Abstract
Gasdermin D (GSDMD), a recently discovered pyroptosis-related protein, has been extensively studied in inflammatory diseases. Research has indicated that inflammation is a causative factor of malignant tumors, including osteosarcoma. Nevertheless, the specific functions of GSDMD in osteosarcoma have not well been studied. This study aimed to explore the clinicopathologic values of GSDMD in osteosarcoma. The expression of GSDMD protein in 41 samples of primary osteosarcoma and 20 normal bone tissues were evaluated by immunohistochemistry and western blot. The χ2 test and Student’s t test were applied to analyze the differences of GSDMD expression between osteosarcoma and normal bone tissues. The χ2 test and Fisher’s exact test were used to assess the associations of GSDMD expression with clinicopathologic characteristics of osteosarcoma patients. Moreover, the Kaplan-Meier and Cox regression model methods were used to analyze the relations between GSDMD expression and disease-free survival (DFS) and overall survival (OS) of osteosarcoma patients. The GSDMD protein was significantly overexpressed in osteosarcoma compared to non-neoplastic bone samples. Additionally, GSDMD overexpression was related to poor chemotherapy response (P = 0.031), distant metastasis (P < 0.001), as well as worse prognosis of osteosarcoma patients. Furthermore, GSDMD protein overexpression was an independent predictor of poor survival time in primary osteosarcoma patients. In conclusion, GSDMD overexpression was related to adverse clinical outcome of osteosarcoma, and could be a therapy target in osteosarcoma. Further study should focus on the related mechanism of GSDMD in osteosarcoma.
Keywords: Osteosarcoma, gasdermin D, immunohistochemistry, prognosis
Introduction
Osteosarcoma, with an annual incidence of 3.4 patients per million worldwide, is a common aggressive tumor of bone which usually affects young populations [1,2]. Osteosarcoma tends to occur in the metaphysis of the distal femur, followed by proximal tibia and humerus [3]. With the application of neoadjuvant chemotherapy incorporated with surgical treatment, the disease-free survival of patients with localized osteosarcoma has greatly improved from under 20% to some 70%. However, patients with metastatic status still suffer from unfavorable clinical outcomes [4-6], attributed to resistance to standard chemotherapy [7,8]. Recently, alkaline phosphatase (AKP) and lactate dehydrogenase (LDH), have been shown by studies to be useful biomarkers for the prognosis of osteosarcoma [9,10]. Unfortunately, these candidate biomarkers are not specific and accurate enough to predict the prognosis and chemosensitivity in osteosarcoma patients. Therefore, the identification of new prognostic biomarkers in osteosarcoma is urgent.
Gasdermin D (GSDMD), one of the gasdermin family members, is mostly expressed in skin and gastrointestinal tract [11]. Under stimulating conditions of intracellular pathogens, GSDMD can be cleaved into fragments of N- and C-terminal domains by pyroptotic caspases [12]. The N domain of GSDMD is a key executioner of pyroptosis, which is a specific programmed cell death with inflammatory involvement [12]. After the initiation of pyroptosis, the N domain translocation of GSDMD to the plasma membrane of cancer cells results in the discharge of inflammatory factors, cellular osmotic pressure change, membrane rupture, and eventually pyroptosis [13-15]. GSDMD-induced pyroptosis exerts critical roles in numerous inflammatory diseases and in cancer [16]. Pyroptosis in tumor cells leads to the activation of the NLRP3 inflammasome, as well as the subsequent pro-inflammatory cytokines, including interleukin-1β (IL-1β) and IL-18 [16,17]. Increased levels of IL-1β and IL-18 could promote cell proliferation and metastasis by the NF-κB pathway in some cancers, such as pancreatic ductal adenocarcinoma, renal cell carcinoma, oral squamous cell carcinoma, and T-cell lymphoma [18-21]. Besides indirectly promoting tumor development by inducing release of inflammatory factors, GSDMD conveys wide prognostic value by regulating signaling pathways. For example, Gao et al. [22] found that GSDMD was overexpressed in lung cancer and contributed to a larger tumor size and advanced tumor stage by promoting the EGFR/Akt signaling pathway.
Although many studies have reported that inflammatory factors play critical roles in osteosarcoma development, apoptosis, tumor initiation and metastasis [23,24], the clinicopathologic significance of GSDMD expression in osteosarcoma is uncertain. In addition to GSDMD roles in pyroptosis, possible functions of GSDMD in osteosarcoma should be fully elucidated. The present study investigated the expression of GSDMD by immunohistochemistry and western blot, and explored its clinicopathologic roles in osteosarcoma patients, so as to provide a new biomarker for predicting the prognosis of osteosarcoma.
Materials and methods
Patients and samples
Our study selected 41 primary osteosarcoma patients who underwent surgical resection in our hospital between 1 January 2012, and 31 December 2015. Histopathologic diagnosis was verified by a senior pathologist. Patients with metastasis at diagnosis, special other than conventional (osteoblastic, chondroblastic and fibroblastic) subtypes and insufficient clinicopathological data or follow-up loss were excluded in this study. All patients underwent conventional treatment with ifosfamide, cisplatin, and doxorubicin. Their clinicopathologic features, including sex, age at diagnosis, tumor size, tumor location, Enneking staging, and chemotherapy response, were reviewed from medical records. Formalin-fixed and paraffin-embedded (FFPE) as well as frozen surgical tumor specimens were obtained for protein determination. Moreover, 20 non-neoplastic bone specimens selected in 2015 were used as controls.
After the surgery, all patients received regular imaging examinations every 3-6 months to monitor disease recurrence and metastasis. Disease-free survival (DFS) and overall survival (OS) time were used to determine the prognosis of patients, which was defined as the duration from diagnosis to first tumor progression and death, respectively. Cases without the occurrence of tumor progression or death were censored at the time of the last follow-up. Informed consent was provided by all participants involved in our study, and the study protocol followed the ethical guidelines of the Declaration of Helsinki and was approved by the Ethics Committee of our hospital (Approve Number: IEC-FOM-013-1.0).
Immunohistochemistry
Protein expression of GSDMD in FFPE osteosarcoma and non-neoplastic bone specimens were examined by applying an ElivisionTM plus Polyer HP Kit (Maixin Biotechnologies, Inc., Fuzhou, China). In brief, serial cross-sections (4 μm thick) of paraffin-embedded specimens underwent deparaffinate and hydration, then incubation with 3% hydrogen peroxide for 10 min at 26°C. After antigen retrieval in boiling citrate buffer, the sections were incubated with anti-GSDMD (cat no. DF12275; 1:100 dilution, Affinity Biosciences, USA) antibody overnight at 4°C. After that, the sections were incubated with horseradish peroxide conjugated antibody for 30 min at room temperature and then diamino-benzidine (DAB) for 5 min. Two investigators explored the immunohistochemical signals of sections under a light microscope (Axioskop 40; Zeiss GmbH, Jena, Germany) at five random fields independently. The GSDMD expression scores were according to the mean signal intensity (0, no staining; 1, weak staining; 2, moderate staining; and 3, strong staining) and the positively stained percentage of tumor cells (0, 0%; 1, 0-25%; 2, 26-50%; 3, 51-75%; and 4, 76-100%) determined the score of GSDMD. Cases with immunohistochemical scores ≤ 2 contained non-expression and low-expression, scores > 2 were defined as the high-expression groups [25] (10889820).
Western blot
Proteins from 61 frozen tissues (100 mg) were extracted by lysis buffer (cat no. 98035; Cell Signaling Technology, Danvers, MA, USA) following the manufacturer’s instructions. After quantification by a Bicinchonininc Acid (BCA) Protein Assay kit (cat no. P0011, Beyotime Biotechnology, China), the lytic proteins (40 μg) underwent separation by 10% SDS-PAGE and transfer to PVDF membranes (Merck Millipore Co. ISEQ00010, Darmstadt, Germany). After that, the membranes were blocked with bovine serum albumin (ST023-50 g; Beyotime Biotechnology, China) for 1 h, and then incubated with primary anti-GSDMD (cat no. DF12275; 1:1000 dilution, Affinity Biosciences, USA) antibody at 4°C overnight, subsequently with a horseradish peroxidase-conjugated secondary antibody (goat anti-rabbit monoclonal antibody, cat no. S0001; 1:3000 dilution, Affinity Biosciences) at room temperature for 1 h. Finally, an Enhanced Chemiluminescent (cat no. P10100; New Cell & Molecular Biotech, China) and FluorChem R detection system (ProteinSimple, USA) were used to visualize the signal. Beta-tubulin (cat no. T0023; 1:100000 dilution, Affinity Biosciences, USA) was simultaneously detected as loading control.
Statistics analysis
GraphPad Prism 7.0 (GraphPad Software Inc., San Diego, CA, USA) and SPSS 19.0 software (IBM Corp., Armonk, NY, USA) were used to analyze the statistical data. The χ2 test and independent-sample Student’s t-test were applied to compare the difference of GSDMD expression between osteosarcoma and normal bone tissues. The χ2 test and Fisher’s exact test were used to evaluate the correlations of GSDMD expression with clinicopathologic characteristics in osteosarcoma patients. The Kaplan-Meier and the log-rank test methods were performed on survival analysis. Moreover, all parameters significant by univariate analysis underwent multivariate analysis by Cox regression model. P < 0.05 was regarded as significant.
Results
Patient features
Table 1 demonstrated the main clinicopathological parameters of osteosarcoma patients. The study included 13 females and 28 males with a mean age of 25.3 years old (range, 5-55 years). A tumor size of < 8 cm and ≥ 8 cm were observed in 23 and 18 patients (8 cm size based on Union for International Cancer Control (UICC) staging). The tumor location was as follows: 22 cases in femur; 3 patients in pelvis; 4 patients in tibia, humerus, radius and others. Regarding the Enneking staging, 12 and 29 patients were classified as IIA and IIB stages. Fifteen patients showed good chemotherapy response and 26 patients had a poor response to chemotherapy. A mean follow-up period of 49.12 months (range, 5-77 months), and with a 5-year survival rate of 61.29%.
Table 1.
Characteristics of osteosarcoma patients
| Clinicopathologic data | n (%) |
|---|---|
| Sex | |
| Male | 28 (68.29) |
| Female | 13 (31.71) |
| Age (years), mean (range) | 25.3 (5-55) |
| ≤ 18 | 18 (43.90) |
| > 18 | 23 (56.10) |
| Tumor size (cm) | |
| ≤ 8 | 23 (56.10) |
| > 8 | 18 (43.90) |
| Tumor location | |
| Femur | 22 (53.65) |
| Tibia | 4 (9.76) |
| Humerus | 4 (9.76) |
| Radius | 4 (9.76) |
| Pelvis | 3 (7.31) |
| Others | 4 (9.76) |
| Enneking staging | |
| IIA | 12 (29.27) |
| IIB | 29 (70.73) |
| Response to chemotherapy* | |
| Good | 15 (36.59) |
| Poor | 26 (63.41) |
| Follow-up duration (month), mean (range) Survival outcome | 49.12 (5-77) |
| Distant metastasis | 17 (41.46) |
| Relapse | 1 (2.44) |
| 5-year survival rate | (61.29) |
Good: tumor necrosis ≥ 90%; poor: tumor necrosis < 90%.
Expression profile of GSDMD in osteosarcoma and normal bone tissues
Immunohistochemical results demonstrated that GSDMD was mainly expressed in the cytoplasm and nucleus of osteosarcoma cells (Figure 1A). The first investigator observed that (score 0, N = 9; score 1, N = 8; score 2, N = 4; score 3, N = 6; score 4, N = 8; score 6, N = 4; score 8, N = 2) in osteosarcoma tissues, (score 0, N = 20) in normal bone tissue. The second investigator observed that (score 0, N = 10; score 1, N = 9; score 2, N = 2; score 3, N = 5; score 4, N = 9; score 6, N = 3; score 8, N = 3) in osteosarcoma tissues, and (score 0, N = 20) in normal bone tissues. The results also achieved good interobserver agreement between the two independent pathologists (kappa coefficient = 0.908, P < 0.001). Therefore, twenty (48.78%) of the 41 osteosarcoma tissues exhibited high GSDMD staining, which was not observed in any 20 normal tissues (Table 2). The expression level of GSDMD was markedly increased in osteosarcoma compared to non-neoplastic bone specimens (P < 0.001). To further confirm the expression difference of GSDMD protein in osteosarcoma and normal bone specimens, western blot assay was performed. GSDMD protein level was validated to be higher in osteosarcoma compared to normal bone tissues (P < 0.001) (Figure 1B, 1C). The above results indicated that GSDMD was overexpressed in surgically resected osteosarcoma specimens.
Figure 1.
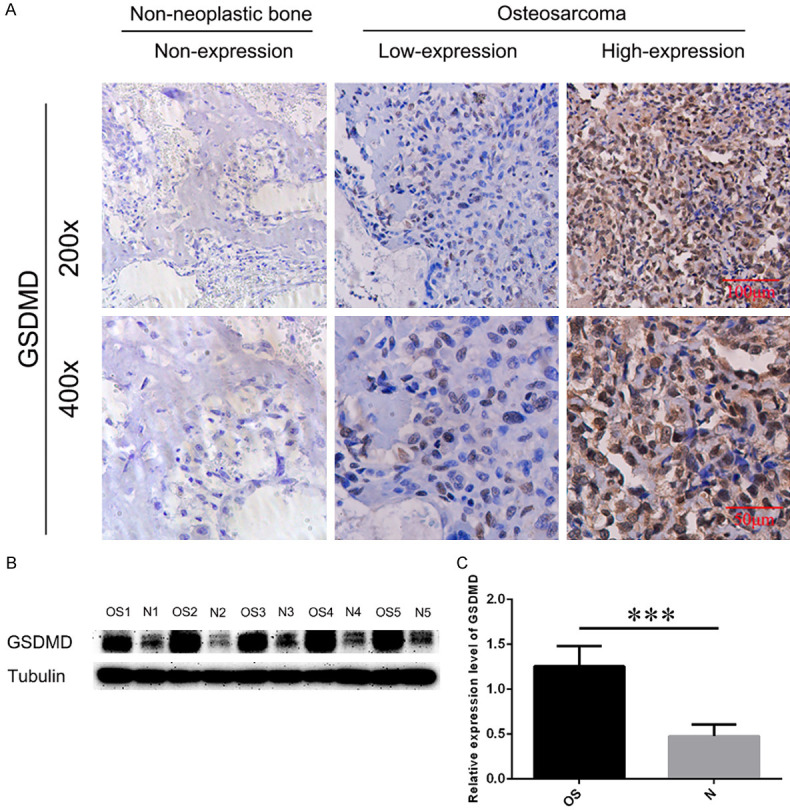
IHC and western blot (WB) analyses of GSDMD expression in non-neoplastic bone tissues and osteosarcoma specimens. A. Representative immunohistochemical staining of GSDMD expression in osteosarcoma and non-neoplastic bone tissues. The cells with positive expression were stained brown. Scale bars, 100 μm and 50 μm. B. WB results of the expression of GSDMD in the lysed osteosarcoma and non-neoplastic bone tissues. Tubulin was used as an internal loading control. C. Relative expression level of GSDMD in the indicated group by t-test. OS: osteosarcoma; N: non-neoplastic bone tissue; ***P < 0.001.
Table 2.
GSDMD status in osteosarcoma
| Osteosarcoma (%) | Non-neoplastic bone tissues (%) | P-value | |
|---|---|---|---|
| GSDMD | |||
| High expression | 20 (48.78) | 0 (0.00) | < 0.001 |
| Low expression | 21 (51.22) | 20 (100.00) |
Association between GSDMD expression in osteosarcoma and clinicopathologic features
Based on the immunohistochemical scores of GSDMD, we explored the correlation of GSDMD expression with clinicopathologic features in osteosarcoma, including sex, age, tumor size, tumor location, Enneking staging, chemotherapy response and distant metastasis. The results demonstrated that GSDMD protein expression was significantly related to poor chemotherapy response (P < 0.05, Table 3) and distant metastasis (P < 0.001, Table 3). None of other clinicopathologic characteristics of osteosarcoma showed significant association with the expression of GSDMD in the present study.
Table 3.
Relationship of GSDMD expression with clinicopathologic data of osteosarcoma
| Clinicopathologic data | Case number | GSDMD | ||
|---|---|---|---|---|
|
| ||||
| High expression (n = 20) | Low expression (n = 21) | P-value | ||
| Sex | ||||
| Male | 28 | 13 | 15 | NS |
| Female | 13 | 7 | 6 | |
| Age (years) | ||||
| ≤ 18 | 18 | 8 | 10 | NS |
| > 18 | 23 | 12 | 11 | |
| Tumor size (cm) | ||||
| ≤ 8 | 23 | 10 | 13 | NS |
| > 8 | 18 | 10 | 8 | |
| Tumor location | ||||
| Tibia or femur | 26 | 13 | 13 | NS |
| Others | 15 | 7 | 8 | |
| Enneking staging | ||||
| IIA | 12 | 3 | 9 | NS |
| IIB | 29 | 17 | 12 | |
| Response to chemotherapy | ||||
| Good | 15 | 4 | 11 | 0.031 |
| Poor | 26 | 16 | 10 | |
| Distant metastasis | ||||
| Yes | 17 | 14 | 3 | < 0.001 |
| No | 24 | 6 | 18 | |
NS: no significance.
Association between GSDMD expression in osteosarcoma and survival
Subsequently, the DFS and OS time were investigated by univariate and multivariate analysis to determine the prognostic role of GSDMD expression in osteosarcoma patients. Univariate analysis of patient survival as a function of GSDMD expression level is presented at Table 4. Patients with high GSDMD expression suffered shorter mean DFS and OS time (DFS, 14.53 months; OS, 38.85 months) when compared to those with low GSDMD expression (DFS, 69.97 months; OS, 79.05 months). Figure 2, shows survival curves of DFS (Figure 2A) and OS (Figure 2B) time in osteosarcoma patients with high and low GSDMD expression groups. The two groups showed a significant difference in survival (DFS, P < 0.001; OS, P < 0.001). It is noteworthy that in univariate analysis, the chemotherapy response and metastasis status also had significant influences on the survival of osteosarcoma patients.
Table 4.
Univariate analysis of GSDMD expression and osteosarcoma patient survival based on the log-rank test
| Characteristic | Case number | Disease-free survival (months) | Overall-free survival (months) | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Mean | SD | 95% CI | p-value | Mean | SD | 95% CI | p-value | ||
| Sex | |||||||||
| Male | 28 | 45.46 | 6.36 | 33.00-57.92 | NS | 57.72 | 5.20 | 47.54-67.91 | NS |
| Female | 13 | 37.65 | 9.83 | 18.39-56.92 | 53.31 | 6.62 | 40.34-66.28 | ||
| Age (years) | |||||||||
| ≤ 18 | 18 | 45.46 | 8.05 | 29.70-61.23 | NS | 57.70 | 5.66 | 46.60-68.79 | NS |
| > 18 | 23 | 42.63 | 7.20 | 28.52-56.73 | 55.03 | 5.94 | 43.39-66.66 | ||
| Tumor size (cm) | |||||||||
| ≤ 8 | 23 | 47.03 | 7.50 | 32.32-61.73 | NS | 56.87 | 5.62 | 45.86-67.88 | NS |
| > 8 | 18 | 37.10 | 7.32 | 22.75-51.44 | 55.32 | 6.12 | 43.33-67.32 | ||
| Tumor location | |||||||||
| Tibia or femur | 26 | 48.81 | 6.64 | 35.80-61.82 | NS | 58.32 | 5.24 | 48.05-68.59 | NS |
| Others | 15 | 34.29 | 9.11 | 16.43-52.14 | 52.25 | 6.61 | 39.29-65.21 | ||
| Enneking staging | |||||||||
| IIA | 12 | 52.63 | 8.15 | 36.65-68.60 | NS | 63.64 | 5.22 | 53.40-73.87 | NS |
| IIB | 29 | 38.36 | 6.60 | 25.44-51.29 | 52.48 | 5.24 | 42.22-62.75 | ||
| Response to chemotherapy | |||||||||
| Good | 15 | 63.76 | 6.39 | 51.24-76.28 | 0.005 | 66.57 | 5.13 | 56.53-76.62 | 0.036 |
| Poor | 26 | 30.71 | 6.73 | 17.51-43.91 | 50.15 | 5.53 | 39.31-60.99 | ||
| Distant metastasis | |||||||||
| Yes | 17 | 21.26 | 6.47 | 8.59-33.93 | < 0.001 | 43.49 | 6.11 | 31.52-55.46 | < 0.001 |
| No | 24 | 61.20 | 5.93 | 49.58-78.81 | 65.72 | 4.93 | 56.05-75.39 | ||
| GSDMD | |||||||||
| High expression | 20 | 14.53 | 4.17 | 6.36-22.70 | < 0.001 | 38.35 | 5.99 | 26.61-50.10 | < 0.001 |
| Low expression | 21 | 65.71 | 5.50 | 54.93-76.48 | 72.62 | 2.91 | 66.91-78.33 | ||
SD: standard deviation; CI: confidence interval; NS: no significance.
Figure 2.

Kaplan-Meier analysis of DFS and OS time by expression of GSDMD in osteosarcoma patients. Significant differences in DFS (A, P < 0.001) and OS (B, P < 0.001) time were observed between patients with high GSDMD expression and low GSDMD expression. DFS: disease-free survival; OS: overall survival.
Upon multivariate analysis, GSDMD expression was included together with chemotherapy response and distant metastasis that significantly predicted worse survival by univariate analysis. The results demonstrated that high expression of GSDMD independently predicted worse DFS (HR = 6.43, P = 0.009) and OS (HR = 4.17, P = 0.041) of patients with osteosarcoma (Table 5). Taken together, these findings demonstrated that GSDMD protein expression was an independent factor for survival status in primary osteosarcoma.
Table 5.
Multivariate analysis of GSDMD expression and osteosarcoma patient survival
| Characteristic | Comparison | Progression-free survival (months) | Overall survival (months) | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| HR | 95% CI | P-value | HR | 95% CI | P-value | ||
| GSDMD | high expression vs. low expression | 6.43 | 1.60-25.77 | 0.009 | 7.44 | 1.97-28.17 | 0.003 |
| Response to chemotherapy | Good vs. poor | 0.31 | 0.08-1.14 | 0.079 | 0.47 | 0.13-1.69 | 0.249 |
| Distant metastasis | Yes vs. no | 3.36 | 1.08-10.43 | 0.036 | 4.13 | 1.19-14.36 | 0.026 |
HR: hazard ratio; CI: confidence interval; NS: no significance.
Discussion
Osteosarcoma is a malignant bone tumor of mainly children and young adults [1,2]. Current conventional treatment methods include neoadjuvant chemotherapy and surgical resection of the localized tumor [4]. Although the combined therapy brings much improvement for the clinical outcome of osteosarcoma, the high rate of tumor metastasis and low 5-year survival rate of metastatic patients remain global issues [4-6,26]. Searching for new prognosis-related biomarkers could improve our understanding of the complex mechanisms of osteosarcoma aggressiveness and progression. The present study observed relationships of the GSDMD protein expression in primary osteosarcoma with poor chemotherapy response and distant metastasis. Moreover, GSDMD was identified as a new prognostic biomarker that independently contributed to the unfavorable survival status of osteosarcoma patients.
Pyroptosis is an inflammatory-mediated cell death activated by certain inflammasomes such as NLRP3, resulting in the cleavage of GSDMD and activation of cytokines including IL-1β and IL-18 [11]. The induction of pyroptosis inhibits the proliferation and progression of several tumor types, including malignant mesothelioma, breast cancer, esophageal cancer, and osteosarcoma [27]. Besides being a tumor suppressor, GSDMD overexpression was found to be closely associated with the proliferation and late TNM stage of non-small lung cancer [22]. Additionally, a previous study reported that CD147 facilitated cell proliferation in bladder cancer by increasing GSDMD expression, indicating that GSDMD protein was involved in tumor cell growth [25]. In the present study, we also observed high expression of GSDMD protein in osteosarcoma by immunohistochemistry and western blot methods. In addition, relationships between GSDMD expression and poor chemotherapy response and distant metastasis, as well as short survival time were also observed in osteosarcoma, which demonstrated that GSDMD protein may play considerable roles in the progression and treatment resistance of osteosarcoma.
It has been reported that GSDMD is overexpressed in immune cells and intestinal epithelial cells, and further studies showed GSDMD is widely expressed in different tissues and cell types [28]. Macrophages play crucial roles in tumor progression. Ségaliny et al. [29] demonstrated that IL-34 is expressed by osteosarcoma cells, is regulated by TNF-α, IL-1β, and contributes to osteosarcoma growth by increasing neo-angiogenesis and the recruitment of M2 macrophages. Furthermore, Wang et al. [30] found that macrophage migration inhibitory factor (MIF), an important cytokine in OS progression, was significantly increased in the tissue and serum samples of OS patients and was associated with tumor size, pulmonary metastasis, and the survival rate of OS patients. Therefore, the function of GSDMD in osteosarcoma may induce the expression of immune cells, such as macrophages. Further studies should focus on the co-expression of GSDMD and immune cells, and their mechanism in osteosarcoma.
There are several limitations in this study. First, current findings, which may be influenced by the limited number of subjects and short follow-up duration of patient survival, need to be confirmed in a larger study with a longer follow-up period. Additionally, preoperative neoadjuvant chemotherapy as the standardized treatment of osteosarcoma may have affected the immunohistochemical fidelity of GSDMD. Further quantitative analysis of GSDMD using biopsy specimens of osteosarcoma may provide more convincing results.
Conclusions
The expression of GSDMD protein is upregulated in osteosarcoma specimens, and its overexpression is significantly related to the unfavorable clinicopathologic features and clinical outcomes in osteosarcoma patients. In addition, GSDMD expression could independently predict reduced survival time in osteosarcoma patients. More functional and related mechanistic studies of GSDMD involvement in osteosarcoma are warranted to improve the survival of osteosarcoma patients.
Acknowledgements
We acknowledge all the orthopedics staff and nursing department who support our study. The study was supported by the National Natural Science Foundation of China (grant number. 81972021 and 31571292), the Special Fund of Youth Top-notch Innovative Talents of Fujian Province (grant number: SQNBJ201601) and Trauma Medical center of Fujian Province.
Disclosure of conflict of interest
None.
References
- 1.Misaghi A, Goldin A, Awad M, Kulidjian AA. Osteosarcoma: a comprehensive review. SICOT J. 2018;4:12. doi: 10.1051/sicotj/2017028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mirabello L, Troisi RJ, Savage SA. International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int J Cancer. 2009;125:229–234. doi: 10.1002/ijc.24320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Isakoff MS, Bielack SS, Meltzer P, Gorlick R. Osteosarcoma: current treatment and a collaborative pathway to success. J. Clin. Oncol. 2015;33:3029–3035. doi: 10.1200/JCO.2014.59.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gianferante DM, Mirabello L, Savage SA. Germline and somatic genetics of osteosarcoma - connecting aetiology, biology and therapy. Nat Rev Endocrinol. 2017;13:480–491. doi: 10.1038/nrendo.2017.16. [DOI] [PubMed] [Google Scholar]
- 5.Raymond AK, Jaffe N. Osteosarcoma multidisciplinary approach to the management from the pathologist’s perspective. Cancer Treat Res. 2009;152:63–84. doi: 10.1007/978-1-4419-0284-9_4. [DOI] [PubMed] [Google Scholar]
- 6.Abate ME, Longhi A, Galletti S, Ferrari S, Bacci G. Non-metastatic osteosarcoma of the extremities in children aged 5 years or younger. Pediatr Blood Cancer. 2010;55:652–654. doi: 10.1002/pbc.22567. [DOI] [PubMed] [Google Scholar]
- 7.Yonemoto T, Hosono A, Iwata S, Kamoda H, Hagiwara Y, Fujiwara T, Kawai A, Ishii T. The prognosis of osteosarcoma occurring as second malignancy of childhood cancers may be favorable: experience of two cancer centers in Japan. Int J Clin Oncol. 2015;20:613–616. doi: 10.1007/s10147-014-0729-8. [DOI] [PubMed] [Google Scholar]
- 8.Hegyi M, Semsei AF, Jakab Z, Antal I, Kiss J, Szendroi M, Csoka M, Kovacs G. Good prognosis of localized osteosarcoma in young patients treated with limb-salvage surgery and chemotherapy. Pediatr Blood Cancer. 2011;57:415–422. doi: 10.1002/pbc.23172. [DOI] [PubMed] [Google Scholar]
- 9.Zumarraga JP, Baptista AM, Rosa LP, Caiero MT, Camargo OP. Serum values of alkaline phosphatase and lactate dehydrogenase in osteosarcoma. Acta Ortop Bras. 2016;24:142–146. doi: 10.1590/1413-785220162403157033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marais LC, Bertie J, Rodseth R, Sartorius B, Ferreira N. Pre-treatment serum lactate dehydrogenase and alkaline phosphatase as predictors of metastases in extremity osteosarcoma. J Bone Oncol. 2015;4:80–84. doi: 10.1016/j.jbo.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang YY, Liu XL, Zhao R. Induction of pyroptosis and its implications in cancer management. Front Oncol. 2019;9:971. doi: 10.3389/fonc.2019.00971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 13.Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, Lieberman J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153–158. doi: 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding J, Wang K, Liu W, She Y, Sun Q, Shi J, Sun H, Wang DC, Shao F. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535:111–116. doi: 10.1038/nature18590. [DOI] [PubMed] [Google Scholar]
- 15.Kuang S, Zheng J, Yang H, Li S, Duan S, Shen Y, Ji C, Gan J, Xu XW, Li J. Structure insight of GSDMD reveals the basis of GSDMD autoinhibition in cell pyroptosis. Proc Natl Acad Sci U S A. 2017;114:10642–10647. doi: 10.1073/pnas.1708194114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broz P, Pelegrin P, Shao F. The gasdermins, a protein family executing cell death and inflammation. Nat Rev Immunol. 2020;20:143–157. doi: 10.1038/s41577-019-0228-2. [DOI] [PubMed] [Google Scholar]
- 17.Shi J, Gao W, Shao F. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci. 2017;42:245–254. doi: 10.1016/j.tibs.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Guo X, Zheng L, Jiang J, Zhao Y, Wang X, Shen M, Zhu F, Tian R, Shi C, Xu M, Li X, Peng F, Zhang H, Feng Y, Xie Y, Xu X, Jia W, He R, Xie C, Hu J, Ye D, Wang M, Qin R. Blocking NF-kappaB is essential for the immunotherapeutic effect of recombinant IL18 in pancreatic cancer. Clin Cancer Res. 2016;22:5939–5950. doi: 10.1158/1078-0432.CCR-15-1144. [DOI] [PubMed] [Google Scholar]
- 19.Lim SW, Ryu KJ, Lee H, Ko YH, Kim WS, Kim SJ. Serum IL18 is associated with hemophagocytosis and poor survival in extranodal natural killer/T-cell lymphoma. Leuk Lymphoma. 2019;60:317–325. doi: 10.1080/10428194.2018.1480772. [DOI] [PubMed] [Google Scholar]
- 20.Xu L, Zhu Y, An H, Liu Y, Lin Z, Wang G, Xu J. Clinical significance of tumor-derived IL-1beta and IL-18 in localized renal cell carcinoma: associations with recurrence and survival. Urol Oncol. 2015;33:68, e69–16. doi: 10.1016/j.urolonc.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 21.Singh P, Verma JK, Singh JK. Validation of salivary markers, IL-1beta, IL-8 and Lgals3bp for detection of oral squamous cell carcinoma in an indian population. Sci Rep. 2020;10:7365. doi: 10.1038/s41598-020-64494-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao J, Qiu X, Xi G, Liu H, Zhang F, Lv T, Song Y. Downregulation of GSDMD attenuates tumor proliferation via the intrinsic mitochondrial apoptotic pathway and inhibition of EGFR/Akt signaling and predicts a good prognosis in nonsmall cell lung cancer. Oncol Rep. 2018;40:1971–1984. doi: 10.3892/or.2018.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang X, Hu H, Zhang W, Shao Z. Prognostic value of prognostic nutritional index and systemic immune-inflammation index in patients with osteosarcoma. J Cell Physiol. 2019;234:18408–18414. doi: 10.1002/jcp.28476. [DOI] [PubMed] [Google Scholar]
- 24.Hong JT, Son DJ, Lee CK, Yoon DY, Lee DH, Park MH. Interleukin 32, inflammation and cancer. Pharmacol Ther. 2017;174:127–137. doi: 10.1016/j.pharmthera.2017.02.025. [DOI] [PubMed] [Google Scholar]
- 25.Peng J, Jiang H, Guo J, Huang J, Yuan Q, Xie J, Xiao K. CD147 expression is associated with tumor proliferation in bladder cancer via GSDMD. Biomed Res Int. 2020;2020:7638975. doi: 10.1155/2020/7638975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Briccoli A, Rocca M, Salone M, Guzzardella GA, Balladelli A, Bacci G. High grade osteosarcoma of the extremities metastatic to the lung: long-term results in 323 patients treated combining surgery and chemotherapy, 1985-2005. Surg Oncol. 2010;19:193–199. doi: 10.1016/j.suronc.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Fang Y, Tian S, Pan Y, Li W, Wang Q, Tang Y, Yu T, Wu X, Shi Y, Ma P, Shu Y. Pyroptosis: a new frontier in cancer. Biomed Pharmacother. 2020;121:109595. doi: 10.1016/j.biopha.2019.109595. [DOI] [PubMed] [Google Scholar]
- 28.Saeki N, Kuwahara Y, Sasaki H, Satoh H, Shiroishi T. Gasdermin (Gsdm) localizing to mouse Chromosome 11 is predominantly expressed in upper gastrointestinal tract but significantly suppressed in human gastric cancer cells. Mamm Genome. 2000;11:718–724. doi: 10.1007/s003350010138. [DOI] [PubMed] [Google Scholar]
- 29.Wang C, Zhou X, Li W, Li M, Tu T, Ba X, Wu Y, Huang Z, Fan G, Zhou G, Wu S, Zhao J, Zhang J, Chen J. Macrophage migration inhibitory factor promotes osteosarcoma growth and lung metastasis through activating the RAS/MAPK pathway. Cancer Lett. 2017;403:271–279. doi: 10.1016/j.canlet.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 30.Segaliny AI, Mohamadi A, Dizier B, Lokajczyk A, Brion R, Lanel R, Amiaud J, Charrier C, Boisson-Vidal C, Heymann D. Interleukin-34 promotes tumor progression and metastatic process in osteosarcoma through induction of angiogenesis and macrophage recruitment. Int J Cancer. 2015;137:73–85. doi: 10.1002/ijc.29376. [DOI] [PubMed] [Google Scholar]


