Abstract
Purpose
Gastrectomy for elderly patients can significantly deteriorate the health-related quality of life (HRQoL). There was no report comparing HRQoL of elderly patients with young patients after gastrectomy for gastric cancer. This study assessed the differences in the changes of HRQoL at one year after gastrectomy according to age.
Methods
From May 2014 to Feb 2016, we prospectively enrolled patients undergoing gastrectomy for gastric cancer. They completed the European Organization for Research and Treatment of Cancer and gastric questionnaires preoperatively and at postoperative 1, 3, 6, 9, and 12 months.
Results
We included 57 elderly patients (≥70 years old) and 74 younger patients. The elderly had similar demographic, surgical, and pathological characteristics with young patients except that elderly had more comorbidity, laparoscopic gastrectomies, and lesser postoperative chemotherapy. One month after gastrectomy, the score of global health status/quality of life, physical, role, and social functioning were significantly impaired in elderly patients. Among them, physical and role functioning were more impaired than those of young patients. The scores of physical functioning, role functioning, cognitive functioning, and social functioning were not fully recovered till 1 year after surgery. There was a significant age group difference in the changes in physical function over the 1-year follow-up.
Conclusion
Elderly patients' global health status/quality of life and social functioning significantly decreased at postoperative 1 month and recovered by 6 months after gastrectomy. There was a significant age-specific difference in physical functioning throughout the 1-year follow-up. Surgeons need to pay more attention to recovery of the elderly patients' HRQoL after gastrectomy.
Keywords: Elderly patients, Gastrectomy, Gastric cancer, Quality of life
INTRODUCTION
The number of elderly patients who underwent gastrectomy increased recently. This trend will be continued because life expectancy has been improved and the proportion of elderly patients is predicted to grow. In Korea, the proportion of elderly patients aged more than 70 years was continuously increased from 9.1% in 1995 to 25.3% in 2014 according to the Information Committee of the Korean Gastric Cancer Association [1]. The Surveillance, Epidemiology, and End Results (SEER) database in the United States also shzowed that elderly patients aged 80 years and more increased from 11.7% in period 1988–2003 to 13.1% in period 2004–2010 [2].
Although old age currently is not considered as an absolute contraindication to surgery for gastric cancer, postoperative morbidity and mortality in elderly patients were usually reported as higher than younger patients [3,4]. Gastrectomy can significantly deteriorate the health-related (HR) quality of life (QoL) [5,6,7,8]. As for surgical outcomes, there were many reports about morbidity, mortality, and survival rate. However, there were few studies about HRQoL in elderly patients.
The purposes of this study were to analyze the changes of the HRQoL in elderly patients who were 70 years old or elder according to the periods (preoperative day, postoperative 1, 3, 6, 9, and 12 months) and to compare them with those of young patients.
METHODS
Patients
From May 2014 to February 2016, we prospectively enrolled patients undergoing gastrectomy. They were asked to complete the HRQoL questionnaires preoperatively and at 5 postoperative intervals up to 1 year (postoperative 1, 3, 6, 9, and 12 months). Patients who postoperatively completed at least 2 questionnaires were included and patients with (1) combined resection except for cholecystectomy and splenectomy, (2) previous or combined malignancies, or (3) neurologic or psychological conditions disable to answer the questionnaires were excluded. This study was approved by Institutional Review Board of SMG-SNU Boramae Medical Center (No. 16-2014-127) and the written informed consent was obtained.
Surgery
All patients underwent surgery first according to Korean Practice Guideline for Gastric Cancer if the tumor is outside of the indication for endoscopic resection or and ≥cT1b or cN+ and M0 gastric cancer [9] and upfront surgery is standard treatment for gastric cancer in Korea. Considering the location and clinical stage of tumor and the length of resectional margin, distal gastrectomy/pylorus-preserving gastrectomy or total gastrectomy was done. Reconstruction was performed with Billroth I or II gastrojejunostomy after distal gastrectomy, and Roux-en-Y esophagojejunostomy after total gastrectomy. D1+lymphadenectomy and D2 lymphadenectomy were performed for early gastric cancer patients and advanced gastric cancer patients, respectively. Laparoscopic gastrectomy was performed if the tumor was not advanced.
After surgery, patients were placed on a diet program that included drinking water on the 3rd postoperative day, followed by a liquid and soft diet. Patients were planned to be discharged on the 7th day, postoperatively.
Health-related quality of life assessment
The HRQoL was assessed using the Korean version of European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaires (QLQ). It consisted of the general module, the EORTC QLQ-C30, and the gastric cancer-specific module, the EORTC QLQ-STO22 [10,11,12]. The EORTC QLQ-C30 included 30 questions; a global health status/QoL scale, 5 functional scales (physical, role, emotional, cognitive, and social), and 9 symptoms scales/items (fatigue, nausea and vomiting, pain, dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial difficulties). The EORTC QLQ-STO22 is a supplement to the QLQ-C30 and is including 22 questions evaluating 9 symptom scales/items (dysphasia, chest and abdominal pain, reflux, eating restriction, dry mouth, taste, body image, anxiety, and hair loss).
The preoperative HRQoL assessment was performed when patients were hospitalized for surgery; alternatively, the postoperative HRQoL assessment was performed at the outpatient department. For global QoL and the functional scales, a higher score indicates better HRQoL, with 100 being perfect. For symptom scales, a lower score indicates better HRQoL, with 0 being perfect or no symptoms reported.
Statistics
Demographic and clinical parameters of both age groups (70 years or older vs. 69 years or younger) were summarized using mean ± standard deviation for continuous variables and frequency (percentage) for categorical variables. The differences in the continuous variables were compared using 2-sample t-test and/or Wilcoxon rank-sum test and Pearson chi-square tests, and exact binomial tests were used to compare the distributions in categorical variables. Normal quantile-quantile plots were examined for checking normality of HRQoL assessment. For comparison between the age groups at each time point, 2-sample t-test were used. For comparison between adjacent time points, 2-sample t-tests were conducted. To account for within-individual correlations, mixed-effects models were fitted for each of 24 HRQoL outcomes adjusting for age, sex, extent of gastrectomy (partial or whole), minimal invasive gastrectomy (open or laparoscopic gastrectomy), TNM stage, postoperative chemotherapy, time, time2, and age × time2, respectively.
To address missing data due to dropouts, we also explored last-observation carried forward approach (LOCF) and the inverse probability weight (IPW) [13]. A 2-sided P-value of <0.05 was considered statistically significant. For statistical analysis, the R program package (R Foundation for Statistical Computing, Vienna, Austria; http://www.r-project.org) and IBM SPSS Statistics ver. 23 (IBM Corp., Armonk, NY, USA) was used.
RESULTS
Patients
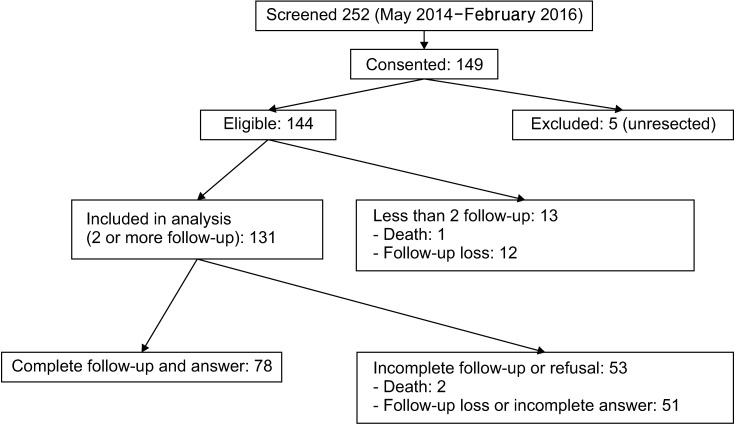
All 252 patients were screened and 144 patients were eligible and finally 57 elderly patients (≥70 years old) and 74 younger patients were included (Fig. 1).
Fig. 1. Patients' registration.
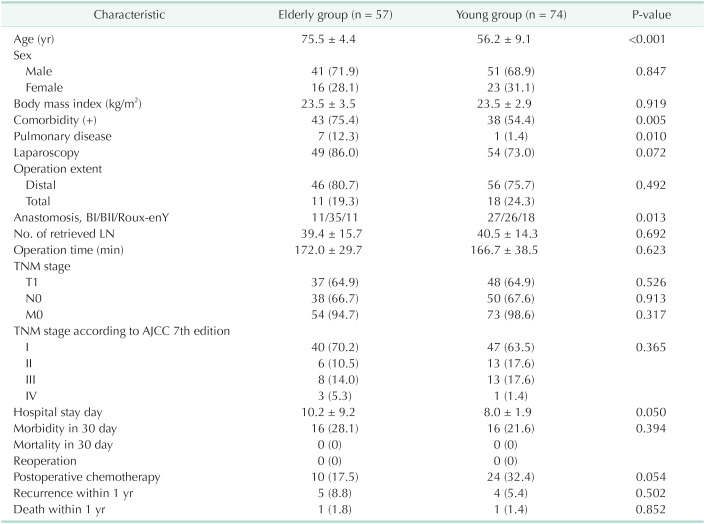
Patients' characteristics were summarized in Table 1. The elderly patients had similar demographic, surgical, and pathological characteristics with young patients while the elderly had more comorbidity (P = 0.005), especially pulmonary disease (P = 0.010). The elderly patients tend to have more laparoscopic surgery, Billroth II anastomosis after distal gastrectomy, longer hospital stay, and more frequent postoperative chemotherapy, which were not significantly different from the younger patients. There were also no significant differences in morbidity, mortality, and reoperation, respectively.
Table 1. Patients' demographic, surgical, and tumor characteristics.
Values are presented as mean ± standard deviation or number (%).
BI, Billroth I; BII, Billroth II; LN, lymph node; AJCC, American Joint Committee on Cancer.
Changes of HRQoL 1 year after gastrectomy
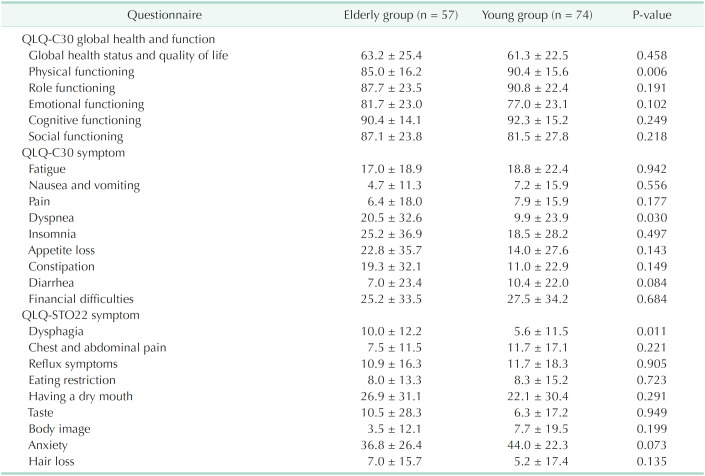
Table 2 showed preoperative HRQoL scores of the age groups. Preoperatively, elderly patients had lower score of physical functioning and more symptoms of dyspnea and dysphagia.
Table 2. Preoperative quality of life.
Values are presented as mean ± standard deviation.
Assessed using European Organization for Research and Treatment of Cancer Quality of Life Questionnaires (QLQ).
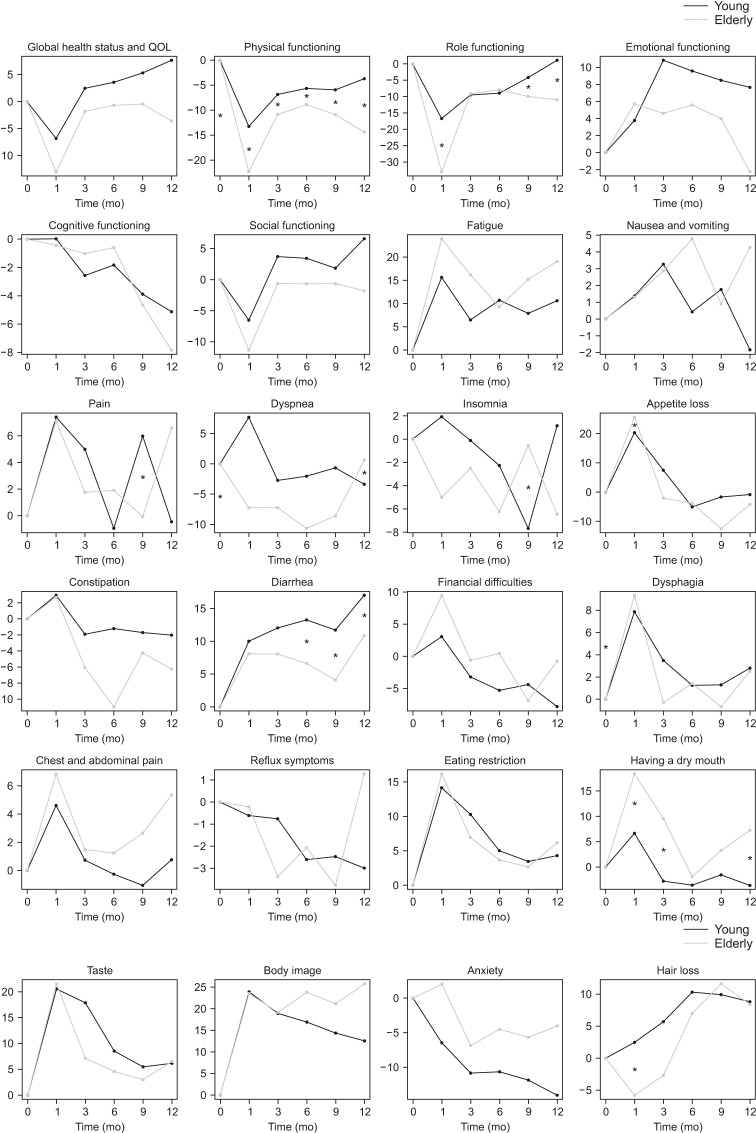
Postoperative longitudinal changes of HRQoL were shown in Fig. 2. The elderly patients' scores of global health status/QoL, physical functioning, role functioning, and social functioning were severely deteriorated one month after gastrectomy. The scores of global health status/QoL and emotional functioning were recovered to some degree of preoperative levels but those of physical functioning, role functioning, cognitive functioning, and social functioning were not fully recovered till 1 year after surgery. Pain, appetite loss, and financial difficulties were worsened 1 month after gastrectomy and improved till 1 year after surgery. Fatigue and diarrhea were continuously worsened after gastrectomy. Dysphagia, chest and abdominal pain, eating restriction, and dry mouth showed similar patterns which were worsened 1 month after gastrectomy and improved till 1 year after surgery. But they were not completely recovered.
Fig. 2. Periodic changes in health-related quality of life (QoL) scores. *Significant difference between the elderly and young patients (P < 0.05).
Predictive factors for HRQoL
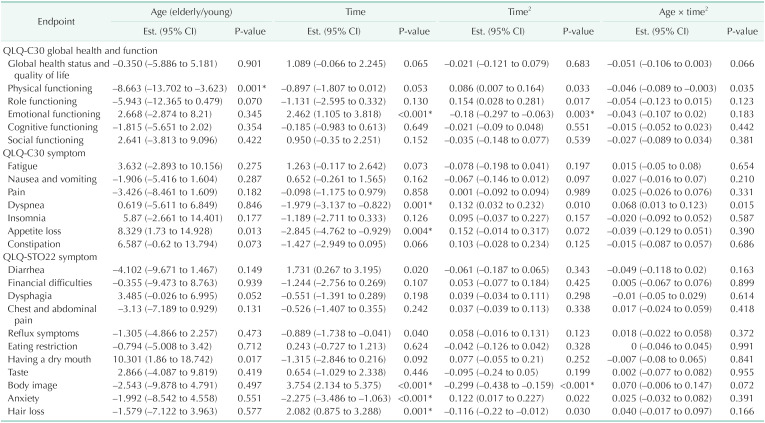
Noting that HRQoL could be confounded with demographical (age and sex) and clinical factors (extent of gastrectomy, laparoscopic gastrectomy, TNM stage, and postoperative chemotherapy), multivariable adjusted regression analysis using mixed-effects models were performed for each HRQoL, respectively (Table 3). Considering the age groups, on average over the 1-year follow-up, the elderly group appeared to show lower scores in physical function compared to the younger group (mean difference [MD], –8.663; 95% confidence interval [CI], –13.702 to –3.623; P = 0.001) (Fig. 2) while there are no statistically significant differences in global health status/QoL (MD, –0.353; 95% CI, –5.886 to 5.181; P = 0.901). Considering the age group and time change together, there was a significant age group difference in the changes in physical function over the 1-year follow-up where the elderly group tends to decrease in their physical function after 9 months (P = 0.035) (Table 3). The score of global health status/QoL shows the similar pattern until the 1st month after surgery; however, there were opposite trends between the 2 groups while this difference did not reach statistical significance (P = 0.066) (Table 3, Fig. 2). Elderly patients had significant appetite loss and dry mouth (P = 0.013 and P = 0.017, respectively).
Table 3. Multivariable adjusted mixed effect model outcomes for 24 health-related quality of life endpoints.
Assessed using European Organization for Research and Treatment of Cancer Quality of Life Questionnaires (QLQ).
EST., estimate; CI, confidence interval.
*Significant difference (P < 0.05).
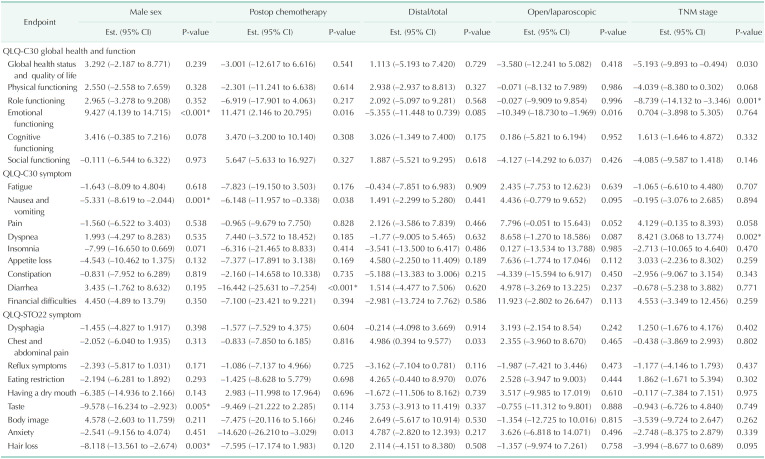
Meanwhile, total gastrectomy, laparoscopic gastrectomy, postoperative chemotherapy, TNM stage, and sex also significantly affect some scores of HRQoL after gastrectomy (Table 4).
Table 4. Parameter estimates for 24 health-related quality of life outcomes except age and time.
Assessed using European Organization for Research and Treatment of Cancer Quality of Life Questionnaires (QLQ).
EST., estimate; CI, confidence interval.
*Significant difference (P < 0.05).
DISCUSSION
Patients after gastrectomy were reported to encounter functional impairments and symptoms, but experience only a slightly impaired global HRQoL [5,8]. HRQoL deteriorations in physical, role, and cognitive functioning scales, and significant reductions lasted till 36-month after total gastrectomy [14]. Persistent QoL deterioration after distal subtotal gastrectomy is primarily due to financial difficulties, eating restrictions, and body image concerns [6].
However, there was no report that compares HRQoL of elderly and young patients after gastrectomy for gastric cancer. This study demonstrated the difference in HRQoL between elderly patients and young patients. Elderly patients' score of global health status/QoL, physical functioning, role functioning, and social functioning were significantly deteriorated at postoperative 1 month. Among them, physical and role functioning were more impaired than those of young patients. Furthermore, the scores of impaired physical functioning, role functioning, cognitive functioning, and social functioning were not restored till postoperative 1 year. This study also demonstrated that there was a significant age group difference in the longitudinal changes in physical function over the 1-year follow-up, but not in global health status/QoL.
Except for age groups, other factors related to HRQoL identified in this study were time trend, sex, total gastrectomy, laparoscopic gastrectomy, and postoperative chemotherapy. Total gastrectomy was known to have more negative effect on HRQoL than distal gastrectomy [5,7,8,14,15,16,17], which preserves more stomach and requires a less extensive lymphadenectomy. The lower HRQoL of women was consistent with other studies [5,18]. Laparoscopic distal gastrectomy resulted in significantly better HRQoL scores on global health status/QoL and most functionings [5,7,19] and many randomized controlled trials to support this are ongoing [20,21,22].
This study has several limitations. First, EORTC QLQ-C30 and QLQ-STO22 tools were not optimized for elderly patients and tended to overestimate the HRQoL of the elderly. For comparison with young patients, using well-established tool like EORTC QLQ was inevitable. Second, a large amount of missing data occurred during the follow-up. Most participants showed up for scheduled visit, but some refused to complete the HRQoL assessments because they felt the QoL questionnaire too burdensome. Twelve patients (8.3%) were excluded owing to incomplete follow-ups and a further 35.4% (51 of 144) were included but had incomplete data. An additional 1.4% of patients died during follow-up. We explored several approaches to account for missing data, including various joint likelihood-factorization techniques for longitudinal studies. For example, we found that result showed similar results when we analyzed our data using the LOCF and IPW approaches (Supplementary Fig. 1) [13]. However, we acknowledge that missing data approaches methods suffered from inherent limitations as we cannot test the performances of each approach in the absence of actual observations. Thus in this study, we excluded missing data points from the analysis and used the completely observed data for transparency. Third, EORTC assessments consist of a lot of questions so that the rate of questionnaire completion is relatively low. To boost the completion rate and the patient retention rate, reward programs can be considered for a future study. Lastly, this study lacked long-term results and the strategy to reduce the missing data or the cross-sectional study design may help this limitation. But when we reviewed the previous studies which showed that time trend is mainly associated with HRQoL in the early recovery period whereas HRQoL remains relatively stable after the 1st year following surgery [5,15,16,23], similar long-term results were expected.
Future research includes (1) nonlinear trend over the course of follow-up using the nonlinear mixed effects model; (2) the time-varying nutrition in the regression model to better understand the impact of the nutrition on the HRQoL of the patient.
In summary, elderly patients had lower score of physical functioning before surgery. Global health status/QoL, physical functioning, role functioning, and social functioning were deteriorated 1 month after gastrectomy and improved 3 months after gastrectomy. But those were not fully recovered. Age group significantly affected the scores of physical functioning and the difference last till 1-year after surgery.
In conclusion, elderly patients' QoL, especially physical functioning, was not completely recovered one year after gastrectomy unlike those of younger patients. Hence surgeons need to pay more attention to the elderly patients' QoL after gastrectomy.
Footnotes
Fund/Grant Support: This work was supported by a clinical research grant-in-aid from the Seoul Metropolitan Government Seoul National University (SMG-SNU) Boramae Medical Center (03-2014-13).
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: HSA.
- Formal Analysis: HSA, JA.
- Investigation: All authors.
- Methodology: HSA, DSH.
- Project Administration: HSA.
- Writing — Original Draft: All authors.
- Writing — Review & Editing: All authors.
SUPPLEMENTARY MATERIALS
Supplementary Fig. 1 can be found via https://doi.org/10.4174/astr.2021.100.1.8.
Age and 24 quality of life marginal/common associations under the different missing data approaches. The 95% confidence interval of the age effects are displayed. CC, complete data analysis; LOCF, last observations carried forward; IPW, inverse probability weighting. GH, global health status and QOL; PF, physical functioning; RF, role functioning; EF, emotional functioning; CF, cognitive functioning; SF, social functioning; FA, fatigue; NV, nausea and vomiting; Dysp, dyspnea; Apploss, appetite loss; Constp, constipation; D, diarrhea; FD, financial difficulties; Spain, chest and abdominal pain; Reflux, reflux symptoms; ERist, eating restriction; DMouth, having a dry mouth; BI, body image; HL, hair loss.
References
- 1.Information Committee of Korean Gastric Cancer Association. Korean Gastric Cancer Association Nationwide Survey on Gastric Cancer in 2014. J Gastric Cancer. 2016;16:131–140. doi: 10.5230/jgc.2016.16.3.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song P, Wu L, Jiang B, Liu Z, Cao K, Guan W. Age-specific effects on the prognosis after surgery for gastric cancer: a SEER population-based analysis. Oncotarget. 2016;7:48614–48624. doi: 10.18632/oncotarget.9548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katai H, Ishikawa T, Akazawa K, Isobe Y, Miyashiro I, Oda I, et al. Five-year survival analysis of surgically resected gastric cancer cases in Japan: a retrospective analysis of more than 100,000 patients from the nationwide registry of the Japanese Gastric Cancer Association (2001-2007) Gastric Cancer. 2018;21:144–154. doi: 10.1007/s10120-017-0716-7. [DOI] [PubMed] [Google Scholar]
- 4.Yang JY, Lee HJ, Kim TH, Huh YJ, Son YG, Park JH, et al. Short- and long-term outcomes after gastrectomy in elderly gastric cancer patients. Ann Surg Oncol. 2017;24:469–477. doi: 10.1245/s10434-016-5482-y. [DOI] [PubMed] [Google Scholar]
- 5.Brenkman HJ, Tegels JJ, Ruurda JP, Luyer MD, Kouwenhoven EA, Draaisma WA, et al. Factors influencing health-related quality of life after gastrectomy for cancer. Gastric Cancer. 2018;21:524–532. doi: 10.1007/s10120-017-0771-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee SS, Chung HY, Kwon O, Yu W. Long-term shifting patterns in quality of life after distal subtotal gastrectomy: preoperative- and healthy-based interpretations. Ann Surg. 2015;261:1131–1137. doi: 10.1097/SLA.0000000000000832. [DOI] [PubMed] [Google Scholar]
- 7.Karanicolas PJ, Graham D, Gönen M, Strong VE, Brennan MF, Coit DG. Quality of life after gastrectomy for adenocarcinoma: a prospective cohort study. Ann Surg. 2013;257:1039–1046. doi: 10.1097/SLA.0b013e31828c4a19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim AR, Cho J, Hsu YJ, Choi MG, Noh JH, Sohn TS, et al. Changes of quality of life in gastric cancer patients after curative resection: a longitudinal cohort study in Korea. Ann Surg. 2012;256:1008–1013. doi: 10.1097/SLA.0b013e31827661c9. [DOI] [PubMed] [Google Scholar]
- 9.Guideline Committee of the Korean Gastric Cancer Association (KGCA), Development Working Group & Review Panel. Korean Practice Guideline for Gastric Cancer 2018: an evidence-based, multi-disciplinary approach. J Gastric Cancer. 2019;19:1–48. doi: 10.5230/jgc.2019.19.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yun YH, Park YS, Lee ES, Bang SM, Heo DS, Park SY, et al. Validation of the Korean version of the EORTC QLQ-C30. Qual Life Res. 2004;13:863–868. doi: 10.1023/B:QURE.0000021692.81214.70. [DOI] [PubMed] [Google Scholar]
- 11.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 12.Vickery CW, Blazeby JM, Conroy T, Arraras J, Sezer O, Koller M, et al. Development of an EORTC disease-specific quality of life module for use in patients with gastric cancer. Eur J Cancer. 2001;37:966–971. doi: 10.1016/s0959-8049(00)00417-2. [DOI] [PubMed] [Google Scholar]
- 13.Ahn J, Ahn HS. Bayesian analysis of longitudinal quality of life measures with informative missing data using a selection model. Stat Methods Med Res. 2020;29:1354–1367. doi: 10.1177/0962280219862001. [DOI] [PubMed] [Google Scholar]
- 14.Lee SS, Chung HY, Kwon OK, Yu W. Quality of life in cancer survivors 5 years or more after total gastrectomy: a case-control study. Int J Surg. 2014;12:700–705. doi: 10.1016/j.ijsu.2014.05.067. [DOI] [PubMed] [Google Scholar]
- 15.Avery K, Hughes R, McNair A, Alderson D, Barham P, Blazeby J. Health-related quality of life and survival in the 2 years after surgery for gastric cancer. Eur J Surg Oncol. 2010;36:148–154. doi: 10.1016/j.ejso.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Park KB, Park JY, Lee SS, Chung HY, Kwon OK. Chronological changes in quality of life and body composition after gastrectomy for locally advanced gastric cancer. Ann Surg Treat Res. 2020;98:262–269. doi: 10.4174/astr.2020.98.5.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park S, Chung HY, Lee SS, Kwon O, Yu W. Serial comparisons of quality of life after distal subtotal or total gastrectomy: what are the rational approaches for quality of life management? J Gastric Cancer. 2014;14:32–38. doi: 10.5230/jgc.2014.14.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van de Poll-Franse LV, Mols F, Gundy CM, Creutzberg CL, Nout RA, Verdonck-de Leeuw IM, et al. Normative data for the EORTC QLQ-C30 and EORTC-sexuality items in the general Dutch population. Eur J Cancer. 2011;47:667–675. doi: 10.1016/j.ejca.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Kim YW, Baik YH, Yun YH, Nam BH, Kim DH, Choi IJ, et al. Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer: results of a prospective randomized clinical trial. Ann Surg. 2008;248:721–727. doi: 10.1097/SLA.0b013e318185e62e. [DOI] [PubMed] [Google Scholar]
- 20.Hur H, Lee HY, Lee HJ, Kim MC, Hyung WJ, Park YK, et al. Efficacy of laparoscopic subtotal gastrectomy with D2 lymphadenectomy for locally advanced gastric cancer: the protocol of the KLASS-02 multicenter randomized controlled clinical trial. BMC Cancer. 2015;15:355. doi: 10.1186/s12885-015-1365-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Straatman J, van der Wielen N, Cuesta MA, Gisbertz SS, Hartemink KJ, Alonso Poza A, et al. Surgical techniques, open versus minimally invasive gastrectomy after chemotherapy (STOMACH trial): study protocol for a randomized controlled trial. Trials. 2015;16:123. doi: 10.1186/s13063-015-0638-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haverkamp L, Brenkman HJ, Seesing MF, Gisbertz SS, van Berge Henegouwen MI, Luyer MD, et al. Laparoscopic versus open gastrectomy for gastric cancer, a multicenter prospectively randomized controlled trial (LOGICA-trial) BMC Cancer. 2015;15:556. doi: 10.1186/s12885-015-1551-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang M, Zhang C, Wu QC. Health-related quality of life and survival among 10-year survivors of esophageal cancer surgery: gastric tube reconstruction versus whole stomach reconstruction. J Thorac Dis. 2019;11:3284–3291. doi: 10.21037/jtd.2019.08.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Age and 24 quality of life marginal/common associations under the different missing data approaches. The 95% confidence interval of the age effects are displayed. CC, complete data analysis; LOCF, last observations carried forward; IPW, inverse probability weighting. GH, global health status and QOL; PF, physical functioning; RF, role functioning; EF, emotional functioning; CF, cognitive functioning; SF, social functioning; FA, fatigue; NV, nausea and vomiting; Dysp, dyspnea; Apploss, appetite loss; Constp, constipation; D, diarrhea; FD, financial difficulties; Spain, chest and abdominal pain; Reflux, reflux symptoms; ERist, eating restriction; DMouth, having a dry mouth; BI, body image; HL, hair loss.