Abstract
Chemotherapy treatment of acute myeloid leukemia (AML) can be compromised due to the multidrug resistance (MDR) of leukemia cells. HOTAIR, a long noncoding RNA (LncRNA), is involved in MDR development of various solid tumors. However, whether it functions in MDR development of leukemia remains unclear. In this study, expressions of HOTAIR in leukemia cell line K562/A02 and bone marrow samples from 10 patients with refractory and relapsed AML were detected by qRT-PCR. The apoptosis, proliferation, and susceptibility of K562/A02 cells to Adriamycin (ADR) were analyzed by flow cytometry and CCK8 assay, respectively. The expression of cell cycle regulator P21 and Notch1 in the K562/A02 cells was examined by qRT-PCR. The accumulation of total AKT and the phosphorylated AKT (pAKTS473) were detected by western blotting. We found that the expression of HOTAIR in drug-resistant cells and patient samples was increased. Inhibition of HOTAIR expression could suppress the proliferation, increase the apoptosis, and promote the doxorubicin sensitivity of K562/A02 cells. Moreover, inhibiting expression of HOTAIR could attenuate the expression of P21 and Notch1 and inhibit the phosphorylation of AKT in drug-resistant cells. In conclusion, our results demonstrated that LncRNA-HOTAIR is involved in MDR development of leukemia cells by regulating the expression of P21 and the AKT/Notch1 signaling pathway.
Keywords: Acute myeloid leukemia, LncRNA-HOTAIR, chemoresistance mechanism
Introduction
Acute myeloid leukemia (AML) is a hematopoietic disease, characterized by accumulation of immature hematopoietic cells in bone marrow and insufficient production of normal blood cells [1]. Currently, anthracycline-based combined chemotherapy is still the main choice of acute myeloid leukemia (AML). 60-80% of patients with AML achieve hematologic remission through chemotherapy, and some patients at low and moderate risk can obtain long-term survival [2]. Multidrug resistance (MDR) of leukemia cells is an important cause of chemotherapy failure and death during the treatment of leukemia [3,4]. Therefore, it is of great value to investigate the mechanism of drug resistance of AML cells and find the biologic markers of multidrug resistance of leukemia cells in order to improve the therapeutic effect of AML.
Long non-coding RNA (LncRNA) is a type of non-coding RNA. Numerous studies have shown that LncRNA plays an important role in gene expression, such as RNA processing, transcription, post-transcriptional modification, regulation of protein activity, and mediation of information transmission. HOX transcript antisense RNA (HOTAIR) is the earliest found and most thoroughly studied LncRNA, with a length of 2158 kb, located on the antisense chain of HOXC loci [5]. Many studies have shown that HOTAIR regulates the development of chemoresistance in various solid tumors such as colorectal cancer, non-small cell lung cancer (NSCLC), and gastric cancer by a variety of pathways [6-8]. However, whether HOTAIR is involved in the development of chemoresistance in AML has not been reported.
In this study, the role of HOTAIR in the development of drug resistance in AML was investigated using the drug-resistant leukemia cell line K562/A02 and validated in clinical samples to explore the correlation between HOTAIR expression in AML cells and clinical efficacy, and find targets for overcoming drug resistance in AML.
Materials and methods
Cell line and cell culture
Human myeloid leukemia cell line K562 and its corresponding drug-resistant cell line K562/A02 were purchased from Tianjin Institute of Hematology (Tianjin, China). Adriamycin (ADR) was added to K562/A02 cell culture medium at a final concentration of 1 μg/mL to maintain cell resistance. The culture medium without ADR was replaced two weeks before each experiment. The two cell lines were cultured in RPMI-1640 complete medium (Gibco BRL, USA) supplemented with 10% fetal bovine serum and inoculated into a 10 cm culture dish. The cells were cultured in an incubator at 37°C, 5% CO2 and saturated humidity. All cells used in the experiment were taken from logarithmic growth phase.
Collection of bone marrow samples from patients with AML
Bone marrow samples were collected from patients with AML in the Department of Hematology, Zhongshan Affiliated Hospital of Xiamen University from June 2016 to June 2018. The diagnostic criteria were in line with the consensus diagnosis and treatment standards of adult acute myeloid leukemia in China. Samples from a total of 10 relapsed and refractory patients, 11 newly treated patients and 13 patients with iron deficiency anemia as control were collected. 3-5 mL of bone marrow samples were collected from patients before chemotherapy in an EDTA anticoagulant tube. Leukemia cells were extracted from bone marrow samples using human lymphocyte separation medium (Human) (Solarbio Life Sciences, China) and red blood cells were lysed with human erythrocyte lysate (China). The cell samples were washed with PBS 2-3 times after lysis and then stored at -80°C.
RNA interference and transfection
RFP fluorescent labeled lentiviral specific small interfering RNA (siRNA) targeting HOTAIR mRNA (si-HOTAIR, 5’-GCAGCACAGAGCAACTCTATA-3’) and negative control (siR-NC) were synthesized by GenaPharma (Shanghai, China), and transfected using Polybrene (GenaPharma Shanghai, China) according to the manufacturer’s protocol. A total of 2×105 K562/A02 cells were seeded into 6-well plates and divided into two groups as follows: cells transfected with the negative control (si-NC group); cells transfected with si-HOTAIR (si-HOTAIR group); After 48 h, the efficacy of transfection was validated by fluorescence microscope and efficacy of gene silencing was validated by reverse transcription-quantitative polymerase chain reaction (RT-qPCR).
Real-time fluorescence quantitative PCR
RNA was extracted from cultured cells and bone marrow sample cells by Trizol (TransGen Biotech, China) lysis. All operations were carried out according to the instructions. cDNA was synthesized using TransScript All-in-One First-Strand cDNA Synthesis SuperMix for qPCR reverse transcription kit (TransGen Biotech, China). TransScript® II Green Two-Step, qRT-PCR SuperMix kit (TransGen Biotech, China) was adopted for the qRT-PCR system. The sequences of primers used were as follows: HOTAIR, 5’-GGAAGCGAAGGGGTTGTGTA-3’ (forward) and 5’-GGCTAGGGCTGGTTTCACTT-3’ (reverse); GAS5, 5’-CGACTCCTGTGAGGTATGGTG-3’ (forward) and 5’-ATCCTTCCTTGGGGACACAAC-3’ (reverse); H19, 5’-CAAAGCCTCCACGACTCTGT-3’ (forward) and 5’-ACTCACGCACACTCGTACTG-3’ (reverse); PVT1, 5’-GCCATAGATCCTGCCCTGTT3’ (forward) and 5’-TCTTGGTGGGGCTTGTGAAT-3’ (reverse); β-actin, 5’-AGCGAGCATCCCCCAAAGTT-3’ (forward) and 5’-GGGCACGAAGGCTCATCATT-3’ (reverse); GAPDH, 5’-CAGGAGGCATTGCTGATGAT-3’ (forward) and 5’-GAAGGCTGGGGCTCATTT-3’ (reverse). The amplification reaction and fluorescence signal acquisition were achieved by ABI Prism 7500 PCR (USA), and the CT values of each target gene were obtained by SDSv1.4.0 software. The relative expression of the target gene was calculated by 2-ΔΔCt formula, and GAPDH was used as internal reference.
Western blotting
Cell proteins were extracted by RIPA lysate (Thermo Fisher Science, USA) and added with broad-spectrum protease inhibitor or phosphatase inhibitor (Thermo Fisher Science, USA) according to different experiments. BCA protein detection kit (Thermo Fisher Science, USA) was used for quantitative analysis. The loading quantity of protein samples was 15 μg. After electrophoresis, the sample protein was separated and transferred to PVDF membranes, and then blocked with 5% nonfat milk at room temperature for 1 h. Then the samples were incubated with primary antibodies overnight at 4°C: anti-Notch1 (1:1000, Abcam); anti-P21 (1:1000, Cell Signaling Technology); anti-AKT (1:1000, Cell Signaling Technology); anti-Phospho-AKTs473 (1:1000, Cell Signaling Technology); anti-β-actinin (1:5000, Applygen, China). Membranes were washed with TBST 2-3 times and then incubated with secondary antibody Horseradish peroxidase labeled goat antirabbit IgG (Multi Sciences, China) for 1 h at room temperature. The membranes were then washed with TBST 2-3 times and exposed in dark room using ECL kit (Sigma Aldrich, USA) according to the instructions.
Proliferation and cytotoxicity analysis by CCK8
Cell proliferation assays: cells in logarithmic growth phase were collected and inoculated into a 96-well plate with 3.5×103 cells/well. Culture medium was used as blank. According to the instructions, CCK8 solution was added at 0, 24, 48, 72 and 96 h, respectively. The absorbance at 450 nm was determined by enzyme labeling after incubation for 2 to 4 h in an incubator.
Cytotoxicity test: cells in the logarithmic growth phase were collected and inoculated into a 96-well culture plate with 3.5×103 cells/well. ADR at different concentrations was added to K562 cells (0.02, 0.04, 0.08, 0.16, 0.32, 0.64 μg/mL) and K562/A02 cells (2, 4, 8, 16, 32, 64 μg/mL). The untreated cells were set as the control group, and the medium was set as the blank group. After 48 h of incubation, the absorbance value was determined at 450 nm according to the instructions of CCK8 kit. Cell growth inhibition rate was calculated as follows: (control group absorbance - experimental group absorbance)/(control group absorbance - blank group absorbance) ×100%. The median inhibitory concentration (IC50) of adriamycin was calculated by SPSS software.
Statistical analysis
Excel, SPSS 17.0 software and Graphpad Prism 5.0 were used for statistical analysis. t-test was used for comparison of measurement data between the two groups. One-way ANOVA was used for comparison of measurement data among multiple groups. P<0.05 was considered significant.
Results
High expression of HOTAIR in drug-resistant cell line K562/A02
First, CCK8 kit was used to detect the toxicity of ADR to drug-resistant cell line K562/A02 and sensitive cell line K562. The results showed that the IC50 of K562/A02 was 170 times higher than that of K562 (0.4 ± 0.08 μg/mL), which confirmed the quality of K562 and K562/A02 cell lines. Next, based on related literature reports, the expressions of four LncRNAs including HOTAIR, H19, GAS5 and PVT1 were detected by qRT-PCR. It was found that the expression of HOTAIR and H19 in K562/A02 cells was significantly higher than that in K562 cells (Figure 1A, 1B), while the expression of GAS5 was lower than that in K562 cells (P = 0.0223) (Figure 1C), and the expression of PVT1 showed no significant difference between the two cell lines (P = 0.3255) (Figure 1D). The results suggested that HOTAIR and H19 might be associated with the development of drug resistance in leukemic cells.
Figure 1.
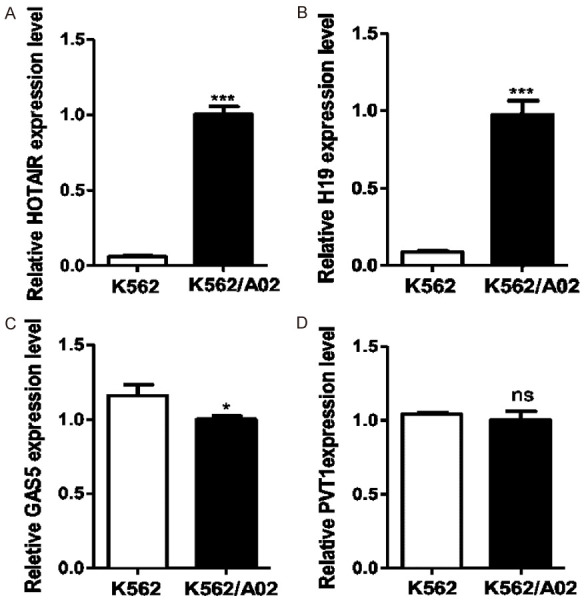
Expressions of four chemoresistance-related LncRNAs in K562/A02 and K562 cell lines were examined. The expressions of HOTAIR and H19 in drug-resistant cell line K562/A02 were significantly higher than those in sensitive cell line K562 (A, B). The expression of GAS5 was lower than that in K562/A02 cells (P = 0.0223) (C), and the expression of PVT1 was not significantly different between the two cell lines (P = 0.3255) (D).
Targeted inhibition of HOTAIR expression suppressed cell proliferation and increased drug sensitivity of K562/A02 cells
In order to find out whether HOTAIR is essential for AML cell survival, we established HOTAIR knockdown cell lines by transfecting a specific siRNA targeting HOTAIR (siHOTAIR) into K562/A02 cells. The results of qRT-PCR showed that the expression of HOTAIR in si-HOTAIR K562/A02 group decreased by about 72% with a significant difference (Figure 2A, 2B). Results of CCK8 assays showed that targeted inhibition of HOTAIR expressions significantly decreased the proliferation rate of cells in the si-HOTAIR group and increased the sensitivity to ADR with the IC50 of 45 ± 1.9 μg/mL for the si-HOTAIR group and 65 ± 2.3 μg/mL for the si-NC group (Figure 2C, 2D).
Figure 2.
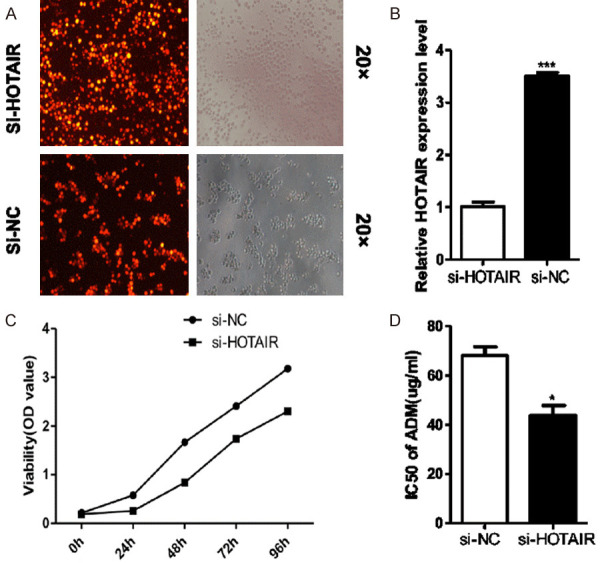
(A) Inhibition of HOTAIR expression reduced drug resistance in K562/A02 cells. (B) After HOTAIR was knocked down by gene transfection, the HOTAIR expression in si-HOTAIR K562/A02 cells was significantly lower than that in si-NC group. The cell proliferation was inhibited (C); and sensitivity to ADR increased (D) in si-HOTAIR K562/A02 cells compared with si-NC cells.
Targeted inhibition of HOTAIR promoted P21 gene expression and inhibited AKT phosphorylation in K562/A02 cells
To explore the mechanism underlying the involvement of HOTAIR in the proliferation of ADR-resistant K562/A02 cells, we then examined the expression of cell cycle-regulation gene P21 and AKT phosphorylation. The qRT-PCR showed that the expression of P21 gene in K562/A02 cell line increased after targeted inhibition of HOTAIR (Figure 3A, 3B), suggesting that HOTAIR could inhibit apoptosis of K562/A02 cells by inhibiting the expression of P21. At the same time, western blotting showed that targeted inhibition of HOTAIR also decreased the phosphorylation of AKT (Figure 3C). These results indicated that HOTAIR overexpression could influence the expression of cell cycle regulatory genes and the apoptosis of leukemia cells.
Figure 3.
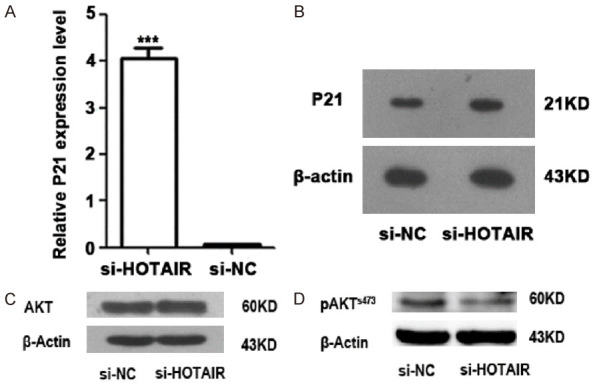
Targeted inhibition of HOTAIR expression affected the expression of cell cycle gene P21 at mRNA level (A) and protein level (B), as well as AKT phosphorylation (C, D) in K562/A02 cells.
Targeted inhibition of HOTAIR inhibited Notch1 gene expression in K562/A02 cells
The expression of Notch1 at mRNA and protein levels in K562/A02 cells was detected by qRT-PCR and western blot, respectively. The results showed that the expression of Notch1 gene and protein in K562/A02 cells decreased after targeted inhibition of HOTAIR (P = 0.0124) (Figure 4B), suggesting that HOTAIR may promote the resistance of K562/A02 cells by promoting Notch1 expression.
Figure 4.
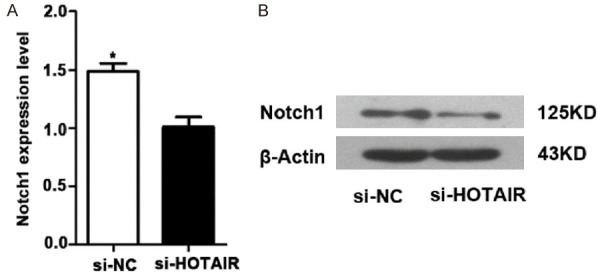
Targeted inhibition of HOTAIR expression inhibited Notch1 expression in K562/A02 cells at mRNA level (A) and protein level (B).
HOTAIR is highly expressed in refractory-relapsed AML cells
To further verify the association between HOTAIR and the chemoresistance of AML cells, the HOTAIR expression was measured in bone marrow samples of leukemia patients. Results showed that the expression of HOTAIR in bone marrow leukemia cells of patients with newly diagnosed (ND) and refractory-relapsed (RR) AML was significantly higher than that of patients with iron deficiency anemia. In addition, the expression of HOTAIR in leukemia cells of patients with refractory AML was significantly higher than that of patients with newly-diagnosed leukemia, and the difference was statistically significant (Figure 5). These results indicated that an elevated HOTAIR expression in acute myeloid leukemia cells may be a biologic marker of chemoresistance, which might be used for diagnosis and evaluation of therapeutic efficacy in patients with acute myeloid leukemia.
Figure 5.
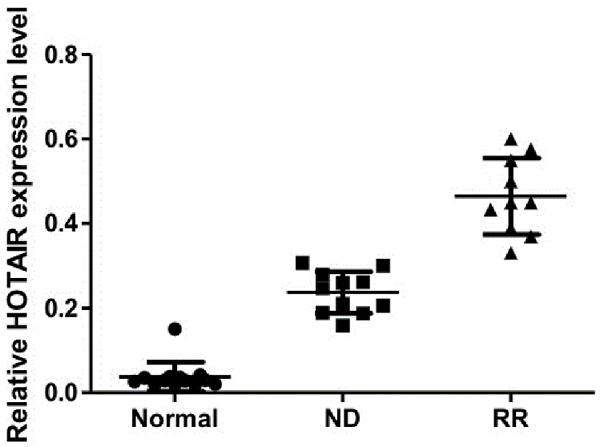
The expression of HOTAIR in leukemia cells of refractory-relapsed leukemia (RR) patients was significantly higher than that of patients with iron deficiency anemia (Normal) and newly diagnosed leukemia (ND). Expression of HOTAIR in RR patients was also significantly higher than that of ND patients.
Discussion
Acute myeloid leukemia (AML) is the most common malignant myeloid disorder of adulthood, and one of the major challenges in AML is high relapse rate after chemotherapy [1]. LncRNA has attracted much attention due to its wide participation in all stages of tumorigenesis and development and has become a new research hotspot of tumor regulatory factors after microRNAs. High expression of HOTAIR, a typical trans-acting LncRNA, is related to the development of drug resistance in various carcinomas. Targeted inhibition of LncRNA HOTAIR expressions sensitized various cancer cells to chemotherapeutic drugs, including gastric cancer [9], breast cancer [10], small-cell lung cancer [11], non-small cell lung cancer [12], colorectal cancer [13] and ovarian cancer [14]. In the context of AML, it was reported that overexpression of HOTAIR predicted a poor prognosis [15]. In this study, we first confirmed that the HORAIR expression in the drug-resistant AML cell line was significantly higher than that in the non-resistant one, and this abnormal expression was further confirmed in the clinical refractory-relapsed AML samples, suggesting that HOTAIR overexpression may be a marker of chemoresistance in leukemia cells and involved in the development of multidrug resistance in leukemia cells. It was then found that after targeted inhibition of HOTAIR, the proliferation of K562/A02 cells was inhibited, the proportion of apoptotic cells was increased, and sensitivity to ADR was enhanced. Similar results were reported in the published literature. An in vitro study of leukemia cells confirmed that HOTAIR could compete with microRNA-193a in c-KIT as a competitive endogenous RNA (ceRNA), thereby reducing the inhibitory effect of microRNA-193a on c-KIT, and thus protecting leukemia cells from apoptosis [16]. Our results suggested that the high expression level of HOTAIR in leukemia cells may be one of the reasons for the continuous proliferation and development of drug resistance of leukemia cells.
Notch signaling is a signaling pathway closely related to the occurrence and development of malignant tumors and their response to chemotherapy. It was found that more than 50% of patients with T-cell acute lymphoblastic leukemia (T-ALL) harbored Notch 1 gene abnormalities [17]. Continuous activation of Notch signaling pathway as an oncogene participated in the pathogenesis of T-ALL [18,19]. Hang et al. found that Notch 1 could promote the drug resistance development of gastric cancer cells by up-regulating the expression of LncRNA AK022798 [20]. Meanwhile, Kamga et al. found that Notch signaling played an important role in AML cell survival mediated by bone marrow stromal cells [21]. In our study, it was found that the expression of Notch1 gene was decreased after targeted inhibition of HOTAIR expressions in drug-resistant leukemia cells. These results suggest that the abnormal expression of Notch1 might be involved in the development of chemoresistance induced by high expression of HOTAIR in leukemia cells, which provides a basis for studying the mechanism of chemoresistance of leukemia cells.
In addition, P21 gene is an important member of the family of cyclin-dependent kinase inhibitors (CDKI), which is located in the downstream of p53 gene and plays an important role in the regulation of drug resistance of cancer cells along with p53. A study on drug resistance of cervical cancer found that overexpression of HOTAIR inhibited the expression of P21 gene and increased the resistance of cervical cancer cells to radiotherapy, indicating that targeting HOTAIR can be used as therapy for cervical cancer [22]. A study on drug resistance of colon cancer confirmed that microRNA-520g participated in the development of drug resistance by regulating the expression of P21 [23]. In this study, we confirmed that targeted inhibition of HOTAIR expression promoted the expression of P21 gene in leukemia drug-resistant cells, thereby affecting the proliferation and apoptosis of drug-resistant cells, and sensitized K562/A02 cells to ADR. Therefore, the P21/AKT pathway plays an important role in HOTAIR-mediated drug resistance.
Conclusions
Through in vitro cytological studies, we found that HOTAIR overexpression could affect the molecular pathway of sensitivity of leukemia cells to ADR, possibly by regulating the Notch signaling and P21 gene. Therefore, it can be speculated that the overexpression of HOTAIR is a molecular marker of chemoresistance in leukemia cells, and downregulation of HOTAIR expression may be a way to overcome chemoresistance in leukemia cells. Nevertheless, more research is needed to further elucidate the complex regulatory mechanism of HOTAIR in the chemoresistance of AML.
Acknowledgements
The authors would like to thank the patients and their families for their contribution to this study. This work was supported by the Science and Technology Project of Science and Technology Department of Guizhou province (Qian Ke He Basics [2016] 1129), the Science and Technology Fund Project of Health and Family Planning Commission of Guizhou Province (gzwjkj2016-1-025) and the Chinese Medicine Science and Technology Research Project of Guizhou Provincial Administration of Traditional Chinese Medicine (QZZYY-2017-035).
Disclosure of conflict of interest
None.
References
- 1.Kumar CC. Genetic abnormalities and challenges in the treatment of acute myeloid leukemia. Genes Cancer. 2011;2:95–107. doi: 10.1177/1947601911408076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmid C, Schleuning M, Schwerdtfeger R, Hertenstein B, Mischak-Weissinger E, Bunjes D, Harsdorf SV, Scheid C, Holtick U, Greinix H, Keil F. Long-term survival in refractory acute myeloid leukemia after sequential treatment with chemotherapy and reduced-intensity conditioning for allogeneic stem cell transplantation. Blood. 2006;108:1092–1099. doi: 10.1182/blood-2005-10-4165. [DOI] [PubMed] [Google Scholar]
- 3.Kunjachan S, Błauż A, Möckel D, Theek B, Kiessling F, Etrych T, Ulbrich K, van Bloois L, Storm G, Bartosz G, Rychlik B. Overcoming cellular multidrug resistance using classical nanomedicine formulations. Eur J Pharm Sci. 2012;45:421–428. doi: 10.1016/j.ejps.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 4.Shaffer BC, Gillet JP, Patel C, Baer MR, Bates SE, Gottesman MM. Drug resistance: still a daunting challenge to the successful treatment of AML. Drug Resist Updat. 2012;15:62–9. doi: 10.1016/j.drup.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mark M, Rijli FM, Chambon P. Homeobox genes in embryogenesis and pathogenesis. Pediatr Res. 1997;42:421–429. doi: 10.1203/00006450-199710000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Xu ZY, Yu QM, Du YA, Yang LT, Dong RZ, Huang L, Yu PF, Cheng XD. Knockdown of long non-coding RNA HOTAIR suppresses tumor invasion and reverses epithelial-mesenchymal transition in gastric cancer. Int J Biol Sci. 2013;9:587–597. doi: 10.7150/ijbs.6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu ZH, Wang XL, Tang HM, Jiang T, Chen J, Lu S, Qiu GQ, Peng ZH, Yan DW. Long non-coding RNA HOTAIR is a powerful predictor of metastasis and poor prognosis and is associated with epithelial-mesenchymal transition in colon cancer. Oncol Rep. 2014;32:395–402. doi: 10.3892/or.2014.3186. [DOI] [PubMed] [Google Scholar]
- 8.Liu MY, Li XQ, Gao TH, Cui Y, Ma N, Zhou Y, Zhang GJ. Elevated HOTAIR expression associated with cisplatin resistance in non-small cell lung cancer patients. J Thorac Dis. 2016;8:3314–3322. doi: 10.21037/jtd.2016.11.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng C, Qin Y, Zhi Q, Wang J, Qin C. Knockdown of long non-coding RNA HOTAIR inhibits cisplatin resistance of gastric cancer cells through inhibiting the PI3K/Akt and Wnt/β-catenin signaling pathways by up-regulating miR-34a. Int J Biol Macromol. 2018;107:2620–2629. doi: 10.1016/j.ijbiomac.2017.10.154. [DOI] [PubMed] [Google Scholar]
- 10.Li Z, Qian J, Li J, Zhu C. Knockdown of LncRNA-HOTAIR downregulates the drug-resistance of breast cancer cells to doxorubicin via the PI3K/AKT/mTOR signaling pathway. Exp Ther Med. 2019;18:435–422. doi: 10.3892/etm.2019.7629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen R, Chen B, Li D, Wang Q, Zhu Y, Li M, Wang Y, Fang S, Guo L. HOTAIR contributes to chemoresistance by activating NF-κB signaling in small-cell lung cancer. Int J Clin Exp Pathol. 2019;12:2997–3004. [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Y, Jiang C, Yang Y, Guo L, Huang J, Liu X, Wu C, Zou J. Silencing of LncRNA-HOTAIR decreases drug resistance of non-small cell lung cancer cells by inactivating autophagy via suppressing the phosphorylation of ULK1. Biochem Biophys Res Commun. 2018;497:1003–1010. doi: 10.1016/j.bbrc.2018.02.141. [DOI] [PubMed] [Google Scholar]
- 13.Xiao Z, Qu Z, Chen Z, Fang Z, Zhou K, Huang Z, Guo X, Zhang Y. LncRNA HOTAIR is a prognostic biomarker for the proliferation and chemoresistance of colorectal cancer via MiR-203a-3p-mediated Wnt/ss-catenin signaling pathway. Cell Physiol Biochem. 2018;46:1275–1285. doi: 10.1159/000489110. [DOI] [PubMed] [Google Scholar]
- 14.Liu S, Lei H, Luo F, Li Y, Xie L. The effect of LncRNA HOTAIR on chemoresistance of ovarian cancer through regulation of HOXA7. Biol Chem. 2018;399:485–497. doi: 10.1515/hsz-2017-0274. [DOI] [PubMed] [Google Scholar]
- 15.Wu S, Zheng C, Chen S, Cai X, Shi Y, Lin B, Chen Y. Overexpression of long non-coding RNA HOTAIR predicts a poor prognosis in patients with acute myeloid leukemia. Oncol Lett. 2015;10:2410–2414. doi: 10.3892/ol.2015.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xing CY, Hu XQ, Xie FY, Yu ZJ, Li HY, Wu JB, Tang LY, Gao SM. Long non-coding RNA HOTAIR modulates c-KIT expression through sponging miR-193a in acute myeloid leukemia. FEBS Lett. 2015;589:1981–1987. doi: 10.1016/j.febslet.2015.04.061. [DOI] [PubMed] [Google Scholar]
- 17.Grabher C, von Boehmer H, Look AT. Notch 1 activation in the molecular pathogenesis of T-cell acute lymphoblastic leukaemia. Nat Rev Cancer. 2006;6:347–359. doi: 10.1038/nrc1880. [DOI] [PubMed] [Google Scholar]
- 18.Eguchi-Ishimae M, Eguchi M, Kempski H, Greaves M. NOTCH1 mutation can be an early, prenatal genetic event in T-ALL. Blood. 2008;111:376–378. doi: 10.1182/blood-2007-02-074690. [DOI] [PubMed] [Google Scholar]
- 19.Liu N, Zhang J, Ji C. The emerging roles of Notch signaling in leukemia and stem cells. Biomark Res. 2013;1:23. doi: 10.1186/2050-7771-1-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hang Q, Sun R, Jiang C, Li Y. Notch 1 promotes cisplatin-resistant gastric cancer formation by upregulating LncRNA AK022798 expression. Anticancer Drugs. 2015;26:632–640. doi: 10.1097/CAD.0000000000000227. [DOI] [PubMed] [Google Scholar]
- 21.Kamga PT, Bassi G, Cassaro A, Midolo M, Di Trapani M, Gatti A, Carusone R, Resci F, Perbellini O, Gottardi M, Bonifacio M. Notch signalling drives bone marrow stromal cell-mediated chemoresistance in acute myeloid leukemia. Oncotarget. 2016;7:21713–21727. doi: 10.18632/oncotarget.7964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jing L, Yuan W, Ruofan D, Jinjin Y, Haifeng Q. HOTAIR enhanced aggressive biological behaviors and induced radio-resistance via inhibiting p21 in cervical cancer. Tumour Biol. 2015;36:3611–3619. doi: 10.1007/s13277-014-2998-2. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Geng L, Talmon G, Wang J. MicroRNA-520g confers drug resistance by regulating p21 expression in colorectal cancer. J Biol Chem. 2015;290:6215–6225. doi: 10.1074/jbc.M114.620252. [DOI] [PMC free article] [PubMed] [Google Scholar]


