Abstract
Humans have altered up to half of the world's land surface. Wildlife living within or close to these human-modified landscapes are presented with opportunities and risks associated with feeding on human-derived foods (e.g., agricultural crops and food waste). Understanding whether and how wildlife adapts to these landscapes is a major challenge, with thousands of studies published on the topic over the past 10 years. In the present article, we build on established theoretical frameworks to understand the behavioral causes of crop and urban foraging by wildlife. We then develop and extend this framework to describe the multifaceted ecological consequences of crop and urban foraging for the individuals and populations in which they arise, with emphasis on social species for which interactions with people are, on balance, negative (commonly referred to as raiding species). Finally, we discuss the management challenges faced by urban and rural land managers, businesses, and government organizations in mitigating human–wildlife conflicts and propose ways to improve the lives of both wildlife and humans living in human-modified landscapes and to promote coexistence.
Keywords: human–wildlife conflict, raiding, behavioral plasticity, movement ecology, time and energy budgets
Up to 50% of the Earth's land surface has been modified by human activities, with 12% dedicated to crops (Ramankutty et al. 2008) and nearly 1% to cities (Liu et al. 2014). Natural environments are shrinking, and transition zones between natural and human-modified spaces are eroding, resulting in increased contact between humans and wildlife (Woodroffe and Ginsberg 1998). Wildlife species living within or close to a human-modified landscape typically experience a drastic change in resource availability, especially food. Traditional food resources can become depleted or destroyed, and agricultural crops or human and pet foods offer potentially novel food sources. Such human-derived foods can be rich in energy and predictable in time and space (Griffin et al. 2017), making crop foraging (table 1; Naughton Treves 1998), or urban foraging (Sol et al. 2013, Barrett et al. 2018, Santini et al. 2019), sometimes known as raiding (table 1), a highly rewarding foraging strategy for wildlife.
Table 1.
Definition of terms.
| Term | Definition | References |
|---|---|---|
| Behavioral plasticity | Plasticity is usually defined as innate phenotypic plasticity that can depend on genetic factors resulting in a constant behavioral trait that can vary across individuals, populations or species. Developmental plasticity refers to learning procedures, in which an animal can learn adaptive behaviors by trial and errors or cognitive abilities when exposed to a new situation. | Snell-Rood 2013 |
| Crop foraging | Entering agricultural landscape in order to consume crops. In the present article, we frequently associate crop foraging with livestock depredation by wild carnivores. It is however distinct from crop damage resulting from moving through the agricultural landscape, which can have distinct causes and consequences on animals’ biology. | Naughton Treves 1998, Davies et al. 2011, Hill 2017 |
| Human–wildlife conflict | Negative interactions between humans and wildlife. For example, disease transmission between humans and wildlife, raiding behavior by wildlife | |
| (crop-foraging or urban-foraging), physical aggression between humans and wildlife. | Donnelly et al. 2006, Acharya et al. 2016, Liu et al. 2011, Woodroffe et al. 2005 | |
| Raiding behavior | Raiding manifests in wildlife entering human landscapes or directly interacting with humans in order to access human food sources. Elephants, primates, wild felids and bears are among the most high profile problematic species, but raiding does manifest in other genus. Throughout the present article, we minimize our use of the term raiding and, instead, favor crop foraging or urban foraging. | Hockings and McLennan 2015, |
| Lewis et al. 2015, Thouless and Sakwa 1995, Zarco-Gonzalez and Monroy-Vilchis 2014 | ||
| Socioecology | Study of social behavior and dynamics (e.g., cohesion, leadership) with regard to species ecology (e.g., predation risks, food availability). | Jarman 1974, Linklater 2000, Wrangham et al. 1993 |
| Movement ecology | Study of the general movement of an animal resulting in patterns of occupation and landscape use, represented by features such as habitat selection or home ranges. | Börger et al. 2006, Burt 1943, Matthiopoulos 2003 |
| Time and energy budgets | The two main budgets or currencies animals have to balance in order to maintain their body condition and reproduce. This notion is used in optimal foraging theories dividing species into time minimizers (species gaining fitness by limiting the time dedicated to foraging such as carnivores) and energy maximizers (species gaining fitness by increases energy intake such as elephants) | Charnov 1976, Hixon 1982, |
| Stephens and Krebs 1986 | ||
| Urban foraging | Urban foraging is broad term to refer to any foraging event happening in the urban or any built environments (residential or commercial property). Food items can be as varied as seeds from bird feeders or people voluntarily feeding wildlife, plant materials from gardens, parks and street trees, food discards from litter bins, food items from restaurants, residential or commercial properties, pets. | Sol et al. 2013, Santini et al. 2019 |
Human–wildlife interactions can bring positive effects for both humans and wildlife, enhancing human well-being (Chan et al. 2007), with a famous example being bird feeding in urban environments (Reynolds et al. 2017). However, when wildlife and humans compete for the same resources or space, conflicts may arise (Bruskotter et al. 2017). Indeed, crop foraging by wildlife can negatively affect local economies (Chan et al. 2007). For example, in rural Uganda, elephants (Loxodonta africana) can damage up to 6510 square meters of crops in a single foraging trip (Naughton Treves 1998). In the urban environment, gulls (Larus spp.) take food directly from people across the globe (Spelt et al. 2019), black bears (Ursus americanus) in North America enter urban environments to forage on garbage (Lewis et al. 2015), and, in Asia, macaques (Macaca fascicularis) enter and damage houses and commercial properties to access human foods (Yeo and Neo 2010). When opportunistic crop and urban foraging by wildlife is not tolerated by people (Carter and Linnell 2016, Bruskotter et al. 2017), this results in conflict with people that compromises both human and wildlife well-being (Barua et al. 2012, Hill 2017).
Studies of animal behavior are increasingly adopting an integrated ecological approach (Nathan et al. 2008) and strive for in situ quantitative studies (King et al. 2018) that link animals’ behavior to the complex environments in which they live, including human-altered landscapes (Caro 1999). Empowered by recent technological (Fehlmann and King 2016), statistical (Franz and Nunn 2009, Koen et al. 2014, Williams et al. 2020), and conceptual (Dingemanse et al. 2010, Sih 2013, Gallagher et al. 2017) advances, the last decade has seen a growing body of literature emphasizing the importance of anthropogenic factors on species’ biology (Tuomainen and Candolin 2011, Sih 2013, Sol et al. 2013, Fleming and Bateman 2018, Santini et al. 2019). However, in only a few studies have addressed the multifaceted aspects of wildlife behavioral adaptations and linked them to the conflicts that can emerge from this adaptability. In the present article, we adopt a behavioral ecology approach to understand crop and urban foraging and discuss how such knowledge is necessary for achieving human coexistence with wildlife when conflicts arise. We apply these established theoretical and statistical frameworks to first understand the behavioral causes of crop and urban foraging by wildlife, describing how and why some individuals (and species) cope better with human-induced changes to their environment (Sih 2013, Sol et al. 2013, Santini et al. 2019). Then, we build on the general approach developed in Tuomainen and Candolin (2011) and review disparate studies in various contexts to define the multifaceted ecological consequences of crop and urban foraging for the spatial and socioecology of terrestrial species. In this section, we mainly focus on species that are typically in conflict with humans over food resources (commonly named raiding species) with a particular emphasis on social species. Finally, we end by discussing a route for mitigating human–wildlife conflict (table 1) and solving some of the management challenges associated with animals foraging in a human-altered landscape. We believe that taking and applying a behavioral ecology approach is key to mitigating human wildlife conflicts in various contexts, reducing its severe consequences on both human and wildlife well-being (Barua et al. 2012, Hill 2017).
The behavioral causes of wildlife foraging in human-modified landscapes
Wildlife exploiting human-derived resources is not new, but a growing number of species have been reported to be colonizing or recolonizing human-altered environments in recent years (Bruskotter et al. 2017). A key challenge is to determine why and how some species and individuals thrive in human-altered environments, whereas others fail. This challenge has been the subject of recent reviews, frameworks, and syntheses (e.g., Tuomainen and Candolin 2011, Sih 2013, Griffin et al. 2017, Santini et al. 2019) that indicate that surviving and reproducing in a dynamic, human-modified environment require a flexible phenotype that alters to match the current environment (Sol et al. 2002, Wright et al. 2010). Flexibility has therefore become central to understanding a species's or individual's responses to altered environments and whether they exploit new food resources. Indeed, although human-derived foods tend to be high in calories and to have short handling times (Griffin et al. 2017, Hill 2017), their inclusion in the diets of species that have access to these resources is not a given (Sih 2013, Barrett et al. 2018).
At a species level, the degree of dietary specialization and niche overlap with human-altered environments can be useful predictors of success in exploiting novel foods. Generalist species that can exploit a wide spectrum of food items and habitats are better at recognizing potential risks and exploiting opportunities associated with human-altered landscapes and are therefore among the first to settle in these environments (Colles et al. 2009, Sih 2013, Santini et al. 2019). However, even specialists can thrive where species are preadapted to human-altered environments (Griffin et al. 2017). For instance, raptor species that nest on cliffs are perfectly suited to exploit high-rise buildings in cities, where they can prey on abundant populations of urban pigeons (generalists) that thrive in such landscapes (Chace and Walsh 2006). The specialist raptor species exploiting high-rise buildings is a good example of where the human-changed landscapes fit within a species's fundamental niche (MacMahon et al. 1981). The overlap between the fundamental niche and the human-altered landscape is therefore another predictor of the likelihood of a given species to use this novel space. In contrast, where species fundamentally change their behavior so that they are able to exploit human-derived resources and landscapes, this requires that they expand their fundamental niche (MacMahon et al. 1981). Indeed, to exploit human-derived resources that are entirely novel requires behavioral flexibility, a trait that characterizes generalist species (Daniels et al. 2019).
The flexibility that characterizes the behavior of generalist species can also help explain within-species differences (interindividual differences) in crop or urban foraging (Wright et al. 2010, Snell-Rood 2013). Interindividual differences in sex, age, size, and personality may determine the propensity (or ability) to forage in human-modified landscapes (Camphuysen et al. 2015, Ducatez et al. 2017, Brooks et al. 2020). For example, sex- and age-specific life history trade-offs are correlated with behavioral risk aversion across species and contexts (see Smith and Blumstein 2008 for a meta-analysis) and may explain why males and older individuals often forage in human spaces more frequently than females and younger individuals (Strum 2010, Chiyo et al. 2012).
Statistically, behavioral plasticity (table 1) may be explained as the slope of an individual's response to some change in environment (when behavior and change are plotted against one another). If an individual's phenotype does not change (i.e., the slope's coefficient is null; figure 1a), the individual is interpreted as not exhibiting flexibility. Where behavior is not flexible but where interindividual differences in behavior do exist (figure 1a), certain behavioral types may show a higher propensity to persist and forage in human-modified landscapes. Where an individual's phenotypes are altered in response to changing environments (i.e., the slope's coefficient is nonnull; figure 1b) and where individuals vary in their type of flexibility (figures 1c and 1d), those individuals whose change in behavior results in novel foraging strategies will be selected for (where this results in net benefits). Furthermore, those individuals that respond quickly (figure 1e) to changes (and in the correct way) should be especially good at exploiting new resources. Several detailed reviews provide in-depth accounts of how to study interindividual differences in personality and plasticity using the statistical and theoretical framework we have described above (Dingemanse et al. 2010, Wright et al. 2010, Snell-Rood 2013).
Figure 1.
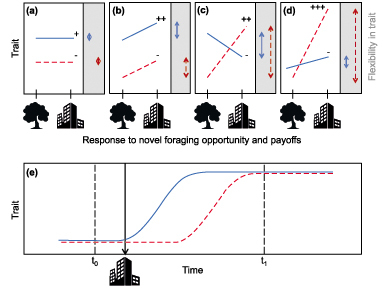
Interindividual differences in responses to a novel foraging opportunity. Behavioral trait expressed by two individuals (plain and dashed lines) before and after exposure to a human-altered landscape. Where the behavior allows an individual to benefit from the human-modified landscape (e.g., via crop or urban foraging), this is indicated by a plus sign; where behaviors result in costs for individuals (e.g., they are unable to access human-derived foods) or an increased risk and cost of injury, they are indicated by a hyphen. The grey box to the right of each panel illustrates the degree of flexibility exhibited by each individual. (a) No flexibility by either individual, but one individual's behavior results in a net benefit and the other does not. (b) Similar flexibility for both individuals, but only one individual benefits because of the different intercept. (c) Between-individual variation in flexibility, correlated with the absolute level of traits; one individual benefits and one incurs a cost. (d) Between-individual variation flexibility, independent of the absolute value of traits; one individual benefits and one incurs a cost. (e) The level of a behavioral trait measured in two individuals at two time points, prior to (t0) and following human changes to the landscape (t1). The rate at which the two individuals react to the change differs, with the individual represented by the solid line reacting more quickly in this scenario.
Learning is also crucial. Over the course of a lifetime, naive individuals can learn to recognize both opportunities and risks presented by human-modified landscapes (Snell-Rood 2013, Sol et al. 2013), and those individuals and populations most frequently exposed to new environments will be the fastest to develop novel foraging strategies by simple trial and error learning (e.g., Ducatez et al. 2013). In particular, species with long life spans and large home ranges will be more likely to experience and learn to exploit human-modified landscapes through their lifetime (e.g., African elephants, Loxodonta africana; Graham et al. 2009). Long-lived, wide-ranging species also tend to have impressive cognitive skills (e.g., Lefebvre et al. 2004), affording navigation in geographically complex environments where resources and risks are dictated by artificial human activity patterns (Griffin et al. 2017). Such skills also allow individuals that forage in agricultural and urban environments to properly assess and update risks that are related to specific locations and specific people (Sol et al. 2013, Bruskotter et al. 2017, Fehlmann et al. 2017b).
Therefore, the discovery of new resources may be challenging, and the number of niches an individual can exploit in the urban environment may be linked to its cognitive abilities. Moreover, if individuals live in social groups with frequent and repeated interactions, novel behaviors can become commonplace via social enhancement (e.g., Aplin et al. 2012), horizontal transmission (i.e., from group mates, e.g., Chiyo et al. 2012), or vertical transmission (i.e., parent to offspring, e.g., Mazur and Seher 2008). Social learning can therefore accentuate or accelerate the new behaviors within a population that allow individuals to derive benefits from urban or crop foraging. The chacma baboon (Papio ursinus) provides an excellent example of a behaviorally flexible, long-lived, wide-ranging, socially and cognitively complex species that thrives in human-modified landscapes (box 1).
Box 1. Raiders of the human realm: Chacma baboons (Papio ursinus).
Baboons, like other long-lived, social species, are well equipped to exploit opportunities presented by human-altered landscapes. First, manual dexterity, agility, and climbing ability allow baboons to enter human landscapes and get access to food items (Hoffman and O'Riain 2012b). Second, large brain size (which is correlated with sociality) may promote innovative behavior (Reader et al. 2011). Third, long life spans provide the opportunity for individual learning and social learning via multiple, long-term individualized social relationships (King et al. 2008). Fourth, overlapping generations allow novel behaviors to be transmitted from older to younger conspecifics more readily (Pereira 1995). Across Africa, baboons are notorious crop and urban foragers. Human-modified landscapes provide baboons the opportunity to forage on high-calorie human crops, foods and waste (Strum 2010). On the Cape Peninsula in the Western Cape Province of South Africa, 1 square kilometer of human-modified habitat (pine plantations and vineyards) can support nearly five times the number of baboons as the same area of natural habitat (Hoffman and O'Riain 2012a). These Cape baboons exploit spaces at the periphery of the city (e.g., vineyards) that are close to refuges (Fehlmann et al. 2017b), resting at the urban edge and waiting for suitable opportunities to exploit the resources in human-modified landscapes, engaging in brief, high-activity raids (Fehlmann et al. 2017a). These altered foraging dynamics result in smaller home ranges than groups elsewhere in the species range, which raid less often or not at all (Altmann and Muruthi 1988, Strum 2010, Hoffman and O'Riain 2012b), and directly alters the Cape baboon time and energy budgets (Doorn et al. 2010, Fehlmann et al. 2017a). High-energy food items and a relaxed time budget in crop- and urban-foraging baboons affords more time resting and improved body condition, which, ultimately is linked to higher biological fitness (Strum 2010). However, as for other primates (Hockings et al. 2015), conflicts with humans can result in severe injuries or death (Beamish 2009) or lead to culling and removal of individuals through management practices (Swan et al. 2017), which can have consequences for population size and stability (Beamish 2009).
Papio ursinus. Photograph: Gaelle Fehlmann.
The theoretical and statistical frameworks for describing why some species and individuals are better able to exploit and to cope with human-induced changes to the environment are therefore well established and describe the overall causes of foraging in human-altered environments (Sol et al. 2002, Sih 2013, Barrett et al. 2018). This provides an excellent platform for understanding crop and urban foraging from both a mechanistic and functional (evolutionary perspective). However, we lack a comparable framework for describing the ecological consequences of crop and urban foraging for the individuals and populations in which they arise.
The ecological consequences of wildlife foraging in human-modified landscapes
In this section of the article, we focus on the ecological consequences of foraging in human-modified landscapes for terrestrial, mainly social species. In particular, we explore the consequences of foraging in human-modified landscapes for activity and energy budgets (table 1), because these are fundamentally linked to species’ foraging ecologies and are therefore predicted to be altered; movement ecology (table 1), because crop and urban foraging requires the exploitation of new landscape features; socioecological dynamics, which can be affected by interindividual variation in space use and propensity to exploit these new foraging opportunities; life history traits, which are likely to be affected as a result of the changes in the first three aspects; and, finally, population dynamics and community ecology, which emerge from the interactions of the first four categories. Although most of these aspects can be relevant for any species foraging in human-altered environments, we mostly build our discussion on those most reported in conflict with humans, which tend to be long-lived social species (Redpath et al. 2013). Descriptions and the relationships among these major factors with respect to foraging in human-modified landscapes are summarized in figure 2.
Figure 2.
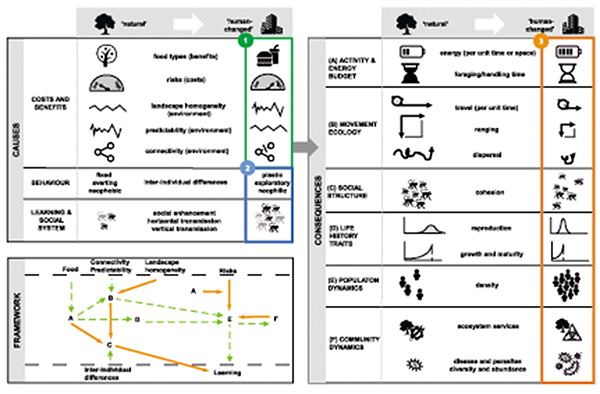
The framework of the ecological causes and consequences of foraging in a human-modified landscape. Environmental (1) and behavioral (2) factors affecting habitat choice at a species level are represented in the box labeled Causes. For individuals choosing to exploit anthropogenic resources, these causes have been shown to affect (A) activity and energy budgets, (B) movement ecology, (C) social structure, (D) life history traits, (E) population dynamics, and (F) community dynamics (see the box labeled “Consequences”). The interconnections between each effect (3) are represented too (see the box labeled “Framework”). Positive effects are represented with dashed arrows, and negative effects are show with plain arrows. For example, the increased energy consumed per unit of time and space and the associated diminished foraging and handling times (A) favor the reduction of the travelling time, decreas home range and the dispersal (B), and alter cohesion of social groups (D). Increased food quality also speeds growth and maturation, and increases reproduction rates (D). In turn, this results in higher population densities (E). These can favor biohazards (e.g., spread of diseases, parasites) and reduce ecosystem services (F).
Activity and energy budgets
Crop and urban foraging strategies have the potential to strongly affect a species's time and energy budgets, because these individuals generally exploit high-energy food items. For some species that require a fixed amount of energy in a day (e.g., time minimizers; Schoener 1971), this means they reach their energetic needs more quickly and with more certainty because of the predictability of food distribution (Fedriani et al. 2001, Beckmann and Berger 2003a). Chacma baboons, for example, are able to massively reduce the time they spend seeking food by foraging for crops or in urban environments (Hoffman and O'Riain 2012a, Fehlmann et al. 2017a). The time released by reduced foraging effort can then be reinvested in other activities, such as vigilance or resting (e.g., Altmann and Muruthi 1988, Fehlmann et al. 2017a) or social interactions such as grooming (e.g., Altmann and Muruthi 1988). The first can contribute to risk mitigation strategies (Roberts 1996, Beckmann and Berger 2003a, Lima et al. 2005), and the second can alleviate stress (Shutt et al. 2007, Hostinar et al. 2014, Wittig et al. 2016), which can be higher for individuals encountering humans (Ahlering et al. 2011, Fourie et al. 2015). However, when human-derived resources are distant from other vital resources (e.g., refuges, water), individuals and groups may actually have to increase their travel times and reduce their time resting in order to exploit these resources (Isaksson et al. 2016, Hill 2017, Enners et al. 2018). Overall, such changes in time and energy budgets can be viewed as a reflection of the differences in availability or quality between natural food items and crop- or urban-foraging options (Spelt et al. 2019).
Movement ecology
Individuals that engage in crop or urban foraging will inevitably range across human-modified landscapes. Although such overlap may be the consequence of human encroachment into the existing home range of wild populations, in several cases, wildlife populations actively alter their space use to exploit new opportunities in human-dominated landscapes (Beckmann and Berger 2003a, Reher et al. 2016, Seiler and Robbins 2016).
Burt (1943) referred to the home range as “the area over which the animal normally travels in search of food.” In human-modified landscapes, species can fulfill their energetic needs faster, reducing the time and effort devoted to both the active and sedentary phases of foraging (Bartumeus et al. 2010, Šálek et al. 2015, Fehlmann et al. 2017a). In addition, crop-foraging species face highly homogeneous landscapes with largely predictable resources (Naughton Treves 1998) that may limit the need for exploration. As a result, the daily path length and home ranges for crop-foraging individuals and groups are typically reduced compared with wild foraging conspecifics (Davison et al. 2009, Bartumeus et al. 2010, Reher et al. 2016). For example, in yellow napped Amazons (Amazona auropalliata), birds foraging in croplands have a home range ten times smaller than birds foraging in pastures (Salinas-Melgoza et al. 2013). Similarly, many resources in the urban environment are at least predictable in space (e.g., refuse bins, bird feeders, restaurants), which can be highly attractive, especially for carnivores (Fleming and Bateman 2018). For example, hyenas (Crocuta crocuta) living next to human lodges in the Kruger National Park, in South Africa, focus their foraging efforts in specific areas and have smaller home range sizes than do groups elsewhere in the species range (Belton et al. 2016).
When individuals begin to forage on crops or in urban spaces, not only does the space they use change, but also what they do in this space. What behavior is performed and where is influenced by landscape attributes (e.g., topography, homogeneity, fragmentation; Shepard et al. 2013, Koen et al. 2014), or species interactions (e.g., predation risks; Fraser and Huntingford 1986). Human-modified areas have, for the most part, been subjected to rapid and fundamental changes in their structure. This includes changes in their topography (via earthmoving works such as for roads, agriculture or via urbanization; e.g., Fleming and Bateman 2018), substrate (from biochemical modifications in fields via fertilization to soil surface changes via concreting), and ecosystems (involving species eradication, displacement or the addition of exogenous species; e.g., Chace and Walsh 2006). Overall, this results in profound changes in connectivity of the environment and affects the costs and benefits associated with crop or urban foraging.
The different barriers animals can encounter in human-altered landscapes are equally likely to impede animal movement (Davison et al. 2009, Tucker et al. 2018). Parks in city centers, backyards in suburbs, and high-density apartment blocks in inner city areas all influence habitat permeability (Cox et al. 2016) and will directly affect foraging strategies in these environments. For terrestrial species, fences, buildings, or ridges can represent barriers that can increase the cost of movement or prevent movement altogether through such environments (Shepard et al. 2013). As a result, terrestrial mammals frequently avoid them (Wall et al. 2006, Kertson et al. 2011, Hoffman and O'Riain 2012b) and often rely on human passages through environments, including transport routes, such as roads or railways, or wildlife corridors carved for conservation purposes. Although these may reduce the time and energy associated with travel (Hägerling and Ebersole 2017), they can introduce risks directly through collision with vehicles (Borkovcová et al. 2012, Murray and Clair 2015) or indirectly through exposure to predators (Fleming and Bateman 2018). Soaring birds, by contrast, have been found to use the updrafts created by buildings to engage in topographic soaring in the urban airscape (Shepard et al. 2016). Fences or flat-roof buildings also offer excellent perching opportunities for predatory birds to exploit when hunting (Chace and Walsh 2006), for colonial birds to use as nesting spaces (Maciusik et al. 2010), and for numerous species to use as refuges away from the reach of people and dogs (Chace and Walsh 2006).
The risks from road traffic (Borkovcová et al. 2012, Murray and Clair 2015) and the risks that emerge from conflict with domesticated animals (e.g., dogs; Kays and Parsons 2014) and people (Woodroffe et al. 2007, Warren 2009, Hockings et al. 2015) also affect a species's movement ecology (Gallagher et al. 2017) and can lead to reduced home ranges (Gehrt et al. 2009). Although such risks are usually predictable in space (e.g., dogs in gardens) and can be associated with specific landmarks (e.g., roads, fences), the magnitude of risk can alter dramatically with artificial and somewhat unpredictable time schedules, depending on human activities (e.g., road traffic) and human attitudes toward wildlife (Gehrt et al. 2009). This is obviously exacerbated in urban environments, and species exposed to such risks are known to adjust their behavior accordingly. For example, birds such as feral pigeons (Columba livia) or mockingbirds (Mimus polygotta) recognize humans that may represent a threat and adjust their responses accordingly (Sol et al. 2013). Commonly, this results in individuals temporarily avoiding human-modified landscapes and restraining their activity in such environments (Gehrt et al. 2009, Fehlmann et al. 2017a, Wilkie and Douglas-Hamilton 2018) but remaining in close proximity to them so that they can exploit foraging opportunities when risks are lower (Graham et al. 2009, Lewis et al. 2015, Šálek et al. 2015). Consequently, hotspots of human–wildlife conflict typically occur at the periphery of cities or farms (Kays and Parsons 2014), especially where these coincide with protected areas or refuges (Naughton Treves 1998, Woodroffe and Ginsberg 1998, Fehlmann et al. 2017b). Within the urban environments, parks can also constitute refuges from which urban-foraging species can commute (Rodewald and Gehrt 2014, Grafius et al. 2017). Therefore, maximizing the interconnectivity of natural resources for wildlife through human-altered landscapes may ease forays between refuges and human-derived resources (Michalski et al. 2006), which can increase contacts with humans and potentially conflicts. More research would be required to fully understand and predict these risks, which can jeopardize the success of conservation measures.
Social structure
Variations in crop and urban foraging between individuals in the same social group can arise because of differences in personality, plasticity, or learning (figure 1). Variations in crop- and urban-foraging propensity might additionally cause or be caused by variations in space use and time budgets (Beckmann and Berger 2003a, Strum 2010), as summarized in the above sections. This can create challenges for the maintenance of group cohesion and synchrony (Ruckstuhl and Kokko 2002, Shannon et al. 2008, King and Cowlishaw 2009), coordination and group decision-making (Dostálková and Špinka 2007, Rands et al. 2008, Herbert-Read 2016), and, more broadly, social structures (Fichtel and Manser 2010, King et al. 2018).
Group cohesion and synchrony are known to depend on the distribution of resources and the risks in the environment. Scarce food sources and their ability to be monopolized, for example, tend to result in an increased spread of the social group (Jarman 1974, Linklater 2000, Nishikawa et al. 2014), and higher risks usually result in groups becoming more cohesive (Jarman 1974, Cowlishaw 1997, Linklater 2000). Because the risks are often higher in human-modified landscapes (Warren 2009, Hockings et al. 2015, Murray and Clair 2015) and because the relevant food sources tend to be of high quality (Hockings and McLennan 2012) and easily monopolized (Kaplan et al. 2011, Flint et al. 2016), collective decisions to exploit human-modified landscapes (as a social unit by the animals) are likely to result in significant consensus costs (Kaplan et al. 2011), borne by lower-ranked or risk-averse individuals (King et al. 2008). As a result, crop- and urban-foraging opportunities are more likely to present a constraint on group cohesion and synchrony and to cause fission and fusion of groups when specific individuals leave the group to forage in human-changed landscapes (Warren et al. 2007, Graham et al. 2009).
At present, we don't properly understand the consequences of reduced cohesion and fission for social groups using human-changed spaces, but we hypothesize that those crop- and urban-foraging individuals that tend to be risk-prone phenotypes (Michl et al. 2000, King et al. 2013) prioritize the exploitation of these new opportunities over social attraction. In the collective behavior literature, individuals’ prioritization of their own travel direction over social attraction has been termed leading according to need or social indifference (Conradt et al. 2009). In such cases, individuals increase their influence on group movement by increasing their assertiveness and decreasing their social attraction range. This has been shown by empirical research with shoaling fish (Ioannou et al. 2015). Where animals favor goal-oriented motion over their tendency to be social, their groups are predicted to fission (Conradt et al. 2009, Sueur et al. 2011). Although we are not aware of any systematic studies on whether and how such fission–fusion dynamics may occur because of crop and urban foraging, in the Cape Peninsula baboons, splinter troops (smaller groups that fission from a large group) are a priority for management, because they drive exceptional levels of urban raiding. Proper understanding of these processes will be particularly important for management policies aimed at removing specific “problem” individuals or groups (Swan et al. 2017), and more research is therefore needed in order to clarify these socioecological processes.
Life history traits and population dynamics
Because individuals acquire energy faster and with more certainty in human-altered landscapes, these individuals tend to show improved body condition (Beckmann and Berger 2003b, Otali and Gilchrist 2004, Chiyo et al. 2011). This, in turn, has a positive influence on individual growth and fitness (Beckmann and Berger 2003b, Chiyo et al. 2011, Rotics et al. 2017). This is particularly true for species defined as energy maximizers, whose fitness is directly related to energy intake (Schoener 1971). In elephants (box 2), for example, crop-foraging males are larger (and potentially mature faster), which can lead to a longer breeding life span (Chiyo et al. 2011). For time minimizers, the time freed by faster foraging bouts can also be directly reallocated to finding mates, maximizing individual fitness. This, coupled with less ranging effort and smaller home ranges, allows for general population densities to increase drastically. Such phenomena have been observed in the periphery of human settlements, particularly in medium size carnivores (Chace and Walsh 2006, Yirga et al. 2013, Šálek et al. 2015), but these observations should be nuanced. In urban environments, higher densities may occur only in green spaces in which urban individuals may be condensed (Rodewald and Gehrt 2014). Indeed, although foraging opportunities might be plenty, other resources such as nesting sites may be limited and may become a limiting factor in the urban matrix (Charter et al. 2016, Hernández-Brito et al. 2018).
Box 2. Nocturnal field trips: The case of elephants.
As humans encroach into natural areas, African elephant (Loxodonta africana) populations encounter human settlements and fields more frequently, which often leads to chronic conflicts (Osborn 2004, Graham et al. 2009). Elephants frequently target crops at specific times of the year: when they are the most palatable (Chiyo et al. 2005, Jackson et al. 2008), when the quality of natural forage decreases (Osborn 2004), or when seasonal migratory routes take them past agricultural areas (Adams et al. 2017). When crops are mature, this foraging opportunity can provide elephants with dense, high calorie and easy to process food items (Osborn 2004). Elephants can be classified as energy maximizers (Hixon 1982), and the crop-foraging individuals, which are most frequently males (Jackson et al. 2008, Ahlering et al. 2011, Chiyo et al. 2012), tend to be bigger and benefit from a prolonged musth phase (Chiyo et al. 2011). Male biased raiding in elephants is likely to be explained by their general boldness (Sitati et al. 2003), their independent ranging behavior, and their size and strength (Shannon et al. 2008), which can allow them to more easily break through physical barriers (e.g., fences). High nutrient intake allows males to allocate more time and effort to secure mating opportunities, through increased walking and reduced time devoted to resting (Shannon et al. 2008). Overall, crop foraging might therefore have positive effects on biological fitness and population size may increase with the large amount of high-quality forage that crops make available to them (Mramba et al. 2019). However, because elephant–human conflicts frequently result in fatalities (Kioko et al. 2008), crop foraging can have severe repercussions on elephant population dynamics and can create an ecological trap. The high risks associated with encountering humans result in appropriate (adaptive) behavioral responses from the elephants, typically avoiding human settlements (Pozo et al. 2018), moving faster where there are higher chances of conflicts (Graham et al. 2009), predominantly at night (Sitati et al. 2003, Graham et al. 2009, Wilkie and Douglas-Hamilton 2018), and preferentially crop foraging close to refuge areas (Jackson et al. 2008).
Loxodonta africana. Photograph: Gaelle Fehlmann.
So far, we have generally portrayed anthropogenic food as high-energy, predictable food sources, but they can vary hugely in nutritional value and availability. Food refuse or discards, for example, although they are readily accessible, can be nutrient and protein deficient and temporarily unavailable (e.g., after refuse collection; Grémillet et al. 2008, Murray et al. 2015); contain nonfood items that can be ingested, such as plastic or rubber bands (Henry et al. 2011); and pose health risks because of pathogens (Drewe et al. 2012, Serieys et al. 2019a). Bin or landfill foraging can therefore be associated with reduced body condition, as has been reported for gannets (Grémillet et al. 2008) and coyotes (Murray et al. 2015). However, it is difficult to disentangle cause and effect in these data—whether frequent raiding reduces individual condition or whether low-quality individuals preferentially source human-derived food items (Murray et al. 2015). Another hypothesis could be that the abundance and predictability of food items, coupled with reduced predation risk in human-altered landscape, allow for the survival of lesser quality individuals resulting in larger population densities but composed of many low quality individuals and a handful of winners (Shochat 2004). More research is clearly needed to disentangle the positive and negative impacts that human-derived food has for individuals at different life stages (Duhem et al. 2005, Grémillet et al. 2008). Identifying the situations in which individuals are worse off by foraging on crops or in cities is important, because it allows us to define the potential ecological traps that may arise from crop- or urban-foraging behaviors (Šálek et al. 2015), and result in drastic population decreases, particularly in species whose reproductive strategies are particularly slow (Hockings et al. 2015).
Community dynamics
We have reviewed the consequences of crop and urban foraging on several aspects of biology and although there is a general positive impact on individual's fitness and population dynamics (figure 1), these can also have broader consequences at a community level. These can alter trophic cascades, particularly if the exploitation of novel foods results in reduced predation on natural prey or if the increased population density leads to the overexploitation of an ecological niche (Hebblewhite et al. 2005, Jones et al. 2016). In fact, the increased population density of species thriving in human-altered landscape is also likely to affect competitors. For example, kleptoparasitism by hyenas is known to challenge wild dogs’ survival because of the costs of losing a prey item (Gorman et al. 1998). As hyenas’ density increases, it is likely that these dynamics may be reinforced and cause other sympatric carnivores such as wild dog and cheetah to decrease (Green et al. 2019). In addition, other ecological services provided by these crop or urban foraging species would be affected. For example, reduced movement through natural landscapes can reduce the dissemination of seeds (Chapman and Onderdonk 1998, Naoe et al. 2016, Sebastián-González et al. 2019) or the maintenance of open habitats or corridors (Cumming et al. 1997). Decreased interactions between species is then likely to increase fragmentation of resources and habitats (Berger-Tal and Saltz 2019). In human-altered landscapes, the presence and increased movement of wild species can increase the spread of exotic and invasive species (Mellado and Zamora 2014) or diseases to humans or domestic species (Daszak et al. 2000, Donnelly et al. 2006, Flint et al. 2016). These challenges will need to be met with urgency by conservation biologists and ecologists.
Globally, disease transmission between humans and wildlife is occurring at an increasing rate, posing a substantial global threat to public health and biodiversity conservation (Jones et al. 2008). Human activities linked to urban and rural land use can interact with disease agents (May 1988) and may result in the alteration of parasite transmission rates, host range, and virulence (Daszak et al. 2000, Patz et al. 2000). Together, these changes may pose a significant conservation risk to wildlife populations living at the urban edge (Serieys et al. 2019b) and particularly to nonhuman primate populations (Wallis and Rick Lee 1999, Drewe et al. 2012, Olarinmoye et al. 2017). With higher population density, which is of central importance to infection rates in the hosts of directly transmitted parasites, the risks are multiplied (Vitone et al. 2004, Silk et al. 2019). Close human contact may also drive higher parasite prevalence in wildlife, notably in baboons (Ghandour et al. 1995, Müller-Graf et al. 1996, Munene et al. 1998), whereas anthropogenic disturbances, such as logging and forest fragmentation, have been shown to alter the dynamics of parasite infection in primate populations (Chapman et al. 2005, Gillespie et al. 2005). Identifying general principles governing disease occurrence is critical for managing vulnerable wildlife populations and mitigating risks to human and animal health. However, it is currently unclear what aspects of anthropogenic changes to the physical environment facilitate the transmission of infectious agents among wildlife and humans (Hassell et al. 2017). The development of improved conservation strategies to deal with established and changing patterns of disease demands that we increase our efforts to understand the interplay between the alteration of ecosystems and disease transmission probabilities.
The challenge of managing wildlife foraging in human-modified landscapes
A change of societal values in developed countries has led to a greater tolerance toward wildlife, and this tolerance and desire for coexistence—rather than conflict—may explain why some species are currently colonizing or recolonizing human-altered landscapes (Carter and Linnell 2016, Bruskotter et al. 2017). Despite these changes, conflicts between wildlife and people emerge as a result of negative interaction that can cause economic loss (e.g., crop depredation or damage to properties; Ogada et al. 2003), disruptive behaviors (e.g., dissemination of trash when foraging on discards Belant 1997, Kaplan et al. 2011, Flint et al. 2016), or increased anxiety (e.g., when the encounter of the wild species result in a risk for humans; Beamish 2009, Lewis et al. 2015, Acharya et al. 2016). In all these ways, conflicts can affect people's perception of biodiversity, hinder conservation goals and ultimately create tensions among people themselves (Chan et al. 2007, Barua et al. 2012).
To mitigate human–wildlife conflict across the globe, people have traditionally inserted barriers in the landscape (table 2; Woodroffe et al. 2007), actively chased wildlife away (table 2; Ogada et al. 2003, Warren 2009), or selectively removed individuals, groups, or populations of species that pose a chronic threat (table 2; Donnelly et al. 2006, Katsvanga et al. 2006). Less frequently, individuals or entire groups may be relocated (Swan et al. 2017) to areas where the source of conflict is absent. These methods are still widely used today and vary in their effectiveness, scalability, and level of public acceptability. Shepherding and fencing livestock, for example, are widely supported as effective nonlethal methods for reducing conflicts with carnivores such as lions (Panthera leo), leopards (Panthera pardus), and cheetahs (Acinonyx jubatus; Woodroffe et al. 2007, Hayward and Kerley 2009). However, fencing may have limited success with burrowing and climbing species (Kioko et al. 2008, Osipova et al. 2018) and can have devastating effects on migratory species such as antelopes (Harris et al. 2009, Hayward and Kerley 2009). Culling or harvesting is often not realistic because of the risk it poses to the viability and demography of local populations (Milner et al. 2007) and the growing opposition from the general public to indiscriminate lethal methods (Shivik et al. 2003, Treves and Naughton-Treves 2005, Slagle et al. 2017). Guarding has been shown to significantly reduce conflicts but is costly to sustain by professionals (table 2; Woodroffe et al. 2007a, Davies et al. 2011) and may lead to social inequalities and hinder people's well-being. For example, crop guarding by children in rural communities hinders school attendance (Barua et al. 2012). Other methods may involve the use of repellents (table 2; Hill and Wallace 2012, Zarco-Gonzalez and Monroy-Vilchis 2014) and supplementary feeding patches (table 2; Oro et al. 2008, Kaplan et al. 2011), but none has yet proven effective in the long term.
Table 2.
Overview of potential management strategies.
| Method | Examples | Limitations | Costs | Labor | Durability | Precision | Public acceptability | Refs | |
|---|---|---|---|---|---|---|---|---|---|
| Nonlethal deterrents | Disruptive | Fencing | Cost | + + + | + | + + | —- | + + | 1–3 |
| Repellents or fladry | Habituation | - | + | - | + | - | 4–6 | ||
| Field ranger | Costs, human flaws | + + | + + + | + + | + + + | + + | 7–9 | ||
| Population control | Translocation | Moving the problem | + + | - | + + | + + + | - | 10 | |
| Contraception or sterilization | Labor intensive | + + | + + + | - | + + + | - | 11 | ||
| Aversive | Conditioned taste aversion | Labor intensive | - | + + + | + + | + + + | - | 12 | |
| Nonlethal attractants | Provisioning | Supplemental feeding patch | Increased feeding | + | + | - | —- | + + | 13–14 |
| Lethal | Population control | Removal of individuals or groups | Identifying problem individuals | + + | + | - - | + + + | + + | 15–17 |
| ++ | + | - - | - | + + + |
Note: Considerations and positive (+) and negative (-) outcomes associated with the various aspects or processes related to the management strategies. References cited: 1–3. Woodroffe et al. 2007, Hayward and Kerley 2009, Osipova et al. 2018, 4–6., 7–9. Ogada et al. 2003, Warren 2009, Fehlmann et al. 2017b, 10–12. Swan et al. 2017, Snijders et al. 2019, 11. Massei and Cowan 2014, 12. Snijders et al. 2019, 13–14. Kaplan et al. 2011, 15–17. Treves and Naughton-Treves 2005, Milner et al. 2007, Swan et al. 2017.
Selecting the correct strategy to manage the impact of crop- and urban-foraging wildlife and the conflicts that emerge from this behavior (human–wildlife and among people) is critical, because an inappropriate or ineffective management strategy may result in an arms race between humans and crop- or urban-foraging species (Ogada et al. 2003, Davies et al. 2011, Kaplan et al. 2011). When conflicts emerge, we suggest a focus on simultaneously raising the costs and reducing the rewards related to these foraging behaviors. This might be particularly challenging because the time and energy constraints of populations foraging on human-derived foods are relaxed, and the additional time and energy available may potentially outweigh failed foraging attempts and exacerbate natural behavioral plasticity (figure 1). Specifically, additional time and energy available to crop- and urban-foraging individuals can potentially reduce the efficacy of fences and guarding methods used in isolation of other methods. We therefore believe there is no single or simple solution. Instead, management strategies require proper understanding of the biological problems and development of bespoke solutions. Indeed, there exists the need for a two-pronged response to human–wildlife conflict (table 1), where scientific studies accurately measure the extent of wildlife damage and how biological factors influence this, whereas social studies look to examine the human component of the conflict, taking into account the varying views of directly affected parties as well as third parties and the prevailing socioeconomic environment. Collaboration between these two endeavors will ensure that mitigation strategies have the best chance of success (Atwood and Breck 2012, Carter and Linnell 2016).
Conclusions
Human activities now dominate the natural environment and most species encounter human-altered features on a daily basis. Although most of these changes have adverse impacts on wildlife communities, a growing number of studies around the world provide empirical evidence for the successes of species foraging in human-altered environments (Chace and Walsh 2006, Hockings et al. 2015, Fleming and Bateman 2018). Species characterized by phenotypic plasticity and a generalist diet appear well adapted to exploit human-modified environments (Griffin et al. 2017). Furthermore, long lived species that live in socially complex groups and possess higher cognitive skills are likely to learn to successfully exploit human-derived food in croplands and urban areas (Barrett et al. 2018). In the present article, we proposed that foraging in human-modified landscapes not only results in a rapid dietary change but presents a novel selective pressure that may lead to important changes in key behavioral traits such as movement patterns, activity, and energy budgets to social dynamics within groups and life history traits. We demonstrated how these aspects of species biology are intertwined, resulting in new population and community dynamics and challenges. We highlighted how these changes can affect management of these individuals, populations and species in conflict with humans in altered environments. How changes in these traits influence reproductive strategies and mate choice remains an important area for future research, which will influence both population dynamics and the spread of such behaviors in populations.
Understanding how wildlife species respond to novel selection pressures in the Anthropocene is important from both a biological and wildlife management perspective. Devising novel approaches and methods to ensuring that more wildlife has better welfare and conservation status within and adjacent to urban and rural landscapes is highlighted as a critical future goal. Realizing this goal will require that we continue to innovate when seeking to understand the drivers of human–wildlife conflict and its mitigation while continuing to develop a theoretical framework for understanding responses of wildlife to diverse global human-modified landscapes.
Acknowledgments
Thanks to the Baboon Technical Team in Cape Town for providing real-life inspiration for the present article and to Emily Shepard, Damien Farine, Lucy Aplin, Jim Bull, Stuart Semple, Anne Scharf, Elham Nourani, Yachang Cheng, Martina Scacco, Teja Curk, Iris Bontekoe, and Valentina Iesari for discussion and comments that improved the manuscript. During preparation of this manuscript AJK was supported by Natural Environment Research Council (NERC) grant (NE/M015351/1) and JO'R acknowledges National Research Foundation (NRF) incentive funding. GF was supported by a Swansea University PhD scholarship and acknowledges Dr Lucy Aplin, Martin Wikelski and the Brigitte-Schlieben-Lange-Programm for supporting her during the latest stage of the preparation of the manuscript.
Author Biographical
Gaelle Fehlmann (fehlmanng@gmail.com) is a postdoctoral fellow at the Max Planck Institute for Animal Behavior, in Bodensee, Germany. Justin O'Riain is a professor and director of the Institute for Communities and Wildlife in Africa, in the Department of Biological Sciences at the University of Cape Town, in Cape Town, South Africa. Ines Fürtbauer is a senior lecturer and head of the Behavioural Ecology and Endocrinology Laboratory and Andrew King is an associate professor and head of the SHOAL group in the Department of Biosciences at Swansea University, in Swansea, Wales, in the United Kingdom.
Contributor Information
Gaelle Fehlmann, Max Planck Institute for Animal Behavior, Bodensee, Germany.
M Justin O'riain, Institute for Communities and Wildlife, Africa, Department of Biological Sciences, University of Cape Town, Cape Town, South Africa.
Ines FÜrtbauer, Behavioural Ecology and Endocrinology Laboratory and Andrew King is an associate professor and head of the SHOAL group in the Department of Biosciences at Swansea University, Swansea, Wales, United Kingdom.
Andrew J King, Max Planck Institute for Animal Behavior, Bodensee, Germany.
References
- Acharya KP, Paudel PK, Neupane PR, Köhl M. 2016. Human–wildlife conflicts in Nepal: Patterns of human fatalities and injuries caused by large mammals. PLOS ONE 11: e0161717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams TSF, Chase MJ, Rogers TL, Leggett KEA. 2017. Taking the elephant out of the room and into the corridor: Can urban corridors work? Oryx 51: 347–353. [Google Scholar]
- Ahlering MA, Millspaugh JJ, Woods RJ, Western D, Eggert LS. 2011. Elevated levels of stress hormones in crop-raiding male elephants. Animal Conservation 14: 124–130. [Google Scholar]
- Altmann J, Muruthi P.. 1988. Differences in daily life between semiprovisioned and wild-feeding baboons. American Journal of Primatology 15: 213–221. [DOI] [PubMed] [Google Scholar]
- Aplin LM, Farine DR, Morand-Ferron J, Sheldon BC. 2012. Social networks predict patch discovery in a wild population of songbirds. Proceedings of the Royal Society B 279: 4199–4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood T, Breck S.. 2012. Carnivores, Conflict, and Conservation: Defining the Landscape of Conflict. US Department of Agriculture National Wildlife Research Center. [Google Scholar]
- Barrett LP, Stanton LA, Benson-Amram S. 2018. The cognition of “nuisance” species. Animal Behaviour 147: 167–177. [Google Scholar]
- Bartumeus F, Giuggioli L, Louzao M, Bretagnolle V, Oro D, Levin SA. 2010. Fishery discards impact on seabird movement patterns at regional scales. Current Biology 20: 215–222. [DOI] [PubMed] [Google Scholar]
- Barua M, Bhagwat S, Jadhav S. 2012. The hidden dimensions of human–wildlife conflict: Health impacts, opportunity and transaction costs. Biological Conservation 157: 309–316. [Google Scholar]
- Belant JL. 1997. Gulls in urban environments: Landscape-level management to reduce conflict. Landscape and Urban Planning 38: 245–258. [Google Scholar]
- Beamish EK. 2009. Causes and Consequences of Mortality and Mutilation in the Cape Peninsula Baboon Population, South Africa. Masters thesis, University of Cape Town, South Africa. [Google Scholar]
- Beckmann JP, Berger J.. 2003a. Rapid ecological and behavioural changes in carnivores: The responses of black bears (Ursus americanus) to altered food. Journal of Zoology 261: 207–212. [Google Scholar]
- Beckmann JP, Berger J.. 2003b. Using black bears to test ideal-free distribution models experimentally. Journal of Mammalogy 84: 594–606. [Google Scholar]
- Belton LE, Cameron EZ, Dalerum F. 2016. Spotted hyaena space use in relation to human infrastructure inside a protected area. PeerJ 4: e2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger-Tal O, Saltz D.. 2019. Invisible barriers: Anthropogenic impacts on inter- and intra-specific interactions as drivers of landscape-independent fragmentation. Philosophical Transactions of the Royal Society B 374: 20180049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkovcová M, Mrtka J, Winkler J. 2012. Factors affecting mortality of vertebrates on the roads in the Czech Republic. Transportation Research Part D: Transport and Environment 17: 66–72. [Google Scholar]
- Brooks J, Kays R, Hare B. 2020. Coyotes living near cities are bolder: Implications for dog evolution and human–wildlife conflict. Behaviour 157: 289–313. [Google Scholar]
- Bruskotter JT, et al. 2017. . Modernization, risk, and conservation of the world's largest carnivores. BioScience 67: 646–655. [Google Scholar]
- Burt WH. 1943. Territoriality and home range concepts as applied to mammals. Journal of Mammalogy 24: 346–352. [Google Scholar]
- Camphuysen KCJ, Shamoun-Baranes J, Loon EE, Bouten W. 2015. Sexually distinct foraging strategies in an omnivorous seabird. Marine Biology 162: 1417–1428. [Google Scholar]
- Caro T. 1999. The behaviour–conservation interface. Trends in Ecology and Evolution 14: 366–369. [DOI] [PubMed] [Google Scholar]
- Carter NH, Linnell JDC.. 2016. Co-adaptation is key to coexisting with large carnivores. Trends in Ecology and Evolution 31: 575–578. [DOI] [PubMed] [Google Scholar]
- Chace JF, Walsh JJ.. 2006. Urban effects on native avifauna: A review. Landscape and Urban Planning 74: 46–69. [Google Scholar]
- Chan KMA, Pringle RM, Ranganathan J, Boggs CL, Chan YL, Ehrlich PR, Haff PK, Heller NE, Al-Khafaji K, Macmynowski DP. 2007. When agendas collide: Human welfare and biological conservation. Conservation Biology 21: 59–68. [DOI] [PubMed] [Google Scholar]
- Chapman CA, Gillespie TR, Goldberg TL. 2005. Primates and the ecology of their infectious diseases: How will anthropogenic change affect host–parasite interactions? Evolutionary Anthropology: Issues, News, and Reviews 14: 134–144. [Google Scholar]
- Chapman CA, Onderdonk DA.. 1998. Forests without primates: Primate/plant codependency. American Journal of Primatology 45: 127–141. [DOI] [PubMed] [Google Scholar]
- Charter M, Izhaki I, Ben Mocha Y, Kark S. 2016. Nest-site competition between invasive and native cavity nesting birds and its implication for conservation. Journal of Environmental Management 181: 129–134. [DOI] [PubMed] [Google Scholar]
- Chiyo PI, Cochrane EP, Naughton L, Basuta GI. 2005. Temporal patterns of crop raiding by elephants: A response to changes in forage quality or crop availability? African Journal of Ecology 43: 48–55. [Google Scholar]
- Chiyo PI, Lee PC, Moss CJ, Archie EA, Hollister-Smith JA, Alberts SC. 2011. No risk, no gain: Effects of crop raiding and genetic diversity on body size in male elephants. Behavioral Ecology 22: 552–558. [Google Scholar]
- Chiyo PI, Moss CJ, Alberts SC. 2012. The influence of life history milestones and association networks on crop-raiding behavior in male African elephants. PLOS ONE 7: e31382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colles A, Liow LH, Prinzing A. 2009. Are specialists at risk under environmental change? Neoecological, paleoecological and phylogenetic approaches. Ecology Letters 12: 849–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradt L, Krause J, Couzin ID, Roper TJ. 2009. “Leading according to need” in self-organizing groups. American Naturalist 173: 304–312. [DOI] [PubMed] [Google Scholar]
- Cowlishaw G. 1997. Trade-offs between foraging and predation risk determine habitat use in a desert baboon population. Animal Behaviour 53: 667–686. [DOI] [PubMed] [Google Scholar]
- Cox DTC, Inger R, Hancock S, Anderson K, Gaston KJ. 2016. Movement of feeder-using songbirds: The influence of urban features. Scientific Reports 6: 37669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumming DHM, Fenton MB, Rautenbach IL, Taylor RD, Cumming GS, Cumming MS, Dunlop JM, Ford AG, Hovorka MD, Johnston DS, Kalcounis M, Mahlangu Z, Portfors CVR. 1997. Elephants, woodlands and biodiversity in southern Africa. South African Journal of Science 93: 231–236. [Google Scholar]
- Daniels SE, Fanelli RE, Gilbert A, Benson-Amram S. 2019. Behavioral flexibility of a generalist carnivore. Animal Cognition 22: 387–396. [DOI] [PubMed] [Google Scholar]
- Daszak P, Cunningham AA, Hyatt AD. 2000. Emerging infectious diseases of wildlife threats to biodiversity and human health. Science 287: 443–449. [DOI] [PubMed] [Google Scholar]
- Davies TE, Wilson S, Hazarika N, Chakrabarty J, Das D, Hodgson DJ, Zimmermann A. 2011. Effectiveness of intervention methods against crop-raiding elephants. Conservation Letters 4: 346–354. [Google Scholar]
- Davison J, Huck M, Delahay RJ, Roper TJ. 2009. Restricted ranging behaviour in a high-density population of urban badgers. Journal of Zoology 277: 45–53. [Google Scholar]
- Dingemanse NJ, Kazem AJN, Réale D, Wright J. 2010. Behavioural reaction norms: Animal personality meets individual plasticity. Trends in Ecology and Evolution 25: 81–89. [DOI] [PubMed] [Google Scholar]
- Donnelly CA, et al. 2006. Positive and negative effects of widespread badger culling on tuberculosis in cattle. Nature 439: 843–846. [DOI] [PubMed] [Google Scholar]
- Doorn AC, O'Riain MJ, Swedell L. 2010. The effects of extreme seasonality of climate and day length on the activity budget and diet of semi-commensal chacma baboons (Papio ursinus) in the Cape Peninsula of South Africa. American Journal of Primatology 72: 104–112. [DOI] [PubMed] [Google Scholar]
- Dostálková I, Špinka M.. 2007. Synchronization of behaviour in pairs: The role of communication and consequences in timing. Animal Behaviour 74: 1735–1742. [Google Scholar]
- Drewe JA, O'Riain MJ, Beamish E, Currie H, Parsons S. 2012. Survey of infections transmissible between baboons and humans, Cape Town, South Africa. Emerging Infectious Diseases 18: 298–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducatez S, Audet JN, Lefebvre L. 2013. Independent appearance of an innovative feeding behaviour in Antillean bullfinches. Animal Cognition 16: 525–529. [DOI] [PubMed] [Google Scholar]
- Ducatez S, Audet J-N, Rodriguez JR, Kayello L, Lefebvre L. 2017. Innovativeness and the effects of urbanization on risk-taking behaviors in wild Barbados birds. Animal Cognition 20: 33–42. [DOI] [PubMed] [Google Scholar]
- Duhem C, Vidal E, Roche P, Legrand J. 2005. How is the diet of yellow-legged gull chicks influenced by parents’ accessibility to landfills? Waterbirds 28: 46–52. [Google Scholar]
- Enners L, Schwemmer P, Corman A-M, Voigt CC, Garthe S. 2018. Intercolony variations in movement patterns and foraging behaviors among herring gulls (Larus argentatus) breeding in the eastern Wadden Sea. Ecology and Evolution 8: 7529–7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedriani JM, Fuller TK, Sauvajot RM. 2001. Does availability of anthropogenic food enhance densities of omnivorous mammals? An example with coyotes in southern California. Ecography 24: 325–331. [Google Scholar]
- Fehlmann G, King AJ. 2016. Bio-logging. Current Biology 26: R830–R831. [DOI] [PubMed] [Google Scholar]
- Fehlmann G, O'Riain MJ, Kerr-Smith C, Hailes S, Luckman A, Shepard ELC, King AJ. 2017a. Extreme behavioural shifts by baboons exploiting risky, resource-rich, human-modified environments. Scientific Reports 7: 15057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehlmann G, O'Riain MJ, Kerr-Smith C, King AJ. 2017b. Adaptive space use by baboons (Papio ursinus) in response to management interventions in a human-changed landscape. Animal Conservation 20: 101–109. [Google Scholar]
- Fichtel C, Manser M.. 2010. Communication in social groups. Pages 29–54 in Kappeler PM, ed. Animal Behaviour: Evolution and Mechanisms. Springer. [Google Scholar]
- Fleming PA, Bateman PW.. 2018. Novel predation opportunities in anthropogenic landscapes. Animal Behaviour 138: 145–155. [Google Scholar]
- Flint BF, Hawley DM, Alexander KA. 2016. Do not feed the wildlife: Associations between garbage use, aggression, and disease in banded mongooses (Mungos mungo). Ecology and Evolution 6: 5932–5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourie NH, Turner TR, Brown JL, Pampush JD, Lorenz JG, Bernstein RM. 2015. Variation in vervet (Chlorocebus aethiops) hair cortisol concentrations reflects ecological disturbance by humans. Primates 56: 365–373. [DOI] [PubMed] [Google Scholar]
- Franz M, Nunn CL.. 2009. Network-based diffusion analysis: A new method for detecting social learning. Proceedings of the Royal Society B 276: 1829–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser DF, Huntingford FA.. 1986. Feeding and avoiding predation hazard: The behavioral response of the prey. Ethology 73: 56–68. [Google Scholar]
- Gallagher AJ, Creel S, Wilson RP, Cooke SJ. 2017. Energy landscapes and the landscape of fear. Trends in Ecology and Evolution 32: 88–96. [DOI] [PubMed] [Google Scholar]
- Gehrt SD, Anchor C, White LA. 2009. Home range and landscape use of coyotes in a metropolitan landscape: Conflict or coexistence? Journal of Mammalogy 90: 1045–1057. [Google Scholar]
- Ghandour AM, Zahid NZ, Banaja AA, Kamal KB, Bouq AI. 1995. Zoonotic intestinal parasites of hamadryas baboons Papio hamadryas in the western and northern regions of Saudi Arabia. The Journal of tropical medicine and hygiene 98: 431–439. [PubMed] [Google Scholar]
- Gillespie TR, Chapman CA, Greiner EC. 2005. Effects of logging on gastrointestinal parasite infections and infection risk in African primates. Journal of Applied Ecology 42: 699–707. [Google Scholar]
- Gorman ML, Mills MG, Raath JP, Speakman JR. 1998. High hunting costs make African wild dogs vulnerable to kleptoparasitism by hyaenas. Nature 391: 479–481. [Google Scholar]
- Grafius DR, Corstanje R, Siriwardena GM, Plummer KE, Harris JA. 2017. A bird's eye view: Using circuit theory to study urban landscape connectivity for birds. Landscape Ecology 32: 1771–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham MD, Douglas-Hamilton I, Adams WM, Lee PC. 2009. The movement of African elephants in a human-dominated land-use mosaic. Animal Conservation 12: 445–455. [Google Scholar]
- Green DS, Farr MT, Holekamp KE, Strauss ED, Zipkin EF. 2019. Can hyena behaviour provide information on population trends of sympatric carnivores? Philosophical Transactions of the Royal Society B 374: 20180052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grémillet D, Pichegru L, Kuntz G, Woakes AG, Wilkinson S, Crawford RJM, Ryan PG. 2008. A junk-food hypothesis for gannets feeding on fishery waste. Proceedings of the Royal Society B 275: 1149–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin AS, Netto K, Peneaux C. 2017. Neophilia, innovation and learning in an urbanized world: A critical evaluation of mixed findings. Current Opinion in Behavioral Sciences 16: 15–22. [Google Scholar]
- Hägerling HG, Ebersole JJ. 2017. Roads as travel corridors for mammals and ground birds in Tarangire National Park, Tanzania. African Journal of Ecology 55: 701–704. [Google Scholar]
- Harris G, Thirgood S, Hopcraft JGC, Cromsigt JPGM, Berger J. 2009. Global decline in aggregated migrations of large terrestrial mammals. Endangered Species Research 7: 55–76. [Google Scholar]
- Hassell JM, Begon M, Ward MJ, Fèvre EM. 2017. Urbanization and disease emergence: Dynamics at the wildlife–livestock–human interface. Trends in Ecology and Evolution 32: 55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward MW, Kerley GIH.. 2009. Fencing for conservation: Restriction of evolutionary potential or a riposte to threatening processes? Biological Conservation 142: 1–13. [Google Scholar]
- Hebblewhite M, White CA, Nietvelt CG, McKenzie JA, Hurd TE, Fryxell JM, Bayley SE, Paquet PC. 2005. Human activity mediates a trophic cascade caused by wolves. Ecology 86: 2135–2144. [Google Scholar]
- Henry P-Y, Wey G, Balança G. 2011. Rubber band ingestion by a rubbish dump dweller, the White Stork (Ciconia ciconia). Waterbirds 34: 504–508. [Google Scholar]
- Herbert-Read JE. 2016. Understanding how animal groups achieve coordinated movement. Journal of Experimental Biology 219: 2971–2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Brito D, Carrete M, Ibáñez C, Juste J, Tella JL. 2018. Nest-site competition and killing by invasive parakeets cause the decline of a threatened bat population. Royal Society Open Science 5: 172477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CM. 2017. Primate crop feeding behavior, crop protection, and conservation. International Journal of Primatology 38: 385–400. [Google Scholar]
- Hill CM, Wallace GE.. 2012. Crop protection and conflict mitigation: Reducing the costs of living alongside non-human primates. Biodiversity and Conservation 21: 2569–2587. [Google Scholar]
- Hixon MA. 1982. Energy maximizers and time minimizers: Theory and reality. The American Naturalist 119: 596–599. [Google Scholar]
- Hockings KJ, McLennan MR.. 2012. From forest to farm: Systematic review of cultivar feeding by chimpanzees: Management implications for wildlife in anthropogenic landscapes. PLOS ONE 7: e33391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockings KJ, et al. 2015. Apes in the Anthropocene: Flexibility and survival. Trends in Ecology and Evolution 30: 215–222. [DOI] [PubMed] [Google Scholar]
- Hoffman TS, O'Riain MJ.. 2012a. Troop size and human-modified habitat affect the ranging patterns of a chacma baboon population in the cape peninsula, South Africa. American Journal of Primatology 74: 853–863. [DOI] [PubMed] [Google Scholar]
- Hoffman TS, O'Riain MJ.. 2012b. Landscape requirements of a primate population in a human-dominated environment. Frontiers in Zoology 9: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Sullivan RM, Gunnar MR. 2014. Psychobiological mechanisms underlying the social buffering of the HPA axis: A review of animal models and human studies across development. Psychological Bulletin 140: 256–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannou CC, Singh M, Couzin ID. 2015. Potential leaders trade off goal-oriented and socially oriented behavior in mobile animal groups. American Naturalist 186: 284–293. [DOI] [PubMed] [Google Scholar]
- Isaksson N, Evans TJ, Shamoun-Baranes J, Åkesson S. 2016. Land or sea? Foraging area choice during breeding by an omnivorous gull. Movement Ecology 4: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson TP, Mosojane S, Ferreira SM, Aarde RJ. 2008. Solutions for elephant Loxodonta africana crop raiding in northern Botswana: Moving away from symptomatic approaches. Oryx 42: 83–91. [Google Scholar]
- Jarman PJ. 1974. The social organisation of antelope in relation to their ecology. Behaviour 48: 215–267. [Google Scholar]
- Jones BM, Cove MV, Lashley MA, Jackson VL. 2016. Do coyotes Canis latrans influence occupancy of prey in suburban forest fragments? Current Zoology 62: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P. 2008. Global trends in emerging infectious diseases. Nature 451: 990–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan BS, O'Riain MJ, Eeden R, King AJ. 2011. A low-cost manipulation of food resources reduces spatial overlap between baboons (Papio ursinus) and humans in conflict. International Journal of Primatology 32: 1397–1412. [Google Scholar]
- Katsvanga CAT, Mudyiwa SM, Gwenzi D. 2006. Bark stripping and population dynamics of baboon troops after chemical control in pine plantations of Zimbabwe. African Journal of Ecology 44: 413–416. [Google Scholar]
- Kays R, Parsons AW.. 2014. Mammals in and around suburban yards, and the attraction of chicken coops. Urban Ecosystems 17: 691–705. [Google Scholar]
- Kertson BN, Spencer RD, Marzluff JM, Hepinstall-Cymerman J, Grue CE. 2011. Cougar space use and movements in the wildland–urban landscape of western Washington. Ecological Applications 21: 2866–2881. [Google Scholar]
- King AJ, Cowlishaw G.. 2009. All together now: Behavioural synchrony in baboons. Animal Behaviour 78: 1381–1387. [Google Scholar]
- King AJ, Douglas CMS, Huchard E, Isaac NJB, Cowlishaw G. 2008. Dominance and affiliation mediate despotism in a social primate. Current Biology 18: 1833–1838. [DOI] [PubMed] [Google Scholar]
- King AJ, Fehlmann G, Biro D, Ward AJ, Fürtbauer I. 2018. Re-wilding collective behaviour: An ecological perspective. Trends in Ecology and Evolution 33: 347–357. [DOI] [PubMed] [Google Scholar]
- King AJ, Fürtbauer I, Mamuneas D, James C, Manica A. 2013. Sex-differences and temporal consistency in stickleback fish boldness. PLOS ONE 8: e81116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kioko J, Muruthi P, Omondi P, Chiyo PI. 2008. The performance of electric fences as elephant barriers in Amboseli, Kenya. South African Journal of Wildlife Research 38: 52–58. [Google Scholar]
- Koen EL, Bowman J, Sadowski C, Walpole AA. 2014. Landscape connectivity for wildlife: Development and validation of multispecies link maps. Methods in Ecology and Evolution 5: 626–633. [Google Scholar]
- Lefebvre L, Reader SM, Sol D. 2004. Brains, innovations and evolution in birds and primates. Brain Behavior and Evolution 63: 233–246. [DOI] [PubMed] [Google Scholar]
- Lewis DL, Baruch-Mordo S, Wilson KR, Breck SW, Mao JS, Broderick J. 2015. Foraging ecology of black bears in urban environments: Guidance for human-bear conflict mitigation. Ecosphere 6: 1–18. [Google Scholar]
- Lima SL, Rattenborg NC, Lesku JA, Amlaner CJ. 2005. Sleeping under the risk of predation. Animal Behaviour 70: 723–736. [Google Scholar]
- Linklater WL. 2000. Adaptive explanation in socio-ecology: Lessons from the Equidae. Biological Reviews 75: 1–20. [DOI] [PubMed] [Google Scholar]
- Liu Z, He C, Zhou Y, Wu J. 2014. How much of the world's land has been urbanized, really? A hierarchical framework for avoiding confusion. Landscape Ecology 29: 763–771. [Google Scholar]
- Maciusik B, Lenda M, Skórka P. 2010. Corridors, local food resources, and climatic conditions affect the use of the urban environment by the black-headed gull Larus ridibundus in winter. Ecological Research 25: 263–272. [Google Scholar]
- MacMahon JA, Schimpf DJ, Andersen DC, Smith KG, Bayn RL. 1981. An organism-centered approach to some community and ecosystem concepts. Journal of Theoretical Biology 88: 287–307. [DOI] [PubMed] [Google Scholar]
- Massei G, Cowan D.. 2014. Fertility control to mitigate human–wildlife conflicts: A review. Wildlife Research 41: 1–21. [Google Scholar]
- May RM. 1988. Conservation and Disease. Conservation Biology 2: 28–30. [Google Scholar]
- Mazur R, Seher V.. 2008. Socially learned foraging behaviour in wild black bears, Ursus americanus. Animal Behaviour 75: 1503–1508. [Google Scholar]
- Mellado A, Zamora R.. 2014. Generalist birds govern the seed dispersal of a parasitic plant with strong recruitment constraints. Oecologia 176: 139–147. [DOI] [PubMed] [Google Scholar]
- Michalski F, Boulhosa RLP, Faria A, Peres CA. 2006. Human–wildlife conflicts in a fragmented Amazonian forest landscape: Determinants of large felid depredation on livestock. Animal Conservation 9: 179–188. [Google Scholar]
- Michl G, Torok J, Garamszegi LZ, Toth L. 2000. Sex-dependent risk taking in the collared flycatcher, Ficedula albicollis, when exposed to a predator at the nestling stage. Animal Behaviour 59: 623–628. [DOI] [PubMed] [Google Scholar]
- Milner JM, Nilsen EB, Andreassen HP. 2007. Demographic Side Effects of Selective Hunting in Ungulates and Carnivores. Conservation Biology 21: 36–47. [DOI] [PubMed] [Google Scholar]
- Mramba RP, Andreassen HP, Mlingi V, Skarpe C. 2019. Activity patterns of African elephants in nutrient-rich and nutrient-poor savannas. Mammalian Biology 94: 18–24. [Google Scholar]
- Müller-Graf CDM, Collins DA, Woolhouse MEJ. 1996. Intestinal parasite burden in five troops of olive baboons (Papio cynocephalus anubis) in Gombe Stream National Park, Tanzania. Parasitology 112: 489–497. [DOI] [PubMed] [Google Scholar]
- Munene E, Otsyula M, Mbaabu DAN, Mutahi WT, Muriuki SMK, Muchemi GM. 1998. Helminth and protozoan gastrointestinal tract parasites in captive and wild-trapped African non-human primates. Veterinary Parasitology 78: 195–201. [DOI] [PubMed] [Google Scholar]
- Murray M, Cembrowski A, Latham ADM, Lukasik VM, Pruss S, St Clair CC. 2015. Greater consumption of protein-poor anthropogenic food by urban relative to rural coyotes increases diet breadth and potential for human–wildlife conflict. Ecography 38: 1235–1242. [Google Scholar]
- Murray MH, Clair CCS.. 2015. Individual flexibility in nocturnal activity reduces risk of road mortality for an urban carnivore. Behavioral Ecology 26: 1520–1527. [Google Scholar]
- Naoe S, Tayasu I, Sakai Y, Masaki T, Kobayashi K, Nakajima A, Sato Y, Yamazaki K, Kiyokawa H, Koike S. 2016. Mountain-climbing bears protect cherry species from global warming through vertical seed dispersal. Current Biology 26: R315–R316. [DOI] [PubMed] [Google Scholar]
- Nathan R, Getz WM, Revilla E, Holyoak M, Kadmon R, Saltz D, Smouse PE. 2008. A movement ecology paradigm for unifying organismal movement research. Proceedings of the National Academy of Sciences 105: 19052–19059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naughton Treves L. 1998. Predicting patterns of crop damage by wildlife around kibale national park, Uganda. Conservation Biology 12: 156–168. [Google Scholar]
- Nishikawa M, Suzuki M, Sprague DS. 2014. Activity and social factors affect cohesion among individuals in female Japanese macaques: A simultaneous focal-follow study. American Journal of Primatology 76: 694–703. [DOI] [PubMed] [Google Scholar]
- Ogada MO, Woodroffe R, Oguge NO, Frank LG. 2003. Limiting depredation by African carnivores: The role of livestock husbandry. Conservation Biology 17: 1521–1530. [Google Scholar]
- Olarinmoye AO, Olugasa BO, Niphuis H, Herwijnen RV, Verschoor E, Boug A, Ishola OO, Buitendijk H, Fagrouch Z, Al-Hezaimi K. 2017. Serological evidence of coronavirus infections in native hamadryas baboons (Papio hamadryas hamadryas) of the Kingdom of Saudi Arabia. Epidemiology and Infection 145: 2030–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oro D, Margalida A, Carrete M, Heredia R, Donázar JA. 2008. Testing the goodness of supplementary feeding to enhance population viability in an endangered vulture. PLOS ONE 3: e4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn FV. 2004. Seasonal variation of feeding patterns and food selection by crop-raiding elephants in Zimbabwe. African Journal of Ecology 42: 322–327. [Google Scholar]
- Osipova L, Okello MM, Njumbi SJ, Ngene S, Western D, Hayward MW, Balkenhol N. 2018. Fencing solves human–wildlife conflict locally but shifts problems elsewhere: A case study using functional connectivity modelling of the African elephant. Journal of Applied Ecology 55: 2673–2684. [Google Scholar]
- Otali E, Gilchrist JS.. 2004. The effects of refuse feeding on body condition, reproduction, and survival of banded mongooses. Journal of Mammalogy 85: 491–497. [Google Scholar]
- Patz JA, Graczyk TK, Geller N, Vittor AY. 2000. Effects of environmental change on emerging parasitic diseases. International Journal for Parasitology 30: 1395–1405. [DOI] [PubMed] [Google Scholar]
- Pereira ME. 1995. Development and social dominance among group-living primates. American Journal of Primatology 37: 143–175. [DOI] [PubMed] [Google Scholar]
- Pozo RA, Cusack JJ, McCulloch G, Stronza A, Songhurst A, Coulson T. 2018. Elephant space-use is not a good predictor of crop-damage. Biological Conservation 228: 241–251. [Google Scholar]
- Ramankutty N, Evan AT, Monfreda C, Foley JA. 2008. Farming the planet: 1. Geographic distribution of global agricultural lands in the year 2000. Global Biogeochemical Cycles 22: GB1003. [Google Scholar]
- Rands SA, Cowlishaw G, Pettifor RA, Rowcliffe JM, Johnstone RA. 2008. The emergence of leaders and followers in foraging pairs when the qualities of individuals differ. BMC Evolutionary Biology 8: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reader SM, Hager Y, Laland KN. 2011. The evolution of primate general and cultural intelligence. Philosophical Transactions of the Royal Society B 366: 1017–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redpath SM, Young J, Evely A, Adams WM, Sutherland WJ, Whitehouse A, Amar A, Lambert RA, Linnell JDC, Watt A, Gutiérrez RJ. 2013. Understanding and managing conservation conflicts. Trends in Ecology and Evolution 28: 100–109. [DOI] [PubMed] [Google Scholar]
- Reher S, Dausmann KH, Warnecke L, Turner JM. 2016. Food availability affects habitat use of Eurasian red squirrels (Sciurus vulgaris) in a semi-urban environment. Journal of Mammalogy 97: 1543–1554. [Google Scholar]
- Reynolds SJ, Galbraith JA, Smith JA, Jones DN. 2017. Garden bird feeding: Insights and prospects from a North-South comparison of this global urban phenomenon. Frontiers in Ecology and Evolution 5 10.3389/fevo.2017.00024. [DOI] [Google Scholar]
- Roberts G. 1996. Why individual vigilance declines as group size increases. Animal Behaviour 51: 1077–1086. [Google Scholar]
- Rodewald AD, Gehrt SD.. 2014. Wildlife Population Dynamics in Urban Landscapes. Pages 117–147 in McCleery RA, Moorman CE, Peterson MN, eds. Urban Wildlife conservation: Theory and Practice. Springer. [Google Scholar]
- Rotics S, et al. 2017. Wintering in Europe instead of Africa enhances juvenile survival in a long-distance migrant. Animal Behaviour 126: 79–88. [Google Scholar]
- Ruckstuhl KE, Kokko H.. 2002. Modelling sexual segregation in ungulates: Effects of group size, activity budgets and synchrony. Animal Behaviour 64: 909–914. [Google Scholar]
- Šálek M, Drahníková L, Tkadlec E. 2015. Changes in home range sizes and population densities of carnivore species along the natural to urban habitat gradient. Mammal Review 45: 1–14. [Google Scholar]
- Salinas-Melgoza A, Salinas-Melgoza V, Wright TF. 2013. Behavioral plasticity of a threatened parrot in human-modified landscapes. Biological Conservation 159: 303–312. [Google Scholar]
- Santini L, González‐Suárez M, Russo D, Gonzalez‐Voyer A, Hardenberg A, Ancillotto L. 2019. One strategy does not fit all: Determinants of urban adaptation in mammals. Ecology Letters 22: 365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoener TW. 1971. Theory of feeding strategies. Annual Review of Ecology and Systematics 2: 369–404. [Google Scholar]
- Sebastián-González E, et al. 2019. The extent, frequency, and ecological functions of food wasting by parrots. Scientific Reports 9: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler N, Robbins MM.. 2016. Factors influencing ranging on community land and crop raiding by mountain gorillas. Animal Conservation 19: 176–188. [Google Scholar]
- Serieys LEK, Bishop J, Okes N, Broadfield J, Winterton DJ, Poppenga RH, Viljoen S, Wayne RK, O'Riain MJ. 2019a. Widespread anticoagulant poison exposure in predators in a rapidly growing South African city. Science of The Total Environment 666: 581–590. [DOI] [PubMed] [Google Scholar]
- Serieys LEK, Hammond-Aryee K, Bishop J, Broadfield J, O'Riain MJ, Helden PD. 2019b. High seroprevalence of Toxoplasma gondii in an urban caracal (Caracal caracal) population in South Africa. Journal of Wildlife Diseases 55: 951–953. [PubMed] [Google Scholar]
- Shannon G, Page BR, Mackey RL, Duffy KJ, Slotow R. 2008. Activity budgets and sexual segregation in African elephants (Loxodonta africana). Journal of Mammalogy 89: 467–476. [Google Scholar]
- Shepard ELC, Williamson C, Windsor SP. 2016. Fine-scale flight strategies of gulls in urban airflows indicate risk and reward in city living. Philosophical Transactions of the Royal Society B 371: 20150394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard ELC, Wilson RP, Rees WG, Grundy E, Lambertucci SA, Vosper SB. 2013. Energy Landscapes Shape Animal Movement Ecology. American Naturalist 182: 298–312. [DOI] [PubMed] [Google Scholar]
- Shivik JA, Treves A, Callahan P. 2003. Nonlethal techniques for managing predation: Primary and secondary repellents. Conservation Biology 17: 1531–1537. [Google Scholar]
- Shochat E. 2004. Credit or debit? Resource input changes population dynamics of city-slicker birds. Oikos 106: 622–626. [Google Scholar]
- Shutt K, MacLarnon A, Heistermann M, Semple S. 2007. Grooming in Barbary macaques: better to give than to receive? Biology Letters 3: 231–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sih A. 2013. Understanding variation in behavioural responses to human-induced rapid environmental change: A conceptual overview. Animal Behaviour 85: 1077–1088. [Google Scholar]
- Silk MJ, Hodgson DJ, Rozins Croft DP, Delahay RJ, Boots M, McDonald RA. 2019. Integrating social behaviour, demography and disease dynamics in network models: Applications to disease management in declining wildlife populations. Philosophical Transactions of the Royal Society B 374: 20180211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitati NW, Walpole MJ, Smith RJ, Leader‐Williams N. 2003. Predicting spatial aspects of human–elephant conflict. Journal of Applied Ecology 40: 667–677. [Google Scholar]
- Slagle K, Bruskotter JT, Singh AS, Schmidt RH. 2017. Attitudes toward predator control in the United States: 1995 and 2014. Journal of Mammalogy 98: 7–16. [Google Scholar]
- Smith BR, Blumstein DT.. 2008. Fitness consequences of personality: A meta-analysis. Behavioral Ecology 19: 448–455. [Google Scholar]
- Snell-Rood EC. 2013. An overview of the evolutionary causes and consequences of behavioural plasticity. Animal Behaviour 85: 1004–1011. [Google Scholar]
- Snijders L, Greggor AL, Hilderink F, Doran C. 2019. Effectiveness of animal conditioning interventions in reducing human–wildlife conflict: A systematic map protocol. Environmental Evidence 8: 10. [Google Scholar]
- Sol D, Lapiedra O, González-Lagos C. 2013. Behavioural adjustments for a life in the city. Animal Behaviour 85: 1101–1112. [Google Scholar]
- Sol D, Timmermans S, Lefebvre L. 2002. Behavioural flexibility and invasion success in birds. Animal Behaviour 63: 495–502. [Google Scholar]
- Spelt A, Williamson C, Shamoun-Baranes J, Shepard E, Rock P, Windsor S. 2019. Habitat use of urban-nesting lesser black-backed gulls during the breeding season. Scientific Reports 9: 10527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strum SC. 2010. The development of primate raiding: Implications for management and conservation. International Journal of Primatology 31: 133–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueur C, King AJ, Conradt L, Kerth G, Lusseau D, Mettke‐Hofmann C, Schaffner CM, Williams L, Zinner D, Aureli F. 2011. Collective decision-making and fission–fusion dynamics: A conceptual framework. Oikos 120: 1608–1617. [Google Scholar]
- Swan GJF, Redpath SM, Bearhop S, McDonald RA. 2017. Ecology of problem individuals and the efficacy of selective wildlife management. Trends in Ecology and Evolution 32: 518–530. [DOI] [PubMed] [Google Scholar]
- Treves A, Naughton-Treves L.. 2005. Evaluating lethal control in the management of human–wildlife conflict. Pages 86–106 in Woodroffe R, Thirgood S, Rabinowitz A, eds. People and Wildlife, Conflict or Co-existence? Cambridge University Press. [Google Scholar]
- Tucker MA, et al. 2018. . Moving in the Anthropocene: Global reductions in terrestrial mammalian movements. Science 359: 466–469. [DOI] [PubMed] [Google Scholar]
- Tuomainen U, Candolin U.. 2011. Behavioural responses to human‐induced environmental change. Biological Reviews 86: 640–657. [DOI] [PubMed] [Google Scholar]
- Vitone ND, Altizer* S, Nunn CL. 2004. Body size, diet and sociality influence the species richness of parasitic worms in anthropoid primates. Evolutionary Ecology Research 6: 183–199. [Google Scholar]
- Wall J, Douglas-Hamilton I, Vollrath F. 2006. Elephants avoid costly mountaineering. Current Biology 16: R527–R529. [DOI] [PubMed] [Google Scholar]
- Wallis J, Rick Lee D. 1999. Primate conservation: The prevention of disease transmission. International Journal of Primatology 20: 803–826. [Google Scholar]
- Warren Y. 2009. Crop-raiding baboons (Papio anubis) and defensive farmers: A West African perspective. West African Journal of Applied Ecology 14: 1–11. [Google Scholar]
- Warren Y, Buba B, Ross C. 2007. Patterns of crop-raiding by wild and domestic animals near Gashaka Gumti National Park, Nigeria. International Journal of Pest Management 53: 207–216. [Google Scholar]
- Wilkie RD, Douglas-Hamilton I.. 2018. High-resolution tracking technology reveals distinct patterns in nocturnal crop raiding behaviour of an African elephant (Loxodonta africana) in Amboseli, Kenya. Pachyderm 0: 41–48. [Google Scholar]
- Williams HJ, et al. 2020. . Optimizing the use of biologgers for movement ecology research. Journal of Animal Ecology 89: 186–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittig RM, Crockford C, Weltring A, Langergraber KE, Deschner T, Zuberbühler K. 2016. Social support reduces stress hormone levels in wild chimpanzees across stressful events and everyday affiliations. Nature Communications 7: 13361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodroffe R, Frank LG, Lindsey PA, Ranah SMK, Romanach S. 2007. Livestock husbandry as a tool for carnivore conservation in Africa's community rangelands: A case-control study. Biodiversity and Conservation 16: 1245–1260. [Google Scholar]
- Woodroffe R, Ginsberg JR.. 1998. Edge effects and the extinction of populations inside protected areas. Science 280: 2126–2128. [DOI] [PubMed] [Google Scholar]
- Wright TF, Eberhard JR, Hobson EA, Avery ML, Russello MA. 2010. Behavioral flexibility and species invasions: The adaptive flexibility hypothesis. Ethology Ecology and Evolution 22: 393–404. [Google Scholar]
- Yeo J-H, Neo H.. 2010. Monkey business: Human–animal conflicts in urban Singapore. Social and Cultural Geography 11: 681–699. [Google Scholar]
- Yirga G, Ersino W, De Iongh HH, Leirs H, Gebrehiwot K, Deckers J, Bauer H. 2013. Spotted hyena (Crocuta crocuta) coexisting at high density with people in Wukro district, northern Ethiopia. Mammalian Biology 78: 193–197. [Google Scholar]
- Zarco-Gonzalez MM, Monroy-Vilchis O.. 2014. Effectiveness of low-cost deterrents in decreasing livestock predation by felids: A case in Central Mexico. Animal Conservation 17: 371–378. [Google Scholar]




