Abstract
Background: In the development of several human cancers, it has been established that neutrophil cytosolic factor 2 (NCF2) plays a major part. Therefore, possible functions of NCF2 in ESCC are investigated in this paper. Methods: The mRNA/protein expression of NCF-2 in ESCC cell lines and tissues were found based on quantitative real-time reverse transcription PCR (qRT-PCR), western blotting, and immunohistochemistry (IHC). A large cohort consisting of 194 postoperative ESCC samples was used for IHC. These data were analyzed based on Chi-square test, Kaplan-Meier analysis, and Cox regression modelling. For the purpose of confirming its role in ESCC cells, we used short hairpin RNA (ShRNA) interfering method to suppress endogenous NCF2 expression. Results: NCF2 was significantly up-regulated for in ESCC tissues and cell lines in at mRNA and protein levels; and NCF-2 expression was absent for all normal esophageal epithelium detected by IHC. Furthermore, the knockdown of NCF-2 compromised the proliferation and invasion of ESCC cells in vitro. Conclusion: Positive NCF2 expression in ESCC may facilitate an aggressive phenotype. This may be an independent biomarker in ESCC.
Keywords: Neutrophil cytosolic factor 2 (NCF2), Esophageal squamous cell carcinoma (ESCC), biomarker, proliferation, and invasion
Introduction
As one of the most common malignancies, esophageal squamous cell carcinoma (ESCC) is ranked number six among cancer-associated deaths [1,2]. Though there has been progress in the diagnosis and ESCC treatment, the prognosis of patients is poor, mainly on account of the advanced level at diagnosis and the lack of effective therapy. The changed expression of oncogenes and tumor suppressors has an association with the development and progression of ESCC based on accumulating evidence [3]. So far, however, ESCC remains unavailable for diagnosis and personal treatment because its biomarkers are highly sensitive and specific.
Neutrophil cytosolic factor 2 (NCF2), as the p67phox gene, is positioned on chromosome 1q25 consisting of 16 exons with 40 kb [4-7]. Till now, among the patients with Chronic granulomatous disease in HGMD Professional, it has been published that there are in total 56 various mutations in the NCF2 gene (Human Gene Mutation Database 2011.3; http://www.hgmd.cf.ac.uk/ac/all.php). It has been demonstrated in previous studies that NCF2 regulates cell growth, malignant transformation and differentiation [8]. In recent studies, it also been demonstrated that NCF2 high expression has a close relation with human disease, including cervical cancer and inflammatory bowel disease [9-12]. NCF2 has been found to be highly expressed in gastric cancer and promoted tumor metastasis and invasion by triggering NF-kB signaling [13]. However, there is no report on the mode of expression and clinical meaning of NCF2 in human ESCC.
This paper examines NCF2 expression in ESCC tissues and adjacent normal nasopharyngeal tissues by using immunohistochemistry (IHC). The ability of proliferation and invasion of ESCC cells was suppressed by silencing NCF2. In addition, the prognostic impacts of NCF2 in ESCC patients were investigated by evaluation of the relation between NCF2 expression and clinicopathologic measures.
Materials and methods
Cell lines and cell cultures
We cultured human esophageal cancer cell lines (NE1, TE1, K30, HK, K180, K510, Eca109 and K520) in DMEM medium (Gibco, Invitrogen, Carlsbad, California, USA) with the supplement of 10% fetal bovine serum (FBS; Gibco). We incubated all the cell lines at 37°C in a 5% CO2 incubator.
Patients and tissue specimen
Between December 2007 and December 2008, there were in total 194 pretreatment formalin-fixed paraffin-embedded (FFPE) specimens of ESCC patients from surgical resection at Sun Yat-sen University Cancer Center; and we obtained 12 freshly-frozen esophageal cancer specimens and 9 normal esophageal epithelium samples from that Center as well. During surgery resection, all patients were diagnosed with stage I-III disease, and before operation all of them did not receive any treatment. All patients were followed up on a regular basis, and it lasted 40.10 months as a median follow-up time for the whole cohort (range, 2.63-83.13). Table 1 summarized patients’ clinicopathologic findings in each cohort. The Institutional Ethical Review Board of the Center supported the study, while from each patient, we obtained written informed consent.
Table 1.
Correlation of NCF2 expression in tissue with patients’ clinicopathological variables in 194 cases of esophageal cancer
| Variables | All cases (N=194) | NCF2 expression (%) | ||
|---|---|---|---|---|
|
| ||||
| Normal expression (N=82) | High expression (N=112) | P-value | ||
| Age (years) | 0.134 | |||
| ≤60 | 95 (48.9) | 35 (36.8) | 60 (63.2) | |
| >60 | 99 (51.1) | 47 (47.5) | 52 (52.5) | |
| Gender | 0.144 | |||
| male | 178 (91.7) | 78 (43.8) | 100 (56.2) | |
| female | 16 (8.3) | 4 (25.0) | 12 (75.0) | |
| Smoking history | 0.52 | |||
| yes | 175 (90.2) | 70 (40.0) | 105 (60.0) | |
| no | 19 (9.8) | 12 (63.2) | 7 (36.8) | |
| Tumor multiplicity | 0.760 | |||
| Unifocal | 116 (59.7) | 48 (41.4) | 68 (58.6) | |
| Multifocal | 78 (40.3) | 34 (43.6) | 44 (56.4) | |
| Tumor size | <0.001 | |||
| ≤3 cm | 73 (37.6) | 47 (64.4) | 26 (35.6) | |
| >3 cm | 121 (62.4) | 35 (28.9) | 86 (71.1) | |
| pT status | <0.001 | |||
| pT1 | 27 (13.9) | 17 (63.0) | 10 (37.0) | |
| pT2 | 70 (36.1) | 41 (58.6) | 29 (41.4) | |
| PT3 | 97 ((50.0) | 24 (24.7) | 73 (75.3) | |
| pN status | 0.553 | |||
| pN- | 27 (13.9) | 10 (37.0) | 17 (63.0) | |
| pN+ | 167 (86.1) | 72 (43.1) | 95 (56.9) | |
| Recurrence | <0.001 | |||
| NO | 127 (65.4) | 68 (53.5) | 59 (46.5) | |
| Yes | 67 (34.6) | 14 (20.9) | 53 (79.1) | |
| P<0.05 | ||||
Quantitative real-time polymerase chain reaction (qPCR)
From cell lines, we extracted all the RNA using TRIzol reagent (Invitrogen, Grand Island, NY, USA). On the basis of the product maker’s instructions, RNA was then transcribed reversely according to Super Script First Strand cDNA System. Therefore, the NCF2 sense primer was 5’-GCGCTAGGCTGGGACCTTGAAGCC-3’, and the antisense primer was 5’-GTCTTGAAGAAGGGCAGTGATAAC-3’.
The sense primer was 5-ATAGCACAGCCTGGATAGCAACGTAC-3’ and the antisense primer was 5-CACCTTCTACAATGAGCTGCGTGTG-3’ for β-actin gene. Using SYBR Green PCR master mix, qRT-PCR was done with a combined volume of 20 μl on the 7900HT fast Real-time.
The cycles of PCR system (Applied Biosystems) were as follows: 50°C for 2 min, 95°C for 10 min, 40 cycles of 95°C for 15 s, and 60°C for 60 s. A melting curve for the confirmation of the amplification specificity was generated by the performance of a dissociation procedure. β-actin was the reference gene.
Western blotting
According to the immunohistochemical analysis, the NCF2 expression levels was using 2 steps in 194 ESCC tissue samples. We cut the paraffin-embedded ESCC specimens into 5-μm sections after whichthey were baked at 65°C for 2 h. Through a series of graded ethanols, xylene was then used to deparaffinised these sections and then they were hydrated. To prevent endogenous peroxidase activity and search the antigenicity slides were continuously boiled in citrate buffer solution (pH 6.5) in a microwave oven for 20 minutes. 3% solution of hydrogen peroxide was used to immerse these sections for 10 minutes. Then, a primary antibody against NCF2 was placed on sections (1:500 dilution; Abcam, Cambridge, MA, USA).Sections were washed three times for a period of five minutes in phosphate buffered saline (PBS) at 4°C overnight. A secondary antibody was successively incubated for one hour at indoor temperature based on tissue sections. After three more times of washing in PBS, for these sections, 3,3-diaminobenzidine (DAB) were used to stained them, after which another Mayer’s haematoxylin was used to counterstain them. They were dehydrated and mounted. The slides were considered as positive controls and negative controls. The ones with positive immunohistochemical staining were positive, and those thatimmunoreacted with PBS for use were negative.
Immunohistochemical (IHC) evaluation
IHC was evaluated by three independent pathologists. The score of cytoplasmic immunoreactivity for NCF2 protein was performed based on evaluation of the staining intensity and positive tumor cell proportion. Briefly, the grades of the staining intensity were: 0 (representing no stain), 1 (representing poor stain, with a light yellow color), 2 (representing moderate stain, with a yellowish brown), and 3 (representing strongly stained, with a brown color). The amount of positively stained tumor cells was scored according to the following standards: 0 (0%), 1 (1-10%), 2 (11-50%), 3 (51-80%), and 4 (81-100%). Then, the scores of IHC were based on multiplying the scores of its intensity and proportion. By using this way, the scores of IHC ultimately can be sorted as follows: “-” (IHC scores 0), “+” (IHC scores 1-4), “++” (IHC scores 5-8), and “+++” (IHC scores 9-12). It divided a group of 194 patients with ESCC into low NCF2 expression group (“-”, “+”) and high NCF2 expression group (“++”, “+++”) for further statistical analysis.
Transfection
When its confluence was 80%, TE1 cells were transfected with siRNA, which is then diluted in serum-free opti-MEM medium and permitted to stand for five minutes, after which a mixture with Lipofectamine 2000 was done in a gentle way. Before the mixture of the transfection was added to the cells, it was stored at indoor temperature for 20 minutes. Transfected cells were placed into the incubator again for 8 h, and then we replaced the medium with DMEM which contained 10% FBS without any antibiotics. The ultimate density of siRNA (labeled with a fluorescent probe) was 50 nmol/ml. In case of transfection, the fluorescence between 8 and 24 hours was monitored and recorded as the transfection efficiency.
Wound healing assay
Transfected TE-1 cells were seeded into 6-well plates. Then the serum was starved in a serum-free media for 24 h, by using a standard 200 μl plastic pipette tip, that results in creating an artificial wound on the confluent monolayer cell. The cells were moved into the scratch place as individual cells from the confluent sides, and viewed and photographed at the width of the scratch gap at 0 h as well as 24 h under the miscroscope. In this experiment, a six-well plate was utilized for three replicate wells.
Transwell invasion assay
Transwell chambers were used to perform invasion assays, and the chambers were coated with Matrigel on the upper surface of membrane with 8 μm pores. Briefly, we harvested and suspended the transfected TE-1 cells in serum free medium and plated 1 × 105, transfected cells into the upper chamber for the invasion assays and placed media which had the supplement of 10% FBS into the bottom chamber. After 24 hours incubation, we fixed the cells which had migrated through the membrane onto the bottom surface, then stained and counted cells under an inverted microscope. Each chamber analyzed five random fields of view; and each assay was conducted not less than in three independently different experiments.
Colony formation assay
We placed six-well plate containing 500 infected cells were cultured for 2 weeks for 20 hours after infection, we fixed colonies with methanol, and stained with 0.1% crystal violet in 20% methanol for a period of 15 minutes.
CCK-8 assay
Through cell proliferation and cytotoxicity reagent WST-8, we measured the cell growth and viability. In brief, we cultured the TE-1 cells in a 96-well plate. After 12 h, at the harvest time, we added 10 μl of CCK8 into each well (ten wells each group for statistics) and based on the measurement of the absorbance of the converted dye at 450 nm, we viability was determined two hours later.
Statistical analysis
The statistics were analyzed based on the SPSS statistical software package (standard version 19.0; SPSS, Chicago, IL). We evaluated the relation of NCF2 expression with ESCC patients’ clinicopathologic characteristics through a Pearson’s chi-square test or Fisher’s exact test. ESCC cell lines’ mRNA expression levels were analyzed using the Student’s t-test. For functional analyses, data were presented as ± standard deviation and Student t-test were used to determine the significance of the differences between two groups. The Kaplan-Meier method was used to estimate OS and DFS, and the differences were compared using the log-rank test. We defined the disease-free survival (DFS) from the initial treatment to cancer progression, metastasis, or death. We defined the overall survival (OS) within period of the initial treatment to death. The Cox proportional hazards regression model was utilized to analyze the survival of Univariate and multivariate models. In addition, Cox regression model provided the Hazard ratio (HR) as well as the CI of 91%. Finally, the Kaplan-Meier method was utilized to plot survival curves which were compared through the log-rank test. A P-value <0.05 based on a two-tailed test was considered significant.
Results
Detection of NCF2 expression in ESCC tissues and cell lines
Based on western blotting and qRT-PCR, the expression of NCF2 protein and mRNA were evaluated in ESCC cell lines including TE1, K30, Eca109, K180, K510, HK, K520 and NE1, which belongs to a normal esophageal cell line. In Figure 1A there was highly expressed NCF2 protein and mRNA in ESCC cell lines, compared to NE1. Furthermore, ESCC tissues and normal esophageal epithelial tissues were paired by mRNA and protein. The balance of NCF2 in eight (Figure 1C, 1D) were assessed, and both NCF2 mRNA and protein which were expressed at higher levels in ESCC tissues (Figure 1B) were observed. This suggests that overexpression of NCF2 may be involved in the progression of ESCC.
Figure 1.
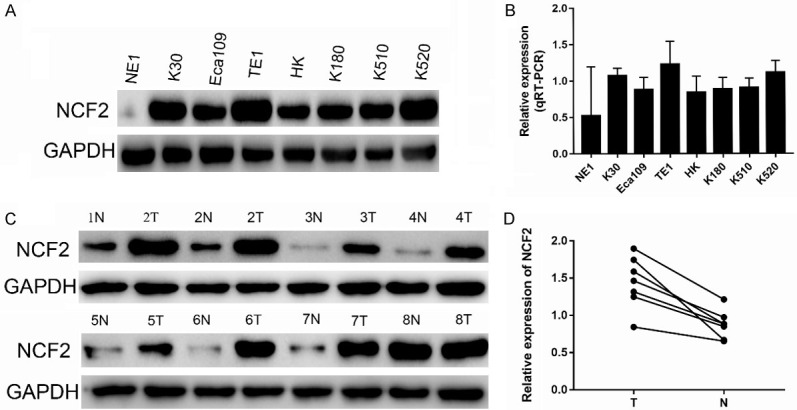
Expression and amplification of NCF2 in ESCC cell lines. A. The level of NCF2 protein examined by Western blotting in 8 human HCC cell lines (i.e., K30, Eca109, TE1, HK, K180, K510 and K520) and one immortalized primary hepatocellular epithelial cell line NE1. B. The expression level of NCF2 mRNA in 8 human ESCC cell lines was evaluated by q-PCR. C. Among 10 ESCC cases, increased expression of NCF2 was detected via western blotting in 8 pairs of ESCC tissues compared with the matched non-cancerous tissues. The expression levels were normalised to those of NCF2. D. The mRNA expression of NCF2 was significantly up-regulated in 8/10 pairs of HCC tissues based on q-PCR. The expression levels were normalised to those of GAPDH. (N, adjacent normal tissue; T, ESCC tissue).
Detection of NCF2 expression in ESCC by immunohistochemistry (IHC)
NCF2 expression standard in 194 pairs of ESCC and adjoining tissues without tumors, was additionally examined by IHC. There were mainly high levels of NCF2 in the cytoplasm of carcinoma cells. By comparison, regarding the adjacent normal tissues (Figure 2), we observed weak or negative NCF2 staining. We described (Figure 3) the four categories of the intensity of NCF2 immunostaining. Through immunohistochemical scores (IHC scores), two groups of patients divisions evolved: one is low NCF2 expression group (IHC score ≤4), and another is high group (IHC score >4), and we detected high expression of NCF2 protein in 112/194 (60.2%) of ESCC tissues.
Figure 2.
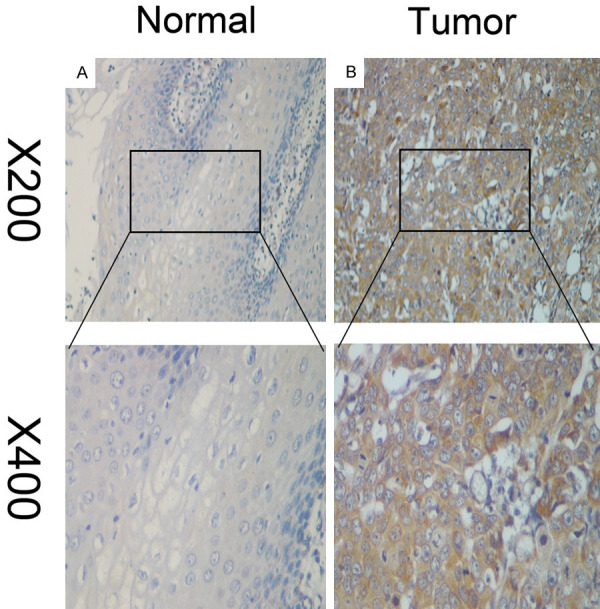
The Representative images of NCF2 expression in ESCC tissues via IHC. NCF2 was absent from or only weakly detected in adjacent normal cells (A), whereas its up-regulation was mainly detected in ESCC tissues (B) (original magnification, ×200 and ×400).
Figure 3.
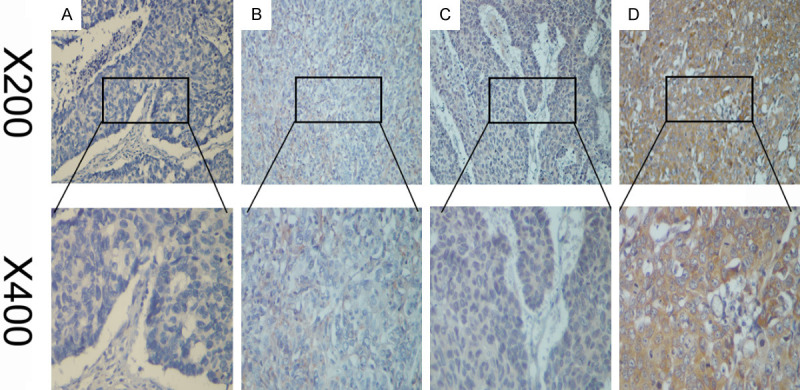
The representative images show different staining intensities of NCF2: (A) negative staining, (B) weak staining, (C) moderate staining, and (D) strong staining (original magnification, ×200 and ×400).
Association between NCF2 expression and the measures of clinicopathology
The association between NCF2 expression and the features of clinicopathology in 194 cases with ESCC was used to investigate Pearson’s chi-square (χ2) test. This paper analyzed significant correlations between NCF2 expression and its three features consisting Tumor size (P<0.01), TNM stage (P<0.001) and Recurrence (P<0.01). No meaningful correlation was found between the NCF2 formulation and the rest of the characteristics of clinicopathology, like the age, gender, smoking history, andtumor multiplicity of the patient (Table 1), showing the relation of the expression of the high NCF2 alongside ESCC patients exhibiting poor survival.
In relation to Kaplan-Meier analysis as well as the log-rank test, we analyzed the relation between the expression of the NCF2 in ESCC patients and their survival time. The period of survival for both high and low NCF2 groups in the log-rank test was different. The average overall survival (OS) period for the high NCF2 expression group was 36.1 months, which is less than the low NCF2 expression group (43.9 months) (log-rank test, P<0.01). Moreover, the higher NCF2 expression patients showed that they had shorter DFS as compared to low NCF2 expression of patients (log-rank test, P=0.001) (Figure 4). However, the NCFF2 standard expression was deployed in ESCC patients’ subgroups to deduce a survival analysis with respect to size of tumor, and TNM stages as well as Recurrence. Furthermore, we analyzed the NCF2 expression’s prognostic value for DFS and OS by means of a univariate analysis model based on the clinicopathologic measures. Through this analysis it was found that the survival was affected by NCF2 expression, tumor size, tumor multiplicity, TNM stage, and recurrence. We further examined these important statistical measures by employing a multivariate Cox regression analysis for evaluating the importance of the expression of NCF2 in the prognosis of ESCC. It was observed that NCF2 expression, TNM stage, and tumor size were independent prognostic elements for OS, while NCF2 expression and tumor size, were independent prognostic elements for DFS (Table 2). Thus, it is suggested that there was significant association of the NCF2 expression level with the prognosis of ESCC.
Figure 4.
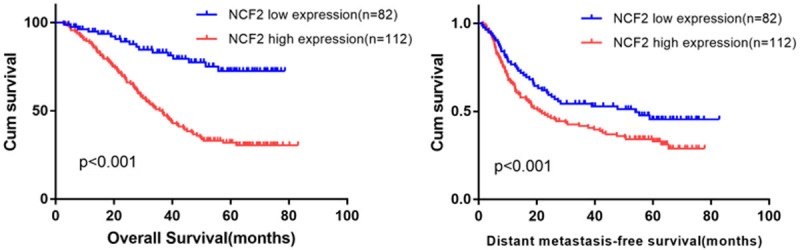
Kaplan-Meier analysis indicating the correlation of NCF2 overexpression with poorer overall survival and distant metastasis-free survival rates of 194 ESCC patients (log-rank test).
Table 2.
Univariate and multivariate Cox regression analysis of prognostic factors in 194 ESCC patients for overall survival and distant metastasis-free survival
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Overall survival | ||||||
| Age | ||||||
| >60 vs ≤60 | 1.153 | 0.868-1.531 | 0.325 | |||
| Sex | ||||||
| male vs female | 0.99 | 0.884-1.966 | 0.09 | |||
| Smoking history | ||||||
| yes vs no | 1.33 | 0.944-2.742 | 1.02 | |||
| Tumor Multiplicity | ||||||
| Unifocal vs Multifocal | 2.14 | 1.124-3.65 | 0.72 | |||
| TNM stage | ||||||
| I-II vs III | 1.291 | 0.827-0.1.893 | 0.003 | 1.453 | 1.028-2.041 | 0.035 |
| Tumor size | ||||||
| ≤3 cm vs >3 cm | 1.321 | 0.969-1.851 | 0.001 | 0.344 | 0.178-0.664 | 0.001 |
| Recurrence | ||||||
| yes vs no | 0.941 | 0.480-1.786 | 0.001 | 1.058 | 0.648-1.537 | 0.059 |
| NCF2 expression | ||||||
| high vs low | 1.33 | 0.966-1.877 | 0.01 | 1.751 | 1.284-2.590 | 0.001 |
| Distant metastasis-free survival | ||||||
| Age | ||||||
| >60 vs ≤60 | 1.081 | 0.815-1.434 | 0.589 | |||
| Sex | ||||||
| male vs female | 0.95 | 0.724-1.623 | 0.94 | |||
| Smoking history | ||||||
| yes vs no | 0.74 | 0.474-1.426 | 0.88 | |||
| Tumor Multiplicity | ||||||
| Unifocal vs Multifocal | 1.34 | 0.432-2.104 | 0.55 | 0.382 | 0.172-0.847 | 0.325 |
| TNM stage | ||||||
| I-II vs III | 0.701 | 0.516-0.952 | 0.023 | 4.00 | 0.182-0.881 | 0.057 |
| Tumor size | ||||||
| ≤3 cm vs >3 cm | 0.59 | 0.299-1.011 | 0.001 | 3.681 | 0.259-6.386 | 0.015 |
| Recurrence | ||||||
| yes vs no | 0.647 | 0.485-0.864 | 0.65 | |||
| NCF2 expression | ||||||
| high vs low | 0.39 | 0.199-1.233 | 0.39 | 1.511 | 1.024-2.531 | 0.001 |
| P<0.05 | ||||||
Effect of NCF2 low-expression on cell proliferation and invasion
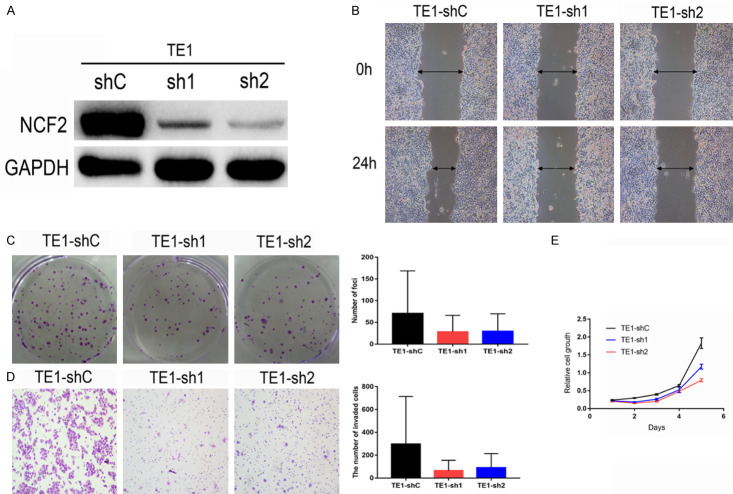
To determine whether cell proliferation and invasion in ESCC cells were affected by NCF2, the NCF2 expression by siRNA in NCF2-high-expressing ESCC cells, TE1 was knocked down. In the CCK8 assays and colony formation assays, the inhibition of NCF2 would produce lower cell proliferation compared to the control group. In addition, it was shown from the invasion assay (In the Wound healing assay and Transwell invasion assay) the ability of cell invasion was tremendously decreased with the knockdown of NCF2 in TE1 cells (Figure 5).
Figure 5.
NCF2 has strong oncogenic function in ESCC cells. A. Western blotting reveals that NCF2 was efficiently knocked down by the treatment of NCF2-shRNA-1 or NCF2-shRNA-2 in TE1 ESCC cells. B. Wound-healing show that NCF2-ilenced TE1 had lower invasive capacity (down) as compared with that in control TE1 cells. C. Representative images of decreased foci formation in monolayer culture induced by NCF2 silenced in ESCC cells. Data are the means ± sd of three independent experiments. P<0.01 by Student’s t-test. D. Transwell invasion assays show that NCF2-silenced TE1 had lower invasive capacity (down) as compared with that in control TE1 cells. Data are the mean ± sd of three independent experiments. P<0.05, P<0.05 by Student’s t-test. E. Rate of cell growth of TE-sh1, TE1-sh2 and sh-Control ESCC cells by CCK-8 kit. P<0.05, P<0.05 by Student’s t-test.
Discussion
At present, TNM stage is the most generally used predictive factor for prognosis of ESCC patients [14,15]. TNM staging alone is inadequate for the accurate prediction of the clinical outcomes of patients with ESCC [16-18]. The clinical prognostic needs are not fully satisfied based on the existing molecular markers for ESCC [19-22]. So novel diagnostic and prognostic markers are needed.
It is proven from the accumulating evidence that there was significantly increasing in the expression of NCF2 in several types of malignancies [23-25]. According to these findings, it was revealed that it plays a viable carcinogenic function of NCF2 in many human virulence [26-29]. So far, however, it has not been elucidated about the stratum of NCF2 formulation in ESCC and its relationship with the characteristics of the clinicopathology. In this research the status of NCF2 expression in a cohort of 194 ESCC patients is evaluated by the performance of immunohistochemistry. Compared with the ones in adjacent normal tissues, it obviously up-regulated NCF2 in ESCC paraffin-embedded tissues. Particularly, it detected the overexpression of NCF2 protein in 50.5% (98/194) of ESCC tissues. By comparison, it was low or absent for the NCF2 expression level in the adjacent nasopharyngeal tissues. Compared with NE1, it was also found that NCF2 was highly expressed at both the protein and mRNA levels in ESCC cell lines. Based on these findings, it is suggested that during the ESCC tumorigenic processes, a selective advantage may be provided by high expression of NCF2.
It was found in the previous studies thatevaluated the NCF2 expression that there was an obvious and close association with tumor progression and unfavorable prognosis in various different types of malignancies. It is demonstrated that high NCF2 expression frequently occurred in colorectal cancer tissues and might play an important role in colorectal carcinogenesis. In a recent study, it was observed that there is significantly association of the increased expression of NCF2 with TNM stage, recurrence which showed that the over expression of NCF2 might be involved in the ESCC progression. In addition, according to the Kaplan-Meier analysis and log rank test, it was proven that there are obvious correlations between enhanced NCF2 expression and the characteristics of shorter OS and poorer DFS in patients with ESCC. It was revealed in the further stratified analysis that the poor outcome in ESCC patients with TNM stage I/II and III could be predicted by the elevated expression of NCF2. Notably, through the multivariate Cox regression analysis, it was shown that the high NCF2 expression was a prognostic independent factor for OS and DFS in ESCC. Therefore, in addition to existing classifications, this paper suggested the use of NCF2 expression for the prediction of OS and DFS in patients with ESCC.
In conclusion, it was shown for the first time that NCF2 protein expression was obvious in ESCC, and that a close relation existed between the expression of NCF2 with unfavorable prognosis in ESCC. It is suggested from these data that the levels of NCF2 protein expression, according to the detection based on IHC, can be considered as a prognostic clinical biomarker outcome of ESCC patients, and serve as viable therapy target in ESCC. Further investigation will indicate the molecular mechanisms on the oncogenic function of NCF2 for ESCC.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 81772514, and 81772513), Pearl River S&T Nova Program of Guangzhou (201806010005).
Informed consent was obtained from all individual participants included in the study.
Disclosure of conflict of interest
None.
References
- 1.Domper Arnal MJ, Ferrández Arenas Á, Lanas Arbeloa Á. Esophageal cancer: risk factors, screening and endoscopic treatment in Western and Eastern countries. World J Gastroenterol. 2015;21:7933–7943. doi: 10.3748/wjg.v21.i26.7933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400–412. doi: 10.1016/S0140-6736(12)60643-6. [DOI] [PubMed] [Google Scholar]
- 4.Gauss KA, Nelson-Overton LK, Siemsen DW, Gao Y, DeLeo FR, Quinn MT. Role of NF-κB in transcriptional regulation of the phagocyte NADPH oxidase by tumor necrosis factor-α. J Leukoc Biol. 2007;82:729–741. doi: 10.1189/jlb.1206735. [DOI] [PubMed] [Google Scholar]
- 5.Quinn MT, Gauss KA. Structure and regulation of the neutrophil respiratory burst oxidase: comparison with non-phagocyte oxidases. J Leukoc Biol. 2004;76:760–781. doi: 10.1189/jlb.0404216. [DOI] [PubMed] [Google Scholar]
- 6.Freeman JL, Lambeth JD. NADPH oxidase activity is independent of p47phox in vitro. J Biol Chem. 1996;271:22578–22582. doi: 10.1074/jbc.271.37.22578. [DOI] [PubMed] [Google Scholar]
- 7.Koshkin V, Lotan O, Pick E. The cytosolic component p47phox is not a sine qua non participant in the activation of NADPH oxidase but is required for optimal superoxide production. J Biol Chem. 1996;271:30326–30329. doi: 10.1074/jbc.271.48.30326. [DOI] [PubMed] [Google Scholar]
- 8.Gauss KA, Bunger PL, Larson TC, Young CJ, Nelson-Overton LK, Siemsen DW, Quinn MT. Identification of a novel tumor necrosis factor α-responsive region in the NCF2 promoter. J Leukoc Biol. 2005;77:267–278. doi: 10.1189/jlb.0604329. [DOI] [PubMed] [Google Scholar]
- 9.Badalzadeh M, Fattahi F, Fazlollahi MR, Tajik S, Bemanian MH, Behmanesh F, Movahedi M, Houshmand M, Pourpak Z. Molecular analysis of four cases of chronic granulomatous disease caused by defects in NCF-2: the gene encoding the p67-phox. Iran J Allergy Asthma Immunol. 2012;11:340–344. [PubMed] [Google Scholar]
- 10.Lomnytska I, Becker S, Hellman K, Hellström AC, Souchelnytskyi S, Mints M, Hellman U, Andersson S, Auer G. Diagnostic protein marker patterns in squamous cervical cancer. Proteomics Clin. 2010;4:17–31. doi: 10.1002/prca.200900086. [DOI] [PubMed] [Google Scholar]
- 11.Jacob CO, Eisenstein M, Dinauer MC, Ming W, Liu Q, John S, Quismorio FQ, Reiff A, Myones BL, Kaufman KM. Lupus-associated causal mutation in neutrophil cytosolic factor 2 (NCF2) brings unique insights to the structure and function of NADPH oxidase. Proc Natl Acad Sci U S A. 2012;109:E59–E67. doi: 10.1073/pnas.1113251108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu G, Hong DK, Dionis KY, Rae J, Heyworth PG, Curnutte JT, Lewis DB. Focus on FOCIS: the continuing diagnostic challenge of autosomal recessive chronic granulomatous disease. Clin Immunol. 2008;128:117–126. doi: 10.1016/j.clim.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Zhang JX, Chen ZH, Chen DL, Tian XP, Wang CY, Zhou ZW, Gao Y, Xu Y, Chen C, Zheng ZS. LINC01410-miR-532-NCF2-NF-kB feedback loop promotes gastric cancer angiogenesis and metastasis. Oncogene. 2018;37:2660–2675. doi: 10.1038/s41388-018-0162-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Nie S, Chen T, Yang X, Huai P, Lu M. Association of helicobacter pylori infection with esophageal adenocarcinoma and squamous cell carcinoma: a meta-analysis. Dis Esophagus. 2014;27:645–653. doi: 10.1111/dote.12194. [DOI] [PubMed] [Google Scholar]
- 15.Zhang HZ, Jin GF, Shen HB. Epidemiologic differences in esophageal cancer between Asian and Western populations. Chin J Cancer. 2012;31:281–286. doi: 10.5732/cjc.011.10390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devesa SS, Blot WJ, Fraumeni JF Jr. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83:2049–2053. [PubMed] [Google Scholar]
- 17.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241–2252. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 18.Jankowski JA, Provenzale D, Moayyedi P. Esophageal adenocarcinoma arising from Barrett’s metaplasia has regional variations in the west. Gastroenterology. 2002;122:588–590. doi: 10.1053/gast.2002.31599. [DOI] [PubMed] [Google Scholar]
- 19.Small AJ, Sutherland SE, Hightower JS, Guarner-Argente C, Furth EE, Kochman ML, Forde KA, Bewtra M, Falk GW, Ginsberg GG. Comparative risk of recurrence of dysplasia and carcinoma after endoluminal eradication therapy of high-grade dysplasia versus intramucosal carcinoma in Barrett’s esophagus. Gastrointest Endosc. 2015;81:1158–1166. e1–4. doi: 10.1016/j.gie.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 20.Leiserson MD, Wu HT, Vandin F, Raphael BJ. CoMEt: a statistical approach to identify combinations of mutually exclusive alterations in cancer. Genome Biol. 2015;16:160. doi: 10.1186/s13059-015-0700-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hammad H, Kaltenbach T, Soetikno R. Endoscopic submucosal dissection for malignant esophageal lesions. Curr Gastroenterol Rep. 2014;16:386. doi: 10.1007/s11894-014-0386-0. [DOI] [PubMed] [Google Scholar]
- 22.Pech O, Behrens A, May A, Nachbar L, Gossner L, Rabenstein T, Manner H, Guenter E, Huijsmans J, Vieth M. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett’s oesophagus. Gut. 2008;57:1200–1206. doi: 10.1136/gut.2007.142539. [DOI] [PubMed] [Google Scholar]
- 23.Khuakoonratt N, Tangjitgamol S, Manusirivithaya S, Khunnarong J, Pataradule K, Thavaramara T, Suekwattana P. Prevalence of high grade squamous intraepithelial lesion (HSIL) and invasive cervical cancer in patients with low grade squamous intraepithelial lesion (LSIL) at cervical pap smear. Asian Pac J Cancer Prev. 2008;9:253–257. [PubMed] [Google Scholar]
- 24.Gunnell AS, Ylitalo N, Sandin S, Sparén P, Adami HO, Ripatti S. A longitudinal Swedish study on screening for squamous cell carcinoma and adenocarcinoma: evidence of effectiveness and overtreatment. Cancer Epidemiol Biomarkers Prev. 2007;16:2641–2648. doi: 10.1158/1055-9965.EPI-07-0278. [DOI] [PubMed] [Google Scholar]
- 25.Castellsagué X. Natural history and epidemiology of HPV infection and cervical cancer. Gynecol Oncol. 2008;110:S4–S7. doi: 10.1016/j.ygyno.2008.07.045. [DOI] [PubMed] [Google Scholar]
- 26.Jacob CO, Reiff A, Armstrong DL, Myones BL, Silverman E, Klein-Gitelman M, McCurdy D, Wagner-Weiner L, Nocton JJ, Solomon A. Identification of novel susceptibility genes in childhood-onset systemic lupus erythematosus using a uniquely designed candidate gene pathway platform. Arthritis Rheum. 2007;56:4164–4173. doi: 10.1002/art.23060. [DOI] [PubMed] [Google Scholar]
- 27.El Kares R, Barbouche MR, Elloumi-Zghal H, Bejaoui M, Chemli J, Mellouli F, Tebib N, Abdelmoula M, Boukthir S, Fitouri Z. Genetic and mutational heterogeneity of autosomal recessive chronic granulomatous disease in Tunisia. J Hum Genet. 2006;51:887–895. doi: 10.1007/s10038-006-0039-8. [DOI] [PubMed] [Google Scholar]
- 28.Malech HL, Hickstein DD. Genetics, biology and clinical management of myeloid cell primary immune deficiencies: chronic granulomatous disease and leukocyte adhesion deficiency. Curr Opin Hematol. 2007;14:29–36. doi: 10.1097/00062752-200701000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Schäppi MG, Jaquet V, Belli DC, Krause KH. Hyperinflammation in chronic granulomatous disease and anti-inflammatory role of the phagocyte NADPH oxidase. Semin Immunopathol. 2008;30:255–271. doi: 10.1007/s00281-008-0119-2. [DOI] [PubMed] [Google Scholar]