Abstract
Background: Undifferentiated carcinoma with osteoclast-like giant cells (UCOGC) of the pancreas is a very rare variant of pancreatic malignant neoplasm. It is regarded as a highly aggressive tumor with a worse prognosis than conventional pancreatic ductal adenocarcinoma. Case presentation: A 54-year-old male patient presented with 3-month recurrent epigastric distress. Computed tomography of the abdomen showed a large cystic mass in the distal pancreas. On macroscopic examination, the lesion had numerous multiloculated cystic cavities. Microscopically, the tumor predominantly comprised a considerable number of evenly distributed non-neoplastic osteoclast-like giant cells and a few neoplastic pleomorphic cells. Although extensive histologic sampling was conducted, a classic ductal adenocarcinoma component was not identified. The patient received no further treatment after his surgery and has been doing well with no evidence of recurrence or metastasis for >7 years. Conclusions: Our results suggest that pure UCOGC has a significantly better prognosis and supports that pure UCOGC may represent a biologically distinct variant of pancreatic carcinoma and it should be separated from other undifferentiated pancreatic carcinomas.
Keywords: Undifferentiated carcinoma, pancreas, osteoclast-like giant cells, prognosis
Background
Giant cell tumors of the pancreas are rare neoplasms; they were originally described by Rosai [1]. Historically, they were classified into three histopathologic types: osteoclastic, pleomorphic and a mixture of the two [1-3]. At the benign end of the spectrum, osteoclast-type giant cell tumors (OGCTs) are characterised by osteoclast-like giant cells and mononuclear stromal cells identical to those observed in giant cell tumors of bone. At the malignant end of the spectrum, pleomorphic giant cell tumors of the pancreas are highly anaplastic neoplasms consisting of bizarre pleomorphic mononucleated and multinucleated giant cells. Further, a combination of these two cell types defines a third type.
The current World Health Organization (WHO) classification groups the three aforementioned types into a single category, undifferentiated carcinoma with osteoclast-like giant cells (UCOGC) [4]. UCOGC is different from plain undifferentiated carcinoma and is regarded as a highly aggressive tumor with an even worse prognosis than that of conventional pancreatic ductal adenocarcinoma [4]. Owing to the rarity of this tumor, its clinicopathologic features and prognosis remain elusive.
Here, we present a case of UCOGC in a 54-year-old male; the tumor did not harbor ductal adenocarcinoma despite extensive sampling. The patient received no adjuvant therapy after his surgery and has been well without disease recurrence or metastasis 7 years after his surgery. Our results are consistent with recent studies that have reported a survival of ≥5 years for such patients [3,5-7]. We hope, with more such cases are reported in the literature, the clinicopathologic features and prognosis of UCOGC will become clear so that patients will be treated accordingly.
Case presentation
A 54-year-old male was admitted to the department of gastroenterology in our hospital due to recurrent epigastric distress, nausea, and edema of the lower limbs for 3 months. He had no significant past medical history or family history. Physical examination revealed a vaguely palpable abdominal mass with mild and deep tenderness. A laboratory examination at the admission only revealed mild anemia with a hemoglobin level of 10.2 g/dL.
A computed tomography (CT) scan of the abdomen revealed a large cystic lesion measuring 20.4 × 15.5 × 9.0 cm in size in the tail of the pancreas (Figure 1). Laparotomy revealed that this large, distally located, pancreatic cystic lesion partially closely adhered to the transverse colon and the spleen. There was lymphadenopathy. The patient underwent subtotal pancreatectomy, splenectomy, and segmental resection of the transverse colon.
Figure 1.
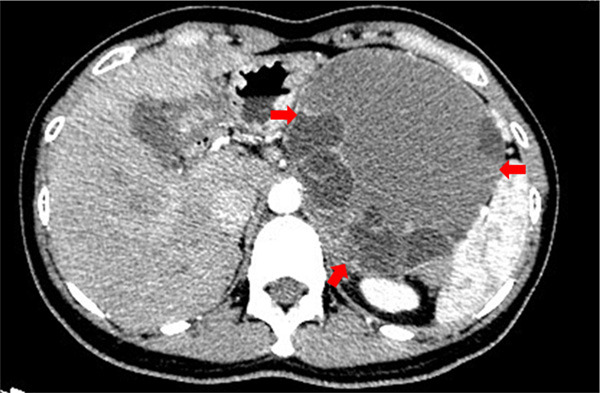
Abdominal computed tomography showing a large cystic lesion in the distal pancreas as indicated by the red arrows.
Macroscopically, the mass was encapsulated, multiloculated, and comprised numerous cysts. The capsule was thick and rough. The cysts contained bloody fluid and necrosis were observed on the cut surface. The mass was adherent to the transverse colon and the spleen. The tumor was extensively sampled for histology.
Histologic examination revealed that the tumor comprised numerous, evenly distributed, non-neoplastic osteoclast-like giant cells and few neoplastic pleomorphic cells (Figure 2A). Extensive hemorrhage was present. The osteoclast-like giant cells contained multiple, relatively uniform and bland nuclei and abundant eosinophilic cytoplasm. The neoplastic pleomorphic cells were medium-sized and contained round to spindle-shaped single nuclei. About 2 mitotic figures were observed per 10 high-power fields in the pleomorphic cells. Although extensive histologic sampling was performed, classic ductal adenocarcinoma components were not identified. In addition, the macroscopic impression of necrosis was confirmed to be old hemorrhage. Histologic examination confirmed that the tumor was confined in the pancreas, and had no invasion into the transverse colon and spleen. By immunohistochemistry, the osteoclast-like giant cells were positive for CD68 but negative for cytokeratin (Figure 2B and 2C). The neoplastic pleomorphic cells were focally and weakly positive for cytokeratin (Figure 2C) and had a low Ki-67 labeling index (<5%) (Figure 2D). The final diagnosis was UCOGC according to the current WHO Blue Book series. All surgical resection margins were negative. Eleven lymph nodes retrieved from the resection specimen were negative for tumor. Appropriate imaging studies did not reveal metastases. The final pathologic stage of the tumor was Union for International Cancer Control T3N0M0 stage IIa.
Figure 2.
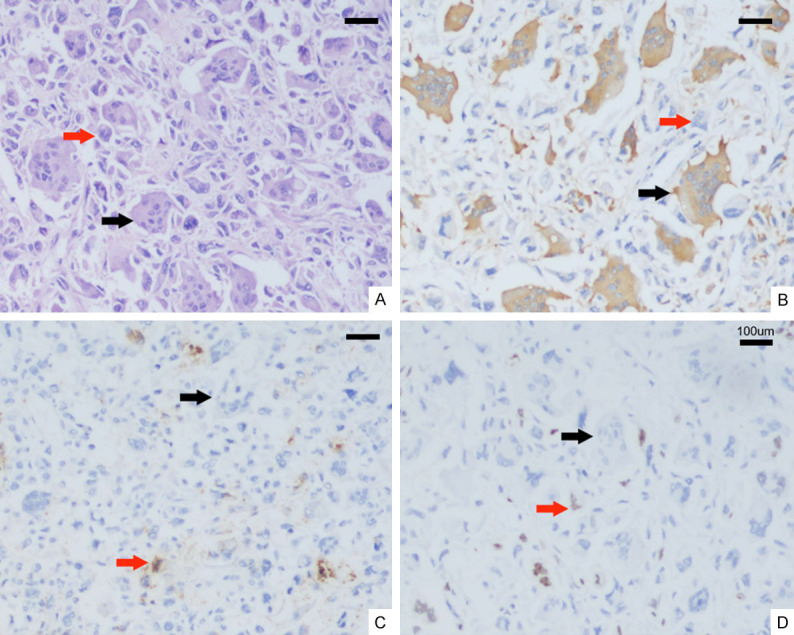
Hematoxylin-eosin and immunohistochemical findings of UCOGC. Red arrow: Neoplastic pleomorphic cells. Black arrow: Non-neoplastic osteoclast-like giant cells. A: A few neoplastic pleomorphic cells and considerable number of evenly distributed osteoclast-like giant cells in this microscopic field (×200). B: Strong CD68 expression in osteoclast-like giant cells (×200). C: Focally weak cytokeratin (CK) staining in neoplastic pleomorphic cells; negative CK staining in osteoclast-like giant cells (×200). D: Low Ki-67 staining (<5%) in neoplastic pleomorphic cells; negative Ki-67 staining in osteoclast-like giant cells (×200). Scale bars = 100 µm.
The patient’s postoperative course was uneventful. He was discharged home on postoperative day 10. He refused chemotherapy or radiotherapy. The patient has been doing well with no evidence of recurrence or metastasis for >7 years.
Discussion
Undifferentiated carcinoma with osteoclast-like giant cells (UCOGC) of the pancreas was not widely recognized until it was included in the current WHO classification; it is now regarded as a highly aggressive tumor [4]. Multiple terms have been used previously for UCOGC, such as pancreatic giant cell tumour, pancreatic osteoclastoma, mixed pleomorphic-osteoclast-like tumour of the pancreas, and osteoclastic and pleomorphic giant cell tumours of the pancreas [1-3,8-11]. UCOGC comprises a wide spectrum of tumors originating from cells ranging from mononuclear stromal cells to pleomorphic/anaplastic giant cells with osteoclast-like giant cells. Additionally, UCOGC often coexists with ductal adenocarcinomas or mucinous cystic neoplasms [3,5,12]. Currently, there are no criteria regarding the amount of the concomitant component, e.g. conventional ductal adenocarcinoma component, allowed in UCOGC [3].
Owing to the rarity of UCOGC, there are insufficient data on the clinicopathologic characteristics or prognosis of this tumor. The prognosis of UCOGC ranges from several months to >10 years [6]. Kobayashi et al. demonstrated that patients with UCOGC in the short-term survivor group had concomitant components of ductal adenocarcinoma, positive lymph node metastasis, and smaller tumor size [6]. The survival rates of patients with histologically pure UCOGC tend to be significantly higher than those of patients with an associated adenocarcinoma [3,5]. This finding highlights the importance of extensive histologic sampling to exclude associated conventional ductal adenocarcinoma [11,12]. The morphologic criteria of osteoclasts is important. For example, large, multinucleated, pleomorphic, giant cells that mimic osteoclasts with intracellular neutrophils or eosinophils are not in fact osteoclasts [3]. In addition, micropapillary carcinoma clusters can mimic osteoclastic cells and pose diagnostic challenges [3]. It has been speculated that the good prognosis in pure UCOGC may be due to the considerable chemotaxis of osteoclastic giant cells leading to a strong immune response [5]. The characteristics and treatment of UCOGC that have been described in the literature are summarized in Table 1.
Table 1.
Characteristics and survival in patients with osteoclast-like giant cell-containing pancreatic undifferentiated carcinoma
| Reference | Gender/Age, years | Location | Maximal tumor dimension, cm | Associated with AC | IHC | Therapy | Survival, months | Invasion of other organ | lymph node | LVI | Resection margin |
|---|---|---|---|---|---|---|---|---|---|---|---|
| F/82 | tail | 13 | No | NA | NA | >4 | NA | Neg | NA | NA | |
| 1 | F/74 | head | 10 | No | NA | NA | >10 | NA | Neg | Yes | NA |
| 2 | M/60 | body, tail | 14 | No | GC: vimentin(+), syn(+), CK(-), EMA(-) | NA | 4 | NA | Neg | NA | NA |
| 6 | F/37 | head | 4 | Yes | GC: vimentin(+), p53(-), AC: p53(+) | Gemcitabine | >66 | NA | Neg | NA | No |
| 7 | F/61 | tail | 10.4 | NA | NA | Gemcitabine | >72 | Left diaphragm | Neg | Yes | No |
| 9 | F/55 | body | 2.5 | Yes | PC&MC: CK(+), viminetin(+), OGC: vimentin(-), AC: CA199(+), CK(+) | NA | 3 | Liver | Neg | NA | NA |
| 9 | F/71 | head | 4.5 | NA | OGC&PGC: EMA(-), vimentin(-), CK(-)desmin(-), SMA(-) | NA | 2 | No | Neg | Yes | NA |
| 9 | M/50 | body | 7 | NA | OGC: CK(-), CD68(+), PGC: CK(+), CD68(-) | NA | 7 | NA | NA | NA | NA |
| 10 | F/72 | head | 6.5 | No | NA | NA | Dead 10 days after admission | Liver, adrenal gland | NA | NA | NA |
| 11 | F/56 | tail | 24 | Yes (focal) | vimentin(+), EMA(-) | Radiation | >6 | NA | NA | Yes | NA |
| 12 | F/68 | neck, body | 6 | No | PC&MC: CK8/18(+), CK19(+), vimentin(+), ki-67(+, 30%); OGC: CD68(+), vimentin(+), CK8/18(-), CK19(-), ki-67(-) | Gemcitabine | 10 | No | Neg | No | No |
| 13 | M/49 | head | 2 | Yes | OGC, MC: CD68(+), AE1/AE3(-) | NA | NA | No | NA | NA | NA |
| 13 | F/68 | body, tail | 14 | No | OGC, MC: CD68(+), AE1/AE3(-) | NA | NA | Liver | NA | NA | NA |
| 13 | M/51 | head | 3.8 | Yes | PC: CD68(-), AE1/AE3(-) | NA | NA | NA | Pos | NA | NA |
| Present case | M/54 | tail | 20.4 | No | OGC: CD68(+), PC: focal weak CK(+), ki-67(+, <5%) | No | >84 | No | Neg | No | Neg |
Note: AC: adenocarcinoma; CK: cytokeratin; EMA: epithelial membrane antigen; F: female; GC: giant cell; IHC: immunohistochemistry; LVI: lymphovascular invasion; M: male; MC: mononuclear cell; NA: not available; Neg: negative; OGC: osteoclast-like giant cell; PC: pleomorphic cell; PGC: pleomorphic giant cell; Pos: positive; Ref: reference; SMA: smooth muscle actin; Syn: synaptophysin.
The histogenesis of OGCT has been debated. Sakai et al. performed microscopic, immunohistochemical, and K-ras gene mutation analyses using a microdissection approach to clarify the origin of neoplastic pleomorphic cells and non-neoplastic osteoclast-like giant cells [13]. They observed that non-neoplastic osteoclast-like giant cells lacked K-ras gene mutations, suggesting that they were of mesenchymal origin, whereas neoplastic pleomorphic cells harboured K-ras gene mutations, probably because they were derived from epithelial cells [13]. Recent research has reported similar genetic alterations in UCOGC and conventional pancreatic ductal adenocarcinoma, including activating mutations of K-ras gene and inactivating mutations of CDKN2A, P53 and Smad4, respectively; however, the study did not specifically examine the molecular changes in the pleomorphic tumor cells and osteoclast-like giant cells [5].
Here, we report a case of pure UCOGC in a 54-year-old man with >7 year disease-free survival after curative surgery. Our current case, along with previously reported cases, suggests that UCOGC lacking a ductal adenocarcinoma component has a significantly better prognosis than what is implied in the current WHO blue book. Wide recognition and additional reports of this entity in the near future or a multicentric study using a more uniform definition for osteoclast-like giant cells, and precise molecular analysis of pleomorphic tumor cells and osteoclast-like giant cells may shed light on the characteristics, and confirm the good prognosis of a subset of UCOGCs.
Acknowledgements
We appreciate the assistance of Feng Yang of the Department of Pathlogy, Anhui Medical University for his technical help. We also thank Dr. Chuan-Ye Xu from the Department of Gastroenterology, The First Affiliated Hospital of Anhui Medical University for his information about the clinical treatment of the patient.
Disclosure of conflict of interest
None.
Abbreviations
- CT
Computed tomography
- OGCTs
Osteoclast-type giant cell tumors
- UCOGC
Undifferentiated carcinoma with osteoclast-like giant cells
- WHO
World Health Organization
References
- 1.Rosai J. Carcinoma of pancreas simulating giant cell tumor of bone. Electron-microscopic evidence of its acinar cell origin. Cancer. 1968;22:333–344. doi: 10.1002/1097-0142(196808)22:2<333::aid-cncr2820220210>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 2.Lewandrowski KB, Weston L, Dickersin GR, Rattner DW, Compton CC. Giant cell tumor of the pancreas of mixed osteoclastic and pleomorphic cell type: evidence for a histogenetic relationship and mesenchymal differentiation. Hum Pathol. 1990;21:1184–1187. doi: 10.1016/0046-8177(90)90157-z. [DOI] [PubMed] [Google Scholar]
- 3.Muraki T, Reid MD, Basturk O, Jang KT, Bedolla G, Bagci P, Mittal P, Memis B, Katabi N, Bandyopadhyay S, Sarmiento JM, Krasinskas A, Klimstra DS, Adsay V. Undifferentiated carcinoma with osteoclastic giant cells of the pancreas: clinicopathologic analysis of 38 cases highlights a more protracted clinical course than currently appreciated. Am J Surg Pathol. 2016;40:1203–1216. doi: 10.1097/PAS.0000000000000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosman F, Carneiro F, Hruban R, Theise N. WHO classification of tumours of the digestive system. Lyon: International Agency for Research on Cancer; 2010. pp. 541–542. [Google Scholar]
- 5.Luchini C, Pea A, Lionheart G, Mafficini A, Nottegar A, Veronese N, Chianchiano P, Brosens LA, Noë M, Offerhaus GJA, Yonescu R, Ning Y, Malleo G, Riva G, Piccoli P, Cataldo I, Capelli P, Zamboni G, Scarpa A, Wood LD. Pancreatic undifferentiated carcinoma with osteoclast-like giant cells is genetically similar to, but clinically distinct from, conventional ductal adenocarcinoma. J Pathol. 2017;243:148–154. doi: 10.1002/path.4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobayashi S, Nakano H, Ooike N, Oohashi M, Koizumi S, Otsubo T. Long-term survivor of a resected undifferentiated pancreatic carcinoma with osteoclast-like giant cells who underwent a second curative resection: a case report and review of the literature. Oncol Lett. 2014;8:1499–1504. doi: 10.3892/ol.2014.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saito H, Kashiyama H, Murohashi T, Sasaki K, Misawa R, Ohwada S. Case of six-year disease-free survival with undifferentiated carcinoma of the pancreas. Case Rep Gastroenterol. 2016;10:472–478. doi: 10.1159/000448878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gocke CD, Dabbs DJ, Benko FA, Silverman JF. KRAS oncogene mutations suggest a common histogenetic origin for pleomorphic giant cell tumor of the pancreas, osteoclastoma of the pancreas, and pancreatic duct adenocarcinoma. Hum Pathol. 1997;28:80–83. doi: 10.1016/s0046-8177(97)90283-5. [DOI] [PubMed] [Google Scholar]
- 9.Imai Y, Morishita S, Ikeda Y, Toyoda M, Ashizawa T, Yamamoto K, Inoue T, Ishikawa T. Immunohistochemical and molecular analysis of giant cell carcinoma of the pancreas: a report of three cases. Pancreas. 1999;18:308–315. doi: 10.1097/00006676-199904000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Jalloh SS. Giant cell tumour (osteoclastoma) of the pancreas--an epithelial tumour probably of pancreatic acinar origin. J Clin Pathol. 1983;36:1171–1175. doi: 10.1136/jcp.36.10.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Temesgen WM, Wachtel M, Dissanaike S. Osteoclastic giant cell tumor of the pancreas. Int J Surg Case Rep. 2014;5:175–179. doi: 10.1016/j.ijscr.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiarelli M, Guttadauro A, Gerosa M, Marando A, Gabrielli F, De Simone M, Cioffi U. An indeterminate mucin-producing cystic neoplasm containing an undifferentiated carcinoma with osteoclast-like giant cells: a case report of a rare association of pancreatic tumors. BMC Gastroenterol. 2015;15:161. doi: 10.1186/s12876-015-0391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakai Y, Kupelioglu AA, Yanagisawa A, Yamaguchi K, Hidaka E, Matsuya S, Ohbuchi T, Tada Y, Saisho H, Kato Y. Origin of giant cells in osteoclast-like giant cell tumors of the pancreas. Hum Pathol. 2000;31:1223–1229. doi: 10.1053/hupa.2000.18491. [DOI] [PubMed] [Google Scholar]


