal side effects. Subsequently, a variety of atypical antipsychotics became available as first-line treatments. On a pharmacological level, these atypical antipsychotics commonly act as antagonists on both the dopaminergic D2 receptor and the serotoninergic 5-HT2A receptor, as well as interacting with multiple other receptors11–13. For example, antipsychotics such as risperidone or olanzapine display the desired two-fold action (D2 and 5-HT2A) and exhibit efficacy against positive symptoms with improved activity on negative and cognitive symptoms and reduced side effects, such as extra-pyramidal symptoms (EPS)11–13. Nonetheless, there are still unmet needs in the treatment of schizophrenia, including patients who are resistant to treatment with current antipsychotics14,15, the need to more efficaciously improve cognitive deficits, alleviate affective disorders (e.g., anxiety and depression) and reduce antipsychotic‐induced EPS, metabolic and endocrine side effects16.
It is notable that some atypical antipsychotic drugs that primarily target D2 and 5-HT2A receptors, such as risperidone and olanzapine, also indirectly activate 5-HT1A receptors17. Other antipsychotics, including clozapine, ziprasidone and quetiapine, act directly as partial agonists at 5-HT1A receptors18. Moreover, ‘third generation’ antipsychotics, such as aripiprazole, cariprazine and brexpiprazole, exhibit prominent direct-acting agonist properties at 5-HT1A receptors19–21. Indeed, 5‐HT1A receptors are now an important therapeutic target for selection of novel antipsychotics based on a range of observations12,22. For example, 5-HT1A receptor activation has been proposed as a target for the development of cognitive enhancers for treatment of cognitive dysfunction in schizophrenia, notably via stimulation of dopamine release in key regions such as the frontal cortex11,23. Thus, some authors proposed that verbal memory and executive functioning were improved in schizophrenia patients that received the 5-HT1A partial agonist, tandospirone, in addition to neuroleptic treatment24,25. Based on molecular, neurochemical, behavioural and clinical data, it has been suggested that agonism of the 5-HT1A receptor may ameliorate the therapy of negative symptoms and comorbid mood deficits, attenuate cognitive deficits and oppose EPS elicited by D2 receptor blockade. Future antipsychotics may therefore be identified that possess an optimized dual D2/5-HT1A mechanism which retains efficacy against positive symptoms via D2 antagonism, whilst improving cognitive impairment and negative symptoms associated with schizophrenia and functional outcome by activation of 5-HT1A receptors26,27.
In addition to pharmacological factors such as those described above, genetic factors could also influence the role of 5-HT1A receptors in schizophrenia28. Indeed, accumulating evidence indicates that a common 5-HT1A gene promoter single-nucleotide polymorphism (SNP rs6295, C-1019G), which occurs in about 5–10% in Oriental populations and 40% in Caucasian population29, leads to changes in 5-HT1A receptor function that markedly affect vulnerability to psychiatric disorders, including schizophrenia30. Thus, several studies highlighted the correlation between 5-HT1A receptor polymorphisms and the presence of schizophrenia symptoms and their response to treatments31–33. This single-nucleotide polymorphism is proposed to have a relevant impact on 5-HT1A receptor density and/or activity. In raphe nuclei, the C-G change linked to the polymorphism impairs the binding of nuclear proteins (e.g., Deaf1) to a palindromic DNA sequence located at this polymorphism. As a result, the rs6295 G allele is associated with a reduced efficiency of Deaf-1 binding, expressed in an increase of 5-HT1A autoreceptor density in the raphe nucleus. In contrast, the 5-HT1A receptor expression is proposed to be decreased on the postsynaptic part, such as in the prefrontal cortex. This lowered postsynaptic 5-HT1A receptor density is linked to a reduced serotonergic signal transduction, which may in turn reduce dopamine release in the prefrontal cortex34. As a consequence, this serotonin imbalance may have implications regarding response to antipsychotics (notably as concerns negative symptoms) and treatment options for patients with schizophrenia29. Taken together, these findings reinforce the assertion that the 5-HT1A receptor is a relevant target for treatment of schizophrenia and other psychiatric disorders.
The exploration of 5-HT1A receptors in schizophrenia by molecular imaging and its contradictory results
The development of molecular imaging techniques with selective radioligands allows direct measurement of serotonin receptors in vitro on brain tissue by autoradiography, or in vivo, in the living human brain by positron emission tomography (PET). Several compounds have been radiolabelled for studies of the 5-HT1A receptor. The most commonly used radioligand for in vitro studies is [3H]-8-OH-DPAT and the most frequent PET radiopharmaceuticals for quantification of 5-HT1A receptors in the living human brain are [11C]-WAY100635 and [18F]-MPPF.
Post-mortem studies and their shortcomings
The first exploration of the 5-HT1A receptor densities in the brain of schizophrenic patients was carried out by in vitro autoradiography on post-mortem brain tissues. The aim was to investigate the role of the 5-HT1A receptor in the pathophysiology of schizophrenia. Other studies followed and, in total, there have been eleven post-mortem studies exploring the G-protein-coupled serotonin 5-HT1A receptor in patients with schizophrenia compared to healthy controls by homogenate or in situ autoradiographic binding techniques (for review, see ref. 35). Although the majority of these post-mortem studies described an increase of 5-HT1A receptor densities in various cortical regions, several articles reported contradictory results. Six studies showed an increase in 5-HT1A receptor density in the prefrontal cortex (PFC) of patients with schizophrenia compared with healthy controls36–41 whereas three did not42–44; one study found a significant increase in the temporal cortex38 and two did not36,44. Other contradictory results mainly concerned the anterior cingulate cortex and the hippocampus36–38,40,44,45. One study showed an increase in 5-HT1A receptor density in the cerebellum of patients with schizophrenia compared with controls46. No studies found any decrease of the 5-HT1A receptors in any brain region in patients with schizophrenia compared to controls. Overall, meta-analysis from Selvaraj and colleagues35 found that the pooled results were in favour of a significant increase in 5-HT1A receptors in the prefrontal cortex of patients with schizophrenia compared to healthy controls, with an effect size of 0.60 (CI: 0.17–1.03, p = 0.007)35. For detailed results, see Table 1.
Table 1.
Post-mortem studies exploring 5-HT1A receptors in schizophrenia.
| Reference | Schizophrenia group | Control group | Radiotracer | Region of interest | Main result |
|---|---|---|---|---|---|
| 36 | n = 13; mean age 55 +/−16 yrs; 8 M/5 F; suicide 2/13; antipsychotics 12/13 | n = 15; mean age 59 + /−15 yrs; 9 M/6 F; suicide 2/15 | [3H]-8-OH-DPAT | DLPFC (BA 46) | Increase (23%) |
| ACC (BA 24) | NS | ||||
| STG; striatum, cortex; hippocampus | NS | ||||
| 37 | n = 9; mean age 53 +/−15 yrs; 4 M/5 F; suicide 1/9; antipsychotics 8/9 | n = 9; mean age 58 +/−17 yrs; 5 M/4 F; no suicide | [3H]-WAY-100635 | DLPFC (BA 46) | Increase (17%) |
| ACC (BA 24) | NS | ||||
| 43 | n = 10; mean age 60 +/−3.7 yrs; 7 M/3 F; no suicide; antipsychotics 8/10 | n = 10; mean age 60 +/−3.8 yrs; 7 M/3 F; no suicide | [3H]-8-OH-DPAT | DLPFC (BA 8, 9, 10) | NS |
| 42 | n = 8; 5 M/3 F; no suicide; antipsychotics 8/8 |
Healthy controls: n = 8; 5 M/3 F; no suicide Bipolar I disorder: n = 8; 4 M/4 F; no suicide; antipsychotics 5/8. |
[3H]-8-OH-DPAT | Frontal cortex (BA 9,10, 40, 46) | NS |
| 40 | n = 10; mean age 75.4 +/−1.5 yrs; 6 M/4 F; antipsychotics 4/10; no suicide | n = 13; mean age 63.3 +/−3.3 yrs; 7 M/4 F; no suicide | [3H]-8-OH-DPAT | ACC (BA 24) | Increase |
| PFC (BA 6,9a and 44) | Increase | ||||
| Motor cortex; sensori-motor cortex; infra-parietal cortex; PCC | NS | ||||
| 38 | n = 10; mean age 67.3 +/−3.8 yrs; 6 M/4 F; antipsychotics 5/10 | n = 11; mean age 63.6 +/−4 yrs; 4 M/7 F | [3H]-8-OH-DPAT | PFC (BA 10) | Increase (40%) |
| Temporal cortex (BA 22) | Increase (60%) | ||||
| Parietal cortex; motor cortex; occipital cortex; cingulum; amygdala; hippocampus | NS | ||||
| 44 | n = 10; mean age 49 +/−18 yrs; 7 M/3 F; suicide 4/10; antipsychotics 7/10 |
Control group: n = 8; mean age 68 +/−15 yrs; 4 M/4 F; no suicide Suicide/affective illness group: n = 8; mean age 38 +/−13 yrs; 5 M/3 F; medication 4/10 |
[3H]-8-OH-DPAT | Motor cortex (BA 4) | Increase |
| ACC (BA 24) | Increase | ||||
| PCC (BA 23) | Increase | ||||
| Hippocampus | Increase | ||||
| Temporal cortex; striatum; frontal (BA9) | NS | ||||
| 45 | n = 20; mean age 47.35 +/−3.7 yrs; 11 M/9 F; suicide 5/20; antipsychotics 20/20 | n = 20; mean age 46.2 +/−3.4 yrs; 13 M/7 F; no suicide | [3H]-8-OH-DPAT | Hippocampus | NS |
| 39 | n = 12; mean age 59 +/−6 yrs; 9 M/3 F; suicide 4/10; antipsychotics 10/12 | n = 18; mean age 63 +/−2 yrs; 13 M/5 F; no suicide | [3H]-8-OH-DPAT | Medial orbital gyrus | Increase |
| Straight gyrus | Increase | ||||
| Olfactory sulcus | Increase | ||||
| 46 | n = 19; mean age 54.3/−1.4 yrs; 14 M/5 F; antipsychotics 19/19 | n = 16; mean age 71.5 +/−3.9 yrs; 10 M/6 F | [3H]-8-OH-DPAT | Cerebellar vermis lobules | Increase |
| 41 | n = 12; mean age 37.8 + /−2.9 yrs; 7 M/5 F; suicide 5/12; antipsychotics 10/12 | n = 12; mean age 37.1 +/−3.4 yrs; 10 M/1 F; no suicide; medication 3/10 | [3H]-8-OH-DPAT | PFC (BA10) | Increase (79%) |
ACC anterior cingulate cortex, BA Brodman’s area, DLPFC dorsolateral prefrontal cortex, F females, M men, NS non-significant, PFC prefrontal cortex, PCC posterior cingulate cortex, STG superior temporal gyrus, yrs years.
PET studies and their shortcomings
There have been few in vivo 5-HT1A positron emission tomography (PET) imaging studies in schizophrenia patients. PET imaging theoretically has great advantages over the in vitro approach. Indeed, in vivo imaging allows the exploration of live patients, allowing for possible longitudinal follow-up. Moreover, PET imaging permits exploration of the entire brain, potentially revealing regions of interest not examined in the brain tissue sections available in brain banks. However, results from PET studies on the 5-HT1A receptor in schizophrenia were mainly non-significant47,48 or contradictory49,50. For example, a PET study focused on 5-HT1A receptor availability in schizophrenia patients treated with ziprasidone and found no significant difference in their distribution before or after receiving medication but found a significant association between 5-HT1A binding and improvement in negative symptoms51. For detailed results, see Table 2.
Table 2.
PET imaging studies exploring 5-HT1A receptors in schizophrenia.
| Reference | Schizophrenia group | Control group | Radiotracer | Reference region | Main result |
|---|---|---|---|---|---|
| 47 |
Non-clozapine group: n = 11; 11M/0F; mean age 41.5 +/−12 yrs Clozapine group: n = 11; 11M/0F; mean age 36.9 +/−10.5 yrs |
n = 11; mean age 37.3 +/−8 yrs; 11M/0F | [11C]-WAY100635 | Cerebellar hemisphere regions | NS |
| 48 | n = 22; mean age 32 +/−10 yrs; 16M/6F; 14 drug free/8 drug naive; 15 schizophrenia/7 schizoaffective disorder diagnosis | n = 18; mean age 32 + /−8 yrs; 15M/3F | [11C]-WAY100635 | None (Kinetic modelling with arterial input modelling strategy) | NS |
| 49 | Drug naive first psychosis episode patients: n = 14; mean age 26 +/−5 yrs; 8M/6F | n = 14; mean age 28 +/−5 yrs; 6M/8F | [11C]-WAY100635 | Cerebellum | Increase (+7.1% +/-6.4%; p = 0.02) |
| 50 | n = 11; 9 M/2 F; mean age 31.1 +/−8.7 yrs; 9 M/2 F; 3 drug free/8 drug naive; 5 schizophrenia/6 schizophreniform diagnosis | n = 22; mean age 31 +/−8.5 yrs; 18M/4F | [11C]-WAY100635 | Cerebellum | Decrease in the amygdala (−19%; p = 0.023) |
F female, M male, NS non-significant, yrs years.
It is striking that although molecular imaging studies of the 5-HT1A receptor in schizophrenia report disparate results, none of them have yet confirmed post-mortem findings. The contrast between in vitro and in vivo findings may be attributable to several factors, such as variations in the sample populations (age, duration of illness, pharmacological treatments…) but also to the methodological approaches. Thus, there are significant pharmacological differences between the radiotracers used for quantification of in vitro and in vivo studies. The 5-HT1A receptor can exist in a high (G-protein-coupled, functionally active) or low (G-protein-decoupled, non-functionally active) affinity state with respect to endogenous binding. As mentioned before, most post-mortem studies have used the agonist [3H]-8-OH-DPAT, which binds with high affinity only to receptors in high-affinity state and it also has limited specificity, binding to both 5-HT1A and 5-HT7 receptors52. On the contrary, PET studies used antagonist radiotracers, [11C]-WAY100635 or [18F]-MPPF, that are more specific (although WAY100635 also has nanomolar range binding affinity for dopamine D4 receptors, rarely taken into account53), and that bind with comparable affinity to 5-HT1A receptors in both high- and low-affinity states54. While these characteristics are well known to neuropharmacologists, they are seldom given consideration in the nuclear medicine and neuroscience communities.
The concept of functionally active 5-HT1A receptor imaging
Principle: antagonist versus agonist
The vast majority of PET radiopharmaceuticals currently used for receptor neuroimaging are antagonists. However, about 30 agonists have been used for PET imaging of various G-protein-coupled receptors: adrenergic, dopaminergic, serotonergic, muscarinic, cannabinoid, and opioid receptors55–57. Among them, a dozen of PET agonist radiotracers were developed and translated to humans for these receptors, but very few studies58–60 directly compared the in vivo density of receptors targeted by an agonist versus an antagonist radiotracer in the same subject (in these cases, animal models). Several interpretations have been given to in vivo binding differences based on the pharmacological agonist/antagonist properties of the ligands. The internalization of certain receptors has been evoked, as well as the modification of their allosteric conformations, or the influence of the extracellular concentration of the endogenous neurotransmitter, all of which could influence differently the binding of an antagonist and an agonist for the same family of receptors (see ref. 56 for a review on this subject).
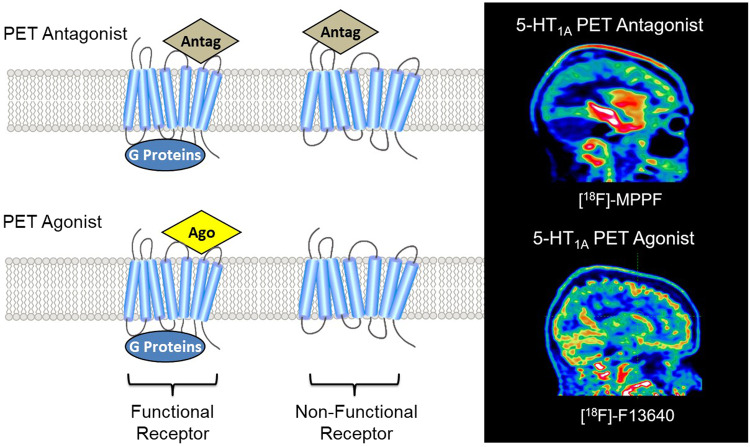
We have proposed a different interpretation, largely based on pharmacological facts and supported by experimental results. Thus it was shown several decades ago that 5-HT1A receptors can exist in a high or a low-affinity state, depending on whether they are coupled or not to their G proteins61,62. This property implies that whereas 5-HT1A receptor antagonists bind to the total pool of receptors, 5-HT1A receptor agonists preferentially bind to a subpopulation of receptors in their high-affinity state63. Consequently, the radiolabelled antagonists, i.e. the current PET radiopharmaceuticals, allow PET neuroimaging of the total pool of receptors, regardless of their functional state. In contrast, and according to this pharmacological mechanism, agonists bind preferentially to the functionally active state of receptors (see Fig. 1).
Fig. 1. Comparison of PET antagonist and agonist radiotracer binding.
The antagonist radiotracer binds to all states of the receptors, i.e. functional (bound to G protein) and non-functional (uncoupled form) whereas the agonist only binds to functional receptors (bound to G protein). As a result, the binding pattern at 5-HT1A receptors differs significantly between the antagonist [18F]-MPPF (binding in hippocampus, cingulate cortex and raphe nuclei) and the agonist [18F]-F13640 (binding mainly in cortical and cerebellar regions).
[18F]-F13640, a new radiopharmaceutical validated in human subjects
In order to specifically target 5-HT1A receptors in their G-protein-coupled functionally active state, we have developed a highly selective 5-HT1A receptor agonist, [18F]-F1364064. F13640, also known as befiradol or NLX-112, is a potent ligand with nanomolar affinity at 5-HT1A receptors, and exceptional selectivity over a wide range of more than fifty receptor and transporter subtypes65,66. Its pharmacology has been extensively investigated, showing that it efficaciously activates 5-HT1A receptors both in vitro and in vivo. Its specificity for 5-HT1A receptors in vivo is supported by the observation that its effects in various experimental models are abolished by co-administration of the 5-HT1A receptor antagonist, WAY100635.
The use of [18F]-labelled F13640 as an agonist PET tracer for functionally active 5-HT1A receptors was recently investigated and validated in animal models (rat, cat and non-human primate). The results showed that the in vivo distribution pattern of [18F]-F13640 is very different from that of an antagonist radiotracer, e.g. [18F]-MPPF. There were also striking distribution differences for [18F]-F13640 between in vitro and in vivo results in rat brain. A probable explanation for the distinct binding patterns between 5-HT1A agonist and antagonist radiotracers could be that the ratio of G-protein-coupled versus uncoupled receptors might be much lower in vivo compared to in vitro findings. In several animal species, although the blockade of labelling by the antagonist WAY-100635 was incomplete, [18F]-F13640 binding was almost completely blocked by the agonist 8-OH-DPAT. This may be due to a more effective competition between agonists as they both compete for receptors in the high-affinity state only. Notably, in vitro [18F]-F13640 binding was markedly reduced by Gpp(NH)p, an agent that switches receptors into an uncoupled state, demonstrating that [18F]-F13640 binds preferentially to G-protein-coupled 5-HT1A receptors, a property that is consistent with its agonist activity64. In contrast, uncoupling of 5-HT1A receptors from G proteins tends to increase the binding of the antagonist [18F]-MPPF, as previously reported67,68. These results highlight the fact that although both [18F]-F13640 and [18F]-MPPF are selective 5-HT1A receptor radioligands, they bind to different pools of receptors depending on their coupling state. This proof of concept of the specific binding of [18F]-F13640 to functionally active receptors was supported by a post-mortem study in Alzheimer subjects68. This study showed that the in vitro binding of [18F]-F13640 differs from that of [18F]-MPPF, the prototypical radiopharmaceutical. [18F]-F13640 is therefore a promising PET tracer for in vivo imaging and quantification of functionally active 5-HT1A receptors in the human brain, and a first-in-man study with this radiopharmaceutical is currently underway (study registration: EudraCT 2017-002722-21). The first human in vivo brain images with [18F]-F13640 showed a binding pattern different from that seen with conventional antagonist 5-HT1A radiopharmaceuticals69. Although the binding regions were well correlated with the density of 5-HT1A receptors, the regions of interest were mainly cortical, indicating that these brain regions are enriched in functionally active receptors. The [18F]-F13640 binding in cerebellum is less frequent, since this region is known to be poor in 5-HT1A receptors, although PET studies in volunteers have revealed the presence of 5-HT1A receptors in the cerebellar vermis of the cerebellum70.
PET imaging of functionally active 5-HT1A receptors: how to revisit this receptor in schizophrenia?
The development of [18F]-F13640 as an agonist PET radiopharmaceutical opens new opportunities for nuclear medicine. Until now, in vivo exploration of 5-HT1A receptor in schizophrenia has mostly focused on the total pool of receptors with the default use of antagonist PET radiopharmaceuticals. We propose that the specific exploration of “functional” 5-HT1A receptors, coupled to their G proteins, opens the way to revisiting the in vivo exploration of this receptor. Comparison between agonist and antagonist PET radiotracer binding could provide a promising and unprecedented receptor mapping strategy, potentially revealing new pathophysiological mechanisms. The availability of a radiopharmaceutical able to measure 5-HT1A agonist sites, enables the undertaking of different protocols to explore 5-HT1A receptors in schizophrenia: either using [18F]-F13640 alone, or by comparing the labelling of [18F]-F13640 with that of [18F]-MPPF or [11C]-WAY1006365. By opening the possibility to specifically explore functionally active receptors instead of total receptor densities, use of [18F]-F13640 could help to investigate issues that have remained unresolved by previous neuroimaging protocols.
Revisiting pathophysiological processes
Protocols based on the concept of functionally active receptors may help to better define how variations in the expression of 5-HT1A receptors could represent a biological trait marker of schizophrenia. First, comparing subjects with schizophrenia with healthy controls matched for sex and age could bring greater precision in the mapping of receptor densities. This could help to clarify the contradictory results previously found in the limbic and frontal cortices in post-mortem and in vivo studies, notably because the pronounced cortical distribution of [18F]-F13640 binding is favourable to the exploration of these brain areas. As a next step, the identification of new functionally active receptor patterns could be associated to cognitive status and illness state. The question of the presence of depression in schizophrenia, considered by some authors as more than a comorbidity71 could also be explored, leading to more subtle phenotyping of different populations of schizophrenic patients. In particular, these patterns could also be linked to suicidal behaviour for which 5-HT1A receptor status is a key player72, life-time prevalence of suicide being around 5% in patients with schizophrenia73. Other notable clinical presentations such as catatonia that, according to the DSM-5, is not linked as a subtype to schizophrenia anymore, could be explored through the prism of the functionally active receptor pattern. As gender differences have been little studied so far or only provided preliminary results that deserved further investigation39,40,42, additional analysis could also be carried out concerning this question. Such protocols should be extended to schizophrenia patients’ relatives, since this disorder is defined by a high heritability and genetic vulnerability28. Finally, genetic polymorphisms of the 5-HT1A receptor, as described earlier, could be at the origin of variation in the density of functionally active receptors and in treatment response to antipsychotics. All these neuroimaging protocols and the extension of this functionally active receptor approach to other neurotransmitters, will undoubtedly strengthen the concept of Research Domain Criteria (RDoC) proposed as an alternative method of classification in psychiatry with more emphasis on neurobiology74.
Revisiting pharmacological mechanisms
The use of protocols based on the concept of functionally active receptor would also provide new insights into the understanding of pharmacological responses. In a previous PET study performed with the radiolabelled antagonist [11C]WAY100635, no difference in total 5-HT1A receptor densities was found in schizophrenia patients before and after treatment with the antipsychotic ziprasidone, but there was a significant association between 5-HT1A binding and improvement in negative symptoms51. Longitudinal studies based on the examination of functionally active receptor densities before and after treatment with antipsychotic drugs could revisit these previous results and show some changes in receptor patterns. Do drug-resistant subjects have a specific pattern of functionally active receptors? Do these receptors change differently over the course of treatment, depending on its efficacy? These protocols will be demanding because of the possible 5-HT1A receptor occupancy rate of some antipsychotics having affinity for this receptor. To avoid such potential confounding factors related to drug occupancy, it may therefore be advisable to initially focus investigation on the effects of antipsychotics having a weak affinity for 5-HT1A receptors. These results could then be correlated to the treatment response rate and may define new profiles between the responders and the non-responders. Moreover, there may be distinct patterns of functionally active receptor densities in drug-resistant patients.
Integrating new multimodal imaging approaches
The literature in biological psychiatry is rich in clinical and research studies that have been performed using different imaging modalities on both separate positron emission tomography (PET) and magnetic resonance (MR) scanners. However, the current emergence of PET/MR cameras could broaden this approach75. While neurologists already make extensive use of this hybrid imaging modality, there is little research currently published in psychiatry using PET/MR. Although PET is able to quantify receptor densities, receptor occupancy by antipsychotics, and, with the development of agonist PET tracers such as [18F]-F13640, also quantify receptors specifically coupled to their G proteins, this approach cannot measure dynamically functional information. On the contrary, functional MRI offers the opportunity to map changes in cerebral activity occurring at resting state or during cognitive tasks and after administration of drugs; but it lacks mechanistic specificity as it provides no information on the receptors primarily targeted76,77. Combination of regional patterns of 5-HT1A functionally active receptors, functional connectivity, and grey matter volume, derived from the disease characteristic networks, can potentially discriminate individual patients with different phenotypes. By analysing the functional connectivity of resting-state fMRI data in schizophrenia, the cortico–cerebellar–striatal–thalamic loop abnormalities, classically described could be correlated with the cortical binding of [18F]-F13640, which in turn could be associated with the cognitive performance of the subjects. Here again, the possibility of longitudinal follow-up, and thus intra-individual comparisons, would allow a distinction to be made between those subjects who respond and those who do not respond to pharmacological or psychotherapeutic strategies.
Conclusions
The exploration of 5-HT1A receptors in psychiatry has given rise to numerous studies. Although this receptor is associated with the pathophysiological mechanisms of schizophrenia and the pharmacological mechanisms of new-generation antipsychotics, its characterization remains incomplete. The contributions of molecular imaging, first in vitro on brain tissue, then in vivo, thanks to PET imaging, have not permitted significant advances but the recent concept of PET neuroimaging of G-protein-coupled receptors makes it possible to revisit PET brain exploration by enabling totally new research paradigms. These will make it possible to follow changes in the pattern of functionally active 5-HT1A receptors in relation to disease symptomatology, such as cognitive dysfunction or mood deficits, both to better distinguish phenotypes and also to understand the basis of the therapeutic response to antipsychotic drugs. It is likely that the strategy of targeting functionally active receptors, as described above for the 5-HT1A receptor, could also be usefully extended to other crucial receptors in pathophysiology or therapy of schizophrenia.
Acknowledgements
O.R. was awarded a scholarship from the French Congress of Psychiatry (Congrès Français de Psychiatrie). This work was supported by the LABEX PRIMES (ANR-11-LABX-0063) of Université de Lyon and the programme “Investissements d’Avenir” (ANR-11-IDEX-0007) operated by the French National Research Agency (ANR) and by the imaging platform, CERMEP (Lyon, France).
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Popova NK, Naumenko VS. 5-HT1A receptor as a key player in the brain 5-HT system. Rev. Neurosci. 2013;24:191–204. doi: 10.1515/revneuro-2012-0082. [DOI] [PubMed] [Google Scholar]
- 2.Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/S0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 3.Haleem DJ. 5-HT1A receptor-dependent control of nigrostriatal dopamine neurotransmission in the pharmacotherapy of Parkinson’s disease and schizophrenia. Behav. Pharm. 2015;26:45–58. doi: 10.1097/FBP.0000000000000123. [DOI] [PubMed] [Google Scholar]
- 4.James SL, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rössler W, Joachim Salize H, van Os J, Riecher-Rössler A. Size of burden of schizophrenia and psychotic disorders. Eur. Neuropsychopharmacol. 2005;15:399–409. doi: 10.1016/j.euroneuro.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Laursen TM, Nordentoft M, Mortensen PB. Excess early mortality in schizophrenia. Annu. Rev. Clin. Psychol. 2014;10:425–448. doi: 10.1146/annurev-clinpsy-032813-153657. [DOI] [PubMed] [Google Scholar]
- 7.Brisch, R. et al. The role of dopamine in schizophrenia from a neurobiological and evolutionary perspective: old fashioned, but still in vogue. Front. Psychiatry5, 47 (2014). [DOI] [PMC free article] [PubMed]
- 8.Meltzer HY, Li Z, Kaneda Y, Ichikawa J. Serotonin receptors: their key role in drugs to treat schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2003;27:1159–1172. doi: 10.1016/j.pnpbp.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 9.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th edn. (Washington, DC, 2013).
- 10.Carbon M, Correll CU. Thinking and acting beyond the positive: the role of the cognitive and negative symptoms in schizophrenia. CNS Spectr. 2014;19:35–53. doi: 10.1017/S1092852914000601. [DOI] [PubMed] [Google Scholar]
- 11.McCreary A, Newman-Tancredi A. Serotonin 5-HT1A receptors and antipsychotics—an update in light of new concepts and drugs. Curr. Pharm. Des. 2015;21:3725–3731. doi: 10.2174/1381612821666150605105215. [DOI] [PubMed] [Google Scholar]
- 12.Newman-Tancredi A. The importance of 5-HT1A receptor agonism in antipsychotic drug action: rationale and perspectives. Curent Opin. Investig. Drugs. 2010;11:2040–3429. [PubMed] [Google Scholar]
- 13.Stahl SM, Shayegan DK. The psychopharmacology of ziprasidone: receptor-binding properties and real-word psychiatric practice. Clin. Psychiatry. 2003;64:6–12. doi: 10.4088/JCP.v64n0102. [DOI] [PubMed] [Google Scholar]
- 14.McIlwain ME, Harrison J, Wheeler AJ, Russell BR. Pharmacotherapy for treatment-resistant schizophrenia. Neuropsychiatr. Dis. Treat. 2011;7:135–149. doi: 10.2147/NDT.S12769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McNabb CB, et al. Functional network dysconnectivity as a biomarker of treatment resistance in schizophrenia. Schizophr. Res. 2018;195:160–167. doi: 10.1016/j.schres.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 16.Howes OH, Kaar SJ. Antipsychotic drugs: challenges and future directions. World Psychiatry. 2018;17:170–171. doi: 10.1002/wps.20522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ichikawa J, et al. 5-HT2A and D2 receptor blockade increases cortical DA release via 5-HT1A receptor activation: a possible mechanism of atypical antipsychotic-induced cortical dopamine release: atypical antipsychotics and 5-HT1A activation. J. Neurochem. 2001;76:1521–1531. doi: 10.1046/j.1471-4159.2001.00154.x. [DOI] [PubMed] [Google Scholar]
- 18.Newman-Tancredi A, et al. Agonist and antagonist actions of antipsychotic agents at 5-HT1A receptors: a [35S]GTPgammaS binding study. Eur. J. Pharm. 1998;355:245–256. doi: 10.1016/S0014-2999(98)00483-X. [DOI] [PubMed] [Google Scholar]
- 19.Maeda K, et al. Brexpiprazole I: in vitro and in vivo characterization of a novel serotonin-dopamine activity modulator. J. Pharm. Exp. Ther. 2014;350:589–604. doi: 10.1124/jpet.114.213793. [DOI] [PubMed] [Google Scholar]
- 20.Stark AD, et al. Interaction of the novel antipsychotic aripiprazole with 5-HT1A and 5-HT2A receptors: functional receptor-binding and in vivo electrophysiological studies. Psychopharmacology. 2007;190:373–382. doi: 10.1007/s00213-006-0621-y. [DOI] [PubMed] [Google Scholar]
- 21.Kiss B, et al. Cariprazine (RGH-188), a dopamine D3 receptor-preferring, D3/D2 dopamine receptor antagonist–partial agonist antipsychotic candidate: in vitro and neurochemical profile. J. Pharm. Exp. Ther. 2010;333:328–340. doi: 10.1124/jpet.109.160432. [DOI] [PubMed] [Google Scholar]
- 22.Jones CA, McCreary AC. Serotonergic approaches in the development of novel antipsychotics. Neuropharmacology. 2008;55:1056–1065. doi: 10.1016/j.neuropharm.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 23.Meltzer HY, Sumiyoshi T. Does stimulation of 5-HT1A receptors improve cognition in schizophrenia? Behav. Brain Res. 2008;195:98–102. doi: 10.1016/j.bbr.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 24.Sumiyoshi T, et al. The effect of tandospirone, a serotonin(1A) agonist, on memory function in schizophrenia. Biol. Psychiatry. 2001;49:861–868. doi: 10.1016/S0006-3223(00)01025-8. [DOI] [PubMed] [Google Scholar]
- 25.Sumiyoshi T, et al. Enhancement of cognitive performance in schizophrenia by addition of tandospirone to neuroleptic treatment. Am. J. Psychiatry. 2001;158:1722–1725. doi: 10.1176/appi.ajp.158.10.1722. [DOI] [PubMed] [Google Scholar]
- 26.Newman-Tancredi A, Kleven MS. Comparative pharmacology of antipsychotics possessing combined dopamine D2 and serotonin 5-HT1A receptor properties. Psychopharmacology. 2011;216:451–473. doi: 10.1007/s00213-011-2247-y. [DOI] [PubMed] [Google Scholar]
- 27.Ye N, Song Z, Zhang A. Dual ligands targeting dopamine D2 and serotonin 5-HT1A receptors as new antipsychotical or anti-Parkinsonian agents. Curr. Med. Chem. 2014;21:437–457. doi: 10.2174/09298673113206660300. [DOI] [PubMed] [Google Scholar]
- 28.Harrison PJ. Recent genetic findings in schizophrenia and their therapeutic relevance. J. Psychopharmacol. 2015;29:85–96. doi: 10.1177/0269881114553647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newman-Tancredi, A. & Albert, P. R. In Schizophrenia Research: Recent Advances (ed. Sumiyoshi, T.) Ch. 15 (Nova Science Publishers, New York, 2012).
- 30.Huang YY, et al. Human 5-HT1A receptor C(−1019)G polymorphism and psychopathology. Int. J. Neuropsychopharmacol. 2004;7:441–451. doi: 10.1017/S1461145704004663. [DOI] [PubMed] [Google Scholar]
- 31.Reynolds GP, Arranz B, Templeman LA, Fertuzinhos S, San L. Effect of 5-HT1A receptor gene polymorphism on negative and depressive symptom response to antipsychotic treatment of drug-naive psychotic patients. Am. J. Psychiatry. 2006;163:1826–1829. doi: 10.1176/ajp.2006.163.10.1826. [DOI] [PubMed] [Google Scholar]
- 32.Wang L, et al. The 1019 C/G polymorphism of the 5-HT1A receptor gene is associated with negative symptom response to risperidone treatment in schizophrenia patients. J. Psychopharmacol. 2008;22:904–909. doi: 10.1177/0269881107081522. [DOI] [PubMed] [Google Scholar]
- 33.Bosia M, et al. Effect of 5-HT1A-receptor functional polymorphism on Theory of Mind performances in schizophrenia. Psychiatry Res. 2011;188:187–190. doi: 10.1016/j.psychres.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 34.Albert PR. Transcriptional regulation of the 5-HT1A receptor: implications for mental illness. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012;367:2402–2415. doi: 10.1098/rstb.2011.0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selvaraj S, Arnone D, Cappai A, Howes O. Alterations in the serotonin system in schizophrenia: a systematic review and meta-analysis of postmortem and molecular imaging studies. Neurosci. Biobehav. Rev. 2014;45:233–245. doi: 10.1016/j.neubiorev.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 36.Burnet PW, Eastwoord SL, Harrison PJ. 5-HT1A and 5-HT2A Receptor mRNAs and binding site densities are differentially altered in schizophrenia. Neuropsychopharmacology. 1996;15:442–455. doi: 10.1016/S0893-133X(96)00053-X. [DOI] [PubMed] [Google Scholar]
- 37.Burnet PW, Eastwood SL, Harrison PJ. [3H]WAY-100635 for 5-HT1A receptor autoradiography in human brain: a comparison with [3H]-8OH-DPAT and demonstration of increased binding in the frontal cortex in schizophrenia. Neurochem. Int. 1997;30:565–574. doi: 10.1016/S0197-0186(96)00124-6. [DOI] [PubMed] [Google Scholar]
- 38.Hashimoto T, Nishino N, Nakai H, Tanaka C. Increase in serotonin 5-HT1A receptors in prefrontal and temporal cortices of brains from patients with chronic schizophrenia. Life Sci. 1991;48:355–363. doi: 10.1016/0024-3205(91)90556-Q. [DOI] [PubMed] [Google Scholar]
- 39.Simpson MDC, Lubman DI, Slater P, Deakin JF. Autoradiography with [3H]8-OH-DPAT reveals increases in 5-HT(1A) receptors in ventral prefrontal cortex in schizophrenia. Biol. Psychiatry. 1996;39:919–928. doi: 10.1016/0006-3223(95)00026-7. [DOI] [PubMed] [Google Scholar]
- 40.Gurevich EV, Joyce JN. Alterations in the cortical serotonergic system in schizophrenia: a postmortem study. Biol. Psychiatry. 1997;42:529–545. doi: 10.1016/S0006-3223(97)00321-1. [DOI] [PubMed] [Google Scholar]
- 41.Sumiyoshi T, Stockmeier CA, Overholser JC, Dilley GE, Meltzer HY. Serotonin1A receptors are increased in postmortem prefrontal cortex in schizophrenia. Brain Res. 1996;708:209–214. doi: 10.1016/0006-8993(95)01361-X. [DOI] [PubMed] [Google Scholar]
- 42.Gray L, Scarr E, Dean B. Serotonin 1a receptor and associated G-protein activation in schizophrenia and bipolar disorder. Psychiatry. 2006;143:111–120. doi: 10.1016/j.psychres.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 43.Dean B, Tomaskovic-Crook E, Opeskin K, Keks N, Copolov D. No change in the density of the serotonin1A receptor, the serotonin4 receptor or the serotonin transporter in the dorsolateral prefrontal cortex from subjects with schizophrenia. Neurochem. Int. 1999;34:109–115. doi: 10.1016/S0197-0186(98)00074-6. [DOI] [PubMed] [Google Scholar]
- 44.Joyce JN, et al. Serotonin uptake sites and serotonin receptors are altered in the limbic system of schizophrenics. Neuropsychopharmacology. 1993;8:315–336. doi: 10.1038/npp.1993.32. [DOI] [PubMed] [Google Scholar]
- 45.Scarr E, Pavey G, Copolov D, Dean B. Hippocampal 5-hydroxytryptamine receptors: abnormalities in postmortem brain from schizophrenic subjects. Schizophr. Res. 2004;71:383–392. doi: 10.1016/j.schres.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 46.Slater P, Doyle CA, Deakin JF. Abnormal persistence of cerebellar serotonin-1A receptors in schizophrenia suggests failure to regress in neonates. J. Neural Transm. 1998;105:305–315. doi: 10.1007/s007020050060. [DOI] [PubMed] [Google Scholar]
- 47.Bantick RA, et al. A positron emission tomography study of the 5-HT1A receptor in schizophrenia and during clozapine treatment. J. Psychopharmacol. 2004;18:346–354. doi: 10.1177/026988110401800304. [DOI] [PubMed] [Google Scholar]
- 48.Frankle WG, et al. Serotonin 1A receptor availability in patients with schizophrenia and schizo-affective disorder: a positron emission tomography imaging study with [11C]WAY 100635. Psychopharmacology. 2006;189:155–164. doi: 10.1007/s00213-006-0543-8. [DOI] [PubMed] [Google Scholar]
- 49.Tauscher J, et al. Brain serotonin 5-HT1A receptor binding in schizophrenia measured by positron emission tomography and [11C]WAY-100635. Arch. Gen. Psychiatry. 2002;59:514. doi: 10.1001/archpsyc.59.6.514. [DOI] [PubMed] [Google Scholar]
- 50.Yasuno F, et al. Decreased 5-HT1A receptor binding in amygdala of schizophrenia. Biol. Psychiatry. 2004;55:439–444. doi: 10.1016/j.biopsych.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 51.Frankle WG, et al. Measurement of the serotonin 1A receptor availability in patients with schizophrenia during treatment with the antipsychotic medication ziprasidone. J. Psychopharmacol. 2011;25:734–743. doi: 10.1177/0269881110388329. [DOI] [PubMed] [Google Scholar]
- 52.Hedlund PB, et al. 8-OH-DPAT acts on both 5-HT1A and 5-HT7 receptors to induce hypothermia in rodents. Eur. J. Pharm. 2004;487:125–132. doi: 10.1016/j.ejphar.2004.01.031. [DOI] [PubMed] [Google Scholar]
- 53.Chemel BR, Roth BL, Armbruster B, Watts VJ, Nichols DE. WAY-100635 is a potent dopamine D4 receptor agonist. Psychopharmacology. 2006;188:244–251. doi: 10.1007/s00213-006-0490-4. [DOI] [PubMed] [Google Scholar]
- 54.Aznavour N, et al. A comparison of in vivo and in vitro neuroimaging of 5-HT1A receptor binding sites in the cat brain. J. Chem. Neuroanat. 2006;31:226–232. doi: 10.1016/j.jchemneu.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 55.Zimmer, L. Pharmacological agonists for more-targeted CNS radio-pharmaceuticals. Oncotarget7, 49 (2016). [DOI] [PMC free article] [PubMed]
- 56.Colom M, Vidal B, Zimmer L. Is there a Role for GPCR agonist radiotracers in PET neuroimaging? Front. Mol. Neurosci. 2019;12:255. doi: 10.3389/fnmol.2019.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shalgunov V, et al. Hunting for the high‐affinity state of G‐protein‐coupled receptors with agonist tracers: theoretical and practical considerations for positron emission tomography imaging. Med. Res. Rev. 2019;39:1014–1052. doi: 10.1002/med.21552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Narendran R, et al. Measurement of the proportion of D2 receptors configured in state of high affinity for agonists in vivo: a positron emission tomography using [11C]N-propyl-norapomorphine and [11C]raclopride in baboons. J. Pharm. Exp. Ther. 2005;315:80–90. doi: 10.1124/jpet.105.090068. [DOI] [PubMed] [Google Scholar]
- 59.Ginovart N, et al. Binding characteristics and sensitivity to endogenous dopamine of [11C]-(+)-PHNO, a new agonist radiotracer for imaging the high-affinity state of D2 receptors in vivo using positron emission tomography. J. Neurochem. 2006;97:1089–1103. doi: 10.1111/j.1471-4159.2006.03840.x. [DOI] [PubMed] [Google Scholar]
- 60.Placzek MS, et al. Discrepancies in kappa opioid agonist binding revealed through PET imaging. ACS Chem. Neurosci. 2019;10:384–395. doi: 10.1021/acschemneuro.8b00293. [DOI] [PubMed] [Google Scholar]
- 61.Mongeau R, Welner SA, Quirion R, Suranyi-Cadotte BE. Further evidence for differential affinity states of the serotonin1A receptor in rat hippocampus. Brain Res. 1992;590:229–238. doi: 10.1016/0006-8993(92)91100-S. [DOI] [PubMed] [Google Scholar]
- 62.Emerit MB, el Mestikawy S, Gozlan H, Rouot B, Hamon M. Physical evidence of the coupling of solubilized 5-HT1A binding sites with G regulatory proteins. Biochem. Pharm. 1990;39:7–18. doi: 10.1016/0006-2952(90)90642-X. [DOI] [PubMed] [Google Scholar]
- 63.Gozlan H, Thibault S, Laporte AM, Lima L, Hamon M. The selective 5HT1A antagonist radioligand [3H]WAY 100635 labels both G-protein-coupled and free 5HT1A receptors in rat brain membranes. Eur. J. Pharm. 1995;288:173–186. doi: 10.1016/0922-4106(95)90192-2. [DOI] [PubMed] [Google Scholar]
- 64.Vidal B, et al. 18F-F13640 preclinical evaluation in rodent, cat and primate as a 5-HT1A receptor agonist for PET neuroimaging. Brain Struct. Funct. 2018;223:2973–2988. doi: 10.1007/s00429-018-1672-7. [DOI] [PubMed] [Google Scholar]
- 65.Newman-Tancredi A, et al. Distinctive in vitro signal transduction profile of NLX-112, a potent and efficacious serotonin 5-HT1A receptor agonist. J. Pharm. Pharm. 2017;69:1178–1190. doi: 10.1111/jphp.12762. [DOI] [PubMed] [Google Scholar]
- 66.Colpaert FC, et al. Large-amplitude 5-HT1A receptor activation: a new mechanism of profound, central analgesia. Neuropharmacology. 2002;43:945–958. doi: 10.1016/S0028-3908(02)00119-3. [DOI] [PubMed] [Google Scholar]
- 67.Lemoine L, et al. [18F]F15599, a novel 5-HT1A receptor agonist, as a radioligand for PET neuroimaging. Eur. J. Nucl. Med. Mol. Imaging. 2010;37:594–605. doi: 10.1007/s00259-009-1274-y. [DOI] [PubMed] [Google Scholar]
- 68.Vidal B, et al. Agonist and antagonist bind differently to 5-HT1A receptors during Alzheimer’s disease: a post-mortem study with PET radiopharmaceuticals. Neuropharmacology. 2016;109:88–95. doi: 10.1016/j.neuropharm.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 69.Colom M, et al. 18F-F13640 PET imaging of functional receptors in humans. Eur. J. Nucl. Med. Mol. Imaging. 2020;47:220–221. doi: 10.1007/s00259-019-04473-7. [DOI] [PubMed] [Google Scholar]
- 70.Parsey RV, et al. Regional heterogeneity of 5-HT1A receptors in human cerebellum as assessed by positron emission tomography. J. Cereb. Blood Flow. Metab. 2005;25:785–793. doi: 10.1038/sj.jcbfm.9600072. [DOI] [PubMed] [Google Scholar]
- 71.Upthegrove R, Marwaha S, Birchwood M. Depression and Schizophrenia: cause, consequence or trans-diagnostic issue? Schizophr. Bull. 2017;43:240–244. doi: 10.1093/schbul/sbw097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Underwood MD, et al. Serotonin receptors and suicide, major depression, alcohol use disorder and reported early life adversity. Transl. Psychiatry. 2018;8:279. doi: 10.1038/s41398-018-0309-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hor K, Taylor M. Suicide and schizophrenia: a systematic review of rates and risk factors. J. Psychopharmacol. 2010;24:81–90. doi: 10.1177/1359786810385490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cuthbert BN. The role of RDoC in future classification of mental disorders. Dialogues Clin. Neurosci. 2020;22:81–85. doi: 10.31887/DCNS.2020.22.1/bcuthbert. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vidal B, et al. In vivo biased agonism at 5-HT1A receptors: characterisation by simultaneous PET/MR imaging. Neuropsychopharmacology. 2018;43:2310–2319. doi: 10.1038/s41386-018-0145-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jenkins BG. Pharmacologic magnetic resonance imaging (phMRI): Imaging drug action in the brain. NeuroImage. 2012;62:1072–1085. doi: 10.1016/j.neuroimage.2012.03.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mwansisya TE, et al. Task and resting-state fMRI studies in first-episode schizophrenia: a systematic review. Schizophr. Res. 2017;189:9–18. doi: 10.1016/j.schres.2017.02.026. [DOI] [PubMed] [Google Scholar]