Transarterial chemoembolization (TACE) has emerged as one of the main primary treatment modalities for unresectable hepatocellular carcinoma (HCC) in patients with cirrhosis, based on HCC predominantly arterial vascularization. Indeed, TACE is still the standard of care for intermediate stage HCC and is currently widely used for early stage tumors ineligible for percutaneous ablation. TACE improves survival since it delays tumor progression, sometimes through a complete response following an extensive necrosis, and treatment response is an independent predictor of survival after TACE (1). TACE performance and safety are based on nodules number, size and morphology, radiologist’s expertise and liver function. Chemoembolization with drug-eluting beads (TACE-DEB) resulting in more sustained drug release with concomitant embolization may be applied, while it simplifies and harmonizes TACE modality, but performances are broadly similar to that of conventional TACE (cTACE) with iodized oil (2). Despite serious improvements in patient’s selection, TACE modalities, efficacy [using modified Response Evaluation Criteria in Solid Tumors (mRECIST)] and discontinuation criteria (3), this treatment usually fails to achieve sustained control of the disease, despite an objective response in about 50% of patients as mentioned by a systematic review of cTACE efficacy (4). Local and/or distant intrahepatic recurrences are commonly observed including in patients who achieved complete response following TACE (5), even under a more invasive pattern with non-smooth tumors margin (6), and then TACE resistant. Many reasons are involved: the lack of a safety margin related to the procedure, capsular tumor invasions, microsatellite lesions (7), and well differentiated tumor portions within early-stage HCC (8) are usually both fed by the hepatic artery and portal vein. Moreover, portal venous supply of HCC nodules resulting from impaired arterial flow following repeated TACE may help tumor survival (9). Neo-angiogenesis is an established response to hypoxia, and hypoxia induced by TACE stimulates vascular endothelial growth factor (VEGF) production by the residual tumor cells (10), and increase tumor angiogenesis and formation of collateral circulation contributing to TACE failure. Thus, antiangiogenic therapy could enhance the TACE efficacy (11). The rationale was clear to combine TACE with sorafenib, that multikinase inhibitor targeting among others the anti-angiogenic factors VEGFR2 and PDGFR. Accordingly, the combination of TACE with sorafenib was explored in phase 2 and 3 studies, with poor results in terms of efficacy (12-14) (Table 1). However, the study design (12), the course (13) or the populations enrolled have been challenged. In a new prospective randomized controlled study, Kudo et al. report that combining cTACE plus sorafenib improves progression-free survival (PFS) based on a new definition of progression in patients with unresectable HCC (15). This study included HCC patients with slightly altered performance status (PS-1) like two studies assessing this combination (Table 1). These patients are considered as advanced stage HCC within Barcelona Clinic Liver Cancer (BCLC) staging system, but we know the heterogeneity of this population (16) and this status may not be related to the tumor burden. Some authors suggest a re-assignment for this population (17). Outcomes of TACTICS trial are not surprising given the failures of previous studies. Hence, the authors propose another drug management and a new definition of progression as a result of the lack of consensus and the challenge to define “unsuccessful TACE” over the past few years (Figure 1).
Table 1. Randomized phase II, phase III clinical trials of combination therapy with TACE ± sorafenib in unresectable HCC.
| Trial name | Number of patients and trial design | Primary end point | Timing of sorafenib | Duration of Sor (weeks)/daily dose of Sor (mg) (median) | TACE session | HFSR (any grade (%)/permanent discontinuation (%) due to AE | ORRs | Results |
|---|---|---|---|---|---|---|---|---|
| Post TACE (Double blind), Kudo et al. (12) | Sor n=229 (400 mg twice daily) vs. Plb n=229; PS-1: 12% | TTP (recurrence after CR, or ≥25% tumor size or new lesion after PR) | After (P/C) response to conventional TACE | 17 weeks/385 mg | (Prior TACE) one: 65%, two: 35% | 82%/45% | (prior TACE) CR: 62%, PR: 38% |
Negative |
| SPACE (Double blind), Lencioni et al. (13) | Sor n=154 (400 mg twice daily) vs. Plb n=153; BCLC B | TTP (according to mRECIST criteria) | On day 1 (4-week cycle) + DEB-TACE on C1, C3, C7, C13 | 21 weeks/566 mg | One: 36%, two: 35%, >two: 26% vs. one: 19%, two: 38%, >two: 40% | 46%/27% | 55.9% vs. 41.3% | Negative |
| TACE-2 (Double blind), Meyer et al. (14) | Sor n=157 (400 mg twice daily) vs. Plb n=156; PS-1: 37% | PFS (progression: RECIST v1.1; or death) | On day 1 + DEB-TACE | 17 weeks/660 mg | One: 41%, Two: 26%, >two: 22% vs. one: 28%, two: 35%, >two: 29% | 42%/19% | 54% vs. 52% | Negative |
| TACTICS, Kudo et al. (15) | Sor n=80 (400 mg/day) vs. Plb n=76; BCLC A: 38%, PS-1: 12% | PFS (progression: [unTACEable criteria or TACE failure/refractoriness criteria (JSH); or death] | 2–3 weeks prior to first conventional TACE | 38.7 weeks/355 mg | – | 53.2%/2.5% | 71.3% vs. 61.8% | Positive |
Sor, sorafenib; TACE, transarterial chemoembolization; HCC, hepatocellular carcinoma; HFSR, hand-foot skin reaction; AE, adverse events; ORR, overall response rate; Plb, placebo; PS, performance status; TTP, time to progression; CR, complete response; mRECIST, modified Response Evaluation Criteria in Solid Tumors; DEB-TACE, drug-eluting beads TACE; PFS, progression free survival; JSH, Japan Society of Hepatology.
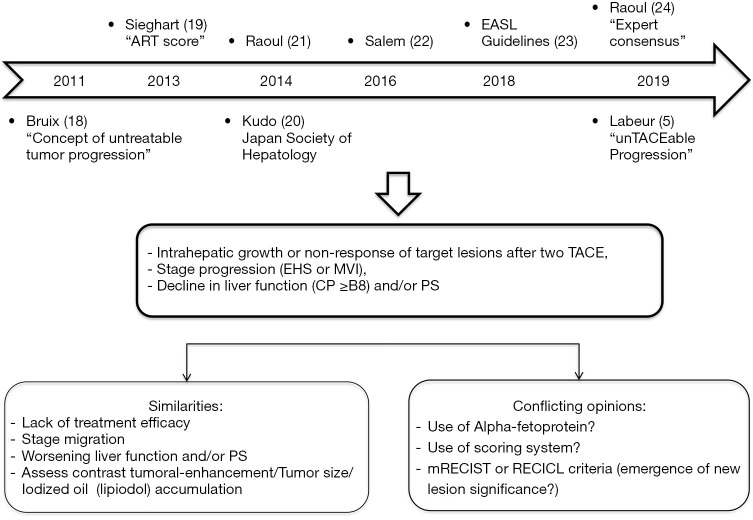
Figure 1.
“unTACEable Progression” concept: an emerging consensus (5,18-24). ART, Assessment for Retreatment with Transarterial Chemoembolization; EASL, European Association for the Study of the Liver; TACE, transarterial chemoembolization; EHS, extra hepatic spread; MVI, macro vascular invasion; CP, Child-Pugh; PS, performance status; mRECIST, modified Response Evaluation Criteria in Solid Tumors; RECICL, Response Evaluation Criteria in Cancer of the Liver.
Regarding drug management, the timing of antiangiogenic therapy with TACE is a key feature and probably one of the reasons for this outcome. By contrast with other studies, sorafenib was started 2 to 3 weeks prior to the first TACE in an attempt to decrease VEGF upregulation. Delivery of sorafenib in an adjuvant setting did not show benefit in the first controlled trial assessing this association (12). The daily dose of sorafenib was 400 mg/day (vs. 800 mg/day in the other three studies), including a possibility to re-escalate, and the use of a lower sorafenib therapy dose has been suggested in previous clinical (25,26), and pre-clinical studies (27) in particular for its immunomodulatory properties. Accordingly, the discontinuation rate decreased and the duration of treatment with sorafenib was substantially longer. This trend occurs in advanced HCC phase III trials (28) and real-life studies (29,30). The rate of sorafenib discontinuation due to drug-related adverse events decreased over time (sharp: 38%; reflect: <10%), while median overall survival (OS) of HCC patients who received sorafenib has increased (sharp: 10.7 months; reflect: 14.2 months). Similarly, Raoul (29) and Tovoli (30) found that the treatment duration with sorafenib and OS were higher over time, with more frequent dose modifications. In other words, the management of sorafenib has improved over time.
In TACTICs trial, repeated cTACE sessions could be performed upon the demonstration of viable tumour or local and/or distant intrahepatic recurrences in patients suitable to cTACE, whereas it is a natural course of the disease. Kudo et al. used a different progression endpoint meeting the Response Evaluation Criteria in Cancer of the Liver (RECICL) and the Japan Society of Hepatology (JSH) criteria for TACE failure/refractoriness (15). This is one of the main criticisms of the study with the sample size, but until now the definition of progression was not clear. Moreover, this policy of retreatment is consistent with current practice. Bruix et al. (18) first introduced the concept of untreatable progression (major progression or decline in liver function and/or PS). Few years later the concept of TACE failure (lack of treatment efficacy)/refractoriness [worsening of tumor (size/enhancement) or stage migration] appeared in Asian and European guidelines. In addition, other studies recommended either the use of a scoring system (19) to decide whether TACE should be continued, or a new definition of “unTACEable progression” (Figure 1).
Progression free survival (PFS) as the primary endpoint in intermediate stage HCC is supported by the subsequent treatment options now available, making it ever more challenging to assess the benefit of TACE based exclusively on OS. OS estimation may be confounded by prolonged post-progression survival. Thus, both this (15) and other studies (5) support the use of new endpoints such as time to “unTACEable progression” for treatment guidance and future trials.
Another point deserves mention, response rate and PFS were notably high in TACTICs trial, especially in the cTACE group alone (Table 1). This raises again the problem of careful patient selection within this heterogeneous intermediate stage. In other words, should we treat the whole patient population using a combination TACE plus sorafenib? This study suggests new insights. Complete response criteria following TACE are known, while some intermediate stages HCC patients show a higher risk of recurrence, and others may not achieve benefit from TACE and therefore may require earlier initiation of systemic treatment. The “Six & Twelve” score (sum of tumor size and number) proposed by Wang et al. (31) can classify survival among recommended TACE candidates, using a simple risk stratification into three subgroups. Moreover, this new model has clear boundaries unlike intermediate stage subclassifications that divide tumor burden according to the up-to-seven criteria (within/out) and probably reduces the scope of patients who could benefit from TACE.
In summary, this study provides unexpected results. Sorafenib could still remain a primary therapeutic option as a combination linked to its VEGFR-inhibitory property. It outlines an attractive approach in combining treatments, which is becoming the new standard in advanced HCC. Other significant results are pending, TACE impact the immune microenvironment and may augment the effects of immune checkpoint inhibitors. Our practices are going to evolve. If these findings are supported, the guidelines will have to be updated to integrate these results and specify the patient categories that could benefit from them.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Translational Medicine. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-4268). XA reports other from Bayer, other from Ipsen, other from Eisai, outside the submitted work. RA reports other from Gliead, other from Bayer, other from Eisai, other from Intercept, other from Abbvie, other from MSD, other from Ipsen, outside the submitted work. MB reports other from Merck-Schering Plough, other from Gliead, other from Janssen, other from Vertex, other from Boehringer-Ingelheim, other from BMS, other from Roche, other from Abbvie, other from GSK, outside the submitted work.
References
- 1.Gillmore R, Stuart S, Kirkwood A, et al. EASL and mRECIST responses are independent prognostic factors for survival in hepatocellular cancer patients treated with transarterial embolization. J Hepatol 2011;55:1309-16. 10.1016/j.jhep.2011.03.007 [DOI] [PubMed] [Google Scholar]
- 2.Golfieri R, Giampalma E, Renzulli M, et al. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer 2014;111:255-64. 10.1038/bjc.2014.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raoul JL, Sangro B, Forner A, et al. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat Rev 2011;37:212-20. 10.1016/j.ctrv.2010.07.006 [DOI] [PubMed] [Google Scholar]
- 4.Lencioni R, de Baere T, Soulen MC, et al. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: A systematic review of efficacy and safety data. Hepatology 2016;64:106-16. 10.1002/hep.28453 [DOI] [PubMed] [Google Scholar]
- 5.Labeur TA, Takkenberg RB, Klumpen HJ, et al. Reason of Discontinuation After Transarterial Chemoembolization Influences Survival in Patients with Hepatocellular Carcinoma. Cardiovasc Intervent Radiol 2019;42:230-8. 10.1007/s00270-018-2118-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adhoute X, Penaranda G, Sellier F, et al. Response to 'the flexible therapeutic approach to the BCLC B stage': Time for scoring systems? J Hepatol 2017. [Epub ahead of print]. doi: . 10.1016/j.jhep.2017.07.038 [DOI] [PubMed] [Google Scholar]
- 7.Kuroda C, Sakurai M, Monden M, et al. Limitation of transcatheter arterial chemoembolization using iodized oil for small hepatocellular carcinoma. A study in resected cases. Cancer 1991;67:81-6. [DOI] [PubMed] [Google Scholar]
- 8.Tajima T, Honda H, Taguchi K, et al. Sequential hemodynamic change in hepatocellular carcinoma and dysplastic nodules: CT angiography and pathologic correlation. AJR Am J Roentgenol 2002;178:885-97. 10.2214/ajr.178.4.1780885 [DOI] [PubMed] [Google Scholar]
- 9.Miyayama S, Matsui O, Zen Y, et al. Portal blood supply to locally progressed hepatocellular carcinoma after transcatheter arterial chemoembolization: Observation on CT during arterial portography. Hepatol Res 2011;41:853-66. 10.1111/j.1872-034X.2011.00836.x [DOI] [PubMed] [Google Scholar]
- 10.Li X, Feng GS, Zheng CS, et al. Expression of plasma vascular endothelial growth factor in patients with hepatocellular carcinoma and effect of transcatheter arterial chemoembolization therapy on plasma vascular endothelial growth factor level. World J Gastroenterol 2004;10:2878-82. 10.3748/wjg.v10.i19.2878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang H, Meng Q, Tan H, et al. Antiangiogenic therapy enhances the efficacy of transcatheter arterial embolization for hepatocellular carcinomas. Int J Cancer 2007;121:416-24. 10.1002/ijc.22655 [DOI] [PubMed] [Google Scholar]
- 12.Kudo M, Imanaka K, Chida N, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer 2011;47:2117-27. 10.1016/j.ejca.2011.05.007 [DOI] [PubMed] [Google Scholar]
- 13.Lencioni R, Llovet JM, Han G, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J Hepatol 2016;64:1090-8. 10.1016/j.jhep.2016.01.012 [DOI] [PubMed] [Google Scholar]
- 14.Meyer T, Fox R, Ma YT, et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol 2017;2:565-75. 10.1016/S2468-1253(17)30156-5 [DOI] [PubMed] [Google Scholar]
- 15.Kudo M, Ueshima K, Ikeda M, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut 2020;69:1492-501. 10.1136/gutjnl-2019-318934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu CY, Lee YH, Hsia CY, et al. Performance status in patients with hepatocellular carcinoma: determinants, prognostic impact, and ability to improve the Barcelona Clinic Liver Cancer system. Hepatology 2013;57:112-9. 10.1002/hep.25950 [DOI] [PubMed] [Google Scholar]
- 17.Giannini EG, Bucci L, Garuti F, et al. Patients with advanced hepatocellular carcinoma need a personalized management: A lesson from clinical practice. Hepatology 2018;67:1784-96. 10.1002/hep.29668 [DOI] [PubMed] [Google Scholar]
- 18.Bruix J, Reig M, Rimola J, et al. Clinical decision making and research in hepatocellular carcinoma: pivotal role of imaging techniques. Hepatology 2011;54:2238-44. 10.1002/hep.24670 [DOI] [PubMed] [Google Scholar]
- 19.Sieghart W, Hucke F, Pinter M, et al. The ART of decision making: retreatment with transarterial chemoembolization in patients with hepatocellular carcinoma. Hepatology 2013;57:2261-73. 10.1002/hep.26256 [DOI] [PubMed] [Google Scholar]
- 20.Kudo M, Matsui O, Izumi N, et al. Transarterial chemoembolization failure/refractoriness: JSH-LCSGJ criteria 2014 update. Oncology 2014;87 Suppl 1:22‐31. 10.1159/000368142 [DOI] [PubMed] [Google Scholar]
- 21.Raoul JL, Gilabert M, Piana G. How to define transarterial chemoembolization failure or refractoriness: a European perspective. Liver Cancer 2014;3:119‐24. 10.1159/000343867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salem R, Gordon AC, Mouli S, et al. Y90 Radioembolization Significantly Prolongs Time to Progression Compared With Chemoembolization in Patients With Hepatocellular Carcinoma. Gastroenterology 2016;151:1155‐1163.e2. 10.1053/j.gastro.2016.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.European Association for the Study of the Liver . Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182‐236. 10.1016/j.jhep.2018.03.019 [DOI] [PubMed] [Google Scholar]
- 24.Raoul JL, Forner A, Bolondi L, et al. Updated use of TACE for hepatocellular carcinoma treatment: How and when to use it based on clinical evidence. Cancer Treat Rev 2019;72:28‐36. 10.1016/j.ctrv.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 25.Nishikawa H, Osaki Y, Endo M, et al. Comparison of standard-dose and halfdose sorafenib therapy on clinical outcome in patients with unresectable hepatocellular carcinoma in field practice: A propensity score matching analysis. Int J Oncol 2014;45:2295-302. 10.3892/ijo.2014.2654 [DOI] [PubMed] [Google Scholar]
- 26.Iavarone M, Cabibbo G, Piscaglia F, et al. Field-practice study of sorafenib therapy for hepatocellular carcinoma: a prospective multicenter study in Italy. Hepatology 2011;54:2055-63. 10.1002/hep.24644 [DOI] [PubMed] [Google Scholar]
- 27.Chuang HY, Chang YF, Liu RS, et al. Serial low doses of sorafenib enhance therapeutic efficacy of adoptive T cell therapy in a murine model by improving tumor microenvironment. PLoS One 2014;9:e109992. 10.1371/journal.pone.0109992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018;391:1163-73. 10.1016/S0140-6736(18)30207-1 [DOI] [PubMed] [Google Scholar]
- 29.Raoul JL, Adhoute X, Penaranda G, et al. Sorafenib: Experience and Better Management of Side Effects Improve Overall Survival in Hepatocellular Carcinoma Patients: A Real-Life Retrospective Analysis. Liver Cancer 2019;8:457-67. 10.1159/000497161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tovoli F, Ielasi L, Casadei-Gardini A, et al. Management of adverse events with tailored sorafenib dosing prolongs survival of hepatocellular carcinoma patients. J Hepatol 2019;71:1175-83. 10.1016/j.jhep.2019.08.015 [DOI] [PubMed] [Google Scholar]
- 31.Wang Q, Xia D, Bai W, et al. Development of a prognostic score for recommended TACE candidates with hepatocellular carcinoma: A multicentre observational study. J Hepatol 2019;70:893-903. 10.1016/j.jhep.2019.01.013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as