Abstract
Objective:
To quantify the extent to which payments for laparoscopic and open colectomy are influenced by a surgeon’s experience with laparoscopy.
Background:
Numerous studies suggest that healthcare costs for laparoscopic colectomy are lower than open surgery. None have assessed the importance of surgeon experience on the relative financial benefits of laparoscopy.
Methods:
We conducted a study of 182,852 national Medicare beneficiaries undergoing laparoscopic or open colectomy between 2010 and 2012. Using instrumental variable methods to account for selection bias, we compared Medicare payments for laparoscopic and open colectomy. We stratified our analysis by surgeons’ annual experience with laparoscopic colectomy to determine the influence of provider experience on payments.
Results:
In the fully adjusted analysis, average episode payments per patient were $2640 [95% confidence interval (CI) −$4091 to −$1189] lower with the laparoscopic approach versus open. Surgeons in the highest quartile of laparoscopic experience demonstrated an average payment savings of $5456 per patient (CI −$7918 to −$2994) in their laparoscopic versus open cases. Among surgeons in the lowest quartile of laparoscopic experience, there was, however, no difference between laparoscopic and open cases (difference: $954, 95% CI −$731 to $2639). Differences in payments were explained by differences in complications rates. Both groups had similar rates of complications for open procedures (least experience, 21%, most experience, 21%; P = 0.45), but differed significantly on rates of complications for laparoscopic cases (least experience, 28%, most experience, 15%; P < 0.01).
Conclusions:
This population-based study demonstrates that differences in payments between laparoscopic and open colectomy are influenced by surgeon experience. The laparoscopic approach does not reduce payments for patients whose surgeons have limited experience with the procedure.
Keywords: instrumental variable analysis, surgeon experience and healthcare costs, laparoscopic colectomy
There is a considerable amount of research suggesting that laparoscopic operations have lower costs compared to traditional open surgery. Several studies focusing on colectomy, and other major abdominal operations, demonstrate a 20% to 50% reduction in costs with the laparoscopic versus open approach.1–5 Lower costs are attributable to shorter lengths of stay and lower complication rates.6,7 These studies contribute to perceptions that laparoscopic surgery is always less expensive, despite concerns about technology and equipment costs. There are growing efforts to reduce the costs of surgical hospitalizations, including numerous policies that use financial penalties to motivate change (eg, bundled payments or accountable care organizations).8–10
It is unclear whether all surgeons, particularly those with limited laparoscopic experience, attain the full financial benefits of the minimally invasive approach. In other domains, such as patient safety or readmissions, the importance of surgeon experience or proficiency has received significant attention.11–14 Few studies have assessed the direct relationship between surgeon experience and the absolute or relative costs of laparoscopic and traditional open surgery. This information is important because the use of minimally invasive technologies may represent 1 leverage point through which surgeons can augment current practices to reduce the costs of care.
To address this question, we conducted a population-based study of Medicare beneficiaries undergoing laparoscopic or open colectomy. We used instrumental variable methods to account for selection bias between patients undergoing each approach. After grouping surgeons by their annual experience with laparoscopic colectomy, we assessed the differences in Medicare payments between laparoscopic versus open surgery. We then evaluated payments for complications, readmissions, and postacute care to determine the mechanisms of payment differences between approaches for surgeons with differing levels of experience.
METHODS
Data Source and Study Population
We used national data from the 100% Medicare Provider Analysis and Review (MEDPAR) files for the years 2010 to 2012. The Centers for Medicare & Medicaid Services maintains this database from claims submitted by hospitals where Medicare beneficiaries receive care. We collected data on age, demographic information, and comorbidities. We linked patient records to other Medicare files containing claims relevant to the surgical episode of care. These data included durable medical equipment, home health, long-stay hospitalizations, outpatient, and skilled nursing facility claims. We selected patients undergoing colon resection using International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) codes (45.73, 17.33, 17.32, 45.75, 45.76, 17.35, 17.36, 45.74, 17.34, 45.82, 45.83, 45.81, 48.50, 48.51, 48.52, and 48.53). We excluded patients younger than 65 or older than 99 years of age. We further excluded patients not continuously enrolled in Medicare Part A and B at the time of operation.
Outcomes
Our primary outcome was price-standardized and risk-adjusted total episode payments. Payments, in contrast to charges or cost-to-charge ratios, reflect actual Medicare spending for surgical care. We extracted payment information for all service types for the index hospitalization and for all payments made by Medicare in the year after surgery. Payments were grouped into categories to identify mechanisms of savings associated with the use of laparoscopy. Categories included index hospitalization, physician, readmission, and postacute care payments. Postacute care payments include payments made by Medicare to skilled nursing or subacute rehabilitation facilities. Because geographic variation in spending can augment financial estimates, we price-standardized all payment amounts using methods that have been previously described and validated.15
Postoperative complications were also identified using ICD-9-CM codes using predefined categories including pulmonary failure (518.81, 518.4, 518.5, 518.8), pneumonia (481, 482.0–482.9, 483, 484,485,507.0), myocardial infarction (410.00–410.91), deep venous thrombosis/pulmonary embolism (415.1, 451.11, 451.19, 451.2, 451.81, 453.8), renal failure (584), surgical site infection (958.3, 998.3, 998.5, 998.59, 998.51), gastrointestinal bleeding (530.82, 531.00–531.21, 531.40, 531.41, 531.60, 531.61, 532.00–532.21, 532.40, 532.41, 532.60, 532.61, 533.00–533.21, 533.40, 533.41, 533.60, 533.61, 534.00–534.21, 534.40, 534.41, 534.60, 534.61, 535.01, 535.11, 535.21, 535.31, 535.41, 535.51, 535.61, 578.9), and hemorrhage (998.1).These complications represent a subset of ICD-9 codes with the highest sensitivity and specificity as has been previously described.16 For certain analyses we grouped complications as medical or surgical. Medical complications included pulmonary failure, pneumonia, myocardial infarction, deep venous thrombosis/pulmonary embolism, and renal failure. Surgical complications were surgical site infection (superficial, deep, and organ space), hemorrhage, and any reoperation. Finally, we determined discharge destinations using discharge codes contained in the Medicare Provider Analysis and Review file. Of particular interest were patients discharged to rehabilitation, skilled nursing, or another inpatient facility.
Statistical Analysis
Baseline patient characteristics were compared after stratifying about the median regional use of laparoscopy (23%). We further stratified patients by the annual experience of their surgeon with laparoscopic colectomy. All comparisons were made by calculating standardized differences for both continuous and dichotomous variables. These methods have been previously described.17 An absolute standardized difference (ASD) of more than 10 is indicative of significant differences between comparison groups. We used analysis of variance, Mann-Whitney U, and t tests where appropriate to evaluate differences in characteristics.
Our primary objective was to evaluate the independent effect of laparoscopy on Medicare payments. For all models with payments as outcomes, we adjusted for differences in patient illness using Hierarchical Condition Categories (HCC).18–20 These categories are assembled from data on patient’s age and medical comorbidities. They were designed and validated by Centers for Medicare & Medicaid Services for risk adjustment of Medicare payments and are considered more accurate comorbidity counts when the outcome of interest is financial data.19 For models adjusting for complications, readmission, or post-acute care services we used standard Elixhauser comorbidities.21 We further adjusted for year-to-year differences in payment using categorical dummy variables. We also accounted for regional differences in payments not otherwise accounted for by price standardization using categorical dummy variables for each hospital referral region (HRR) as a fixed effect.
We used instrumental variable methods to address selection bias not accounted for by conventional multivariable analysis.22 This is necessary because we hypothesize that patients selected for laparoscopic operations will trend toward having lower payments and better outcomes because patients may have more favorable anatomy or are healthier in general. This would incorrectly inflate the financial benefits of laparoscopy over open surgery. Instrumental variable methods are an econometric technique used to balance measured and unmeasured differences in characteristics between 2 or more comparison groups. Instrumental variables must correlate with the exposure (laparoscopy), but cannot be associated with the outcome (Medicare payments) except through the relationship to the exposure. This latter criterion is referred to as exogeneity. Our instrumental variable was the regional use of the laparoscopic approach. We calculated the proportion of colon resections performed laparoscopically for each HRR. To ensure that the instrument was exogenous, we excluded the hospital in which the patient received their operation.
The interpretation of instrumental variable results is unique. These estimates apply to the marginal patient. This is an individual who would be considered a candidate for either operation. Instrumental variable estimates do not apply to patients who are clearly a candidate for 1 approach or the other. These analyses capitalize on the nature variation with which laparoscopy is performed across HRRs to pseudorandomize patients and balance both known and unknown characteristics.
We evaluated the instrumental variable in several ways. We confirmed its relationship to our exposure, the receipt of laparoscopic colectomy (F statistic = 244). An F statistic of more than 10 is suggestive of a strong instrument. We confirmed that the instrumental variable approach was necessary using the Durbin-Wu-Hausman tests of endogeneity. These were significant for all instrumental variable models. This indicates that standard multivariable regression resulted in biased estimates when compared with the instrumental variable model.22
We employed a 2-stage least squares method for our instrumental variable analysis. This method has been validated in financial analyses with healthcare data.23 The first stage model assessed the association between receipt of laparoscopic colectomy and the instrumental variable. We adjusted for HCCs (or Elixhauser comorbidities depending on the outcome), year of operation, and regional effects using HRRs. We used the linear prediction from this model in the second stage multiple linear regression model. This value replaces the categorical variable for laparoscopic or open surgery. The second stage model is used to generate estimates of local average treatment effect for laparoscopic compared to open colectomy. In this setting, the local average treatment effect is the coefficient (β) for the covariate.
Because we were interested in the influence of surgeons’ experience with laparoscopy, we calculated each surgeon’s annual number of laparoscopic and open colectomy procedures with Medicare beneficiaries. We identified surgeons by unique provider identification numbers. We selected those on record as the primary operation using a method previously described.24 We grouped surgeons in to quartiles groups based on their annual experience with laparoscopic colectomy. For the analyses, we combined the middle quartiles to improve generalizability and simplify reporting of the data. The quartiles therefore represent the bottom 25% (1–3 cases), middle 50% (4–8 cases), and top 25% (9–55 cases) with respect to annual experience of laparoscopic colectomy.
For all estimates, we used bootstrapping to generate confidence intervals (CIs) and the corresponding t statistics. The t statistics were generated from normal-based CIs derived from bootstrapping with 1000 replications, where draws were made at the hospital level to deal with clustering at the hospital level. We then used marginal means to generate absolutes estimates of Medicare payments based on surgical approach. Several sensitivity analyses were performed in an identical manner, restricting the patient population by clinical diagnosis, elective procedure status, or surgeon specialty.
All statistical analyses were performed using STATA statistical software version 14 (College Station, TX). We employed a 2-sided approach at the 5% significance level for all hypothesis testing. The present study was deemed exempt by the institutional review board at the University of Michigan.
RESULTS
Patient Characteristics
We compared patient characteristics, unadjusted outcome rates, and unadjusted total episode payments stratified by the median of our instrumental variable (Table 1). We report results across quartiles of surgeon experience to confirm that the instrumental variable balanced patient characteristics within each strata of surgeon experience. For patients treated by surgeons with the bottom quartile (least experience), for example, the proportion surgeries for cancer was similar above and below the median instrumental variable (44% vs 46%; ASD = 4.4). Results were similar for patients treated by surgeons in the middle (46% vs 47%; ASD = 6.1) and top quartiles (47% vs 47%; ASD = 2.9). This trend persisted for all measured patient characteristics, and thus confirming that the instrumental variable balanced patient factors both overall and within the predefined groups of surgeons. The distribution of diagnosis-related groups (DRGs) was evaluated in 2 ways. DRGs of 329, 330, and 331 were distributed evenly across surgeons stratified by experience. Furthermore, the proportion of DRGs was further balanced about the median IV within each stratification of experience.
TABLE 1.
Patient Characteristics by Surgeon Experience and Regional Use of Laparoscopy
| Bottom Quartile (Least Experience) | Middle Quartiles | Top Quartile (Most Experience) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Regional Use of Laparoscopy* | Regional Use of Laparoscopy* | Regional Use of Laparoscopy* | |||||||
| <Median (n = 29,277) | >Median (n = 35,718) | ASD‡ | <Median (n = 43,096) | > Median (n = 33,152) | ASD‡ | <Median (n = 26,601) | >Median (n = 11,834) | ASD‡ | |
| Age, yr | |||||||||
| Mean (SD) | 76 (8) | 76 (8) | 5.5 | 76 (7) | 76 (7) | 5.8 | 75 (7) | 76 (7) | 7.2 |
| Median (IQR) | 75 (12) | 76 (12) | 75 (12) | 76 (12) | 75 (11) | 76 (12) | |||
| Race/ethnicity, n (%) | |||||||||
| White | 25,946 (88) | 31,111 (87) | 6.5 | 38,780 (89) | 29,101 (88) | 4.4 | 26,601 (91) | 10,811 (91) | 8.1 |
| Black | 2347 (8) | 3065 (9) | 5.4 | 2996 (7) | 2545 (8) | 4.9 | 1821 (6) | 680 (6) | 3.7 |
| Comorbid conditions | |||||||||
| Mean (SD) | 3 (2) | 3 (2) | 2.2 | 2(2) | 2(2) | 4.3 | 2 (2) | 2 (2) | 3.1 |
| Median (IQR) | 2 (2) | 2 (2) | 2 (2) | 2 (2) | 2 (2) | 2 (2) | |||
| Length of stay, day | |||||||||
| Median (IQR) | 7 (7) | 7 (7) | 1.8 | 6 (6) | 6 (6) | 3.9 | 6 (5) | 6 (6) | 5.2 |
| Specific comorbidities, n (%) | |||||||||
| Congestive heart failure | 2957 (10) | 3552 (10) | 1.4 | 3934 (9) | 2876 (9) | 1.1 | 2255 (8) | 881 (7) | 2.2 |
| Diabetes mellitus | 5647 (19) | 6685 (19) | 1.2 | 8248 (19) | 6409 (19) | 1.1 | 5453 (18) | 2215 (19) | 2.7 |
| Liver disease | 380 (1) | 419 (1) | 1.0 | 517(1) | 442 (1) | 1.0 | 387 (1) | 154(1) | 1.4 |
| Renal failure | 2398 (8) | 2787 (8) | 1.1 | 3282 (8) | 2414 (7) | 2.2 | 1976 (7) | 763 (6) | 1.6 |
| Obesity | 2168 (7) | 2398 (7) | 2.1 | 3168 (7) | 2317 (7) | 1.8 | 2238 (8) | 830 (7) | 1.9 |
| Depression | 1781 (6) | 2150 (6) | 1.0 | 2730 (6) | 2019 (6) | 1.3 | 1732 (6) | 716 (6) | 2.0 |
| Operative indication, n (%) | |||||||||
| Malignancy | 12,848 (44) | 16,370 (46) | 4.4 | 19,690 (46) | 15,705 (47) | 6.1 | 13,773 (47) | 5641 (47) | 2.9 |
| Diverticular disease | 6704 (23) | 8109 (23) | 6.3 | 8534 (20) | 6683 (20) | 2.4 | 4998 (18) | 2213 (18) | 4.4 |
| Presentation, n (%) | |||||||||
| Elective | 17,424 (59) | 20,048 (56) | 9.2 | 29,156 (67) | 21,275 (64) | 9.5 | 22,759 (77) | 8605 (75) | 8.3 |
| Postoperative events, n (%) | |||||||||
| Complications | 7643 (26) | 9171 (26) | 6.2 | 9128 (21) | 7465 (22) | 6.4 | 4891 (17) | 2229 (18) | 9.1 |
| Death within 30 days | 1714 (6) | 2308 (6) | 6.0 | 1879 (4) | 1634 (5) | 4.0 | 867 (3) | 359 (3) | 7.0 |
| Costs, USD† | |||||||||
| Unadjusted total episode payments | $28,602 | $29,813 | 7.5 | $26,811 | $28,664 | 8.7 | $25,601 | $25,523 | 2.5 |
Regional use of laparoscopy—the instrumental variable is the proportion of colectomies performed laparoscopically within an HRR in a given year, excluding the hospital in which the specific beneficiary had their operation.
United States Dollars (USD, $).
Absolute standardized difference (ASD); an absolute standardized difference >10 (approximately equivalent to P < 0.05) indicates significant imbalance of baseline covariates. IQR indicates interquartile range; SD, standard deviation.
Total Episode Payments by Surgeon Experience and Approach
Next, we compared fully adjusted total episode payments between laparoscopic and open surgeries across each quartile of surgeon experience. Figure 1 shows that surgeon experience modified the effect of laparoscopy on total episode payments. The use of laparoscopy did not alter episode payments for surgeons with the least experience ($954, 95% CI −$731 to $2639). For these surgeons, payments were higher after laparoscopic ($26,915) compared to open surgery ($23,312, P < 0.01) as outlined in Table 2. For surgeons with the most experience, however, laparoscopy was associated with a sizable and statistically significant reduction in total episode payments (−$5456, 95% CI −$7918 to −$2994). As a result, average episode payments were lower for laparoscopic ($20476) compared to open surgery ($23793, P < 0.01) for these surgeons. Figure 1 demonstrates the stepwise reduction in payments with the use of laparoscopy (vs open) that is independently associated with surgeon experience.
FIGURE 1.
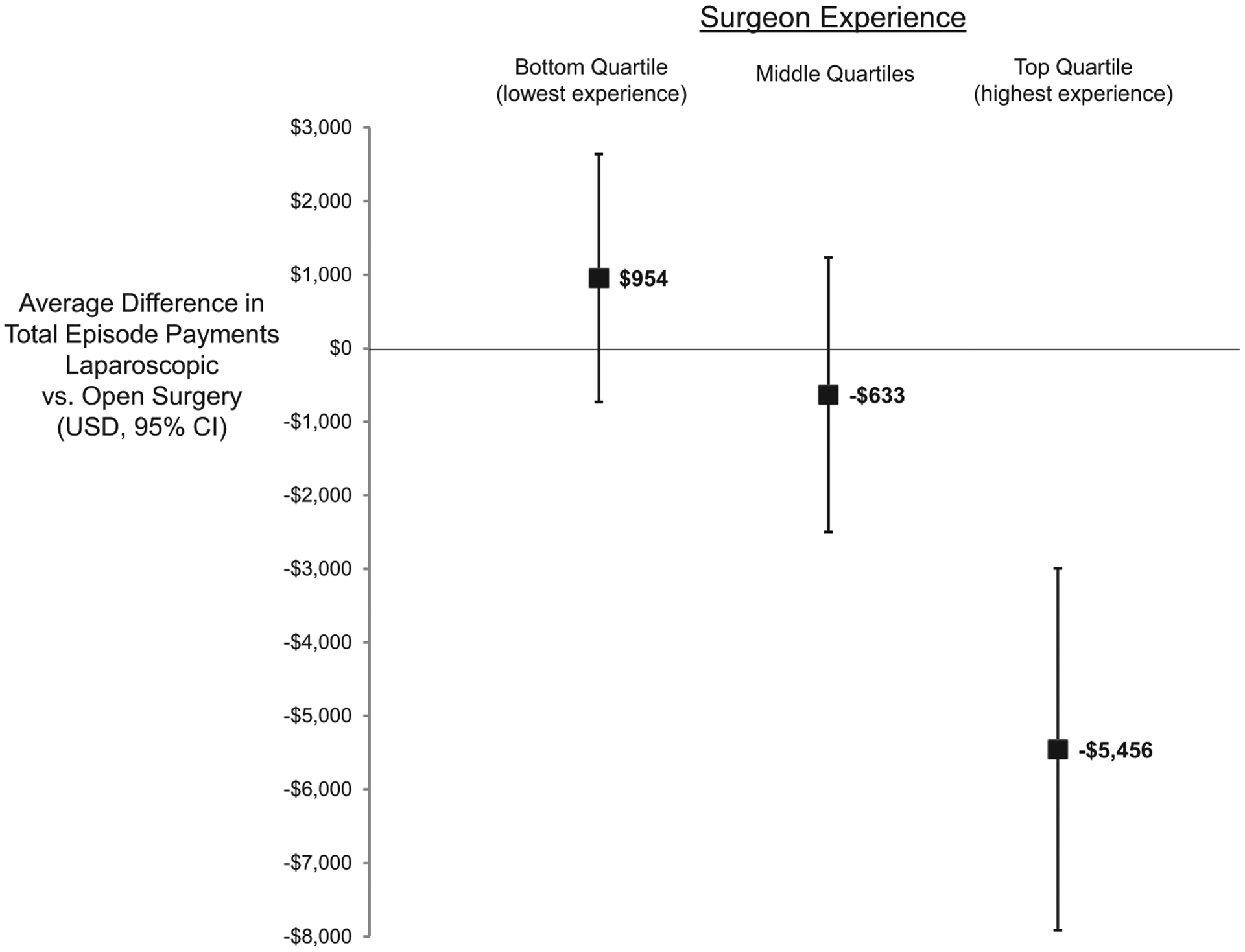
Independent influence of laparoscopic (vs open) surgery on total episode payments stratified by surgeon experience with laparoscopy.
TABLE 2.
Estimated Average Medicare Payments Laparoscopic and Open Surgery, Stratified by Surgeon Experience
| Laparoscopic Surgery | Open Surgery | |||||
|---|---|---|---|---|---|---|
| Type of Payment | No. Patients (%) | Payment (USD) | No. Patients (%) | Payment (USD) | Difference from Use of Laparoscopy | P |
| Bottom quartile (least experience) | ||||||
| Index hospitalization | 22,886 (100) | $17,760 | 42,201 (100) | $15,744 | $1851 | <0.01 |
| Physician payments when present | 22,315 (98) | $4045 | 42,136 (99) | $3490 | $898 | <0.01 |
| Average overall | 22,886 (100) | $3448 | 42,201 (100) | $4015 | $759 | <0.01 |
| Readmission payments when present | 2514 (11) | $10224 | 6820 (16) | $10434 | −$1457 | 0.28 |
| Average overall | 22,886 (100) | $1366 | 42,201 (100) | $1556 | −$252 | 0.29 |
| Postacute care payments when present | 11,548 (51) | $4556 | 29,482 (70) | $5442 | −$256 | 0.58 |
| Average overall | 22,886 (100) | $2722 | 42,201 (100) | $3582 | −$1403 | <0.01 |
| Total episode | 22886 (100) | $26915 | 42201 (100) | $23312 | $954 | 0.27 |
| Middle quartiles | ||||||
| Index hospitalization | 39,724 (100) | $14,764 | 36,784 (100) | $16,807 | $278 | 0.62 |
| Physician payments when present | 39,617 (99) | $3435 | 36,723 (99) | $4046 | $971 | 0.01 |
| Average overall | 39,724 (100) | $3426 | 36,784 (100) | $4038 | $970 | <0.01 |
| Readmission payments when present | 4000 (10) | $9811 | 6011 (16) | $9990 | −$2,650 | 0.06 |
| Average overall | 39,724 (100) | $1207 | 36,784 (100) | $1397 | −$558 | 0.03 |
| Postacute care payments when present | 21,567 (54) | $4095 | 25,920 (70) | $5039 | −$881 | 0.12 |
| Average overall | 39,724 (100) | $2430 | 36,784 (100) | $3334 | −$1323 | <0.01 |
| Total episode | 39,724 (100) | $21,828 | 36,784 (100) | $25,577 | −$633 | 0.51 |
| Top quartile (most experience) | ||||||
| Index hospitalization | 28,202 (100) | $14,003 | 13,055 (100) | $15,784 | −$2559 | 0.04 |
| Physician payments when present | 28,086 (99) | $3291 | 13,035 (99) | $3877 | $61 | 0.85 |
| Average overall | 28,202 (100) | $3282 | 13,055 (100) | $3860 | −$356 | 0.48 |
| Readmission payments when present | 2759 (10) | $9236 | 2298 (18) | $9392 | $1119 | 0.68 |
| Average overall | 28,202 (100) | $1090 | 13,055 (100) | $1256 | −$564 | 0.16 |
| Postacute care payments when present | 4499 (51) | $3756 | 9290 (71) | $4553 | −$927 | 0.24 |
| Average overall | 28,202 (100) | $2100 | 13,055 (100) | $2892 | −$1975 | 0.02 |
| Total episode | 28,202 (100) | $20,476 | 13,055 (100) | $23,793 | −$5456 | 0.01 |
USD indicates United States Dollar.
Episode Payment Components by Surgeon Experience and Approach
To better understand the source of payment differences, we assessed each component of the total Medicare payment (Table 2). For surgeons with the least experience, the use of laparoscopy (vs open) was associated with higher payments for the index hospitalization ($1851, 95% CI $803–$2899). In contrast, the use of laparoscopy (vs open) was associated with lower index hospitalization payments for surgeons with the most experience (−$2559, 95% CI −$4972 to −$147).
Surgeon experience had a variable effect on the relationship between laparoscopy and payments for physician services. Payments for physician services were higher for laparoscopic surgery (vs open) for surgeons with the least experience ($759, 95% CI $415 to $1102). The use of laparoscopy did not significantly change payments for physician services for surgeons with the most experience (−$356, 95% CI −$612-$27).
Although no difference was seen in readmission payments comparing laparoscopic versus open cases across all 3 levels of experience, payments for postacute care were significantly lower for surgeons with the most experience (−$1975, 95% CI −$3241 to −$710). Stratifying surgeons by total colectomy volume did not show similar results. In general, total volume attenuated the relative effect of laparoscopic versus open surgery on total episode payments.
Complications, Readmissions, and Discharges
We evaluated the incidence of postoperative events to further delineate why payments were different between laparoscopic and open surgery and across quartiles of surgeon experience. Although surgeons with the least and most experience with laparoscopy had similar rates of complications for their open procedures (least experience: 21%, most experience: 21%; P = 0.45), a clear stepwise decrease in complications was observed for laparoscopic cases (least experience: 28%, medium experience: 18%, most experience: 15%; P < 0.01). Similar patterns were seen for rates of readmission and discharge to another inpatient facility (Figs. 2A–C).
FIGURE 2.
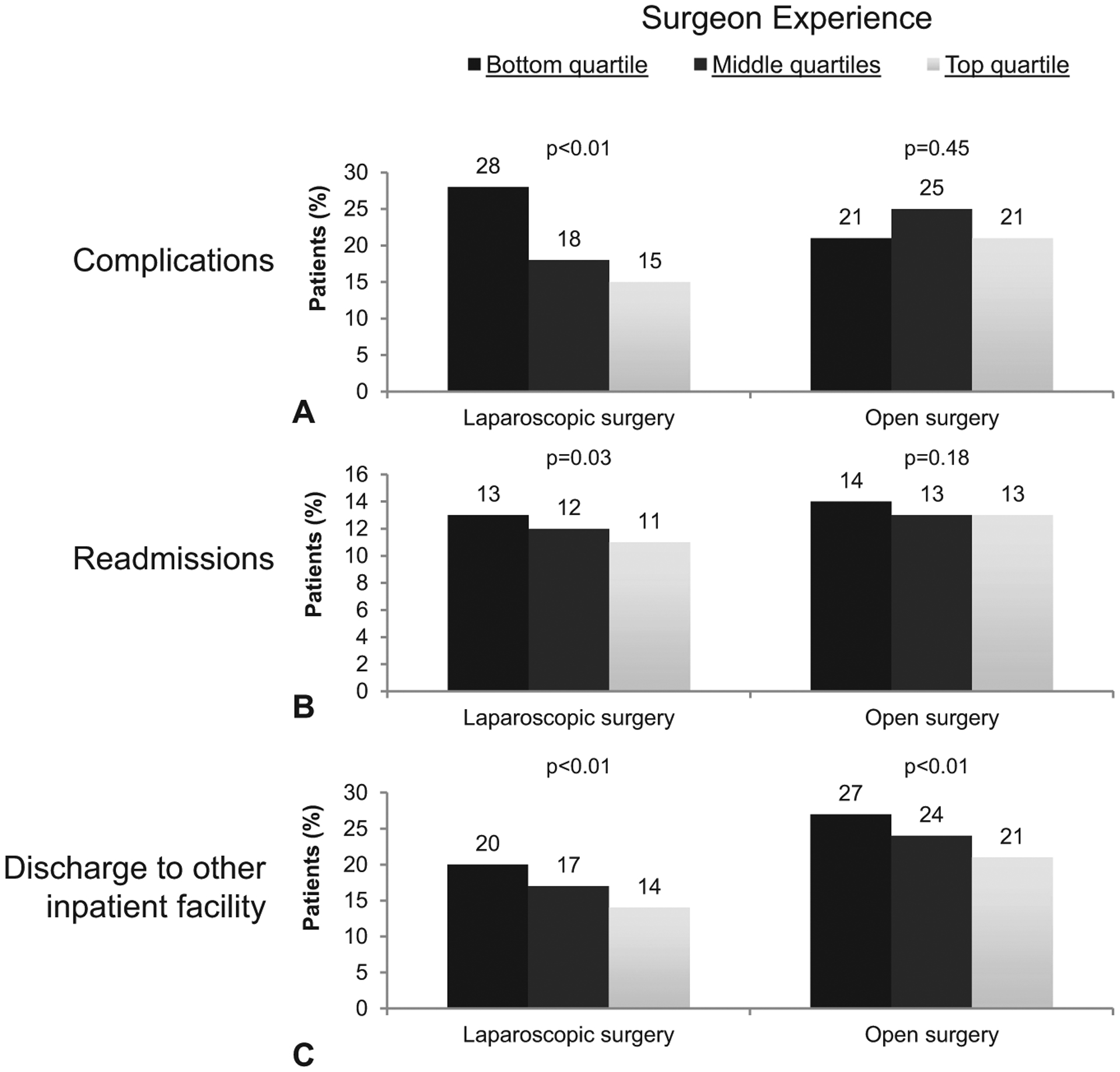
A-C. Adjusted rates of complications, readmissions, and discharges to other facilities derived from our instrumental variable models. Rates are reported across quartiles of surgeon experience. P values reflect the comparison of rates between top and bottom quartile surgeons.
Comparing Payments for Complications
Finally, we sought to characterize the extent to which Medicare payments for postoperative complications are influenced by surgeon experience (Table 3). The independent increase in Medicare payments related to the occurrence of any complication was similar between surgeons with the most ($15,461) and least ($15,166, P = 0.26) experience following laparoscopic surgery. Results were similar for open surgery, in which payment increases were not statistically different between quartiles of surgeons. Taken together, the financial savings from an experienced laparoscopic surgeon is the result of fewer complications, not the payments made per complication.
TABLE 3.
Average Impact of Postoperative Events on Total Episode Payments Across Surgeon Experience and Operative Approach
| Bottom Quartile (Least Experience) | Middle Quartile | Top Quartile (Most Experience) | P | |
|---|---|---|---|---|
| Laparoscopic surgery | ||||
| Any postoperative complication | $15,166 | $15,250 | $15,461 | 0.26 |
| Surgical complication | $12,183 | $11,899 | $11,962 | 0.88 |
| Medical complication | $19,444 | $19,349 | $19,798 | 0.29 |
| Any readmission | $16,166 | $15,502 | $14,914 | 0.04 |
| Postacute care needs | $7691 | $6902 | $6790 | 0.02 |
| Open surgery | ||||
| Any postoperative complication | $15,956 | $16,704 | $16,239 | 0.47 |
| Surgical complication | $13,625 | $13,702 | $13,108 | 0.53 |
| Medical complication | $17,529 | $18,334 | $18,003 | 0.28 |
| Any readmission | $16,218 | $15,999 | $14,615 | 0.01 |
| Postacute care needs | $8951 | $9096 | $9147 | 0.52 |
Payments are in United States Dollar (USD) and reflect the average increase in Medicare payments when the event occurs. P values reflect the comparison between the top and bottom quartile.
DISCUSSION
In this population-based study, surgeons’ experience with laparoscopy was associated with significant differences in both absolute and relative Medicare payments between laparoscopic and open colectomy. Although laparoscopic surgery was, on average, less expensive than open surgery, surgeons with the least laparoscopic experience actually had higher payments with the minimally invasive approach. This difference was driven by higher complication rates and greater utilization of postacute care services (eg, skilled nursing facilities) among less experienced surgeons.
Previous comparative cost studies of laparoscopic and open procedures using administrative data are methodologically limited. For example, a large study of 60,000 cancer patients from the National Inpatient Sample database found that laparoscopic surgery was associated with a $2000 absolute reduction in charges compared to open surgery.25 The present study and others like it benefit from the inclusion of many patients from different demographics and regions. They are limited, however, by inaccurate cost measurements based on estimated charges and an inability to adequately account for selection bias from unmeasured patient characteristics. Although these are known and accepted limitations of large administrative databases, they limit the generalizability and accuracy of the findings.18 The present study was designed to specifically fill these 2 previous methodological shortcomings. By using actual Medicate payments (rather than charges or costs derived from cost-to-charge ratios), the estimates included herein reflect the real-life resources needed to provide this service to beneficiaries. The instrumental variable methodology addresses selection bias to provide more accurate estimates of the relationship between surgeon experience and healthcare payments. Taken together, the present study gives more confidence and insight into cost benefit of using laparoscopy for colectomy procedures.
Previous work by our group supports the use of this analytic approach and showed that surgeon experience is an important factor in the relative safety of laparoscopic versus open colectomy.26
In that context, there is a complex relationship between surgeon experience, postoperative complications, and healthcare payments. The present study confirmed prior work showing that complications significantly increase total episode payments.27 Medical complications, such as pneumonia or prolonged ventilation, are more expensive than surgical complications such as a wound infection. Surgeon experience, however, did not influence the extent to which a complication increased total payments. In other words, the financially important event was the occurrence of the complication and not variation in subsequent management. Complications are similar in cost regardless of the index operation (laparoscopic vs open), but on average laparoscopic surgery results in fewer complications overall.
The present study underscores and clarifies the complex relationship between surgeon experience, postoperative complications, and healthcare payments. It builds on prior analyses of surgical cohorts that demonstrate an association of higher complications rates with significantly increased total episode payments.27–30 Moreover, it gives insight into the mechanism by which these complication differences occur. Although the payment for individual complications was similar across different surgeon experience levels, the rate of at which complications occurred was lower among surgeons with higher laparoscopic experience. In other words, the financially important event was the occurrence of the complication and not variation in payments for subsequent management. Complications are similar in cost regardless of the index operation (laparoscopic vs open), but on average laparoscopic surgery results in fewer complications overall.
The present study should be interpreted within the context of its limitations. Our results may not be generalizable to all patients because we use Medicare data that included only patients older than 65 years. Colon surgery is nonetheless more common in this demographic and Medicare patients consume more resources than younger and healthier populations. It is also unlikely that technical aspects of the operations differ in younger patient populations. Our study is designed to address the issue of selection bias that is a common limitation of financial analysis using administrative data. We confirmed that this was a valid and appropriate approach through rigorous testing of our instrumental variable. It is also possible that our determination of surgical experience is a proxy for other factors related to the safety and cost of colectomy. For example, low experience surgeons may only work in regions with low socioeconomic status patients or in worse hospitals, thereby leading to higher Medicare payments by association. We addressed this issue in 2 ways. First, our instrumental variable balanced patient characteristics (both known and unknown) within strata of surgeon experience. Second, we adjust for clustering of outcomes within hospitals and for regional trends in surgical payments and safety at the level of the hospital referral region. It is also possible that our results may be influenced by DRG distribution for the index hospitalization. We confirmed that DRGs were evenly distributed across strata of experience. In order to address the potential for “upcoding” from complications we stratified our analysis by patients with and without complications. We also adjusted for HCCs, which are intended to account for patient characteristics that result in higher DRG payments. Nonetheless, our findings underscore the importance of postacute care and readmission on payments, which would not be influenced by the index DRG. Finally, surgeon experience likely reflects the more accurate determinate of outcomes, surgical skill. Although we are unable to measure skill in an analysis of this scope, other work has shown that more experience is associated with overall higher technical skill.31
The present study has important implications for surgeons and clinical leaders in surgery. Consistent with prior work on clinical outcomes, we have shown that surgeon experience influences the benefits, in terms of health care expenditures, from minimally invasive surgery. Like improvements in patient safety, the financial benefits of laparoscopy are only realized when the surgeon has appropriate experience or proficiency. This finding is important to surgical leaders for several reasons: First, this work supports the need for more rigorous credentialing standards for individual surgeons. It highlights the need to improve continuing education through more extensive proctoring or coaching to enhance surgical skills. This must be done in a careful manner by surgeons with sufficient firsthand experience or access to experienced individuals for proctoring until proficiency can be achieved. Second, the present study makes a business case for investing in the training and re-training surgeons in practice. New procedures are continually introduced into practice and surgeons need to take the time to learn them safely. Taking time to learn new procedures is expensive. Increasing the number of surgeons at a given hospital who are, however, proficient with complex laparoscopy has an important beneficial impact on the financial bottom line for hospitals and health care payers.
CONCLUSIONS
In the present study, we found that surgeons’ experience with laparoscopy was associated with significant differences in Medicare payments for laparoscopic and open colectomy. Although laparoscopic surgery was, on average, less expensive than open surgery, surgeons with the least experience with laparoscopy actually had higher costs with the minimally invasive approach. This difference was driven by higher complication and readmission rates and greater utilization of postacute care services (eg, skilled nursing facilities) among less experienced surgeons.
Disclosure:
This work was support by R01AG039434 (J.B.D.), R01HS023597 (J.B.D.), and K08AG047252 (S.E.R.). The authors declare no conflict of interests.
REFERENCES
- 1.Senagore AJ, Brannigan A, Kiran RP, et al. Diagnosis-related group assignment in laparoscopic and open colectomy: financial implications for payer and provider. Dis Colon Rectum. 2005;48:1016–1020. [DOI] [PubMed] [Google Scholar]
- 2.Crawshaw BP, Chien HL, Augestad KM, et al. Effect of laparoscopic surgery on health care utilization and costs in patients who undergo colectomy. JAMA Surg. 2015;150:410–415. [DOI] [PubMed] [Google Scholar]
- 3.Cleary SP, Han HS, Yamamoto M, et al. The comparative costs of laparoscopic and open liver resection: a report for the 2nd International Consensus Conference on Laparoscopic Liver Resection. Surg Endosc. 2016;30:4691–4696. [DOI] [PubMed] [Google Scholar]
- 4.Grenda TR, Pradarelli JC, Thumma JR, et al. Variation in hospital episode costs with bariatric surgery. JAMA Surg. 2015;150:1109–1115. [DOI] [PubMed] [Google Scholar]
- 5.Salem L, Devlin A, Sullivan SD, et al. Cost-effectiveness analysis of laparoscopic gastric bypass, adjustable gastric banding, and nonoperative weight loss interventions. Surg Obes Relat Dis. 2008;4:26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwon S, Billingham R, Farrokhi E, et al. Adoption of laparoscopy for elective colorectal resection: a report from the Surgical Care and Outcomes Assessment Program. J Am Coll Surg. 2012;214:909.e1–918.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsui C, Klein R, Garabrant M. Minimally invasive surgery: national trends in adoption and future directions for hospital strategy. Surg Endosc. 2013;27:2253–2257. [DOI] [PubMed] [Google Scholar]
- 8.Zuckerman RB, Sheingold SH, Orav EJ, et al. Readmissions, observation, and the hospital readmissions reduction program. NEngl J Med. 2016;374:1543–1551. [DOI] [PubMed] [Google Scholar]
- 9.Scarborough JE, Schumacher J, Kent KC, et al. Associations of specific postoperative complications with outcomes after elective colon resection: a procedure-targeted approach toward surgical quality improvement. JAMA Surg. 2016;152:e164681. [DOI] [PubMed] [Google Scholar]
- 10.Celio AC, Kasten KR, Burruss MB, et al. Surgeon case volume and readmissions after laparoscopic Roux-en-Y gastric bypass: more is less. Surg Endosc. 2017;31:1402–1406. [DOI] [PubMed] [Google Scholar]
- 11.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346: 1128–1137. [DOI] [PubMed] [Google Scholar]
- 12.Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117–2127. [DOI] [PubMed] [Google Scholar]
- 13.Wennberg DE, Lucas FL, Birkmeyer JD, et al. Variation in carotid endarterectomy mortality in the Medicare population: trial hospitals, volume, and patient characteristics. JAMA. 1998;279:1278–1281. [DOI] [PubMed] [Google Scholar]
- 14.Yeo HL, Abelson JS, Mao J, et al. Surgeon annual and cumulative volumes predict early postoperative outcomes after rectal cancer resection. Ann Surg. 2016. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 15.Gottlieb DJ, Zhou W, Song Y, et al. Prices don’t drive regional Medicare spending variations. Health Aff. 2010;29:537–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iezzoni LI, Daley J, Heeren T, et al. Identifying complications of care using administrative data. Med Care. 1994;32:700–715. [DOI] [PubMed] [Google Scholar]
- 17.Xian Y, Holloway RG, Chan PS, et al. Association between stroke center hospitalization for acute ischemic stroke and mortality. JAMA. 2011;305:373–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wagner TH, Upadhyay A, Cowgill E, et al. Risk adjustment tools for learning health systems: a comparison of DxCG and CMS-HCC V21. Health Services Res. 2016;51:2002–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pope GC, Kautter J, Ellis RP, et al. Risk adjustment of Medicare capitation payments using the CMS-HCC model. Health Care Financ Rev. 2004;25: 119–141. [PMC free article] [PubMed] [Google Scholar]
- 20.Kautter J, Pope GC, Ingber M, et al. The HHS-HCC risk adjustment model for individual and small group markets under the Affordable Care Act. Medicare Medicaid Res Rev. 2014;4:mmrr2014-004-03-a03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. [DOI] [PubMed] [Google Scholar]
- 22.Newhouse JP, McClellan M. Econometrics in outcomes research: the use of instrumental variables. Annu Rev Public Health. 1998;19:17–34. [DOI] [PubMed] [Google Scholar]
- 23.Terza JV, Bradford WD, Dismuke CE. The use of linear instrumental variables methods in health services research and health economics: a cautionary note. Health Serv Res. 2008;43:1102–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller ME, Welch WP, Welch HG. The impact of practicing in multiple hospitals on physician profiles. Med Care. 1996;34:455–462. [DOI] [PubMed] [Google Scholar]
- 25.Vaid S, Tucker J, Bell T, et al. Cost analysis of laparoscopic versus open colectomy in patients with colon cancer: results from a large nationwide population database. Am Surg. 2012;78:635–641. [PubMed] [Google Scholar]
- 26.Sheetz KH, Norton EC, Birkmeyer JD, et al. Provider Experience and the Comparative Safety of Laparoscopic and Open Colectomy. Health Serv Res. 2017;52:56–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dimick JB, Chen SL, Taheri PA, et al. Hospital costs associated with surgical complications: a report from the private-sector National Surgical Quality Improvement Program. J Am Coll Surg. 2004;199:531–537. [DOI] [PubMed] [Google Scholar]
- 28.Birkmeyer JD, Gust C, Dimick JB, et al. Hospital quality and the cost of inpatient surgery in the United States. Ann Surg. 2012;255:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eappen S, Lane BH, Rosenberg B, et al. Relationship between occurrence of surgical complications and hospital finances. JAMA. 2013;309: 1599–1606. [DOI] [PubMed] [Google Scholar]
- 30.Healy MA, Mullard AJ, Campbell DA Jr, et al. Hospital and payer costs associated with surgical complications. JAMA Surg. 2016;151: 823–830. [DOI] [PubMed] [Google Scholar]
- 31.Birkmeyer JD, Finks JF, O’Reilly A, et al. Surgical skill and complication rates after bariatric surgery. N Engl J Med. 2013;369:1434–1442. [DOI] [PubMed] [Google Scholar]


