Abstract
Breast mucoepidermoid carcinoma (MEC) is clinically rare, with an estimated incidence of 0.2-0.3% of all primary breast tumors. To date, only 41 cases have been reported in the literature. Herein, we present a case of breast MEC diagnosed at our hospital. The clinicopathologic features were preliminarily discussed by reviewing the literature. A 42-year-old Chinese woman presented with a lump in her right breast that was detected approximately three months prior. A microscopic examination showed that the breast MEC was composed of different proportions of mucinous cells, intermediate cells, and epidermoid cells. Most mucinous cells were positive for cytokeratin 7, while the epidermoid and intermediate cells were positive for p63 and cytokeratin 5/6. All tumor cells were negative for other myoepithelial markers, such as calponin. Tumor cells did not express estrogen, progesterone, or the HER-2/neu protein. After the patient underwent mastectomy, she was diagnosed with a low-grade mucoepidermoid carcinoma based on the clinical, histologic, and immuno-phenotypic characteristics. Our findings provide further insight into the pathologic mechanism of MEC, as correct diagnosis is essential for patient management.
Keywords: Breast, mucoepidermoid carcinoma, pathologic morphology, immunohistochemistry
Introduction
Breast cancers are the most common type of malignant tumor among Chinese women, of which the most common subtype is nonspecific invasive carcinoma. Salivary gland-like tumors of the breast are extremely rare and resemble primary salivary gland tumors. Mucoepidermoid carcinoma (MEC) is rarely diagnosed in the breast, and due to its rarity, the understanding of its clinicopathologic features is limited. The estimated incidence is 0.2 to 0.3% of all breast tumors [1]. Herein, we report a new case of MEC arising in the breast. We review the clinicopathologic correlation of 41 cases with breast MEC reported in English literature between 1979 and 2020. Our aim was to deepen the understanding of this rare, primary tumor subtype in order to improve its diagnosis.
Materials and methods
Paraffin-embedded tissue samples from the MEC patient were obtained from the Department of Pathology at the Affiliated Hospital of Southwest Medical University in December 2018. The diagnosis was confirmed by histopathology. The patient’s clinicopathologic data, including age, tumor size, gender, tumor grade, operative approach, and follow-up information, were also obtained. The specimens were fixed with 4% formaldehyde solution, and paraffin embedding was performed routinely. After enzymatic digestion, continuous sections were performed and stained using hematoxylin, eosin, and Alcian blue (AB) (pH 2.5). For immunohistochemistry, a traditional EnVision method was used. All procedures were supervised and granted approval by the Ethics Committee of the Affiliated Hospital of Southwest Medical University (KY2019242).
Results
Clinical findings
A 42-year-old Chinese woman was hospitalized with complaints of a painful mass in her right breast that was detected three months prior. On physical examination, a well-circumscribed mass was detected by palpation of the outer and lower quadrant of the right breast. No nipple discharge or additional abnormalities were observed. The physical examination of the left breast was normal. Upon axillary examination, lymph nodes were not detected. Additionally, the patient had no family history of breast carcinoma. The patient developed menarche at age 15. Breast ultrasonography reported an irregular 2.6×1.8 cm cystic and solid lesion of the right breast (Figure 1) with poor sound transmission in the dark area, thick walls, and visible light band separation. The lesion was considered likely to be benign. Therefore, the patient underwent a lumpectomy. Half a year later, the patient underwent an improved radical mastectomy on the right side of the breast and axillary lymph node dissection at another hospital. There was no axillary lymph node metastasis, and no surgical complications were identified. After surgery, the patient took oral chemotherapy, of which the details are unknown. At the 12-month follow-up, the patient did not present with any local or distant recurrence.
Figure 1.
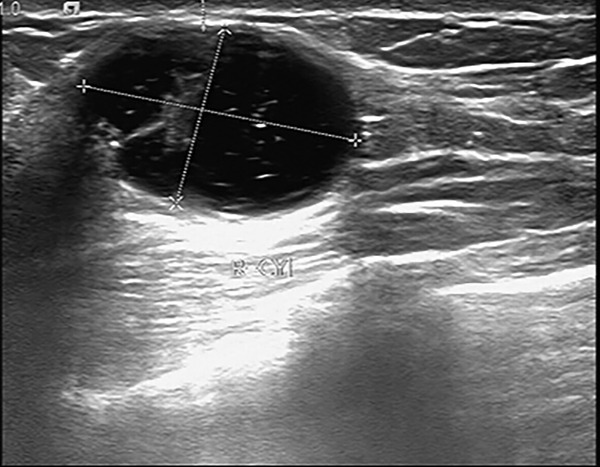
Ultrasonography showing a cystic lesion of the right breast.
Histopathological findings
Visual inspection of the excised tumor revealed a solid cystic, well-circumscribed lesion with a gray and white cut surface that measured 2.6×1.8×0.8 cm in volume. Microscopically, the tumor was comprised of solid neoplastic nests and cystic cavities filled with mucoid material (Figure 2A). Additionally, the cystic cavities accounted for approximately 30% of the tumor. Similar to salivary glands, the breast MEC was formed of different proportions of mucinous cells, intermediate cells, and epidermoid cells (Figure 2B). The neoplastic nests consisted largely of epidermoid cells. Mucinous cells were round-to-oval in shape, the cytoplasm contained mucoid vacuoles, and their nuclei were round and had coarse chromatin. Intermediate cells were few, small, and round in shape, with unclear boundaries, oval nuclei, and small nucleoli. Epidermoid cells were larger in size, polygonal in shape, had clear boundaries, and abundant eosinophilic cytoplasm. Keratosis was not observed. All the tumor cells lacked cell atypia. Furthermore, mitotic activity was scanty, and necrosis was absent. No invasion of nerves or lymphatic vessels was observed.
Figure 2.
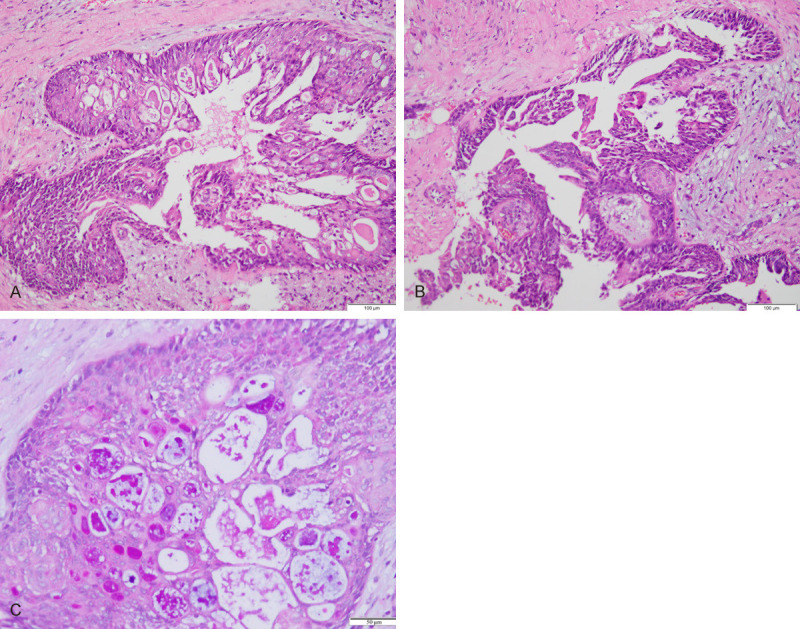
Histopathologic features of mucoepidermoid carcinoma of the breast. A. The lesions are constituted by solid neoplastic nests and scattered cystic spaces filled with mucoid material; (×100). B. Three cell types were observed in mucoepidermoid carcinoma. Intermediate cells are on the left (red arrow); epidermoid cells are localized centrally (black arrow); (×100). C. AB-PAS stains show numerous mucinous cells in the invasive component (The arrow points to mucoid material); (×100).
Immunohistochemical findings
For breast MEC, each type of tumor cell has its own unique immune markers. Thus, immunohistochemistry was utilized to help identify cell types. Most intermediate and epidermoid cells were positive for p63 (Figure 3A) and cytokeratin 5/6. Mucinous cells strongly expressed cytokeratin 7 (Figure 3B). Myoepithelial markers, such as calponin were negative across all tumor cells (Figure 3C). Tumor cells did not express estrogen, progesterone, or the HER-2/neu protein. The mucoid material that filled cysts was positive for AB-PAS (Figure 2C). Additionally, 10% of tumor cells were positive for Ki-67 and p53.
Figure 3.
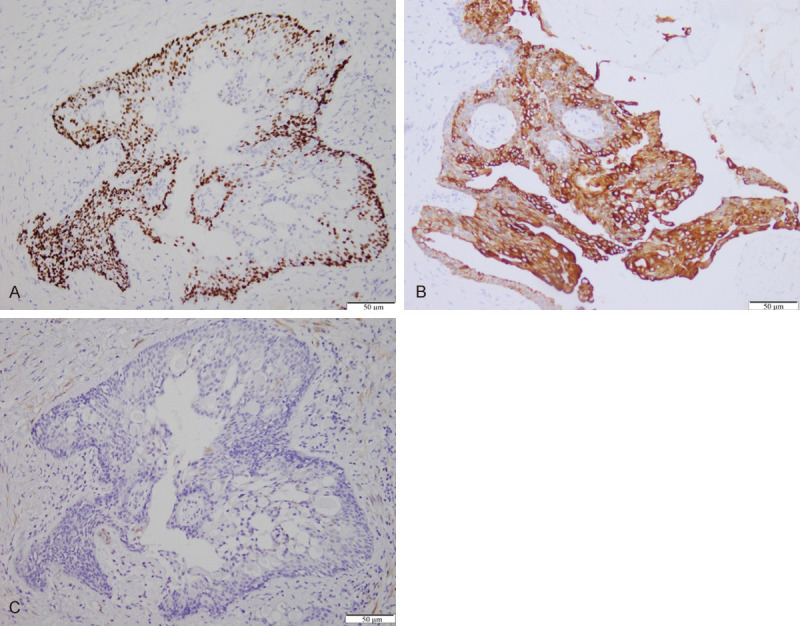
Immunohistochemistry of mucoepidermoid breast carcinoma. A. Epidermoid and intermediate cells are positive for p63, whereas mucinous cells around the microcysts and toward the cystic lumen are mostly negative (The arrow points to a positive stain); (×100). B. Glandular cells are positive for CK 7 (The arrow points to a positive stain); (×200). C. Tumor cells are negative for calponin; (×100).
Pathological diagnosis
Combining histopathologic and immunohistochemical results, this patient was diagnosed with a case of breast MEC with no evidence of lymphatic or vascular permeation. There were no tumor cells found in surgical margins. According to the Elston and Ellis grading system [2], this case was classified as low-grade.
Discussion
First described by Foote et al. [3] in 1945, MEC is a malignant tumor that occurs in the salivary glands. It can also occur in the lungs, bronchus, esophagus, thyroid, and other parts [4-6]. In 1979, Patchefsky et al. were the first to report primary MEC of the breast [7]. MEC belongs to the salivary gland-like neoplasms group. It is exceedingly rare and has an estimated incidence of 0.2% to 0.3% of all breast tumors. Some authors believe that the true incidence is higher than that reported in literature as some cases are misdiagnosed as carcinomas with squamous differentiation [8].
At present, only 41 cases have been reported in English literature. We reviewed all cases and found that all patients were female, aged 29-86 years, and had a maximum tumor diameter ranging from 0.5 to 10 cm, with an average of 3.4 cm. Grossly, MEC can present as a solid nodule or a cystic lesion that is well-circumscribed or ill-defined. Table 1 summarizes the clinicopathologic characteristics of the 41 previously reported cases, as well as this case.
Table 1.
Summary of reported cases of breast mucoepidermoid carcinoma from 1979 to 2020
| No. | Author [ref.] | Year | Age (years) | Site | Size (cm) | Grade | Surgical Approach | Lymph node metastasis | Distant Metastasis | Follow-up, (months) | Status |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Present case | 2020 | 42 | Right | 2.6 | LG | MRM | NA | No | 12 | Alive |
| 2 | Mingfei Yan et al. [14] | 2019 | 60 | Right | 1.9 | LG | lumpectomy | NA | No | 60 | Alive |
| 3 | Burghel et al. [15] | 2018 | 73 | Left | NA | LG | NA | 0/2 | No | NA | NA |
| 4 | Sherwell-Cabello et al. [16] | 2017 | 86 | Left | 6 | LG | MRM | NA | No | 3 | Alive |
| 5 | Cheng et al. [13] | 2017 | 39 | Right | 1.5 | LG | MRM | 3/18 | No | 156 | Alive |
| 6 | 49 | Left | 1.5 | LG | MRM | 0/17 | No | 41 | Alive | ||
| 7 | 66 | Left | 1.3 | LG | Mastectomy + SLD | 0/6 (SLD) | No | 9 | Alive | ||
| 8 | 61 | Left | 3 | LG | Mastectomy + SLD | 0/3 (SLD) | No | 4 | Alive | ||
| 9 | Fujino et al. [17] | 2016 | 71 | Right | 1.7 | IG | Mastectomy + SLD | 0/NA | No | NA | NA |
| 10 | Palermo et al. [18] | 2013 | 80 | Right | 4 | HG | NA | 0/NA | No | NA | NA |
| 11 | Turk et al. [19] | 2013 | 40 | Right | 5.5 | NA | MRM | 1/24 | No | 5 | Alive |
| 12 | Basbug et al. [20] | 2011 | 69 | Left | 10 | HG | MRM | 0/12 | No | 12 | Alive |
| 13 | Camelo-Piragua et al. [1] | 2009 | 49 | Right | 4 | IG | MRM | 1/3 | No | 8 | Alive |
| 14 | Hornychova et al. [9] | 2007 | 63 | Right | 1.8 | HG | SM + LND | 0/17 | No | 18 | Alive |
| 15 | 30 | Left | 8 | LG | MRM | 0/NA | No | 60 | Alive | ||
| 16 | Horii et al. [21] | 2006 | 54 | Left | 2.5 | LG | Mastectomy + LND | 0/NA | No | 36 | Alive |
| 17 | Gomez-Aracil et al. [22] | 2006 | 69 | Right | 7.5 | HG | MRM + LND | 24/28 | No | 54 | Alive |
| 18 | Di Tommaso et al. [23] | 2004 | 80 | Left | 0.5 | LG | Excision | NA | No | 5 | Alive |
| 19 | 29 | Left | 0.8 | LG | Excision | NA | No | 90 | Alive | ||
| 20 | 54 | Left | 1.5 | LG | Quadrantectomy + LND | NA | No | 13 | Alive | ||
| 21 | 55 | Left | 1.1 | IG | Quadrantectomy + LND | NA | No | 3 | Alive | ||
| 22 | 36 | Left | 0.6 | HG | Quadrantectomy + LND | NA | No | 18 | Alive | ||
| 23 | Terzi et al. [24] | 2004 | 79 | Right | 8 | HG | MRM | 4/14 | No | NA | NA |
| 24 | Tjalma et al. [25] | 2002 | 58 | Right | 3.5 | HG | RM | 1/17 | Yes | 156 | Alive |
| 25 | Berry et al. [26] | 1998 | 51 | Left | 3.5 | HG | Mastectomy + LND | 0/NA | No | NA | NA |
| 26 | Markopoulos et al. [27] | 1998 | 40 | Right | 2 | HG | Wide local excision + LND | 0/NA | No | 60 | Alive |
| 27 | Chang et al. [28] | 1998 | 54 | Left | 4.5 | HG | MRM | 0/9 | No | 48 | Alive |
| 28 | Luchtrath and Moll [29] | 1989 | 60 | NA | 5 | HG | RM | 12/18 | Yes, (bone) | 30 | DOD |
| 29 | Pettinato et al. [30] | 1989 | 72 | Right | 7 | HG | MRM | 16/19 | Yes, (lung) | 10 | DOD |
| 30 | Hanna and Kahn [10] | 1985 | 51 | Left | 2 | NA | MRM | 0/NA | No | 8 | Alive |
| 31 | 31 | NA | NA | NA | MRM | 2/18 | No | 14 | Alive | ||
| 32 | Hastrup and Sehested [31] | 1985 | 59 | Left | 1 | HG | RM | 0/4 | Yes, (lung and liver) | 25 | DOD |
| 33 | Leong and Williams [32] | 1985 | 57 | Left | 3.5 | HG | SM | 0/20 | Yes | 7 | DOD |
| 34 | Ratanarapee et al. [33] | 1983 | 27 | NA | NA | HG | NA | 6/15 | Yes | 14 | DOD |
| 35 | Fisher et al. [8] | 1983 | 65 | Right | 2 | LG | Lumpectomy | NA | No | 60 | Alive |
| 36 | 71 | Left | 2 | LG | MRM | 0/19 | No | 48 | Alive | ||
| 37 | 57 | Right | 2.5 | LG | MRM | 0/11 | No | 120 | Alive | ||
| 38 | 49 | Right | 3.7 | LG | RM | 0/13 | No | 108 | Alive | ||
| 39 | 60 | Left | 4 | LG | SM | NA | No | 48 | Alive | ||
| 40 | Kovi et al. [34] | 1981 | 46 | Left | 11 | HG | MRM | 17/19 | NA | NA | NA |
| 41 | Patchefsky et al. [7] | 1979 | 66 | Right | 1.3 | LG | RM | 0/20 | No | 94 | DOR |
| 42 | 70 | Right | 5 | LG | Quadrantectomy | NA | No | 10 | Alive |
Abbreviations: LG = low grade; HG = high grade; IG = intermediate grade; MRM = modified radical mastectomy; SLD = sentinel lymph node; DOD = died of disease; DOR = died of other reasons; SM = simple mastectomy; RM = radical mastectomy; LND = lymph node dissection; NA = not applicable.
Histologically, normal mammary gland tissue and salivary glands are exocrine glands that are composed of tubules and acini that is derived from the embryonic ectoderm and shares a similar cellular composition. Both consist of luminal epithelial cells that are surrounded by myoepithelial cells. These structural similarities generate comparable neoplastic lesions. Thus, breast MEC shares morphologic features and an immunophenotype with salivary gland MEC [9].
Microscopically, several types of cells can be seen in varying proportions including basaloid, intermediate, epidermoid, and mucinous cells. Basaloid cells are typically small and oval. They have oval nuclei and appear at the circumference of neoplastic nests. Mucinous cells are large and have a pale cytoplasm, and their nuclei are located in the margin of neoplastic nests. Intermediate cells are large and have eosinophilic cytoplasm; their nuclei are elliptic, and their nucleoli are small. Epidermoid cells are larger than intermediate cells, polygonal, and have eosinophilic cytoplasm. Their nuclei are round to elliptic, and the cells are rarely keratinized [10]. Low-grade breast MEC mainly consists of mucinous cells, which can account for more than 50% of the tumor. The MEC tumor cells can form irregular sheets and often form cystic cavities of different sizes. Mucous cells can cover the epidermoid cells or be mixed in with the epidermoid cells. High-grade breast MEC consists mainly of intermediate and epidermoid cells, with few mucous cells, the latter of which usually account for less than 10% of the tumor. The neoplastic cells show obvious atypia, with more mitosis, and the tumor can be seen infiltrating into the surrounding tissues.
The immunohistochemistry of breast MEC is unique. It often demonstrates a basal-like and a typically triple-negative immunophenotype, with the absence of estrogen, progesterone, and HER2 receptors [11]. However, unlike other triple-negative breast cancers, they have a better prognosis [12]. We have found reports [13] that tumor cells exhibit lower levels of hormonal receptor expression, but all cases present a good prognosis. CK7 was mainly expressed in cells in the center of the tumor nest and cyst cavity. CK14 was mainly expressed in cells in the outer layers of neoplastic nests. Basaloid and intermediate cells were positive for P63, and were negative for smooth muscle actin and calponin.
Regarding treatment, there is currently no standard therapeutic regimen for breast MEC, probably due to its rarity. Treatment can involve surgery, radiotherapy, and chemotherapy. For patients with high-grade malignancy and strong invasiveness, whole-breast radical surgery and axillary lymph node dissection should be performed. In the case of low-grade malignancy, the tumor must be excised by complete resection of the tumor.
The prognosis of breast MEC is favorable, but the histologic grade is an important prognostic factor. Tumors of high grade have poorer prognosis, while those with low grade are opposite. Yan [14] summarized the characteristics of high-grade MEC, which include rare presence of cystic components (less than 20%), neural invasion, tumor necrosis, four or more mitotic figures per 10 high-power field, and anaplasia. Among the 41 cases we reviewed, there were 16 high-grade cases, 19 low-grade, 3 intermediate, and 3 that had unclear grading. Among those with high-grade MEC, five patients died from the disease within 7 to 30 months after diagnosis. Importantly, each of these 5 cases developed distant metastasis. On the other hand, none of the cases with low-grade MEC in the literature died of breast MEC. Thus, low-grade MEC does not display aggressive behavior, whereas high-grade usually exhibits aggressive behavior, with frequent metastasis to axillary lymph nodes and distant organs.
Conclusion
In summary, breast MEC is extremely rare. Its pathologic characteristics and biologic behavior are different from that of invasive carcinoma. Although most cases present with a triple-negative subtype, a good prognosis is expected.
Acknowledgements
The patient’s informed consent was obtained at the time of publication of this case report and associated images. This study was partly supported by operating research grants from Sichuan Science and Technology Project, no. 2020YJ0494.
Disclosure of conflict of interest
None.
References
- 1.Camelo-Piragua SI, Habib C, Kanumuri P, Lago CE, Mason HS, Otis CN. Mucoepidermoid carcinoma of the breast shares cytogenetic abnormality with mucoepidermoid carcinoma of the salivary gland: a case report with molecular analysis and review of the literature. Hum Pathol. 2009;40:887–892. doi: 10.1016/j.humpath.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 3.Stewart FW, Foote FW, Becker WF. Muco-epidermoid tumors of salivary glands. Ann Surg. 1945;122:820–844. doi: 10.1097/00000658-194511000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huo Z, Wu H, Li J, Li S, Wu S, Liu Y, Luo Y, Cao J, Zeng X, Liang Z. Primary pulmonary mucoepidermoid carcinoma: histopathological and moleculargenetic studies of 26 cases. PLoS One. 2015;10:e0143169. doi: 10.1371/journal.pone.0143169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koide N, Hamanaka K, Igarashi J, Hanazaki K, Adachi W, Hosaka S, Uehara T, Amano J. Co-occurrence of mucoepidermoid carcinoma and squamous cell carcinoma of the esophagus: report of a case. Surg Today. 2000;30:636–642. doi: 10.1007/s005950070104. [DOI] [PubMed] [Google Scholar]
- 6.Lai CY, Chao TC, Lin JD, Hsueh C. Sclerosing mucoepidermoid carcinoma with eosinophilia of thyroid gland in a male patient: a case report and literature review. Int J Clin Exp Pathol. 2015;8:5947–5951. [PMC free article] [PubMed] [Google Scholar]
- 7.Patchefsky AS, Frauenhoffer CM, Krall RA, Cooper HS. Low-grade mucoepidermoid carcinoma of the breast. Arch Pathol Lab Med. 1979;103:196–198. [PubMed] [Google Scholar]
- 8.Fisher ER, Palekar AS, Gregorio RM, Paulson JD. Mucoepidermoid and squamous cell carcinomas of breast with reference to squamous metaplasia and giant cell tumors. Am J Surg Pathol. 1983;7:15–27. doi: 10.1097/00000478-198301000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Hornychova H, Ryska A, Betlach J, Bohac R, Cizek T, Tomsova M, Obermannova R. Mucoepidermoid carcinoma of the breast. Neoplasma. 2007;54:168–172. [PubMed] [Google Scholar]
- 10.Hanna W, Kahn HJ. Ultrastructural and immunohistochemical characteristics of mucoepidermoid carcinoma of the breast. Hum Pathol. 1985;16:941–946. doi: 10.1016/s0046-8177(85)80133-7. [DOI] [PubMed] [Google Scholar]
- 11.Jones C, Ford E, Gillett C, Ryder K, Merrett S, Reis-Filho JS, Fulford LG, Hanby A, Lakhani SR. Molecular cytogenetic identification of subgroups of grade III invasive ductal breast carcinomas with different clinical outcomes. Clin Cancer Res. 2004;10:5988–5997. doi: 10.1158/1078-0432.CCR-03-0731. [DOI] [PubMed] [Google Scholar]
- 12.Pia-Foschini M, Reis-Filho JS, Eusebi V, Lakhani SR. Salivary gland-like tumours of the breast: surgical and molecular pathology. J Clin Pathol. 2003;56:497–506. doi: 10.1136/jcp.56.7.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng M, Geng C, Tang T, Song Z. Mucoepidermoid carcinoma of the breast: four case reports and review of the literature. Medicine (Baltimore) 2017;96:e9385. doi: 10.1097/MD.0000000000009385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan M, Gilmore H, Harbhajanka A. Mucoepidermoid carcinoma of the breast with MAML2 rearrangement: a case report and literature review. Int J Surg Pathol. 2020;28:787–792. doi: 10.1177/1066896920916779. [DOI] [PubMed] [Google Scholar]
- 15.Burghel GJ, Abu-Dayyeh I, Babouq N, Wallace A, Abdelnour A. Mutational screen of a panel of tumor genes in a case report of mucoepidermoid carcinoma of the breast from Jordan. Breast J. 2018;24:1102–1104. doi: 10.1111/tbj.13142. [DOI] [PubMed] [Google Scholar]
- 16.Sherwell-Cabello S, Maffuz-Aziz A, Rios-Luna NP, Pozo-Romero M, Lopez-Jimenez PV, Rodriguez-Cuevas S. Primary mucoepidermoid carcinoma of the breast. Breast J. 2017;23:753–755. doi: 10.1111/tbj.12819. [DOI] [PubMed] [Google Scholar]
- 17.Fujino M, Mori D, Akashi M, Yamamoto H, Aibe H, Matake K, Shirahane K. Mucoepidermoid carcinoma of the breast found during treatment of lymphoma. Case Rep Oncol. 2016;9:806–814. doi: 10.1159/000452792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palermo MH, Pinto MB, Zanetti JS, Ribeiro-Silva A. Primary mucoepidermoid carcinoma of the breast: a case report with immunohistochemical analysis and comparison with salivary gland mucoepidermoid carcinomas. Pol J Pathol. 2013;64:210–215. doi: 10.5114/pjp.2013.38141. [DOI] [PubMed] [Google Scholar]
- 19.Turk E, Karagulle E, Erinanc OH, Soy EA, Moray G. Mucoepidermoid carcinoma of the breast. Breast J. 2013;19:206–208. doi: 10.1111/tbj.12080. [DOI] [PubMed] [Google Scholar]
- 20.Basbug M, Akbulut S, Arikanoglu Z, Sogutcu N, Firat U, Kucukoner M. Mucoepidermoid carcinoma in a breast affected by burn scars: comprehensive literature review and case report. Breast Care (Basel) 2011;6:293–297. doi: 10.1159/000331316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horii R, Akiyama F, Ikenaga M, Iwase T, Sakamoto G. Muco-epidermoid carcinoma of the breast. Pathol Int. 2006;56:549–553. doi: 10.1111/j.1440-1827.2006.02004.x. [DOI] [PubMed] [Google Scholar]
- 22.Gomez-Aracil V, Mayayo Artal E, Azua-Romeo J, Mayayo Alvira R, Azua-Blanco J, Arraiza Goicoechea A. Fine needle aspiration cytology of high grade mucoepidermoid carcinoma of the breast: a case report. Acta Cytol. 2006;50:344–348. doi: 10.1159/000325967. [DOI] [PubMed] [Google Scholar]
- 23.Di Tommaso L, Foschini MP, Ragazzini T, Magrini E, Fornelli A, Ellis IO, Eusebi V. Mucoepidermoid carcinoma of the breast. Virchows Arch. 2004;444:13–19. doi: 10.1007/s00428-003-0923-y. [DOI] [PubMed] [Google Scholar]
- 24.Terzi ASA, Uner A. A 79 year-old woman with a mass in the right breast. Turk J Cancer. 2004;34:38–39. [Google Scholar]
- 25.Tjalma WA, Verslegers IO, De Loecker PA, Van Marck EA. Low and high grade mucoepidermoid carcinomas of the breast. Eur J Gynaecol Oncol. 2002;23:423–425. [PubMed] [Google Scholar]
- 26.Berry MG, Caldwell C, Carpenter R. Mucoepidermoid carcinoma of the breast: a case report and review of the literature. Eur J Surg Oncol. 1998;24:78–80. doi: 10.1016/s0748-7983(98)80135-2. [DOI] [PubMed] [Google Scholar]
- 27.Markopoulos C, Gogas H, Livaditou A, Floros D. Mucoepidermoid carcinoma of the breast. Eur J Gynaecol Oncol. 1998;19:291–293. [PubMed] [Google Scholar]
- 28.Chang LC, Lee N, Lee CT, Huang JS. High-grade mucoepidermoid carcinoma of the breast: case report. Changgeng Yi Xue Za Zhi. 1998;21:352–357. [PubMed] [Google Scholar]
- 29.Luchtrath H, Moll R. Mucoepidermoid mammary carcinoma. Immunohistochemical and biochemical analyses of intermediate filaments. Virchows Arch A Pathol Anat Histopathol. 1989;416:105–113. doi: 10.1007/BF01606314. [DOI] [PubMed] [Google Scholar]
- 30.Pettinato G, Insabato L, De Chiara A, Manco A, Petrella G. High-grade mucoepidermoid carcinoma of the breast. Fine needle aspiration cytology and clinicopathologic study of a case. Acta Cytol. 1989;33:195–200. [PubMed] [Google Scholar]
- 31.Hastrup N, Sehested M. High-grade mucoepidermoid carcinoma of the breast. Histopathology. 1985;9:887–892. doi: 10.1111/j.1365-2559.1985.tb02873.x. [DOI] [PubMed] [Google Scholar]
- 32.Leong AS, Williams JA. Mucoepidermoid carcinoma of the breast: high grade variant. Pathology. 1985;17:516–521. doi: 10.3109/00313028509105513. [DOI] [PubMed] [Google Scholar]
- 33.Ratanarapee S, Prinyar-Nussorn N, Chantarakul N, Pacharee P. High-grade mucoepidermoid carcinoma of the breast. A case report. J Med Assoc Thai. 1983;66:642–648. [PubMed] [Google Scholar]
- 34.Kovi J, Duong HD, Leffall LS. High-grade mucoepidermoid carcinoma of the breast. Arch Pathol Lab Med. 1981;105:612–614. [PubMed] [Google Scholar]


