Abstract
Objectives
It has been widely reported that maternal diabetes impairs oocyte quality. However, the responsible mechanisms remain to be explored. In the present study, we focused on whether SIRT3‐GSK3β pathway mediates the meiotic defects in oocytes from diabetic mice.
Materials and methods
GSK3β functions in mouse oocyte meiosis were first detected by targeted siRNA knockdown. Spindle assembly and chromosome alignment were visualized by immunostaining and analysed under the confocal microscope. PCR‐based site mutation of specific GSK3β lysine residues was used to confirm which lysine residues function in oocyte meiosis. siRNA knockdown coupled with cRNA overexpression was performed to detect SIRT3‐GSK3β pathway functions in oocyte meiosis. Immunofluorescence was performed to detect ROS levels. T1DM mouse models were induced by a single intraperitoneal injection of streptozotocin.
Results
In the present study, we found that specific depletion of GSK3β disrupts maturational progression and meiotic apparatus in mouse oocytes. By constructing site‐specific mutants, we further revealed that acetylation state of lysine (K) 15 on GSK3β is essential for spindle assembly and chromosome alignment during oocyte meiosis. Moreover, non–acetylation‐mimetic mutant GSK3β‐K15R is capable of partly preventing the spindle/chromosome anomalies in oocytes with SIRT3 knockdown. A significant reduction in SIRT3 protein was detected in oocytes from diabetic mice. Of note, forced expression of GSK3β‐K15R ameliorates maternal diabetes‐associated meiotic defects in mouse oocytes, with no evident effects on oxidative stress.
Conclusion
Our data identify GSK3β as a cytoskeletal regulator that is required for the assembly of meiotic apparatus, and discover a beneficial effect of SIRT3‐dependent GSK3β deacetylation on oocyte quality from diabetic mice.
Keywords: diabetes, meiosis, oocyte, oxidative stress, Sirtuin
Diabetic oocytes display a reduction in SIRT3 protein and show higher frequency of spindle/chromosome defects and excessive ROS. Deacetylation‐mimetic mutant GSK3β‐K15R alleviates the meiotic defects in oocytes from diabetic mice, while it was unable to decrease the ROS level in diabetic oocytes.
1. INTRODUCTION
Type 1 diabetes mellitus is a global health issue, and its pooled prevalence has already reached to 2.69‰ and increased at the rate of 1.8% annually. 1 , 2 Animal models show that diabetes induces abnormal redistribution of endoplasmic reticulum, 3 mitochondrial dysfunction, meiotic apparatus disorganization, 4 , 5 reduced thickness of zona pellucida 6 and epigenetic changes in oocytes. 6 , 7 , 8 , 9 In addition, diabetes condition leads to defective energy metabolism in oocyte 5 , 10 , 11 and preimplantation embryo. 12 Within cumulus cells, inappropriate apoptosis and mitochondrial dysfunction are also observed in diabetes mouse. 13 These factors contribute to the impaired oocyte quality, consequently influencing embryo development and pregnancy outcomes. Although the defective phenotypes have been identified in oocytes from diabetic animals, the underlying molecular pathways have yet to be determined.
Sirtuins (SIRT1‐7), a family of NAD+‐dependent deacetylases, are involved in multiple biological processes including energy metabolism, oxidative stress, cellular ageing and longevity. 14 , 15 , 16 , 17 , 18 , 19 Sirtuins are increasingly identified as key mediators in the control of gametogenesis, fertilization and embryo development since a sterile phenotype was observed in SIR2α (silent information regulator 2) null mice. 15 , 20 Among Sirtuin family, SIRT3 is located in mitochondrial matrix 21 and maintains metabolism homeostasis under basal or stress condition through deacetylating various enzymes, such as AcsCS2, LCAD, OTC and HMGCS2. 22 , 23 In addition, SIRT3 has been shown to be able to control the antioxidant system via regulating superoxide dismutase 2 (SOD2) during mammalian oocyte maturation. 24 , 25 , 26 , 27 Recently, Nagalingam et al 28 revealed that SIRT3 could deacetylate glycogen synthase kinase‐3 beta (GSK3β), enhancing its enzymatic activity. GSK3β is a highly conserved serine/threonine protein kinase and linked to several cellular events and some prevalent diseases. 29 , 30 Genetic mutation study demonstrated that loss of function of GSK3β in Drosophila neuroblasts causes mitotic apparatus anomalies and delayed metaphase‐to‐anaphase transition. 31 Baluch et al 32 found that GSK3β is accumulated at both spindle poles and kinetochore region in metaphase oocytes. In addition, inhibition of GSK3β leads to massive apoptosis of meiotic prophase I oocyte via premature TAp63 expression. 33 However, to date, little is known about the function of GSK3β and its interaction with SIRT3 during oocyte maturation.
In the present study, by knockdown and overexpression experiments, we uncovered the role of GSK3β in oocyte meiosis and showed that SIRT3‐GSK3β deacetylation pathway functions in the defective phenotypes of oocytes from diabetic mice.
2. MATERIALS AND METHODS
All chemicals and culture media were purchased from Sigma unless stated otherwise.
2.1. Mice and ethics statement
Female ICR mice (3‐4 weeks old) were sacrificed in this study. Mouse models of T1DM were induced by a single intraperitoneal injection of streptozotocin (S0130; Sigma‐Aldrich) at a dose of 190 mg/kg after fasting 12 hours. Four days later, blood glucose was measured via a glucometer. Mice with glucose level higher than 300 mg/dL (16.7 mmol/L) were selected for further analysis. Controls were injected with an equivalent volume of sodium‐citrate solution to account for the solvent (S4641; Sigma‐Aldrich). All procedures and animal care followed guidelines stipulated by the Care and Use Committee of Nanjing Medical University.
2.2. Antibodies
The following antibodies were used in this study: rabbit polyclonal anti‐SIRT3 (Cat#:ab8667; Abcam); rabbit monoclonal anti‐GSK3β (Cat#:ab32391; Abcam); mouse monoclonal FITC‐conjugated anti‐α‐tubulin antibody (Cat#: F2168; Sigma); rabbit monoclonal anti‐Myc antibody (Cat#: 2278; Cell Signaling Technology); mouse monoclonal HRP‐conjugated anti‐α‐tubulin antibody (Cat#: HRP‐66031; Proteintech).
2.3. Oocyte collection and culture
Female mice were superovulated by intraperitoneal injection with 5 IU pregnant mares' serum gonadotrophin (PMSG) ~48 hours prior to oocyte collection. Cumulus‐oocytes complexes (COCs) were dissociated by repeatedly puncturing of the ovary surface using syringe needle and collected using mouth‐controlled micropipette. Cumulus cells surrounding oocyte were separated by pipetting the complex up and down using micropipette with inner diameter of ~100 μm. For in vitro maturation, GV oocytes were cultured in paraffin oil covered M16 medium in incubator (37°C, 5% CO2, 5% O2, 90% N2).
2.4. Immunofluorescence
Visualization of spindle and chromosomes was conducted as described previously. 34 In brief, oocytes were fixed in 4% fresh‐prepared paraformaldehyde for 30 minutes and permeabilized in 0.5% Triton X‐100 for 20 minutes at room temperature. Following incubation in blocking buffer (PBS, 0.5% Triton X‐100, 1% BSA) for 1 hour, oocytes were labelled with FITC‐conjugated anti‐tubulin antibody overnight at 4°C. After three washes, oocytes were stained with propidium iodide (PI) for 20 minutes to visualize chromosome. Finally, oocytes were transferred to microscope slides and examined under a laser scanning confocal microscope in time (LSM 700, Zeiss).
2.5. Measurement of intracellular ROS
Intracellular ROS level was detected by CM‐H2DCFDA probe (Cat#: C6827; Life Technologies). Oocytes were incubated in HEPES buffer containing 5 µmol/L CM‐H2DCFDA for 30 minutes. After three washes with fresh HEPES buffer, 10 oocytes were loaded on Nunc™ Glass Bottom Dishes (Cat#:15082; Thermo Scientific) and covered with mineral oil. The images are acquired using a laser scanning confocal microscope (LSM 700, Zeiss).
2.6. Plasmid construction and mRNA synthesis
Total RNA from oocyte sample was extracted and reverse‐transcribed into cDNA as we described previously. 35 PCR products were purified and digested with FseI and AscI (NEB Inc), and then inserted into the pCS2 + plasmid vector encoding N‐terminal Myc‐tags. GSK3β mutant plasmids (K15Q, K15Q, K36Q and K36R) were generated through PCR using Phusion High‐Fidelity DNA Polymerase (Cat#: M0530S, NEB). For mRNA synthesis, the plasmids were linearized by Not I and capped RNAs were produced using SP6 mMESSAGE mMACHINE (Ambion) according to the manufacturer's instruction. Synthesized RNA was purified by Arcturus PicoPure RNA Isolation Kit (Applied Biosystems) and stored at −80°C. The related primers can be found in Table S1.
2.7. Knockdown or overexpression experiments
Microinjection experiments were conducted using a Narishige microinjector for SIRT3 knockdown or overexpression of GSK3β mutants. siRNA duplexes against SIRT3 and GSK3β were purchased from Gene Pharma and diluted to 20 µmol/L stock solutions. Five picolitre solution of siRNA or capped RNA was injected into the cytoplasm of oocytes. Control oocytes were injected with the same amount of negative control or PBS in parallel. After injections, oocytes were cultured in M16 medium supplemented with 2.5 µmol/L milrinone for 20‐22 hours to make oocytes arrest at GV stage, facilitating the degradation or translation of targeted RNA, and then moved to milrinone‐free medium for further analysis. The related siRNA sequences are listed in Table S1.
2.8. Western blot
The samples containing sufficient number of oocytes (at least 100) are lysed in Laemmli buffer and denatured by boiling for 5 minutes before electrophoresis. The denatured proteins were then separated by 12% precast SDS‐PAGE gel and electrically transferred to polyvinylidene fluoride (PVDF) membranes. After blocking with 5% skimmed milk for 1 hour, membranes were incubated with primary antibodies overnight at 4°C (anti‐SIRT3 pAb, 1:500; anti‐GSK3β mAb, 1:1000; anti‐Myc‐Tag mAb, 1:1000), followed by three washes with PBST (PBS containing 0.1% Tween 20) and incubation with HRP‐conjugated secondary antibody for 1 hour at room temperature. The protein bands were visualized by an ECL Plus Western Blotting Detection System (GE Healthcare). For loading control, membranes were rinsed in stripping buffer and re‐probed with anti‐α‐tubulin antibody (1:2000).
2.9. Quantitative real‐time PCR
Quantitative real‐time PCR (qRT‐PCR) was performed to verify the knockdown efficiency of siRNA. In brief, total RNA was isolated from control or knockdown oocytes using an Arcturus PicoPure RNA Isolation Kit (Applied Biosystems), and reverse‐transcribed with PrimeScript RT Master Mix (TaKaRa). The cDNA was quantified by SYBR Green Mix (Vazyme) using StepOnePlus™ Real‐Time PCR System (Applied Biosystems). GAPDH was used as an internal control. Primer sequences are listed in Table S1.
2.10. Statistical analysis
GraphPad Prism software was used to analyse data. Data are presented as mean ± SD, unless otherwise indicated. Differences were analysed by Student's t test. P values <.05 were considered to be significant. At least three replicates were conducted for each treatment.
3. RESULTS
3.1. GSK3β is required for mouse oocyte meiosis
Accumulation of GSK3β at both spindle poles and kinetochore region has been reported in mouse oocytes. 32 To explore the role of GSK3β during oocyte maturation, we microinjected GSK3β siRNA into fully grown mouse oocytes. This led to a significant knockdown (KD) of both GSK3β protein and mRNA (Figure 1A,B). Although oocytes from control and GSK3β‐KD group had comparable germinal vesicle breakdown (GVBD) rate (77.7 ± 5.7% vs 80.3 ± 5.6% control; P > .05; Figure 1D), markedly reduced polar body (Pb1) extrusion rate was detected in GSK3β‐KD oocytes (39.0 ± 4.5% vs 78.7 ± 4.0% control; P < .05; Figure 1C,E). Spindle assembly is important for correct chromosome segregation and asymmetric division in oocytes. 36 , 37 Given that most oocytes fail to complete meiosis I and extrude Pb1 in the GSK3β‐KD group, we speculated that the assembly of meiotic apparatus is disturbed. To this end, oocytes were immunolabelled with anti‐tubulin antibody to visualize the spindle and co‐stained with propidium iodide for chromosomes. As shown in Figure 1F,G, the percentage of oocytes exhibiting disorganized spindle with randomly scattered chromosomes in the GSK3β‐KD group was considerably higher than control oocytes showing chromosomes tightly align at the equator plane of barrel‐shaped spindle (45.4 ± 2.6% vs 12.3 ± 2.2% control; P < .05). Oxidative stress has been suggested to induce microtubule instability. 38 Hence, we decided to assess reactive oxygen species (ROS) level in GSK3β‐KD oocytes using CM‐H2DCFDA fluorescence dye. Nevertheless, as shown in Figure 1H,I, both control and GSK3β‐KD oocytes displayed negligible fluorescence signal (9.40 ± 3.34 vs 9.35 ± 3.71 control; P > .05), indicating that GSK3β depletion has no effects on redox homeostasis in mouse oocytes. Altogether, the results suggest that proper spindle assembly and chromosome alignment in oocytes requires GSK3β.
FIGURE 1.
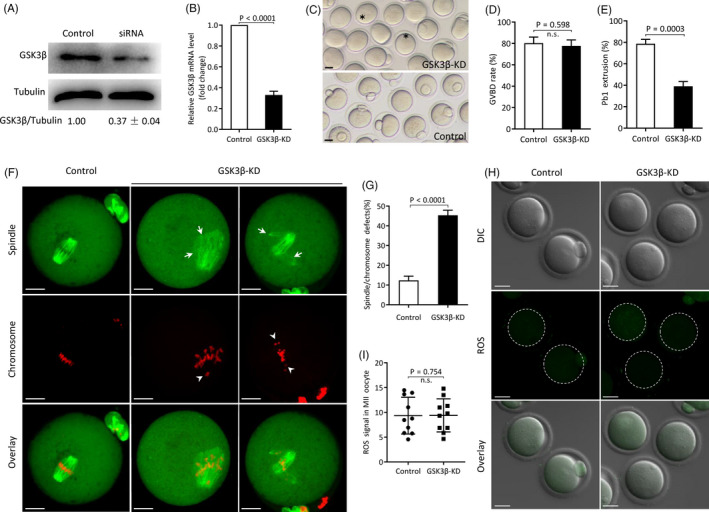
Effects of GSK3β knockdown on oocyte maturation. A, B, Knockdown efficiency of GSK3β‐siRNA was verified by Western blot and qRT‐PCR, respectively. Tubulin or GAPDH served as an internal control. Western blot experiments were repeated at least three times, with a representative gel image shown. C, Phase‐contrast images of control and GSK3β‐KD oocytes. Asterisks indicate the oocytes that fail to extrude polar body, Scale bars: 30 μm. D, E, Rate of GVBD and Pb1 extrusion in control and GSK3β‐KD oocytes. F, Representative confocal images of control and GSK3β‐KD oocytes stained with α‐tubulin antibody (green) and propidium iodide (red) to visualize spindle and chromosome, respectively. Oocyte with typical bipolar spindle and well‐aligned chromosome is regarded as normal. Oocytes showing spindle disorganization (arrows) and chromosome misalignment (arrowheads) are easily observed in GSK3β‐KD oocytes. Scale bars: 20 μm. G, Percentage of oocytes with abnormal spindle/chromosomes in control and GSK3β‐KD group. Data are expressed as mean ± SD from three independent experiments in which at least 100 oocytes were examined. H, ROS signal was detected by CM‐H2DCFDA fluorescence dye and captured using laser confocal microscope. Representative images of ROS signal (green) in control and GSK3β‐KD oocytes. Scale bars: 30 μm. I, Quantification of ROS fluorescence intensity (n = 10 for each group)
3.2. Acetylation‐mimetic mutant GSK3β‐K15Q disrupts meiotic apparatus in mouse oocytes
Recent work indicated that GSK3β activity is regulated by reversible acetylation at specific lysine (K) reside. 28 To investigate whether GSK3β acetylation, and if so, which lysine residues function in oocyte meiosis, site‐specific mutants targeting K15 and K36 were constructed and microinjected into fully grown oocytes for phenotypic analysis. Substitution of lysine (K) with a glutamine (Q) mimics an acetylated amino acid state, while substitution with an arginine (R) mimics deacetylation. 39 As shown in Figure 2A, GSK3β mutants (K15Q, K15R, K36Q and K36R) were ectopically expressed in oocytes, confirmed by Western blotting. For brevity, these oocytes are called ‘K15Q/R oocytes’ and ‘K36Q/R oocytes’ here. We found that control and all mutant oocytes resume meiosis normally after 3 hours culture (Figure 2B); however, more than half of K15Q oocytes fail to exclude Pb1 following 14 hours culture (44.7 ± 2.1% vs 77.5 ± 1.5% control; P < .05; Figure 2C). In line with this, K15Q oocytes displayed higher frequency of spindle defects and chromosome misalignment than controls (34.9 ± 4.0% vs 13.1 ± 1.5% control; P < .05; Figure 2D,E). In contrast, overexpression of all mutants had little effects on ROS generation in oocytes (Figure 2F,G). Collectively, these data strongly suggest that acetylation status of K15 is important for GSK3β function in oocyte meiosis.
FIGURE 2.
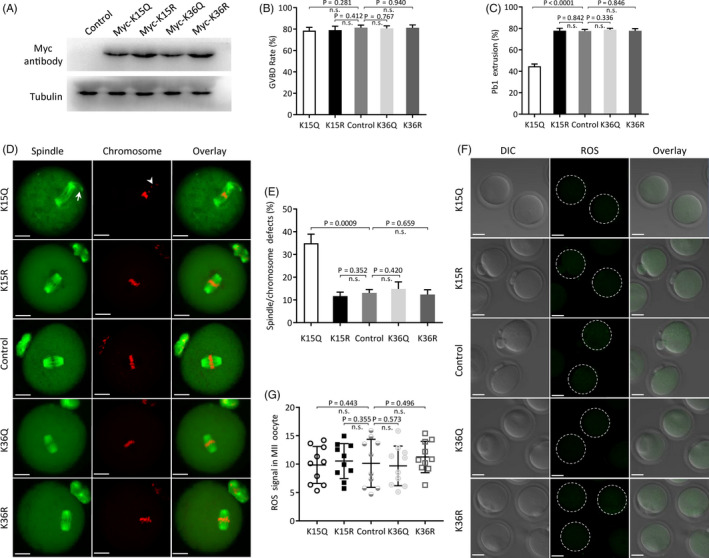
Effects of GSK3β acetylation on oocyte maturation. Acetylation‐mimetic mutant GSK3β‐K15Q/K36Q or deacetylation‐mimetic mutant GSK3β‐K15R/K36R was microinjected into fully grown oocytes to evaluate meiotic apparatus and redox homeostasis. A, Western blotting shows that four mutants of GSK3β were efficiently overexpressed, probing with anti‐Myc antibody. B, C, Quantitative analysis of GVBD (control: 81.3 ± 2.3%, K15Q: 78.6 ± 3.1%, K15R: 79.0 ± 3.7%, K36Q: 80.7 ± 2.3%, K36R: 81.2 ± 2.7%)and Pb1 extrusion rate (control: 77.5 ± 1.5%, K15Q: 44.7 ± 2.1%, K15R: 77.8 ± 2.3%, K36Q: 78.8 ± 1.3%, K36R: 77.8 ± 2.0%) in control and GSK3β mutant oocytes. D, Representative images of spindle/chromosomes in control and GSK3β mutant oocytes. Arrows indicate spindle defects, and arrowheads indicate chromosome misalignment. Scale bars: 20 μm. E, Percentage of oocytes with abnormal spindle/chromosomes in control and GSK3β mutant groups (control: 13.1 ± 1.5%, K15Q: 34.9 ± 4.0%, K15R: 11.7 ± 1.8%, K36Q: 14.9 ± 3.1%, K36R: 12.4 ± 2.1%). Data are expressed as mean ± SD from three independent experiments in which at least 100 oocytes were examined. F, Representative images of ROS signal (green) in control and GSK3β mutant oocytes. Scale bars: 30 μm. G, Quantification of fluorescence intensity (control: 10.14 ± 4.24, K15Q: 9.85 ± 3.25, K15R: 10.53 ± 3.10, K36Q: 9.69 ± 3.49, K36R: 11.25 ± 2.76; n = 10 for each group)
3.3. SIRT3 promotes the assembly of meiotic apparatus in mouse oocytes through deacetylation of GSK3β‐K15
SIRT3 was shown to be able to deacetylate GSK3β and thereupon upregulate its catalytic activity. 28 Previously, we observed the meiotic defects and oxidative stress in oocytes depleted of SIRT3. 24 Given the disorganized spindle/chromosomes in GSK3β‐K15Q oocytes, we speculated that SIRT3‐GSK3β deacetylation pathway may be involved in oocyte meiosis. To do this, we conducted a functional rescue experiment. Specifically designed siRNAs were injected into fully grown oocytes to knock down SIRT3 (SIRT3‐KD; Figure 3A,B). Notably, as shown in Figure 3C,D, we found that the spindle defects and chromosome misalignment in SIRT3‐KD oocytes were partially prevented by the overexpression of non‐acetylated GSK3β‐K15R mutant (38.7 ± 4.1% SIRT3‐KD vs 18.4 ± 3.5% SIRT3‐KD + K15R; P < .05). In contrast, GSK3β‐K15R did not lower the elevated ROS level in SIRT3‐KD oocytes (22.22 ± 8.23 SIRT3‐KD vs 24.03 ± 9.82 SIRT3‐KD + K15R; P > .05; Figure 3E,F). Together, these results indicate that K15 is an important deacetylation site on GSK3β mediating the effects of SIRT3 on meiotic apparatus in oocytes.
FIGURE 3.
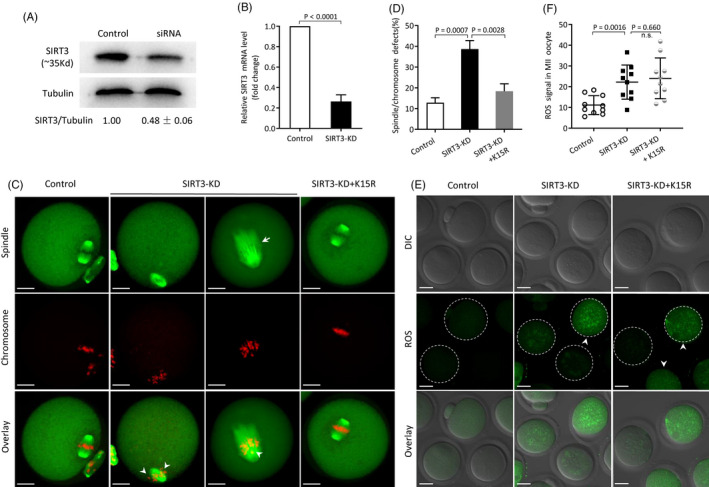
GSK3β‐K15R partly rescues the meiotic defects in SIRT3‐KD oocytes. A, B, Efficiency of SIRT3 knockdown was verified by Western blot and qRT‐PCR, respectively. Western blot experiments were repeated at least three times, with a representative gel image shown. C, Representative images of spindle/chromosomes in control, SIRT3‐KD and SIRT3‐KD + K15R oocytes, respectively. Arrows indicate spindle defects, and arrowheads indicate chromosome misalignment. Scale bars: 20 μm. D, Percentage of oocyte with abnormal spindle/chromosomes in control, SIRT3‐KD and SIRT3‐KD + K15R group. Data are expressed as mean ± SD from three independent experiments in which at least 100 oocytes were examined. E, Representative images of ROS signal (green) in control, SIRT3‐KD and SIRT3‐KD + K15R oocytes. Scale bars: 30 μm. Arrowhead indicates the significantly elevated fluorescence intensity. F, Quantification of ROS fluorescence intensity (n = 10 for each group)
3.4. Deacetylation‐mimetic mutant GSK3β‐K15R alleviates the meiotic defects in oocytes from diabetic mice
Numerous reports have shown that oocytes from diabetic mice have metabolic dysfunction and meiotic defects, 40 but the responsible mechanisms remain to be explored. Consistent with previous data, we found that diabetic oocytes display higher frequency of spindle/chromosome defects (38.0 ± 2.7% vs 13.2 ± 2.8% control; P < .05; Figure 4C,D) and excessive ROS (23.59 ± 6.87 vs 12.34 ± 5.20 control; P < .05; Figure 4E,F) in relative to control cells. On the other hand, we detected a reduction in SIRT3 protein in oocytes from diabetic mice(Figure 4A), and importantly, forced expression of exogenous SIRT3 protein (Figure 4B) significantly ameliorates the meiotic defects (38.0 ± 2.7% diabetic vs 18.2 ± 1.6% diabetic + SIRT3; P < .05; Figure 4C,D) and oxidative stress observed (23.59 ± 6.87 diabetic vs 15.01 ± 5.18 diabetic + SIRT3; Figure 4E,F) in diabetic oocytes. Next, we asked whether GSK3β mutant could partly reduce the incidence of meiotic defects in diabetic oocytes. For this purpose, non–acetylation‐mimetic mutant of GSK3β (GSK3β‐K15R) was injected into fully grown oocytes from diabetic mice. Following in vitro maturation, the relevant phenotypes were evaluated. As presented in Figure 4C,D, confocal microscopy revealed that GSK3β‐K15R markedly decreases the percentage of spindle/chromosome defects in diabetic oocytes (38.0 ± 2.7% diabetic vs 19.6 ± 3.1% diabetic + K15R; P < .05). In line with the data mentioned above, this constitutively non‐acetylated form of GSK3β was unable to decrease the ROS level in diabetic oocytes (23.59 ± 6.87 diabetic vs 24.33 ± 5.50 diabetic + K15R; P > .05 Figure 4E,F). Altogether, these results suggest that SIRT3‐modulated GSK3β‐K15 deacetylation is directly linked to meiotic defects in oocytes from diabetic mice.
FIGURE 4.
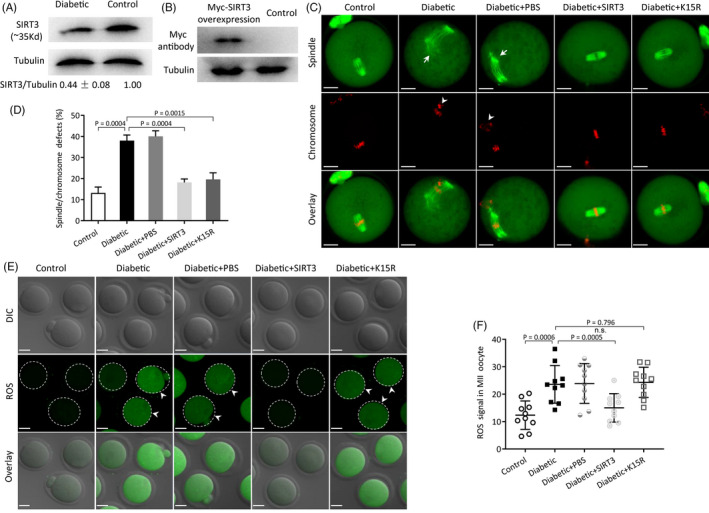
GSK3β‐K15R alleviates meiotic defects in oocytes from diabetic mice. A, Western blot analysis showed the reduced SIRT3 expression in oocytes from diabetic mice compared with controls. Western blot experiments were repeated at least three times, with a representative gel image shown. B, Western blot analysis showed that exogenous SIRT3 protein was efficiently overexpressed. C, Representative images of spindle/chromosomes from control, diabetic, diabetic + PBS, diabetic + SIRT3 and diabetic + K15R oocytes, respectively. Scale bars: 20 μm. D, Percentage of oocyte with abnormal spindle/chromosomes in control, diabetic, diabetic + PBS, diabetic + SIRT3 and diabetic + K15R group. Data are expressed as mean ± SD from three independent experiments in which at least 100 oocytes were examined. E, Representative images of ROS signal (green) in control, diabetic, diabetic + PBS, diabetic + SIRT3 and diabetic + K15R oocytes, respectively. Scale bars: 30 μm. Arrowhead indicates the significantly elevated fluorescence intensity. F, Quantification of ROS fluorescence intensity (n = 10 for each group)
4. DISCUSSION
GSK3 was linked to glycogen metabolism during insulin signalling in past decades since it was first identified in 1980. 29 , 41 , 42 Recently, increasing evidence demonstrated that GSK3β acts as a signal transduction hub participating in many cellular events, such as apoptosis, 43 embryonic development, 44 tumorigenesis 45 and microtubule dynamics. 46 Using ATP‐competitive inhibitors, GSK3β has been implicated in spindle assembly and chromosome segregation during mitosis. 31 , 47 , 48 In the present study, we utilized the specifically designed siRNAs to investigate the role of GSK3β in oocyte meiosis. Proper formation of meiotic structure is crucial for maintaining oocyte quality. This study identified GSK3β as a cytoskeletal regulator that is required for this process. Substantial reports have suggested that GSK3β activity is regulated by various mechanisms, such as intracellular localization, binding protein and post‐translational modifications including phosphorylation, ADP‐ribosylation and ubiquitination. 29 , 49 , 50 Most recently, Sundaresan et al 28 discovered that GSK3β activity is closely associated with its acetylation status controlled by SIRT3. Here, we found that, in normal mouse oocytes, only GSK3β‐K15Q mutant disrupts the maturational progression and assembly of meiotic apparatus (Figure 2), whereas GSK3β‐K15R is able to suppress the abnormal spindle/chromosomes in oocytes depleted of SIRT3 (Figure 3). Our findings support a concept that SIRT3‐controlled GSK3β‐K15 deacetylation plays an important role in the formation of meiotic structure. We cannot rule out that SIRT3 may act on other targets in its function during oocyte maturation. In addition, because of the limited materials and technical reason, we are currently incapable of directly dissecting the relationship between GSK3β acetylation and SIRT3 activity in mouse oocytes. Additional experiments will be required to uncover these details.
Maternal diabetes has been demonstrated to adversely affect preimplantation embryo development and pregnancy outcomes. Emerging evidence has implicated that these effects are associated with compromised oocyte competence. 51 Specifically, an increased frequency of spindle disorganization and chromosome congress failure was detected in oocytes from diabetic mice. 4 The molecular mechanism by which maternal diabetes affects meiotic structure in mammalian oocytes has yet to be investigated. Here, we noted that expression of the non–acetylation‐mimetic mutant GSK3β‐K15R effectively lowered the frequency of meiotic defects in diabetic oocytes (Figure 4), which may open a new area for future application of GSK3β deacetylation agonists to treat diabetic patient with reproduction issues. Besides, diabetic oocytes also experience abnormal cellular metabolism and mitochondrial dysfunction. 4 , 52 Formation of ROS is a by‐product of oxidative phosphorylation in the mitochondria. It has been widely reported that ROS level was markedly increased in oocytes from diabetic mice when compared to those from control mice. 51 We have previously revealed a protective mechanism of SIRT3 against oxidative stress in diabetic oocytes via deacetylating SOD at K68. 26 Interestingly, in the present study, we noticed that ectopic expression of GSK3β‐K15R has little effects on the ROS overproduction in diabetic oocytes (Figure 4). We speculated that SIRT3 plays its protective role through SIRT3‐GSK3β deacetylation pathway and antioxidant system, respectively, in diabetes mice, and ROS appears not be a mediator in this deacetylation pathway. Further studies are imperative to screen candidates that serve as downstream targets of GSK‐3β in SIRT3‐GSK3β deacetylation pathway. Based on these observations, we propose a model where maternal diabetes induces the loss of SIRT3 in oocytes, which, in turn, increases the acetylation of GSK3β‐K15 and impairs its enzymatic activity, consequently resulting in the deficient meiotic apparatus during oocyte maturation. Given the involvements of SIRT3‐GSK3β pathway in diabetic oocytes, prevention of these related deficits transmission may provide therapeutic opportunities for the treatment of reproductive complications and birth defects of diabetic patient.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interests.
AUTHOR CONTRIBUTIONS
QW conceived and designed the experiments. YX, YJ, JG, ZH, CL, DW, SZ and LH performed the research and analysed the data. QW and YX interpreted the data and wrote the paper. All authors have read and approved the final manuscript.
Supporting information
Table S1
ACKNOWLEDGEMENTS
This work was supported by the National Key Scientific Research Projects (2018YFC1004000 to QW), National Natural Science Foundation of China (NO. 81925014 and 31771657 to QW) and Science Foundation for Distinguished Young Scholars of Jiangsu Province (BK20180035 to QW).
Xin Y, Jin Y, Ge J, et al. Involvement of SIRT3‐GSK3β deacetylation pathway in the effects of maternal diabetes on oocyte meiosis. Cell Prolif. 2021;54:e12940 10.1111/cpr.12940
Yongan Xin, Yifei Jin and Juan Ge contributed equally to this work.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Logel SN, Bekx MT, Rehm JL. Potential association between type 1 diabetes mellitus and gender dysphoria. Pediatr Diabetes. 2019;21:266‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mayer‐Davis EJ, Lawrence JM, Dabelea D, et al. Incidence trends of type 1 and type 2 diabetes among youths, 2002–2012. N Engl J Med. 2017;376(15):1419‐1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang CH, Qian W‐P, Qi S‐T, et al. Maternal diabetes causes abnormal dynamic changes of endoplasmic reticulum during mouse oocyte maturation and early embryo development. Reprod Biol Endocrinol. 2013;11:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang Q, Ratchford AM, Chi MM‐Y, et al. Maternal diabetes causes mitochondrial dysfunction and meiotic defects in murine oocytes. Mol Endocrinol. 2009;23(10):1603‐1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jiang G, Zhang G, An T, et al. Effect of type I diabetes on the proteome of mouse oocytes. Cell Physiol Biochem. 2016;39(6):2320‐2330. [DOI] [PubMed] [Google Scholar]
- 6. Li L, Jing Y, Dong M‐Z, et al. Type 1 diabetes affects zona pellucida and genome methylation in oocytes and granulosa cells. Mol Cell Endocrinol. 2019;500:110627. [DOI] [PubMed] [Google Scholar]
- 7. Ge ZJ, Liang X‐W, Guo L, et al. Maternal diabetes causes alterations of DNA methylation statuses of some imprinted genes in murine oocytes. Biol Reprod. 2013;88(5):117. [DOI] [PubMed] [Google Scholar]
- 8. Ge ZJ, Zhang C‐L, Schatten H, Sun Q‐Y. Maternal diabetes mellitus and the origin of non‐communicable diseases in offspring: the role of epigenetics. Biol Reprod. 2014;90(6):139. [DOI] [PubMed] [Google Scholar]
- 9. Shi Z, Zhao C, Long W, Ding H, Shen R. Microarray expression profile analysis of long non‐coding RNAs in umbilical cord plasma reveals their potential role in gestational diabetes‐induced macrosomia. Cell Physiol Biochem. 2015;36(2):542‐554. [DOI] [PubMed] [Google Scholar]
- 10. Colton SA, Humpherson PG, Leese HJ, Downs SM. Physiological changes in oocyte‐cumulus cell complexes from diabetic mice that potentially influence meiotic regulation. Biol Reprod. 2003;69(3):761‐770. [DOI] [PubMed] [Google Scholar]
- 11. Ratchford AM, Chang AS, Chi MM‐Y, Sheridan R, Moley KH. Maternal diabetes adversely affects AMP‐activated protein kinase activity and cellular metabolism in murine oocytes. Am J Physiol Endocrinol Metab. 2007;293(5):E1198‐E1206. [DOI] [PubMed] [Google Scholar]
- 12. Moley KH, Chi MM, Mueckler MM. Maternal hyperglycemia alters glucose transport and utilization in mouse preimplantation embryos. Am J Physiol. 1998;275(1):E38‐E47. [DOI] [PubMed] [Google Scholar]
- 13. Wang Q, Frolova AI, Purcell S, et al. Mitochondrial dysfunction and apoptosis in cumulus cells of type I diabetic mice. PLoS One. 2010;5(12):e15901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guarente L, Franklin H. Epstein Lecture: Sirtuins, aging, and medicine. N Engl J Med. 2011;364(23):2235‐2244. [DOI] [PubMed] [Google Scholar]
- 15. Tatone C, Di Emidio G, Barbonetti A, et al. Sirtuins in gamete biology and reproductive physiology: emerging roles and therapeutic potential in female and male infertility. Hum Reprod Update. 2018;24(3):267‐289. [DOI] [PubMed] [Google Scholar]
- 16. Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J. 2007;404(1):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haigis MC, Guarente LP. Mammalian sirtuins–emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20(21):2913‐2921. [DOI] [PubMed] [Google Scholar]
- 18. Schumacker PT. A tumor suppressor SIRTainty. Cancer Cell. 2010;17(1):5‐6. [DOI] [PubMed] [Google Scholar]
- 19. Guan KL, Xiong Y. Regulation of intermediary metabolism by protein acetylation. Trends Biochem Sci. 2011;36(2):108‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McBurney MW, Yang X, Jardine K, et al. The mammalian SIR2alpha protein has a role in embryogenesis and gametogenesis. Mol Cell Biol. 2003;23(1):38‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Onyango P, Celic I, McCaffery JM, Boeke JD, Feinberg AP. SIRT3, a human SIR2 homologue, is an NAD‐dependent deacetylase localized to mitochondria. Proc Natl Acad Sci USA. 2002;99(21):13653‐13658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Giralt A, Villarroya F. SIRT3, a pivotal actor in mitochondrial functions: metabolism, cell death and aging. Biochem J. 2012;444(1):1‐10. [DOI] [PubMed] [Google Scholar]
- 23. Finley LW, Haigis MC. Metabolic regulation by SIRT3: implications for tumorigenesis. Trends Mol Med. 2012;18(9):516‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang L, Han L, Ma R, et al. Sirt3 prevents maternal obesity‐associated oxidative stress and meiotic defects in mouse oocytes. Cell Cycle. 2015;14(18):2959‐2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Han L, Wang H, Li L, Li X, Ge J, Reiter RJ, Wang Q. Melatonin protects against maternal obesity‐associated oxidative stress and meiotic defects in oocytes via the SIRT3‐SOD2‐dependent pathway. J Pineal Res. 2017;63(3);e12431. [DOI] [PubMed] [Google Scholar]
- 26. Liu X, Zhang L, Wang P, et al. Sirt3‐dependent deacetylation of SOD2 plays a protective role against oxidative stress in oocytes from diabetic mice. Cell Cycle. 2017;16(13):1302‐1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kawamura Y, Uchijima Y, Horike N, et al. Sirt3 protects in vitro‐fertilized mouse preimplantation embryos against oxidative stress‐induced p53‐mediated developmental arrest. J Clin Invest. 2010;120(8):2817‐2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sundaresan NR, Bindu S, Pillai VB, et al. SIRT3 blocks aging‐associated tissue fibrosis in mice by deacetylating and activating glycogen synthase kinase 3beta. Mol Cell Biol. 2015;36(5):678‐692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase‐3. Trends Biochem Sci. 2004;29(2):95‐102. [DOI] [PubMed] [Google Scholar]
- 30. Rayasam GV, Tulasi VK, Sodhi R, Davis JA, Ray A. Glycogen synthase kinase 3: more than a namesake. Br J Pharmacol. 2009;156(6):885‐898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wojcik EJ. A mitotic role for GSK‐3beta kinase in Drosophila. Cell Cycle. 2008;7(23):3699‐3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Baluch DP, Capco DG. GSK3 beta mediates acentromeric spindle stabilization by activated PKC zeta. Dev Biol. 2008;317(1):46‐58. [DOI] [PubMed] [Google Scholar]
- 33. Wen J, Yan H, He M, et al. GSK‐3beta protects fetal oocytes from premature death via modulating TAp63 expression in mice. BMC Biol. 2019;17(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang L, Hou X, Ma R, Moley K, Schedi T, Wang Q. Sirt2 functions in spindle organization and chromosome alignment in mouse oocyte meiosis. FASEB J. 2014;28(3):1435‐1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Qiu D, Hou X, Han L, Li X, Ge J, Wang Q. Sirt2‐BubR1 acetylation pathway mediates the effects of advanced maternal age on oocyte quality. Aging Cell. 2018;17(1):e12698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bennabi I, Terret ME, Verlhac MH. Meiotic spindle assembly and chromosome segregation in oocytes. J Cell Biol. 2016;215(5):611‐619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dumont J, Desai A. Acentrosomal spindle assembly and chromosome segregation during oocyte meiosis. Trends Cell Biol. 2012;22(5):241‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Harkcom WT, Ghosh AK, Sung MS, et al. NAD+ and SIRT3 control microtubule dynamics and reduce susceptibility to antimicrotubule agents. Proc Natl Acad Sci USA. 2014;111(24):E2443‐E2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Choi E, Choe H, Min J, Choi JY, Kim J, Lee H. BubR1 acetylation at prometaphase is required for modulating APC/C activity and timing of mitosis. EMBO J. 2009;28(14):2077‐2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cheng PP, Xia J‐J, Wang H‐L, et al. Islet transplantation reverses the effects of maternal diabetes on mouse oocytes. Reproduction. 2011;141(4):417‐424. [DOI] [PubMed] [Google Scholar]
- 41. Frame S, Cohen P. GSK3 takes centre stage more than 20 years after its discovery. Biochem J. 2001;359(Pt 1):1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Doble BW, Woodgett JR. GSK‐3: tricks of the trade for a multi‐tasking kinase. J Cell Sci. 2003;116(Pt 7):1175‐1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Watcharasit P, Bijur GN, Zmijewski JW, et al. Direct, activating interaction between glycogen synthase kinase‐3beta and p53 after DNA damage. Proc Natl Acad Sci USA. 2002;99(12):7951‐7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. He X, Saint‐Jeannet J‐P, Woodgett JR, Varmus HE, Dawid IB. Glycogen synthase kinase‐3 and dorsoventral patterning in Xenopus embryos. Nature. 1995;374(6523):617‐622. [DOI] [PubMed] [Google Scholar]
- 45. Walz A, Ugolkov A, Chandra S, et al. Molecular pathways: revisiting glycogen synthase kinase‐3beta as a target for the treatment of cancer. Clin Cancer Res. 2017;23(8):1891‐1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Buttrick GJ, Wakefield JG. PI3‐K and GSK‐3: Akt‐ing together with microtubules. Cell Cycle. 2008;7(17):2621‐2625. [DOI] [PubMed] [Google Scholar]
- 47. Wakefield JG, Stephens DJ, Tavare JM. A role for glycogen synthase kinase‐3 in mitotic spindle dynamics and chromosome alignment. J Cell Sci. 2003;116(Pt 4):637‐646. [DOI] [PubMed] [Google Scholar]
- 48. Tighe A, Ray‐Sinha A, Staples OD, Taylor SS. GSK‐3 inhibitors induce chromosome instability. BMC Cell Biol. 2007;8:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Forde JE, Dale TC. Glycogen synthase kinase 3: a key regulator of cellular fate. Cell Mol Life Sci. 2007;64(15):1930‐1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cormier KW, Woodgett JR. Recent advances in understanding the cellular roles of GSK‐3. F1000Res. 2017;6:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang Q, Moley KH. Maternal diabetes and oocyte quality. Mitochondrion. 2010;10(5):403‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang Q, Frolova AI, Purcell S, et al. Mitochondrial dysfunction and apoptosis in cumulus cells of type I diabetic mice. PLoS One. 2010;5(12):e15901. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


