Abstract
Background: C-X-C motif chemokine ligand 5 (CXCL5), an important chemokine, has been validated to promote human tumorigenesis. However, the clinical significance and the underlying molecular mechanisms of CXCL5 have not been completely explored in cervical cancer. Herein, the aim was to investigate miR-577-mediated CXCL5 signaling in cervical tumorigenicity. Material and methods: Sixty-one pairs of cervical cancer specimens and para-carcinoma tissues were collected to measure miR-577 and CXCL5 expression levels. miR-577 mimics and/or si-CXCL5 were transfected into cervical cancer cell lines, Hela, and SiHa cells, to determine their effect on cell proliferation, migration and apoptosis. Results: Our results demonstrated that CXCL5 is overexpressed in cervical cancer tissues and cell lines. Knockdown of CXCL5 with specific siRNA transfection in Hela and SiHa cells significantly inhibited cell proliferation and migration and induced apoptosis in vitro. We also report that CXCL5 is a direct target of miR-577. Additionally, transfection of miR-577 mimics can inhibit CXCL5 protein expression, but not mRNA in Hela cells. miR-577 mimic transfection significantly inhibits migration and induces apoptosis in Hela and SiHa cells. However, the antineoplastic activities of miR-577 are reversed by overexpression of CXCL5 in vitro. Conclusions: Overexpression of CXCL5 is involved in tumor development of cervical cancer. Inhibition of CXCL5 by its post-transcriptional regulator, miR-577, may provide a promising therapeutic strategy for patients with cervical cancer.
Keywords: CXCL5, cervical cancer, miR-577, prognosis, oncogene
Introduction
C-X-C motif chemokine ligand 5 (CXCL5) is an important chemokine and has been reported to accelerate tumor growth, metastasis and angiogenesis [1,2]. Compared with normal tissues or para-carcinoma tissues, overexpression of CXCL5 has been disclosed in more than fourteen different cancer tissues [3]. Some researchers reveal that CXCL5 is secreted by tumor cells into the tumor microenvironment and confers the malignant phenotypes on tumor cells [4-6] by mediating multiple signaling pathways, including epithelial-mesenchymal transition, GSK3β/β-catenin and ERK/MSK1/Elk-1/snail [7-9]. However, the clinical significance and underlying molecular mechanisms of CXCL5 have not been completely explored in the progression of cervical cancer.
microRNAs (miRs), a class of non-coding RNAs, are approximately 22 nucleotides in length, and are involved in post-transcriptional and epigenetic regulation by partial or entire complementary base pairing with the 3’-untranslated regions (3’-UTR) of target mRNA [10,11]. To our knowledge, dysregulation of miRs expression is observed in the overwhelming majority of human malignant tumors [10,12,13]. Numerous miR studies are devoted to elaborating the function of each miR in the pathogenesis of cervical cancer [14-16]. For example, miR-106b-5p and miR-146b-3p are up-regulated and exacerbate cervical cancer progression [16,17]. However, miR-134-5p and miR-144-3p are down-regulated and serve as tumor-suppressive miRNAs to repress cell growth and metastasis in cervical cancer [18,19]. Herein, we aimed to explore whether CXCL5 can be regulated by miRs in the progression of cervical cancer.
miR-577 has been identified as a tumor suppressor in various cancers, including prostate cancer, glioblastoma, and colorectal cancer [20-22]. In cervical cancer, the abrogation of miR-577 facilitates proliferation and migration of cervical cancer cells [14]. Our results indicate that miR-577 is decreased and negatively correlated with CXCL5 in cervical cancer. CXCL5 is identified as a direct target of miR-577. The knockdown of CXCL5 or overexpression of miR-577 can inhibit proliferation and migration and induces apoptosis of cervical cancer cells in vitro.
Material and methods
Clinical specimens
Sixty-one pairs of cervical cancer specimens and para-carcinoma tissues were collected from the First Affiliated Hospital of the Air Force Medical University of PLA (Xi’an, China) to evaluate miR-577 and CXCL5 expression. Informed consent was obtained from the patients. This study was authorized by the Ethics Committee of the First Affiliated Hospital of the Air Force Medical University of PLA.
Cell culture and transfection
Human normal cervical squamous cell line Ect1/E6E7 (CHI Scientific, Inc., Maynard, MA, USA) and cervical cancer cell lines Hela and SiHa (ATCC, USA) were maintained in DMEM; (Gibco, USA) containing 10% FBS (Gibco, USA) with 5% CO2 at 37°C. miR-Con, miR-577 mimics, miR-577-Mut, and overexpressed CXCL5 plasmids were purchased from GenePharm (Shanghai, China) and transfected into Hela and SiHa cells using lipofectamine 2000 (Invitrogen).
Immunohistochemical (IHC) staining and histologic scoring
IHC analysis was performed to evaluate CXCL5-positive staining in cervical cancer specimens and para-carcinoma tissues. Primary antibody to CXCL5 (dilution: 1:100; R&D Systems, MN, USA) was used to incubate paraffin sections. Histologic scoring was evaluated by two pathologists as described previously [23].
Western blot
Western blot was performed to analyze CXCL5 protein expression as described previously [23]. CXCL5 primary antibody (dilution: 1:1000; R&D Systems) was used to incubate the protein membrane.
Cell proliferation, migration and apoptosis assays
Cell viability was analyzed using a cell counting kit-8 (CCK-8; Dojindo, Japan) as described previously [23]. Cell migration was detected using the transwell chamber (8 μ pore size) without the Matrigel matrix. Cell apoptosis was evaluated using TUNEL staining (Roche) as described previously [24]. Cells with TUNEL-positive staining (red) indicated apoptosis.
Luciferase reporter assay
Wild-type (WT) and mutant-type (Mut) 3’-UTR of CXCL5 were obtained from GenePharm (Shanghai, China) and inserted into the multiple cloning sites of the luciferase-expressing pMIR-REPORT vector (Ambion). Hela cells were co-transfected with the WT or Mut 3’-UTR of CXCL5 (0.5 μg) into miR-con, miR-577 mimic (100 nm) or miR-577-mut transfected cells. The luciferase activity was measured using a luciferase reporter assay kit (Promega Corporation, Madison, WI, USA).
Statistical analysis
Data are shown as mean ± standard deviation. The student t-test (or Mann-Whitney U test) was used to analyze two-group differences. Inter-group differences were analyzed by one-way ANOVA. Overall survival was performed using the Kaplan-Meier method with the log-rank test. Pearson correlation analysis was performed to evaluate the correlation between miR-577 and CXCL5 in cervical cancer tissues. SPSS (version 23.0) was used to perform statistical analysis. A p-value < 0.05 was considered significant.
Results
CXCL5 is up-regulated in cervical cancer tissues and cell lines
IHC staining uncovered that CXCL5 positive staining is significantly elevated in cervical tissues compared with corresponding para-carcinoma tissues (Figure 1A and 1B). Compared with human normal cervical squamous cell line Ect1/E6E7, CXCL5 protein expression exhibits a significant increase in Hela and SiHa cells (Figure 1C and 1D).
Figure 1.
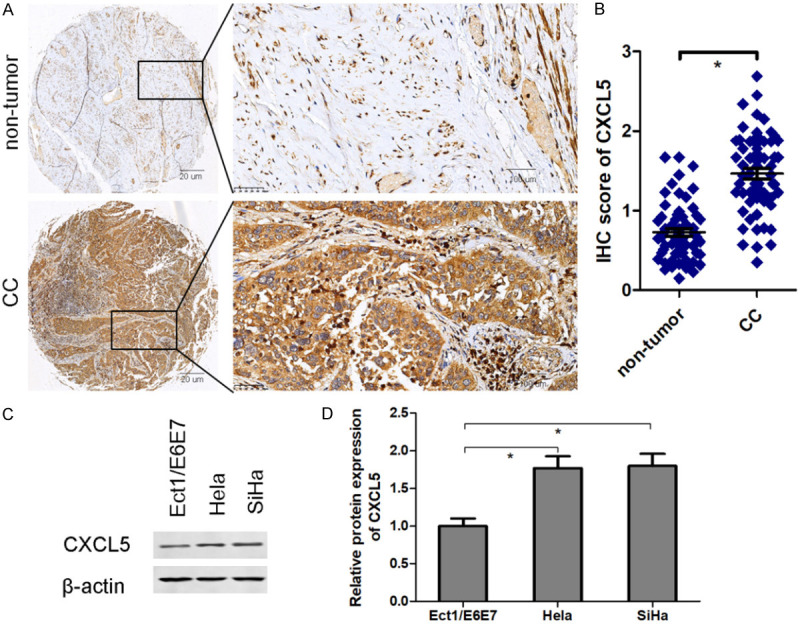
CXCL5 is up-regulated in cervical cancer tissues and cell lines. IHC staining was performed to analyze CXCL5 positive staining in cervical cancer tissues and para-carcinoma tissues (A: left: magnification 40×; right: magnification 200×) and quantitative analysis (B). Western blot was used to measure CXCL5 protein expression in human normal cervical squamous cell line Ect1/E6E7 and cervical cancer cell lines Hela and SiHa (C and D). *P < 0.05. CC, cervical cancer.
High CXCL5 expression is correlated with poor overall survival of cervical cancer patients
High CXCL5 expression (n = 37) in cervical cancer is defined as 2-fold higher than that of in para-carcinoma tissues. Following the Kaplan-Meier method with log-rank test, cervical cancer patients with high CXCL5 expression have shorter overall survival than those of patients with low CXCL5 expression (n = 24) (Figure 2A). In addition, FIGO stage (Figure 2B), lymph node metastasis (Figure 2C), and invasion depth (Figure 2D) are related to OS. Our findings suggest that CXCL5 is a poor prognostic indicator of cervical cancer.
Figure 2.
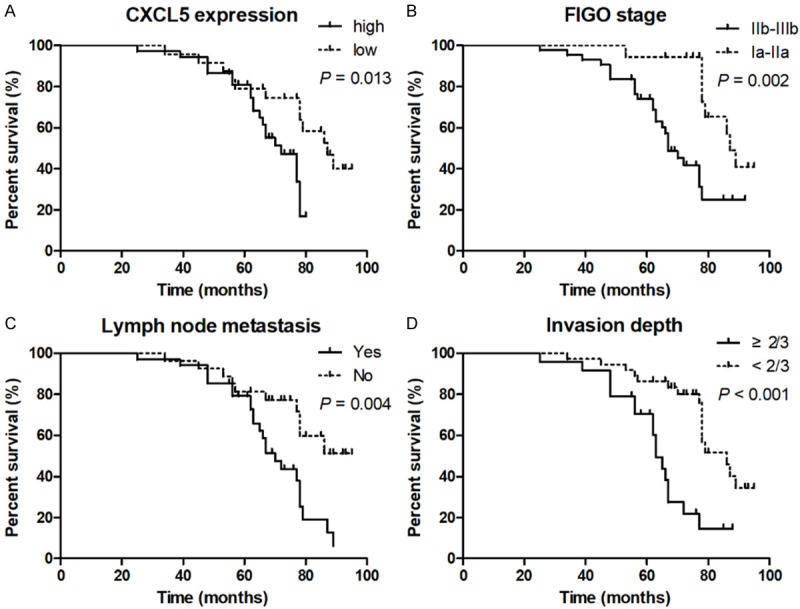
High CXCL5 expression is correlated with poor overall survival of cervical cancer patients. Cervical cancer patients were divided into two groups: high CXCL5 expression (n = 37) and low CXCL5 expression (n = 24). Cervical cancer patients with high CXCL5 expression are associated with shorter overall survival (A), poorer FIGO stage (B), lymph node metastasis (C), and deeper invasion depth (D).
Inhibition of CXCL5 impedes cervical cancer cell proliferation and migration in vitro
After following specific si-CXCL5 transfection, cell growth, migration and apoptosis were analyzed using CCK-8, transwell assay and TUNEL staining in Hela and SiHa cells. CCK-8 analysis revealed that CXCL5 knockdown leads to a significant inhibition of the proliferation rate of Hela and SiHa cells (Figure 3A and 3B). Compared with si-Con transfection, transfection with si-CXCL5 into Hela and SiHa cells significantly represses cell migration in vitro (Figure 3C and 3D). As shown in Figure 3E and 3F, the silencing of CXCL5 significantly increases TUNEL-positive staining cells, suggesting that suppression of CXCL5 can induce cervical cancer cell apoptosis.
Figure 3.

CXCL5 inhibition impedes the tumorigenicity of cervical cancer in vitro. After si-CXCL5 transfection, cell growth, migration and apoptosis were analyzed using CCK-8 (A and B), transwell assay (C and D) and TUNEL staining (E and F) in cervical cancer cell lines Hela and SiHa cells. n = 3 in each group. *P < 0.05.
miR-577 directly targets CXCL5
Based on online bioinformatics software, miR-577 can bind with the 3’-UTR of CXCL5 (Figure 4A). To further validate whether miR-577 directly targets CXCL5, a luciferase reporter gene was constructed containing either CXCL5 WT-type or Mut-type 3’-UTR. After transfection with miR-577 mimics, but not the miR-577-Mut sequence, luciferase reporter activity is significantly decreased in WT-type cells. However, transfection with miR-577 mimics or miR-577-Mut sequence has no obvious effect on luciferase reporter activity in Mut-type cells (Figure 4B). RT-qPCR results suggested that transfection with miR-577 mimics or miR-577-Mut sequence has no impact on CXCL5 mRNA expression (Figure 4C), but CXCL5 protein expression is significantly inhibited following miR-577 mimic transfection, but not miR-577-Mut, in Hela cells (Figure 4D and 4E), suggesting that miR-557 functions as a post-transcriptional regulator to directly target CXCL5.
Figure 4.
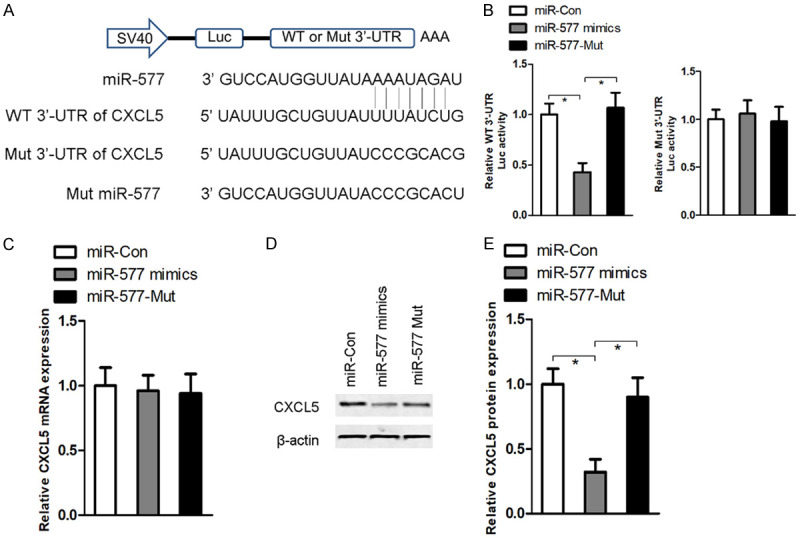
CXCL5 is a direct target of miR-77. Based on online bioinformatics software, miR-577 can bind with the 3’-UTR of CXCL5 (A). A luciferase reporter gene was constructed containing either a CXCL5 WT-type or Mut-type 3’-UTR of the miR-577 binding site (B). After transfection with miR-577 mimics or miR-577-Mut sequence into Hela cells, RT-qPCR (C) and western blot (D and E) were performed to measure CXCL5 mRNA and protein expression. n = 3 in each group. *P < 0.05.
miR-577 is decreased in cervical cancer tissues
miR-577 expression levels were measured using RT-qPCR assay in sixty-one pairs of cervical cancer and para-carcinoma tissues. Our results indicated that miR-577 expression levels are significantly decreased in cervical cancer tissues compared with para-carcinoma tissues (Figure 5A). Interestingly, Pearson correlation analysis revealed that miR-577 expression is inversely correlated with CXCL5 protein expression in cervical cancer tissues (Figure 5B).
Figure 5.
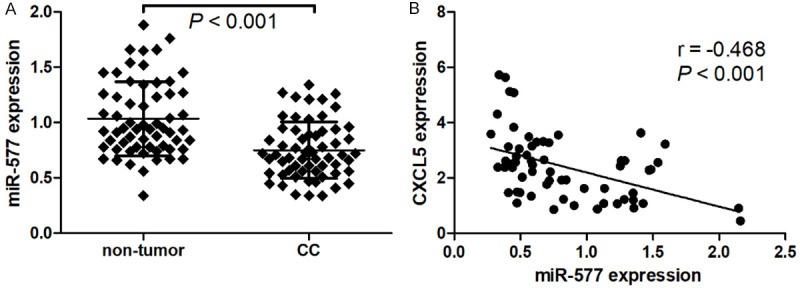
miR-577 is decreased in cervical cancer tissues. miR-577 expression levels were measured using RT-qPCR assay in sixty-one pairs of cervical cancer and para-carcinoma tissues (A). Pearson correlation analysis was performed to evaluate the correlation between miR-577 and CXCL5 in cervical cancer tissues (B).
Overexpression of CXCL5 restrains miR-577-induced migratory inhibition and apoptosis
After transfection with miR-577 mimics into Hela and SiHa cells, cell migration is significantly inhibited (Figure 6A and 6B), and cell apoptosis is significantly elevated in vitro (Figure 6C and 6D). However, co-expression of CXCL5 and miR-577 mimics in Hela and SiHa cells, by miR-577 mimic transfection results in migratory inhibition. Apoptosis is neutralized by overexpression of CXCL5, reflecting that CXCL5 is a downstream target of miR-577 to regulate migration and apoptosis of cervical cancer cells.
Figure 6.
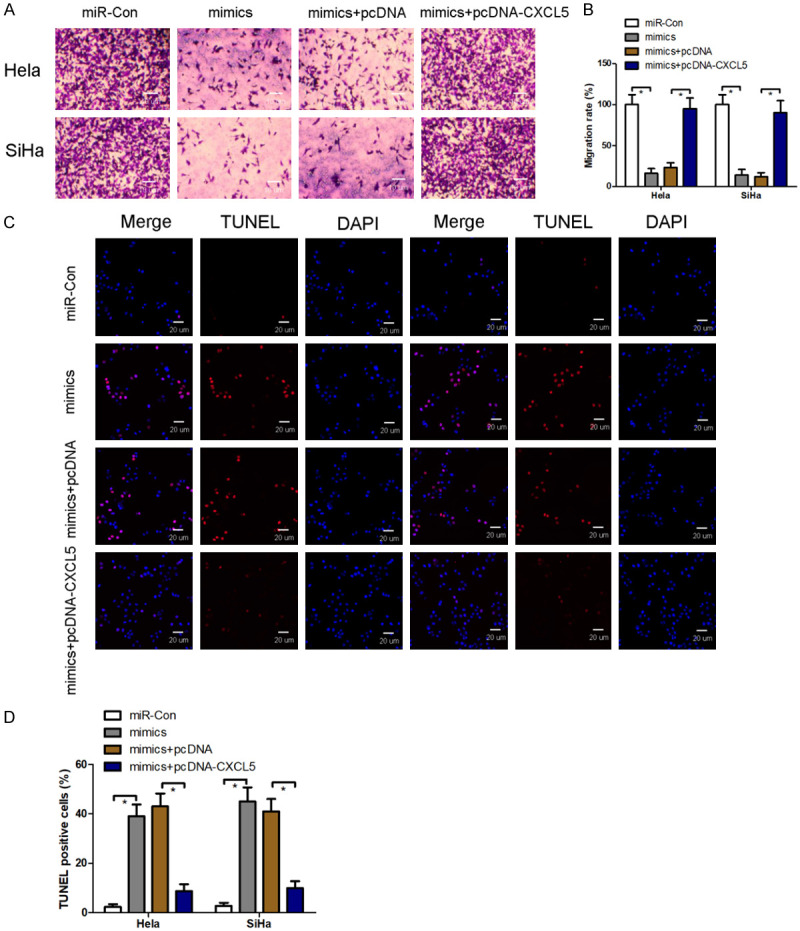
Overexpression of CXCL5 restrains miR-577-induced migratory inhibition and apoptosis. After co-transfection with miR-577 mimics and overexpressed CXCL5 plasmids into Hela and SiHa cells, cell migration and apoptosis were analyzed using transwell assay (A and B) and TUNEL staining (C and D). n = 3 in each group. *P < 0.05.
Discussion
In our study, experimental evidence suggests that CXCL5 is a direct target of miR-577 and is implicated in the progression of cervical cancer. Overexpression of CXCL5 is observed in cervical cancer tissues and cell lines. CXCL5 loss-of-function significantly inhibits cell proliferation and migration and induces apoptosis in Hela and SiHa cells. These findings suggest that CXCL5 may serve as a cancerogenic action in cervical cancer progression.
Previous studies exhibit that CXCL5 functions as an oncogene in diverse human malignancies [1,8,25,26]. For example, serum CXCL5 and tumorous CXCL5 are markedly higher in nasopharyngeal carcinoma (NPC) patients than those of in the corresponding control group [25]. CXCL5 knockdown inhibits NPC cell growth in vivo and in vitro and impedes cell migration and invasion in vitro [25]. IHC staining confirms that CXCL5 protein expression is overexpressed in tumor specimens and positively correlated with poor tumor stages and prognosis in patients with colorectal cancer [8]. CXCL5 recombinant protein promotes breast cancer cell proliferation and metastasis to bone [1]. A comprehensive meta-analysis, including 19 cohorts and 5070 patients, highlights that elevation of CXCL5 may be an adverse prognostic marker for poor overall survival, progression-free survival, and recurrence-free survival [26]. Consistent with these previous studies [1,8,25,26], our study found that cervical cancer patients with high CXCL5 expression get poor overall survival, high FIGO stage, lymph node metastasis, and tissue invasiveness. Based on high CXCL5 expression in cervical cancer cells, specific siRNA was established to silence CXCL5 expression in in vitro experiments. After transfection with si-CXCL5 into Hela and SiHa cells, the migratory and invasive abilities of cervical cancer cells are significantly inhibited, and cell apoptosis is enhanced in vitro. These findings suggest that overexpression of CXCL5 possesses cancer-promoting roles in cervical cancer.
Usually, miR mediates multiple targets to modulate pathophysiologic processes [27,28]. Overexpression of miR-577 acutely represses the invasiveness and EMT of breast cancer by regulating Rab25 [29]. miR-577 exacerbates metastasis and chemoresistance of gastric cancer by targeting SDPR [30]. The knockdown of miR-577 restores RAB14 to preserve the oncological behavior of cervical cancer [14]. Herein, we report that CXCL5 is a target of miR-577. Additionally, post-transcriptional mechanistic investigation corroborates that miR-577 can inhibit CXCL5 protein expression, but not mRNA. In mammals, miRs preferentially regulate protein translation by partially binding with the 3’-UTR of mRNA [31,32]. miRs may have not the function of degrading mRNA, but the binding sites can prevent mRNA translation to reduce protein synthesis [31,32]. miR-577 mimic transfection significantly inhibits migration and induces apoptosis in Hela and SiHa cells. However, the antineoplastic activities of miR-577 are reversed by overexpression of CXCL5 in vitro. These results reveal that the miR-577/CXCL5 signaling axis contributes to the pathogenesis of cervical cancer.
In conclusion, our results exhibit that overexpression of CXCL5 is an aggressiveness factor for cervical cancer. We also present a novel signaling pathway of miR-577/CXCL5 to mediate cervical cancer cell migration and apoptosis. Inhibition of CXCL5 by its post-transcriptional regulator, miR-577, may provide a promising therapeutic strategy for cervical cancer patients.
Disclosure of conflict of interest
None.
References
- 1.Romero-Moreno R, Curtis KJ, Coughlin TR, Miranda-Vergara MC, Dutta S, Natarajan A, Facchine BA, Jackson KM, Nystrom L, Li J, Kaliney W, Niebur GL. The CXCL5/CXCR2 axis is sufficient to promote breast cancer colonization during bone metastasis. Nat Commun. 2019;10:4404. doi: 10.1038/s41467-019-12108-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roca H, Jones JD, Purica MC, Weidner S, Koh AJ, Kuo R, Wilkinson JE, Wang Y, Daignault-Newton S, Pienta KJ, Morgan TM, Keller ET, Nör JE, Shea LD, McCauley LK. Apoptosis-induced CXCL5 accelerates inflammation and growth of prostate tumor metastases in bone. J Clin Invest. 2018;128:248–266. doi: 10.1172/JCI92466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang W, Wang H, Sun M, Deng X, Wu X, Ma Y, Li M, Shuoa SM, You Q, Miao L. CXCL5/CXCR2 axis in tumor microenvironment as potential diagnostic biomarker and therapeutic target. Cancer Commun (Lond) 2020;40:69–80. doi: 10.1002/cac2.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Z, Zhou J, Zhang J, Li S, Wang H, Du J. Cancer-associated fibroblasts promote PD-L1 expression in mice cancer cells via secreting CXCL5. Int J Cancer. 2019;145:1946–1957. doi: 10.1002/ijc.32278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forsthuber A, Lipp K, Andersen L, Ebersberger S, Graña C, Ellmeier W, Petzelbauer P, Lichtenberger BM, Loewe R. CXCL5 as regulator of neutrophil function in cutaneous melanoma. J Invest Dermatol. 2019;139:186–194. doi: 10.1016/j.jid.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Zhang D, Zhou J, Tang D, Zhou L, Chou L, Chou KY, Tao L, Lu LM. Neutrophil infiltration mediated by CXCL5 accumulation in the laryngeal squamous cell carcinoma microenvironment: a mechanism by which tumour cells escape immune surveillance. Clin Immunol. 2017;175:34–40. doi: 10.1016/j.clim.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Mao Z, Zhang J, Shi Y, Li W, Shi H, Ji R, Mao F, Qian H, Xu W, Zhang X. CXCL5 promotes gastric cancer metastasis by inducing epithelial-mesenchymal transition and activating neutrophils. Oncogenesis. 2020;9:63. doi: 10.1038/s41389-020-00249-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao J, Ou B, Han D, Wang P, Zong Y, Zhu C, Liu D, Zheng M, Sun J, Feng H, Lu A. Tumor-derived CXCL5 promotes human colorectal cancer metastasis through activation of the ERK/Elk-1/Snail and AKT/GSK3β/β-catenin pathways. Mol Cancer. 2017;16:70. doi: 10.1186/s12943-017-0629-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu YL, Hou MF, Kuo PL, Huang YF, Tsai EM. Breast tumor-associated osteoblast-derived CXCL5 increases cancer progression by ERK/MSK1/Elk-1/snail signaling pathway. Oncogene. 2013;32:4436–4447. doi: 10.1038/onc.2012.444. [DOI] [PubMed] [Google Scholar]
- 10.Png KJ, Halberg N, Yoshida M, Tavazoie SF. A microRNA regulon that mediates endothelial recruitment and metastasis by cancer cells. Nature. 2011;481:190–194. doi: 10.1038/nature10661. [DOI] [PubMed] [Google Scholar]
- 11.Cheng CJ, Bahal R, Babar IA, Pincus Z, Barrera F, Liu C, Svoronos A, Braddock DT, Glazer PM, Engelman DM, Saltzman WM, Slack FJ. MicroRNA silencing for cancer therapy targeted to the tumour microenvironment. Nature. 2015;518:107–110. doi: 10.1038/nature13905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16:203–222. doi: 10.1038/nrd.2016.246. [DOI] [PubMed] [Google Scholar]
- 13.He L, He X, Lim LP, de Stanchina E, Xuan Z, Liang Y, Xue W, Zender L, Magnus J, Ridzon D, Jackson AL, Linsley PS, Chen C, Lowe SW, Cleary MA, Hannon GJ. A microRNA component of the p53 tumour suppressor network. Nature. 2007;447:1130–1134. doi: 10.1038/nature05939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang J, Liang B, Hou S. TMPO-AS1 promotes cervical cancer progression by upregulating RAB14 via sponging miR-577. J Gene Med. 2019;21:e3125. doi: 10.1002/jgm.3125. [DOI] [PubMed] [Google Scholar]
- 15.Pardini B, De Maria D, Francavilla A, Di Gaetano C, Ronco G, Naccarati A. MicroRNAs as markers of progression in cervical cancer: a systematic review. BMC Cancer. 2018;18:696. doi: 10.1186/s12885-018-4590-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yi Y, Liu Y, Wu W, Wu K, Zhang W. The role of miR-106p-5p in cervical cancer: from expression to molecular mechanism. Cell Death Discov. 2018;4:36. doi: 10.1038/s41420-018-0096-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yao S, Xu J, Zhao K, Song P, Yan Q, Fan W, Li W, Lu C. Down-regulation of HPGD by miR-146b-3p promotes cervical cancer cell proliferation, migration and anchorage-independent growth through activation of STAT3 and AKT pathways. Cell Death Dis. 2018;9:1055. doi: 10.1038/s41419-018-1059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu J, Zhao Y, Li F, Qiao B. MiR-144-3p: a novel tumor suppressor targeting MAPK6 in cervical cancer. J Physiol Biochem. 2019;75:143–152. doi: 10.1007/s13105-019-00681-9. [DOI] [PubMed] [Google Scholar]
- 19.Jin X, Chen X, Hu Y, Ying F, Zou R, Lin F, Shi Z, Zhu X, Yan X, Li S, Zhu H. LncRNA-TCONS_00026907 is involved in the progression and prognosis of cervical cancer through inhibiting miR-143-5p. Cancer Med. 2017;6:1409–1423. doi: 10.1002/cam4.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li T, Xing Y, Yang F, Sun Y, Zhang S, Wang Q, Zhang W. LncRNA SNHG3 sponges miR-577 to up-regulate SMURF1 expression in prostate cancer. Cancer Med. 2020;9:3852–3862. doi: 10.1002/cam4.2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang W, Shen C, Li C, Yang G, Liu H, Chen X, Zhu D, Zou H, Zhen Y, Zhang D, Zhao S. miR-577 inhibits glioblastoma tumor growth via the Wnt signaling pathway. Mol Carcinog. 2016;55:575–585. doi: 10.1002/mc.22304. [DOI] [PubMed] [Google Scholar]
- 22.Jiang H, Ju H, Zhang L, Lu H, Jie K. microRNA-577 suppresses tumor growth and enhances chemosensitivity in colorectal cancer. J Biochem Mol Toxicol. 2017;31 doi: 10.1002/jbt.21888. [DOI] [PubMed] [Google Scholar]
- 23.Wang F, Ying HQ, He BS, Pan YQ, Deng QW, Sun HL, Chen J, Liu X, Wang SK. Upregulated lncRNA-UCA1 contributes to progression of hepatocellular carcinoma through inhibition of miR-216b and activation of FGFR1/ERK signaling pathway. Oncotarget. 2015;6:7899–7917. doi: 10.18632/oncotarget.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Q, Wang W, Cui J. Melatonin enhances TNF-α-mediated cervical cancer HeLa cells death via suppressing CaMKII/Parkin/mitophagy axis. Cancer Cell Int. 2019;19:58. doi: 10.1186/s12935-019-0777-2. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Qiu WZ, Zhang HB, Xia WX, Ke LR, Yang J, Yu YH, Liang H, Huang XJ, Liu GY, Li WZ, Xiang YQ, Kang TB, Guo X, Lv X. The CXCL5/CXCR2 axis contributes to the epithelial-mesenchymal transition of nasopharyngeal carcinoma cells by activating ERK/GSK-3β/snail signalling. J Exp Clin Cancer Res. 2018;37:85. doi: 10.1186/s13046-018-0722-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu B, Fan H, Lv X, Chen S, Shao Z. Prognostic significance of CXCL5 expression in cancer patients: a meta-analysis. Cancer Cell Int. 2018;18:68. doi: 10.1186/s12935-018-0562-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He Y, Hwang S, Cai Y, Kim SJ, Xu M, Yang D, Guillot A, Feng D, Seo W, Hou X, Gao B. MicroRNA-223 ameliorates nonalcoholic steatohepatitis and cancer by targeting multiple inflammatory and oncogenic genes in hepatocytes. Hepatology. 2019;70:1150–1167. doi: 10.1002/hep.30645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee GJ, Hyun S. Multiple targets of the microRNA miR-8 contribute to immune homeostasis in Drosophila. Dev Comp Immunol. 2014;45:245–251. doi: 10.1016/j.dci.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 29.Yin C, Mou Q, Pan X, Zhang G, Li H, Sun Y. MiR-577 suppresses epithelial-mesenchymal transition and metastasis of breast cancer by targeting Rab25. Thorac Cancer. 2018;9:472–479. doi: 10.1111/1759-7714.12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo Y, Wu J, Wu Q, Li X, Wu J, Zhang J, Rong X, Rao J, Liao Y, Bin J, Huang N, Liao W. miR-577 regulates TGF-β induced cancer progression through a SDPR-modulated positive-feedback loop with ERK-NF-κB in gastric cancer. Mol Ther. 2019;27:1166–1182. doi: 10.1016/j.ymthe.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaudet AD, Fonken LK, Watkins LR, Nelson RJ, Popovich PG. MicroRNAs: roles in regulating neuroinflammation. Neuroscientist. 2018;24:221–245. doi: 10.1177/1073858417721150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu L, Deng H, Hu J, Huang S, Xiong J, Deng J. The promising role of miR-296 in human cancer. Pathol Res Pract. 2018;214:1915–1922. doi: 10.1016/j.prp.2018.09.026. [DOI] [PubMed] [Google Scholar]


