Abstract
Our previous study demonstrated increased expression of Heat shock protein (Hsp) 90 in the skin of patients with systemic sclerosis (SSc). We aimed to evaluate plasma Hsp90 in SSc and characterize its association with SSc-related features. Ninety-two SSc patients and 92 age-/sex-matched healthy controls were recruited for the cross-sectional analysis. The longitudinal analysis comprised 30 patients with SSc associated interstitial lung disease (ILD) routinely treated with cyclophosphamide. Hsp90 was increased in SSc compared to healthy controls. Hsp90 correlated positively with C-reactive protein and negatively with pulmonary function tests: forced vital capacity and diffusing capacity for carbon monoxide (DLCO). In patients with diffuse cutaneous (dc) SSc, Hsp90 positively correlated with the modified Rodnan skin score. In SSc-ILD patients treated with cyclophosphamide, no differences in Hsp90 were found between baseline and after 1, 6, or 12 months of therapy. However, baseline Hsp90 predicts the 12-month change in DLCO. This study shows that Hsp90 plasma levels are increased in SSc patients compared to age-/sex-matched healthy controls. Elevated Hsp90 in SSc is associated with increased inflammatory activity, worse lung functions, and in dcSSc, with the extent of skin involvement. Baseline plasma Hsp90 predicts the 12-month change in DLCO in SSc-ILD patients treated with cyclophosphamide.
Subject terms: Pathogenesis, Rheumatology, Biomarkers
Introduction
Systemic sclerosis (scleroderma, SSc) is an autoimmune connective tissue disease characterized by vasculopathy and fibrotic changes in multiple organs, particularly the skin and the lungs. It is a rare chronic rheumatic disorder of a complex aetiopathogenesis1. Genetic and environmental factors and epigenetics most probably underlie susceptibility to this disease. Scleroderma is traditionally seen as a prototypical multi-system fibrotic disorder mediated by transforming growth factor (TGF)-β and fibroblasts, which produce excessive extracellular matrix proteins such as collagen. However, another hypothesis attributes this disease to the malfunction of the connective tissue repair mechanism in response to injury1,2. Patients with SSc can be categorized into two subgroups based on the affected regions: diffuse cutaneous (dc) SSc with proximal involvement, and limited cutaneous (lc) SSc with fibrosis of the skin distal to the elbows or knees, and occasionally of the face and neck1. Internal organ complications associated with SSc contribute to its highest morbidity and mortality amongst inflammatory rheumatic disorders3. Up to 80% of SSc patients develop interstitial lung disease (ILD), and 25–30% of these patients present with the progressive phenotype. The underlying mechanisms of fibrotic changes in ILD associated with SSc (SSc-ILD) include mesenchymal cell activation, structural changes to epithelial and endothelial cells, and cellular lesions4. Currently, ILD is the leading cause of death for SSc patients, accounts for up to 30% of deaths, and has a 10-year mortality of up to 40%5. Clinical management of SSc-ILD is challenging and relies on the identification of clinically meaningful disease, assessment of response to pharmacological therapy, and implementation of routine monitoring3. Circulating biomarkers reflect relevant physiological or pathological processes, thus may serve as tools for diagnosis, risk evaluation, and prediction of treatment response or safety monitoring. However, disease-specific biomarkers that could be used in routine clinical practice are lacking6. Recently, several new promising or effective therapies for SSc-ILD have become available. Therefore, there is an unmet need for disease- or organ-specific biomarkers to assess and to predict treatment response6,7.
Heat shock proteins (Hsp) are chaperone proteins first described in 1962 in Drosophila based on their increased expression at elevated temperatures8. More recent studies found that Hsp are also induced by other forms of cellular stress (e.g. increased pH, disruption of homeostasis, viral infection, reparative healing, and tissue remodeling, etc.)9,10. Under physiological conditions as well as during stress response, Hsp recognize and adhere to misfolded proteins in non-native conformations and protect these client proteins against irreversible degradation11. In this study, we focused on Hsp90, which is highly conserved and ubiquitously expressed in eukaryotic organisms and in bacteria. It is crucial for many physiological processes, e.g., the cell cycle regulation, cellular apoptosis, protein degradation, and other signalling pathways12–14. The abundant Hsp90 activates and stabilizes its various client proteins, such as transcription factors, kinases, and ubiquitin ligases15. Some client proteins of Hsp90 are involved in the progression of a diverse group of disorders, e.g., malignancies, neurodegenerative diseases, and bacterial or viral infections16. Moreover, Hsp90 is also believed to function as a cytokine in the immune response, and participates in innate and adaptive immunity. Nevertheless, the cytokine-like properties of Hsp90 are yet to be elucidated17–19. Of particular interest is the significance of Hsp90 in stabilizing and folding TGF-β receptors and Src kinases that promote fibrosis in SSc20–22. Consequently, inhibition of Hsp90 results in proteasomal degradation of TGF-β receptors and Src23. We have previously shown that the Hsp90 expression is increased in SSc skin and dermal fibroblasts and is critical for TGF-β signalling. Pharmacological inhibition of Hsp90 effectively blocked the pro-fibrotic effects of TGF-β in cultured fibroblasts and three different preclinical models of SSc24. These results have translational implications because several Hsp90 inhibitors have already been used in clinical trials for other indications, mostly tumorous conditions16,25.
Therefore, we aimed to assess the systemic levels of Hsp90 in patients with SSc and age-/sex-matched healthy individuals, and analyze its association with SSc-related clinical features with a focus on fibrotic manifestations. Our next aim was to evaluate the potential of systemic Hsp90 as a predictor of response to cyclophosphamide treatment in a group of SSc-ILD patients.
Results
Patients and healthy controls: clinical and demographic characteristics
In the cross-sectional analysis, a total of 92 patients and 92 healthy controls were included. Clinical and demographic characteristics of the patients and healthy controls are stated in Table 1.
Table 1.
Clinical and demographic characteristics of SSc patients and healthy controls: cross-sectional analysis.
| Parameters | SSc patients (n = 92) | Healthy controls (n = 92) | p-value |
|---|---|---|---|
| Sex: female/male, n (%) | 79 (86)/13 (14) | 79 (86)/13 (14) | 1.000 |
| Age, median (IQR) (years) | 55.0 (45.0–60.5) | 51.0 (44.0–61.0) | 0.806 |
| Disease duration, median (IQR) (years) | 4.0 (1.3–8.0) | ||
| SSc subtype: dcSSc/lcSSc, n (%) | 38 (41)/54 (59) | ||
| ANA positivity, n (%) | 85 (92) | ||
| ACA positivity, n (%) | 16 (17) | ||
| Anti-Scl70 positivity, n (%) | 41 (45) | ||
| Raynaud’s phenomenon, n (%) | 91 (99) | ||
| Gastrointestinal involvement, n (%) | 55 (60) | ||
| Arthralgia and/or arthritis, n (%) | 33 (30) | ||
| Interstitial lung disease, n (%) | 58 (63) | ||
| Pulmonary arterial hypertension, n (%) | 19 (21) | ||
| Cardiac involvement, n (%) | 16 (17) | ||
| Renal involvement, n (%) | 3 (3) | ||
| Digital ulcers, n (%) | 24 (26) | ||
| CRP, median (IQR) (mg/L) | 3.2 (1.6–6.5) | ||
| ESR, median (IQR) (mm/h) | 13.5 (9.0–28.0) | ||
| mRSS median (IQR) | 10.0 (5.0–19.0) | ||
| ESSG activity score median (IQR) | 3.3 (2.0–4.0) | ||
| FVC, median (IQR) % predicted | 76.0 (62.8–100.4) | ||
| FEV1, median (IQR) % predicted | 72.4 (59.9–94.5) | ||
| DLCO, median (IQR) % predicted | 61.5 (46.2–75.5) | ||
| SpO2, median (IQR) % | 96.0 (94.0–97.0) | ||
| Current treatment: GC/MTX/CPA/AZA/MMF, n (%) | 48 (44)/10 (9)/4 (4)/4 (4)/1 (1) |
SSc systemic sclerosis, lcSSc limited cutaneous SSc, dcSSc diffuse cutaneous SSc, IQR interquartile range, ANA antinuclear antibodies, Anti-Scl-70 anti–DNA-topoisomerase I antibodies, ACA anticentromere antibodies, ESSG European Scleroderma Study Group, mRSS modified Rodnan skin score, CRP C-reactive protein, ESR erythrocyte sedimentation rate, FVC forced vital capacity, FEV1 forced expiratory volume in 1 s, DLCO diffusing capacity for carbon monoxide, SpO2 blood oxygen saturation level, GC low dose glucocorticoids (i.e. ≤ 10 mg/day of prednisone), MTX methotrexate, CPA cyclophosphamide, AZA azathioprine, MMF mycophenolate mofetil.
In the longitudinal analysis, a total of 30 patients with SSc-ILD with active alveolitis without pulmonary arterial hypertension who underwent a routine 6-month (n = 16) or 12-month (n = 14) treatment with i.v. cyclophosphamide were included. Clinical and demographic attributes of SSc-ILD patients treated with cyclophosphamide are presented in Table 2. The lung function tests FVC, and FEV1 in these SSc-ILD patients remained stable for both 6 and 12 months upon treatment with cyclophosphamide; no statistical differences from baseline were found. However, a trend towards improvement in DLCO from baseline was found after 12 months (Table 2).
Table 2.
Baseline clinical parameters of patients with SSc-ILD treated with cyclophosphamide: longitudinal analysis.
| Parameters | Baseline (month 0) (n = 30) | Month 1 (n = 30) | Month 6 (n = 30) | Month 12 (n = 30) | p-value (p0–1; p0–6; p0–12) |
|---|---|---|---|---|---|
| Sex: female/male, n (%) | 23 (77)/7 (23) | ||||
| Age, median (IQR) (years) | 57.7 (46.0–66.0) | ||||
| Disease duration, median (IQR) (years) | 2.8 (0.9–6.0) | ||||
| SSc subtype: dcSSc/lcSSc, n (%) | 14 (47)/16 (53) | ||||
| ANA positivity, n (%) | 25 (83) | ||||
| ACA positivity, n (%) | 2 (6) | ||||
| Anti-Scl70 positivity (%) | 16 (53) | ||||
| Raynaud’s phenomenon, n (%) | 29 (97) | ||||
| GI, n (%) | 16 (53) | ||||
| Arthralgia and/or arthritis, n (%) | 11 (37) | ||||
| ILD, n (%) | 30 (100) | ||||
| PAH, n (%) | 0 (0) | ||||
| CI, n (%) | 3 (10) | ||||
| RI, n (%) | 1 (3) | ||||
| Digital ulcers, n (%) | 6 (20) | ||||
| mRSS median (IQR) | 12.0 (4.0–19.0) | ||||
| ESSG activity score median (IQR) | 2.5 (1.0–4.0) | ||||
| Hsp90, median (IQR) (ng/mL) | 13.9 (8.6–20.6) | 18.0 (9.5–23.3) | 12.0 (8.7–21.3) | 12.5 (8.9–16.7)a | 0.683; 0.827; 0.285 |
| CRP, median (IQR) (mg/L) | 6.2 (2.7–13.4) | 6.7 (2.8–12.4) | 5.6 (2.5–14.5) | 4.7 (2.2–18.7) | 0.564; 0.117; 1.000 |
| ESR, median (IQR) (mm/h) | 14.0 (9.5–22.3) | 15.5 (10.8–25.8) | 14.5 (11.3–23.8) | 14.0 (10.0–21.0) | 0.275; 0.394; 0.467 |
| FVC, median (IQR) % predicted | 76.0 (62.8–100.4) | 74.5 (63.0–102.2) | 74.4 (60.0–98.3) | –; 0.532; 0.346 | |
| FEV1, median (IQR) % predicted | 72.4 (59.9–94.5) | 76.0 (68.0–96.5) | 69.0 (64.5–93.0) | –; 0.532; 0.467 | |
| DLCO, median (IQR) % predicted | 61.5 (46.2–75.5) | 60.0 (47.0–79.0) | 64.5 (55.5–79.0) | –; 0.297; 0.090 | |
| Current treatment: GC, n (%) | 16 (53) |
SSc systemic sclerosis, lcSSc limited cutaneous SSc, dcSSc diffuse cutaneous SSc, IQR interquartile range, ANA antinuclear antibodies, Anti-Scl-70 anti–DNA-topoisomerase I antibodies, ACA anticentromere antibodies, GI gastrointerstinal ivolvement, ILD interstitial lung disease, PAH pulmonary arterial hypertension, CI cardiac involvement, RI renal involvement, ESSG European Scleroderma Study Group, mRSS modified Rodnan skin score, CRP C-reactive protein, ESR erythrocyte sedimentation rate, FVC forced vital capacity, FEV1 forced expiratory volume in one second, DLCO diffusing capacity for carbon monoxide, GC low dose glucocorticoids (i.e. ≤ 10 mg/day of prednisone), p0–1 difference between month 0 and 1, p0–6 difference between month 0 and 6, p0–12 difference between month 0 and 12 tested by Friedman's test (for all comparisons).
aValues for plasma Hsp90 at month 12 are available only for 14 patients who underwent a 12-month cyclophosphamide treatment.
Plasma Hsp90 in patients with systemic sclerosis and healthy controls: a cross-sectional analysis
The plasma levels of Hsp90 were elevated in all SSc patients compared to healthy individuals [12.5 (9.6–17.9) vs 9.8 (7.7–12.4) ng/mL, p < 0.001]. After dividing the patients into two basic SSc subtypes, patients with lcSSc [13.4 (9.6–17.9) ng/mL, p < 0.001], as well as patients with dcSSc [11.4 (9.6–17.3) ng/mL, p = 0.012] showed a significant increase in Hsp90 compared to healthy controls. However, no significant difference between lcSSc and dcSSc was detected (p = 0.311) (Fig. 1).
Figure 1.
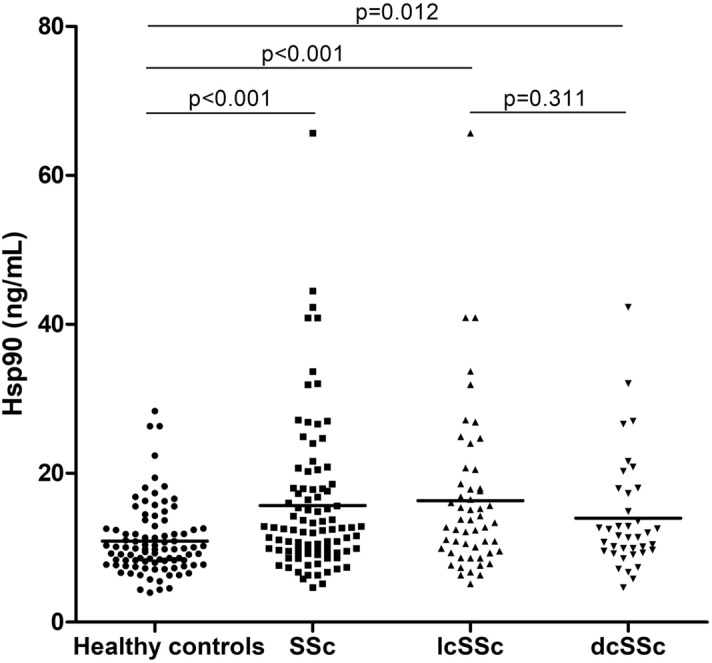
Plasma levels of Hsp90 are significantly elevated in SSc patients and both subsets of SSc (lcSSc and dcSSc) compared with healthy controls. The levels of plasma Hsp90 are comparable in lcSSc and dcSSc patients. Horizontal bars represent the median.
Hsp90 plasma levels in relation to clinical parameters of SSc: a cross-sectional analysis
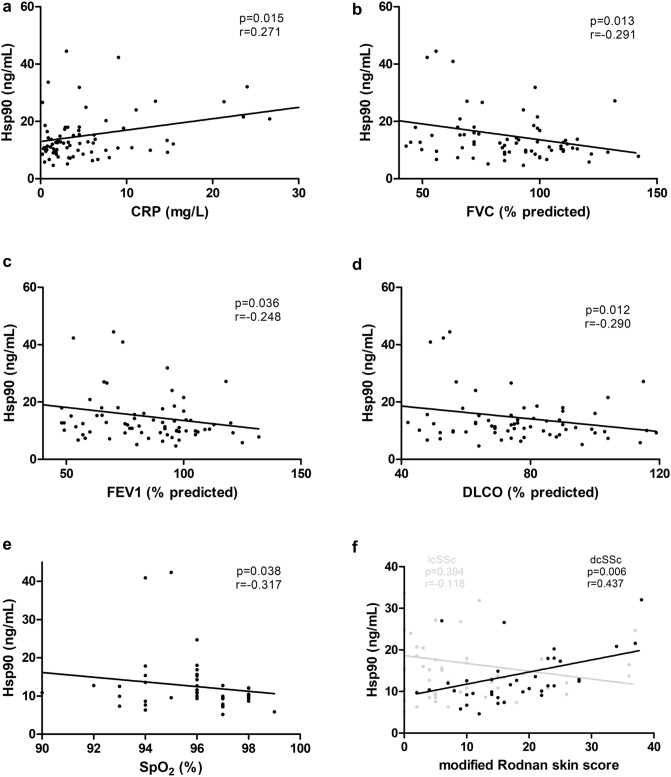
In all SSc patients, plasma levels of Hsp90 positively correlated with C-reactive protein (CRP) (Fig. 2a). Furthermore, extracellular Hsp90 concentrations were increased in patients with deteriorated lung functions: a negative correlation with individual functional parameters of SSc-ILD was detected, such as FVC, FEV1, DLCO, and SpO2 (Fig. 2b–e). When adjusted for CRP, correlations with FVC, DLCO, and SpO2 (in decreasing order) remained significant in multivariate analysis (Table 3). Of particular interest, only in patients with dcSSc, Hsp90 plasma levels positively correlated with the modified Rodnan skin score (mRSS) (Fig. 2f). Concentrations of extracellular Hsp90 were not significantly affected by other main clinical parameters of SSc (data not shown).
Figure 2.
Bivariate correlations of Hsp90 with systemic inflammation, lung function tests, and skin involvement. In all SSc patients, plasma Hsp90 positively correlated with C-reactive protein (CRP) (a), and negatively correlated with lung function parameters, such as forced vital capacity (FVC) (b), forced expiratory volume in one second (FEV1) (c), diffusing lung capacity for carbon monoxide (DLCO) (d), and oxygen saturation assessed by pulse oximetry (SpO2) (e). In patients with diffuse cutaneous (dc)SSc, plasma Hsp90 positively correlated with the extent and severity of skin involvement (black dots), whereas in limited cutaneous (lc)SSc, no significant correlation was found (grey dots) (f).
Table 3.
Univariate and multivariate regression analysis predicting plasma Hsp90 levels based on lung function parameters, unadjusted and adjusted for CRP levels.
| Parameter | Unadjusted | Adjusted for CRP | ||
|---|---|---|---|---|
| b (95% CI) | p-value | b (95% CI) | p-value | |
| FVC | − 0.14 (− 0.23; − 0.04) | 0.005 | − 0.12 (− 0.22; − 0.03) | 0.012 |
| FEV1 | − 0.11 (− 0.22; − 0.01) | 0.043 | − 0.10 (− 0.21; 0.01) | 0.079 |
| DLCO | − 0.12 (− 0.22; − 0.02) | 0.017 | − 0.12 (− 0.22; − 0.02) | 0.020 |
| SpO2 | − 1.15 (− 2.29; − 0.02) | 0.047 | − 1.13 (− 2.25; − 0.01) | 0.049 |
FVC forced vital capacity, FEV1 forced expiratory volume in one second, DLCO diffusing capacity for carbon monoxide, SpO2 oxygen saturation assessed by pulse oximetry, CRP C-reactive protein, b y intercept of a line, CI confidence interval. Statistically significant differences (p < 0.05) are marked in bold.
Plasma levels of Hsp90 in patients with SSc-ILD treated with cyclophosphamide: a longitudinal analysis
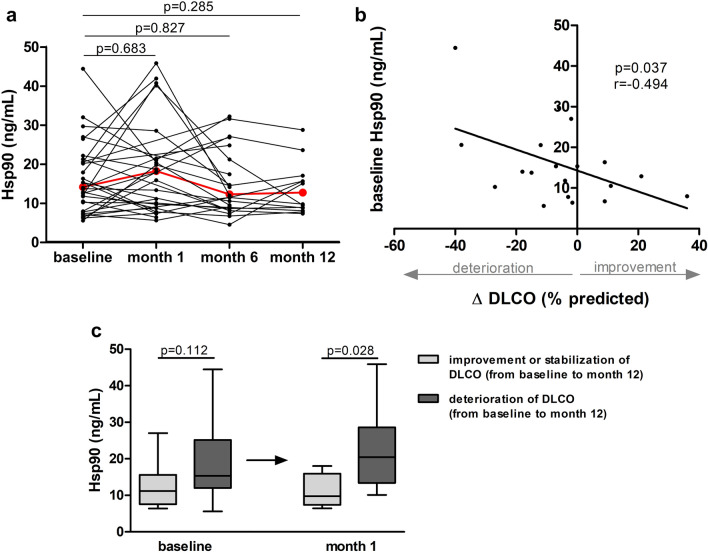
In the prospective analysis of patients with SSc-ILD treated with 6 or 12 monthly i.v. pulses of cyclophosphamide we did not observe any significant differences between baseline Hsp90 and after 1, 6, and 12 months (Fig. 3a). However, baseline Hsp90 was able to predict the 12-month absolute change in DLCO% predicted upon cyclophosphamide treatment (DLCOmonth12–month0; r = -0.494, p = 0.037) (Fig. 3b). Moreover, plasma Hsp90 levels at month 1 predicted the deterioration (absolute decrease by ≥ 5%), or stabilization (absolute increase or decrease by < 5%) and improvement (absolute increase by ≥ 5%) of DLCO % predicted at month 12 with respect to baseline (Fig. 3c).
Figure 3.
Longitudinal analysis of Hsp90 plasma levels in patients with SSc-ILD treated with cyclophosphamide. (a) No significant differences in plasma Hsp90 were detected between baseline and after 1, 6, and 12 months of therapy with cyclophosphamide in patients with systemic sclerosis-associated interstitial lung disease (SSc-ILD). The red line represents the median. Friedman's test was used for all comparisons. (b) Lower baseline levels of plasma Hsp90 were significantly associated with larger improvement in diffusing lung capacity for carbon monoxide at month 12 from baseline (ΔDLCO% predicted) upon cyclophosphamide treatment. (c) Differences in plasma Hsp90 levels at baseline and one month thereafter in patients grouped according to the course of pulmonary function during the observation time, classified by deterioration and stabilization or improvement of DLCO% predicted at month 12 with respect to baseline. Descriptions of deterioration, stabilization, and improvement are stated in the text. Values are presented as box plots: the boxes represent the 25th–75th percentiles, the lines within the boxes represent the median, and the whiskers represent minimum to maximum.
Discussion
In this study, we determined the concentration of plasma Hsp90 in a cross-sectional cohort of patients with SSc compared to healthy controls and analyzed its possible association with disease-specific features. Furthermore, we evaluated the association between plasma Hsp90 and treatment response to cyclophosphamide in a longitudinal cohort of SSc-ILD patients.
For the first time, we report a modest increase in plasma levels of Hsp90 in SSc patients compared to healthy controls, which was statistically significant. However, no significant differences between diffuse and limited cutaneous SSc were observed. To date, systemic levels of Hsp90 have been demonstrated to be elevated in some rheumatic conditions (e.g., systemic lupus erythematosus, axSpA and inflammatory myopathies), as well as in type 1 diabetes and several tumorous conditions (e.g., melanoma, liver and lung cancer)26–32.
Our analysis demonstrated a positive correlation between Hsp90 and C-reactive protein (CRP). IL-6 and CRP play an important role in both the pathogenesis and clinical manifestations of SSc and may be useful indicators of disease activity, severity, and poor prognosis33. Hsp90 mediates a nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) dependent inflammatory pathway associated with increased expression and secretion of IL-618,19. Thus, the positive correlation between Hsp90 and CRP could reflect the possible interaction between Hsp90 and IL-6, which induces CRP production.
Furthermore, increased Hsp90 was associated with impaired lung function, indicated by a decrease in FVC, FEV1, DLCO, and SpO2. All parameters, except for FEV1, remained statistically significantly associated with Hsp90 in a multivariate regression model adjusted for CRP. Recently, Sontake et al. demonstrated increased Hsp90 expression in lung fibrosis and elevated Hsp90 ATPase activity in fibroblasts isolated from fibrotic lung lesions. Moreover, inhibition of Hsp90 exerted potent anti-fibrotic effects both in vitro and in murine models of pulmonary fibrosis34,35. In addition, Hsp90 could also contribute to the development of lung fibrosis through IL-6. Interleukin-6 signaling is necessary for collagen production and substantially depends on the proper function of the transcription factor Signal Transducer and Activator of Transcription (STAT)336–39. Hsp90 stabilizes and activates STAT3, thus may play a crucial role in the progression of lung fibrosis mediated by IL-640. In our longitudinal analysis of SSc-ILD patients treated with i.v. cyclophosphamide, we did not find any statistically significant changes in the lung function tests or in plasma Hsp90 levels. We observed stabilization of FVC, and a trend towards improvement in DLCO at month 12 from baseline, which are consistent with other observational studies on cyclophosphamide41. Nevertheless, baseline plasma Hsp90 was able to predict a 12-month absolute change in DLCO% predicted. In line with this finding, plasma Hsp90 measured after one month of cyclophosphamide treatment predicted the deterioration, stabilization, or improvement of DLCO at month 12. Since there are a few treatment options currently available for SSc-ILD, and novel therapeutic targets are currently under evaluation in ongoing clinical trials, these results might have practical implications in the stratification of SSc-ILD patients according to potential therapeutic response.
In our cross-sectional analysis, we also established a positive correlation between Hsp90 and the extent and severity of skin involvement. However, this was only found in patients with dcSSc, possibly due to more extensive and severe skin involvement compared to lcSSc. This is in line with our previous findings demonstrating increased local expression of Hsp90 in the involved skin and cultured fibroblasts of patients with dcSSc, as well as in the fibrotic skin of three preclinical models of SSc. In that study, inhibition of Hsp90 indicated potent anti-fibrotic properties in three murine models of dermal fibrosis, which mimic different clinical stages of SSc24. Moreover, a recent study showed that Hsp90 interacts very closely with fibronectin and may regulate matrix assembly, which is upregulated in SSc2,42.
Our study is limited by small numbers, especially in the longitudinal analysis, and the lack of other longitudinal SSc-ILD cohorts treated with other currently available pharmacological agents. Therefore, further studies are needed to confirm and validate our data. However, based on our results, extracellular Hsp90 could become an attractive soluble biomarker of skin and lung involvement in SSc with the potential to predict treatment response in SSc-ILD patients.
Conclusion
Our study demonstrates that Hsp90 is elevated in the peripheral blood of patients with systemic sclerosis compared to healthy individuals. Plasma Hsp90 levels in SSc patients rise with decreased pulmonary function and increased systemic inflammation. In diffuse cutaneous SSc, Hsp90 is associated with more extensive skin involvement. Of particular interest, in SSc-ILD patients, baseline Hsp90 predicts the 12-month change in DLCO upon cyclophosphamide treatment. These data further support the important role of Hsp90 as a regulator of fibroblast activation and tissue fibrosis in SSc and might have a direct practical application in the real-life stratification of patients with SSc-ILD according to potential therapeutic response.
Methods
Patients and healthy controls
All patients involved in this study fulfilled the ACR/EULAR classification criteria for SSc43. Plasma samples from all patients and healthy individuals were prepared from the whole blood collected into commercially available EDTA-treated tubes. For the cross-sectional analysis, plasma samples were obtained from 92 Caucasian patients with systemic sclerosis (SSc) and 92 age- and sex-matched Caucasian healthy controls. For the longitudinal analysis, plasma samples were obtained prospectively at baseline, and 1, 6, and 12 months thereafter from 30 Caucasian patients with SSc-ILD with active alveolitis without pulmonary arterial hypertension who underwent a routine 6-month (n = 16) or 12-month (n = 14) treatment with i.v. cyclophosphamide (CPA, 500 mg/m2 monthly). Active alveolitis was defined as the presence of areas of ground-glass attenuation on high-resolution computed tomography (HRCT) and reduced levels of diffusing lung capacity for carbon monoxide (DLCO) and/or forced vital capacity (FVC), as described elsewhere44. Other SSc-related clinical features were assessed according to generally accepted definitions and recorded, such as the presence of pulmonary arterial hypertension, renal, cardiac and gastrointestinal involvement, Raynaud's phenomenon, and digital ulcers45. Skin involvement was evaluated using the modified Rodnan skin score (mRSS)46. Disease activity was determined by the European Scleroderma Study Group (ESSG) SSc activity score47. Pulmonary function tests (PFT) were routinely performed using standard methods, in accordance with the ATS recommendations48. The DLCO was measured by a single-breath method using a gas mixture of 0.2% CO and 8% helium, with correction for hemoglobin. Peripheral oxygen saturation (SpO2) was measured by a handheld pulse oximeter (CR-100, Noramedica, Czech Republic). In the longitudinal analysis PFTs were performed at baseline, and 6 and 12 months thereafter, and the results are expressed as a percentage of the normal predicted values based on the patient’s sex, age, and height. The research was confirmed by the local ethics committee at the Institute of Rheumatology in Prague, and each patient signed an informed consent form. All methods were performed in accordance with the relevant guidelines and regulations.
Laboratory measurements
All samples were stored at − 80 °C until use. Serum levels of C-reactive protein (CRP) were determined by an immuno-turbidimetric technique using an Olympus AU 400 biochemical analyzer (Olympus Optical, Tokyo, Japan), and erythrocyte sedimentation rate (ESR) was measured according to the Fahreus and Westergren method. ANAs were detected using indirect immunofluorescence on HEP2 cells, and the autoantibodies of the ENA complex (anti-U1RNP, anti-Ro, anti-La, anti-DNA-topoisomerase I, anti-Jo-1, anti-P protein, anti-Sm, and anti-centromere) were assayed by immunoblot. Plasma levels of Hsp90 were assessed by a high-sensitivity ELISA kit (eBioscience, Vienna, Austria) according to the manufacturer's protocol. The assay recognizes human Hsp90 alpha. The calculated sensitivity is 0.03 ng/mL. The absorbance value was established at 450 nm by an ELISA reader (SUNRISE; Tecan, Grödig, Austria).
Statistical analysis
Basic descriptive statistics (mean, median, standard deviation, interquartile range, skewness, and kurtosis) were computed for all variables, which were subsequently tested for normality using the Kolmogorov–Smirnov and Shapiro–Wilk tests. Differences in interval variables (e.g. Hsp90, age, etc.) were tested using the Mann–Whitney U test, while the chi-square test was used to compare frequency counts of categorical variables (e.g. gender). The bivariate relationships between variables under study were assessed using the Spearman correlation coefficient. Linear regression analysis was used to predict patients' Hsp90 levels by a set of predictors (FEV1, FVC, DLCO, SpO2) while adjusting for a confounder (CRP). Friedman's test was used to analyze repeated longitudinal measurements taken: (a) at baseline, (b) after 1 month, (c) after 6 months, and (d) after 12 months of therapy with cyclophosphamide. Data are presented as median (IQR) unless stated otherwise. Statistical significance was set at p < 0.05. All analyses were conducted using SPSS version 25 (SPSS, Inc., Chicago, IL, USA). Graphs were created using GraphPad Prism 5 (version 5.02; GraphPad Software, La Jolla, CA, USA).
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the Institute of Rheumatology in Prague. All study participants were ≥ 18 years of age, and each of them signed an informed consent form. No individual personal data are included.
Acknowledgements
This work was supported by the Ministry of Health of the Czech Republic 00023728, Grant nos. 16-33542A, 16-33574A, NV18-01-00161A, and SVV 260523. We thank Xiao Fu for language editing.
Abbreviations
- Hsp90
Heat shock protein 90
- SSc
Systemic sclerosis
- lcSSc
Limited cutaneous SSc
- dcSSc
Diffuse cutaneous SSc
- TGF-β
Transforming growth factor β
- TβRI and TβRII
Transforming growth factor β receptors
- IQR
Interquartile range
- ANA
Antinuclear antibodies
- Anti-Scl-70
Anti-DNA-topoisomerase I antibodies
- ACA
Anticentromere antibodies
- ATS
American Thoracic Society
- ESSG
European Scleroderma Study Group
- mRSS
Modified Rodnan skin score
- CRP
C-reactive protein
- ESR
Erythrocyte sedimentation rate
- HRCT
High-resolution computed tomography
- PFT
Pulmonary function tests
- FVC
Forced vital capacity
- FEV1
Forced expiratory volume in one second
- DLCO
Diffusing capacity for carbon monoxide
- SpO2
Blood oxygen saturation level
- GI
Gastrointerstinal ivolvement
- ILD
Interstitial lung disease
- PAH
Pulmonary arterial hypertension
- CI
Cardiac involvement
- RI
Renal involvement
- GC
Low dose glucocorticoids (i.e. ≤ 10 mg/day of prednisone)
- MTX
Methotrexate
- CPA
Cyclophosphamide
- AZA
Azathioprine
- MMF
Mycophenolate mofetil
- RA
Rheumatoid arthritis
- axSpA
Axial spondyloarthritis
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- TLR
Toll-like receptor
- ERK
Extracellular signal-regulated kinases
- IL-6R
Interleukin 6 receptor
- STAT
Signal transducer and activator of transcription
Author contributions
H.S. and M.T. were responsible for the study concept and design. H.S. performed laboratory analysis. M.K., H.S. and M.T. carried out statistical analysis. S.O., M.S., B.H. and K.B. collected patients’ data. M.T. and H.S. were responsible for data interpretation and manuscript preparation. J.V., L.Š., K.P., R.B. and J.H.W.D. revised the manuscript and gave their final approval of the version to be published. All authors read and approved the final manuscript.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Denton CP, Khanna D. Systemic sclerosis. Lancet. 2017;390:1685–1699. doi: 10.1016/S0140-6736(17)30933-9. [DOI] [PubMed] [Google Scholar]
- 2.Distler JHW, Györfi AH, Ramanujam M, Whitfield ML, Königshoff M, Lafyatis R. Shared and distinct mechanisms of fibrosis. Nat. Rev. Rheumatol. 2019;15:705–730. doi: 10.1038/s41584-019-0322-7. [DOI] [PubMed] [Google Scholar]
- 3.Denton CP, Wells AU, Coghlan JG. Major lung complications of systemic sclerosis. Nat. Rev. Rheumatol. 2018;14:511–527. doi: 10.1038/s41584-018-0062-0. [DOI] [PubMed] [Google Scholar]
- 4.Khanna D, Tashkin DP, Denton CP, Renzoni EA, Desai SR, Varga J. Etiology, risk factors, and biomarkers in systemic sclerosis with interstitial lung disease. Am. J. Respir. Crit. Care. Med. 2020;201:650–660. doi: 10.1164/rccm.201903-0563CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perelas A, Silver RM, Arrossi AV, Highland KB. Systemic sclerosis-associated interstitial lung disease. Lancet. Respir. Med. 2020;8:304–320. doi: 10.1016/S2213-2600(19)30480-1. [DOI] [PubMed] [Google Scholar]
- 6.Elhai M, Avouac J, Allanore Y. Circulating lung biomarkers in idiopathic lung fibrosis and interstitial lung diseases associated with connective tissue diseases: Where do we stand? Semin. Arthritis. Rheum. 2020 doi: 10.1016/j.semarthrit.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Distler JH, Feghali-Bostwick C, Soare A, Asano Y, Distler O, Abraham DJ. Review: Frontiers of antifibrotic therapy in systemic sclerosis. Arthritis. Rheumatol. 2017;69:257–267. doi: 10.1002/art.39865. [DOI] [PubMed] [Google Scholar]
- 8.Schlesinger MJ. Heat shock proteins. J. Biol. Chem. 1990;256:12111–12114. [PubMed] [Google Scholar]
- 9.Santoro MG. Heat shock factors and the control of the stress response. Biochem. Pharmacol. 2000;59:55–63. doi: 10.1016/S0006-2952(99)00299-3. [DOI] [PubMed] [Google Scholar]
- 10.Guo J, Chang C, Li W. The role of secreted heat shock protein-90 (Hsp90) in wound healing—How could it shape future therapeutics? Expert Rev. Proteomics. 2017;14:665–675. doi: 10.1080/14789450.2017.1355244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindquist S, Craig EA. The heat-shock proteins. Annu. Rev. Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- 12.Burrows F, Zhang H, Kamal A. Hsp90 activation and cell cycle regulation. Cell Cycle. 2004;3:1530–1536. doi: 10.4161/cc.3.12.1277. [DOI] [PubMed] [Google Scholar]
- 13.Echeverria PC, Picard D. Molecular chaperones, essential partners of steroid hormone receptors for activity and mobility. Biochim. Biophys. Acta. Mol. Cell. Res. 2010;1803:641–649. doi: 10.1016/j.bbamcr.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 14.Pearl LH, Prodromou C. Structure and in vivo function of Hsp90. Curr. Opin. Struct. Biol. 2000;10:46–51. doi: 10.1016/S0959-440X(99)00047-0. [DOI] [PubMed] [Google Scholar]
- 15.McClellan AJ, Xia Y, Deutschbauer AM, Davis RW, Gerstein M, Frydman J. Diverse cellular functions of the Hsp90 molecular chaperone uncovered using systems approaches. Cell. 2007;131:121–135. doi: 10.1016/j.cell.2007.07.036. [DOI] [PubMed] [Google Scholar]
- 16.Zuehlke AD, Moses MA, Neckers L. Heat shock protein 90: Its inhibition and function. Philos. Trans. R. Soc. B. Biol. Sci. 2018;373:20160527. doi: 10.1098/rstb.2016.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsan MF, Gao B. Cytokine function of heat shock proteins. AJP Cell. Physiol. 2004;286:C739–744. doi: 10.1152/ajpcell.00364.2003. [DOI] [PubMed] [Google Scholar]
- 18.Bohonowych JE, Hance MW, Nolan KD, Defee M, Parsons CH, Isaacs JS. Extracellular Hsp90 mediates an NF-κB dependent inflammatory stromal program: Implications for the prostate tumor microenvironment. Prostate. 2014;74:395–407. doi: 10.1002/pros.22761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung SW, et al. Extracellular heat shock protein 90 induces interleukin-8 in vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2009;378:444–449. doi: 10.1016/j.bbrc.2008.11.063. [DOI] [PubMed] [Google Scholar]
- 20.Beyer C, Distler JHW. Tyrosine kinase signaling in fibrotic disorders. Biochim. Biophys. Acta. Mol. Basis. Dis. 2013;1832:897–904. doi: 10.1016/j.bbadis.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Koga F, Xu W, Karpova TS, McNally JG, Baron R, Neckers L. Hsp90 inhibition transiently activates Src kinase and promotes Src-dependent Akt and Erk activation. Proc. Natl. Acad. Sci. 2006;103:11318–11322. doi: 10.1073/pnas.0604705103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skhirtladze C, et al. Src kinases in systemic sclerosis: Central roles in fibroblast activation and in skin fibrosis. Arthriti. Rheum. 2008;58:1475–1484. doi: 10.1002/art.23436. [DOI] [PubMed] [Google Scholar]
- 23.Wrighton KH, Lin X, Feng XH. Critical regulation of TGFbeta signaling by Hsp90. Proc. Natl. Acad. Sci. USA. 2008;105:9244–9249. doi: 10.1073/pnas.0800163105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomcik M, et al. Heat shock protein 90 (Hsp90) inhibition targets canonical TGF-β signalling to prevent fibrosis. Ann. Rheum. Dis. 2014;73:1215–1222. doi: 10.1136/annrheumdis-2012-203095. [DOI] [PubMed] [Google Scholar]
- 25.Gao C, et al. Inhibition of heat shock protein 90 as a novel platform for the treatment of cancer. Curr. Pharm. Des. 2019;25:849–855. doi: 10.2174/1381612825666190503145944. [DOI] [PubMed] [Google Scholar]
- 26.Norton PM, Isenberg DA, Latchman DS. Elevated levels of the 90 kd heat shock protein in a proportion of SLE patients with active disease. J. Autoimmun. 1989;2:187–195. doi: 10.1016/0896-8411(89)90154-6. [DOI] [PubMed] [Google Scholar]
- 27.Bubova, K. et al. Plasma Hsp90 levels in patients with spondyloarthritis and their relation to structural changes: A cross-sectional study. Biomark. Med.10.2217/bmm-2020-0360. [DOI] [PubMed]
- 28.Storkanova H, et al. Increased Hsp90 in muscle tissue and plasma associates with disease activity and skeletal muscle involvement in patients with idiopathic inflammatory myopathies. Ann. Rheum. Dis. 2020;79(supplement 1):410. [Google Scholar]
- 29.Ocaña GJ, et al. Inflammatory stress of pancreatic beta cells drives release of extracellular heat-shock protein 90α. Immunology. 2017;151:198–210. doi: 10.1111/imm.12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tas F, Bilgin E, Erturk K, Duranyildiz D. Clinical significance of circulating serum cellular heat shock protein 90 (HSP90) level in patients with cutaneous malignant melanoma. Asian. Pacific. J. Cancer Prev. 2017;18:599–601. doi: 10.22034/APJCP.2017.18.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fu Y, et al. Plasma heat shock protein 90alpha as a biomarker for the diagnosis of liver cancer: An official, large-scale, and multicenter clinical trial. E. Bio. Medicine. 2017;24:56–63. doi: 10.1016/j.ebiom.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi Y, et al. Plasma levels of heat shock protein 90 alpha associated with lung cancer development and treatment responses. Clin. Cancer. Res. 2014;20:6016–6022. doi: 10.1158/1078-0432.CCR-14-0174. [DOI] [PubMed] [Google Scholar]
- 33.Muangchan C, Pope JE. The significance of interleukin-6 and C-reactive protein in systemic sclerosis: A systematic literature review. Clin. Exp. Rheumatol. 2013;31:122–134. [PubMed] [Google Scholar]
- 34.Sontake V, et al. Hsp90 regulation of fibroblast activation in pulmonary fibrosis. JCI Insight. 2017;2:e91454. doi: 10.1172/jci.insight.91454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koh RY, et al. Inhibition of transforming growth factor-β via the activin receptor-like kinase-5 inhibitor attenuates pulmonary fibrosis. Mol. Med. Rep. 2015;11:3808–3813. doi: 10.3892/mmr.2015.3193. [DOI] [PubMed] [Google Scholar]
- 36.O’Reilly S, Cant R, Ciechomska M, Van Laar JM. Interleukin-6: A new therapeutic target in systemic sclerosis? Clin. Transl. Immunol. 2013;2:e4. doi: 10.1038/cti.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khanna D, et al. Safety and efficacy of subcutaneous tocilizumab in adults with systemic sclerosis (faSScinate): A phase 2, randomised, controlled trial. Lancet. 2016;387:2630–2640. doi: 10.1016/S0140-6736(16)00232-4. [DOI] [PubMed] [Google Scholar]
- 38.Narváez J, Lluch J, Alegre Sancho JJ, Molina-Molina M, Nolla JM, Castellví I. Effectiveness and safety of tocilizumab for the treatment of refractory systemic sclerosis associated interstitial lung disease: A case series. Ann. Rheum. Dis. 2019;78:e123. doi: 10.1136/annrheumdis-2018-214449. [DOI] [PubMed] [Google Scholar]
- 39.O’Reilly S, Ciechomska M, Cant R, Van Laar JM. Interleukin-6 (IL-6) trans signaling drives a STAT3-dependent pathway that leads to hyperactive transforming growth factor-β (TGF-β) signaling promoting SMAD3 activation and fibrosis via gremlin protein. J. Biol. Chem. 2014;289:9952–9960. doi: 10.1074/jbc.M113.545822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sato N, et al. Involvement of heat-shock protein 90 in the interleukin-6-mediated signaling pathway through STAT3. Biochem. Biophys. Res. Commun. 2003;300:847–852. doi: 10.1016/S0006-291X(02)02941-8. [DOI] [PubMed] [Google Scholar]
- 41.Nannini C, West CP, Erwin PJ, Matteson EL. Effects of cyclophosphamide on pulmonary function in patients with scleroderma and interstitial lung disease: A systematic review and meta-analysis of randomized controlled trials and observational prospective cohort studies. Arthritis Res. Ther. 2008;10:R124. doi: 10.1186/ar2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chakraborty A, Boel NM-E, Edkins AL. HSP90 interacts with the fibronectin N-terminal domains and increases matrix formation. Cells. 2020;9:272. doi: 10.3390/cells9020272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Den Hoogen F, et al. 2013 classification criteria for systemic sclerosis: An American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 2013;65:2737–2747. doi: 10.1002/art.38098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silver RM, Miller KS, Kinsella MB, Smith EA, Schabel SI. Evaluation and management of scleroderma lung disease using bronchoalveolar lavage. Am. J. Med. 1990;88:470–476. doi: 10.1016/0002-9343(90)90425-D. [DOI] [PubMed] [Google Scholar]
- 45.Bombardieri S, Medsger TA, Jr, Silman AJ, Valentini G. The assessment of the patient with systemic sclerosis. Introduction. Clin. Exp. Rheumatol. 2003;21:S2–S4. [PubMed] [Google Scholar]
- 46.Clements PJ, et al. Skin thickness score in systemic sclerosis: An assessment of interobserver variability in 3 independent studies. J. Rheumatol. 1993;20:1892–1896. [PubMed] [Google Scholar]
- 47.Valentini G, Silman AJ, Veale D. Assessment of disease activity. Clin. Exp. Rheumatol. 2003;21:S39–41. [PubMed] [Google Scholar]
- 48.Crapo O, et al. Standardization of spirometry, 1994 update. American Thoracic Society. Am. J. Respir. Crit. Care. Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.