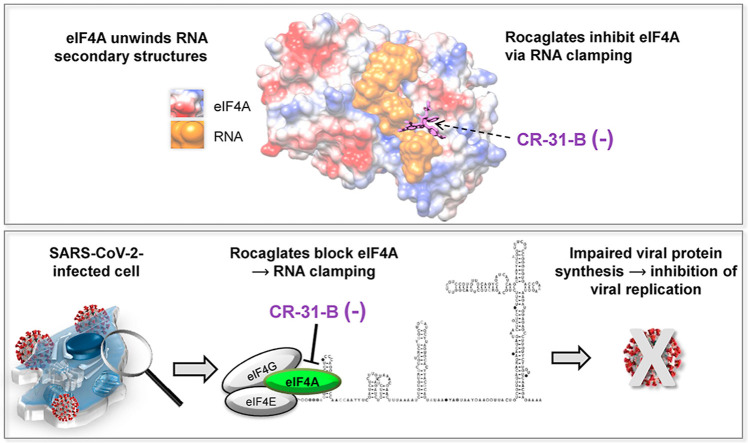
Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the causative agent of COVID-19, a severe respiratory disease with varying clinical presentations and outcomes, and responsible for a major pandemic that started in early 2020. With no vaccines or effective antiviral treatments available, the quest for novel therapeutic solutions remains an urgent priority. Rocaglates, a class of plant-derived cyclopenta[b]benzofurans, exhibit broad-spectrum antiviral activity against multiple RNA viruses including coronaviruses. Specifically, rocaglates inhibit eukaryotic initiation factor 4A (eIF4A)-dependent mRNA translation initiation, resulting in strongly reduced viral RNA translation. Here, we assessed the antiviral activity of the synthetic rocaglate CR-31-B (−) against SARS-CoV-2 using both in vitro and ex vivo cell culture models. In Vero E6 cells, CR-31-B (−) inhibited SARS-CoV-2 replication with an EC50 of ~1.8 nM. In primary human airway epithelial cells, CR-31-B (−) reduced viral titers to undetectable levels at a concentration of 100 nM. Reduced virus reproduction was accompanied by substantially reduced viral protein accumulation and replication/transcription complex formation. The data reveal a potent anti-SARS-CoV-2 activity by CR-31-B (−), corroborating previous results obtained for other coronaviruses and supporting the idea that rocaglates may be used in first-line antiviral intervention strategies against novel and emerging RNA virus outbreaks.
Keywords: Rocaglate, SARS-CoV-2, COVID-19, Antiviral activity, eIF4A, Translation initiation
Graphical abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a member of the genus Betacoronavirus (Gorbalenya et al., 2020), causes a potentially severe respiratory illness, coronavirus disease 2019 (COVID-19), with case fatality rates ranging from 0.5% to 1% according to recent estimates (Rajgor et al., 2020; Meyerowitz-Katz and Merone, 2020). Following its rapid global spread, the World Health Organization declared COVID-19 a pandemic in March 2020 (WHO, 2020). To date, no approved therapeutic is available against any human coronavirus but a number of investigational antiviral compounds that target viral functions have entered clinical trials (Brown et al., 2019; de Wit et al., 2020; Sheahan et al., 2017; de Wilde et al., 2014; Choy et al., 2020; Sanders et al., 2020). Moreover, compounds that modulate the human immune system and/or have an anti-inflammatory effect are also being tested for their potential to reduce the severity of COVID-19 progression (Oldenburg and Doan, 2020; Guaraldi et al., 2020; Jalkanen et al., 2020; Lucas et al., 2009). Finally, antivirals that target host mechanisms critical to viral replication are being developed, including inhibitors targeting host proteases required to activate the fusogenic activity of the SARS-CoV-2 spike protein (Bestle et al., 2020; Hoffmann et al., 2020).
We and others have focused on approaches that inhibit viral protein synthesis, another host function critical to viral proliferation. Incidentally, a recently published SARS-CoV-2 protein interaction map identified the host translational machinery as a top target for repurposing drugs to block SARS-CoV-2 (Gordon et al., 2020). In particular, two eukaryotic factors involved in the initiation phase of translation, eukaryotic initiation factor-1A (eIF1A) and eukaryotic initiation factor-4A (eIF4A), have been singled out for further development. eIF1A is a small protein that binds to the 40S ribosome subunit-mRNA complex (Passmore et al., 2007), and eIF4A is a DEAD-box RNA helicase central to the activity of the eukaryotic translation initiation complex eIF4F (Chu and Pelletier, 2015). As of this writing, the eIF1A inhibitor aplidin (PharmaMar) has entered a Phase 2 clinical trial for the treatment of COVID-19, and the eIF4A inhibitor zotatifin (eFFECTOR Therapeutics) is undergoing evaluation for potential COVID-19 clinical development (Harrison, 2020; Bronstrup and Sasse, 2020).
We recently demonstrated the antiviral potency of rocaglates, a class of natural and synthetic compounds, against MERS-CoV and the common cold human coronavirus HCoV-229E in vitro and ex vivo (Müller et al., 2018a, 2020). Natural (e.g. rocaglamide A, silvestrol) and synthetic rocaglates (CR-31-B (−), zotatifin) are highly specific nanomolar eIF4A inhibitors. Rocaglates form stacking interactions with polypurine sequences in the 5′-untranslated regions (UTRs) of capped mRNAs, clamping the mRNAs onto eIF4A and stalling mRNA unwinding, which results in a dissociation of the mRNA-eIF4A complex from eIF4E and eIF4G. Because eIF4A-mediated translation is key to the activation of a majority of oncogenes, several of these selective rocaglates are in preclinical cancer studies and at least one compound, zotatifin, has been advanced into early stage clinical studies (Bordeleau et al., 2008; Lucas et al., 2009; Kogure et al., 2013; Patton et al., 2015; Ernst et al., 2020).
Many viral RNAs contain 5′-UTRs with stable RNA structures (Madhugiri et al., 2016; Schlereth et al., 2016) and are thus dependent on eIF4A for translation. Silvestrol exhibits broad-spectrum antiviral activity against a range of RNA viruses such as Ebola virus, Zika virus, Chikungunya virus, Crimean Congo hemorrhagic fever virus, Lassa virus, hepatitis E and several coronaviruses (Biedenkopf et al., 2017; Elgner et al., 2018; Henss et al., 2018; Müller et al., 2018a, 2020; Glitscher et al., 2018; Todt et al., 2018). While silvestrol and other natural rocaglates represent promising therapeutic leads due to their activities in the low nanomolar range and high selectivity indices (≥100) in primary cells, their natural availability is limited (Pannell, 1998) and their chemical synthesis is challenging due to their complex structures (Adams et al., 2009; Pan et al., 2014). As an alternative, a variety of synthetic rocaglate analogs have been generated that exhibit similar or enhanced eIF4A-targeting characteristics and can be produced to high purity and in large quantities.
Recently, we compared the antiviral activity of silvestrol with that of CR-31-B (−), which lacks the dioxane moiety of silvestrol and makes it structurally less complex and more straightforward to synthesize (Wolfe et al., 2014). We were able to show that, similar to silvestrol, CR-31-B (−) inhibits the replication of a range of RNA viruses both in vitro and ex vivo (Müller et al., 2020). Here, we evaluated the potential activity of the synthetic rocaglate CR-31-B (−) against SARS-CoV-2 using both in vitro and human ex vivo cell culture systems.
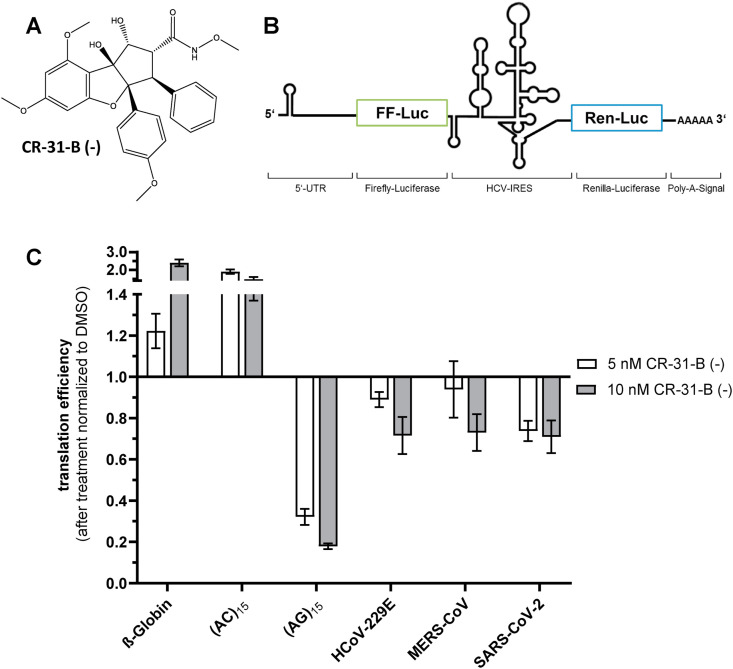
In a first set of experiments, we analyzed potential inhibitory effects of CR-31-B (−) (Fig. 1 A) on 5′-UTRs of different coronaviruses including SARS-CoV-2 in a dual luciferase reporter assay (Fig. 1B) to assess whether translation of mRNAs containing these viral 5′-UTRs depends on eIF4A. Cap-dependent translation of the firefly luciferase gene was measured and transfection efficiencies were normalized to renilla luciferase expression, which is under control of an eIF4A-independent IRES element from HCV. The short and unstructured 5′-UTR of the beta-globin gene and an unstructured (AC)15 sequence served as negative controls, since these 5′-UTR sequences were shown to be not repressible upon inhibition of eIF4A (Müller et al., 2018a, 2020). The polypurine sequence (AG)15 was used as a positive control since this sequence can be efficiently clamped onto the surface of eIF4A by different rocaglates due to π-π stacking interactions (Iwasaki et al., 2019; Müller et al., 2020). Using this experimental setup, eIF4A-dependency can be inferred from sensitivity of firefly luciferase mRNA translation to the presence of a specific eIF4A inhibitor. The data revealed that the 5′-UTRs of SARS-CoV-2, HCoV-229E and MERS-CoV were similarly sensitive to eIF4A-dependent translation inhibition by CR-31-B (−) in the dual luciferase reporter assay, when 10 nM CR-31-B (−) was used (Fig. 1C), indicating that CR-31-B (−) may have also antiviral activity against SARS-CoV-2.
Fig. 1.
Effect of CR-31-B (−) on dual reporter gene expression from constructs containing different 5′-UTRs. (A) Structure of the synthetic rocaglate CR-31-B (−). (B) Schematic illustration of the dual luciferase reporter construct used to determine eIF4A-dependent translation of coronavirus 5′-UTRs. (C) Effects of 5 and 10 nM CR-31-B (−) on reporter gene expression in the context of 5′-UTRs from three human coronaviruses, HCoV-229E, MERS-CoV and SARS-CoV-2. The 5′-UTR of the human β-globin mRNA and the unstructured (AC)15 sequence served as negative controls, while the (AG)15 polypurine sequence served as a positive control. Experiments were performed using previously described protocols (Müller et al., 2018a, 2020) with at least three independent biological replicates.
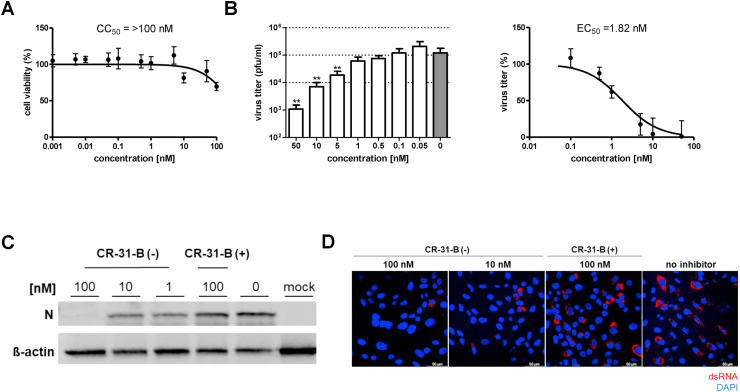
To analyze possible antiviral effects of CR-31-B (−) in cell culture, African green monkey Vero E6 cells were used (Ogando et al., 2020). No major cytotoxicity was detected for concentrations of up to 100 nM, with cell viability being reduced by about 10–25% at this highest concentration tested (Fig. 2 A). Moreover, the production of infectious SARS-CoV-2 progeny was found to be reduced in a dose-dependent manner with an EC50 of ~1.8 nM (Fig. 2B), which is in a similar range as the CR-31-B (−) EC50 values reported previously for other coronaviruses (~2.9 nM for HCoV-229E; ~1.9 nM for MERS-CoV) (Müller et al., 2020). The selectivity index (CC50/EC50) for SARS-CoV-2-infected Vero E6 cells was determined to be > 50. Next, we analyzed the effect of CR-31-B (−) on SARS-CoV-2 protein accumulation and the formation of viral replication/transcription complexes in Vero E6 cells. Viral nucleocapsid (N) protein levels were found to be drastically reduced in the presence of 100 nM CR-31-B (−), and moderately reduced at concentrations of 10 or 1 nM (Fig. 2C). As expected, viral protein accumulation was not affected in the presence of 100 nM of the inactive (+)-enantiomer CR-31-B (+) nor was it affected in cells treated with solvent only. In line with this, the formation of SARS-CoV-2 replication/transcription complexes was impaired in infected cells treated with CR-31-B (−) (Fig. 2D).
Fig. 2.
Dose-dependent antiviral activity of the synthetic rocaglate CR-31-B (−) in SARS-CoV-2 infected Vero E6 cells. (A) Vero E6 cells were treated for 24 h with the indicated CR-31-B (−) concentrations. Cell viability (compared to that of untreated cells) was determined by MTT assay (n = 8) as described previously (Müller et al., 2018a). (B) SARS-CoV-2 titers in supernatants collected from infected Vero E6 cells (MOI = 0.1) treated with the indicated CR-31-B (−) concentrations were collected at 24 h p.i. (n = 6) and virus titers were determined by plaque assay. Significance levels compared to the results for untreated cells were determined by the two-tailed Mann Whitney U test and are indicated as follows: *, P < 0.05; **, P < 0.005. Data from six independent experiments were used to calculate the EC50 value by non-linear regression analysis. (C) Representative Western blot analysis of SARS-CoV-2 N protein accumulation (top panel) after treatment with CR-31-B (−) and CR-31-B (+). Vero E6 cells were infected with SARS-CoV-2 (MOI = 1) or left uninfected and treated with the indicated CR-31-B concentrations for 24 h p.i. Protein accumulation was analyzed by Western blotting using polyclonal rabbit anti-SARS nucleocapsid protein antibody (Rockland) and mouse-anti actin antibody (abcam), respectively, each diluted 1:500 in PBS containing 1% bovine serum albumin (BSA). Beta-actin (lower panel) was used as a loading control (n = 3). (D) Representative immunofluorescence analysis to determine the effects of CR-31-B (+) and CR-31-B (−) on viral dsRNA accumulation in SARS-CoV-2-infected Vero E6 cells. Cells were infected (MOI = 1) and cultivated in medium containing the indicated CR-31-B concentrations. Cells were fixed at 24 h p.i. and analyzed by confocal microscopy using a mouse anti-dsRNA mAb (J2, SCICONS English & Scientific Consulting Kft, red) that detects a viral RNA replication intermediate (red) (Müller et al., 2018b). Cell nuclei were stained with DAPI (blue) (n = 2). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
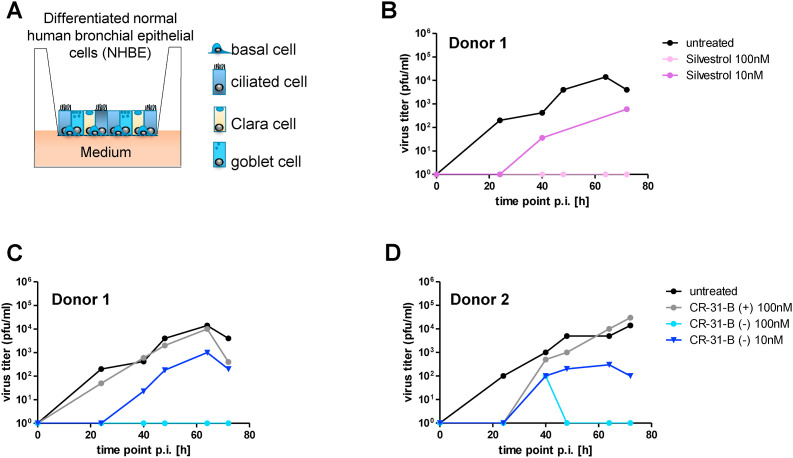
To further evaluate the antiviral potential of CR-31-B (−) in a biologically relevant ex vivo respiratory cell culture system, we analyzed air/liquid interface (ALI) cultures of differentiated primary normal human bronchial epithelial (NHBE) cells obtained from two different donors. ALI cultures are increasingly recognized as an excellent culture model mimicking the tracheobronchial region of the human respiratory tract and thus enabling respiratory infection research in a physiologically relevant cellular environment (Jonsdottir and Dijkman, 2016). Differentiated NHBE cells (Fig. 3 A) were infected with SARS-CoV-2 in the presence of inhibitor, CR-31-B (−) or silvestrol (obtained from the Sarawak Biodiversity Center, Kuching; North-Borneo, Malaysia; purity > 99%), the inactive enantiomer CR-31-B (+) or solvent control (untreated). Silvestrol was included as a reference in this experiment because this rocaglate has previously been tested extensively against a broad range of viruses. Silvestrol treatment reduced SARS-CoV-2 titers at different time points p.i. about 10 to 100-fold when used at a concentration of 10 nM, while virus replication in NHBE cells was completely abolished at a concentration of 100 nM (Fig. 3B). Next, we analyzed the effects of CR-31-B on viral replication in NHBE cells. CR-31-B (−) reduced the production of infectious virus progeny by ~1.5 log steps at a concentration of 10 nM in differentiated NHBE cells obtained from two different donors. At 100 nM, CR-31-B (−) reduced SARS-CoV-2 titers to undetectable levels, whereas the enantiomer CR-31-B (+) did not affect viral replication compared to the solvent control (Fig. 3C and D). No obvious cytotoxicity could be observed at this concentration using light microscopy.
Fig. 3.
Comparison of antiviral effects of CR-31-B (−) and silvestrol using differentiated NHBE cells infected with SARS-CoV-2. (A) NHBE cells (Lonza, CC-2540) obtained from a 13-year-old Caucasian boy (Donor 1) and a 36-year-old Caucasian man (Donor 2), which both were non-smoking and lacking respiratory pathology, were seeded on collagen IV-coated transwell plates (Corning Costar, CLS3470-48 EA) and grown in a mixture of DMEM (Invitrogen) and BEGM (Lonza; CC-4175) supplemented with retinoic acid (75 nM). After reaching confluence, the cells were cultivated under air-liquid conditions for at least four additional weeks to allow differentiation into pseudostratified human airway epithelia. (B and C) Differentiated NHBE cells were infected with SARS-CoV-2 (MOI = 3) and treated with silvestrol (B), CR-31-B (−) or CR-31-B (+) (C) at the indicated concentrations. At the indicated time points p.i., the apical surface of the cells was incubated with 100 μl PBS for 15 min and SARS-CoV-2 titers in the cell culture supernatants were determined by virus plaque assay.
The discovery and development of antivirals that target host mechanisms critical to viral proliferation is of growing importance. The rationale behind this approach is twofold: first, host mechanisms are not virus-specific, but are used by a broad range of viruses, and second, targeting a host mechanism preempts the risk for developing resistance typically associated with targeting viral structures or mechanisms. Several host-targeting approaches against SARS-CoV-2 are currently under investigation. Most of these represent efforts to repurpose approved drugs with known safety profiles.
Here, we added to the antiviral toolbox against SARS-CoV-2 the synthetic rocaglate CR-31-B (−), a specific inhibitor of eIF4A-dependent mRNA translation. We identified CR-31-B (−) as a potent and non-cytotoxic inhibitor of SARS-CoV-2 replication in vitro and ex vivo. The observed antiviral activities are directly comparable to those that we reported previously for other coronaviruses, namely HCoV-229E and MERS-CoV, and a range of highly pathogenic positive- and negative-sense RNA viruses. To our knowledge, this is the first report of a rocaglate inhibiting SARS-CoV-2 proliferation in a relevant ex vivo human bronchial cell system.
Against the backdrop of a number of reports we and others have published over the past three years documenting the broad-spectrum antiviral activity of CR-31-B (−) and related rocaglates, the data we report here strengthen the case for the potential use of rocaglates as first-line, broad-spectrum ‘pan-antivirals’. The necessary next step is the in vivo evaluation of CR-31-B (−) and other rocaglates to confirm safety and determine appropriate dosing regimens. Extensive preclinical work with CR-31-B (−), zotatifin, and other rocaglates as cancer therapeutics is a priori highly encouraging for the potential antiviral application of rocaglates in humans. Minimal or no toxicities were reported for long-term dosing regimens in animal models, along with favorable safety, ADME- and bioavailability profiles (Wolfe et al., 2014; Chan et al., 2019; Rodrigo et al., 2012). In addition, the advancement of zotatifin into the clinic supports the potential of this class of molecules to be used as antivirals in humans.
In summary, the path to clinical implementation is still long and will most likely require further optimization of CR-31-B (−) or other lead rocaglates currently under investigation. Nevertheless, the antiviral efficacy against viruses from diverse families, the low toxicity of this class of compounds both in vitro and ex vivo, and the ongoing advanced efforts to harness their activity as cancer therapeutics lead us to suggest that rocaglates represent a potentially powerful addition to the toolbox of therapeutic options for mitigating the consequences of global outbreaks by newly emerging RNA viruses.
Funding
The work was supported by the LOEWE Center DRUID (projects A2 and B2 to A.G. and J.Z.), the German Center for Infection Research (DZIF), partner site Giessen-Marburg-Langen (TTU Emerging Infections, to J.Z. and S.P.), Deutsche Forschungsgemeinschaft (SFB 1021 ‘RNA viruses: RNA metabolism, pathogenesis and host response’; projects A01 and A02, to J.Z. and R.K.H., respectively and project C01 to S.P.; CRU KFO309, project P3 to J.Z.), and the BMBF project HELIATAR (A.G. and J.Z). Further support to H.G.W. were from the NCI Cancer Center Support Grant to MSKCC (CCSG, P30 CA08748), the Starr Cancer Consortium (GC230724) and from NIH grants RO1CA183876-05, RO1CA207217–03, R35 CA252982-01, P50 CA192937-03, P50 CA217694), LLS 7014-17, LLS 1318-15.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Ulrike Wend for her excellent technical assistance.
References
- Adams T.E., El Sous M., Hawkins B.C., et al. Total synthesis of the potent anticancer Aglaia metabolites (-)-silvestrol and (-)-episilvestrol and the actice analogue (-)-4'-desmethoxyepisilvestrol. J. Am. Chem. Soc. 2009;131:1607–1616. doi: 10.1021/ja808402e. [DOI] [PubMed] [Google Scholar]
- Bestle D., Heindl M.R., Limburg H., et al. TMPRSS2 and furin are both essential for proteolytic activation of SARS-CoV-2 in human airway cells. Life Sci Alliance. 2020;3 doi: 10.26508/lsa.202000786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedenkopf N., Lange-Grünweller K., Schulte F.W., et al. The natural compound silvestrol is a potent inhibitor of Ebola virus replication. Antivir. Res. 2017;137:76–81. doi: 10.1016/j.antiviral.2016.11.011. [DOI] [PubMed] [Google Scholar]
- Bordeleau M.E., Robert F., Gerard B., et al. Therapeutic suppression of translation initiation modulates chemosensitivity in a mouse lymphoma model. J. Clin. Invest. 2008;118(7):2651–2660. doi: 10.1172/JCI34753. PMID: 18551192; PMCID: PMC2423864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronstrup M., Sasse F. Natural products targeting the elongation phase of eukaryotic protein biosynthesis. Nat. Prod. Rep. 2020;37:752–762. doi: 10.1039/d0np00011f. [DOI] [PubMed] [Google Scholar]
- Brown A.J., Won J.J., Graham R.L., et al. Broad spectrum antiviral remdesivir inhibits human endemic and zoonotic deltacoronaviruses with a highly divergent RNA dependent RNA polymerase. Antirviral Res. 2019;169:104541. doi: 10.1016/j.antiviral.2019.104541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K., Robert F., Oertlin C., et al. eIF4A supports an oncogenic translation program in pancreatic ductal adenocarcinoma. Nat. Commun. 2019;10:5151. doi: 10.1038/s41467-019-13086-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy K.T., Wong A.Y., Kaewpreedee P., et al. Remdesivir, Iopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antivir. Res. 2020;178:104786. doi: 10.1016/j.antiviral.2020.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J., Pelletier J. Targeting the eIF4A RNA helicase as an anti-neoplastic approach. Biochim. Biophys. Acta. 2015;1849:781–791. doi: 10.1016/j.bbagrm.2014.09.006. [DOI] [PubMed] [Google Scholar]
- de Wilde A.H., Jochmans D., Posthuma C.C., et al. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob. Agents Chemother. 2014;58:4875–4884. doi: 10.1128/AAC.03011-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit E., Feldmann F., Cronin J., et al. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc. Natl. Acad. Sci. U. S. A. 2020;117 doi: 10.1073/pnas.1922083117. 67771-6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgner F., Sabino C., Basic M., et al. Inhibition of Zika virus replication by silvestrol. Viruses. 2018;10:149. doi: 10.3390/v10040149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst J.T., Thompson P.A., Nilewski C., et al. Design of development candidate eFT226, a first in class inhibitor of eukaryotic initiation factor 4A RNA helicase. J. Med. Chem. 2020;11:5879–5955. doi: 10.1021/acs.jmedchem.0c00182. [DOI] [PubMed] [Google Scholar]
- Glitscher M., Himmelsbach K., Woytinek K., et al. Inhibition of hepatitis E virus spread by the natural compound silvestrol. Viruses. 2018;10:301. doi: 10.3390/v10060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A.E., Baker S.C., Baric R.S., et al. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon E.G., Jang G.M., Bouhaddou M., et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guaraldi G., Meschiari M., Cozzi-Lepri A., et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020;2:e474–e484. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison C. Drug researchers pursue new lines of attack against COVID-19. Nat. Biotechnol. 2020;38:659–662. doi: 10.1038/d41587-020-00013-z. [DOI] [PubMed] [Google Scholar]
- Henss L., Scholz T., Grünweller A., Schnierle B.S. Silvestrol inhibits Chikungunya virus replication. Viruses. 2018;10:592. doi: 10.3390/v10110592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman M., Kleine-Weber H., Schroeder S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki S., Iwasaki W., Takahashi M., et al. The translation inhibitor rocaglamide targets a bimolecular cavity between eIF4A and polypurine RNA. Mol. Cell. 2019;73:738–748. doi: 10.1016/j.molcel.2018.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalkanen J., Hollmén M., Jalkanen S. Interferon beta-1a for COVID-19: critical importance of the administration route. Crit. Care. 2020;24:335. doi: 10.1186/s13054-020-03048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsdottir H.R., Dijkman R. Coronaviruses and the human airway: a universal system for virus-host interaction studies. Virol. J. 2016;13:24. doi: 10.1186/s12985-016-0479-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogure T., Kinghorn A.D., Yan I., et al. Therapeutic potential of the translation inhibitor silvestrol in hepatocellular cancer. PLoS One. 2013;8 doi: 10.1371/journal.pone.0076136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas D.M., Edwards R.B., Lozanski G., et al. The novel plant-derived agent silvestrol has B-cell selective activity in chronic lymphocytic leukemia and acute lymphoblastic leukemia in vitro and in vivo. Blood. 2009;113:4656–4666. doi: 10.1182/blood-2008-09-175430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhugiri R., Fricke M., Marz M., Ziebuhr J. Coronavirus cis-acting RNA elements. Adv. Virus Res. 2016;96:127–163. doi: 10.1016/bs.aivir.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerowitz-Katz G., Merone L. A systematic review and meta-analysis of published research data on COVID-19 infection fatality rates. Int. J. Infect. Dis. 2020;101:138–148. doi: 10.1016/j.ijid.2020.09.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller C., Schulte F.W., Lange-Grünweller K., et al. Broad-spectrum antiviral activity of the eIF4A inhibitor silvestrol against corona- and picornaviruses. Antivir. Res. 2018;150:123–129. doi: 10.1016/j.antiviral.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller C., Hardt M., Schwudke D., et al. Inhibition of cytosolic phospholipase A2alpha impairs an early step of coronavirus replication in cell culture. J. Virol. 2018;92 doi: 10.1128/JVI.01463-17. e01463-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller C., Obermann W., Schulte F.W., et al. Comparison of broad-spectrum antiviral activities of the synthetic rocaglate CR-31-B (-) and the eIF4A-inhibitor Silvestrol. Antivir. Res. 2020;175:104706. doi: 10.1016/j.antiviral.2020.104706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogando N.S., Dalebout T.J., Zevenhoven-Dobbe J.C., et al. SARS-coronavirus-2 replication in Vero E6 cells: replication kinetics, rapid adaptation and cytopathology. J. Gen. Virol. 2020;101:925–940. doi: 10.1099/jgv.0.001453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenburg C.E., Doan T. Azithromycin for severe COVID-19. Lancet. 2020;6736:31863–31868. doi: 10.1016/S0140-6736(20)31863-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L., Wooard J.L., Lucas D.M., et al. Rocaglamide, silvestrol and structurally related bioactive compounds from Aglaia species. Nat. Prod. Rep. 2014;31:924–939. doi: 10.1039/c4np00006d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannell C.M. 1998. Aglaia Foveolate. The IUCN Red List of Threatened Species. [DOI] [Google Scholar]
- Passmore L.A., Schmeing T.M., Maag D. The eukaryotic translation initiation factors eIF1 and eIF1A induce an open conformation of the 40S ribosome. Mol. Cell. 2007;26:41–50. doi: 10.1016/j.molcel.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Patton J.T., Lustberg M.E., Lozanski G., et al. The translation inhibitor silvestrol exhibits direct anti-tumor activity while preserving innate and adaptive immunity against EBV-driven lymphoproliferative disease. Oncotarget. 2015;6:2693–2708. doi: 10.18632/oncotarget.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajgor D.D., Lee M.H., Archuleta S., et al. The many estimates of the COVID-19 case fatality rate. Lancet Infect. Dis. 2020;20:776–777. doi: 10.1016/S1473-3099(20)30244-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigo C.M., Cencic R., Roche S.P., et al. Synthesis of rocaglamide hydroxamates and related compounds as eukaryotic translation inhibitors: synthetic and biological studies. J. Med. Chem. 2012;55:558–562. doi: 10.1021/jm201263k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. J. Am. Med. Assoc. 2020;323:1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- Schlereth J., Grünweller A., Biedenkopf N., et al. RNA binding specificity of Ebola virus transcription factor VP30. RNA Biol. 2016;13:783–798. doi: 10.1080/15476286.2016.1194160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan T.P., Sims A.C., Graham R.L., et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017;9:396. doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todt D., Moeller N., Praditya D., et al. The natural compound silvestrol inhibits hepatitis E virus (HEV) replication in vitro and in vivo. Antivir. Res. 2018;157:151–158. doi: 10.1016/j.antiviral.2018.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . World Health Organization. Coronavirus Sease 2019 (COVID-19) Situation Report- 51. 2020. [Google Scholar]
- Wolfe A.L., Singh K., Zhong Y., et al. RNA G-quadruplexes cause eIF4A-dependent oncogene translation in cancer. Nature. 2014;513:65–70. doi: 10.1038/nature13485. [DOI] [PMC free article] [PubMed] [Google Scholar]