Abstract
Tissue stem cell senescence leads to stem cell exhaustion, which results in tissue homeostasis imbalance and a decline in regeneration capacity. However, whether neural stem cell (NSC) senescence occurs and causes neurogenesis reduction during aging is unknown. In this study, mice at different ages were used to detect age-related hippocampal NSC (H-NSC) senescence, as well as the function and mechanism of embryonic stem cell-derived small extracellular vesicles (ESC-sEVs) in rejuvenating H-NSC senescence. We found a progressive cognitive impairment, as well as age-related H-NSC senescence, in mice. ESC-sEV treatment significantly alleviated H-NSC senescence, recovered compromised self-renewal and neurogenesis capacities, and reversed cognitive impairment. Transcriptome analysis revealed that myelin transcription factor 1 (MYT1) is downregulated in senescent H-NSCs but upregulated by ESC-sEV treatment. In addition, knockdown of MYT1 in young H-NSCs accelerated age-related phenotypes and impaired proliferation and differentiation capacities. Mechanistically, ESC-sEVs rejuvenated senescent H-NSCs partly by transferring SMAD family members 4 (SMAD4) and 5 (SMAD5) to activate MYT1, which downregulated egl-9 family hypoxia inducible factor 3 (Egln3), followed by activation of hypoxia inducible factor 2 subunit α (HIF-2α), nicotinamide phosphoribosyl transferase (NAMPT), and sirtuin 1 (Sirt1) successively. Taken together, our results indicated that H-NSC senescence caused cellular exhaustion, neurogenesis reduction, and cognitive impairment during aging, which can be reversed by ESC-sEVs. Thus, ESC-sEVs may be promising therapeutic candidates for age-related diseases.
Keywords: aging, hippocampal NSCs, senescence, ESC-sEVs, MYT1
Graphical Abstract
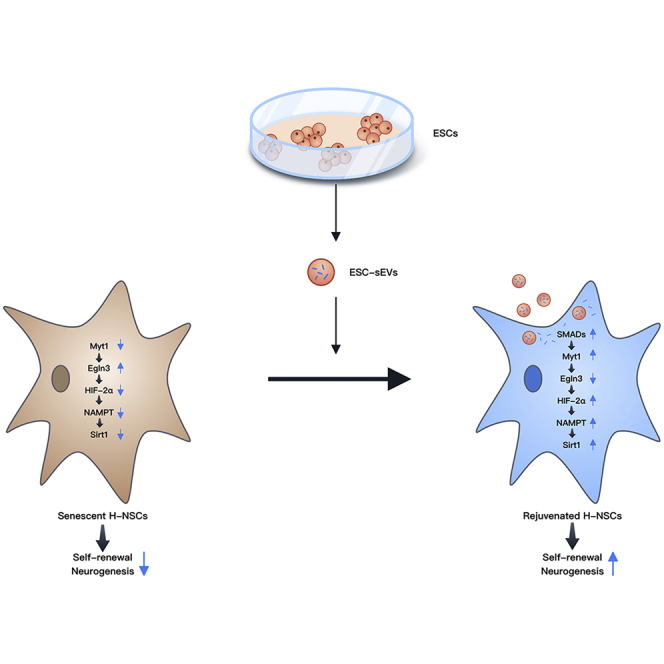
Neural stem cell (NSC) senescence gradually occurs during aging, which leads to neurogenesis reduction and cognitive dysfunction. ESC-sEVs rejuvenate senescent NSCs and thereby improve cognitive function partially through the delivery of SMAD4 and SMAD5 to activate MYT1, which leads to Egln3 downregulation, followed by upregulation of HIF-2α, NAMPT, and Sirt1 successively.
Introduction
With the increase of lifespan, aging populations are progressively growing, which is also accompanied by an increased risk of age-related neurodegenerative diseases, including Alzheimer’s disease (AD) and Parkinson’s disease (PD), causing severe social and economic burdens.1 Adult neurogenesis mainly occurs in the subventricular zone (SVZ) of the lateral ventricle and dentate gyrus (DG) of the hippocampus and declines gradually with age,2,3 primarily due to the loss of the proliferative capability of neural stem cells (NSCs),4 consequently giving rise to age-related memory loss and cognitive dysfunction. Cellular senescence, which is defined as an essentially irreversible cell cycle arrest caused by various biological and pathological conditions, has been demonstrated to account for tissue homeostasis impairment.5 Age-associated stem cell senescence has been confirmed as an important part of the pathogenesis of multiple disorders.6 For example, hematopoietic stem cells became senescent gradually with aging, lost their self-renewal and regenerative potential, and weakened the adaptive immune system.7 Multiple studies have demonstrated that NSC senescence was associated with the loss of stem cell markers,8 as well as self-renewal and neurogenic capacity impairment.9, 10, 11 However, whether NSCs undergo the senescence process with natural aging, the potential underlying mechanism, and the relationship with neurogenesis are not well elucidated.
Rejuvenation of stem cell senescence can enhance their self-renewal and regenerative capacities, which has been proposed as a promising therapeutic approach to restore tissue structure and function.6 For example, rejuvenation of senescent oligodendrocyte progenitor cells by fasting or metformin can restore the differentiation potential as well as remyelination ability.12 Small extracellular vesicles (sEVs) are natural nanosized particles that participate in intracellular communication and influence the behavior of recipient cells via the delivery of functional biomolecules.13 Stem cell-derived sEVs are regarded as an attractive therapeutic strategy in regenerative medicine due to their promising pro-regenerative effects, no risk of aneuploidy, and low possibility of immune rejection.14, 15, 16 Embryonic stem cell-derived sEVs (ESC-sEVs) contain bioactive factors from their parental cells that possess unique capacities, including infinite proliferation ability, pluripotency, and an intrinsic youthful barrier to aging,17 suggesting they may be beneficial for treating aging-associated disease. Recent studies from our group and others have demonstrated that ESC-sEVs can rejuvenate somatic cell senescence and promote regeneration,18,19 suggesting they may be beneficial to aging-associated disease.
In this study, we reported that hippocampal NSC (H-NSC) senescence is accompanied by the progression of aging. Myelin transcription factor 1 (MYT1) is downregulated in senescent H-NSCs, and knockdown of MYT1 in young H-NSCs promotes age-related phenotypes and impairs proliferation as well as differentiation capacities. We also found that ESC-sEVs can rejuvenate senescent H-NSCs partly by transferring SMAD family members 4 (SMAD4) and 5 (SMAD5) to activate MYT1, inhibiting egl-9 family hypoxia inducible factor 3 (Egln3) expression, improving hypoxia inducible factor 2 subunit α (HIF-2α) levels, promoting nicotinamide phosphoribosyl transferase (NAMPT) expression, and upregulating sirtuin 1 (Sirt1). In this study, for the first time, we identify a MYT1-dependent transcriptional program involved in H-NSC senescence that contributed to cognitive impairment with aging. We also demonstrate that ESC-sEVs have the potential to reverse cognitive dysfunction by rejuvenating H-NSC senescence.
Results
Hippocampal NSCs Exhibited a Senescent Phenotype with Aging
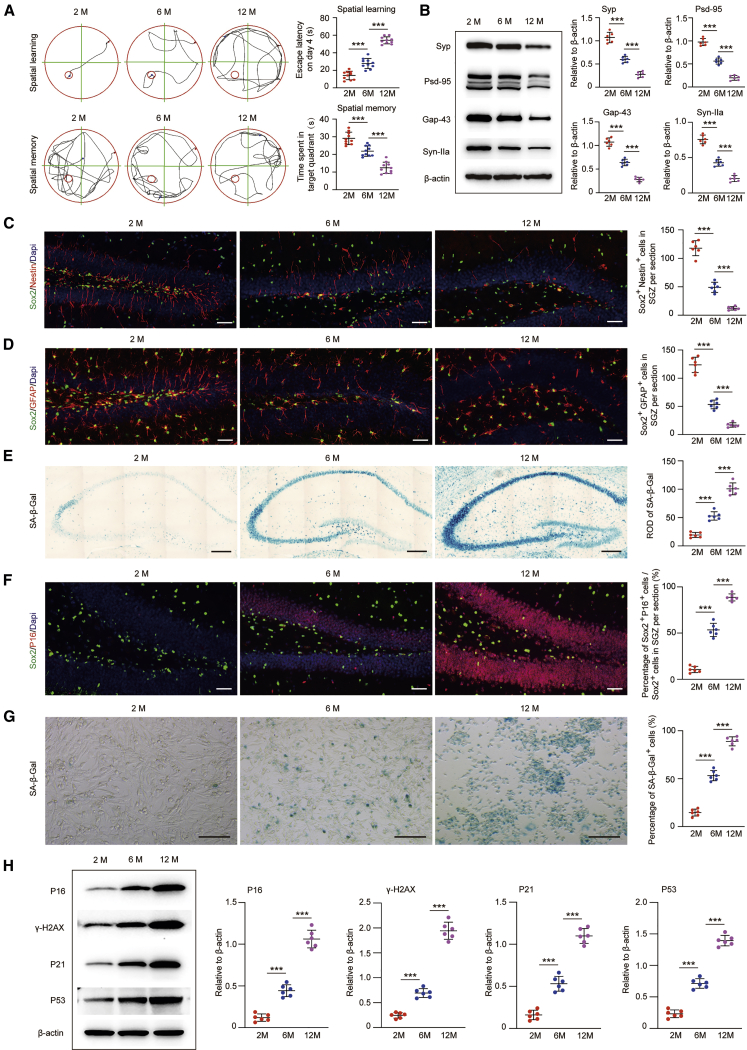
In this study, senescence-accelerated mouse prone 8 (SAMP8) mice were used to evaluate H-NSC senescence with aging. As cognitive aging is a lifelong process of ongoing and progressive cognitive functional decline in the senior,20 we first evaluated the spatial learning and memory abilities of SAMP8 mice of different ages (2 months, young; 6 months, middle-aged; 12 months, aged) using a Morris water maze (MWM). As shown in Figure 1A, the escape latency on day 4 was markedly increased from the 2 month group to the 12 month group. The time spent in the target quadrant on day 5 was gradually decreased from the 2 month group to the 12 month group. As synaptic plasticity is the neurobiological basis of cognitive function,21 we then detected the expression of hippocampal synapse-related proteins, including synaptophysin (Syp), postsynaptic density protein-95 (PSD-95), growth-associated protein-43 (Gap-43), and synapsin IIa (Syn-IIa). As shown in Figure 1B, among the three groups, the expression level of Syp, PSD-95, Gap-43, and Syn-IIa decreased significantly with the increase in age. These data confirmed an age-related reduction of cognitive function in mice.
Figure 1.
Cognitive Impairment, H-NSC Depletion, and Senescence in SAMP8 Mice during Aging
(A) Spatial learning and memory abilities in mice at an age of 2, 6, and 12 months were tested by the MWM. n = 10 per group. ∗∗∗p < 0.001. (B) Western blot analysis and quantification of Syp, Gap-43, PSD-95, and Syn-IIa (relative to β-actin) in the hippocampus of mice at an age of 2, 6, and 12 months. n = 6 per group. ∗∗∗p < 0.001. (C) IF images for Sox2+ (green) and Nestin+ (red) cells and the number of Sox2+/Nestin+ double-stained cells in the SGZ of mice at an age of 2, 6, and 12 months. Scale bars, 50 μm. n = 6 per group. ∗∗∗p < 0.001. (D) IF images for Sox2+ (green) and GFAP+ (red) cells and the number of Sox2+/GFAP+ double-stained cells in the SGZ of mice at an age of 2, 6, and 12 months. Scale bars, 50 μm. n = 6 per group. ∗∗∗p < 0.001. (E) SA-β-gal staining of the hippocampus and quantification of hippocampal SA-β-gal activity in mice at an age of 2, 6, and 12 months. Scale bars, 250 μm. n = 6 per group. ∗∗∗p < 0.001. (F) IF images of p16INK4a+ (red)/Sox2+ (green) senescent H-NSCs and the quantification of Sox2+/p16INK4a+ double-stained cells of all Sox2+ cells in the SGZ of mice at an age of 2, 6, and 12 months. Scale bars, 50 μm. n = 6 per group. ∗∗∗p < 0.001. (G) Representative images of SA-β-gal staining and quantification of SA-β-gal+ cells from isolated H-NSCs from SAMP8 mice at an age of 2, 6, and 12 months. Scale bars, 100 μm. n = 6 per group. ∗∗∗p < 0.001. (H) Western blot analysis and quantification of p16INK4a, γ-H2AX, p21, and p53 in isolated neurospheres in SAMP8 mice at an age of 2, 6, and 12 months. n = 6 per group. ∗∗∗p < 0.001.
As H-NSCs play a crucial role in maintaining and restoring cognition, their loss directly leads to the decline of neuroplasticity and cognitive function.22,23 We calculated the number of H-NSCs in the subgranular zone (SGZ) among the three groups. Antibodies against Sox2 and Nestin as well as Sox2 and glial fibrillary acidic protein (GFAP) were used to label H-NSCs. As shown in Figures 1C and 1D, Sox2 and Nestin coexpressing cells as well as Sox2 and GFAP coexpressing cells were found to be densely present in the SGZ of 2 month mice, and the gradually diminished with an increase of age. The number of Sox2+/Nestin+ and Sox2+/GFAP+ NSCs in the 6 month group was significantly lower than that in the 2 month group, and these cells almost disappeared in 12 month mice. We further isolated H-NSCs from different ages of mice, and we found that the neurosphere size (Figure S1A) and β-tubulin III+ cells (Figure S1B) decreased with the increase of age in mice, meaning that the proliferation and neuronal differentiation abilities of H-NSCs decreased with aging. In addition, the correlation analysis showed that the numbers of Sox2+/Nestin+ cells and Sox2+/GFAP+ cells in the SGZ were negatively correlated with escape latency on day 4, and positively correlated with the time spent in the target quadrant on day 5, with the expression level of hippocampal synapse-related proteins (Syp, PSD-95, Gap-43, Syn-IIa) (Figure S2). Overall, the age-dependent loss of H-NSCs correlated well with age-related cognitive function decline, which led us to investigate the internal change of H-NSCs with aging.
Since stem cells could become senescent with aging, which impairs their intrinsic functions,6 we further detected the senescent status of H-NSCs in mice of different ages. As shown in Figure 1E, compared to the 2 month group, the activity of senescence-associated β-galactosidase (SA-β-gal) gradually increased in the hippocampus in the 6 month and 12 month group. These results indicated an aggravation of hippocampal senescence with aging in mice. Then, we performed p16INK4a (senescence marker) and Sox2 (NSC marker) double staining to identify senescent NSCs in the hippocampus. As shown in Figure 1F, among the three groups, the percentage of Sox2 and p16INK4a coexpressing cells of the total Sox2+ cells in the SGZ was the lowest in the 2 month group, and gradually increased with age. Additionally, we isolated H-NSCs from the hippocampus of mice at different ages, and we found that the percentage of SA-β-gal+ cells gradually increased from 2 months to 12 months (Figure 1G). Moreover, the expression level of senescence-related proteins (p16INK4a, γ-H2AX, p21, and p53) in isolated hippocampal neurospheres also gradually increased with age (Figure 1H). Taken together, these findings indicated an age-related H-NSC senescence in mice and suggested that H-NSC senescence during aging may result in loss of H-NSCs and subsequently cognitive dysfunction.
ESC-sEVs Ameliorate Hippocampal NSC Senescence and Enhanced NSC Activity in SAMP8 Mice during Aging
As described above, H-NSC senescence could be an important reason for the loss of H-NSCs during aging. Thus, amelioration of H-NSC senescence may reverse hippocampal neurogenesis reduction and cognitive impairment. In our previous study, we demonstrated that ESC-sEVs possessed an excellent ability to rejuvenate endothelial senescence.19 Therefore, we explored whether ESC-sEVs could rejuvenate H-NSC senescence during aging. As shown in Figures S3A and S3B, ESC colonies were identified by the expression of alkaline phosphatase (ALP) and pluripotency-related markers, including OCT4, Nanog, TRA-1-81, TRA-1-60, and SSEA4. Then, ESC-sEVs were purified from the conditioned medium (CM). ESC-sEVs exhibited a size distribution of approximately 100 nm with a characteristic cup-shaped morphology under transmission electron microscopy (TEM) (Figure S3C). Flow nanoanalyzer analysis indicated particles with a mean diameter of 70.3 ± 17.7 nm and a concentration of 1.82 × 1011 ± 0.17 × 1011 particles/mL (Figure S3D). Western blot showed that ESC-sEVs express exosomal markers CD9, CD63, and TSG101, but not the Golgi matrix protein GM130, β-actin, and Lamin A/C (Figure S3E), which means there was no contamination of cellular components in the isolated ESC-sEVs. We further evaluated the yield of ESC-sEVs by particle concentration and protein concentration. As shown in Figures S3F–S3I, the mean particle concentration was 7.06 × 108 ± 0.93 × 108 particles/mL in CM and 817.92 ± 94.13 particles/cell. The mean protein concentration was 1,127.49 ± 81.47 ng/mL in CM and 11.43 × 10−7 ± 1.57 × 10−7 ng/particle. All relevant data of our experiments have been submitted to the EV-TRACK knowledgebase (EV-TRACK ID: EV200069).24
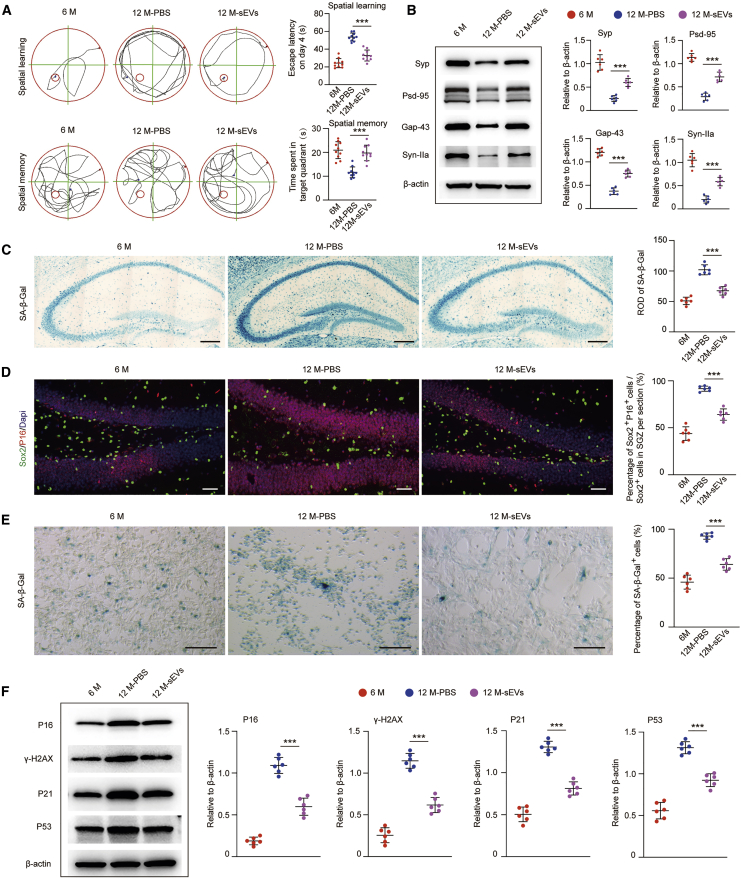
Next, ESC-sEVs were injected intravenously into 6-month-old male SAMP8 mice for 6 months. To track the biodistribution of ESC-sEVs in mice, DiR-labeled ESC-sEVs (1 × 1010 particles/mL, 100 μL) were injected into SAMP8 mice via tail vein injection, followed by euthanasia and ex vivo imaging of dissected organs 24 h after injection. As shown in Figure S4A, high intensity of fluorescent signals could be observed in the brain, heart, lung, liver, spleen, and kidneys. Meanwhile, no DiR signal was detected in naive SAMP8 mice. These results demonstrated the ability of ESC-sEVs to migrate into the brain and other organs in mice. We then investigated the anti-aging effects of ESC-sEVs in SAMP8 mice. As shown in Figure 2A, the escape latency was shorter while the duration in the target quadrant was much longer in the 12 month mice treated with ESC-sEVs than in the 12 month mice treated with PBS. Moreover, the decreased expression of hippocampal Syp, PSD-95, Gap-43, and Syn-IIa in 12 month mice was rescued by ESC-sEV treatment (Figure 2B). These data indicated that the application of ESC-sEVs could reverse cognitive deficits in aged mice. To investigate whether reversion of cognitive dysfunction in aging mice by ESC-sEVs was through ameliorating H-NSC senescence, we tested senescence-associated hallmarks in the hippocampus. As shown in Figure 2C, hippocampal SA-β-gal activity in 12 month mice treated with ESC-sEVs was much lower than in 12 month mice treated with PBS. The percentage of Sox2+/p16INK4a+ cells of total Sox2+ cells in the SGZ also decreased in ESC-sEV-treated aged mice (Figure 2D). Subsequently, H-NSCs were isolated from the hippocampus and tested for senescence phenotypes. As shown in Figure 2E, the percentage of SA-β-gal+ H-NSCs from ESC-sEV-treated aged mice was much lower than from PBS-treated aged mice. In addition, the expression levels of p16INK4a, γ-H2AX, p21, and p53 proteins in the ESC-sEVs group were also reduced compared to the PBS group (Figure 2F). These results indicated that ESC-sEV treatment could decelerate the process of H-NSC senescence in mice during aging.
Figure 2.
ESC-sEVs Reverse Cognitive Aging and Rejuvenate H-NSC Senescence in SAMP8 Mice
(A) Spatial learning and memory abilities were tested by an MWM test in 6 month, 12 month-PBS, and 12 month-sEV mice. n = 10 per group. ∗∗∗p < 0.01. (B) Western blot analysis and quantification of Syp, Gap-43, PSD-95, and Syn-IIa in hippocampus of 6 month, 12 month-PBS, and 12 month-sEV mice. n = 6 per group. ∗∗∗p < 0.001. (C) SA-β-gal staining of the hippocampus and quantification of hippocampal SA-β-gal activity in 6 month, 12 month-PBS, and 12 month-sEV mice. Scale bars, 250 μm. n = 6 per group. ∗∗∗p < 0.001. (D) IF images of senescent H-NSCs (p16INK4a+, red; Sox2+, green) and the percentage of Sox2+/p16INK4a+ double-stained cells in whole Sox2+ cells in the SGZ of 6 month, 12 month-PBS, and 12 month-sEV mice. Scale bars, 50 μm. n = 6 per group. ∗∗∗p < 0.001. (E) SA-β-gal staining of isolated H-NSCs and the percentage of SA-β-gal+ cells in 6 month, 12 month-PBS, and 12 month-sEV mice. Scale bars, 100 μm. n = 6 per group. ∗∗∗p < 0.001. (F) Western blot analysis and quantification of p16INK4a, γ-H2AX, p21, and p53 in isolated neurospheres of 6 month, 12 month-PBS, and 12 month-sEV mice. n = 6 per group. ∗∗∗p < 0.001.
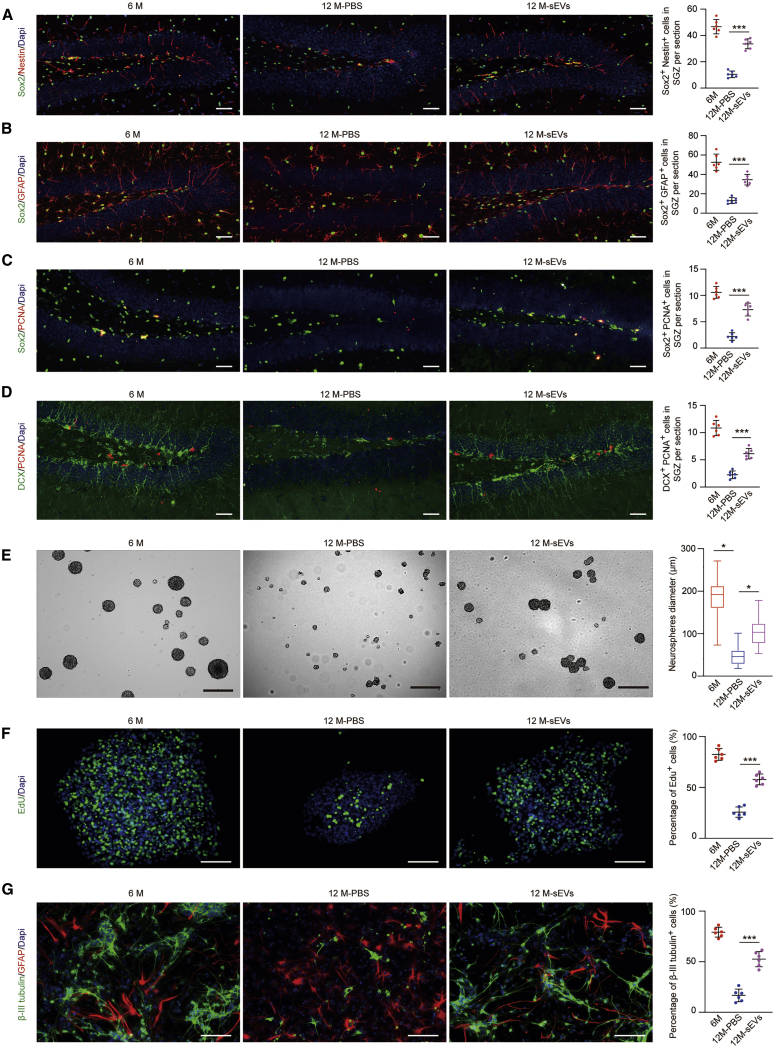
Furthermore, we detected the number of H-NSCs and immature neurons (DCX+ cells) in the SGZ. As shown in Figures 3A and 3B, the number of Sox2+/Nestin+ cells and Sox2+/GFAP+ cells was much higher in ESC-sEV-treated mice than in PBS-treated mice. The number of proliferating H-NSCs (Sox2+/PCNA+ cells) was also increased in ESC-sEV-treated aged mice compared to PBS-treated aged mice (Figure 3C). Moreover, the number of DCX+ cells and DCX+/PCNA+ cells was also markedly higher in ESC-sEV-treated mice than in PBS-treated mice (Figure 3D), which indicated that ESC-sEVs can promote hippocampal neurogenesis in aged mice. In addition, compared to H-NSCs isolated from PBS-treated mice, H-NSCs isolated from ESC-sEV-treated mice exhibited higher proliferation (Figures 3E and 3F) and neuronal differentiation (Figure 3G) abilities. Altogether, these results revealed that ESC-sEVs can restore the cognitive function of aged mice, and this effect may be mediated by rejuvenating the senescence of H-NSCs.
Figure 3.
ESC-sEVs Reverse H-NSC Depletion and Promote Neurogenesis in the SGZ of SAMP8 Mice
(A) IF images for hippocampal Sox2+ (green) and Nestin+ (red) cells and the number of Sox2+/Nestin+ double-stained cells in the SGZ of 6 month, 12 month-PBS, and 12 month-sEV mice. Scale bars, 50 μm. n = 6 per group. ∗∗∗p < 0.001. (B) IF images for hippocampal Sox2+ (green) and GFAP+ (red) cells and the number of Sox2+/GFAP+ double-stained cells in the SGZ of 6 month, 12 month-PBS, and 12 month-sEV mice. Scale bars, 50 μm. n = 6 per group. ∗∗∗p < 0.001. (C) IF images for proliferated H-NSCs (Sox2+, green; PCNA+, red) and the number of Sox2+/PCNA+ double-stained cells in the SGZ of 6 month, 12 month-PBS, and 12 month-sEV mice. Scale bars, 50 μm. n = 6 per group. ∗∗∗p < 0.001. (D) IF images for hippocampal DCX+ (green) and PCNA+ (red) cells and the number of DCX+/PCNA+ double-stained cells in the SGZ of 6 month, 12 month-PBS, and 12 month-sEV mice. Scale bars, 50 μm. n = 6 per group. ∗∗∗p < 0.001. (E) Neurosphere formation and quantification of neurosphere diameters in 6 month, 12 month-PBS, and 12 month-sEV mice. Scale bars, 300 μm. n = 6 per group. ∗p < 0.05. (F) IF images for EdU incorporation in isolated neurospheres and the percentage of EdU+/DAPI+ double-stained cells from total DAPI+ cells in 6 month, 12 month-PBS, and 12 month-sEV mice. Scale bars, 100 μm. n = 6 per group. ∗∗∗p < 0.001. (G) IF images for β-tubulin III+ (green) and GFAP+ cells and the percentage of β-tubulin III+ cells in whole cells in 6 month, 12 month-PBS, 12 month-ESC-sEV mice. Scale bars, 100 μm. n = 6 per group. ∗∗∗p < 0.001.
ESC-sEVs Rejuvenate Hippocampal NSC Senescence and Enhance NSC Activity In Vitro
The potential for self-renewal and differentiation in stem cells gradually decreases with continuous passaging, namely replicative senescence, which is regarded as an important in vitro model to explore stem cell function during aging.25,26 In our study, we isolated H-NSCs from 2-week-old male C57BL/6 mice and applied the continuous passaging model to investigate the function and mechanism of ESC-sEVs in rejuvenating H-NSC senescence. Under culture conditions, primary H-NSCs can be passaged at most to passage 15, when the cells can hardly form neurospheres (Figure S5A). The neurospheres gradually decreased starting at passage 6 (Figures S5A and S5B). The Cell Counting Kit-8 (CCK8) assay also showed that the viability of H-NSCs before passage 6 is very high, gradually decreasing with passage number (Figure S5C). The SA-β-gal activity of H-NSCs from passage 2 to passage 6 is low and sharply increased with continuous passaging, reaching a plateau at passage 10 (Figure S5D). The expression level of p16INK4a in H-NSCs also reached the highest level at passage 10 (Figure S5E). Therefore, H-NSCs at passage 10 were used to test the effects of ESC-sEVs on senescence.
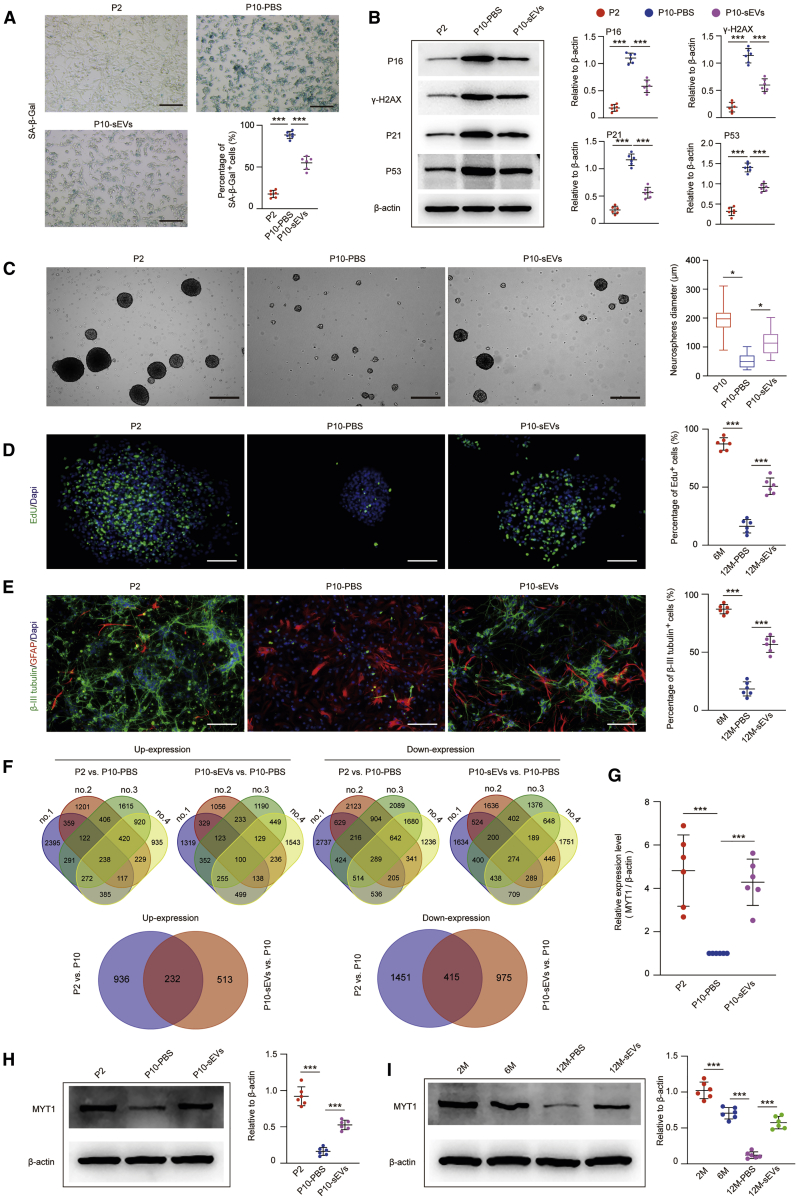
First, ESC-sEVs were labeled with DiO and cocultured with H-NSCs. We found that DiO-labeled ESC-sEVs can be internalized by H-NSCs (Figure S4B). Next, we applied ESC-sEVs to treat H-NSCs from passage 2 until passage 10. As shown in Figure 4A, ESC-sEV treatment reversed the increase of SA-β-gal activity in H-NSCs after continuous passaging. The expression level of p16INK4a, γ-H2AX, p21, and p53 in H-NSCs was also decreased by the treatment with ESC-sEVs (Figure 4B). The application of ESC-sEVs also enhanced the proliferation and neuronal differentiation abilities of H-NSCs in passage 10 (Figures 4C–4E). These data further confirmed that H-NSC senescent phenotypes can be reversed by ESC-sEVs.
Figure 4.
ESC-sEVs Upregulate MYT1 in H-NSCs and Ameliorate H-NSC Senescence
(A) Representative images of SA-β-gal staining and the percentage of SA-β-gal+ cells in passage 2 (P2) H-NSCs, passage 10 H-NSCs treated with PBS (P10-PBS), and passage 10 H-NSCs treated with ESC-sEVs (P10-sEVs). Scale bars, 100 μm. n = 6 per group. ∗∗∗p < 0.001. (B) Western blot analysis and quantification of p16INK4a, γ-H2AX, p21, and p53 in H-NSCs of P2, P10-PBS, and P10-sEVs. n = 6 per group. ∗∗∗p < 0.001. (C) Neurosphere formation and quantification of neurosphere diameters in H-NSCs of P2, P10-PBS, and P10-sEVs. Scale bars, 300 μm. n = 6 per group. ∗p < 0.05. (D) IF images for EdU incorporation in neurospheres and the percentage of EdU+/DAPI+ double-stained cells in whole DAPI+ cells in H-NSCs of P2, P10-PBS, and P10-sEVs. Scale bars, 100 μm. n = 6 per group. ∗∗∗p < 0.001. (E) IF images for β-tubulin III+ (green) and GFAP+ cells and the percentage of β-tubulin III+ cells in whole cells in H-NSCs of P2, P10-PBS, and P10-sEVs. Scale bars, 100 μm. n = 6 per group. ∗∗∗p < 0.001. (F) RNA-seq data from H-NSCs of P2, P10-PBS, and P10-sEVs were analyzed by the “Calculate and draw custom Venn diagrams” online tool; 232 genes were co-upregulated and 415 genes were co-downregulated in P2 versus P10-PBS and P10-sEVs versus P10-PBS. n = 4 per group. (G) The relative expression level of MYT1 in H-NSCs of P2, P10-PBS, and P10-sEVs was detected by qRT-PCR. n = 6 per group. ∗∗∗p < 0.001. (H) Western blot analysis and quantification of MYT1 in H-NSCs of P2, P10-PBS, and P10-sEVs. n = 6 per group. ∗∗∗p < 0.001. (I) Western blot analysis and quantification of MYT1 in isolated neurospheres of 2 month, 6 month, and 12 month-PBS and 12 month-sEV mice. n = 6 per group. ∗∗∗p < 0.001.
To elucidate the underlying mechanism involved in rejuvenating H-NSC senescence by ESC-sEVs, we performed RNA sequencing (RNA-seq) analysis to explore the change of gene expression in H-NSCs among passage 2 (NSCs-P2), passage 10 treated with ESC-sEVs (1 × 1010 particles/mL ESC-sEVs treated from passage 2 to passage 10 [NSCs-P10-sEVs]), and passage 10 treated with PBS (the same amount of PBS treated from passage 2 to passage 10 [NSCs-P10-PBS]), and the results were listed in Data S1. We applied the “Calculate and draw custom Venn diagrams” online tool (http://bioinformatics.psb.ugent.be/webtools/Venn/) to identify the significantly upregulated or downregulated genes across NSCs-P2 against NSCs-P10-PBS, as well as NSCs-P10-sEVs against NSCs-P10-PBS, and selected the co-upregulated or co-downregulated genes between NSCs-P2 against NSCs-P10-PBS and NSCs-P10-sEVs against NSCs-P10-PBS. As shown in Figure 4F, we identified 232 co-upregulated genes (>1.5-fold, p < 0.05) and 415 co-downregulated genes (< 0.75-fold, p < 0.05). Among them, 23 genes (10 genes were co-upregulated and 13 genes were co-downregulated) were involved in the nervous system and were selected as candidate genes that may function in regulating NSC senescence (Figures S6A and S6B). qRT-PCR was used to verify the expression level of these genes (Figures S6C and S6D). MYT1 is expressed in differentiating progenitors and postmitotic neuronal precursors of the central nervous system and peripheral nervous system, and it has been demonstrated to regulate neurogenesis in mice.27,28 Our RNA-seq analysis as well as later qRT-PCR and western blot showed that MYT1 is highly expressed in H-NSCs at passage 2 and significantly downregulated at passage 10, while coculture with ESC-sEVs upregulated MYT1 expression in H-NSCs at passage 10 (Figures 4G and 4H). The expression level of MYT1 in H-NSCs isolated from 2 month, 6 month, and 12 month SAMP8 mice was also gradually decreased, while the application of ESC-sEVs significantly increased MYT1 expression in the H-NSCs of 12 month mice when compared to PBS-treated 12 month mice (Figure 4I). Therefore, we supposed that MYT1 may possess a potential role in regulating H-NSC senescence to influence neurogenesis, and ESC-sEVs rejuvenated H-NSC senescence in aged mice likely via upregulation of MYT1.
ESC-sEVs Rejuvenate Senescent Hippocampal NSCs by Upregulating MYT1
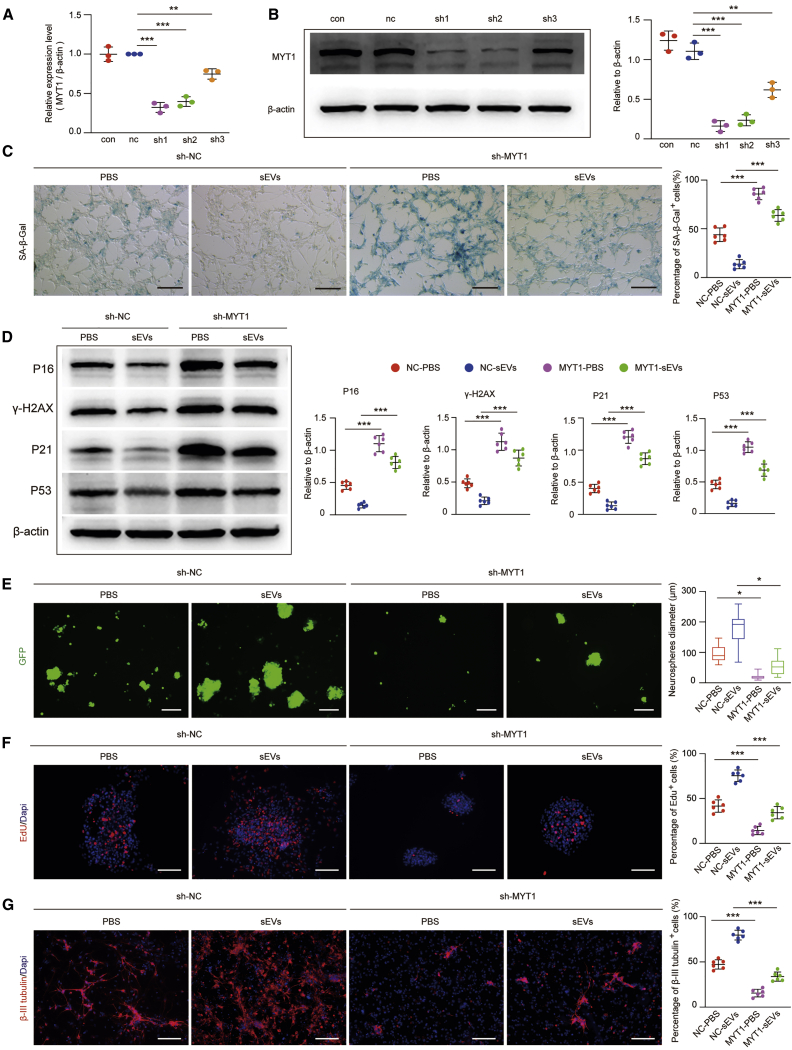
To address whether MYT1 is involved in regulating H-NSC senescence, we generated young H-NSCs (passage 2 from 2-week-old male C57BL/6 mice) with constitutive knockdown of either a MYT1 transgene (short hairpin RNA [shRNA] [sh]-MYT1) or a GFP control transgene (sh-negative control [sh-NC]) and identified their senescent phenotype. qRT-PCR and western blot showed the downregulation of MYT1 at both the RNA and protein level, and sh-MYT1 showed the highest efficiency and was chosen for the following experiments (Figures 5A and 5B). SA-β-gal staining (Figure S7A) and western blot for p16INK4a and γ-H2AX (Figure S7B) showed an increase of the senescent phenotype in H-NSCs after MYT1 knockdown. The diameter of sh-NC neurospheres was larger (Figure S7C), and the percentage of differentiated neurons was higher (Figure S7D) than that of the sh-MYT1 group. These results suggested that downregulation of MYT1 levels may induce senescence and reduce the proliferation and neuronal differentiation capacity of H-NSCs.
Figure 5.
Knockdown of MYT1 Accelerates H-NSC Senescence and Abolishes the Anti-aging Function of ESC-sEVs
(A) The knockdown efficiency of MYT1 shRNAs in H-NSCs was detected by qRT-PCR. n = 3 per group. ∗∗p < 0.01, ∗∗∗p < 0.001. (B) Western blot analysis and quantification of MYT1 in H-NSCs showing the knockdown efficiency of MYT1 shRNAs. n = 3 per group. ∗∗p < 0.01, ∗∗∗p < 0.001. (C) SA-β-gal staining and the percentage of SA-β-gal+ cells in sh-NC NSCs treated with PBS or ESC-sEVs (sh-NC-PBS or sh-NC-sEVs) and sh-MYT1 NSCs treated with PBS or ESC-sEVs (sh-MYT1-PBS or sh-MYT1-sEVs). Scale bars, 100 μm. n = 6 per group. ∗∗∗p < 0.001. (D) Western blot analysis and quantification of p16INK4a, γ-H2AX, p21, and p53 in H-NSCs of sh-NC-PBS, sh-NC-sEVs, sh-MYT1-PBS, and sh-MYT1-sEVs. n = 6 per group. ∗∗∗p < 0.001. (E) IF images for GFP-labeled neurospheres and the diameter of neurospheres in H-NSCs of sh-NC-PBS, sh-NC-sEVs, sh-MYT1-PBS, and sh-MYT1-sEVs. Scale bars, 200 μm. n = 6 per group. ∗p < 0.05. (F) IF images for EdU incorporation in neurospheres and the percentage of EdU+/DAPI+ double-stained cells in all DAPI+ cells in sh-NC-PBS, sh-NC-sEVs, sh-MYT1-PBS, and sh-MYT1-sEVs. Scale bars, 100 μm. n = 6 per group. ∗∗∗p < 0.001. (G) IF images for β-tubulin III+ and the percentage of β-tubulin III+ cells in whole cells in H-NSCs of sh-NC-PBS, sh-NC-sEVs, sh-MYT1-PBS, and sh-MYT1-sEVs. Scale bars, 100 μm. n = 6 per group. ∗∗∗p < 0.001.
To further determine whether MYT1 is a crucial factor for the anti-senescence effect of ESC-sEVs on H-NSCs, we applied ESC-sEVs or PBS to treat sh-MYT1 NSCs and sh-NC NSCs for three passages (passage 3 to passage 5). As shown in Figures 5C and 5D, ESC-sEV treatment reduced SA-β-gal activity and the expression of p16INK4a, γ-H2AX, p21, and p53 in H-NSCs after passaging, while knockdown of MYT1 abolished the downregulation effects of ESC-sEVs on the activity of SA-β-gal and the expression of p16INK4a, γ-H2AX, p21, and p53. In addition, knockdown of MYT1 also resulted in the functional impairment of ESC-sEVs in promoting H-NSC self-renewal and neurogenesis (Figures 5E–5G). Taken together, these results confirmed that MYT1 was involved in the regulation of H-NSC senescence, and ESC-sEVs rejuvenated senescent H-NSCs and promoted their proliferation as well as neuronal differentiation partly by the upregulation of MYT1.
ESC-sEVs Rejuvenate Senescent Hippocampal NSCs by Regulating the MYT1-Egln3-Sirt1 Axis
We further investigated the mechanism of how MYT1 regulates H-NSC senescence. Vasconcelos et al.28 performed transcriptome sequencing for neural stem cell line NS5 with MYT1 overexpression, and they identified 1,002 downregulated genes and 761 upregulated genes. By analyzing these data with our RNA-seq results, we selected four upregulated genes (Pou5f2, Pcdhga11, Pde5a, and Tspan2) and nine downregulated genes (Dusp6, Wls, Il33, Kif21a, Pcdhb5, S100b, Frk, Egln3, and Abca9) (Figure S8A). qRT-PCR showed that Egln3 was significantly upregulated in sh-MYT1 NSCs compared with that in sh-NC NSCs (Figure S8B). Western blot also confirmed the results at the protein level (Figure S8C). These results indicated that MYT1 can inhibit Egln3 in H-NSCs.
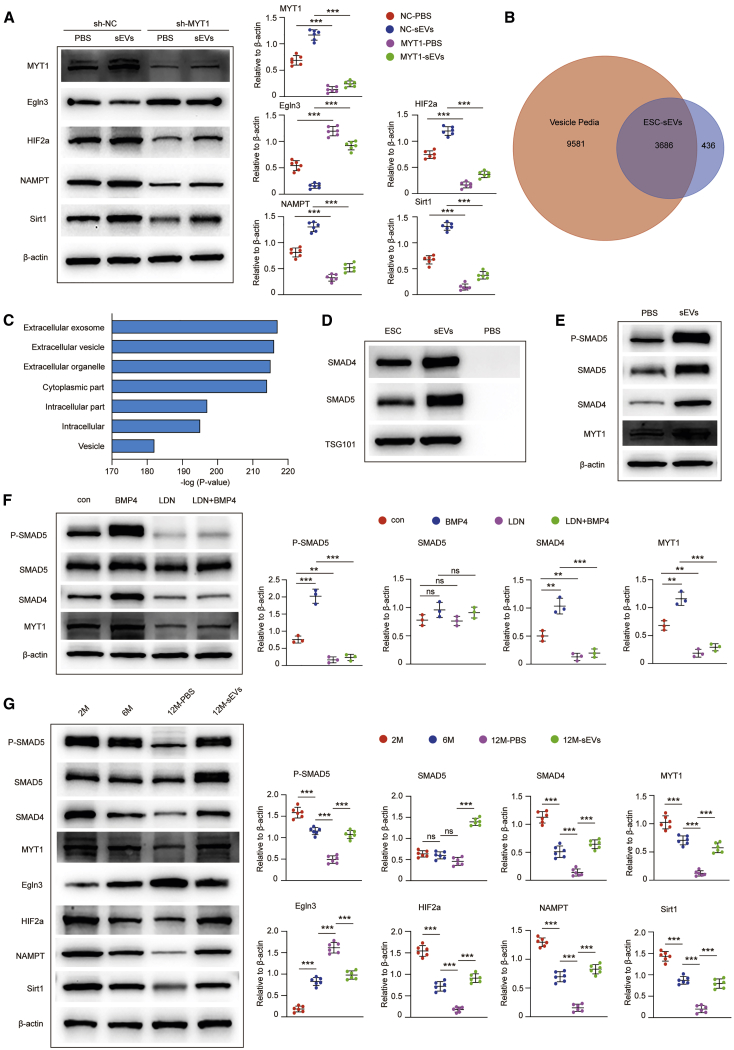
Egln3 (also known as PHD3) encodes an intracellular prolyl hydroxylase that is involved in the cellular response to oxygen availability by stabilizing HIF-2α.29 Moreover, it has been demonstrated that HIF-2α can directly target NAMPT (also known as visfatin) and positively regulate the expression of NAMPT,30 which is involved in the amelioration of senescence via upregulation of Sirt1.31,32 In our study, we found that the expression levels of HIF-2α, NAMPT, and Sirt1 were decreased significantly in sh-MYT1 NSCs compared with sh-NC NSCs (Figure S8D). These results confirmed that MYT1 may inhibit Egln3 and promote the expression of its downstream molecules, including HIF-2α, NAMPT, and Sirt1 in H-NSCs. Furthermore, we showed that ESC-sEV treatment reduced the expression of Egln3 and increased the expression of HIF-2α, NAMPT, and Sirt1, while knockdown of MYT1 impaired these effects of ESC-sEVs (Figure 6A). These results indicated that MYT1 mediated the effects of ESC-sEVs on the expression of Egln3 and its downstream molecules in H-NSCs.
Figure 6.
ESC-sEVs Rejuvenate Senescent H-NSCs by Transferring SMADs to Activate MYT1, Inhibit Egln3, and Upregulate HIF-2α, NAMPT, and Sirt1 Successively
(A) Western blot analysis and quantification of MYT1, Egln3, HIF-2α, NAMPT, and Sirt1 in H-NSCs of sh-NC-PBS, sh-NC-sEVs, sh-MYT1-PBS, and sh-MYT1-sEVs. n = 6 per group. ∗∗∗p < 0.001. (B) Comparison of proteins in ESC-sEVs identified by LC-MS/MS with the EV proteome database Vesiclepedia. (C) GO analysis of proteins in ESC-sEVs showed mostly that proteins belonged to extracellular vesicles. (D) Western blot analysis of SMAD4, SMAD5, and TSG101 in ESCs, ESC-sEVs, and PBS. (E) Western blot analysis and quantification of p-SMAD5, SMAD5, SMAD4, and MYT1 in H-NSCs treated with PBS and ESC-sEVs. (F) Western blot analysis and quantification of p-SMAD5, SMAD5, SMAD4, and MYT1 in H-NSCs, H-NSCs treated with 25 ng/mL BMP4, H-NSCs treated with 200 nM LDN-193189, and H-NSCs treated with LDN-193189 and BMP4. n = 3 per group. ∗∗p < 0.01, ∗∗∗p < 0.001. (G) Western blot analysis and quantification of p-SMAD5, SMAD5, SMAD4, MYT1, Egln3, HIF-2α, NAMPT, and Sirt1 in isolated neurospheres of 2 month, 6 month, and 12 month-PBS and 12 month-sEV mice. n = 6 per group. ∗∗∗p < 0.001.
ESC-sEVs Ameliorate Hippocampal NSC Senescence by Transferring SMADs to Regulate the MYT1-Egln3-Sirt1 Axis
Encapsulated proteins are one of the key molecules in sEVs that modulate recipient cell function.33 From our previous liquid chromatography-tandem mass spectrometry (LC-MS/MS) data identifying the protein content in ESC-sEVs (ProteomeXchange Consortium dataset identifier PXD018655),34 we found 4,122 proteins in ESC-sEVs, and 3,686 proteins can be aligned to the EV proteome database Vesiclepedia (Figure 6B). Cellular component analysis by Gene Ontology (GO) analysis also showed that most of the proteins belonged to the EVs (Figure 6C). Sherry-Lynes et al.35 had suggested that bone morphogenetic proteins (BMPs) can activate the SMAD signaling cascade to upregulate the expression of Myt1. As LC-MS/MS analysis had identified that SMAD4 and SMAD5 were encapsulated in ESC-sEVs, western blot also confirmed the enrichment of SMAD4 and SMAD5 in ESC-sEVs (Figure 6D), and the expression of SMAD4, SMAD5, and phosphorylated SMAD5 (p-SMAD5) were enhanced in H-NSCs when treated by ESC-sEVs (Figure 6E). Thus, we supposed that ESC-sEVs may transfer SMAD4 and SMAD5 to upregulate MYT1 in H-NSCs.
As shown in Figure 6F, compared with the control group, H-NSCs treated with recombinant BMP4 (25 ng/mL, PeproTech) for 6 h exhibited higher expression of p-SMAD5, SMAD4, and MYT1, while treatment with LDN-193189 (LDN, 200 nM, Selleck Chemicals) for 30 min significantly inhibited the expression of p-SMAD5, SMAD4, and MYT1, which were hardly reversed by treatment with BMP4 for 6 h. These results indicated that MYT1 activation was associated with the activation of SMAD4 and SMAD5 in H-NSCs. We further detected the expression of these proteins in H-NSCs from a replicative senescence model and SAMP8 mice. The protein levels of p-SMAD5, SMAD5, SMAD4, MYT1, HIF-2α, NAMPT, and Sirt1 were decreased and Egln3 was activated after continuous passaging of H-NSCs, while this tendency was reversed after treatment with ESC-sEVs (Figure S9). In addition, the date from isolated H-NSCs of SAMP8 mice also confirmed the decreasing tendency of p-SMAD5, SMAD4, MYT1, HIF-2α, NAMPT, and Sirt1 and the increasing tendency of Egln3, as well as the function of ESC-sEVs in reversion of the expression tendency of these proteins (Figure 6G).
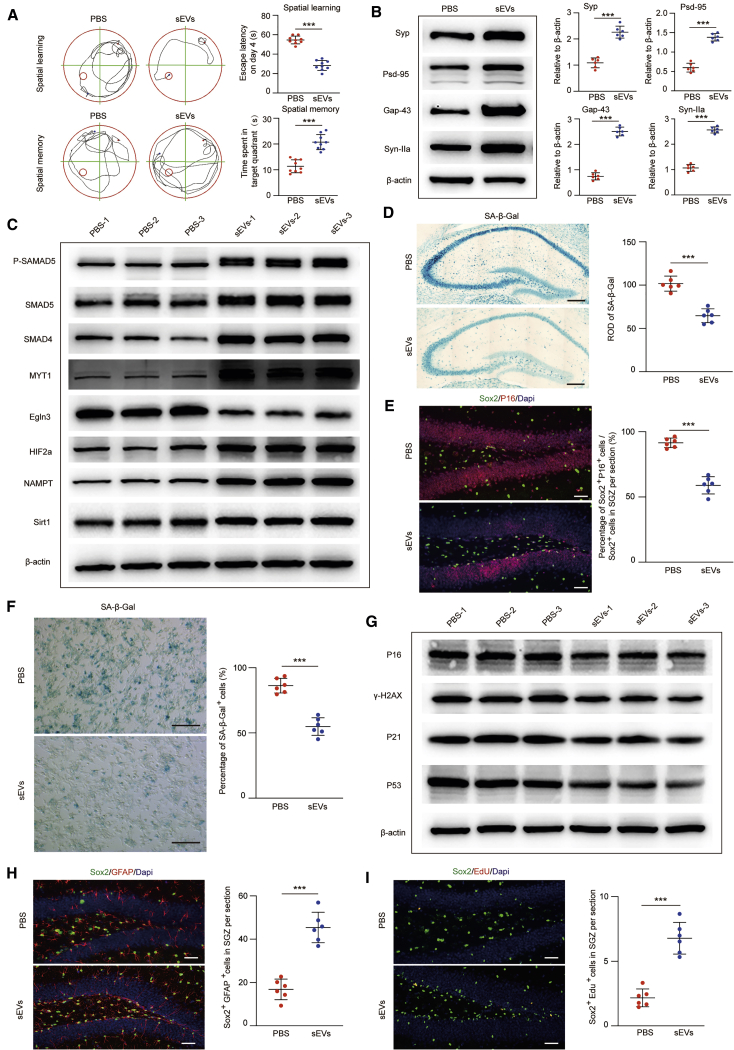
In addition, the anti-senescence effects of ESC-sEVs were further confirmed using C57BL/6 mice by intravenous injection into 12-month-old mice for 8 months. As shown in Figure 7A, the escape latency was shorter while the duration in the target quadrant was much longer in 20 month mice treated with ESC-sEVs than in 20 month mice treated with PBS. The expression of hippocampal Syp, PSD-95, Gap-43, and Syn-IIa in 20 month mice was also enhanced by ESC-sEVs (Figure 7B). Inconsistent with the results from SAMP8 mice, these results indicated that ESC-sEVs can also reverse cognitive dysfunction of natural aged C57BL/6 mice. Western blot showed that the protein levels of p-SMAD5, SMAD5, SMAD4, MYT1, HIF-2α, NAMPT, and Sirt1 were increased, while Egln3 was decreased, in H-NSCs isolated from ESC-sEV-treated C57BL/6 mice (Figure 7C). In addition, hippocampal SA-β-gal activity and the percentage of Sox2+/p16INK4a+ cells from the total Sox2+ cells in the SGZ were decreased in ESC-sEV-treated aged C57BL/6 mice (Figures 7D and 7E). The percentage of SA-β-gal+ H-NSCs and the expression levels of p16INK4a, γ-H2AX, p21, and p53 in H-NSCs were also reduced from ESC-sEV-treated C57BL/6 mice (Figures 7F and 7G). Additionally, the number of Sox2+/GFAP+ cells and Sox2+/5-ethynyl-2′-deoxyuridine (EdU)+ cells were found to be significantly higher in ESC-sEV-treated C57BL/6 mice than in PBS-treated mice (Figures 7H and 7I). Taken together, these results demonstrated that ESC-sEVs can transfer SMAD4 and SMAD5 to upregulate MYT1 in H-NSCs, and then inhibit Egln3 and promote HIF-2α, NAMPT and Sirt1 expression, which resulted in senescent H-NSC rejuvenation, as well as self-renewal and neurogenesis recovery, followed by cognitive recovery.
Figure 7.
ESC-sEVs Reverse Cognitive Aging and Rejuvenate H-NSC Senescence in C57BL/6 Mice
(A) Spatial learning and memory abilities were tested by an MWM test in 20 month-PBS and 20 month-sEV mice. n = 9 per group. ∗∗∗p < 0.001. (B) Western blot analysis and quantification of Syp, Gap-43, PSD-95, and Syn-IIa in hippocampus of 20 month-PBS and 20 month-sEV mice. n = 6 per group. ∗∗∗p < 0.001. (C) Western blot analysis of p-SMAD5, SMAD5, SMAD4, MYT1, Egln3, HIF-2α, NAMPT, and Sirt1 in isolated neurospheres of 20 month-PBS and 20 month-sEV mice. n = 3 per group. (D) SA-β-gal staining of hippocampus and quantification of hippocampal SA-β-gal activity in 20 month-PBS and 20 month-sEV mice. Scale bars, 250 μm. n = 6 per group. ∗∗∗p < 0.001. (E) IF images for senescent H-NSCs (p16INK4a+, red; Sox2+, green) and the percentage of Sox2+/p16INK4a+ double-stained cells in whole Sox2+ cells in the SGZ of 20 month-PBS and 20 month-sEV mice. Scale bars, 50 μm. n = 6 per group. ∗∗∗p < 0.001. (F) SA-β-gal staining of isolated H-NSCs and the percentage of SA-β-gal+ cells in 20 month-PBS and 20 month-sEV mice. Scale bars, 100 μm. n = 3 per group. ∗∗∗p < 0.001. (G) Western blot analysis of p16INK4a, γ-H2AX, p21, and p53 in isolated neurospheres of 20 month-PBS and 20 month-sEV mice. n = 3 per group. (H) IF images for hippocampal Sox2+ (green) and GFAP+ (red) cells and the number of Sox2+/GFAP+ double-stained cells in the SGZ of 20 month-PBS and 20 month-sEV mice. Scale bars, 50 μm. n = 6 per group. ∗∗∗p < 0.001. (I) IF images for proliferating H-NSCs (Sox2+, green; EdU+, red) and the number of Sox2+/EdU+ double-stained cells in the SGZ of 20 month-PBS and 20 month-sEV mice. Scale bars, 50 μm. n = 6 per group. ∗∗∗p < 0.001.
Discussion
Stem cells exhibited senescence phenotypes in many tissues with the advance of aging. These senescent stem cells showed similar features, such as blunted responsiveness to tissue injury and dysregulation of proliferation, differentiation, and function, which resulted in the reduction of effective cellular replacement and tissue regeneration in aged organisms.36,37 Accordingly, therapeutic strategies, including cellular reprogramming,38 stem cell transplantation,39 and senolytics (i.e., ABT263),40 have been demonstrated to effectively rejuvenate or eliminate senescent tissue-specific stem cells to promote functional recovery. Research on stem cell aging and anti-aging suggested that senescent stem cells were important pathogenesis as well as therapeutic targets for age-related diseases. In this study, we reported that H-NSC senescence is accompanied with the progress of aging. In accordance with other studies,9, 10, 11 senescent H-NSCs exhibited significant senescent phenotypes with impairment of proliferation and neuronal differentiation capacities, which partly caused the aging-related decline of H-NSCs and neurogenesis in the hippocampus.23
Stem cell-derived sEVs have been reported to have therapeutic potential in the treatment of diseases from various organs, including the brain,41 heart,14 and kidney,42 which indicated that sEVs derived from stem cells hold great potential in regenerative medicine. Recent evidence has demonstrated that ESC-sEVs possess the ability to promote tissue recovery via amelioration of somatic cell senescence.18,19 However, whether ESC-sEVs could rejuvenate aging-related senescent H-NSCs and restore their capacity is still unknown. In our study, we found that chronic application of ESC-sEVs could markedly reduce the senescence hallmarks, recover the compromised self-renewal and neurogenesis capacity of H-NSCs, and result in cognitive recovery in mice during aging. Our study suggested that ESC-sEVs can rejuvenate age-related senescence in H-NSCs and thereby reverse cognitive dysfunction with aging.
We further applied RNA-seq to identify the intrinsic changes of H-NSCs in a replicative senescence model. Hundreds of genes could be candidate regulators of H-NSC aging as well as the target for ESC-sEVs to rejuvenate senescence. As an initial step, we identified MYT1 as a putative age-associated regulator of H-NSC senescence and functional decline. MYT1 was downregulated in senescent H-NSCs, and knockdown of MYT1 in young H-NSCs promoted aging-related phenotypes, including an increase of SA-β-gal activity and the expression of p16INK4a, γ-H2AX, p21, p53, and the reduction of proliferation and neuronal differentiation abilities. Furthermore, we identified that Egln3, which was found to be increased in aged mouse and human hearts43 and upregulated in senescent H-NSCs, is negatively regulated by MYT1, because knockdown of MYT1 expression in young H-NSCs significantly increased the expression level of Egln3. Egln3 encodes intracellular prolyl hydroxylase that regulates HIF-α expression,44 and it has been demonstrated to mainly regulate the expression of HIF-2α.29 Meanwhile, HIF-2α can directly target NAMPT, and it positively regulates the expression of NAMPT,30 which ameliorates senescence via upregulation of Sirt1.31,32 In addition, NAMPT and Sirt1 are important regulators involved in NSC maintenance. NAMPT is the main source of nicotinamide adenine dinucleotide (NAD)+ and is required for G1/S progression in NSCs. Stein and Imai45 revealed that the expression level of NAD+ and NAMPT decreased with age in the hippocampus. The adult NSC pool was significantly exhausted in vivo when NAMPT was ablated during aging, while it can be rescued by enhancing hippocampal NAMPT levels. Sirt1 can positively modulate the Wnt signaling pathway,46 which plays the key role in the regulation of NSC homeostasis during development and in the adult brain. Additionally, genes and proteins involved in Wnt/β-catenin signaling within the SVZ and the SGZ niches were markedly downregulated in aging, inflammation/oxidative stress, and neurodegeneration diseases, while most of the neurogenesis-promoting factors (including Sirt1, BMP/SMAD, and HIF-α) or treatments that reverse neurogenesis downregulation act, at least in part, in cooperation/synergy with Wnt/β-catenin signaling intermediacy.47, 48, 49 In our study, we found that HIF-2α, NAMPT, and Sirt1 were downregulated in senescent H-NSCs as well as in MYT1 knockdown of young H-NSCs. In addition, ESC-sEV treatment significantly rescued the decrease of MYT1, HIF-2α, NAMPT, and Sirt1, and it reversed the increase of Egln3 in senescent H-NSCs, while this function was abolished by the knockdown of MYT1. Taken together, these data suggested that the therapeutic effect of ESC-sEVs in rejuvenating H-NSC senescence was partly attributed to upregulation of MYT1 to inhibit Egln3, improving the expression of HIF-2α, NAMPT, and Sirt1.
Recently, many studies have demonstrated that sEVs acted as a delivery system partly by transferring their proteins to recipient cells to alter their gene expression and bioactivity.50,51 For example, Rong et al.50 revealed that NSC-sEVs could deliver 14-3-3t protein to interact with Beclin-1 to activate autophagy in injured spinal cords, and they promoted their functional behavior recovery. Therefore, we hypothesized that ESC-sEVs ameliorate H-NSC senescence by delivering functional proteins to upregulate MYT1 in senescent H-NSCs during aging. In our LC-MS/MS data,34 4,122 proteins were identified from ESC-sEVs, and 3,686 proteins were aligned to the EV proteome database EVpedia, and most of the proteins belonged to the EVs. It has been previously reported that activation of the SMAD signaling cascade can upregulate the expression of MYT1.35 Generally, the activation of the SMAD pathway can be defined as phosphorylation of the C terminus of SMAD1, SMAD5, or SMAD8, leading to the formation of a complex with SMAD4 and translocating into the nucleus to regulate the expression of specific genes.52 As activation of the SMAD signaling cascade can also promote neurogenesis,53 we then identified whether ESC-sEVs transfer SMADs to regulate MYT1 in H-NSCs. In our study, we found that SMAD4 and SMAD5 are highly enriched in ESC-sEVs, and they can be delivered into H-NSCs to improve the expression levels of SMAD4, SMAD5, and p-SMAD5. We further detected the relationship between SMAD4, SMAD5, and MYT1 in H-NSCs, and we demonstrated that activation of SMAD4 and p-SMAD5 can upregulate MYT1 in H-NSCs. Collectively, our study reveals a close link between proteins enriched in ESC-sEVs and the anti-aging genes in H-NSCs, and it highlights the critical role of SMAD4 and SMAD5 in ESC-sEVs to positively regulate MYT1 to reverse the senescence of H-NSCs. However, as sEVs are complex “living” structures that encapsulate proteins, trophic factors, miRNAs, and RNAs are important molecules in sEVs to modulate recipient cell function.51,54 Previous studies have demonstrated that ESC-sEVs can transfer highly enriched miR-200a to ameliorate endothelial senescence.19 In our study, we found that MYT1 knockdown cannot totally reverse the anti-senescence function of ESC-sEVs. Therefore, taken together, we think that other mechanisms, including encapsulation of miRNAs in ESC-sEVs, can also function to rejuvenate H-NSC senescence.
Furthermore, neurons, astrocytes, oligodendrocytes, vasculature, extracellular matrix and associated molecules, and structural elements and molecules in NSC niches are important in supporting the lifelong self-renewal of NSCs and the production of differentiated cells.55 The alteration of structural elements (such as inflammation) during aging plays a crucial role in inducing NSC senescence, dysfunction, and exhaustion. For example, Yousef et al.56 demonstrated that the blood-brain barrier was damaged, microglia were activated, and inflammatory cytokines (tumor necrosis factor [TNF]-α, interleukin [IL]-1β, and IL-6) were overexpressed in the aged hippocampus, which can cause H-NSC exhaustion and neurogenesis reduction. Notch1 signaling downregulation in the NSC niche of the aged brain also results in NSC loss and neurogenesis reduction.57 Our in vivo results in Figures 2C and 2D show that the hippocampal SA-β-gal activity and the p16INK4a fluorescence intensity of hippocampus in 12 month SAMP8 mice treated with ESC-sEVs was much lower than 12 month mice treated with PBS, indicating that ESC-sEV treatment could also improve the senescent status of non-NSCs in hippocampus, suggesting that the microenvironment in the NSC niches was switched toward youth by the ESC-sEV treatment. Thus, ESC-sEVs may also modify the microenvironment in the NSC niche to rejuvenate H-NSCs, and this still needs further investigation.
In summary, our data demonstrated that the downregulation of MYT1 in H-NSCs during aging can induce cell senescence, which impairs their proliferation and neuronal differentiation capacities, and may be one of the factors that results in H-NSC exhaustion, neurogenesis reduction, and cognitive impairment. ESC-sEVs exhibited a positive effect on rejuvenating H-NSC senescence partly by transferring their highly enriched SMAD4 and SMAD5 to activate MYT1, inhibit Egln3, and increase the expression of HIF-2α, NAMPT, and Sirt1 successively. Thus, these findings highlight a novel cell-free therapeutic tool for rejuvenating senescent resident stem cells to treat age-related diseases.
Materials and Methods
Details of the materials and experimental procedures are available in the Supplemental Information, and the models and methods used for each in vivo/ex vivo/in vitro study are summarized in Table S3. The data that support the findings of this study are available from the corresponding authors on reasonable request.
ESC Culture and ESC-sEV Isolation and Identification
The human ESCs (H9) were provided by the Institute of Biochemistry and Cell Biology of Chinese Academy of Sciences, and they were cultured in ncEpic hPSC medium (Nuwacell Biotechnologies). ESC-sEVs were isolated by differential centrifugation/ultracentrifugation protocols and identified by TEM, nano-flow cytometry, and western blot. Further details are provided in the Supplemental Information.
Animal Experimental Procedures
Animal care and experimental procedures were approved by the Animal Research Committee of the Shanghai Jiao Tong University Affiliated Sixth People’s Hospital (approval code: DWSY2018-118). Male SAMP8 mice and male C57BL/6 mice were housed under pathogen-free conditions and provided a standard diet and water. For the in vivo study, mice were treated with 100 μL of PBS or ESC-sEVs (1 × 1010 particles dissolved in 100 μL of PBS) by tail vein injection twice weekly. 6-month-old male SAMP8 mice were treated for 6 months until sacrifice at 12 months of age, and 12-month-old male C57BL/6 mice were treated for 8 months until sacrifice at 20 months of age. C57BL/6 mice were injected with EdU (50 mg/kg per mouse) 5, 3, and 1 days before sacrifice to label the proliferated cells.
H-NSC Isolation and Culture, and the Effects of ESC-sEVs on H-NSC Senescence, Proliferation, and Differentiation
H-NSCs were isolated from the hippocampus of mice and cultured in complete NSC medium as described previously.58 Briefly, H-NSCs were incubated with 1 × 1010 particles/mL ESC-sEVs or an equal volume of PBS from passage 2 to passage 10. Then, neurospheres were collected for experiments including SA-β-gal staining and western blot. For the Edu incorporation assay, neurospheres were dissociated and 20,000 cells were incubated in complete NSC medium for 4 days, and then EdU (10 μM) was added to culture medium for 4 h. Next, neurospheres were fixed with 4% paraformaldehyde (PFA), and immunofluorescence (IF) staining with EdU and DAPI was used to evaluate cell proliferation (percentage of EdU+ cells in total cells). For the differentiation assay, neurospheres were dissociated and 50,000 cells were plated on poly-l-lysine-coated 48-well plates. Cells were cultured with the differentiation medium for 5 days. Next, cells were fixed and IF double staining of β-tubulin III and GFAP were applied to calculate the percentage of β-tubulin III+ cells in total cells. Further details are provided in the Supplemental Information.
SA-β-gal Staining
SA-β-gal staining of brain sections and cultured NSCs was performed using an SA-β-gal staining kit. The activity of SA-β-gal for hippocampus in brain sections was evaluated by means of the ROD (relative optical density) value. In cell cultures, the activity of SA-β-gal was defined as the ratio of SA-β-gal+ cells, which was determined by counting the blue cells and dividing by the total number of observed cells. Further details are provided in the Supplemental Information.
RNA-Seq Analysis of H-NSCs and Proteomic Analysis of ESC-sEVs
RNA-seq analysis was performed by Shanghai Biotechnology (Shanghai, China). Purified libraries were quantified by a Qubit 2.0 fluorometer (Life Technologies, USA) and validated by an Agilent 2100 bioanalyzer (Agilent Technologies, USA), and then sequenced on an Illumina HiSeq sequencer (Illumina, USA). The RNA-seq datasets have been deposited in the Gene Expression Omnibus (GEO) database under GEO: GSE158000. Proteomic analysis was performed by Shanghai Applied Protein Technology (Shanghai, China). LC-MS/MS analysis was performed on a Q Exactive mass spectrometer (Thermo Scientific). Further details are provided in the Supplemental Information.
RNA Pull-Down Assay
For MYT1 interference experiments, three pairs of shRNA sequences were designed and sequences are listed in Table S1. MYT1 silencing lentiviral particle (sh-MYT1) and sh-NCs were purchased from Hanbio Biotechnology (Shanghai, China). The shRNA was inserted into the lentivirus vector pHBLV-U6-MCS-CMV-ZsGreen-PGK-Puro (Hanbio Biotechnology) to silence MYT1. H-NSCs were transfected with sh-MYT1 or sh-NC under standard conditions using Polybrene, and 72 h later cells were subjected to selection with 1 μg/mL puromycin. Assays were performed after at least 10 days in selection.
Other Methods
Procedures related to the MWM test, IF staining, western blot analysis, qRT-PCR analysis, ESC-sEV uptake assay, and others are provided in the Supplemental Information.
Statistical Analysis
All data are presented as mean ± SD. An unpaired Student’s t test was used to examine the inter-group differences, whereas one-way analysis of variance (ANOVA) was utilized to explore the heterogeneity among different groups, followed by a Bonferroni post hoc test in the absence of equivalent variance. A Kruskal-Wallis test and Mann-Whitney U test were applied for nonparametric analysis. A difference of p <0.05 was deemed to be statistically significant.
Author Contributions
G.H. and Y.X. performed the experiments, ESC-sEV isolation, and proteomic analysis, and prepared the manuscript; B.C. and J.Z. performed animal experiments; L.G. and Y.C. performed data collection and analysis; and Q.L., Y.W., and Z.D. conceptualized the project, provided funding, supported the study material, and provided final approval of the manuscript.
Conflicts of Interests
The authors declare no competing interests.
Acknowledgments
We sincerely thank Dr. Bin Hu, Xin Niu, Ji Yuan (Institute of Microsurgery on Extremities, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital), Dr. Qingwei Zhu, and Dr. Xiaozheng Ling (Department of Neurosurgery, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital) for technical assistance. This work was supported by the National Natural Science Foundation of China (project nos. 82071371, 81671209, and 81871833).
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.ymthe.2020.09.037.
Contributor Information
Qing Li, Email: liqing_236@aliyun.com.
Yang Wang, Email: wangyang63@sjtu.edu.cn.
Zhifeng Deng, Email: zfdeng@sjtu.edu.cn.
Supplemental Information
References
- 1.Nguyen H., Zarriello S., Coats A., Nelson C., Kingsbury C., Gorsky A., Rajani M., Neal E.G., Borlongan C.V. Stem cell therapy for neurological disorders: a focus on aging. Neurobiol. Dis. 2019;126:85–104. doi: 10.1016/j.nbd.2018.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Capilla-Gonzalez V., Herranz-Pérez V., García-Verdugo J.M. The aged brain: genesis and fate of residual progenitor cells in the subventricular zone. Front. Cell. Neurosci. 2015;9:365. doi: 10.3389/fncel.2015.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Encinas J.M., Michurina T.V., Peunova N., Park J.H., Tordo J., Peterson D.A., Fishell G., Koulakov A., Enikolopov G. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell. 2011;8:566–579. doi: 10.1016/j.stem.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouab M., Paliouras G.N., Aumont A., Forest-Bérard K., Fernandes K.J. Aging of the subventricular zone neural stem cell niche: evidence for quiescence-associated changes between early and mid-adulthood. Neuroscience. 2011;173:135–149. doi: 10.1016/j.neuroscience.2010.11.032. [DOI] [PubMed] [Google Scholar]
- 5.Childs B.G., Durik M., Baker D.J., van Deursen J.M. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat. Med. 2015;21:1424–1435. doi: 10.1038/nm.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ren R., Ocampo A., Liu G.H., Izpisua Belmonte J.C. Regulation of stem cell aging by metabolism and epigenetics. Cell Metab. 2017;26:460–474. doi: 10.1016/j.cmet.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 7.de Haan G., Lazare S.S. Aging of hematopoietic stem cells. Blood. 2018;131:479–487. doi: 10.1182/blood-2017-06-746412. [DOI] [PubMed] [Google Scholar]
- 8.Raabe E.H., Lim K.S., Kim J.M., Meeker A., Mao X.G., Nikkhah G., Maciaczyk J., Kahlert U., Jain D., Bar E. BRAF activation induces transformation and then senescence in human neural stem cells: a pilocytic astrocytoma model. Clin. Cancer Res. 2011;17:3590–3599. doi: 10.1158/1078-0432.CCR-10-3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Molofsky A.V., He S., Bydon M., Morrison S.J., Pardal R. Bmi-1 promotes neural stem cell self-renewal and neural development but not mouse growth and survival by repressing the p16Ink4a and p19Arf senescence pathways. Genes Dev. 2005;19:1432–1437. doi: 10.1101/gad.1299505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molofsky A.V., Slutsky S.G., Joseph N.M., He S., Pardal R., Krishnamurthy J., Sharpless N.E., Morrison S.J. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443:448–452. doi: 10.1038/nature05091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishino J., Kim I., Chada K., Morrison S.J. Hmga2 promotes neural stem cell self-renewal in young but not old mice by reducing p16Ink4a and p19Arf expression. Cell. 2008;135:227–239. doi: 10.1016/j.cell.2008.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neumann B., Baror R., Zhao C., Segel M., Dietmann S., Rawji K.S., Foerster S., McClain C.R., Chalut K., van Wijngaarden P., Franklin R.J.M. Metformin restores CNS remyelination capacity by rejuvenating aged stem cells. Cell Stem Cell. 2019;25:473–485.e8. doi: 10.1016/j.stem.2019.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Théry C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R., Antoniou A., Arab T., Archer F., Atkin-Smith G.K. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adamiak M., Cheng G., Bobis-Wozowicz S., Zhao L., Kedracka-Krok S., Samanta A., Karnas E., Xuan Y.T., Skupien-Rabian B., Chen X. Induced pluripotent stem cell (iPSC)-derived extracellular vesicles are safer and more effective for cardiac repair than iPSCs. Circ. Res. 2018;122:296–309. doi: 10.1161/CIRCRESAHA.117.311769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basu J., Ludlow J.W. Exosomes for repair, regeneration and rejuvenation. Expert Opin. Biol. Ther. 2016;16:489–506. doi: 10.1517/14712598.2016.1131976. [DOI] [PubMed] [Google Scholar]
- 16.Khan M., Nickoloff E., Abramova T., Johnson J., Verma S.K., Krishnamurthy P., Mackie A.R., Vaughan E., Garikipati V.N., Benedict C. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ. Res. 2015;117:52–64. doi: 10.1161/CIRCRESAHA.117.305990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yabut O., Bernstein H.S. The promise of human embryonic stem cells in aging-associated diseases. Aging (Albany NY) 2011;3:494–508. doi: 10.18632/aging.100328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bae Y.U., Son Y., Kim C.H., Kim K.S., Hyun S.H., Woo H.G., Jee B.A., Choi J.H., Sung H.K., Choi H.C. Embryonic stem cell-derived mmu-miR-291a-3p inhibits cellular senescence in human dermal fibroblasts through the TGF-β receptor 2 pathway. J. Gerontol. A Biol. Sci. Med. Sci. 2019;74:1359–1367. doi: 10.1093/gerona/gly208. [DOI] [PubMed] [Google Scholar]
- 19.Chen B., Sun Y., Zhang J., Zhu Q., Yang Y., Niu X., Deng Z., Li Q., Wang Y. Human embryonic stem cell-derived exosomes promote pressure ulcer healing in aged mice by rejuvenating senescent endothelial cells. Stem Cell Res. Ther. 2019;10:142. doi: 10.1186/s13287-019-1253-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith G.E. Healthy cognitive aging and dementia prevention. Am. Psychol. 2016;71:268–275. doi: 10.1037/a0040250. [DOI] [PubMed] [Google Scholar]
- 21.Liu B., Liu J., Zhang J., Mao W., Li S. Effects of autophagy on synaptic-plasticity-related protein expression in the hippocampus CA1 of a rat model of vascular dementia. Neurosci. Lett. 2019;707:134312. doi: 10.1016/j.neulet.2019.134312. [DOI] [PubMed] [Google Scholar]
- 22.Sorrells S.F., Paredes M.F., Cebrian-Silla A., Sandoval K., Qi D., Kelley K.W., James D., Mayer S., Chang J., Auguste K.I. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature. 2018;555:377–381. doi: 10.1038/nature25975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Villeda S.A., Luo J., Mosher K.I., Zou B., Britschgi M., Bieri G., Stan T.M., Fainberg N., Ding Z., Eggel A. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Deun J., Mestdagh P., Agostinis P., Akay Ö., Anand S., Anckaert J., Martinez Z.A., Baetens T., Beghein E., Bertier L., EV-TRACK Consortium EV-TRACK: transparent reporting and centralizing knowledge in extracellular vesicle research. Nat. Methods. 2017;14:228–232. doi: 10.1038/nmeth.4185. [DOI] [PubMed] [Google Scholar]
- 25.Campisi J. The biology of replicative senescence. Eur. J. Cancer. 1997;33:703–709. doi: 10.1016/S0959-8049(96)00058-5. [DOI] [PubMed] [Google Scholar]
- 26.Wang X., Liu C., Li S., Xu Y., Chen P., Liu Y., Ding Q., Wahapu W., Hong B., Yang M. Effects of continuous passage on immunomodulatory properties of human adipose-derived stem cells. Cell Tissue Bank. 2015;16:143–150. doi: 10.1007/s10561-014-9451-z. [DOI] [PubMed] [Google Scholar]
- 27.Matsushita F., Kameyama T., Kadokawa Y., Marunouchi T. Spatiotemporal expression pattern of Myt/NZF family zinc finger transcription factors during mouse nervous system development. Dev. Dyn. 2014;243:588–600. doi: 10.1002/dvdy.24091. [DOI] [PubMed] [Google Scholar]
- 28.Vasconcelos F.F., Sessa A., Laranjeira C., Raposo A.A.S.F., Teixeira V., Hagey D.W., Tomaz D.M., Muhr J., Broccoli V., Castro D.S. MyT1 counteracts the neural progenitor program to promote vertebrate neurogenesis. Cell Rep. 2016;17:469–483. doi: 10.1016/j.celrep.2016.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taniguchi C.M., Finger E.C., Krieg A.J., Wu C., Diep A.N., LaGory E.L., Wei K., McGinnis L.M., Yuan J., Kuo C.J., Giaccia A.J. Cross-talk between hypoxia and insulin signaling through Phd3 regulates hepatic glucose and lipid metabolism and ameliorates diabetes. Nat. Med. 2013;19:1325–1330. doi: 10.1038/nm.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang S., Ryu J.H., Oh H., Jeon J., Kwak J.S., Kim J.H., Kim H.A., Chun C.H., Chun J.S. NAMPT (visfatin), a direct target of hypoxia-inducible factor-2α, is an essential catabolic regulator of osteoarthritis. Ann. Rheum. Dis. 2015;74:595–602. doi: 10.1136/annrheumdis-2013-204355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma C., Pi C., Yang Y., Lin L., Shi Y., Li Y., Li Y., He X. Nampt expression decreases age-related senescence in rat bone marrow mesenchymal stem cells by targeting Sirt1. PLoS ONE. 2017;12:e0170930. doi: 10.1371/journal.pone.0170930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanokawa-Akakura R., Akakura S., Tabibzadeh S. Replicative senescence in human fibroblasts is delayed by hydrogen sulfide in a NAMPT/SIRT1 dependent manner. PLoS ONE. 2016;11:e0164710. doi: 10.1371/journal.pone.0164710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colombo M., Raposo G., Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 34.Gong L., Chen B., Zhang J., Sun Y., Yuan J., Niu X., Hu G., Chen Y., Xie Z., Deng Z. Human ESC-sEVs alleviate age-related bone loss by rejuvenating senescent bone marrow-derived mesenchymal stem cells. J. Extracell. Vesicles. 2020;9:1800971. doi: 10.1080/20013078.2020.1800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sherry-Lynes M.M., Sengupta S., Kulkarni S., Cochran B.H. Regulation of the JMJD3 (KDM6B) histone demethylase in glioblastoma stem cells by STAT3. PLoS ONE. 2017;12:e0174775. doi: 10.1371/journal.pone.0174775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ermolaeva M., Neri F., Ori A., Rudolph K.L. Cellular and epigenetic drivers of stem cell ageing. Nat. Rev. Mol. Cell Biol. 2018;19:594–610. doi: 10.1038/s41580-018-0020-3. [DOI] [PubMed] [Google Scholar]
- 37.Maryanovich M., Zahalka A.H., Pierce H., Pinho S., Nakahara F., Asada N., Wei Q., Wang X., Ciero P., Xu J. Adrenergic nerve degeneration in bone marrow drives aging of the hematopoietic stem cell niche. Nat. Med. 2018;24:782–791. doi: 10.1038/s41591-018-0030-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ocampo A., Reddy P., Martinez-Redondo P., Platero-Luengo A., Hatanaka F., Hishida T., Li M., Lam D., Kurita M., Beyret E. In vivo amelioration of age-associated hallmarks by partial reprogramming. Cell. 2016;167:1719–1733.e12. doi: 10.1016/j.cell.2016.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neves J., Sousa-Victor P., Jasper H. Rejuvenating strategies for stem cell-based therapies in aging. Cell Stem Cell. 2017;20:161–175. doi: 10.1016/j.stem.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang J., Wang Y., Shao L., Laberge R.M., Demaria M., Campisi J., Janakiraman K., Sharpless N.E., Ding S., Feng W. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 2016;22:78–83. doi: 10.1038/nm.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kodali M., Castro O.W., Kim D.K., Thomas A., Shuai B., Attaluri S., Upadhya R., Gitai D., Madhu L.N., Prockop D.J., Shetty A.K. Intranasally administered human MSC-derived extracellular vesicles pervasively incorporate into neurons and microglia in both intact and status epilepticus injured forebrain. Int. J. Mol. Sci. 2019;21:181. doi: 10.3390/ijms21010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aghajani Nargesi A., Lerman L.O., Eirin A. Mesenchymal stem cell-derived extracellular vesicles for kidney repair: current status and looming challenges. Stem Cell Res. Ther. 2017;8:273. doi: 10.1186/s13287-017-0727-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rohrbach S., Simm A., Pregla R., Franke C., Katschinski D.M. Age-dependent increase of prolyl-4-hydroxylase domain (PHD) 3 expression in human and mouse heart. Biogerontology. 2005;6:165–171. doi: 10.1007/s10522-005-7950-9. [DOI] [PubMed] [Google Scholar]
- 44.Epstein A.C., Gleadle J.M., McNeill L.A., Hewitson K.S., O’Rourke J., Mole D.R., Mukherji M., Metzen E., Wilson M.I., Dhanda A. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 45.Stein L.R., Imai S. Specific ablation of Nampt in adult neural stem cells recapitulates their functional defects during aging. EMBO J. 2014;33:1321–1340. doi: 10.1002/embj.201386917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holloway K.R., Calhoun T.N., Saxena M., Metoyer C.F., Kandler E.F., Rivera C.A., Pruitt K. SIRT1 regulates Dishevelled proteins and promotes transient and constitutive Wnt signaling. Proc. Natl. Acad. Sci. USA. 2010;107:9216–9221. doi: 10.1073/pnas.0911325107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lie D.C., Colamarino S.A., Song H.J., Désiré L., Mira H., Consiglio A., Lein E.S., Jessberger S., Lansford H., Dearie A.R., Gage F.H. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 48.Marchetti B., Pluchino S. Wnt your brain be inflamed? Yes, it Wnt! Trends Mol. Med. 2013;19:144–156. doi: 10.1016/j.molmed.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marchetti B., Tirolo C., L’Episcopo F., Caniglia S., Testa N., Smith J.A., Pluchino S., Serapide M.F. Parkinson’s disease, aging and adult neurogenesis: Wnt/β-catenin signalling as the key to unlock the mystery of endogenous brain repair. Aging Cell. 2020;19:e13101. doi: 10.1111/acel.13101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rong Y., Liu W., Lv C., Wang J., Luo Y., Jiang D., Li L., Zhou Z., Zhou W., Li Q. Neural stem cell small extracellular vesicle-based delivery of 14-3-3t reduces apoptosis and neuroinflammation following traumatic spinal cord injury by enhancing autophagy by targeting Beclin-1. Aging (Albany NY) 2019;11:7723–7745. doi: 10.18632/aging.102283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Théry C., Zitvogel L., Amigorena S. Exosomes: composition, biogenesis and function. Nat. Rev. Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 52.Hill C.S. Nucleocytoplasmic shuttling of Smad proteins. Cell Res. 2009;19:36–46. doi: 10.1038/cr.2008.325. [DOI] [PubMed] [Google Scholar]
- 53.Jovanovic V.M., Salti A., Tilleman H., Zega K., Jukic M.M., Zou H., Friedel R.H., Prakash N., Blaess S., Edenhofer F., Brodski C. BMP/SMAD pathway promotes neurogenesis of midbrain dopaminergic neurons in vivo and in human induced pluripotent and neural stem cells. J. Neurosci. 2018;38:1662–1676. doi: 10.1523/JNEUROSCI.1540-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xin H., Li Y., Chopp M. Exosomes/miRNAs as mediating cell-based therapy of stroke. Front. Cell. Neurosci. 2014;8:377. doi: 10.3389/fncel.2014.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Riquelme P.A., Drapeau E., Doetsch F. Brain micro-ecologies: neural stem cell niches in the adult mammalian brain. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008;363:123–137. doi: 10.1098/rstb.2006.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yousef H., Czupalla C.J., Lee D., Chen M.B., Burke A.N., Zera K.A., Zandstra J., Berber E., Lehallier B., Mathur V. Aged blood impairs hippocampal neural precursor activity and activates microglia via brain endothelial cell VCAM1. Nat. Med. 2019;25:988–1000. doi: 10.1038/s41591-019-0440-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun F., Mao X., Xie L., Ding M., Shao B., Jin K. Notch1 signaling modulates neuronal progenitor activity in the subventricular zone in response to aging and focal ischemia. Aging Cell. 2013;12:978–987. doi: 10.1111/acel.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kitayama T., Yoneyama M., Tamaki K., Yoneda Y. Regulation of neuronal differentiation by N-methyl-d-aspartate receptors expressed in neural progenitor cells isolated from adult mouse hippocampus. J. Neurosci. Res. 2004;76:599–612. doi: 10.1002/jnr.20095. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.